- 1Fourth Department of Liver Disease, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 2Beijing Municipal Key Laboratory of Liver Failure and Artificial Liver Treatment Research, Beijing, China
Introduction: Extracellular vesicles (EVs) carrying functional cargoes are emerging as biomarkers and treatment strategies in multiple liver diseases. Nevertheless, the potential of EVs in liver failure remains indistinct. In this systematic review, we comprehensively analyzed the potential of EVs as biomarkers of liver failure and the therapeutic effects and possible mechanisms of EVs for liver failure.
Methods: We conducted a systematic review by comprehensively searching the following electronic databases: PubMed, Web of Science, Embase and Cochrane Central Register of Controlled Trials from inception to March 2022. The used text words (synonyms and word variations) and database-specific subject headings included “Extracellular Vesicles”, “Exosomes”, “Liver Failure”, “Liver Injury”, etc.
Results: A total of 1479 studies were identified. After removing 680 duplicate studies and 742 irrelevant studies, 57 studies were finally retained and analyzed. Fourteen studies revealed EVs with functional cargoes could be used to make the diagnosis of liver failure and provide clues for early warning and prognostic assessment of patients with liver failure. Forty-three studies confirmed the administration of EVs from different sources alleviated hepatic damage and improved survival through inhibiting inflammatory response, oxidative stress as well as apoptosis or promoting hepatocyte regeneration and autophagy.
Conclusions: EVs and their cargoes can be used not only as superior biomarkers of early warning, early diagnosis and prognostic assessments for liver failure, but also as potentially effective treatment options for liver failure. In the future, large-scale studies are urgently needed to verify the diagnostic, predictive and therapeutic value of EVs for liver failure.
1 Introduction
Liver failure is a severe liver disease syndrome caused by multiple precipitating factors, which accompanies by grievous liver dysfunction or decompensation. The typical clinical manifestations of this syndrome include jaundice, coagulation dysfunction, ascites, hepatorenal syndrome, and hepatic encephalopathy (1, 2). At present, effective treatment for liver failure is lacking except for liver transplantation, although it still faces challenges of graft rejection, high cost and donor shortage. The transplant-free survival in patients with liver failure is rather low, usually less than 50% (3–6). Especially, diverse etiologies, complex clinical manifestations, undefined pathogenesis, high mortality and lack of effective treatments make it more difficult to accurately diagnose and properly treat patients with liver failure. In this context, it is extremely important to seek candidate biomarkers for early warning, early diagnosis and prognostic assessment of liver failure (7, 8). On the other hand, exploring potentially effective therapies for liver failure is also essential for improving survival rate, which is the scientists have been working on all the time (9–11).
Extracellular vesicles (EVs) are nanoscale vesicles with proteolipid bilayers. They can be secreted by almost all cells into the extracellular milieu, which makes them widely distributed in various biological fluids, such as blood, urine, milk, sputum, ascites, and cerebrospinal fluid (12). EVs are broadly divided into two main subgroups depending on their biogenesis: exosomes (Exos, 50-150 nm in diameter) and microvesicles or microparticles (MVs or MPs, 50-500 nm in diameter, up to 1000 nm) (13, 14). EVs have been considered metabolic waste. Over the past decade, scientists have demonstrated that EVs contain various bioactive substances or signal transduction molecules that exert crucial roles in homeostasis maintenance, antigen presentation, gene regulation, and so on (15, 16). Considering that EVs can reflect the pathophysiological state of the cells from which they are derived, EVs have the great potential to emerge as non-invasive biomarkers for early diagnosis and prognosis assessment of liver diseases (17–21). In addition, EVs possess the following advantages: stable membrane structure, low immunogenicity, good histocompatibility, easy chemical and genetic programming. Therefore, it is feasible to utilize EVs as drugs or drug carriers to treat refractory diseases such as cancer, neurological and cardiovascular disorders (22–24).
So far, there isn’t a universal ideal animal model for liver failure in view of multiple etiologies and complex mechanisms related to this syndrome (25, 26). Currently available animal models of liver failure have their strengths and weaknesses. Animal models of acute liver failure (ALF) enrolled in the present systematic review mainly involve hepatotoxic drug-induced, surgically induced, and mixed models. Virus-induced ALF models are rare and unsatisfactory (27). The commonly used drugs and chemical reagents for ALF induction include D-galactosamine (D-GalN) with or without lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-α), acetaminophen (APAP), carbon tetrachloride (CCl4), concanavalin A (Con A) and thioacetamide (TAA) (28, 29). The surgical ALF models can be divided into hepatectomy (total or partial), devascularization (total or partial) and a combination of both (30, 31). Hepatic ischemia-reperfusion injury (IRI), as an inevitable local sterile inflammatory response following surgery, is one of the main causes of early organ dysfunction and failure after liver transplantation (32). And hepatic IRI model accounts for a large proportion of surgically induced ALF models. Animal models of acute-on-chronic liver failure (ACLF) are usually induced by acute insult [such as LPS and/or D-GaIN, or ethyl alcohol (EtOH)] in the setting of chronic liver injury (for example, CCl4 or bile duct ligation) (33).
Till now, studies on EVs as biomarkers or therapeutic options for liver failure are still in the infancy. This systematic review summarized the current available studies including preclinical animal studies and clinical studies in this field, and hopes to provide novel ideas and directions for the early warning, diagnosis, treatment and prognostic assessment of liver failure.
2 Methods
Our systematic review was prepared according to the Cochrane recommendations for study methodology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (34).
2.1 Literature search strategy
The search strategy was developed by two informatics specialists. We comprehensively and systematically searched the following electronic databases: PubMed, Web of Science, Embase, and Cochrane Central Register of Controlled Trials from inception to March 2022. The used text words (synonyms and word variations) and database-specific subject headings included the followings: “Extracellular Vesicles”, “Exosome”, “Liver Failure”, “Liver Injury”, and so on. The search strategy for PubMed was summarized in Table S1, and it was also applicable to all other databases.
2.2 Eligibility criteria
We enrolled the studies according to the following criteria: (1) studies on EVs or modified EVs. (2) studies on preclinical or clinical studies of liver failure. (3) studies on EVs as potential biomarkers and treatment options for liver failure.
We excluded the studies according to the following criteria: (1) studies unrelated to EVs. (2) studies on the application of EVs in diseases other than liver failure and liver injury we described in the Introduction. (3) conference abstracts, reviews, mechanism studies, non-English articles and in vitro studies.
2.3 Data extraction
The following data were extracted from each study: experimental model, EV source, EV separation techniques, EV administration including dosage and route, EV cargoes, EV functions and others (See Tables 1–6 for details). To select articles potentially eligible for inclusion, two authors independently screened the title and abstract of each article, then reviewed the full texts of all retaining studies and analyzed the related information. The divergence between these two authors was judged by the corresponding author. We did not conduct a meta-analysis due to the limited number of available randomized controlled trials. All included studies recruited patients that provided informed consent before enrollment.
2.4 Quality assessment
We used the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool to evaluate the risk of bias for preclinical studies (94). And the risk of bias graph was drawn using the RevMan 5.3.2 software provided by the Cochrane Collaboration Network. The risk of bias for clinical studies was assessed using the Newcastle-Ottawa scale (NOS). The Confidence in the Evidence from Reviews of Qualitative research (CERQual) tool was used to assess the evidence quality of outcomes in this systematic review (95). The quality assessment was done independently by two authors and the divergence between these two authors was judged by the corresponding author. A PRISMA figure was created.
3 Results
3.1 Literature selection
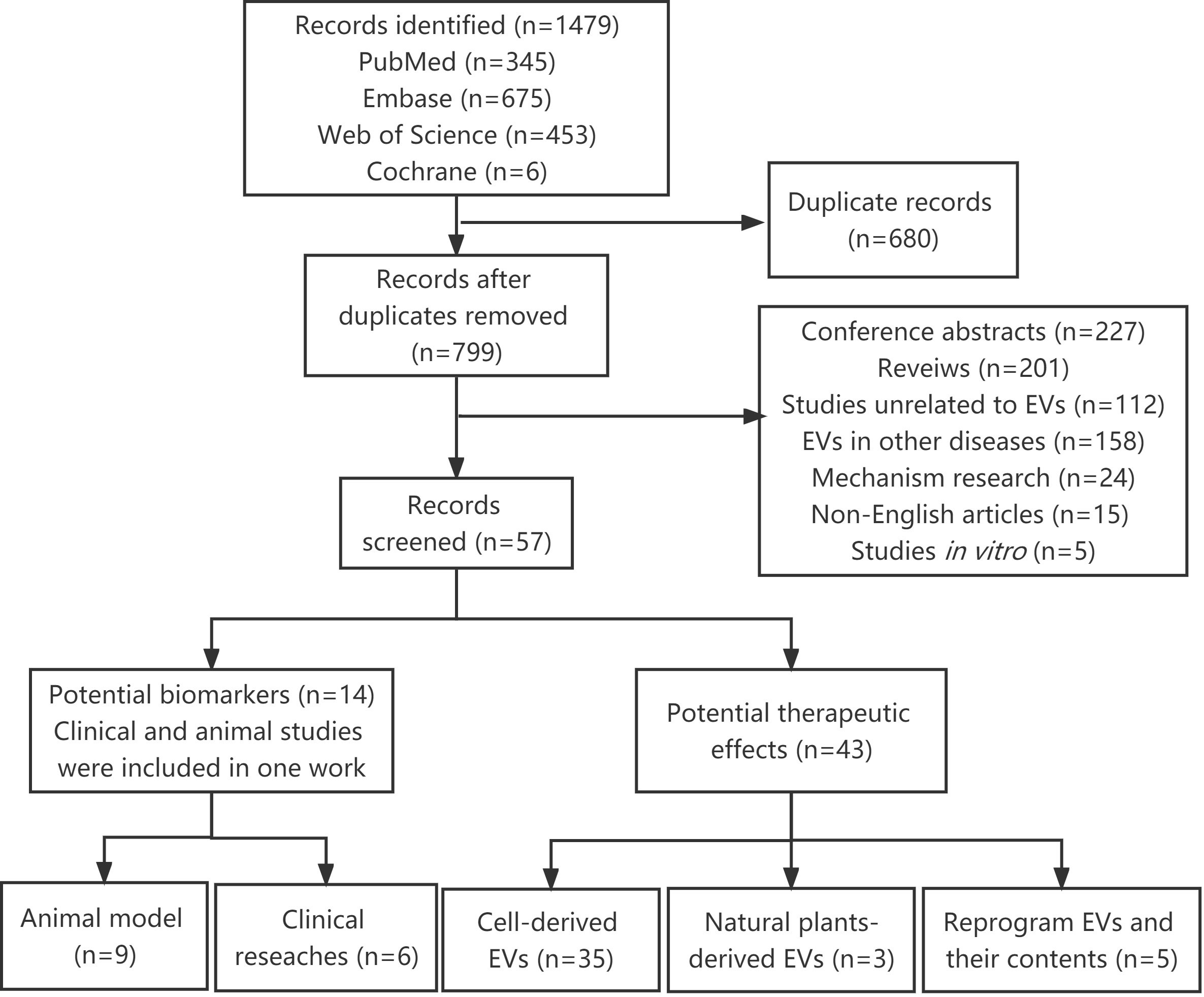
A total of 1479 articles were identified using our search strategy. After removing 680 duplicates, 799 articles were submitted to the title, abstract or full-text assessment by two independent authors. Among these, 742 irrelevant articles were further excluded, including conference abstracts, reviews, studies unrelated to EVs, EVs in other diseases, mechanism research, non-English articles and in vitro studies. Finally, 57 articles were retained and analyzed in this systematic review (Figure 1).
3.2 EVs as potential biomarkers for early warning, diagnosis and prognostic assessment of liver failure
We summarized the studies on EVs as potential biomarkers of liver failure. Tables 1, 2 listed the relevant preclinical and clinical studies, respectively. This review revealed that EVs not only could be utilized as the early warning or diagnosis markers of liver failure, but also as biomarkers for prognostic assessment of this syndrome.
3.2.1 EVs as potential biomarkers for early warning or diagnosis of liver failure
3.2.1.1 Preclinical studies
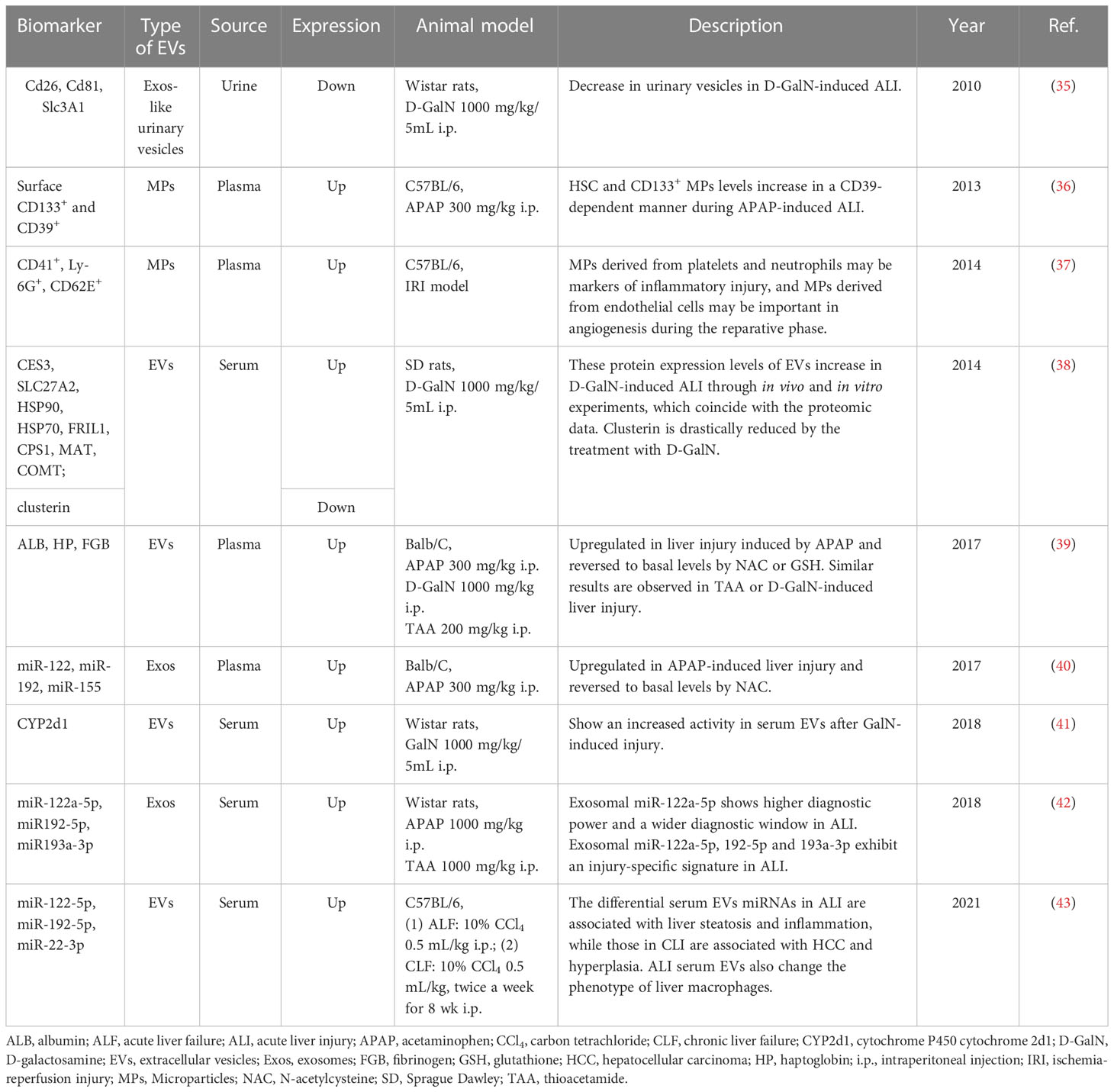
Conde-Vancells J et al. investigated the proteome of urinary vesicles derived from D-GalN-treated rats and attempted to identify potential biomarkers for acute liver injury (ALI). They found several proteins normally present in urinary vesicles including CD26, SLC3A1 and CD81 were dramatically reduced in urinary samples obtained from D-GalN-treated rats. And the authors believed that these three proteins had the potential to be used as candidate non-invasive urinary indicators of acute liver damage (35). CD133 and CD39 are expressed by hematopoietic stem cells (HSCs) and mobilized after liver injury. In APAP-induced experimental ALI, HSC and plasma CD133+ MPs levels were increased in a CD39-dependent manner. And differentially increased plasma CD39+ CD133+ MPs were helpful for monitoring critically ill patients with hepatic dysfunction and identifying patients who urgently needed for liver transplantation (36). In IRI, platelet- and neutrophil-derived MPs were acutely elevated following injury, and could serve as markers of inflammatory injury. In contrast, MPs derived from endothelial cells increased after injury response during the reparative phase, suggesting angiogenesis in the regenerating liver. Hence, MPs might be regarded as markers of acute inflammatory injury or regeneration in IRI (37). Eva Rodríguez-Suárez et al. analyzed the proteome of EVs derived from primary hepatocytes, and they found CES3, SLC27A2, HSP90, HSP70, FRIL1, CPS1, MAT and COMT increased in D-GalN-induced ALI through in vivo and in vitro experiments (38). Some unique proteins in EVs reflect the identity and tissue-specific origin of EVs. For instance, liver-specific proteins such as CES1, ADH1, GST, APOA1, ALB, HP and FGB in the EVs increased after hepatotoxin-induced liver injury. And ALB and HP in the circulating EVs were also confirmed to be increased in the alcohol-induced liver injury of the rodent model (39). Cytochrome P450 2D1 was also upregulated in hepatic EVs after GalN-induced injury, and this hepatocyte-specific enzyme could serve as a novel candidate marker to specifically detect and follow DILI (41).
Recently, exosomal miRNAs have emerged as promising biomarkers with diagnostic value. Receiver operating characteristic (ROC) analysis revealed exosomal miR-122a-5p exhibited superior diagnostic performance with an earlier diagnostic potential and a wider diagnostic time window compared to the corresponding serum counterpart in two animal models of ALI. In addition, exosomal miRNAs showed a higher correlation with ALT activity. Notably, exosomal miRNAs-122a-5p, 192-5p and 193a-3p manifested an injury-specific signature in ALI, and could be used not only as diagnostic tools but also to differentiate between different etiologies of hepatic injury (42). Furthermore, the levels of liver-specific miRNAs such as miR-122, miR-192 and miR-155 in circulating Exos were reported to be elevated in APAP-induced liver injury, but significantly decreased and returned to basal levels after treatment with antioxidant N-acetyl-cysteine (NAC), suggesting the levels of exosomal miR-122, miR-192 and miR-155 mirrored the severity of hepatocyte damage and might be used as potential sensitive diagnostic biomarkers for liver injury. High levels of circulating miRNAs are produced within certain cells in a tissue-specific manner, making them good candidate biomarkers for particular types of tissue injury. In this regard, nine miRNAs were identified as signatures of ALI. Of which, five miRNAs (miR-21a-5p, miR-92a-3p, miR-194-5p, miR-17-5p and miR-19b-3p) were increased, four miRNAs (miR-451a, miR-27a-3p, miR-26a-5p and miR-223-3p) were decreased (43).
3.2.1.2 Clinical studies
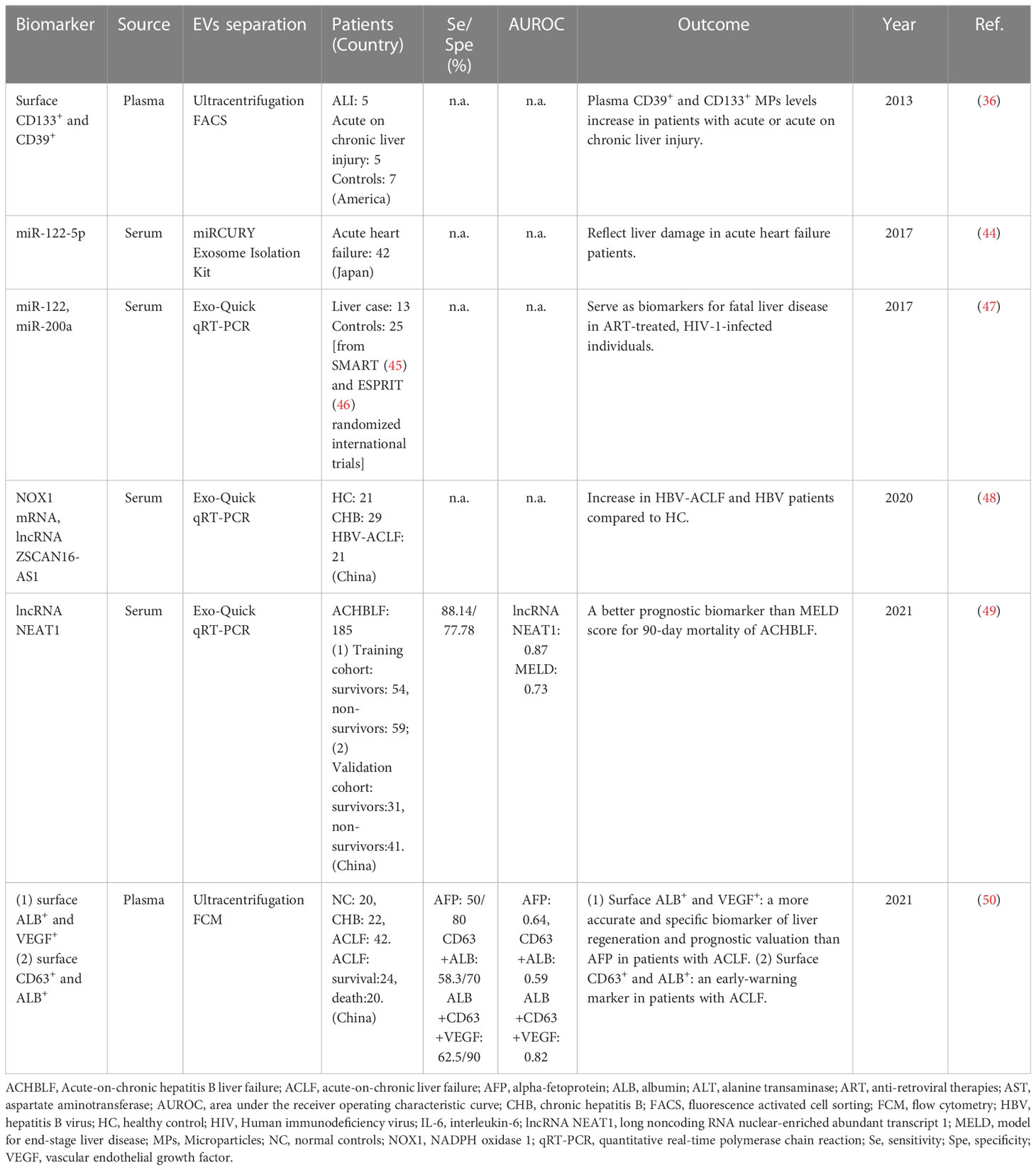
ACLF is defined as an acute deterioration of liver function in patients with chronic liver diseases. It is a life-threatening clinical syndrome with a high mortality of 50–90%. Early diagnosis and recognition of patients who will die without liver transplantation are vitally important. Chen JJ and colleagues investigated differentially expressed messenger RNAs (mRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs)in circulating Exos from patients with ACLF using RNA sequencing. They found higher lncRNA but less circRNA was expressed in HBV-ACLF patients. NADPH oxidase 1 (NOX1) mRNA and lncRNA ZSCAN16-AS1 were highly expressed in patients with HBV-ACLF, and the expression levels of them were positively correlated with the progression or severity of liver injury (48). In addition, CD39+CD133+ MPs were elevated in patients with acute-on-chronic liver decompensation, suggesting acute liver insults and/or acute deterioration of liver function in the setting of chronic liver injury (36).
3.2.2 EVs as potential biomarkers for prognostic assessment of liver failure
A prospective study evaluated the predictive value of serum exosomal long noncoding RNA nuclear-enriched abundant transcript 1 (LncRNA NEAT1) for 90-day mortality in acute-on-chronic hepatitis B liver failure (ACHBLF). The results displayed that lncRNA NEAT1 levels were higher in non-survivors than survivors. In the training cohort, lncRNA NEAT1 was an independent predictor for 90-day mortality of ACHBLF. Meanwhile, lncRNA NEAT1 showed a significantly higher area under the curve of receiver operating characteristic (AUC) than the MELD score in the training and validation cohort. ACHBLF patients with lncRNA NEAT1 levels above 1.92 showed poorer survival conditions than those below. Thus, serum exosomal lncRNA NEAT1 might be a better prognostic biomarker than the MELD score for 90-day mortality in ACHBLF (49). On the other hand, the assessment of liver regeneration is particularly critical for predicting prognosis and improving the quality of life in ACLF patients. Jiao Y et al. reported that the percentage of Exos with ALB, CD63 and VEGF increased in CHB, but decreased in ACLF. Among ACLF patients, the Exos with ALB, CD63 and VEGF were significantly more in the survival group than the dead group. The sensitivity and specificity of Exos with CD63, ALB and VEGF were significantly higher than other markers of liver regeneration and prognostic valuation including AFP. Therefore, the Exos with ALB and VEGF might be more accurate and specific biomarkers of liver regeneration and prognostic valuation than AFP in patients with ACLF (50).
3.2.3 EVs as potential biomarkers for reflecting the severity of hepatic damage in other diseases
In some cases, liver disease is one of the main contributors to the increased morbidity and mortality in other severe diseases. Under these circumstances, circulating miRNAs might be used to reflect liver damage and develop risk assessment. For example, a prospective, observational study reported serum miR-122-5p levels were significantly positively correlated with serum liver function markers in the setting of acute heart failure (44). In anti-retroviral therapies (ART)-treated, HIV-1-infected individuals, circulating levels of miR-122 and miR-200a were elevated in HIV/HCV co-infected individuals, compared to HIV mono-infected individuals. Especially, higher pre-ART levels of circulating miR-122 and miR-200a were noticed in HIV-1 positive individuals who died from liver-related diseases whilst undergoing suppressive ART, compared to matched controls. Thus, circulating miR-122 and miR-200a were considered as promising predictive biomarkers for severe liver disease in the ART-treated, HIV-1-infected populations (47).
3.3 EVs as the potential treatment option for liver failure/injury
Tables 3, 4 listed the studies which exhibited the therapeutic potential of EVs for liver failure or liver injury induced by hepatotoxic drugs and surgery (mainly hepatic IRI), respectively. EVs derived from stem cells were most frequently reported to have therapeutic potential for liver failure or liver injury, including EVs from the human umbilical cord (blood) mesenchymal stem cells [hUC(B)MSCs], adipose-derived stem cells [A(D)SCs], and bone marrow-derived mesenchymal stem cells (BM-MSCs). Moreover, EVs derived from human menstrual blood-derived stem cells (MenSCs) (54), human-induced pluripotent stem cell-derived MSCs (hiPSC-MSCs) (67, 68) and human liver stem cells (HLSCs) (82) were also documented to protect against liver failure. Other sources of EVs included: hepatocytes (65, 66, 83), dendritic cells (DCs) (71), normal or damaged liver tissues (64) and red blood cells (RBCs) (90, 92).
The therapeutic effects of EVs on liver failure were investigated only using rodent models. Overall, different EV therapies were documented to alleviate liver damage and improve the survival of animals.
3.3.1 EVs as the potential treatment option for drug-induced liver failure/injury
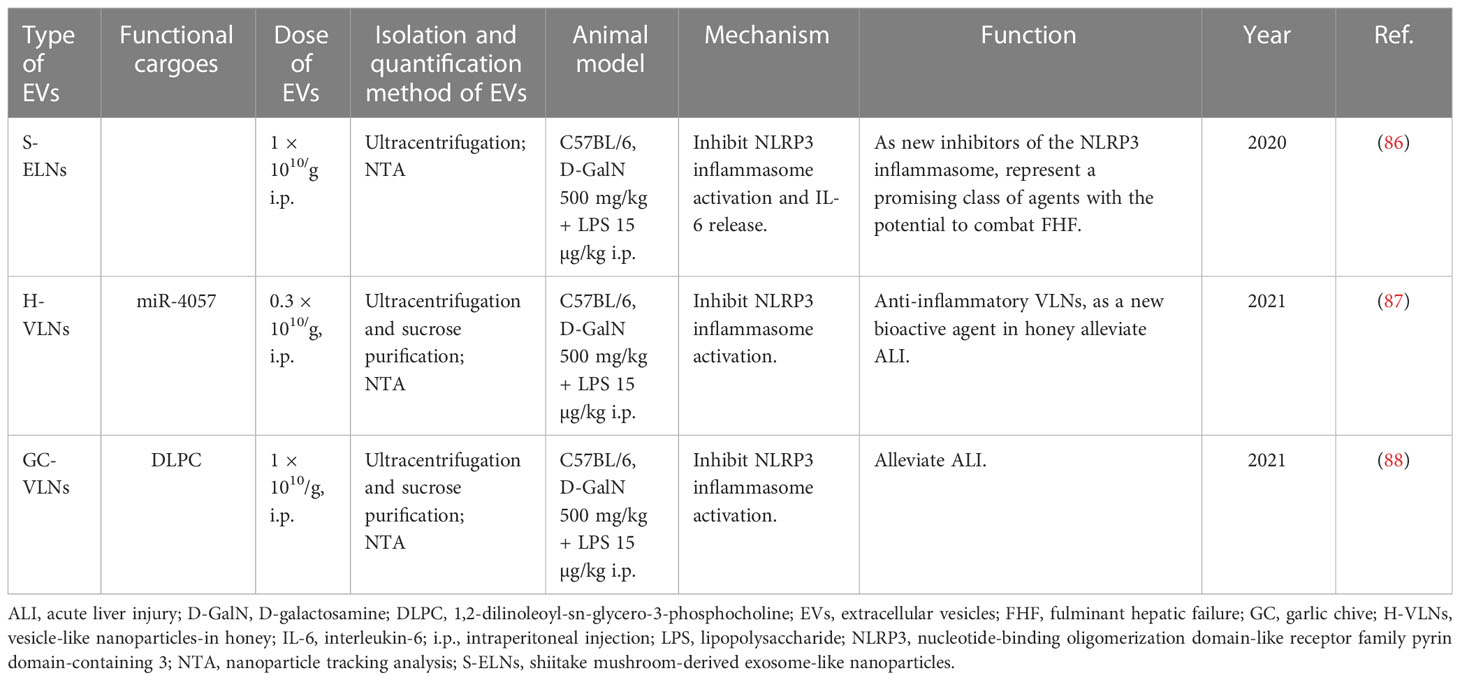
Hepatotoxins D-GalN plus LPS were most often utilized to induce liver failure. Exos derived from hUCMSC with or without TNF-α treatment were reported to alleviate D-GalN/LPS-induced ALF and promote the repair of damaged liver tissues by reducing the activity of the NLRP3 inflammasome in macrophages (60, 61). miR-17 or lncRNA H19 in ASC-derived Exos protected animals from ALF by inhibiting TXNIP/NLRP3 inflammasome activation in macrophages or promoting hepatocyte proliferation mediated by the HGF/c-Met pathway (57, 58). Exos derived from BM-MSCs or human MenSCs were also demonstrated to dramatically improve the survival of mice with lethal hepatic failure by suppressing cell apoptosis (53, 54). Unexpectedly, some foods can also secrete exosome-like nanoparticles (ELNs) or vesicle-like nanoparticles (VLNs) which have protective roles. For example, shiitake mushroom-derived ELNs and honey- or garlic chive-derived VLNs were verified to protect mice against D-GalN/LPS-induced liver failure through inhibiting NLRP3 inflammasome activation (86–88) (Table 5).
CCl4 is another common hepatotoxin to induce liver failure. hUCMSC-Exos were shown to relieve CCl4-induced liver injury through antioxidant effects (55, 56). And miR-455-3p-enriched hUCMSC-Exos were verified to ameliorate ALI through inhibiting inflammatory response by targeting PI3K signaling (62). Exos derived from BM-MSCs were elucidated to elicit the hepatoprotective effects by activating the proliferative and regenerative responses in CCl4-induced liver injury (51, 59). Human hepatocyte-derived EVs were documented to attenuate ALI through modulating inflammatory immune response (65). Interestingly, the administration of both normal and damaged liver EVs was proved to significantly accelerate the recovery of liver tissue from CCl4-induced hepatic necrosis by inducing the production of hepatocyte growth factor at the site of the injury (64).
In addition, hUCMSC-derived EVs were demonstrated to alleviate APAP-induced ALF through activating ERK and IGF-1R/PI3K/AKT signaling pathway (63). Moreover, EVs derived from BM-MSC exerted beneficial protection through immunosuppression in the Con A-induced animal model of liver injury (52).
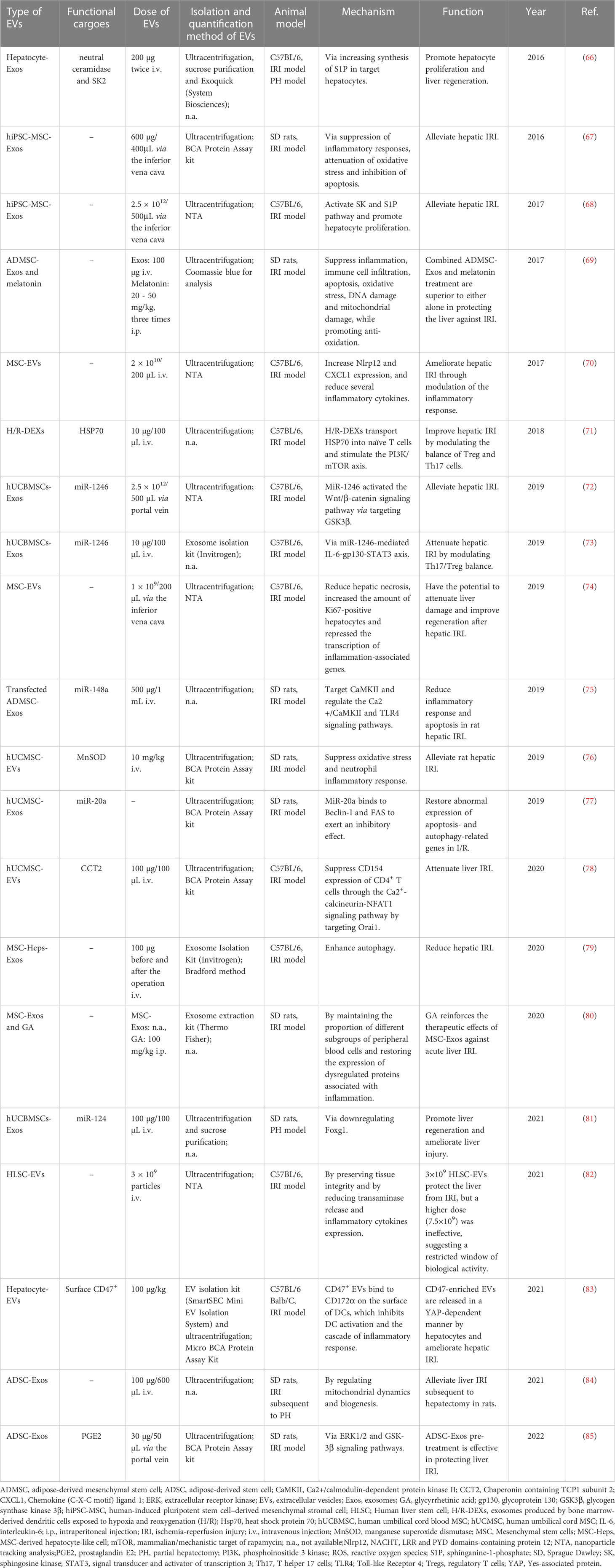
3.3.2 EVs as the potential treatment option for liver failure/injury induced by hepatic IRI
EVs and their bioactive cargoes were confirmed to protect mice or rats against hepatic failure (Table 4) induced by hepatic IRI. Exos derived from ADSCs attenuated hepatic IRI via promoting survival mediated by ERK1/2 and GSK-3β signaling pathways (85) or regulating mitochondrial dynamics and biogenesis (84). The overexpression of miR-148a in ADSC-Exos by transfection inhibited the expressions of CaMKII and TLR4 in liver ischemia-reperfusion tissues and reduced the occurrence of the inflammatory response and apoptosis (75). hUCMSC-Exos also were documented to alleviate hepatic IRI by suppressing oxidative stress and neutrophil inflammatory response (76), inhibiting Beclin1- and FAS-mediated autophagy and apoptosis (77), or modulating inflammatory immune response (78) and promoting liver regeneration by downregulating Foxg1 (81). Exosomal miR-1246 derived from hUCBMSCs exerted anti-apoptosis, pro-survival and anti-inflammatory effects by modulating the GSK3β-mediated Wnt/β-catenin signaling pathway (72) or modulating the balance between Tregs and Th17 cells (73). In addition, EVs originating from BM-MSCs were demonstrated to protect the liver against IRI through modulating inflammatory response (increased anti-inflammatory NLRP12 expression) (70), improving hepatic regeneration (74), or enhancing autophagy (79). Moreover, hiPSC-MSC-derived Exos played a protective role in IRI via inhibiting inflammation, apoptosis, and oxidative stress (67) or promoting cell proliferation via the activation of sphingosine kinase and sphingosine-1-phosphate pathway (68). Furthermore, 3×109 HLSC-EVs were able to modulate hepatic IRI by preserving tissue integrity and reducing transaminase release and inflammatory cytokines expression (82). In addition to stem cell-derived EVs, EVs from other sources also were proved to exert hepatoprotection in IRI. For instance, EVs derived from DCs were documented to attenuate IRI by transporting HSP70 to naïve T cells and stimulating the PI3K/mTOR axis to modulate the balance between Treg and Th17 Cells (71). CD47-enriched EVs released by hepatocytes in a Yes-associated protein (YAP)-dependent manner ameliorated hepatic IRI through inhibiting dendritic cell activation (83).
3.3.3 Modified EVs as the potential treatment option for liver failure/injury
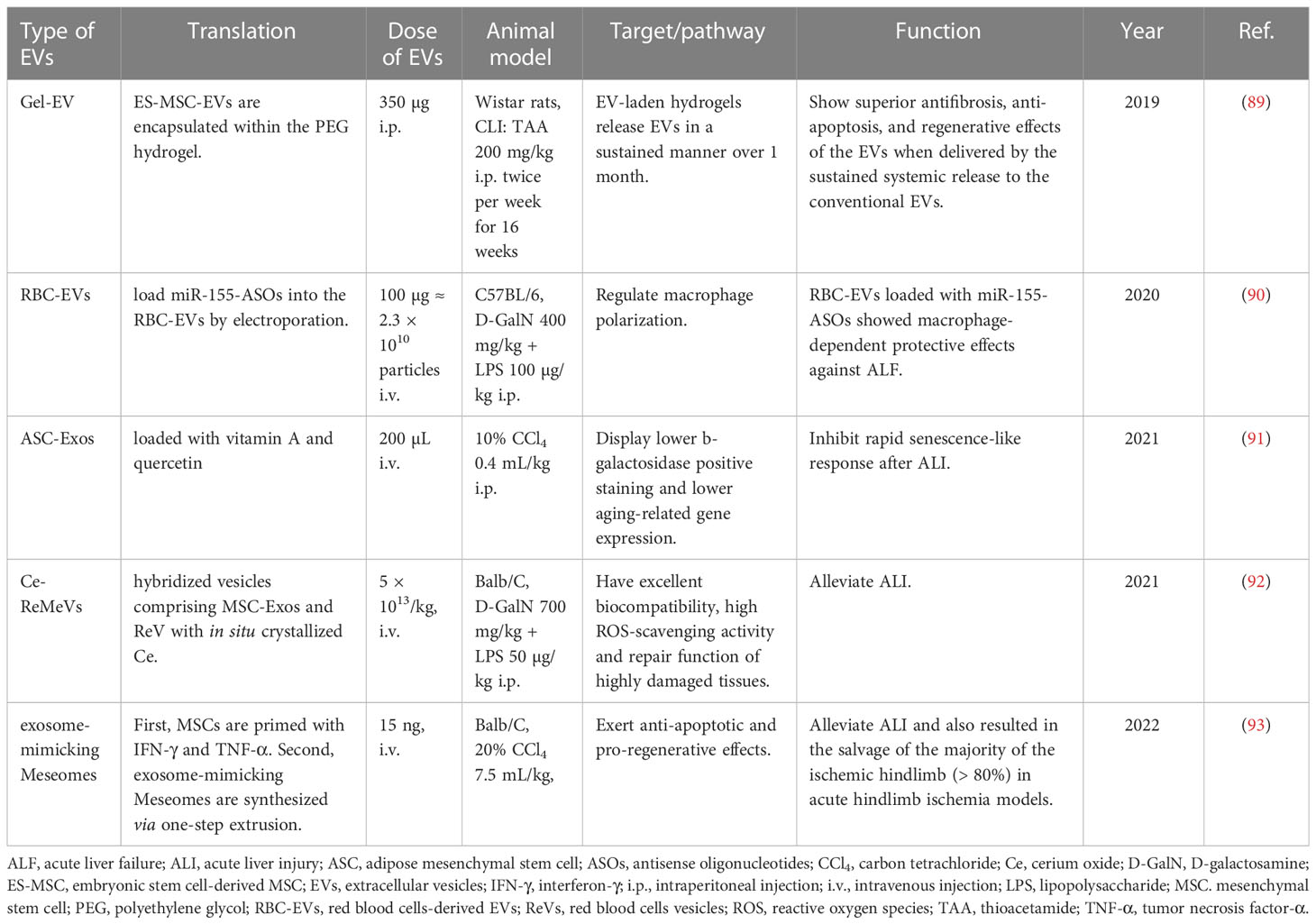
In some cases, EVs from the parental cells are not sufficient to effectively treat diseases. In light of this, scientists are trying their best to modify EVs or combine them with other hepatoprotective agents to optimize their functions. As a result, the protective effects of EVs against liver failure enhanced after modification. For example, the therapeutic effects of ADMSC-EVs on ALI through inhibiting inflammation or rapid senescence-like response were reinforced by combined treatment with melatonin (69) or glycyrrhetinic acid (80), loading with vitamin A and quercetin (91), or even transfection with miR-148a (75). Recently, EVs from red blood cells (RBC-EVs) are considered as preferable drug delivery vehicles because of their characteristics of low immunogenicity, easy availability and liver accumulation. RBC-EVs loaded with antisense oligonucleotides (ASOs) of miR-155 (miR155-ASOs) exhibited excellent protective and therapeutic effects against ALF by regulating macrophage polarization (90). Hybridized Ce-red blood cell vesicles (Ce-ReVs), in situ growth of cerium oxide (Ce) nanocrystals onto nano-sized red blood cell vesicles (ReVs), showed strong reactive oxygen species (ROS) elimination. Upon further hybridization with MSC-Exos, the resulting Ce-ReMeVs conferred superior repair benefit and brought about promising therapeutic outcomes even for models with more severe inflammatory damage, including D-GalN/LPS-induced ALI model (92). Furthermore, scientists have developed a hydrogel-mediated sustained systemic delivery of MSC-EVs to improve liver regeneration in chronic liver failure (89). In addition, systemic administration of exosome-mimicking Meseomes which is composed of membranes and secretome from efficacy-potentiated MSCs alleviated tissue necrosis and promoted the recovery of liver function in ALI models induced by CCl4 (93) (Table 6).
3.4 The quality assessment results
According to the quality assessment result by the SYRCLE tool, the majority of 57 included studies were marked as “low” or “unclear” (66.7%) risk of bias due to insufficient information regarding selection method, allocation concealment, animal housing, blinding, replacement of dropout animals, and so on (Table S2). The risk of bias summary and risk of bias graph were shown in Figure S1. All the 3 case-control studies were considered as “high” quality with a cumulative NOS score of seven points or greater. The risk of bias comprehensive assessment is shown in Table S3. The CERQual tool revealed that the review findings reached a “high” confidence appraisal (Table S4). The PRISMA checklist was completed which included further details for the review scoring (Table S6).
4 Discussion
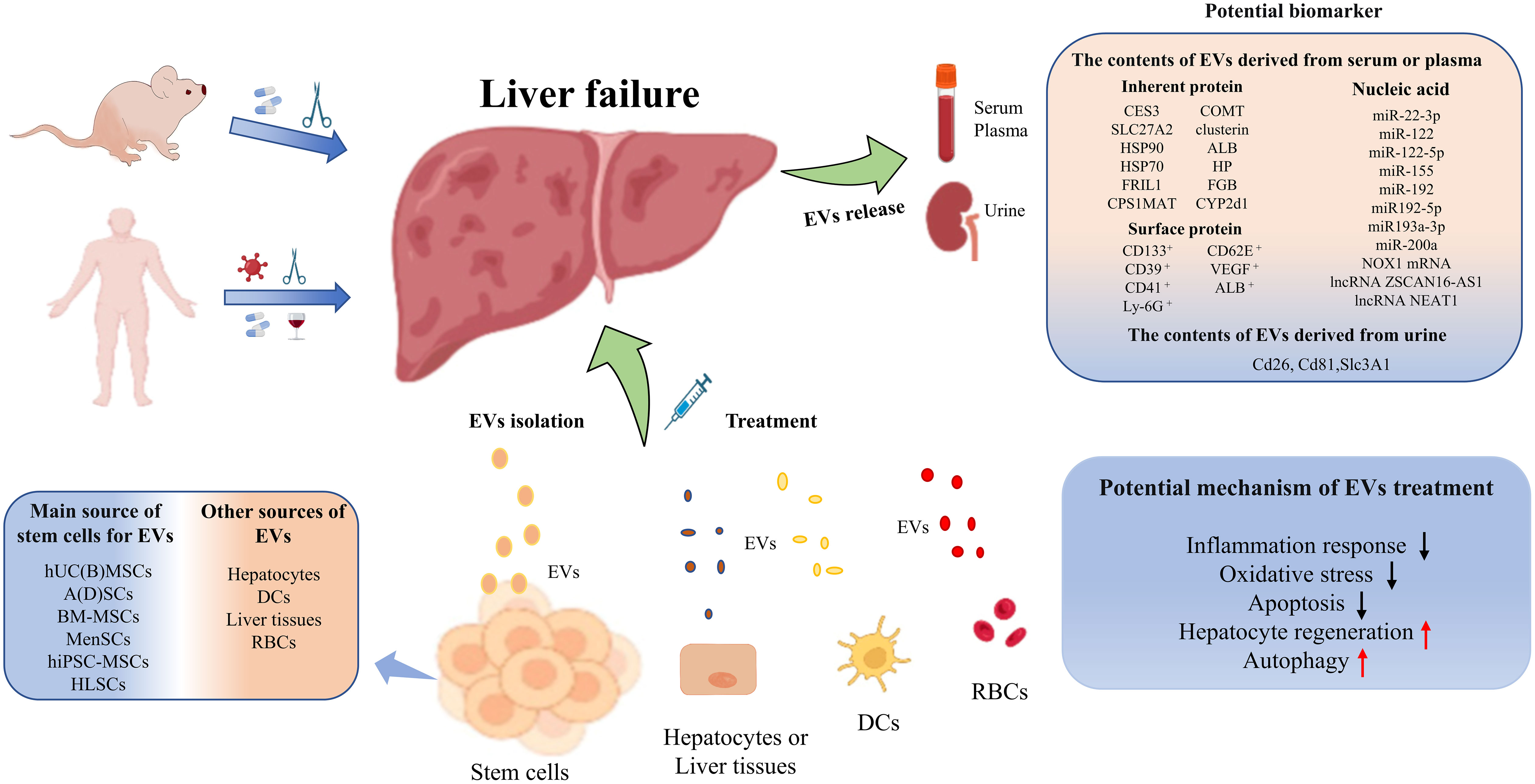
In this review, we systematically and comprehensively summarized published studies on the administration of EVs in experimental animal models or clinical studies related to liver failure, and assessed the potential of EVs as biomarkers and treatment options for liver failure. The results revealed that EVs carrying differentially expressed proteins or nucleic acids (especially surface membrane proteins and various RNAs) can be utilized not only as early warning or diagnostic biomarkers, but also to provide clues for prognostic evaluation of liver failure. In addition, EVs and their functional cargoes have been confirmed to exert important hepatoprotective effects and can be used to treat liver failure.
Emerging studies have documented that EVs with their cargoes can serve as biomarkers for early warning, early or differential diagnosis and prognosis assessment of liver diseases, such as drug-induced liver injury, viral hepatitis, non-alcoholic fatty liver disease, alcoholic liver disease, liver cirrhosis and liver cancer (18–21). Nevertheless, it remains unclear about the potential of EVs as biomarkers of liver failure. And one of the major aims of this systematic review is to clarify this issue.
Differentially expressed proteins in EVs usually reflect the functional properties and tissue-specific origin of EVs, as well as underlying pathophysiological mechanisms, thus they have the potential to act as emerging biomarkers for early warning or diagnosis of liver failure. Multiple studies in this systematic review showed that the unique proteins, including intrinsic proteins or surface membrane proteins carried by EVs, are good early warning or diagnostic biomarkers for liver failure. In terms of preclinical studies, elevated surface protein CD133 (36) or intrinsic proteins ALB, HP and FGB (39) in circulating EVs may serve as early warning biomarkers in APAP-induced liver injury. In addition, several proteins such as CES3, SLC27A2, and HSP90 were found to be more commonly expressed in EVs isolated from sera of D-GalN-induced rats (38). On the contrary, some proteins (such as CD26, SLC3A1 and CD81) that are normally present in urinary vesicles were discovered to be dramatically reduced in urinary samples obtained from D-GalN-treated rats (35). In hepatic IRI, MPs derived from platelets (with surface CD41+) and neutrophils (with surface Ly-6G+) may be markers of hepatic inflammation following injury, and MPs derived from endothelial cells (with surface CD62E+) may reflect angiogenesis during the reparative phase (37).
In addition, differentially expressed RNA, especially miRNA in EVs, can also be considered as early warning, diagnosis or prognosis evaluation biomarkers of liver failure. For instance, miR-122, miR-192 and miR-155 carried by EVs may serve as biomarkers of ALI considering that they were upregulated in APAP-induced liver injury and reversed to basal levels after NAC treatment (40). Notably, exosomal miR-122a-5p was regarded as the best diagnostic biomarker with higher diagnostic power and a wider diagnostic window for ALI (42). Furthermore, a set of miRNAs including miR-122-5p, miR-192-5p and miR-22-3p were considered as potential biomarkers which contribute mainly to liver steatosis and inflammation in ALI (43).
Consistent with findings in experimental animal studies, differentially expressed surface proteins and RNAs carried by EVs also may be considered as potential early warning or prognostic biomarkers for liver failure in clinical studies. ALB and VEGF carried by Exos may be more accurate and specific biomarkers of liver regeneration and prognostic evaluation than AFP in serum for patients with ACLF, and CD63+ALB+Exos may be an early warning marker for ACLF patients (50). Furthermore, NOX1 mRNA and lncRNA ZSCAN16-AS1 were reported to be increased in HBV-ACLF and HBV patients compared to healthy control (48). Especially, lncRNA NEAT1 is a better prognostic biomarker than the MELD score for 90-day mortality in ACHBLF (49). Interestingly, miR-122 has also been found to reflect the severity of liver damage in patients with other diseases, such as patients with acute heart failure and ART-treated, HIV-1-infected individuals (44, 47). However, more comprehensive and large-scale clinical studies are still needed in the further to verify the role of EVs as good biomarkers for liver failure.
Although preclinical and clinical studies have revealed that EVs may be potential early warning, diagnosis or prognosis evaluation biomarkers of liver failure, research in this area remains to be in its infancy and large-scale clinical studies are scarce. This may be ascribed to the followings: (1) there is no widely accepted ideal animal model of liver failure, especially ACLF; (2) high technical requirements and detection costs as well as uncertain results limit the implementation of studies related to Exos; (3) the etiologies of liver failure are diversified, which leads to great individual variation; (4) liver failure is often accompanied by complex complications, which may act as confounding factors and limit the diagnostic and prognostic value of EVs for liver failure in clinical studies.
The other objective of this systematic review is to assess the treatment potential of EVs in liver failure. To this end, we comprehensively summarized the studies in which EVs are utilized to treat liver failure. EVs derived from stem cells, especially MSCs, are most commonly used to treat liver failure. MSCs can be isolated from bone marrow, adipose tissue, umbilical cord, and so on. They have been documented to possess the strong ability of differentiation, regeneration and immunomodulation, and can be used to treat various liver diseases efficiently and safely in clinical studies (96, 97). The therapeutic effect of MSCs can be attributed to paracrine, especially EVs, to a large extent. Compared with MSCs, MSC-EVs have the following advantages: (1) much smaller in size and easier to pass through biological barriers including the blood-brain barrier; (2) can be administered intravenously, and more likely accumulate in the liver; (3) cryopreservation does not affect the clinical efficacy; (4) lower immunogenicity and better histocompatibility. Therefore, EVs are emerging as a novel treatment option.
EVs and their functional cargoes turn out to play important hepatoprotective effects in liver failure by inhibiting inflammatory response, oxidative stress, apoptosis or promoting hepatocyte regeneration and autophagy.
Regarding the inhibition of inflammation response, hUCMSCs-Exos containing miR-299-3p or miR-455-3p ameliorated drug-induced ALF through inhibiting the recruitment and activation of the NLRP3 inflammasomes or modulating PI3K signaling (61, 62). Moreover, hUC(B)MSCs-EVs protected hepatic IRI through suppressing CD154 expression on CD4+ T cells via CCT2 (78) or modulating the balance between Tregs and Th17 cells via miR-1246-mediated IL-6-gp130-STAT3 axis (73). Furthermore, AMSC-derived Exos carrying miR-17 ameliorated GalN/LPS-induced ALF by targeting TXNIP, which is well-known as a key player in the activation of NLRP3 inflammasome (57). In addition, BM-MSC-derived EVs protected against murine hepatic ischemia/reperfusion injury by increasing NLRP12 and CXCL1 expression and reducing several inflammatory cytokines such as IL-6 (70).
In the light of resistance to oxidative stress and apoptosis, hUCMSC-Exos inhibited oxidative stress-induced apoptosis in APAP- or CCl4-induced liver injury by upregulation of ERK and IGF-1R/PI3K/AKT signaling pathways and downregulation of the IKKB/NF-kB/casp-9/-3 pathway (55, 63, 65). ADSC-Exos protected against liver IRI by regulating mitochondrial dynamics and biogenesis or ERK1/2 and GSK3beta signaling pathways (84, 85). Moreover, MenSC-Exos reduced the number of liver mononuclear cells (MNCs) and the amount of the active apoptotic protein caspase-3 in D-GalN/LPS-induced fulminant hepatic failure (54).
EVs have also been proven to treat liver failure through promoting hepatocyte regeneration and proliferation. For example, hASCs-EVs containing lncRNA H19 promoted hepatocyte regeneration in D-GalN-induced ALF via upregulating the HGF/c-Met pathway (58). And hUCBMSC-Exos enriched with miR-124 promoted liver regeneration in partial hepatectomy by downregulating Foxg1 (81). Moreover, hiPSC-MSCs-derived Exos alleviated hepatic IRI through activating sphingosine kinase (SK) and sphingosine-1-phosphate(S1P) signaling pathway and promoting hepatocyte proliferation (68).
With respect to enhancement of autophagy, Exos obtained from MSC‐derived hepatocyte‐like cells treatment enhanced autophagy during hepatic IRI to exert the hepatoprotective effect (79), and hUCMSC‐Exos carried with miR‐20a inhibited Beclin‐1 and FAS to alleviate the abnormal expression of apoptosis‐ and autophagy‐related genes in liver ischemia-reperfusion (77).
In addition to stem cell-derived EVs, EVs derived from other cells or tissues have also been confirmed to protect against liver failure, such as hepatocytes (65, 66, 83), DCs (71) as well as normal or damaged liver tissue (64). Interestingly, researchers also extracted desired EVs from natural foods, such as mushroom, honey and garlic chive, and proved that they may have a protective effect on the liver by inhibiting the activation of inflammasome (86–88). These findings greatly broaden the source of EVs and provide new ideas for the treatment of liver failure.
In recent years, scientists have attempted to modify EVs to achieve superior therapeutic effects for liver failure. For example, red blood cells which have the features of low immunogenicity, easy availability and liver accumulation were transfected with hepatoprotective miRNAs [such as miR155‐ASOs (90)] or hybridized with Ce and MSC-Exos (92) to improve the targeting and therapeutic effect of EVs on ALF. Moreover, the protective effect of ADMSC-Exos on liver failure was improved by loading them with vitamin A and quercetin (91). In addition, EV-encapsulated PEG hydrogels were developed to retard the clearance of MSC-EVs, ultimately improving liver regeneration in chronic liver failure (89).
5 Conclusion
In conclusion, EVs and their cargoes can be used not only as superior biomarkers of early warning, diagnosis and prognostic assessments for liver failure, but also as potentially effective treatment options for patients with liver failure (Figure 2). In the future, large-scale studies are urgently needed to verify the diagnostic, predictive and therapeutic value of EVs for liver failure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
WL and HT drafted the paper. SL provided literature search support. YC and LB revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Beijing Hospitals Authority’s Ascent Plan (DFL20221501); Construction Project of High-level Technology Talents in Public Health (Discipline leader -01-12); the Beijing Municipal Natural Science Foundation (7202068, 72222093, 7222094); Beijing Nova Program (20220484201); Chinese Foundation for Hepatitis Prevention and Control-Tian Qing Liver Disease Research Fund Subject (NO. TQGB20210013); Beijing You’an Hospital, Capital Medical University-Young and middle-aged talents incubation project (NO. YNKTQN2021003); R&D Program of Beijing Municipal Education Commission (KM202310025009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1116518/full#supplementary-material
Abbreviations
ACHBLF, acute-on-chronic hepatitis B liver failure; ACLF, acute-on-chronic liver failure; ADMSC, adipose-derived mesenchymal stem cell; A(D)SC, adipose-derived stem cell; ALF, acute liver failure; ALI, acute liver injury; APAP, acetaminophen; ASOs, antisense oligonucleotides; AUC, area under the curve of receiver operating characteristic; BM-MSC, bone marrow-derived mesenchymal stem cell; CCl4, carbon tetrachloride; Ce-ReMeVs, hybridization Ce-ReVs with MSC-Exos; Ce-ReVs, hybridized Ce-red blood cells vesicles; Con A, concanavalin A; DC, dendritic cell; D-GalN, D-galactosamine; ELNs, exosome-like nanoparticles; EtOH, ethyl alcohol; EVs, Extracellular vesicles; Exos, exosomes; hiPSC-MSC, human-induced pluripotent stem cell-derived MSC; HLSC, human liver stem cell; HSC, hematopoietic stem cell; HSP70, heat shock protein 70; hUC(B)MSC, human umbilical cord (blood) mesenchymal stem cell; IRI, ischemia-reperfusion injury; lncRNA, long noncoding RNA; LPS, lipopolysaccharide; MenSC, menstrual blood-derived stem cell; mRNA, messenger RNA; MSC, mesenchymal stem cell; MVs or MPs, microvesicles or microparticles; NAC, N-acetyl-cysteine; NOX1, NADPH oxidase 1; PEG, polyethylene glycol; RBC, red blood cell; RBC-EVs, EVs from red blood cells; ROS, reactive oxygen species; TAA, thioacetamide; TNF-α, tumor necrosis factor-α; VLNs, vesicle-like nanoparticles; YAP, Yes-associated protein.
References
1. Stravitz RT, Lee WM. Acute liver failure. Lancet (2019) 394:869–81. doi: 10.1016/S0140-6736(19)31894-X
2. Bajaj JS, O'Leary JG, Lai JC, Wong F, Long MD, Wong RJ, et al. Acute-on-Chronic liver failure clinical guidelines. Am J Gastroenterol (2022) 117:225–52. doi: 10.14309/ajg.0000000000001595
3. Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol (2013) 59:74–80. doi: 10.1016/j.jhep.2013.02.010
4. Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, et al. Outcomes in adults with acute liver failure between 1998 and 2013: An observational cohort study. Ann Intern Med (2016) 164:724–32. doi: 10.7326/M15-2211
5. Trebicka J, Sundaram V, Moreau R, Jalan R, Arroyo V. Liver transplantation for acute-on-Chronic liver failure: Science or fiction? Liver Transpl (2020) 26:906–15. doi: 10.1002/lt.25788
6. Mezzano G, Juanola A, Cardenas A, Mezey E, Hamilton JP, Pose E, et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut (2022) 71:148–55. doi: 10.1136/gutjnl-2020-322161
7. Wu H, Xia F, Zhang L, Fang C, Lee J, Gong L, et al. A ROS-sensitive nanozyme-augmented photoacoustic nanoprobe for early diagnosis and therapy of acute liver failure. Adv Mater (2022) 34:e2108348. doi: 10.1002/adma.202108348
8. Sun Z, Liu X, Wu D, Gao H, Jiang J, Yang Y, et al. Circulating proteomic panels for diagnosis and risk stratification of acute-on-chronic liver failure in patients with viral hepatitis b. Theranostics (2019) 9:1200–14. doi: 10.7150/thno.31991
9. Jin Y, Wang H, Yi K, Lv S, Hu H, Li M, et al. Applications of nanobiomaterials in the therapy and imaging of acute liver failure. Nanomicro Lett (2020) 13:25. doi: 10.1007/s40820-020-00550-x
10. Shokravi S, Borisov V, Zaman BA, Niazvand F, Hazrati R, Khah MM, et al. Mesenchymal stromal cells (MSCs) and their exosome in acute liver failure (ALF): a comprehensive review. Stem Cell Res Ther (2022) 13:192. doi: 10.1186/s13287-022-02825-z
11. Ye C, Li W, Li L, Zhang K. Glucocorticoid treatment strategies in liver failure. Front Immunol (2022) 13:846091. doi: 10.3389/fimmu.2022.846091
12. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
13. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol (2018) 19:213–28. doi: 10.1038/nrm.2017.125
14. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol (2019) 21:9–17. doi: 10.1038/s41556-018-0250-9
15. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol (2022) 23:369–82. doi: 10.1038/s41580-022-00460-3
16. Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol (2016) 65:213–21. doi: 10.1016/j.jhep.2016.03.004
17. Azparren-Angulo M, Royo F, Gonzalez E, Liebana M, Brotons B, Berganza J, et al. Extracellular vesicles in hepatology: Physiological role, involvement in pathogenesis, and therapeutic opportunities. Pharmacol Ther (2021) 218:107683. doi: 10.1016/j.pharmthera.2020.107683
18. Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol (2017) 14:455–66. doi: 10.1038/nrgastro.2017.71
19. Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther (2017) 174:63–78. doi: 10.1016/j.pharmthera.2017.02.020
20. Thietart S, Rautou PE. Extracellular vesicles as biomarkers in liver diseases: A clinician's point of view. J Hepatol (2020) 73:1507–25. doi: 10.1016/j.jhep.2020.07.014
21. Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut (2021) 70:784–95. doi: 10.1136/gutjnl-2020-322526
22. Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discovery (2022) 21:379–99. doi: 10.1038/s41573-022-00410-w
23. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol (2021) 16:748–59. doi: 10.1038/s41565-021-00931-2
24. Kostallari E, Valainathan S, Biquard L, Shah VH, Rautou PE. Role of extracellular vesicles in liver diseases and their therapeutic potential. Adv Drug Delivery Rev (2021) 175:113816. doi: 10.1016/j.addr.2021.05.026
25. Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci (1991) 36:770–4. doi: 10.1007/BF01311235
26. Newsome PN, Plevris JN, Nelson LJ, Hayes PC. Animal models of fulminant hepatic failure: a critical evaluation. Liver Transpl (2000) 6:21–31. doi: 10.1002/lt.500060110
27. Tunon MJ, Alvarez M, Culebras JM, Gonzalez-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol (2009) 15:3086–98. doi: 10.3748/wjg.15.3086
28. McGill MR, Jaeschke H. Animal models of drug-induced liver injury. Biochim Biophys Acta Mol Basis Dis (2019) 1865:1031–9. doi: 10.1016/j.bbadis.2018.08.037
29. Maes M, Vinken M, Jaeschke H. Experimental models of hepatotoxicity related to acute liver failure. Toxicol Appl Pharmacol (2016) 290:86–97. doi: 10.1016/j.taap.2015.11.016
30. Martins PN, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int (2008) 28:3–11. doi: 10.1111/j.1478-3231.2007.01628.x
31. Belanger M, Butterworth RF. Acute liver failure: a critical appraisal of available animal models. Metab Brain Dis (2005) 20:409–23. doi: 10.1007/s11011-005-7927-z
32. Hirao H, Nakamura K, Kupiec-Weglinski JW. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat Rev Gastroenterol Hepatol (2022) 19:239–56. doi: 10.1038/s41575-021-00549-8
33. Gama JFG, Cardoso L, Lagrota-Candido JM, Alves LA. Animal models applied to acute-on-chronic liver failure: Are new models required to understand the human condition? World J Clin cases (2022) 10:2687–99. doi: 10.12998/wjcc.v10.i9.2687
34. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
35. Conde-Vancells J, Rodriguez-Suarez E, Gonzalez E, Berisa A, Gil D, Embade N, et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteomics Clin Appl (2010) 4:416–25. doi: 10.1002/prca.200900103
36. Schmelzle M, Splith K, Andersen LW, Kornek M, Schuppan D, Jones-Bamman C, et al. Increased plasma levels of microparticles expressing CD39 and CD133 in acute liver injury. Transplantation (2013) 95:63–9. doi: 10.1097/TP.0b013e318278d3cd
37. Freeman CM, Quillin RC 3rd, Wilson GC, Nojima H, Johnson BL 3rd, Sutton JM, et al. Characterization of microparticles after hepatic ischemia-reperfusion injury. PloS One (2014) 9:e97945. doi: 10.1371/journal.pone.0097945
38. Rodriguez-Suarez E, Gonzalez E, Hughes C, Conde-Vancells J, Rudella A, Royo F, et al. Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity. J Proteomics (2014) 103:227–40. doi: 10.1016/j.jprot.2014.04.008
39. Cho YE, Im EJ, Moon PG, Mezey E, Song BJ, Baek MC. Increased liver-specific proteins in circulating extracellular vesicles as potential biomarkers for drug- and alcohol-induced liver injury. PloS One (2017) 12:e0172463. doi: 10.1371/journal.pone.0172463
40. Cho YE, Kim SH, Lee BH, Baek MC. Circulating plasma and exosomal microRNAs as indicators of drug-induced organ injury in rodent models. Biomol Ther (Seoul) (2017) 25:367–73. doi: 10.4062/biomolther.2016.174
41. Palomo L, Mleczko JE, Azkargorta M, Conde-Vancells J, Gonzalez E, Elortza F, et al. Abundance of cytochromes in hepatic extracellular vesicles is altered by drugs related with drug-induced liver injury. Hepatol Commun (2018) 2:1064–79. doi: 10.1002/hep4.1210
42. Motawi TK, Mohamed MR, Shahin NN, Ali MAM, Azzam MA. Time-course expression profile and diagnostic potential of a miRNA panel in exosomes and total serum in acute liver injury. Int J Biochem Cell Biol (2018) 100:11–21. doi: 10.1016/j.biocel.2018.05.002
43. Lv XF, Zhang AQ, Liu WQ, Zhao M, Li J, He L, et al. Liver injury changes the biological characters of serum small extracellular vesicles and reprograms hepatic macrophages in mice. World J Gastroenterol (2021) 27:7509–29. doi: 10.3748/wjg.v27.i43.7509
44. Koyama S, Kuragaichi T, Sato Y, Kuwabara Y, Usami S, Horie T, et al. Dynamic changes of serum microRNA-122-5p through therapeutic courses indicates amelioration of acute liver injury accompanied by acute cardiac decompensation. ESC Heart Fail (2017) 4:112–21. doi: 10.1002/ehf2.12123
45. Strategies for Management of Antiretroviral Therapy Study G, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med (2006) 355:2283–96. doi: 10.1056/NEJMoa062360
46. Group I-ES, Committee SS, Abrams D, Levy Y, Losso MH, Babiker A, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med (2009) 361:1548–59. doi: 10.1056/NEJMoa0903175
47. Murray DD, Suzuki K, Law M, Trebicka J, Neuhaus Nordwall J, Johnson M, et al. Circulating miR-122 and miR-200a as biomarkers for fatal liver disease in ART-treated, HIV-1-infected individuals. Sci Rep (2017) 7:10934. doi: 10.1038/s41598-017-11405-8
48. Chen J, Xu Q, Zhang Y, Zhang H. RNA Profiling analysis of the serum exosomes derived from patients with chronic hepatitis and acute-on-chronic liver failure caused by HBV. Sci Rep (2020) 10:1528. doi: 10.1038/s41598-020-58233-x
49. Gao S, Fan YC, Han LY, Wang K. Serum exosomal long noncoding RNA nuclear-enriched abundant transcript 1 predicts 90-day mortality in acute-on-chronic hepatitis b liver failure. Expert Rev Clin Immunol (2021) 17:789–97. doi: 10.1080/1744666X.2021.1933442
50. Jiao Y, Lu W, Xu P, Shi H, Chen D, Chen Y, et al. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure. Hepatol Int (2021) 15:957–69. doi: 10.1007/s12072-021-10217-3
51. Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther (2014) 5:76. doi: 10.1186/scrt465
52. Tamura R, Uemoto S, Tabata Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin a-induced liver injury model. Inflammation Regener (2016) 36:26. doi: 10.1186/s41232-016-0030-5
53. Haga H, IK Y, Takahashi K, Matsuda A, Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl Med (2017) 6:1262–72. doi: 10.1002/sctm.16-0226
54. Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther (2017) 8:9. doi: 10.1186/s13287-016-0453-6
55. Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther (2017) 25:465–79. doi: 10.1016/j.ymthe.2016.11.019
56. Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, et al. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int (2018) 2018:6079642. doi: 10.1155/2018/6079642
57. Liu Y, Lou G, Li A, Zhang T, Qi J, Ye D, et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine (2018) 36:140–50. doi: 10.1016/j.ebiom.2018.08.054
58. Jin Y, Wang J, Li H, Gao S, Shi R, Yang D, et al. Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine (2018) 34:231–42. doi: 10.1016/j.ebiom.2018.07.015
59. Damania A, Jaiman D, Teotia AK, Kumar A. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res Ther (2018) 9:31. doi: 10.1186/s13287-017-0752-6
60. Jiang L, Zhang S, Hu H, Yang J, Wang X, Ma Y, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun (2019) 508:735–41. doi: 10.1016/j.bbrc.2018.11.189
61. Zhang S, Jiang L, Hu H, Wang H, Wang X, Jiang J, et al. Pretreatment of exosomes derived from hUCMSCs with TNF-alpha ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci (2020) 246:117401. doi: 10.1016/j.lfs.2020.117401
62. Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther (2020) 11:37. doi: 10.1186/s13287-020-1550-0
63. Wu HY, Zhang XC, Jia BB, Cao Y, Yan K, JY Li, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acetaminophen-induced acute liver failure through activating ERK and IGF-1R/PI3K/AKT signaling pathway. J Pharmacol Sci (2021) 147:143–55. doi: 10.1016/j.jphs.2021.06.008
64. Lee J, SR K, Lee C, YI J, Bae S, YJ Y, et al. Extracellular vesicles from in vivo liver tissue accelerate recovery of liver necrosis induced by carbon tetrachloride. J Extracell Vesicles (2021) 10:e12133. doi: 10.1002/jev2.12133
65. Kakizaki M, Yamamoto Y, Nakayama S, Kameda K, Nagashima E, Ito M, et al. Human hepatocyte-derived extracellular vesicles attenuate the carbon tetrachloride-induced acute liver injury in mice. Cell Death Dis (2021) 12:1010. doi: 10.1038/s41419-021-04204-7
66. Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol (2016) 64:60–8. doi: 10.1016/j.jhep.2015.07.030
67. Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y, et al. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy (2016) 18:1548–59. doi: 10.1016/j.jcyt.2016.08.002
68. Du Y, Li D, Han C, Wu H, Xu L, Zhang M, et al. Exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect liver against hepatic ischemia/ reperfusion injury via activating sphingosine kinase and sphingosine-1-Phosphate signaling pathway. Cell Physiol Biochem (2017) 43:611–25. doi: 10.1159/000480533
69. Sun CK, Chen CH, Chang CL, Chiang HJ, Sung PH, Chen KH, et al. Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Transl Res (2017) 9:1543–60.
70. Haga H, Yan IK, Borrelli DA, Matsuda A, Parasramka M, Shukla N, et al. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl (2017) 23:791–803. doi: 10.1002/lt.24770
71. Zheng L, Li Z, Ling W, Zhu D, Feng Z, Kong L. Exosomes derived from dendritic cells attenuate liver injury by modulating the balance of treg and Th17 cells after ischemia reperfusion. Cell Physiol Biochem (2018) 46:740–56. doi: 10.1159/000488733
72. Xie K, Liu L, Chen J, Liu F. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle (2019) 18:3491–501. doi: 10.1080/15384101.2019.1689480
73. Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life (2019) 71:2020–30. doi: 10.1002/iub.2147
74. Anger F, Camara M, Ellinger E, Germer CT, Schlegel N, Otto C, et al. Human mesenchymal stromal cell-derived extracellular vesicles improve liver regeneration after ischemia reperfusion injury in mice. Stem Cells Dev (2019) 28:1451–62. doi: 10.1089/scd.2019.0085
75. Nong K, Liu S, Zhang D, Chen C, Yang Y, Cai H. The effects of mesenchymal stem cell exosome with an overexpression of mir-148a on hepatic ischemia-reperfusion injury. Int J Clin Exp Med (2019) 12:13325–36.
76. Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J (2019) 33:1695–710. doi: 10.1096/fj.201800131RR
77. Zhang L, Song Y, Chen L, Li D, Feng H, Lu Z, et al. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol (2020) 235:3698–710. doi: 10.1002/jcp.29264
78. Zheng J, Lu T, Zhou C, Cai J, Zhang X, Liang J, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells protect liver Ischemia/Reperfusion injury by reducing CD154 expression on CD4+ T cells via CCT2. Adv Sci (Weinh) (2020) 7:1903746. doi: 10.1002/advs.201903746
79. Yang B, Duan W, Wei L, Zhao Y, Han Z, Wang J, et al. Bone marrow mesenchymal stem cell-derived hepatocyte-like cell exosomes reduce hepatic Ischemia/Reperfusion injury by enhancing autophagy. Stem Cells Dev (2020) 29:372–9. doi: 10.1089/scd.2019.0194
80. Wei X, Zheng W, Tian P, Liu H, He Y, Peng M, et al. Administration of glycyrrhetinic acid reinforces therapeutic effects of mesenchymal stem cell-derived exosome against acute liver ischemia-reperfusion injury. J Cell Mol Med (2020) 24:11211–20. doi: 10.1111/jcmm.15675
81. Song XJ, Zhang L, Li Q, Li Y, Ding FH, Li X. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci (2021) 265:118821. doi: 10.1016/j.lfs.2020.118821
82. Calleri A, Roggio D, Navarro-Tableros V, De Stefano N, Pasquino C, David E, et al. Protective effects of human liver stem cell-derived extracellular vesicles in a mouse model of hepatic ischemia-reperfusion injury. Stem Cell Rev Rep (2021) 17:459–70. doi: 10.1007/s12015-020-10078-7
83. Yuan Z, Ye L, Feng X, Zhou T, Zhou Y, Zhu S, et al. YAP-dependent induction of CD47-enriched extracellular vesicles inhibits dendritic cell activation and ameliorates hepatic ischemia-reperfusion injury. Oxid Med Cell Longev (2021) 2021:6617345. doi: 10.1155/2021/6617345
84. Zhang Q, Piao C, Ma H, Xu J, Wang Y, Liu T, et al. Exosomes from adipose-derived mesenchymal stem cells alleviate liver ischaemia reperfusion injury subsequent to hepatectomy in rats by regulating mitochondrial dynamics and biogenesis. J Cell Mol Med (2021) 25:10152–63. doi: 10.1111/jcmm.16952
85. Zhang Y, Li Y, Wang Q, Zheng D, Feng X, Zhao W, et al. Attenuation of hepatic ischemiareperfusion injury by adipose stem cellderived exosome treatment via ERK1/2 and GSK3beta signaling pathways. Int J Mol Med (2022) 49. doi: 10.3892/ijmm.2021.5068
86. Liu B, Lu Y, Chen X, Muthuraj PG, Li X, Pattabiraman M, et al. Protective role of shiitake mushroom-derived exosome-like nanoparticles in d-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients (2020) 12. doi: 10.3390/nu12020477
87. Chen X, Liu B, Li X, TT An, Zhou Y, Li G, et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J Extracell Vesicles (2021) 10:e12069. doi: 10.1002/jev2.12069
88. Liu B, Li X, Yu H, Shi X, Zhou Y, Alvarez S, et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics (2021) 11:9311–30. doi: 10.7150/thno.60265
89. Mardpour S, Ghanian MH, Sadeghi-Abandansari H, Mardpour S, Nazari A, Shekari F, et al. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl Mater Interfaces (2019) 11:37421–33. doi: 10.1021/acsami.9b10126
90. Zhang G, Huang X, Xiu H, Sun Y, Chen J, Cheng G, et al. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J Extracell Vesicles (2020) 10:e12030. doi: 10.1002/jev2.12030
91. Fang J, Liang W. ASCs -derived exosomes loaded with vitamin a and quercetin inhibit rapid senescence-like response after acute liver injury. Biochem Biophys Res Commun (2021) 572:125–30. doi: 10.1016/j.bbrc.2021.07.059
92. Zhao J, Wang Y, Wang W, Tian Y, Gan Z, He H, et al. In situ growth of nano-antioxidants on cellular vesicles for efficient reactive oxygen species elimination in acute inflammatory diseases. Nano Today (2021) 40. doi: 10.1016/j.nantod.2021.101282
93. Qi CX, Liu XS, Zhi DK, Tai YF, Liu YF, Sun QQ, et al. Exosome-mimicking nanovesicles derived from efficacy-potentiated stem cell membrane and secretome for regeneration of injured tissue. Nano Res (2022) 15:1680–90. doi: 10.1007/s12274-021-3868-z
94. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol (2014) 14:43. doi: 10.1186/1471-2288-14-43
95. Lewin S, Glenton C, Munthe-Kaas H, Carlsen B, Colvin CJ, Gulmezoglu M, et al. Using qualitative evidence in decision making for health and social interventions: An approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PloS Med (2015) 12:e1001895. doi: 10.1371/journal.pmed.1001895
96. Zhang J, HF C, Wang H, Shao D, Tao Y, Li M. Stem cell therapy and tissue engineering strategies using cell aggregates and decellularized scaffolds for the rescue of liver failure. J Tissue Eng (2021) 12:2041731420986711. doi: 10.1177/2041731420986711
Keywords: extracellular vesicles, liver failure, diagnosis, prognosis assessment, treatment, systematic review
Citation: Lu W, Tang H, Li S, Bai L and Chen Y (2023) Extracellular vesicles as potential biomarkers and treatment options for liver failure: A systematic review up to March 2022. Front. Immunol. 14:1116518. doi: 10.3389/fimmu.2023.1116518
Received: 05 December 2022; Accepted: 09 February 2023;
Published: 22 February 2023.
Edited by:
Gerardo Guillen, Center for Genetic Engineering and Biotechnology (CIGB), CubaReviewed by:
Baihai Jiao, University of Connecticut Health Center, United StatesKunkai Su, Zhejiang University, China
Copyright © 2023 Lu, Tang, Li, Bai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Chen, Y2h5YmV5b25kMTA3MUBjY211LmVkdS5jbg==; Li Bai, dGVuZGVyNzhAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work
 Wang Lu
Wang Lu Huixin Tang1,2†
Huixin Tang1,2† Li Bai
Li Bai Yu Chen
Yu Chen