
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 08 March 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1114930
This article is part of the Research TopicMethods in Autoimmune and Autoinflammatory Disorders: 2022View all 11 articles
 Jinying Fang
Jinying Fang Mingxuan Liu
Mingxuan Liu Zhenghui Huang
Zhenghui Huang Yucao Ma
Yucao Ma Yiwen Wang
Yiwen Wang Xiaojia Zheng
Xiaojia Zheng Liu Lv
Liu Lv Chunpin Liu
Chunpin Liu Wei Li
Wei Li Zhenghong Zhu
Zhenghong Zhu Huachao Zhu
Huachao Zhu Jie Hu
Jie Hu Yonghong Wang
Yonghong Wang Hailong Wang*
Hailong Wang*Background: Traditional Chinese medicines (TCMs), such as Tripterygium wilfordii Hook F (TwHF), Glycyrrhiza uralensis, Caulis sinomenii and others have anti-inflammatory effects. They are widely used in China to treat rheumatoid arthritis (RA), but proof of their use as an evidence-based medicine is little. The aim of this network meta-analysis (NMA) was to evaluate the efficacy and safety of TCMs.
Methods: By searching online databases and using a manual retrieval method, randomized controlled trials (RCTs) that met specific selection criteria were included in the meta-analysis. The search included papers that were published between the establishment of the databases and November 10, 2022. Analyses were performed using Stata software (version 14) and Review Manager (version 5.3).
Results: 61 papers with 6316 subjects were included in the current NMA. For ACR20, MTX plus SIN therapy (94.30%) may be a significant choice. For ACR50 and ACR70, MTX plus IGU therapy (95.10%, 75.90% respectively) performed better than other therapies. IGU plus SIN therapy (94.80%) may be the most promising way to reduce DAS-28, followed by MTX plus IGU therapy (92.80%) and TwHF plus IGU therapy (83.80%). In the analysis of the incidence of adverse events, MTX plus XF therapy (92.50%) had the least potential, while LEF therapy (22.10%) may cause more adverse events. At the same time, TwHF therapy, KX therapy, XF therapy and ZQFTN therapy were not inferior to MTX therapy.
Conclusions: TCMs with anti-inflammatory effect were not inferior to MTX therapy in the treatment of RA patients. Combining with TCMs can improve the clinic efficacy and reduce the possibility of adverse events of DMARDs, which may be a promising regimen.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022313569.
Rheumatoid arthritis (RA) is a common chronic inflammatory disease that leads to severe joint damage, disability and low quality of life. What’s more, RA patients may suffer from serious comorbidities, such as lung disease, cardiovascular disease, osteoporosis, etc. RA is a worldwide social and economic burden, as it occurs in approximately 5-10 per 1000 people (1–3). Many studies have shown disease-modifying anti-rheumatic drugs (DMARDs) could prevent or reduce joint damage, and maintain normal joint function. Although, various types of DMARDs have been used for RA patients, including conventional synthetic DMARDs, targeted synthetic DMARDs and biologic DMARDs, more than 40% of patients could not control the progression of RA or tolerate adverse effects after taking DMARDs (4, 5). Therefore, more and more attention has been paid to traditional Chinese medicines (TCMs), which have a long history in the treatment of RA. TCMs, such as Tripterygium wilfordii Hook F (TwHF), Glycyrrhiza uralensis, and Caulis sinomenii, have anti-inflammatory and anti-angiogenic effects (6–9).
TwHF preparation is widely used in RA patients in China. A project (2017YFC0907604) from The Chinese National Key Research R&D Program found that among 82,589 RA patients, 16.5% were taking TwHF, ranking fourth just behind methotrexate (MTX), leflunomide (LEF) and hydroxychloroquine at the end of November 10, 2022. The recent randomized control trial (RCT) reported by Yang-Zhong Zhou et al. (10) illustrates TwHF has similar efficacy in the treatment of RA compared to MTX in the American College of Rheumatology 20% (ACR20), ACR50, ACR70 assessment. Triptolide is the main bioactive component of TwHF and is primarily responsible for the anti-inflammatory effect. Some breakthroughs in molecular biology are related to triptolide, which helps us in using TwHF preparation (11, 12).
Kunxian capsule and Xinfeng capsule are the new generations of TwHF preparations, which could relieve joint pain, joint swelling and morning stiffness. They could reduce toxicity and increase efficacy by adding matrimony vine, Astragalus membranaceus, Epimedium, centipede, etc (13, 14). Sinomenine (SIN) and its new preparation, Zhengqing Fengtongning capsule, also have an anti-inflammatory function to inhibit the progression of RA (9). However, large-scale RCTs are still lacking, especially data on the efficacy and safety of various TCMs compared with other DMARDs.
This network meta-analysis (NMA) differs from a traditional meta-analysis in that it may directly or indirectly compare the efficacy of multiple interventions simultaneously. This NMA aims to compare the efficacy and safety of various TCMs with DMARDs in RA patients. The results will provide a basis for strengthening conclusions to guide and support the clinical use of the TCMs with anti-inflammatory effects in RA patients.
RCTs investigating the treatment of RA with anti-inflammatory TCMs, published in either English or Chinese, regardless of the use of the blind method. The study protocol was registered with PROSPERO (CRD42022313569).
Participants were defined as having been diagnosed with RA according to the 1987 American Rheumatology Association guidelines (15) or the 2010 ACR/European League against Rheumatism (EULAR) criteria (16).
Tripterygium Glycoside Tablet or Tripterygium Tablet (TwHF), Kunxian Capsule (KX), Xinfeng Capsule (XF), Zhengqing Fengtongning Capsule (ZQFTN), SIN, MTX, LEF, Iguratimod (IGU), Sulfasalazine (SSZ) used singly or as a two-drug combination in the treatment of RA. The duration of treatment was not less than 8 weeks. There is to be no limitation on the use of non-steroidal anti-inflammatory drugs, folic acid, calcium tablets, vitamins, and low-dose hormones during the treatment.
ACR20, ACR50, ACR70, disease activity score in 28 joints (DAS-28) and adverse event.
(1) patients with other autoimmune diseases or other serious conditions that could influence the results, such as severe heart failure, cancer, DIC, and severe infections; (2) studies that were abstracts, case reports, reviews, commentaries, and editorials, etc.; (3) literature with repetitive content; or (4) interventions such as herbs containing.
This study used PubMed, Web of Science, the Cochrane Library, Excerpta Medica Database (EMBASE), VIP Information/Chinese Scientific Journals, China Network Knowledge Infrastructure (CNKI), and WANFANG databases to search for relevant studies. The literature search included articles that were published between the establishment of the databases and November 10, 2022.
We conducted electronic searches using exploded Medical Subject Headings (MeSH) terms and various combinations of the keywords. The search terms used were (MeSH exp “Rheumatoid arthritis” and key words “Caplan Syndrome”, “Felty Syndrome”, “Rheumatoid Nodule”, “Rheumatoid Vasculitis”, “Sjogren’s Syndrome”, “Rheumatic Diseases”, “Rheumatic”, and “Rheumatic Diseases*”), (MeSH exp “tripterygium” and key words “Tripterygium* wilfordii”, “Tripterygium* wilfordius”, “Leigong Teng*”, “Leigong Teng*”, and “Thundergod Vine*”), “Kunxian Capsule”, “Xinfeng Capsule”, “Zhengqing Fengtongning Capsule”, “SIN”, “Methotrexate”, “Leflunomide”, “Iguratimod”, “Sulfasalazine”. At the same time, reference lists of included textbooks, all retrieved studies, review articles, and reports of academic congresses were checked manually. A comprehensive search strategy is shown in Supplementary Table S3. For the Chinese databases, free texts were used, such as “Lei gong teng”, “Lei Gong TengZhiji”, “Lei Gong Teng Duo Gan”, “Jia An Die Ling (MTX)”,”Lai Fu Mi Te (LEF)”, “Kun Xian Jiao Nang (Kunxian Capsule)”, “Xin Feng Jiao Nang (Xinfeng Capsule)”, “Zheng Qing Feng Tong Ning (Zhengqing Fengtongning Capsule)”, “Ai La Mo De (Leflunomide)”, “Liu Dan Huang An Pi Ding (Sulfasalazine)”, “Qing Teng Jian (Sinomenine)”, “Sui Ji Dui Zhao Shi Yan (RCT)”, “Lei feng shi guan jie yan (rheumatoid arthritis)”.
Two investigators (Fang and Liu) independently reviewed the studies from the retrieved literature, based on the inclusion criteria, and extracted their analytical results and data. If two investigators had different opinions about the quality of a study, a third author (Huang) reassessed the differences. Data were only included for review only when a consensus was reached among all three investigators.
Two investigators (Fang and Liu) independently assessed the risk of bias using the Cochrane risk-of-bias tool. Each study was reviewed and scored as high (if the answer was yes), low (if the answer was no), or unclear (if there were insufficient details to make a definite judgment), based on the following criteria: (1) selection bias (random sequence generation), (2) selection bias (random sequence generation), (3) performance bias (blinding of participants and personnel), (4) detection bias (blinding of outcome assessment), (5) attrition bias (incomplete outcome data), (6) reporting bias (selective reporting), and (7) other bias.
The endpoints of this NMA were ACR20, ACR50, ACR70, DAS-28 and adverse events. ACR20 is defined as a reduction by 20% or greater reduction in the number of tender and swollen joints plus a 20% improvement in at least three of the following five measures: pain, patient global assessment, physician global assessment, physical disability score, and blood acute-phase reactants. ACR50 is defined as an reduction of 50% or greater reduction in the number of tender and swollen joints, plus 50% improvement in at least three of the aforementioned five measures. ACR70 is defined as an reduction of 70% or greater reduction in the number of tender and swollen joints, plus 70% improvement in at least three of the above five measures. Disease Activity Score in 28 joints (DAS-28) is related to tender and swollen joints, Erythrocyte Sedimentation Rate (ESR) and general health (GH). The formula is as following:
In this meta-analysis, the mean differences (MD) were chosen as the effect sizes for continuous outcomes. The weighted mean difference ((WMD = √SD12 plus SD22 – SD1 × SD2); SD = baseline endpoint) was used to evaluate measured data. For dichotomous outcomes, pooled odds ratios (OR) were calculated with corresponding 95% confidence intervals (CI). Because of the clinical and methodological heterogeneity of both treatments and subjects among those enrolled trials, random-effect models were used in the meta-analysis (17, 18). First, the Bayesian NMA was performed using STATA software (version 14). The network graph could display the relationship among different interventions for each outcome. For example, line thicknesses indicated the number of trials and the node sizes corresponded to the total sample sizes for treatments. The hierarchy of treatment rankings was estimated by the value of surface under the cumulative ranking curve (SUCRA), in which the more enormous SUCRA value of comparisons was regarded as the better efficacy or the lower the adverse event (19). Using the loop-specific approach evaluates the agreement between direct and indirect sources of evidence in each closed loop (20). Publication bias was assessed using funnel plots and consistency tests.
Electronic and manual searches yielded 25895 potentially relevant papers; 2613 from Pubmed, 1657 from Web of Science,1786 from the EMBASE, 1416 from the Cochrane Library, 7213 from CNKI, 5980 from VIP Information/Chinese Scientific Journals, 5179 from WANFANG, and 51 manually. After removing duplicated papers, 18610 papers remained. Of these, 271 papers were selected after reviewing their titles and abstracts. After reviewing the full text of each publication and based on the exclusion criteria, 91 papers were selected. After reviewing the studies included in the qualitative synthesis, 61 papers were selected, including 6316 patients (10, 14, 21–79). The selection process is shown in Figure 1. Detailed information on the included studies is provided in Supplementary Table S1. The entire network plots of different comparisons for all outcomes are shown in Figure 2.
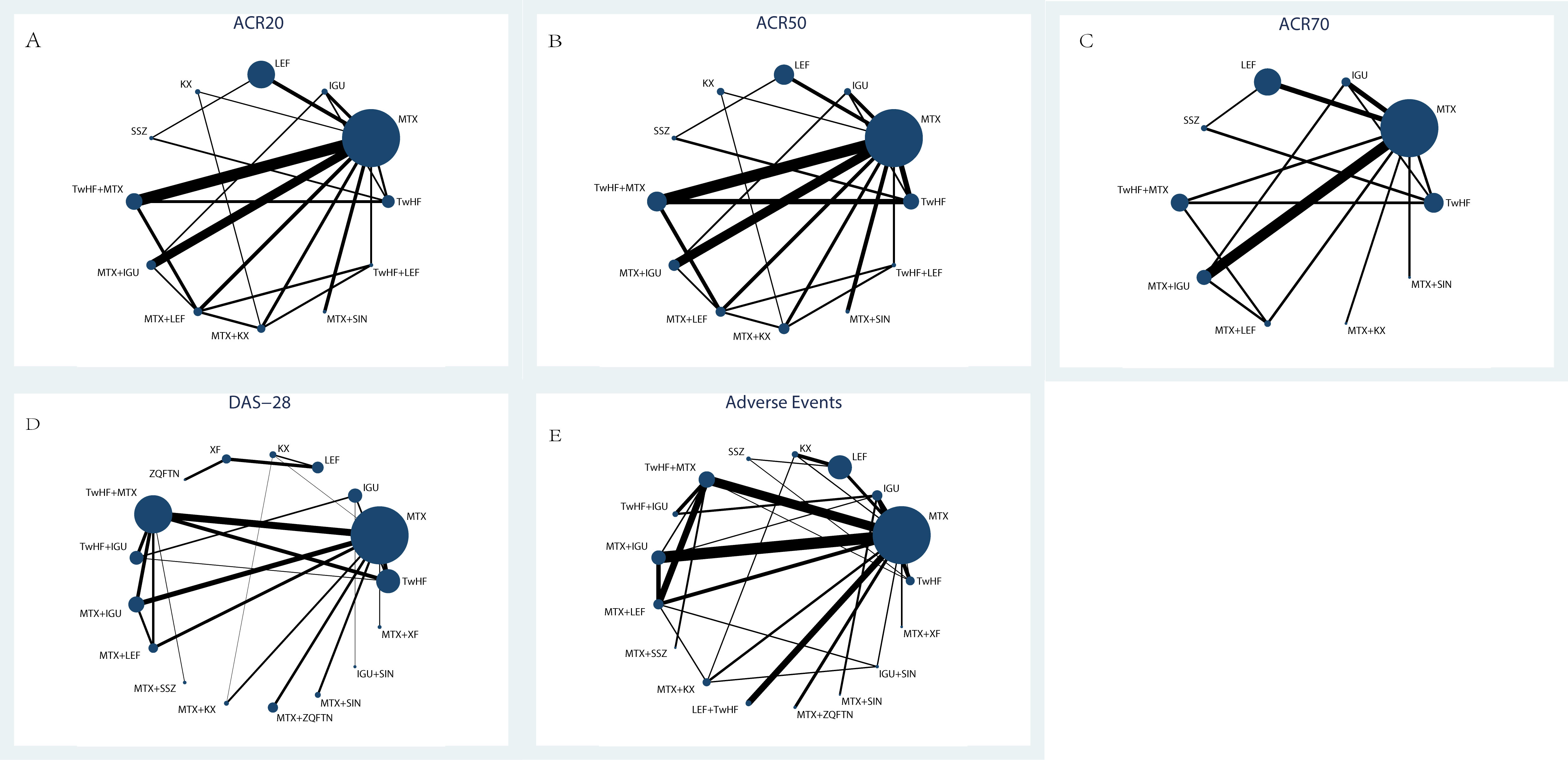
Figure 2 The evidence network of all papers about different treatments. (A) ACR20; (B) ACR50; (C) ACR70; (D) DAS-28; (E) Adverse Events. Line thicknesses corresponded to the number of trials, and node sizes indicated the total sample sizes for treatments.
The overall quality of the studies included in this review was un satisfactory; details of the risk-of-bias assessment are shown in Figure 3. All 61 papers used the random number acquisition method, but none offered a detailed description. Of the 61 papers included, 20 used random number table, 1 used the computer random method and 1 used bicolor random.
In the evaluation of the ACR20, 28 studies were included with a total of 3630 patients. There were 12 types of interventions, including TwHF therapy, MTX therapy, IGU therapy, KX therapy, LEF therapy, SSZ therapy, MTX plus TwHF therapy, MTX plus KX therapy, MTX plus LEF therapy, TwHF plus LEF therapy, MTX plus IGU therapy and MTX plus SIN therapy, as shown in Figure 4A. MTX plus SIN therapy (94.30%) exhibited the most significant possibility of improving ACR20 over other treatments, followed by MTX plus LEF therapy (93.30%) and MTX plus IGU therapy (76.00%). For single drug therapy, KX therapy (28.20%) and TwHF therapy (39.20%) were not inferior to MTX therapy (23.80%) and LEF therapy (23.60%) in improving ACR20, as shown in Table 1.
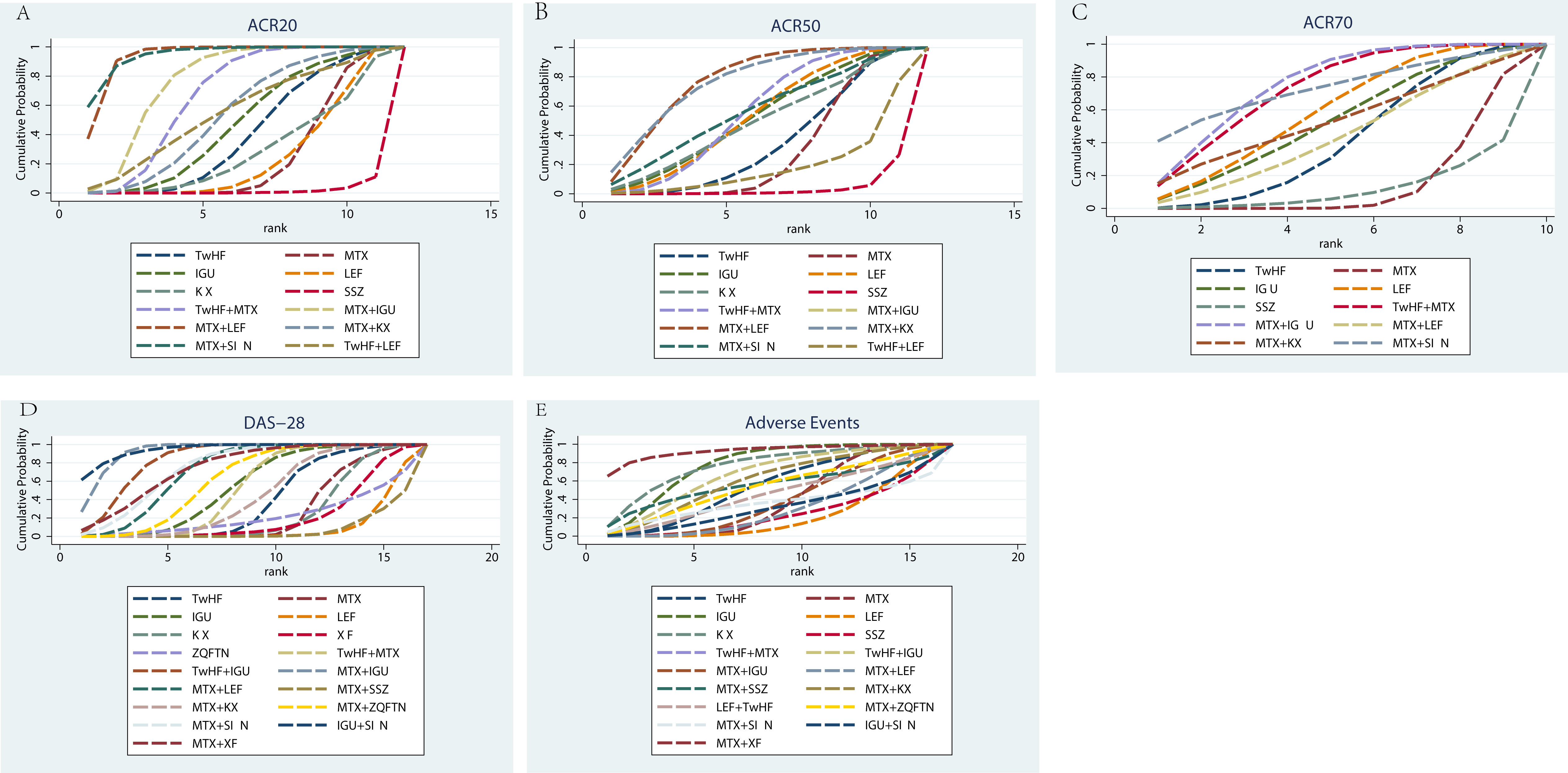
Figure 4 Rank of the cumulative probabilities for basic parameters. (A) ACR20; (B) ACR50; (C) ACR70; (D) DAS-28; (E) Adverse Events.
Regarding the ACR50, 27 studies were included with a total of 3032 patients. There were 12 types of interventions, including TwHF therapy, MTX therapy, IGU therapy, KX therapy, LEF therapy, SSZ therapy, MTX plus TwHF therapy, MTX plus KX therapy, MTX plus LEF therapy, TwHF plus LEF therapy, MTX plus IGU therapy and MTX plus SIN therapy, as shown in Figure 4B. MTX plus IGU therapy (95.10%), MTX plus LEF therapy (77.30%) and MTX plus KX therapy (76.40%) did better in improving ACR50 than other therapies, as shown in Table 1. For single drug therapy, TwHF therapy (34.60%) was not inferior to MTX therapy (29.10%) in improving ACR50. Meanwhile, KX therapy (49.00%) and LEF therapy (53.00%) were similar in ACR50.
In the evaluation of the ACR70, 19 studies were included with a total of 2348 patients. There were 10 types of interventions, including TwHF therapy, MTX therapy, IGU therapy, LEF therapy, SSZ therapy, MTX plus TwHF therapy, MTX plus KX therapy, MTX plus LEF therapy, MTX plus IGU therapy and MTX plus SIN therapy, as shown in Figure 4C. MTX plus IGU therapy (75.90%), MTX plus SIN therapy (73.10%) and TwHF plus MTX therapy (73.00%) did better in improving ACR70 than other therapies, as shown in Table 1.
In the evaluation of the DAS-28, 40 studies were included with a total of 3418 patients. There were 17 types of interventions, including TwHF therapy, MTX therapy, IGU therapy, KX therapy, LEF therapy, ZQFTN therapy, XF therapy, MTX plus TwHF therapy, MTX plus KX therapy, MTX plus LEF therapy, TwHF plus IGU therapy, MTX plus ZQFTN therapy, MTX plus IGU therapy, MTX plus SSZ therapy, MTX plus SIN therapy, IGU plus SIN therapy, and MTX plus XF therapy, as shown in Figure 4D. IGU plus SIN therapy (94.80%) had the most significant possibility of improving DAS-28 over other treatments, followed by MTX plus IGU therapy (92.80%) and TwHF plus IGU therapy (83.80%). For single drug therapy, TwHF therapy (38.10%), KX therapy (24.60%), XF therapy (20.20%) and ZQFTN therapy (21.50%) were not inferior to MTX therapy (26.00%) in improving DAS-28, as shown in Table 1.
In the incidence of adverse events, 47 studies were included with a total of 5111 patients, including gastrointestinal reactions, liver dysfunction, allergic reactions, headache, etc, as shown in Supplementary Table S2. There were 17 types of interventions, including TwHF therapy, MTX therapy, IGU therapy, KX therapy, LEF therapy, SSZ therapy, MTX plus TwHF therapy, MTX plus KX therapy, MTX plus LEF therapy, TwHF plus IGU therapy, MTX plus ZQFTN therapy, MTX plus IGU therapy, MTX plus SSZ therapy, TwHF plus LEF therapy, MTX plus SIN therapy, IGU plus SIN therapy, and MTX plus XF therapy (Figure 4E). The SUCRA values of each treatment for adverse events showed MTX plus XF therapy (92.50%) had the least potential for the incidence of adverse events than other treatments, while LEF therapy (22.10%) may cause more adverse events, as shown in Table 1.
In this study, a forest plot was generated to assess for inconsistency, as shown in Supplementary Figure S1. With the exception of the M-S-T closed loop with all outcomes, there was no apparent inconsistency in any of the other closed loops, as shown in Table 2.
Cluster analysis was used to identify the most promising therapeutic strategies among the different treatments in terms of ACR20, ACR50, ACR70, DAS-28 and the incidence of adverse events simultaneously. As shown in Figure 5A, the results of the cluster analysis showed that MTX plus KX therapy and IGU therapy were associated with favorable benefits both for improving the ACR20 and reducing the incidence of adverse events compared with the other treatments. In contrast, LEF therapy and SSZ therapy were the worst treatments in improving ACR20 and reducing the incidence of adverse events, as shown in Figure 5A. MTX plus KX therapy had the greatest possibility of improving ACR50 and reducing adverse events, while SSZ therapy was the worst treatment, as shown in Figure 5B. For improving ACR70 and reducing the adverse events, IGU therapy was the most effective treatment, while SSZ therapy was the worst treatment, as shown in Figure 5C. For DAS-28 and adverse events, MTX plus XF therapy was the most effective treatment, while LEF therapy was the worst treatments, as shown in Figure 5D.
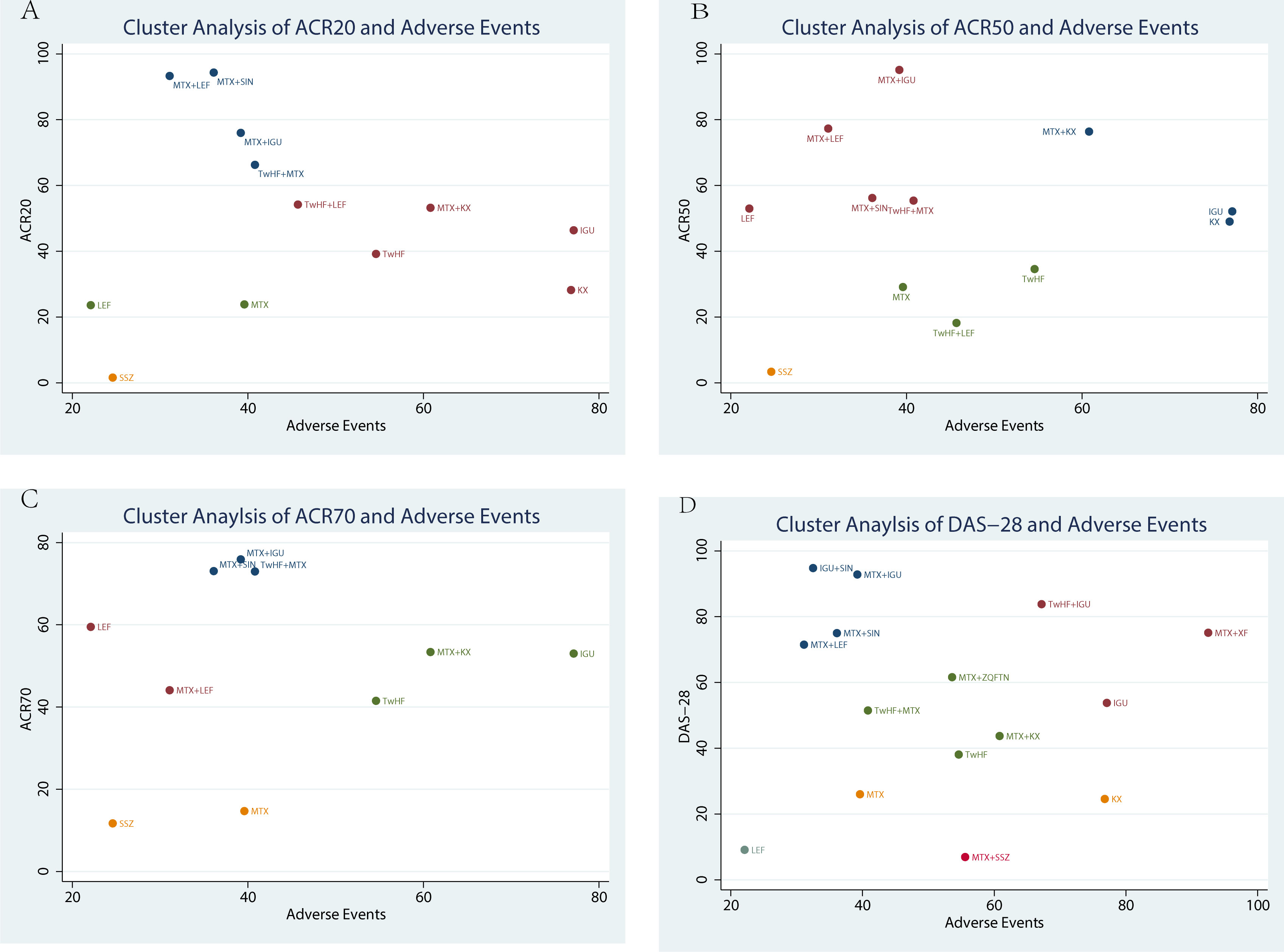
Figure 5 Cluster analysis plot of efficacy and safety. (A) the adverse events (X-axis) and ACR20 (Y-axis); (B) the adverse events (X-axis) and ACR50 (Y-axis); (C) the adverse events (X-axis) and ACR70(Y-axis); (D) the adverse events (X-axis) and DAS-28 (Y-axis).
According to the funnel plot of ACR20, ACR50, ACR70, DAS-28 and adverse events, this NMA may have a potential publication bias in the present study, as shown in Figure 6.
There was no consensus on the use of TCMs in combination with other DMARDs in the treatment of RA. This NMA was conducted to generate a hierarchy of treatment rankings. The ranking probabilities for these treatments were further calculated in terms of their clinical efficacy and safety under in various endpoints to provide a basis for making better optimal choices. This NMA found that for ACR20, MTX plus SIN therapy (94.30%) had the greatest likelihood of achieving the best efficacy among the treatment regimens involved, followed by MTX plus LEF therapy (93.30%) and MTX plus IGU therapy (76.00%). For ACR50 response, MTX plus IGU therapy (95.10%), MTX plus LEF therapy (77.30%), and MTX plus KX therapy (76.40%) performed better than other therapies. For ACR70, MTX plus IGU therapy (75.90%) ranked first. In the assessment of DAS-28, IGU plus SIN therapy (94.80%) had the greatest potential to improve DAS-28 over other treatments, followed by MTX plus IGU therapy (92.80%) and TwHF plus IGU therapy (83.80%). In the analysis of the incidence of adverse events, MTX plus XF therapy (92.50%) had the least potential, while LEF therapy (22.10%) may cause more adverse events. At the same time, TwHF therapy, KX therapy, XF therapy and ZQFTN therapy were not inferior to MTX therapy. According to the results of cluster analysis, MTX plus KX therapy and MTX plus XF therapy may be good choices in terms of both efficacy and safety. We found that combining with TCMs could improve the clinical efficacy and reduce the possibility of adverse events of other DMARDs.
RA is a chronic inflammatory autoimmune disease, but why it occurs is still uncertain as yet. In general, we believe that occurrence of RA is related to genetic, epigenetic and environmental factors (80). However, the most important factor is the inflammatory immune response, which is characterized by an increase in inflammatory cytokines and chemokines, circulating autoantibodies and increasing concentration (81). Synovial hyperplasia is the main pathological manifestation of RA, which is the main cause of joint damage (81). A network pharmacology found the mechanisms of TwHF would treat RA through 31 signaling pathways, and then it inactivates TNF and NF-kappa B signaling pathways to inhibit the inflammatory response (82). RA-synovial fibroblasts (RASFs) are the main factors of joint destruction in RA (83). The main component of Kunxian Capsule and Xinfeng Capsule is triptolide, which is extracted from TwHF. TwHF inhibited PGE2 production by IL-1β-stimulated RASFs and inhibited COX-2 protein expression. At the same time, it can inhibit lipopolysaccharide-induced chemokine CCL5 production by RASFs and induce apoptosis of RASFs by activating caspase-3 activity (84–86). TwHF reduces the production of IL-1, IL-17, and TNF-α, which would protect chondrocytes (87). Also, Tripterygium wilfordii significantly inhibits the transcription and generation of IL-17 from murine splenocytes and purified CD4+ T cells by repressing IL-6-induced phosphorylation of STAT3 (88). The animal experiments also show that it could apparently relieve joint pain and swelling by suppressing the releases of IL-1α, IL-1βIL-4, IL-10 and MMP3 (89).
Sinomenine (SIN) is extracted from CAULIS SINOMENII, which is the main active ingredient of Zhengqing Fengtongning capsule (90). Many studies demonstrated sinomenine could alleviate morning stiffness and joint pain in the RA patients, by modifying neurotransmission, inhibiting cyclooxygenase 2-dependent prostaglandin E2 and NO, TNF, INF-γ, IL-6, IL-1β, and IL-4, and suppressing the activity of P38 MAPK, MMPs, and NF-κB (91, 92). At the same time, SIN could regulate immunity and alleviate inflammation by inhibiting T- lymphocyte and B-lymphocyte activation (93), regulating NLRP3 inflammasome and NF-κB pathway (94). Our NMA showed, Zhengqing Fengtongning capsule combined with MTX could obviously reduce the DAS-28 of RA patients. Thus, Zhengqing Fengtongning capsule may be a good choice for RA patients, who want to reduce morning stiffness and joint pain.
This NMA had some limitations. First, during the literature review, we found that not all studies described the allocation concealment procedure or the randomization process in great detail; so selection bias could not be completely excluded. Second, the number of articles with different treatment methods varies widely, which may lead to relatively imprecise conclusions. In the same time, the sample size of some studies was small and the treatment cycle was short. Next, some treatments had few papers, which may lead to statistical bias. Finally, TCMs are not widely used in other countries. Therefore, almost all selected papers in this NMA were from China, which may have caused regional, language, and racial biases. We hope that in the future there will be large-scale RCTs in different countries to further provide more reliable data.
According to the results of ACR20, MTX plus SIN therapy may be the significant choice for RA patients. According to the results of ACR50 and ACR70, MTX plus IGU therapy may be the significant choice for RA patients. According to the results of DAS-28, IGU plus SIN therapy had the greatest possibility. According to the results of safety, MTX plus XF therapy had the least potential for the incidence of adverse events. MTX plus KX therapy and MTX plus XF therapy may be good choices, considering both efficacy and safety. In conclusion, combining with TCMs can improve the clinical efficacy and reduce the possibility of adverse events of DMARDs, which may be a promising regimen.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The article was designed by H-LW. J-YF drafted the manuscript and data curation with support of H-LW. M-XL and Z-HH contributed to data curation, validation, and writing review. All authors contributed to the scientific discussion of the integrative approach and of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by funding from the National Natural Science Foundation of China (Num: 82174336), National Key R&D Program (Num: 2022YFC3501203), the National Natural Science Foundation of China (Num: 82104652), and Beijing Tongzhou District Science and Technology Project (Num: KJ2022CX045).
This NMA was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA guidelines.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1114930/full#supplementary-material
1. Zhang D, Lyu JT, Zhang B, Zhang XM, Jiang H, Lin ZJ. Comparative efficacy, safety and cost of oral Chinese patent medicines for rheumatoid arthritis: A Bayesian network meta-analysis. BMC complementary Med therapies (2020) 20(1):210. doi: 10.1186/s12906-020-03004-4
2. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: A review. Jama (2018) 320(13):1360–72. doi: 10.1001/jama.2018.13103
3. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ (Clinical Res ed) (2018) 361:k1036. doi: 10.1136/bmj.k1036
4. Lin N, Zhang YQ, Jiang Q, Liu W, Liu J, Huang QC, et al. Clinical practice guideline for tripterygium Glycosides/Tripterygium wilfordii tablets in the treatment of rheumatoid arthritis. Front Pharmacol (2020) 11:608703. doi: 10.3389/fphar.2020.608703
5. Lin YJ, Anzaghe M, Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells (2020) 9(4):880. doi: 10.3390/cells9040880
6. Zhang W, Li F, Gao W. Tripterygium wilfordii inhibiting angiogenesis for rheumatoid arthritis treatment. J Natl Med Assoc (2017) 109(2):142–8. doi: 10.1016/j.jnma.2017.02.007
7. Nong C, Wang XZ, Jiang ZZ, Zhang LY. Progress of effect and mechanisms of tripterygium wilfordii on immune system. Zhongguo Zhong yao za zhi (2019) 44(16):3374–83. doi: 10.19540/j.cnki.cjcmm.20190419.401
8. Sharma T, Sharma P, Chandel P, Singh S, Sharma N, Naved T, et al. Circumstantial insights into the potential of traditional Chinese medicinal plants as a therapeutic approach in rheumatoid arthritis. Curr Pharm design (2022) 28(26):2140–9. doi: 10.2174/1381612828666220324124720
9. Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long H, et al. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and Monocyte/Macrophage subsets. Front Immunol (2018) 9:2228. doi: 10.3389/fimmu.2018.02228
10. Zhou YZ, Zhao LD, Chen H, Zhang Y, Wang DF, Huang LF, et al. Comparison of the impact of tripterygium wilfordii hook f and methotrexate treatment on radiological progression in active rheumatoid arthritis: 2-year follow up of a randomized, non-blinded, controlled study. Arthritis Res Ther (2018) 20(1):70. doi: 10.1186/s13075-018-1563-6
11. Tu L, Su P, Zhang Z, Gao L, Wang JD, Hu TY, et al. Author correction: Genome of tripterygium wilfordii and identification of cytochrome P450 involved in triptolide biosynthesis. Nat Commun (2020) 11(1):5309. doi: 10.1038/s41467-020-19253-3
12. Tong L, Zhao Q, Datan E, Lin GQ, Minn II, Pomper G, et al. Triptolide: reflections on two decades of research and prospects for the future. Natural product Rep (2021) 38(4):843–60. doi: 10.1039/D0NP00054J
13. Yan HX, Xu CF, Yang H, Wen XY, Wang ZP, Chen YH, et al. Network pharmacology-based analysis on the curative effect of kunxian capsules against rheumatoid arthritis. Evidence-Based complementary Altern medicine eCAM (2021) 2021:6812374. doi: 10.1155/2021/6812374
14. Zhang PH, Liu J, Tan B, Zhu FB, Fang L. XinFeng capsule can adjust NF-κB-channel improves hypercoagulability in patients with rheumatoid arthritis. J Immunol (2016) 32(01):49–55. doi: 10.13431/j.cnki.immunol.j.20160010
15. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis rheumatism (1988) 31(3):315–24. doi: 10.1002/art.1780310302
16. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. Rheumatoid arthritis classification criteria: An American college of Rheumatology/European league against rheumatism collaborative initiative. Arthritis rheumatism (2010) 62(9):2569–81. doi: 10.1002/art.27584
17. Jackson D, Turner R, Rhodes K, Viechtbauer W. Methods for calculating confidence and credible intervals for the residual between-study variance in random effects meta-regression models. BMC Med Res Method (2014) 14:103. doi: 10.1186/1471-2288-14-103
18. Chan JS. Bayesian Informative dropout model for longitudinal binary data with random effects using conditional and joint modeling approaches. Biometrical J Biometrische Z (2016) 58(3):549–69. doi: 10.1002/bimj.201400064
19. Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: Application and practice using stata. Epidemiol Health (2017) 39:e2017047. doi: 10.4178/epih.e2017047
20. Cai W, Gu Y, Cui H, Cao Y, Wang X, Yao Y, et al. The efficacy and safety of mainstream medications for patients with cDMARD-naïve rheumatoid arthritis: A network meta-analysis. Front Pharmacol (2018) 9:138. doi: 10.3389/fphar.2018.00138
21. Feng L, Ma L. Study on efficacy and safety of combined methotrexate with tripterygium wilfordii polyglycoside tablets in treatment of patients with rheumatoid arthritis. J Clin Exp Med (2013) 12(09):659–61.
22. Sun W, Liu XM. Comparison of efficacy of methotrexate combined with leflunomide or tripterygium wilfordii polyglycosides in the treatment of early rheumatoid arthritis with positive anti cyclic citrullinated peptide antibody. Shanxi Med J (2010) 39(01):59–60.
23. Tian W, Feng XY, Song J, Zhong W. Clinical efficacy of methotrexate combined with tripterygium wilfordii polyglycoside tablets in elderly patients with active rheumatoid arthritis. ZHONGHUA YANGSHENG BAOJIAN (2021) 39(10):156–8.
24. Zhu L, Chen P. Comparison of the efficacy of methotrexate combined with tripterygium wilfordii polyglycosides and methotrexate alone in the treatment of rheumatoid arthritis. Lab Med Cli (2015) 12(23):3568–70.
25. Yang M, Zhou RH LI, Li BZ, Xu J, Shi YH, Mo HY. Clinical observation on treatment of Rheumatoid arthritis with tripterygium wilfordii polyglycoside combined with methotrexa. Chin J Exp Traditional Med Formulae (2013) 19(17):300–4.
26. Jia CP. Clinical research on the treatment of rheumatoid arthritis with cold and dampness syndromes with the kunxian capsule and the methotrexate. Hubei: Hubei University of Traditional Chinese Medicine (2016).
27. Jiang ZR, Gao ML, Liu YY. Clinical observation on kunxian capsule in treating active rheumatoid arthritis. Guangming Tradit Chin Med (2015) 30(02):279–81.
28. Xu B, Huang BB, Xu GH, Hao B, Yu Y, Lin J. Effect of kunxian capsule on elderly rheumatoid arthritis and its influence on bone metabolism. China Prac Med (2020) 15(34):14–7. doi: 10.14163/j.cnki.11-5547/r.2020.34.004
29. Jiang JX. Clinical observation of leflunomide combined with methotrexate in the treatment of rheumatoid arthritis. Chin J Clin Rational Drug Use (2018) 11(16):31–32+34. doi: 10.15887/j.cnki.13-1389/r.2018.16.015
30. Liu YM, Lei YM, Zhang B, Xia LP, Lu J, Shen H. Comparison of KUN XIAN with leflunomide in the treatment of rheumatoid arthritis. Prog Anatomical Sci (2019) 25(01):17–20. doi: 10.16695/j.cnki.1006-2947.2019.01.006
31. Zhu JQ, Qian JD. Effect of leflunomide combined with methotrexate on rheumatoid arthritis. Med frontier (2016) (4):192–3.
32. Chen P, Zhu L, Zou X, Du HL, Zhou Y, Gu XH. Tripterygium glucosides combined with methotrexate in treatment of rheumatoid arthritis: A randomised controlled trial. J AnHui TCM Coll (2011) 30(06):28–32.
33. Pan ZP, Lin SP, Lin X. Observing the short-term curative effect of tripterygium glycosides combined with methotrexate in treating rheumatoid arthritis. Rheumatism Arthritis (2014) 3(03):17–20.
34. Long CY, Liang Y, Li N, Liu HM, Wang YL. Clinical efficacy of tripterygium wilfordii polyglycosides tablets in the elderly patients with rheumatoid arthritis. Inner Mongolia J Traditional Chin Med (2019) 38(09):72–3. doi: 10.16040/j.cnki.cn15-1101.2019.09.044
35. Wang M, Huang J, Fan H, He D, Zhao SY, Shu YS, et al. Treatment of rheumatoid arthritis using combination of methotrexate and tripterygium glycosides tablets-a quantitative plasma pharmacochemical and pseudotargeted metabolomic approach. Front Pharmacol (2018) 9:1051. doi: 10.3389/fphar.2018.01051
36. Lei SW, Zhou SH, Li ZJ, Wang HJ, Yang D. Effect of methotrexate combined with tripterygium wilfordii polyglycosides on middle-aged and elderly patients with rheumatoid arthritis and on metalloproteinase-3. Gansu Med J (2020) 39(06):513–6. doi: 10.15975/j.cnki.gsyy.2020.06.011
37. Wang WQ. Methotrexate combined with zhengqingfengtongning sustained-release tablets in the treatment of 120 patients with rheumatoid arthritis. Zhejiang Pract Med (2010) 15(04):280–281+305. doi: 10.16794/j.cnki.cn33-1207/r.2010.04.013
38. Lin CS, Yang XY, Dai L, Sun WF, Yang SF, Shen Y, et al. Multi-center clinical study on therapeutic effect of kunxian capsule on rheumatoid arthritis. Chin J Integrated Traditional Western Med (2011) 31(06):769–74.
39. Liu J, Chen RL. Effect of xinfeng capsule on the content of peripheral blood CD4+ CD25+ CD127lo regulatory T cell of rheumatoid arthritis patients in active stage. J Shandong Univ Traditional Chin Med (2009) 33(06):480–3. doi: 10.16294/j.cnki.1007-659x.2009.06.010
40. Sun Y, Liu J, Wan L. Effect of xinfeng capsule on improving pulmonary function in rheumatoid arthritis patients. Chin J Integrated Traditional Western Med (2016) 36(07):814–20.
41. Sun Y, Liu J, Wan L, Wang F, Qi YJ. Xinfeng capsule increases peripheral blood BTLA expression of CD19 + and CD24 + b cells and relieves oxidative stress damage to improve cardiac function of patients with rheumatoid arthritis. Chin J Cell Mol Immunol (2015) 31(01):93–96+99. doi: 10.13423/j.cnki.cjcmi.007216
42. Zhu FX, Zhou RH, Shi YH, Mo HY, Li BZ, Li LM. Clinical study of zhengqing fengtongning combined with methotrexate in treatment of elderly rheumatoid arthritis. Modern Prev Med (2013) 40(15):2944–2946+2949.
43. Lu Y, Sun JM. Observation on the therapeutic effect of zhengqingfengtongning combined with methotrexate on rheumatoid arthritis. Liaoning J Traditional Chin Med (2011) 38(10):2019–21. doi: 10.13192/j.ljtcm.2011.10.102.luy.078
44. Liu YY, Shen HL. Clinical trial iguratimod tablets combined with tripterysium glycosides tablets in the treatment of elderly patients with active rheumatoid arthritis. Chin J Clin Pharmacol (2018) 34(19):2283–6. doi: 10.13699/j.cnki.1001-6821.2018.19.012
45. Wu YY, Fei J, Song DM, Huang Y. Safety and efficacy of iguratimod tablets combined with leigongteng glucosides pills in non-menopausal female with rheumatoid arthritis. J Clin Med Pract (2021) 25(20):115–8.
46. Li J, Chen QP, Liu JY, Wang XY, Liu D. Combination of elamod and methotrexate in the treatment of 84 cases of senile rheumatoid arthritis. Shanxi Med J (2016) 45(01):120–1.
47. Liu CC. Clinical study of iguratimod and tripterygium glycoside tablets in treating damp–heat blockage syndrome of Wang bi(rheumatoid arthritis). Shenyang: Changchun University of Traditional Chinese Medicine (2019).
48. Li HB, Nie DQ, Liu D. Comparative study on tripterygium wilfordii and igutimod in the treatment of active rheumatoid arthritis. Chin Community Physician (2020) 36(18):13–14+16.
49. Li JY, Sun ZM. Analysis of the therapeutic effect of methotrexate combined with alamod on refractory rheumatoid arthritis. J China Prescription Drug (2021) 19(09):109–11.
50. Mo ML, Tang DX, Zhang J, Liu Y, Xie LH. A randomized controlled trial of methotrexate combined with elamod in the treatment of active rheumatoid arthritis. J Fujian Med Univ (2018) 52(04):245–8.
51. Shen QY, Hu GH, Chen MJ, Zhu H. Clinical effects of tripterygium glycosides tablets combined with iguratimod on elderly patients with active rheumatoid arthritis. Chin Traditional Patent Med (2021) 43(02):384–7.
52. Goldbach-Mansky R, Wilson M, Fleischmann R, Olsen N, Silverfield J, Kempf P, et al. Comparison of tripterygium wilfordii hook f versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Internal Med (2009) 151(4):229–240, w249-251. doi: 10.7326/0003-4819-151-4-200908180-00005
53. Fan J. Effect of methotrexate combined with tripterygium wilfordii polyglycoside tablets in patients with rheumatoid arthritis. Zhejiang Clin Med (2018) 20(5):834–835,838.
54. Zhou B, Zhou YL, Lin WM, Shi P, Fu Y, Cao JY. Effect of sinomenine combined with methotrexate on the expression of IL-1, IL-17 and TNF-α in rheumatoid arthritis patients. Modern J Integrated Traditional Chin Western Med (2014) 23(19):2066–8.
55. Dai L, Song XL, Wang J. Effects of sinomenine combined with methotrexate on serum inflammatory factors in patients with rheumatoid arthritis and and its safety. Shaanxi Med J (2018) 47(12):1580–3.
56. Gu F, Sun Y, Chen SW, Wang P, Ding CZ, Sun LY. Clinical study of sinomenine in combination with methotrexate for the treatment of active rheumatoid arthritis. Shanghai journal of traditional Chinese medicine. (2014) 48(06):58–60. doi: 10.16305/j.1007-1334.2014.06.034
57. Cai Q, Jin SX, Chen GJ, Yue T. Efficacy of sinomenine combined with methotrexate on early rheumatoid arthritis and its influence on expressions of MMP-3 and RANKL/OPG. Acad J Shanghai Univ Traditional Chin Med (2019) 33(01):36–41. doi: 10.16306/j.1008-861x.2019.01.009
58. Zhang EZ. Effect of zhengqing fengtongning sustained release tablet combined with eramod in the treatment of rheumatoid arthritis. Heilongjiang Med And Pharm (2022) 45(03):140–1.
59. Lv QW, Zhang W, Shi Q, Zheng WJ, Li X, Chen H, et al. Comparison of tripterygium wilfordii hook f with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): A randomised, controlled clinical trial. Ann rheumatic Dis (2015) 74(6):1078–86. doi: 10.1136/annrheumdis-2013-204807
60. Wang SQ. Effect of eramod combined with MTX on rheumatoid arthritis. J Bethune Military Med Coll (2017) 15(03):309–11. doi: 10.16485/j.issn.2095-7858.2017.03.015
61. Mo H, Ma SB. Clinical effect of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. Internal Med China (2015) 10(02):156–9. doi: 10.16121/j.cnki.cn45-1347/r.2015.02.06
62. Li B. Efficacy and safety of allamod combined with methotrexate in the treatment of rheumatoid arthritis. J Clin rational Drug Use (2022) 15(19):19–21. doi: 10.15887/j.cnki.13-1389/r.2022.19.006
63. Zhao HN, Hao XJ. Clinical effect of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. Clin Res Pract (2018) 3(15):44–5. doi: 10.19347/j.cnki.2096-1413.201815020
64. Zhu H, Song LP, Liu S, Tian W, Feng LH, Nie YK, et al. Efficacy and safety of ilamod in the treatment of senile rheumatoid arthritis. China Foreign Med Treat (2019) 38(20):4–6. doi: 10.16662/j.cnki.1674-0742.2019.20.004
65. Zhu ZH, Wan L, Liu J, Huang CB, Zhao L, Fang YY, et al. Clinical effect of compound tripterygium wilfordii preparation (Xinfeng Capsule) in improving immune inflammation of rheumatoid arthritis due to spleen deficiency and dampness. Chin J Exp Formulas (2023) 29(05):32–8. doi: 10.13422/j.cnki.syfjx.20222171
66. Jin J, Gao H, Lu M. Effect of methotrexate combined with eramod on rheumatoid arthritis. Today Nurse (2020) 27(09):43–6. doi: 10.19792/j.cnki.1006-6411.2020.26.017
67. Meng DQ, Wang GR, Pan WY, Li H, Liu SS, Li YS, et al. Short term clinical efficacy of methotrexate combined with alamod in treatment of refractory rheumatoid arthritis. Chin J Clin Res (2015) 28(12):1586–8. doi: 10.13429/j.cnki.cjcr.2015.12.010
68. Shi XD, Zhang XL, Duan XW. Efficacy and safety of methotrexate combined iguratimod in treatmet of active rheumatoid arthritis. J Nanchang Univ (Medical) (2015) 55(01):33–36+47. doi: 10.13764/j.cnki.ncdm.2015.01.010
69. Chen XY. Clinical efficacy of methotrexate combined with elamod in the treatment of active rheumatoid arthritis. World Latest Med Inf (Electronic Version) (2018) 18(10):86+89. doi: 10.19613/j.cnki.1671-3141.2018.10.066
70. Mo Y. Clinical efficacy and safety of methotrexate combined with leflunomide in the treatment of rheumatoid arthritis. J Clin Rational Drug Use (2017) 10(09):61–2. doi: 10.15887/j.cnki.13-1389/r.2017.09.029
71. Qi DX, Liu Y, Huang DH. Efficacy and safety of iguratimod and methotrexate in treatment of active rheumatoid arthritis. Chin Drug Eval (2019) 36(03):217–20. doi: 10.3969/j.issn.2095-3593.2019.03.015
72. Liu L. Therapeutic effect of leflunomide and methotrexate on rheumatoid arthritis. Guide China Med (2012) 10(23):611–2. doi: 10.3969/j.issn.1671-8194.2012.23.470
73. Huo LP, Li CL, Niu YN, Jia W. Clinical observation of leflunomide combined with methotrexate in the treatment of rheumatoid arthritis. Med Forum (2013) 17(32):4226–8.
74. Bi WH. Effect of alamod combined with methotrexate on serum VEGF level in patients with rheumatoid arthritis. Inner Mongolia: Inner Mongolia Medical University (2019).
75. Scott DL, Smolen JS, Kalden JR, Putte L B, Larsen A, Kvien TK, et al. Treatment of active rheumatoid arthritis with leflunomide: two year follow up of a double blind, placebo controlled trial versus sulfasalazine. Ann rheumatic Dis (2001) 60(10):913–23. doi: 10.1136/ard.60.10.913
76. Emery P, Breedveld FC, Lemmel EM, Kaltwasser JP, Dawes PT, Gömör B, et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatol (Oxford England) (2000) 39(6):655–65. doi: 10.1093/rheumatology/39.6.655
77. Strand V, Cohen S, Schiff M, Weaver A, Fleischmann R, Cannon G, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. leflunomide rheumatoid arthritis investigators group. Arch Internal Med (1999) 159(21):2542–50. doi: 10.1001/archinte.159.21.2542
78. Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, et al. Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. utilization of leflunomide in the treatment of rheumatoid arthritis trial investigator group. Arthritis rheumatism (2001) 44(9):1984–92. doi: 10.1002/1529-0131(200109)44:9<1984::AID-ART346>3.0.CO;2-B
79. Liu ZC, Long SH, Zou Q, Liao XP. Effect of tripterygium wilfordii polyglycoside combined with iramod in the treatment of rheumatoid arthritis. Shenzhen J Integrated Traditional Chin Western Med (2022) 32(14):89–91. doi: 10.16458/j.cnki.1007-0893.2022.14.027
80. Giannini D, Antonucci M, Petrelli F, Bilia S, Alunno A, Puxeddu I. One year in review 2020: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol (2020) 38(3):387–97. doi: 10.55563/clinexprheumatol/3uj1ng
81. Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity (2017) 46(2):183–96. doi: 10.1016/j.immuni.2017.02.006
82. Jiang Y, Zhong M, Long F, Yang R. Deciphering the active ingredients and molecular mechanisms of tripterygium hypoglaucum (Levl.) hutch against rheumatoid arthritis based on network pharmacology. Evidence-Based complementary Altern medicine: eCAM (2020) 2020:2361865. doi: 10.1155/2020/2361865
83. Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis (2015) 18(4):433–48. doi: 10.1007/s10456-015-9477-2
84. Tang Y, Liu Q, Feng Y, Zhang Y, Xu Z, Wen C, et al. Tripterygium ingredients for pathogenicity cells in rheumatoid arthritis. Front Pharmacol (2020) 11:583171. doi: 10.3389/fphar.2020.583171
85. Agere SA, Akhtar N, Watson JM, Ahmed S. RANTES/CCL5 induces collagen degradation by activating MMP-1 and MMP-13 expression in human rheumatoid arthritis synovial fibroblasts. Front Immunol (2017) 8:1341. doi: 10.3389/fimmu.2017.01341
86. Yang CM, Chen YW, Chi PL, Lin CC, Hsiao LD. Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochem Pharmacol (2017) 132:77–91. doi: 10.1016/j.bcp.2017.03.003
87. Liu DD, Zhang BL, Yang JB, Zhou KP. Celastrol ameliorates endoplasmic stress-mediated apoptosis of osteoarthritis via regulating ATF-6/CHOP signalling pathway. J Pharm Pharmacol (2020) 72(6):826–35. doi: 10.1111/jphp.13250
88. Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N, et al. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol Immunol (2010) 47(15):2467–74. doi: 10.1016/j.molimm.2010.06.007
89. Long C, Yang Y, Wang Y, Zhang X, Zhang L, Huang S, et al. Role of glutamine-Glutamate/GABA cycle and potential target GLUD2 in alleviation of rheumatoid arthritis by tripterygium hypoglaucum (levl.) hutch based on metabolomics and molecular pharmacology. J ethnopharmacol (2021) 281:114561. doi: 10.1016/j.jep.2021.114561
90. Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med (2008) 74(12):1423–9. doi: 10.1055/s-2008-1081346
91. Jiang W, Fan W, Gao T, Li T, Yin ZM, Guo HH, et al. Analgesic mechanism of sinomenine against chronic pain. Pain Res Manage (2020) 2020:1876862. doi: 10.1155/2020/1876862
92. Jiang W, Tang M, Yang L, Zhao X, Gao J, Jiao Y, et al. Analgesic alkaloids derived from traditional Chinese medicine in pain management. Front Pharmacol (2022) 13:851508. doi: 10.3389/fphar.2022.851508
93. Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol (2011) 11(3):373–6. doi: 10.1016/j.intimp.2010.11.018
Keywords: traditional Chinese medicines, Tripterygium wilfordii Hook F, Sinomenine, rheumatoid arthritis, network meta-analysis
Citation: Fang J, Liu M, Huang Z, Ma Y, Wang Y, Zheng X, Lv L, Liu C, Li W, Zhu Z, Zhu H, Hu J, Wang Y and Wang H (2023) Efficacy and safety of TCMs with anti-inflammatory effect in patients with rheumatoid arthritis: A network meta-analysis. Front. Immunol. 14:1114930. doi: 10.3389/fimmu.2023.1114930
Received: 03 December 2022; Accepted: 20 February 2023;
Published: 08 March 2023.
Edited by:
Huji Xu, Tsinghua University, ChinaReviewed by:
Amira Kamil Mohammed, University of Baghdad, IraqCopyright © 2023 Fang, Liu, Huang, Ma, Wang, Zheng, Lv, Liu, Li, Zhu, Zhu, Hu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailong Wang, d2FuZ2hhaWxvbmdAdG9tLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.