
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 27 February 2023
Sec. Nutritional Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1113607
This article is part of the Research Topic Understanding the Influence of Nutrition in Modulating Autoimmune Disease View all 6 articles
Neutrophils are considered as core immune cells involve in the early stage of rheumatoid arthritis (RA) and participate in the disease progression. The underlining mechanisms include the elevated chemotaxis and infiltration of neutrophils, the increase in the reactive oxygen species and the promotion of neutrophil extracellular traps formation. Accumulating studies demonstrated the important role of nutrients intake played in the initiation and progression of RA. This study summarized the effects of several macronutrients and micronutrients on regulating RA through the modulation of activated neutrophils and appealed for a healthy diet in RA-risk individuals as well as RA patients.
Rheumatoid arthritis (RA) is one of the most common systemic autoimmune disease, with a worldwide prevalence of around 0.5% (1). This is a chronic progressive disease mostly characterized by synovitis. Autoantibodies and immune cells infiltrate and cumulate in the synovial cavity, causing arthralgia, bone destruction and finally joint deformity. The etiology of RA is complex and not thoroughly clarified, with both susceptibility genes and environmental risk factors involved. Over the past decades, in spite of the decreased mortality, RA has been more and more prevalent especially in developed countries and urban areas, accompanied by earlier disease onset, which emphasizes the importance of environmental triggers (2). Mucosal sites, such as respiratory tracts and intestinal tracts, are thought to be the places where inflammation initially occurs in the hypothesis of ‘mucosal origin’ (3). This hints that air and food may be the most relevant source of environmental triggers. In fact, smoking has been deemed as the most important environmental factor in the risk of RA (4).
On the other side, immune imbalance is regarded as a critical part in the pathogenesis of RA and interacts with environmental factors. The excessive recruitment of neutrophils is thought to be crucial for the initiation of RA and also participate in the progression and perpetuation of RA through several mechanisms. There exists a rising interest on whether nutrients consumption participates in the pathogenesis and progression of RA through modulating the activity of neutrophils. In this study, we aimed to explore and summarize the function of nutrients in regulating RA, especially focusing on how they modulate the infiltration and activation of neutrophils.
In the process of inflammation, neutrophil is the first kind of immune cell recruited via chemotaxis, and always remains to take the highest proportion in the inflamed sites (5). Under normal conditions, neutrophils have a relative short half-life less than 1 day. Whereas during inflammation, activated neutrophils acquire severalfold prolonged life spans (6, 7). As one of the most important cell types in the immune system, neutrophil can defend against pathogens directly by phagocytosis or by releasing granular enzymes such as myeloperoxidase (MPO), matrix metalloproteinase (MMP) and neutrophil elastase (NE). It can also produce reactive oxygen species (ROS) via the activation of membrane-bound NADPH-oxidase, thus promote respiratory burst. Furthermore, the networks that neutrophil forms with granular enzymes and extracellular nuclear contents, nominated as neutrophil extracellular traps (NETs), are able to entrap and eliminate pathogens with great efficiency (8, 9).
Supported by the results of proteomic analysis, neutrophil is the most abundant cell type in the inflamed synovial fluid of RA (10). Neutrophils in the RA synovial fluid presented with elevated expression of chemokines, which further amplified the inflammatory response. In addition, those neutrophils also produced more ROS, exacerbating oxygen stress (11). There was also a strengthened activation of NETs, together with postponed apoptosis (12). Furthermore, anti-citrullinated protein antibodies (ACPAs), which are markable in RA, are considered to be associated with activated neutrophils through the exposure of related antigens in the NETs (13, 14).
In a case-control study which retrospectively assessed the diet consumed 5 years before disease onset based on the Chinese population, RA patients consumed more carbohydrates than healthy controls. Increased carbohydrates intake might make excessive energy absorption, lead to increased body weight and elevate the risk of RA (15, 16).
More pieces of evidence were in the case of monosaccharide. An observational study indicated that the intake of sugar-sweetened soda increased the risk of RA (17). Researchers also found an association between the consumption of high-fructose soft drinks and the onset of RA (18). The background mechanism may be that elevated glucose and fructose ingestion promote the production of advanced glycation end products (AGEs) and enhance autophagy and NETosis (19). Whereas, a recent cohort study based on the French population found no correlation between sugar-sweetened soft drinks and RA risk, but indicated that artificially-sweetened soft drinks increased RA risk (20).
Glutamine, which is a non-essential amino acid relatively abundant in beef, eggs, tofu and other protein-rich foods, is another source of energy in addition to glucose. Glutamine is consumed at the highest rate by neutrophils compared with other immune cells (21). As the substrate of NADPH, glutamine participants in increasing superoxide generation through NADPH oxidase in neutrophils (22). Besides, glutamine is involved in the synthesis of O-linked beta-N-acetylglucosamine (O-GlcNAc), which is increased in activated neutrophils and promotes cellular mobility via the MAPK pathway (23). However, there were rare publications focused on the association of glutamine supplementation and RA, except for one study conducted 17 years ago which showed that supplementation with beta-hydroxy-beta-methylbutyrate, glutamine and arginine had no benefit in reversing cachexia in RA patients (24).
Red meat may exacerbate inflammation through saturated fatty acids and nitrites. But whether red meat intake can increase the risk of RA is still under debate. In a case-control study, a high red meat intake was associated with an increase in the risk of inflammatory polyarthritis (25). Compared to a meat-rich diet, people consuming a vegan diet experienced a decrease in total neutrophil counts (26). In RA patients who underwent a 3-month diet excluding meat, gluten and lactose, the circulating neutrophils were significantly decreased, together with a relief of inflammation symptoms (27). And in a recent cross-sectional study with 707 RA patients recruited, a high intake of red meat was associated with earlier disease onset, especially in those with smoking habits or overweight problems (28). Nevertheless, there were also lots of studies suggesting no effect red meat consumption laid on the risk of RA (29, 30). A recent meta-analysis analyzed 7 cohorts and 6 case-control studies also found no significant association between red meat consumption and the risk of RA (31).
Omega-3 polyunsaturated fatty acid was considered as a protective factor against RA. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are rich in deep-sea fishes, have been shown to be able to inhibit NF-κB signal, thus reducing the production of pro-inflammatory cytokines (32). With the suppression of chemotaxis, the recruitment and infiltration of leucocytes were also inhibited (33). The metabolic product of omega-3, resolvin, was also found able to attenuate inflammation and relieve joint pain via the inhibition of neutrophil recruitment in RA (34). A prospective cohort study showed that a more than 0.21 g per day dietary consumption of long-chain omega-3 polyunsaturated fatty acids was associated with a 35% decline in the risk of RA (35). In a cross-sectional study, fish consumption no less than 2 times per week was able to attenuate the disease severity of RA patients (36).
Previous reports have found that RA patients experience significantly lowered levels of 25-hydroxyvitamin D [25(OH)D] (37, 38). And the deficiency of 25(OH)D was thought to be associated with a higher disease severity (39). The seasonal fluctuation of disease performance might be related to the seasonal variation of the serum 25(OH)D levels (40).
In fact, vitamin D (vit D) took part in the amelioration of inflammation by reducing the synthesis of pro-inflammatory mediators, inhibiting the release of ROS, and decreasing NETosis (41, 42). In mouse models, supplementation of Vitamin D3 was able to promote ATP degradation and revert E-ADA activity in neutrophils, thus ameliorating the joint symptoms (43). And in RA patients with vit D deficiency, supplementation of vit D could rapidly improve the disease activity (44). Moreover, five years of vit D supplementation could reduce the incidence of autoimmune disease by 22% (45).
The serum zinc level is also decreased in RA, probably because of the increased zinc import to the cell under the exposure of pro-inflammatory cytokines (46). As a result, the deficiency of zinc will further promote inflammation by increasing the release of pro-inflammatory mediators and ROS by epigenetic mechanisms (47).
In mice models, supplementation of zinc by injection could decrease the recruitment and activity of neutrophils, thus ameliorating inflammation and tissue damage (48). A meta-analysis of clinical trials indicated that with the increase of serum zinc, neutrophil levels decreased, and so was circulating CRP, hs-CRP, TNF-α and IL-6 (49). However, as for the effect zinc supplementation laid on NETosis, there remains a controversy. Some reports suggested that zinc could inhibit NETosis by inhibiting histone citrullination (48, 50). While some others found an increase in the NETs formation and release after the treatment of zinc (51, 52).
Selenium is another trace element in the human body and showed remarkable anti-inflammation and antioxidant potential in RA. Compared to normal controls, patients with RA presented with significantly lower serum selenium levels (53). Moreover, RA patients with higher serum selenium concentration seemed to have milder inflammation, indicated by lower levels of CRP and ESR (54). Animal studies proposed that selenium-treated RA mice presented with reduced neutrophil counts, decreased NETs production, downregulated pro-inflammatory cytokines, and improved disease severity (55). Additionally, selenium also caused a reduction of ROS and alleviated oxidative stress (56).
Iron plays an important role both in the recruitment and in the physical functions of neutrophils. Compared to normal controls, the serum iron level was significantly lower and the level of soluble transferrin receptor was elevated in RA patients (57). RA aggravated iron redistribution, making a decrease of iron in the blood and an increase in the synovium, and amplifying local inflammation (58). Iron imbalance contributed to RA inflammation. On the one hand, a deficiency of iron might promote the formation of NETs, which could be reversed by iron supplementation. On the other hand, when it came to iron overload, free iron would participate in NETosis and increase inflammation, which was able to be rescued by iron chelators (59).
N-acetylcysteine (NAC) is the acetylated form of L-cysteine. The supplementation of NAC is widely utilized in chronic obstructive pulmonary disease and acetaminophen intoxication, but has not been recommended in RA (60). NAC could remove ROS and inhibit the synthesis of pro-inflammation cytokines, thus reducing the recruitment of neutrophils and other immune cells (61). Up to now, studies about the correlation between NAC and RA are still rare. A clinical trial conducted recently showed that NAC supplementation could reduce the levels of several mediators involved in oxidative stress, but could not reduce disease activity or improve the symptoms of RA patients (62).
Quercetin is an ingredient widely existing in various plants. It has been proven to have numerous protective effects such as antioxidation, reducing inflammation and preventing cancer (63). Recently, a clinical study demonstrated the function of quercetin in ameliorating inflammation and improving symptoms in RA patients (64). Experiments based on animal models further confirmed this and uncovered the fundamental mechanisms (63). Firstly, quercetin could reduce chemokines and pro-inflammation cytokines, thus inhibiting neutrophil infiltration. In addition, quercetin could also increase apoptosis and inhibit the release of pro-inflammatory cytokines by macrophages. Moreover, quercetin could inhibit autophagy and reduce the production of NETs (65–68).
Resveratrol is another ingredient extracted from numerous plants. Animal studies suggested that resveratrol was able to reduce ROS and alleviate RA (69). A meta-analysis of preclinical models showed that resveratrol could decrease the level of several pro-inflammatory cytokines including IL-1, IL-6 and TNF-α (70). The beneficial effect of resveratrol in RA patients was also verified by a clinical trial, where those accepted daily resveratrol supplementation showed improved clinical symptoms and serum inflammation indicators (71).
Icariin is the major ingredient of epimedium, a traditional herb in China. Evidence accumulates that icariin is able to alleviate inflammation and regulate immunology (72). Experiments based on arthritis rat models showed that it could decrease the levels of pro-inflammatory mediators, reduce the density of neutrophils and suppress joint degradation (73).
Moreover, Tetrandrine, a kind of alkaloid separated from Stephania tetrandra S. Moore, was able to mitigate the symptoms of RA in arthritis murine models. Not only could it decrease serum IL-6 level, but it was also capable to inhibit NETs formation (74). Cedrol, which can be found in ginger, was also considered able to attenuate inflammation in RA. It was verified by animal models that cedrol could inhibit the phosphorylation of JAK3 protein, thus inhibiting the secretion of pro-inflammatory cytokines, and decreasing the neutrophil count (75).
The aetiopathogenesis of RA was quite complicated with both genetic risk factors and environmental risk factors involved. In the past decades, more and more researches focused on nutrients in RA and revealed its important role in the prevention and treatment of RA. In this study, we summarized the function of several macronutrients and micronutrients in regulating the onset and disease severity of RA through modulating the migration and activity of neutrophils (Table 1). Although controversies existed on the effects of red meat, zinc, Ferrum, NAC and so on, additional sugar intake and excessive energy consumption were widely accepted as risk factors of RA, and omega-3 polyunsaturated fatty acid, vitamin D supplementations, selenium, as well as ingredients extracted from plants, showed their promising effects on prevention of RA onset and amelioration of disease severity (Figure 1). Whatever, a healthy diet with more vegetables and fruits as well as less red meat and sugar was recommended in RA.
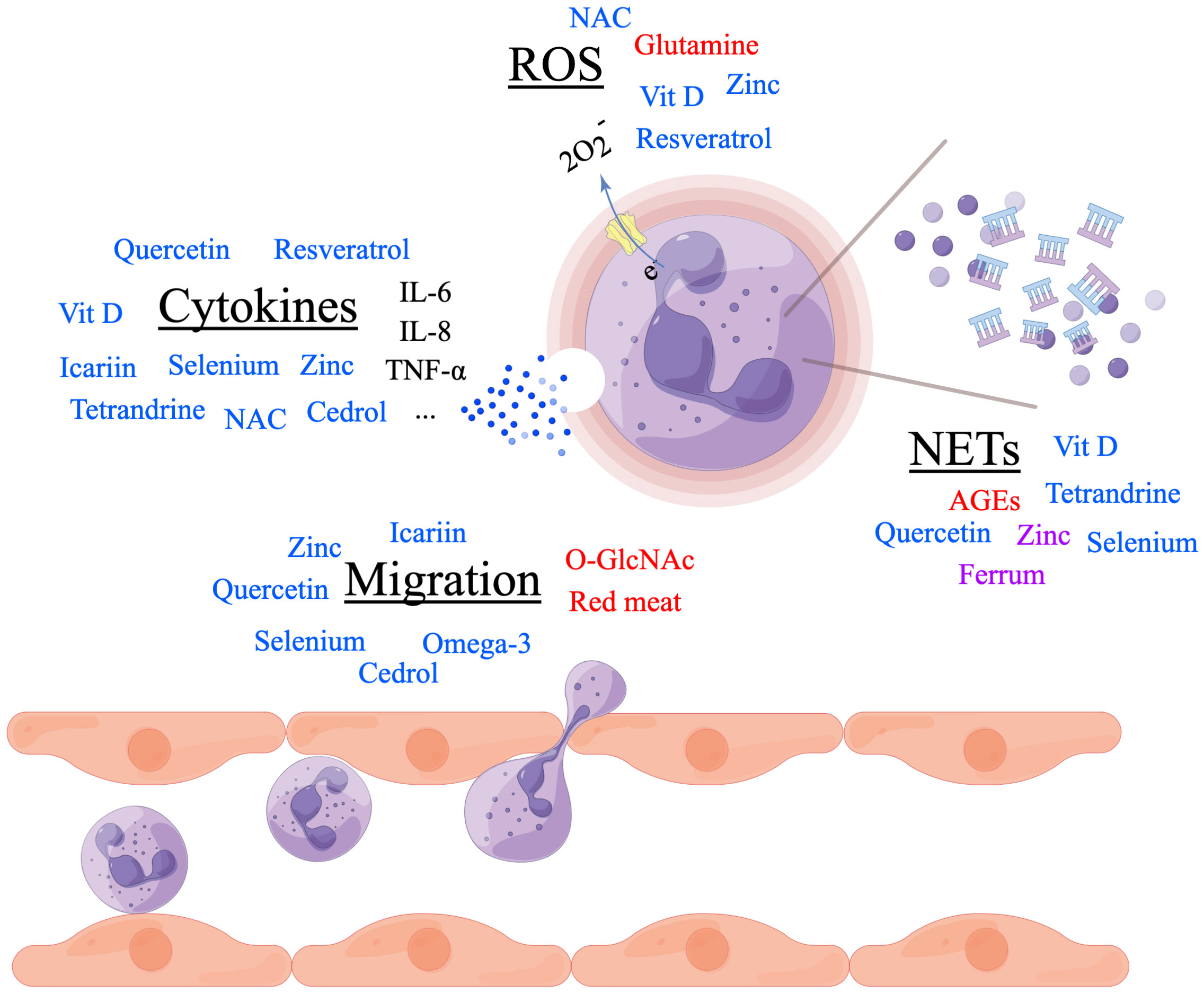
Figure 1 Nutrients in regulating rheumatoid arthritis (RA) through neutrophils. Nutrients in red font promote the process. Nutrients in blue font inhibit the process. Nutrients in purple font controversially influence the process. Made by Figdraw.
Y-RS contributed in literature search and manuscript writing. D-YX revised the manuscript. JL raised the idea for the article and proofread the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (82001710).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol Int (2021) 41:863–77. doi: 10.1007/s00296-020-04731-0
2. Finckh A, Gilbert B, Hodkinson B, Bae SC, Thomas R, Deane KD, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol (2022) 18:591–602. doi: 10.1038/s41584-022-00827-y
3. Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nat Rev Rheumatol (2018) 14:542–57. doi: 10.1038/s41584-018-0070-0
4. Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun (2020) 110:102400. doi: 10.1016/j.jaut.2019.102400
5. Chen W, Wang Q, Ke Y, Lin J. Neutrophil function in an inflammatory milieu of rheumatoid arthritis. J Immunol Res (2018) 2018:8549329. doi: 10.1155/2018/8549329
6. Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
7. Fresneda Alarcon M, McLaren Z, Wright HL. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: Same foe different M.O. Front Immunol (2021) 12:649693. doi: 10.3389/fimmu.2021.649693
8. Hidalgo A, Chilvers ER, Summers C, Koenderman L. The neutrophil life cycle. Trends Immunol (2019) 40:584–97. doi: 10.1016/j.it.2019.04.013
9. Pérez-Figueroa E, Álvarez-Carrasco P, Ortega E, Maldonado-Bernal C. Neutrophils: Many ways to die. Front Immunol (2021) 12:631821. doi: 10.3389/fimmu.2021.631821
10. Birkelund S, Bennike TB, Kastaniegaard K, Lausen M, Poulsen TBG, Kragstrup TW, et al. Proteomic analysis of synovial fluid from rheumatic arthritis and spondyloarthritis patients. Clin Proteomics (2020) 17:29. doi: 10.1186/s12014-020-09292-9
11. Wright HL, Lyon M, Chapman EA, Moots RJ, Edwards SW. Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species, and neutrophil extracellular traps. Front Immunol (2020) 11:584116. doi: 10.3389/fimmu.2020.584116
12. Kumar S, Dikshit M. Metabolic insight of neutrophils in health and disease. Front Immunol (2019) 10:2099. doi: 10.3389/fimmu.2019.02099
13. Apel F, Zychlinsky A, Kenny EF. The role of neutrophil extracellular traps in rheumatic diseases. Nat Rev Rheumatol (2018) 14:467–75. doi: 10.1038/s41584-018-0039-z
14. Liu Y, Kaplan MJ. Neutrophils in the pathogenesis of rheumatic diseases: Fueling the fire. Clin Rev Allergy Immunol (2021) 60:1–16. doi: 10.1007/s12016-020-08816-3
15. He J, Wang Y, Feng M, Zhang X, Jin YB, Li X, et al. Dietary intake and risk of rheumatoid arthritis-a cross section multicenter study. Clin Rheumatol (2016) 35:2901–8. doi: 10.1007/s10067-016-3383-x
16. Feng X, Xu X, Shi Y, Liu X, Liu H, Hou H, et al. Body mass index and the risk of rheumatoid arthritis: An updated dose-response meta-analysis. BioMed Res Int (2019) 2019:3579081. doi: 10.1155/2019/3579081
17. Hu Y, Costenbader KH, Gao X, Al-Daabil M, Sparks JA, Solomon DH, et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am J Clin Nutr (2014) 100:959–67. doi: 10.3945/ajcn.114.086918
18. DeChristopher LR, Uribarri J, Tucker KL. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20-30 years. Nutr Diabetes (2016) 6:e199. doi: 10.1038/nutd.2016.7
19. Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther (2015) 22:326–34. doi: 10.1038/cgt.2015.21
20. Ascione S, Barde F, Artaud F, Nguyen Y, Macdonald C, Mariette X, et al. Association between beverage consumption and risk of rheumatoid arthritis: A prospective study from the French E3N cohort. Rheumatol (Oxford). (2022) keac544. doi: 10.1093/rheumatology/keac544
21. Pithon-Curi TC, De Melo MP, Curi R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: A comparative study. Cell Biochem Funct (2004) 22:321–6. doi: 10.1002/cbf.1109
22. Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients (2018) 10:1564. doi: 10.3390/nu10111564
23. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu Rev Immunol (2012) 30:459–89. doi: 10.1146/annurev-immunol-020711-074942
24. Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: A randomised controlled trial. Clin Nutr (2005) 24:442–54. doi: 10.1016/j.clnu.2005.01.006
25. Pattison DJ, Symmons DP, Lunt M, Welch A, Luben R, Bingham SA, et al. Dietary risk factors for the development of inflammatory polyarthritis: Evidence for a role of high level of red meat consumption. Arthritis Rheum (2004) 50:3804–12. doi: 10.1002/art.20731
26. Lederer AK, Maul-Pavicic A, Hannibal L, Hettich M, Steinborn C, Gründemann C, et al. Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids - a randomized, controlled trial. Clin Nutr (2020) 39:3241–50. doi: 10.1016/j.clnu.2020.02.011
27. Guagnano MT, D’Angelo C, Caniglia D, Di Giovanni P, Celletti E, Sabatini E, et al. Improvement of inflammation and pain after three months’ exclusion diet in rheumatoid arthritis patients. Nutrients (2021) 13:3535. doi: 10.3390/nu13103535
28. Jin J, Li J, Gan Y, Liu J, Zhao X, Chen J, et al. Red meat intake is associated with early onset of rheumatoid arthritis: A cross-sectional study. Sci Rep (2021) 11:5681. doi: 10.1038/s41598-021-85035-6
29. Benito-Garcia E, Feskanich D, Hu FB, Mandl LA, Karlson EW. Protein, iron, and meat consumption and risk for rheumatoid arthritis: A prospective cohort study. Arthritis Res Ther (2007) 9:R16. doi: 10.1186/ar2123
30. Sundström B, Ljung L, Di Giuseppe D. Consumption of meat and dairy products is not associated with the risk for rheumatoid arthritis among women: A population-based cohort study. Nutrients (2019) 11:2825. doi: 10.3390/nu11112825
31. Asoudeh F, Jayedi A, Kavian Z, Ebrahimi-Mousavi S, Nielsen SM, Mohammadi H. A systematic review and meta-analysis of observational studies on the association between animal protein sources and risk of rheumatoid arthritis. Clin Nutr (2021) 40:4644–52. doi: 10.1016/j.clnu.2021.05.026
32. Venter C, Eyerich S, Sarin T, Klatt KC. Nutrition and the immune system: A complicated tango. Nutrients (2020) 12:818. doi: 10.3390/nu12030818
33. Calder PC. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem Soc Trans (2017) 45:1105–15. doi: 10.1042/BST20160474
34. Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-Hayat SM, Bo S, Sahebkar A. Resolvins: Emerging players in autoimmune and inflammatory diseases. Clin Rev Allergy Immunol (2020) 58:82–91. doi: 10.1007/s12016-019-08754-9
35. Di Giuseppe D, Wallin A, Bottai M, Askling J, Wolk A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: A prospective cohort study of women. Ann Rheum Dis (2014) 73:1949–53. doi: 10.1136/annrheumdis-2013-203338
36. Tedeschi SK, Bathon JM, Giles JT, Lin TC, Yoshida K, Solomon DH. Relationship between fish consumption and disease activity in rheumatoid arthritis. Arthritis Care Res (Hoboken) (2018) 70:327–32. doi: 10.1002/acr.23295
37. Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. The association between vitamin d concentration and pain: A systematic review and meta-analysis. Public Health Nutr (2018) 21:2022–37. doi: 10.1017/S1368980018000551
38. Li D, Jeffery LE, Jenkinson C, Harrison SR, Chun RF, Adams JS, et al. Serum and synovial fluid vitamin d metabolites and rheumatoid arthritis. J Steroid Biochem Mol Biol (2019) 187:1–8. doi: 10.1016/j.jsbmb.2018.10.008
39. Mouterde G, Gamon E, Rincheval N, Lukas C, Seror R, Berenbaum F, et al. Association between vitamin d deficiency and disease activity, disability, and radiographic progression in early rheumatoid arthritis: The ESPOIR cohort. J Rheumatol (2020) 47:1624–8. doi: 10.3899/jrheum.190795
40. Cutolo M, Soldano S, Sulli A, Smith V, Gotelli E. Influence of seasonal vitamin d changes on clinical manifestations of rheumatoid arthritis and systemic sclerosis. Front Immunol (2021) 12:683665. doi: 10.3389/fimmu.2021.683665
41. Neve A, Corrado A, Cantatore FP. Immunomodulatory effects of vitamin d in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med (2014) 14:275–83. doi: 10.1007/s10238-013-0249-2
42. Vanherwegen AS, Gysemans C, Mathieu C. Regulation of immune function by vitamin d and its use in diseases of immunity. Endocrinol Metab Clin North Am (2017) 46:1061–94. doi: 10.1016/j.ecl.2017.07.010
43. da Silva JLG, Passos DF, Bernardes VM, Cabral FL, Schimites PG, Manzoni AG, et al. Co-Nanoencapsulation of vitamin D(3) and curcumin regulates inflammation and purine metabolism in a model of arthritis. Inflammation (2019) 42:1595–610. doi: 10.1007/s10753-019-01021-1
44. Chandrashekara S, Patted A. Role of vitamin d supplementation in improving disease activity in rheumatoid arthritis: An exploratory study. Int J Rheum Dis (2017) 20:825–31. doi: 10.1111/1756-185X.12770
45. Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, et al. Vitamin d and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. Bmj (2022) 376:e066452. doi: 10.1136/bmj-2021-066452
46. Bonaventura P, Lamboux A, Albarède F, Miossec P. A feedback loop between inflammation and zn uptake. PloS One (2016) 11:e0147146. doi: 10.1371/journal.pone.0147146
47. Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P. Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. J Nutr Biochem (2013) 24:289–97. doi: 10.1016/j.jnutbio.2012.06.007
48. Wessels I, Pupke JT, von Trotha KT, Gombert A, Himmelsbach A, Fischer HJ, et al. Zinc supplementation ameliorates lung injury by reducing neutrophil recruitment and activity. Thorax (2020) 75:253–61. doi: 10.1136/thoraxjnl-2019-213357
49. Jafari A, Noormohammadi Z, Askari M, Daneshzad E. Zinc supplementation and immune factors in adults: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr (2022) 62:3023–41. doi: 10.1080/10408398.2020.1862048
50. Kuźmicka W, Manda-Handzlik A, Cieloch A, Mroczek A, Demkow U, Wachowska M, et al. Zinc supplementation modulates NETs release and neutrophils’ degranulation. Nutrients (2020) 13:51. doi: 10.3390/nu13010051
51. Zhou X, Wang H, Lian S, Wang J, Wu R. Effect of copper, zinc, and selenium on the formation of bovine neutrophil extracellular traps. Biol Trace Elem Res (2021) 199:3312–8. doi: 10.1007/s12011-020-02477-1
52. Ganatra HA, Varisco BM, Harmon K, Lahni P, Opoka A, Wong HR. Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate Immun (2017) 23:67–76. doi: 10.1177/1753425916677073
53. Ma Y, Zhang X, Fan D, Xia Q, Wang M, Pan F. Common trace metals in rheumatoid arthritis: A systematic review and meta-analysis. J Trace Elem Med Biol (2019) 56:81–9. doi: 10.1016/j.jtemb.2019.07.007
54. Deyab G, Hokstad I, Aaseth J, Småstuen MC, Whist JE, Agewall S, et al. Effect of anti-rheumatic treatment on selenium levels in inflammatory arthritis. J Trace Elem Med Biol (2018) 49:91–7. doi: 10.1016/j.jtemb.2018.05.001
55. Rehman A, John P, Bhatti A. Biogenic selenium nanoparticles: Potential solution to oxidative stress mediated inflammation in rheumatoid arthritis and associated complications. Nanomaterials (Basel) (2021) 11:2005. doi: 10.3390/nano11082005
56. Qamar N, John P, Bhatti A. Emerging role of selenium in treatment of rheumatoid arthritis: An insight on its antioxidant properties. J Trace Elem Med Biol (2021) 66:126737. doi: 10.1016/j.jtemb.2021.126737
57. Tański W, Chabowski M, Jankowska-Polańska B, Jankowska EA. Iron metabolism in patients with rheumatoid arthritis. Eur Rev Med Pharmacol Sci (2021) 25:4325–35. doi: 10.26355/eurrev_202106_26140
58. Chang S, Tang M, Zhang B, Xiang D, Li F. Ferroptosis in inflammatory arthritis: A promising future. Front Immunol (2022) 13:955069. doi: 10.3389/fimmu.2022.955069
59. Ni S, Yuan Y, Song S, Li X. A double-edged sword with a therapeutic target: Iron and ferroptosis in immune regulation. Nutr Rev (2022) nuac071. doi: 10.1093/nutrit/nuac071
60. Tsikas D, Mikuteit M. N-Acetyl-L-cysteine in human rheumatoid arthritis and its effects on nitric oxide (NO) and malondialdehyde (MDA): Analytical and clinical considerations. Amino Acids (2022) 54:1251–60. doi: 10.1007/s00726-022-03185-x
61. Pei Y, Liu H, Yang Y, Yang Y, Jiao Y, Tay FR, et al. Biological activities and potential oral applications of n-acetylcysteine: Progress and prospects. Oxid Med Cell Longev (2018) 2018:2835787. doi: 10.1155/2018/2835787
62. Esalatmanesh K, Jamali A, Esalatmanesh R, Soleimani Z, Khabbazi A, Malek Mahdavi A. Effects of n-acetylcysteine supplementation on disease activity, oxidative stress, and inflammatory and metabolic parameters in rheumatoid arthritis patients: A randomized double-blind placebo-controlled trial. Amino Acids (2022) 54:433–40. doi: 10.1007/s00726-022-03134-8
63. Shen P, Lin W, Deng X, Ba X, Han L, Chen Z, et al. Potential implications of quercetin in autoimmune diseases. Front Immunol (2021) 12:689044. doi: 10.3389/fimmu.2021.689044
64. Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J Am Coll Nutr (2017) 36:9–15. doi: 10.1080/07315724.2016.1140093
65. Kawaguchi K, Kaneko M, Miyake R, Takimoto H, Kumazawa Y. Potent inhibitory effects of quercetin on inflammatory responses of collagen-induced arthritis in mice. Endocr Metab Immune Disord Drug Targets (2019) 19:308–15. doi: 10.2174/1871530319666190206225034
66. Guazelli CFS, Staurengo-Ferrari L, Zarpelon AC, Pinho-Ribeiro FA, Ruiz-Miyazawa KW, Vicentini F, et al. Quercetin attenuates zymosan-induced arthritis in mice. BioMed Pharmacother (2018) 102:175–84. doi: 10.1016/j.biopha.2018.03.057
67. Haleagrahara N, Miranda-Hernandez S, Alim MA, Hayes L, Bird G, Ketheesan N. Therapeutic effect of quercetin in collagen-induced arthritis. BioMed Pharmacother (2017) 90:38–46. doi: 10.1016/j.biopha.2017.03.026
68. Yuan K, Zhu Q, Lu Q, Jiang H, Zhu M, Li X, et al. Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J Nutr Biochem (2020) 84:108454. doi: 10.1016/j.jnutbio.2020.108454
69. Yang G, Chang CC, Yang Y, Yuan L, Xu L, Ho CT, et al. Resveratrol alleviates rheumatoid arthritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways, and suppressing angiogenesis. J Agric Food Chem (2018) 66:12953–60. doi: 10.1021/acs.jafc.8b05047
70. Mittal M, Mehta P, Rajput S, Rajender S, Chattopadhyay N. The pharmacological assessment of resveratrol on preclinical models of rheumatoid arthritis through a systematic review and meta-analysis. Eur J Pharmacol (2021) 910:174504. doi: 10.1016/j.ejphar.2021.174504
71. Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin Rheumatol (2018) 37:2035–42. doi: 10.1007/s10067-018-4080-8
72. Bi Z, Zhang W, Yan X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. BioMed Pharmacother (2022) 151:113180. doi: 10.1016/j.biopha.2022.113180
73. Liu X, Wang Z, Qian H, Tao W, Zhang Y, Hu C, et al. Natural medicines of targeted rheumatoid arthritis and its action mechanism. Front Immunol (2022) 13:945129. doi: 10.3389/fimmu.2022.945129
74. Lu Q, Jiang H, Zhu Q, Xu J, Cai Y, Huo G, et al. Tetrandrine ameliorates rheumatoid arthritis in mice by alleviating neutrophil activities. Evid Based Complement Alternat Med (2022) 2022:8589121. doi: 10.1155/2022/8589121
Keywords: nutrients, rheumatoid arthritis, neutrophil, neutrophil extra cellular traps, inflammation
Citation: Shao Y-R, Xu D-Y and Lin J (2023) Nutrients and rheumatoid arthritis: From the perspective of neutrophils. Front. Immunol. 14:1113607. doi: 10.3389/fimmu.2023.1113607
Received: 01 December 2022; Accepted: 16 February 2023;
Published: 27 February 2023.
Edited by:
Preeti Singh Chauhan, Children’s Hospital of Philadelphia, United StatesReviewed by:
Meraj Ansari, National Institute of Pharmaceutical Education and Research, Mohali, IndiaCopyright © 2023 Shao, Xu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Lin, bGluamluemp1QHpqdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.