
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 17 May 2023
Sec. T Cell Biology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1107298
This article is part of the Research Topic Neuro-Immune Players of Peripheral Pain Signalling View all 7 articles
Neuropathic pain is a common type of chronic pain, primarily caused by peripheral nerve injury. Different T-cell subtypes play various roles in neuropathic pain caused by peripheral nerve damage. Peripheral nerve damage can lead to co-infiltration of neurons and other inflammatory cells, thereby altering the cellular microenvironment and affecting cellular metabolism. By elaborating on the above, we first relate chronic pain to T-cell energy metabolism. Then we summarize the molecules that have affected T-cell energy metabolism in the past five years and divide them into two categories. The first category could play a role in neuropathic pain, and we explain their roles in T-cell function and chronic pain, respectively. The second category has not yet been involved in neuropathic pain, and we focus on how they affect T-cell function by influencing T-cell metabolism. By discussing the above content, this review provides a reference for studying the direct relationship between chronic pain and T-cell metabolism and searching for potential therapeutic targets for the treatment of chronic pain on the level of T-cell energy metabolism.
Chronic pain is defined as pain that lasts or recurs for three months or longer (1). The main clinical manifestations are spontaneous pain, hyperalgesia and allodynia (2), accompanied by existing or potential tissue damage. Neuropathic pain is the most common type of chronic pain, with a prevalence of over 80% (3) and the most comprehensive and established animal models (4). It is characterized as a chronic pain syndrome resulting from a lesion or disease affecting the sensory nervous system (5), comprising central and peripheral neuropathic pain (CNP and PNP) (6). PNP, which can occur after nerve injury, is the most common type of clinical neuropathic pain (7, 8).
The mechanism of neuropathic pain mainly involves peripheral sensitization and central sensitization. Peripheral sensitization refers to the phenomenon that the threshold of nociceptors is reduced due to nerve injury (9), while central sensitization is often manifested by the enhanced response of the pain loop in the central nervous system (CNS) (10). Infiltrated macrophages and T cells, as well as activated microglia and astrocytes, can release several pain regulators, contains proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-17 and interferon-γ (IFN-γ), causing pain hypersensitivity (11–14). Particularly, T cells are able to infiltrate the dorsal root ganglion (DRG) and release the pro-inflammatory mediator leukocyte elastase (LE), leading to mechanical ectopic pain; and are involved in mechanical nociceptive hyperalgesia in the spinal cord, playing an important role in pain in the central and peripheral systems (15).
Cellular energy metabolism primarily relies on three nutrient types: carbohydrates, proteins, and fats. Normal cells break down proteins and fats into smaller molecules that enter the mitochondria to participate in the tricarboxylic acid (TCA) cycle. In contrast, glucose metabolism, a key aspect of carbohydrate metabolism, occurs in two distinct scenarios. Both conditions involve glycolysis, a series of biochemical reactions that convert glucose to pyruvate. Under aerobic conditions, pyruvate enters the mitochondrial TCA cycle for energy production, a process referred to as glucose oxidative phosphorylation (OXPHOS) or aerobic glycolysis. Under anaerobic conditions, pyruvate is converted to lactate in the cytoplasm while simultaneously generating energy, known as anaerobic glycolysis. However, tumor cells exhibit distinct energy metabolism compared to normal mature cells. Regardless of oxygen availability, tumor cells primarily rely on glucose consumption and lactate production for energy, a phenomenon known as the “Warburg effect” or aerobic glycolysis (16). Recent studies have demonstrated that activated T cells share similar energy metabolism with tumor cells, utilizing the “Warburg effect” as a critical energy source (17). To prevent ambiguity, we adopt the terms “anaerobic glycolysis” for lactate production in the cytoplasm and “glucose OXPHOS” for the process of glucose conversion to pyruvate followed by mitochondrial entry and OXPHOS. Moreover, the conversion of glucose to pyruvate is referred to as glycolysis. In recent years, the metabolic regulation of T-cell activity and function has become a focal point of research. This area of study has been widely explored in various diseases, such as oncology (18), metabolic syndrome (19), autoimmune diseases (20), and inflammatory diseases (21). While direct studies on the relationship between T-cell energy metabolism and neuropathic pain are relatively limited, there exist numerous molecules that play a role in T-cell metabolism and are implicated in the development of neuropathic pain.
The review first outlines the functions of different subpopulations of T cells in peripheral neuropathic pain arising from persistent nerve injury. Subsequently, it provides a comprehensive overview of the changes in the microenvironment caused by nerve injury and alterations in cellular energy metabolism that result from these changes. It also discusses the primary metabolic pathways adopted by T cells. We have focused on identifying the crucial metabolic molecules in T cells that have garnered attention in recent five years of research, categorizing them into two groups: (1) molecules of T-cell metabolism that participate in neuropathic pain, and (2) molecules of T-cell metabolism that have yet to play a role in neuropathic pain. These perspectives help to explore the critical role of T-cell metabolic molecules in the development and occurrence of neuropathic pain and provide potential targets for intervening in neuropathic pain.
Different T cell subtypes play different roles. In the nerve injury-induced neuropathic pain model, CD4+ T cells infiltrate into the injured nerve, DRG, and spinal cord (22–26) and facilitate the transition from acute to chronic pain (25) and maintenance of chronic pain with different subpopulations acting distinctively (25, 27). Several studies have revealed that compared with mice from heterozygous litters, interferon-γ receptor 1 (IFN-γR1) deletion mutants and congenital athymic nude mice deficient in mature T cells show significantly less mechanical allodynia and thermal hyperalgesia after chronic constriction injury of the sciatic nerve (CCI) (28, 29). Corresponding transfer of T helper 1 cells (Th1) to nude mice enhances pain hypersensitivity in recipients to levels similar with the heterozygous donor rats (28, 29). In contrast, passive transfer of anti-inflammatory cytokine-producing Th2 into heterozygous rats significantly attenuates their pain hypersensitivity (29). Actually, the circulating levels of Th2-related IL-10 and IL-4 in patients with painless neuropathy are higher than those in patients with painful neuropathy and controls (30). These suggest that a Th2-mediated anti-inflammatory response may play a crucial role in regulating pain development. In a CCI model, IL-17A-positive T cells are detected by immunocytochemistry within the damaged nerve, and lack of Th17 is associated with reduced thermal nociceptive sensitization (26, 31). Depletion of regulatory T cells (Tregs) is achieved by injection of anti-CD25 antibody, and it is observed that Treg-depleted mice exhibit prolonged mechanical hypersensitivity (32). In studies of neuropathic pain resulting from peripheral nerve injury in mice, CD8+ T lymphocytes aggregate to a greater extent in the DRG and dorsal horn by migration after CCI, yet infiltrate mainly in the ventral horn after transection. These cells have been found to produce IL-10, which can relieve chronic pain (33), indicating that they may play a role in the resolution of chronic pain. However, it is worth noting that CD8+ T cells are not the only source of IL-10, as CD4+ T cells and macrophages are also capable of producing this cytokine (34).
After peripheral nerve injury, various cells such as macrophages (35), glial cells (36), and lymphocytes (37) infiltrated the injured nerve, DRG, or spinal cord. These cells, along with the neurons, released numerous mediators that contributed to the alteration of the microenvironment in which they were located. These alterations induced a diverse range of effects on the cells in that environment, such as facilitating the conversion of inflammatory phenotypes (38) and triggering oxidative stress (39). Recent studies showed that metabolic abnormalities could occur in this microenvironment (40), which theoretically could modify neuropathic pain if rectified (41). Interestingly, metabolic abnormalities could also exist in T cells. Notably, in previous studies, it has been found that different types of T cells possess different metabolic patterns, and that their functions can be influenced by altering their metabolic processes.
In response to severe injurious stimuli, peripheral tissues and nerves may undergo a series of changes that induce alterations in the microenvironment surrounding the cells. For instance, nerve cells and other cells release a series of factors, causing to an inflammatory shift in the microenvironment. Specifically, following nerve lesions, neurons release reactive oxygen species/reactive nitrogen species (ROS/RNS) (42) and excess glutamate (43). Mast cells, neutrophils, and macrophages within the microenvironment subsequently release inflammatory factors such as adenosine triphosphate (ATP), bradykinin, prostaglandin E2, histamine, Serotonin (5-HT), IL-1β, IL-6, neural growth factor, and TNF-α, leading to peripheral inflammation. This inflammatory response further activates neurons to release pain-causing factors including substance P, calcitonin gene-related peptide (CGRP), neurokinin A, and nitrous oxide (NO), resulting in neurogenic pain (44). Sustained injurious stimuli lead to a prolonged inflammatory process, with lymphocytes releasing factors to create an “inflammatory soup” that lowers pain thresholds through peripheral sensitization due to inflammation-related changes (45).
In addition to eliciting inflammatory changes, the microenvironment experiences metabolic alterations. Functional neuroimaging studies reveal that glucose metabolism undergoes enhancement and glucose transporter 3 (GLUT3) protein expression increases in the medial prefrontal cortex (mPFC), which plays a pivotal role in neuropathic pain development (46). Classical antineuropathic pain therapeutics, such as gabapentin (47), and transcranial direct current stimulation (48), can mitigate pain responses by modulating glucose metabolism in the mPFC. The enhancement of glucose metabolism implies that there might be a temporary decrease in glucose within the microenvironment. These metabolic alterations result in the accumulation of various intermediates, including phosphoenolpyruvate (PEP) (41, 49) and lactate (41, 49, 50), which subsequently disturb the acid-base equilibrium in the microenvironment, driving a cascade of pathophysiological processes (41). Of note, astrocytes can produce L-lactate through the astrocyte-neuron lactate shuttle (ANLS), playing a crucial role in maintaining synaptic transmission (51, 52). Additionally, it has been shown to increase the concentration of glutamate after excitatory neuronal injury (53).
Alterations in the microenvironment could affect the cells within it and contribute to multiple pathological processes in neuropathic pain. For instance, the binding of ROS/RNS to receptors, such as Transient receptor potential cation channel subfamily M member 2 (TRPM2) expressed by glial cells and leukocytes, triggers the expression of pro-inflammatory mediators through the activation of mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) (54, 55). Moreover, these extracellular changes can also result in alterations to the intracellular metabolism, which will be elaborated upon in the subsequent section.
Due to microenvironment changes, various cellular metabolisms undergo certain alterations (40, 56). The accumulation of ROS/RNS by injury can impair mitochondria in the nociceptive pathway and induce mitochondrial dysfunction and can severely impair OXPHOS, resulting in metabolic disturbances (57), which has a significant impact on the mechanisms of neuropathic pain. It can occur in neurons, glial cells, and immune cells (55, 58), and restoring normal mitochondrial function can alleviate both the induction and persistence of pain (59). Targeting mitochondrial metabolism has also emerged as a potential therapeutic approach for neuroinflammation in progressive multiple sclerosis (60). Lactate can shuttle between glial cells and neurons, acting as one of the essential energy sources for neurons in the context of nerve injury, in order to maintain and promote pain transmission (51). Furthermore, Glutamate can be taken up by various cells and increase the metabolism of glutamine (41, 61, 62). It is noteworthy that, besides enhancing the metabolism of glutamate, glutamine also facilitates anaerobic glycolysis. Glutamate can be taken up by astrocytes via a sodium-dependent mechanism, which increases the intracellular sodium concentration, activates the sodium-potassium-ATPase on the cell membrane, promotes glucose uptake, and thus promotes anaerobic glycolysis (41). Furthermore, the presence of IFN-γ within the microenvironment has been implicated in potential modulatory effects on microglial cells (63). According to recent literature (63), brief exposure to β-amyloid alters the metabolic profile of microglia from OXPHOS to glycolysis by activating the mammalian target of rapamycin/hypoxia-inducible factor-1α (mTOR/HIF-1α) pathway. However, prolonged β-amyloid exposure reduces both glycolysis and OXPHOS, which impairs microglial responsiveness to harmful stimuli. In a murine model of Alzheimer’s disease, exogenous IFN-γ activates the mTOR pathway to promote glycolysis, which mitigates β-amyloid-induced microglial activation and ameliorates resultant neurofunctional deficits (63).
In particular, the metabolism of T cells could be influenced by alterations in the external environment, which in turn may affect a range of T cell functions. Current research has shown that activation of the T cell receptor (TCR) leads to enhanced uptake of GLUT1 on T cells (64). Following enhancement of T-cell glycolysis, the build-up of the metabolic intermediate phosphoenolpyruvate (PEP) can inhibit the endoplasmic reticulum (ER) calcium (Ca2+) channel, leading to impaired Ca2+ uptake by the ER Ca2+ store. This leads to a rise in cytoplasmic Ca2+ concentration, further activating the inflammatory pathway, driving T-cell differentiation towards a pro-inflammatory phenotype, and inducing the transcription of pro-inflammatory cytokines (41, 65). Lactate accumulation can enhance NF-κB pathway-mediated immune responses and inflammatory cascade reactions in Th17 (66). Excessive glutamate may contribute to enhanced glutamine metabolism in T cells, which is a potential outcome of altered T-cell energy metabolism.
It is noteworthy that various T-cell subtypes rely on distinct metabolic pathways, and their energy metabolism plays a pivotal role in their activation, differentiation, and effector functions (67). In a quiescent state, T cells primarily catabolize glucose and fatty acids (FA) via OXPHOS to maintain their basal cellular state and sustain their circulation in lymphoid tissues (17, 68). Correspondingly, when activated, CD4+ T cells and CD8+ cytotoxic T cells predominantly shift their metabolic program towards anaerobic glycolysis and glutaminolysis, allowing for the rapid generation of copious amounts of ATP and biosynthetic precursors necessary to sustain their activation and effector responses (69). Distinct metabolic programs are employed by different effector CD4+ T cell subtypes after activation. Specifically, Th1, Th2, and Th17 predominantly rely on glycolysis as their primary energy-generating pathway (70–72). Notwithstanding the fact that Th1 and Th17 cells predominantly rely on glucose as their principal fuel source and engage in glycolysis, their respective tendencies toward anaerobic glycolysis vs glucose oxidation via phosphorylation are divergent. Specifically, Th17 shows a preference for pyruvate conversion to lactate in order to expeditiously synthesize non-mitochondrial ATP (73), while Th1 cells exhibit a greater proclivity for pyruvate oxidation (20). Tregs predominantly rely on FA oxidation (FAO) and OXPHOS to maintain their function (71, 74). Memory CD4+ T cells primarily utilize glycolysis, while during the transition from activation to memory in CD8+ T cells, cellular metabolism is reprogrammed (75), leading to enhanced OXPHOS, FAO, and mitochondrial maintenance (76). As a result, memory CD8+ T cells primarily rely on FAO as their metabolic pathway. Overall, T cell energy metabolism includes glycolysis, FA OXPHOS, and glutaminolysis, as illustrated in Figure 1B.
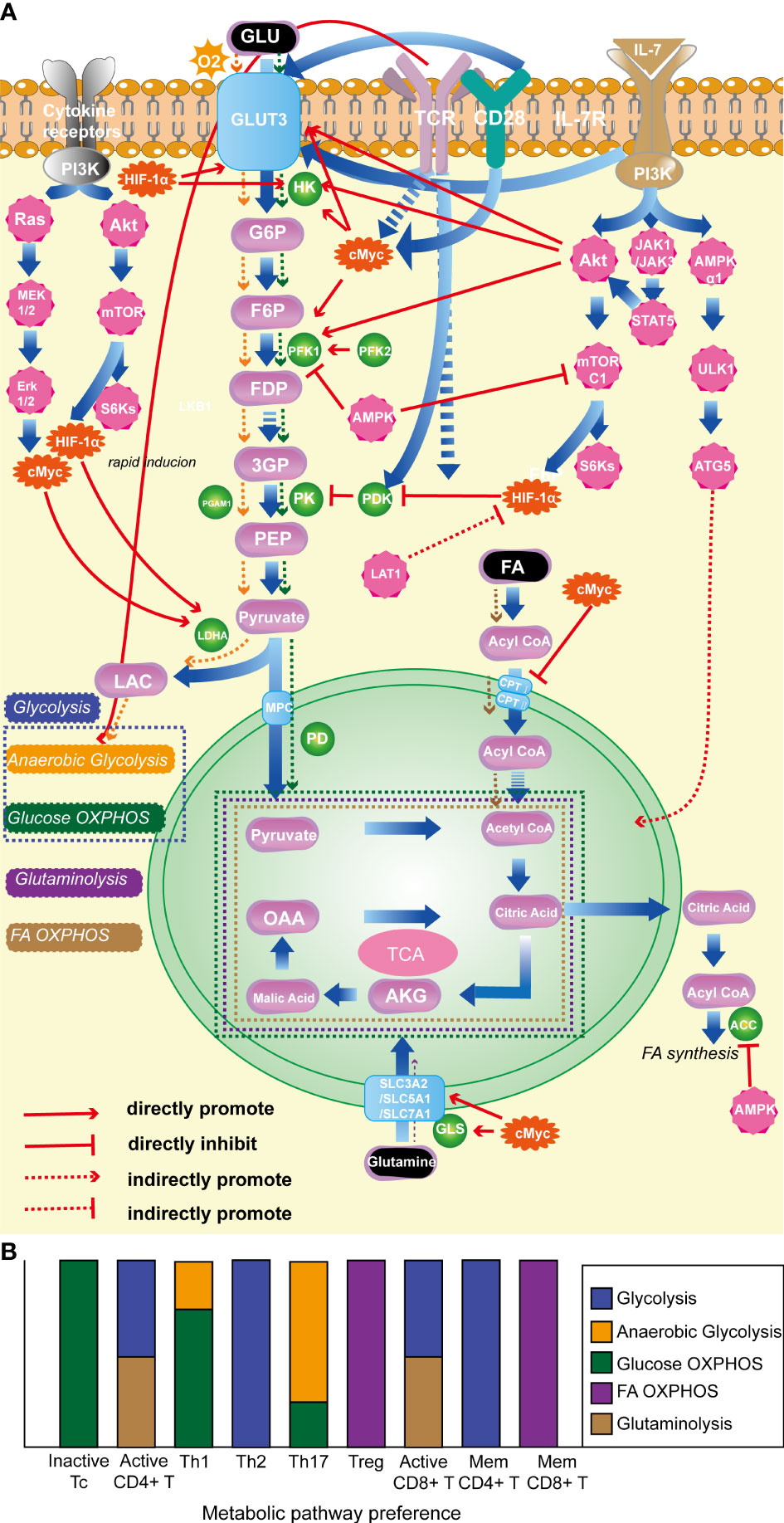
Figure 1 (A). Classical molecules regulating T-cell energy metabolism. (B). The energy metabolism preferences of T cells with different state and subtype. (1) HIF-1α: promotes glycolysis; inhibits OXPHOS of pyruvate; (2) c-Myc: enhances glycolysis; promotes glutamine catabolism. (3) AKT: foster glycolysis; (4) mTORC1: promotes glycolysis. (5) AMPK: prevent lipid synthesis; promote lipid oxidation; inhibit glycolysis. (6) TCR: promote glycolysis. (7) CD28: promote glycolysis. (8) IL-7R facilitates glycolysis; promotes OXPHOS. (9) LDHA: promotes glycolysis. HIF-1α, hypoxia-inducible factor-1α; mTORC1, the mammalian target of rapamycin complex 1; AMPK, AMP-activated protein kinase; TCR, T-cell receptor; IL-7R, interleukin-7 receptor; LDHA, lactate dehydrogenase.
Various enzymes and proteins participate in the metabolic processes discussed above. Among them, the glycolysis pathway is extensively and in-depth researched. The mTOR pathway and the transcription factor HIF-1α are key promoting regulatory factors in the progression of glycolysis (77, 78), while AMP-activated protein kinase (AMPK) and phosphatase and tensin homolog (PTEN) act as negative regulators (79, 80). It is worth noting that T cell surface-specific activation molecules, such as TCR (64), CD28 (81), and IL-7R (82), play important promoting roles in T cell glycolysis. In OXPHOS, FA are converted into fatty acyl-CoA in the cytoplasm and then transported into mitochondria by carnitine palmitoyltransferase I and II to play a crucial role in OXPHOS. C-Myc has an inhibitory effect on this critical enzyme. Glutaminase is a key enzyme in the glutamine metabolism process, and c-Myc has a promoting effect on glutamine metabolism (83). See Figure 1A for the detailed mechanisms of specific molecules and metabolic processes.
The adaptation of specific metabolic phenotypes is critical for T-cell immune function, and the regulation of cellular metabolism plays a pivotal role in shaping the plasticity of T-cell functions. Manipulating classical molecules involved in T-cell metabolism can have a profound impact on the function of these immune cells. For instance, the deficiency or restraint of mTOR can repress glycolysis, hindering the activation of CD4+ T cells to differentiate into Th effector cells, while promoting the generation of Tregs (84). Conversely, augmented the mammalian target of rapamycin complex 1 (mTORC1) signaling can promote Th1 or Th17 cell differentiation by enhancing glycolysis and suppressing Treg differentiation (85). Notably, although the absence of mTORC1 does not affect Treg differentiation, its function is suppressed (86–88). HIF-1α plays a crucial role in Th17 differentiation and function, while inhibiting Treg differentiation by facilitating glycolysis (72, 89). The c-Myc transcription factor mainly affects T-cell activation by modulating glycolysis. T cells deficient in c-Myc exhibit impaired proliferation and IL-2 secretion and are unable to differentiate into effector T cells (90). The AMP-activated protein kinase (AMPK) pathway is a key modulator of energy metabolism that exerts profound effects on T-cell subset differentiation. AMPK has been shown to regulate Th1 and Th17 differentiation by suppressing glycolysis through the inhibition of mTORC1. Additionally, AMPK-mediated FA OXPHOS promotes Treg and memory CD8+ T-cell differentiation and function, indicating the intricate interplay between metabolic pathways and T-cell plasticity (91).
In T-cell metabolism researches, the classical molecules mentioned earlier have been thoroughly explored for their significant impact on T-cell function. We designate these molecules as the classical molecules involved in T-cell energy metabolism. However, with the advancement of research in this field, it has become apparent that certain molecules seemingly unrelated to metabolism also partake in T-cell metabolic pathways and regulate T-cell function via such pathways. Therefore, we classify these molecules as those that affect T-cell energy metabolism and refer to them as T-cell metabolism-related molecules. We conducted a focused screening of T-cell metabolism-related molecules in the literature over the past five years and found that these molecules do not exert their effects in every T-cell, but rather depend on specific T-cell subtypes. Subsequently, we classified T-cell metabolism-related molecules into two distinct categories based on their association with chronic neuropathic pain. The first category encompasses molecules that participate in the underlying mechanisms of chronic neuropathic pain, underscoring their importance in this condition. The second category consists of newly reported molecules that regulate T-cell metabolism, highlighting their critical role in this cellular process. Given their distinct emphases, we refer to them as “molecules affecting neuropathic pain” and “molecules affecting T-cell metabolism”, respectively. These molecules are shown in Figure 2 and Table 1.
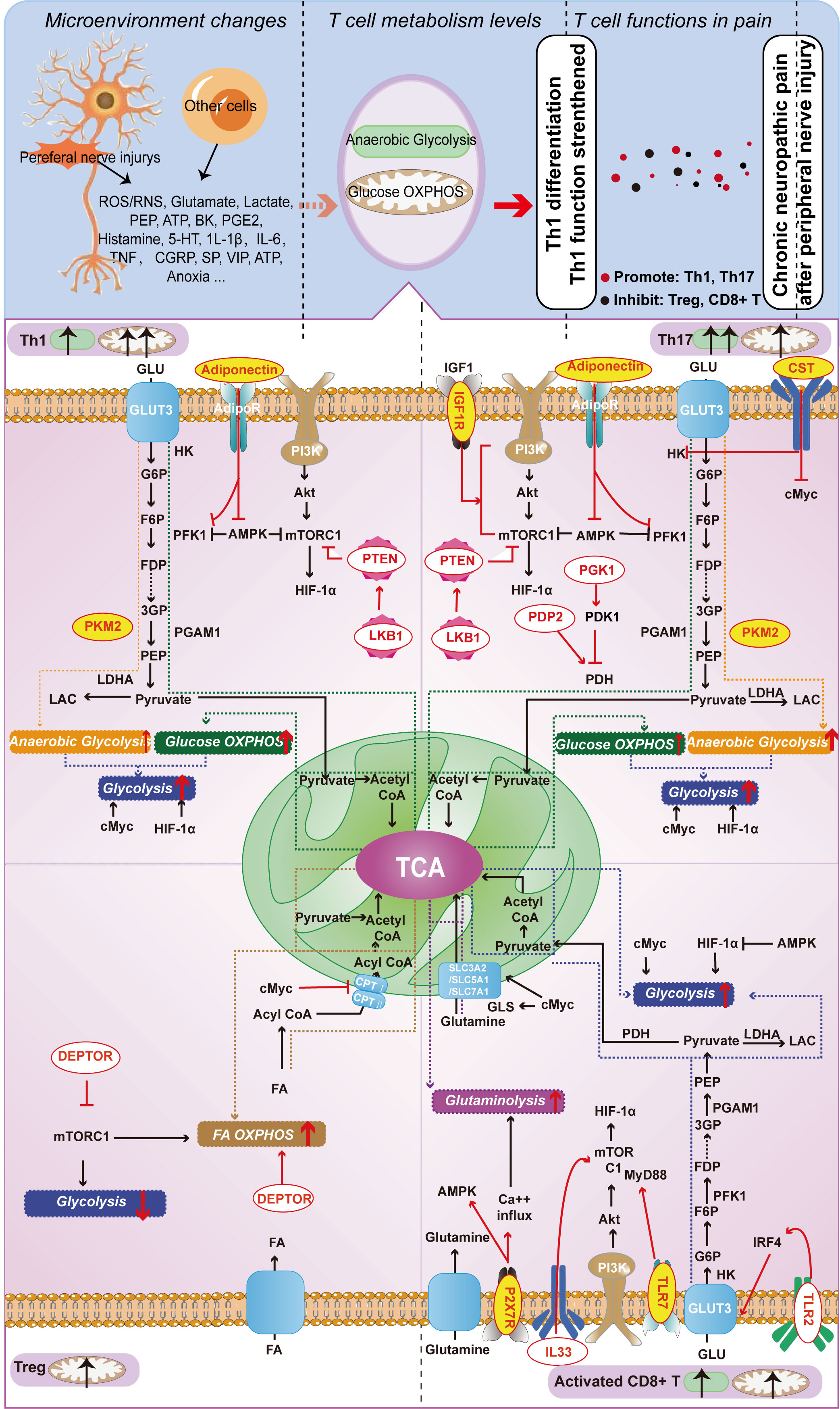
Figure 2 Metabolism-Related Molecules in Different T Cell Subtypes (Th1, Th17, Treg, and Activated CD8+ T Cells) Involved in Neuropathic Pain from Nerve Injury. Red letters on a yellow background indicate molecules capable of playing a role both in T cell function and in chronic neuropathic pain; red letters on a white background indicate molecules that currently play a role only in T cell function, but not in chronic neuropathic pain.
In this section, we provide an overview of T-cell metabolism-related molecules implicated in chronic neuropathic pain over the past five years. These molecules include pyruvate kinase M2 subtype (PKM2) and adiponectin, which mainly function in Th1 cells; insulin-like growth factor 1 receptor (IGF-1R), PKM2, cortistatin (CST), and adiponectin, which primarily work in Th17 cells; and P2X receptor 7 (P2RX7) that acts in CD8+ T cells. We initially present how these molecules modulate the functions of distinct T cell subpopulations by regulating their energy metabolism. Subsequently, we explore the roles and underlying mechanisms of these molecules in relation to chronic neuropathic pain. Based on the discussion presented, it is clear that T cell subtypes are involved in chronic neuropathic pain (as extensively examined in the previous section), and these molecules not only affect T cell functions, but also directly influence the pathological progression of chronic pain. It implies that these molecules may affect T cell function by modulating their metabolism, ultimately contributing to the development of chronic neuropathic pain. Although direct evidence to substantiate this perspective is presently limited, the growing number of associated molecules underscores the significant potential and research value in this domain. In the subsequent sections, we will offer an in-depth analysis of these molecules.
Pyruvate kinase M2 subtype (PKM2), an intermediate in glycolysis, catalyzes the last step of glycolysis by the conversion from PEP to pyruvate (92). As a key enzyme in glycolysis, PKM2 is present in multiple cells and participates in metabolic processes (93, 94). The current studies demonstrate that PKM2 is requisite for Th1 and Th17 differentiation (95). Accordingly, interference with PKM2 expression by small interfering RNA (siRNA) or pharmacological inhibition of PKM2 activity could suppress its involvement in glycolysis and subsequently restrict the function of Th1 and Th17 (96, 97). It has been demonstrated that PKM2 is a potential therapeutic target in various diseases (98–100). For instance, in a recent study, PD-1-targeted particles suppressed activated T cells and alleviated autoimmunity exactly via inhibition of PKM2-mediated glycolysis (98).
The role of PMK2 in neuropathic pain has been studied (101). In the rat animal model of CCI-induced neuropathic pain, peripheral nerve injury significantly raised PKM2 levels in spinal cord. Double immunofluorescence staining displayed co-localization of PKM2 with neurons, microglia and astrocytes. Intrathecal injection of PKM2 siRNA leads to attenuation of CCI-induced extracellular regulated protein kinases (ERK) and signal transducer and activator of transcription 3 (STAT3) activation, as well as CCI-induced mechanical allodynia and thermal hyperalgesia. The above findings suggest that inhibition of PKM2 expression can effectively attenuate CCI-induced neuropathic pain and inflammatory responses in rats, possibly through modulation of ERK and STAT3 signaling pathways (101). This study illustrates that PKM2 is an important contributor to neuropathic pain, however it fails to focus on the impact on T cell metabolism in chronic pain, which would be a valuable direction for future research.
Adiponectin is an adipocytokine primarily secreted by adipose tissue (102), modulating the regulation of glucose and FA oxidation (103, 104). Adiponectin binds to several receptors, including two adiponectin receptors (AdipoR1 and AdipoR2) and one cadherin-like receptor (105). Previous studies have demonstrated that Adiponectin exerts a pivotal regulatory influence on T-cell differentiation and function (106). Recent research has revealed that in Th1, after binding to the adiponectin receptor, adiponectin can interfere with the transcription factor HIF-1α, resulting in the suppression of glycolysis. Similarly, upon binding to AdipoR1, Adiponectin can suppress phosphofructokinase 1 (PFK1), the principal enzyme involved in glycolysis, thereby influencing glycolysis and inhibiting Th17 function (107, 108). These findings suggest that adiponectin can modulate T-cell metabolism to affect its function, thereby suggesting the potential of adiponectin as a therapeutic target for T-cell-related diseases. Notably, in patients with obesity, adiponectin can regulate the activity of pro-inflammatory CD4+ T cells (108), and its effectiveness in treating obesity has been demonstrated (109).
Studies have demonstrated that adiponectin plays a protective role in neuropathic pain (110). L Sun and colleagues conducted experiments on wild-type (WT) and adiponectin-knockout (KO) mice with partial sciatic nerve ligation (pSNL) or sham surgery, assessing pain behavior and protein levels (110). The results revealed that adiponectin-KO mice exhibited significantly lower thermal and mechanical pain thresholds than WT mice under both physiological and pathological conditions. Adiponectin was found to regulate thermal nociception by inhibiting the activation of p38 MAPK and transient receptor potential cation channel subfamily V member 1 (TRPV1) in neurons, microglia, and cortical neurons, underscoring its regulatory role in neuropathic pain. Targeting adiponectin may be a promising therapeutic strategy for reducing thermal sensitivity via inhibiting the activation of DRG neurons, spinal cord microglia, and somatosensory cortical neurons. In addition, adiponectin has been shown to regulate T-cell metabolism and function. Targeting Th1 and Th17 metabolism may inhibit the function of these T cells, which could alleviate neuropathic pain. Although further research is needed to fully explore this treatment approach, it offers a novel direction for developing more effective neuropathic pain treatment strategies.
The IGF system is essential in diverse physiological processes, including cellular metabolism, growth, and differentiation (111). Insulin-like growth factor 1 receptor (IGF-1R) is one of the major signaling receptor tyrosine kinases that mediate the actions of IGF1 and IGF2 (112), which could be expressed in CD4+ T cells, particularly the Th17 (113). One step further research indicates, in CD4+ T cells, IGF1 binding to IGF1R mediates signaling through the PI3K-AKT-mTOR pathway to increase anaerobic glycolysis, which subsequently facilitates Th17 cell differentiation (113). Thus, IGF1 might be considered a decision-making molecule for Th17 differentiation, on the basis of an enhanced pathway of anaerobic glycolysis. This decisional role makes its expression on T cells necessary for the full development of pathogenic Th17-mediated CNS inflammation in EAE, and a potential therapeutic target candidate for new therapies to treat autoimmune diseases. Lenaldekar (LDK), a novel small molecule targeting the IGF-1R signaling pathway, has been studied to reduce T cell proliferation and attenuate disease-related clinical symptoms (114).
Several studies have long focused on IGF1 as a factor contributing to neuropathic pain (115, 116). Following peripheral nerve injury, impaired neurons release this molecule into the synaptic environment, acting on IGF1R on DRG neurons and consequently mediating an enhancement of protein kinase C alpha (PKCα)-dependent T-type calcium currents to increase pain. Interfering with this pathway could potentially reduce mechanical and thermal pain hypersensitivity in rodents (115). A recent study demonstrated that IGF1 is not only produced by cultured neurons, but also by astrocytes (117), while IGF1R is primarily expressed in neurons (118). It focused on the study that IGF1 acting on neurons could promote neuropathic pain by mediating mTOR-related signaling, with intrathecal injection of IGF1R inhibitors or IGF1-neutralizing antibodies attenuating CCI-induced pain behavior (118). Of note, in the previous studies, IGF1R is also expressed in T cells in the CNS (113); thus, the T-cell metabolic pathway mediated by IGF1R is of great potential research value in neuropathic pain.
Cortistatin (CST) is a cyclic neuropeptide with a Cys-Cys ring, which has a strong inhibitory effect on cortical neurons (119) and immune cells (120). In recent years, CST has been found to have immunomodulatory effects in various disease models. For instance, in a rat model of collagen-induced arthritis, berberine can induce CST in the gut to inhibit Th17 cell response and ameliorate arthritis symptoms (121). This suggests that CST plays a crucial role in the regulation of Th17 cells, but its inhibitory mechanism remains poorly explored. To address this issue, Guo et al. conducted a study aimed at elucidating the underlying mechanisms of CST in Th17 (122). It revealed that CST exerts its inhibitory effect on Th17 differentiation by regulating glycolysis. More specifically, CST was found to significantly suppress the glycolytic activity of Th17, while downregulating the mRNA expression of two key glycolytic molecules, namely c-Myc and HK2. Notably, overexpression of c-Myc and HK2 almost completely abolished the inhibitory effect of CST on Th17 cell differentiation, highlighting the critical involvement of the c-Myc-HK2 pathway in the CST-mediated inhibition of Th17 differentiation. Furthermore, the study identified the growth hormone secretagogue receptor 1 (GHSR1) as the mediator of the inhibitory effect of CST on Th17 differentiation. These findings provide important insights into the mechanism of CST in immune regulation and shed light on a novel therapeutic target for the treatment of immune-related disorders.
Correspondingly, amongst studies on neuropathic pain, Mario Delgado’s team proposed in 2014 that CST, a natural analgesic component of the peripheral injury receptor system, produced by peptidergic injury receptor neurons in the DRG in response to inflammation and noxious stimuli (123), plays an essential pain suppressing role in persistent inflammatory pain. Recently, Mario Delgado’s team presented new evidence on the role of CST in neuropathic pain (124). They found that the injection of CST, both peripherally and centrally, was able to alleviate the hyperalgesia and allodynia associated with peripheral nerve injury and diabetic neuropathy. The analgesic effect of CST targeted multiple aspects, regulating hypersensitization of nociceptors, inhibiting neuroinflammatory responses, and enhancing production of neurotrophic factors. Deficiency in CST worsened neuropathic pain responses and peripheral nerve dysfunction. These findings suggest that CST-based treatments may offer an appealing substitute for managing chronic neuropathic pain, and the multi-pronged analgesic effects of CST hint at the possibility of T cell energy metabolism as a forthcoming therapeutic target.
Extracellular ATP (eATP) is a “danger signal” used by eukaryotes to detect cell damage (125). In mice and humans, the release of eATP during inflammation or injury stimulates innate immune activation and neuropathic pain via the purinergic receptor P2X receptor 7 (P2RX7) (126–128). Notably, P2RX7 was expressed in diverse cells and was capable of facilitating both glycolysis (129, 130) and mitochondrial respiration (131). Nevertheless, the regulation of energy metabolism suggests that P2RX7 may be instrumental in leaning immune cell differentiation, which is likely due to the fact that P2RX7 activity may be governed by multiple factors. Therefore, under different circumstances, P2RX7 may predominantly support one metabolic pathway (e.g. respiration) over another (e.g. glycolysis). For example, in mice, eATP and P2RX7 have been shown to be requisite for Th17 differentiation (132). This process could be enhanced by glucose metabolism, which might be facilitated by lower sensitivity to mitochondrial damage, or by the preferential coupling of P2RX7 and glycolysis in this cell type. Recently, it was reported that P2RX7 promoted mitochondrial homeostasis and metabolic function in differentiating memory CD8+ T cells (133). It also showed that P2RX7 is required for the establishment, maintenance and function of long-lived central and tissue-resident memory CD8+ T cell populations in mice. In contrast, P2RX7 is not required for the generation of short-lived effector CD8+ T cells. Specifically, in activated CD8+ T cells, P2RX7 was stimulated by eATP (derived from damaged cells or exported from activated living cells). Calcium influx was subsequently induced, increasing mitochondrial metabolic activity (133), which can be reflected in enhanced glutaminolysis and enhanced FA OXPHOS. It is important to emphasize that memory CD8+ T cells function primarily in the metabolic mode of FA OXPHOS, whereas activated CD8+ T cells function primarily in the metabolic modes of glycolysis and glutaminolysis. Therefore, we speculate that differences in the primary metabolic modality used may contribute to the different roles of P2RX7 in the importance of memory CD8+ T cells and activated CD8+ T cells.
Previous studies have revealed that P2RX7 regulates metabolic processes, for which it is considered as a promising pharmacological target for the treatment of neuropathic pain (132, 134, 135). In a recent study, P2RX7 can guide the metabolic adaptation of long-lived memory CD8+T cells. At the same time, we also observed that the pharmacological inhibitor of P2RX7 stimulated the metabolism and differentiation disorder of activated mouse and human CD8+ T cells in vitro. In vivo, the transient blockage of P2RX7 improved neuropathic pain, but also impaired the production of memory CD8+ T cells (133). It suggests that the mechanism underlying the amelioration of neuropathic pain with suppression of P2RX7 is, at least in part, through inhibiting active CD8+ T cell metabolism to consequently affect the role of active CD8+ T cells in chronic neuropathic pain. Furthermore, besides enhancing mitochondrial stability, AMPK (which inhibits HIF-1α and thus glycolysis) is induced by this kind of T-cell metabolic inhibition. Remarkably, the role of memory CD8+ T cells in neuropathic pain has been neglected and little studied in previous studies. Our focus here is also on the effect of P2RX7 on active CD8+ T metabolism. Thus, the present study also provides a different perspective to examine the role of memory CD8+ T cells in neuropathic pain.
Regarding the molecules that have not been implicated in chronic pain, we concentrate on elucidating their function and specific mechanisms in regulating T-cell metabolism. These molecules include liver kinase B1-phosphatase and tension homolog deleted on chromosome 10 (LKB1-PTEN), which primarily function in Th1 cells; pyruvate dehydrogenase phosphatase catalytic subunit 2 (PDP2), phosphoglycerate kinase 1 (PGK1), and LKB1-PTEN, which primarily function in Th17 cells; DEP domain containing mTOR interacting protein (DEPTOR), which has a significant impact on Treg function; interleukin-33 (IL-33) and Toll-like receptor 2 (TLR2), which are critical participants in CD8+ T cell responses. Despite the lack of demonstrated involvement in chronic neuropathic pain treatment, these molecules hold the potential to emerge as targets in this field, rendering them of significant interest for comprehensive reviews. It should be noted that, in fact, there are additional molecules beyond those presented above. Due to the limitations in space, we have compiled a comprehensive list of these unmentioned molecules in Table S1.
The mTORC is a central regulator of T cell metabolic reprogramming, with the liver kinase B1-phosphatase and tension homolog deleted on chromosome 10 (LKB1-PTEN) signaling pathway being one of its upstream regulators. LKB1 and PTEN directly interact with each other, and LKB1 can phosphorylate PTEN to inhibit the mTORC1 signaling pathway, independent of AMPK. Consequently, LKB1 deficiency results in increased mTORC1 activity and upregulation of glycolysis mediated by HIF-1α. Further experiments have shown that Th17 and Th1 cell bias in LKB1-deficient T cells is mediated by glycolysis. These findings highlight the critical role of the LKB1-PTEN signaling pathway in regulating T cell metabolism and immune balance. Through the modulation of glycolysis, the LKB1-PTEN signaling pathway regulates the differentiation of Th1 and Th17 cells, thereby maintaining the internal homeostasis and immune balance of T cells (136).
Recent studies have identified pyruvate dehydrogenase phosphatase catalytic subunit 2 (PDP2) as a crucial regulator of T cell metabolism, specifically in the glycolysis and glucose OXPHOS pathways (137). While expressed in both Th1 and Th17 cells, PDP2 plays a role in restricting Th17 differentiation by inhibiting glycolysis. This function is closely associated with pyruvate dehydrogenase (PDH). PDH is a critical branching enzyme that converts pyruvate to acetyl-CoA, which is then transported to the mitochondria for OXPHOS. Due to the advantage of Th17 cells to convert pyruvate to lactate for rapid generation of non-mitochondrial ATP, PDH has been shown to uniquely regulate Th17 cells (73). Further, PDP2 has been identified as a key factor that promotes PDH activity in vivo, facilitating glucose metabolism and the subsequent steps of glycolysis and glucose OXPHOS. Taken together, these findings emphasize the critical role of PDP2 in regulating T cell metabolism and function, with a particular focus on Th17.
Phosphoglycerate kinase 1 (PGK1) plays a crucial role in metabolic regulation in CD4+ T cells. Recently, Yang Lu et al. found that both CD4+ T cells and Th17 exhibited increased glycolysis and PGK1 expression in the hearts of mice with myocarditis (138). Treatment of mice with the PGK1 inhibitor NG52 resulted in reduced inflammation and fibrosis in the heart, improved cardiac contractile function, reduced infiltration of Th17 and Th1 in the heart, and increased Treg proportion. In addition, NG52 could inhibit the activation and differentiation of CD4+ T cells from mice with myocarditis and patients with myocarditis in vitro. The mechanism underlying the effects of the PGK1 inhibitor involves the suppression of glycolytic activity and the reduction of the phosphorylation of pyruvate dehydrogenase kinase 1 (PDHK1), leading to increased production of ROS in the mitochondria, which in turn inhibits Th17 differentiation. Previous studies have shown that PDHK1 is a specific enzyme in Th17 that is almost not expressed in Th1 or other T-cell subsets. PDHK1 can inhibit PDH, which suppresses pyruvate entry into mitochondria and promotes Th17 glycolysis (73). In summary, PGK1 plays a metabolic regulatory role in CD4+ T cells and regulates the function of Th17 by modulating their glycolytic metabolism.
DEP domain containing mTOR interacting protein (DEPTOR) is an evolutionarily conserved intracellular binding partner of mTOR and serves as a negative regulator of signal transduction. In this study, we identified the expression of DEPTOR in CD4+ T cells and observed that its relative expression levels modulate differentiation and glucose utilization of CD4+ T effector cells in vitro. Using a knock-in mouse model, we further found that induced expression of DEPTOR in CD4 T regulatory cells stabilizes Foxp3 expression, promotes a shift towards OXPHOS metabolism, and enhances survival and suppressive function. These findings demonstrate the critical regulatory role of DEPTOR in CD4+ T cells, particularly in Tregs, and highlight the close interplay between this process and T cell metabolism (139).
Interleukin-33 (IL-33), a crucial member of the IL family, plays a crucial role in both innate and adaptive immunity. Recent research has revealed that IL-33 induces activation and proliferation of CD8+ T effector cells through extracellular signaling pathways, rather than nuclear signaling pathways (140). Specifically, IL-33 activates the mTORC1 pathway, enhancing glucose uptake and lactate production in CD8+ T cells, thereby promoting accelerated anaerobic glycolysis and increased activation of effector T cells. This discovery not only sheds light on the impact of IL-33 on CD8+ T cell function and underlying metabolic mechanisms but may also have profound implications for the future treatment strategies of CD8+ T cell related diseases.
Recent research has found that Toll-like receptor 2 (TLR2), a co-stimulatory molecule, plays a role in enhancing TCR-dependent activation of CD8+ T cells when activated (141). Specifically, TLR2 agonist Pam3CSK4 was shown to directly enhance TCR-dependent activation of CD8+ T cells (141). Transcriptome analysis further revealed that TLR2 signaling increases the expression of genes related to the cellular energy metabolism of CD8+ T cells, such as Interferon Regulatory Factor 4 (IRF4), leading to improved glycolysis and glutaminolysis. This effect can further increase the expression of genes related to immune regulation and function, such as IFN-γ. The metabolic processes of glycolysis and glutaminolysis are necessary for the enhanced T cell activation mediated by TLR2. Overall, TLR2 promotes CD8+ T cell immune response and function by regulating their energy metabolism.
Chronic neuropathic pain is a complex and multifaceted condition that arises from pathological changes or disorders within the nervous system (142). It can be classified as either central or peripheral neuropathic pain (143), with the latter having relatively well-established researches compared to the former (144, 145). Peripheral neuropathic pain can be caused by a multitude of factors, including peripheral nerve injury, chemotherapy, and nerve inflammation-induced neuropathic pain (146). In this review, we focus on neuropathic pain resulting from peripheral nerve injury, which has been extensively studied in animal models (144, 145) and has well-established mechanisms involving both peripheral and central sensitization.
Numerous studies have explored the energy metabolism of T cells in autoimmune and inflammatory disorders, including but not limited to rheumatoid arthritis (147) and systemic lupus erythematosus (20, 148). In these diseases, pro-inflammatory T cells, including Th1 and Th17 cells (particularly Th17), play a crucial role and rely primarily on glycolysis for their functions (149, 150). Therefore, most researches on T cell metabolism have focused on the glycolytic pathway of Th17 cells, which explains why this review is more detailed in exploring the molecular mechanisms of Th17 glycolysis. It should be emphasized that T cell energy metabolism in cancer has also been extensively studied. Nonetheless, these studies have been excluded from this review due to the distinct energy metabolism of T cells within the tumor microenvironment compared to that of T cells in physiological conditions or other diseases (151).
Previous studies on neuropathic pain resulting from peripheral injuries have identified the release of a series of mediators during nerve damage. This event leads to the infiltration of other inflammatory and immune cells in the area, which then continue to release substances and ultimately form a microenvironment that can profoundly impact the cells present and cause metabolic abnormalities (45). Notably, metabolic reprogramming of glial cells has been reported in chronic neuropathic pain (41). Such conditions may also occur in T cells. This review places particular emphasis on the molecular aspects of T cell energy metabolism and explores its current involvement in neuropathic pain. By establishing a link between T cell metabolism and neuropathic pain resulting from peripheral injuries, this review provides a potential target for intervening in neuropathic pain.
During nerve injury, the release of eATP can activate P2RX7 on CD8+ T cells, leading to mitochondrial homeostasis and affecting energy metabolism pathways, including lipid OXPHOS and glutamine catabolism, which ultimately influence the function of CD8+ T cells. Notably, P2RX7 is essential for the differentiation of memory CD8+ T cells. Moreover, pharmacological inhibition of P2RX7 in vitro induced metabolic and differentiation dysregulation in activated mouse and human CD8+ T cells, while transient P2RX7 blockade improved neuropathic pain in vivo but impaired memory CD8+ T cell production. Hence, in chronic neuropathic pain, P2RX7 can modulate T cell function through alterations in energy metabolism, while blockade of P2RX7 can ameliorate neuropathic pain (133). This study provides a cogent link between neuropathic pain and T cell metabolism, yet the contribution of memory CD8+ T cells in neuropathic pain requires further elucidation. While direct evidence connecting T-cell metabolism and neuropathic pain is limited, this review takes a novel approach by exploring how molecules that impact T-cell function by affecting T-cell energy metabolism and how these molecules can contribute to chronic neuropathic pain. which bridges the connection between T-cell energy metabolism and neuropathic pain. Finally, this review explores some T-cell energy metabolism-associated molecules that have not yet been explored in neuropathic pain. These molecules may represent potential targets for the treatment of neuropathic pain, and the exploration of T cell energy metabolism as a novel avenue for neuropathic pain therapy is an area ripe for further development.
It is essential to highlight that this review was manually searched for relevant studies. Although we have categorized the research according to a certain logic, it still cannot cover all the pertinent studies. We refer to the molecules that play a role in T-cell energy metabolism as T-cell energy metabolism-related molecules and categorize them into molecules that act in neuropathic pain and those that have not yet been found to act in neuropathic pain. Furthermore, we set our screening criteria to new molecules related to T cell metabolism within the last five years to gather as comprehensive a collection of related studies as feasible. It is crucial to note that this article can also serve as a point of reference for exploring other types of neuropathic pain. For instance, the key transcription factor HIF-1α in T cell glycolysis plays a vital role in neuropathic pain. In the initial stages of the CRPS mouse model, inhibiting HIF-1α can produce an anti-abnormal pain effect and suppress the production of inflammatory cytokines (152). Hyaluronic acid (HA)/CD44 regulates Th1 differentiation by activating the Annexin A1-AKT-mTOR signaling pathway, promoting the pathogenesis of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). AKT is involved, which may be linked to its regulation of glycolysis (153). However, the changes in T-cell energy metabolism in the corresponding disease environment must be taken into account. To conclude, exploring the connection between T cell energy metabolism and neuropathic pain is a research area that merits in-depth exploration.
Neuropathic pain, resulting from peripheral nerve damage, leads to microenvironmental changes due to the buildup of substances such as inflammatory cytokines and metabolic byproducts. These changes may influence cellular metabolism and function, thus altering their involvement in chronic pain, as evidenced in glial cells (41). This review intends to investigate the relationship between T-cell metabolic alterations, their function, and their contribution to chronic pain. Firstly, we elucidate the impact of metabolic pathway alterations on T-cell function to link T cell metabolism and function. Subsequently, we investigate how microenvironmental alterations lead to T-cell metabolic changes, connecting chronic pain and T-cell metabolism. Lastly, we focus on recent advances in molecules related to T-cell metabolism over the past five years. Some molecules play pivotal roles in both T-cell metabolism and chronic pain. We clarify how they modulate distinct T-cell subset functions by regulating energy metabolism and further elucidate their roles and potential mechanisms in chronic neuropathic pain. This implies that these molecules can influence T-cell function through metabolic alterations, thus promoting chronic neuropathic pain development. Despite limited direct evidence, the growing number of associated molecules underscores the field’s significant potential and research value. For other molecules not yet linked to chronic pain, we focus on their specific mechanisms and roles in T-cell metabolic regulation. Although not currently employed in the treatment of chronic pain, these molecules show promise as potential targets.
Thus, in future research, comprehensively exploring T-cell metabolism in chronic pain may enhance our understanding of T-cell mechanisms in chronic pain and establish a theoretical basis for novel therapies. Moreover, attention should be focused on T-cell metabolic molecules that have yet to be explored in chronic pain, as they could hold significant roles and potentially serve as future targets for chronic pain treatment. However, the complexity of chronic pain, involving multiple pain types and corresponding microenvironmental changes, along with the intricate nature of T-cell metabolism processes, including numerous metabolic pathways, interactions, and regulations, render the exploration of T-cell metabolism in chronic pain more challenging. Despite these challenges, this research field offers promising prospects and merits further exploration. In summary, delving deeper into T-cell metabolism in chronic pain may offer new insights and therapeutic strategies to address this complex pathological issue in chronic pain.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; XD took part in drafting, revising or critically reviewing the article; RC and JY gave final approval of the version to be published; MD and JL have agreed on the journal to which the article has been submitted; and YL and SS agree to be accountable for all aspects of the work.
This study was supported by grants from the National Natural Science Foundation of China (no. 82071485), and the 2022 Free Innovation Pre-research Fund (No. F016.01003.22003.121).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1107298/full#supplementary-material
1. Fasick V, Spengler RN, Samankan S, Nader ND, Ignatowski TA. The hippocampus and tnf: common links between chronic pain and depression. Neurosci Biobehav Rev (2015) 53:139–59. doi: 10.1016/j.neubiorev.2015.03.014
2. Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology (2018) 129(2):343–66. doi: 10.1097/aln.0000000000002130
3. Asseyer S, Schmidt F, Chien C, Scheel M, Ruprecht K, Bellmann-Strobl J, et al. Pain in Aqp4-Igg-Positive and mog-Igg-Positive neuromyelitis optica spectrum disorders. Multiple sclerosis J - Experimental Trans Clin (2018) 4(3):2055217318796684. doi: 10.1177/2055217318796684
4. Challa SR. Surgical animal models of neuropathic pain: Pros and cons. Int J Neurosci (2015) 125(3):170–4. doi: 10.3109/00207454.2014.922559
5. Doyen PJ, Beckers P, Brook GA, Hermans E. Regulators of g protein signalling as pharmacological targets for the treatment of neuropathic pain. Pharmacol Res (2020) 160:105148. doi: 10.1016/j.phrs.2020.105148
6. Zheng XB, Zhang YL, Li Q, Liu YG, Wang XD, Yang BL, et al. Effects of 1,8-cineole on neuropathic pain mediated by P2x2 receptor in the spinal cord dorsal horn. Sci Rep (2019) 9(1):7909. doi: 10.1038/s41598-019-44282-4
7. Wei J, Zhu X, Yang G, Shen J, Xie P, Zuo X, et al. The efficacy and safety of botulinum toxin type a in treatment of trigeminal neuralgia and peripheral neuropathic pain: A meta-analysis of randomized controlled trials. Brain Behav (2019) 9(10):e01409. doi: 10.1002/brb3.1409
8. Li AL, Zhang JD, Xie W, Strong JA, Zhang JM. Inflammatory changes in paravertebral sympathetic ganglia in two rat pain models. Neurosci Bull (2018) 34(1):85–97. doi: 10.1007/s12264-017-0142-1
9. Wang H, Ehnert C, Brenner GJ, Woolf CJ. Bradykinin and peripheral sensitization. Biol Chem (2006) 387(1):11–4. doi: 10.1515/bc.2006.003
10. Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature (1983) 306(5944):686–8. doi: 10.1038/306686a0
11. Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, et al. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain (2016) 12. doi: 10.1177/1744806916646784
12. Kim CF, Moalem-Taylor G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain (2011) 12(3):370–83. doi: 10.1016/j.jpain.2010.08.003
13. Sorkin LS, Doom CM. Epineurial application of tnf elicits an acute mechanical hyperalgesia in the awake rat. J peripheral nervous system JPNS (2000) 5(2):96–100. doi: 10.1046/j.1529-8027.2000.00012.x
14. Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. Ifn-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci USA (2009) 106(19):8032–7. doi: 10.1073/pnas.0810420106
15. Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, Kavelaars A. T cells as an emerging target for chronic pain therapy. Front Mol Neurosci (2019) 12:216. doi: 10.3389/fnmol.2019.00216
16. Koppenol WH, Bounds PL, Dang CV. Otto warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer (2011) 11(5):325–37. doi: 10.1038/nrc3038
17. Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: Energy metabolism and the t-cell response. Nat Rev Immunol (2005) 5(11):844–52. doi: 10.1038/nri1710
18. Nabe S, Yamada T, Suzuki J, Toriyama K, Yasuoka T, Kuwahara M, et al. Reinforce the antitumor activity of Cd8(+) t cells Via glutamine restriction. Cancer Sci (2018) 109(12):3737–50. doi: 10.1111/cas.13827
19. Bantug GR, Galluzzi L, Kroemer G, Hess C. The spectrum of t cell metabolism in health and disease. Nat Rev Immunol (2018) 18(1):19–34. doi: 10.1038/nri.2017.99
20. Sharabi A, Tsokos GC. T cell metabolism: New insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol (2020) 16(2):100–12. doi: 10.1038/s41584-019-0356-x
21. Yang W, Liu H, Xu L, Yu T, Zhao X, Yao S, et al. Gpr120 inhibits colitis through regulation of Cd4(+) t cell interleukin 10 production. Gastroenterology (2022) 162(1):150–65. doi: 10.1053/j.gastro.2021.09.018
22. Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience (2002) 112(1):23–38. doi: 10.1016/s0306-4522(02)00065-9
23. McLachlan EM, Hu P. Inflammation in dorsal root ganglia after peripheral nerve injury: Effects of the sympathetic innervation. Autonomic Neurosci basic Clin (2014) 182:108–17. doi: 10.1016/j.autneu.2013.12.009
24. Cao L, DeLeo JA. Cns-infiltrating Cd4+ t lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol (2008) 38(2):448–58. doi: 10.1002/eji.200737485
25. Du B, Ding YQ, Xiao X, Ren HY, Su BY, Qi JG. Cd4+ αβ t cell infiltration into the leptomeninges of lumbar dorsal roots contributes to the transition from acute to chronic mechanical allodynia after adult rat tibial nerve injuries. J Neuroinflamm (2018) 15(1):81. doi: 10.1186/s12974-018-1115-7
26. Kleinschnitz C, Hofstetter HH, Meuth SG, Braeuninger S, Sommer C, Stoll G. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol (2006) 200(2):480–5. doi: 10.1016/j.expneurol.2006.03.014
27. Kobayashi Y, Kiguchi N, Fukazawa Y, Saika F, Maeda T, Kishioka S. Macrophage-t cell interactions mediate neuropathic pain through the glucocorticoid-induced tumor necrosis factor ligand system. J Biol Chem (2015) 290(20):12603–13. doi: 10.1074/jbc.M115.636506
28. Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci Off J Soc Neurosci (2009) 29(46):14415–22. doi: 10.1523/jneurosci.4569-09.2009
29. Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience (2004) 129(3):767–77. doi: 10.1016/j.neuroscience.2004.08.035
30. Uçeyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology (2007) 69(1):42–9. doi: 10.1212/01.wnl.0000265062.92340.a5
31. Liu H, Dolkas J, Hoang K, Angert M, Chernov AV, Remacle AG, et al. The alternatively spliced fibronectin Cs1 isoform regulates il-17a levels and mechanical allodynia after peripheral nerve injury. J Neuroinflamm (2015) 12:158. doi: 10.1186/s12974-015-0377-6
32. Austin PJ, Kim CF, Perera CJ, Moalem-Taylor G. Regulatory t cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain (2012) 153(9):1916–31. doi: 10.1016/j.pain.2012.06.005
33. Sun Q, Hu T, Zhang Y, Wang X, Liu J, Chen W, et al. Irg1/Itaconate increases il-10 release to alleviate mechanical and thermal hypersensitivity in mice after nerve injury. Front Immunol (2022) 13:1012442. doi: 10.3389/fimmu.2022.1012442
34. Kavelaars A, Heijnen CJ. T cells as guardians of pain resolution. Trends Mol Med (2021) 27(4):302–13. doi: 10.1016/j.molmed.2020.12.007
35. Naito MG, Xu D, Amin P, Lee J, Wang H, Li W, et al. Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proc Natl Acad Sci USA (2020) 117(9):4959–70. doi: 10.1073/pnas.1916427117
36. Donnelly CR, Andriessen AS, Chen G, Wang K, Jiang C, Maixner W, et al. Central nervous system targets: Glial cell mechanisms in chronic pain. Neurother J Am Soc Exp Neurother (2020) 17(3):846–60. doi: 10.1007/s13311-020-00905-7
37. Bethea JR, Fischer R. Role of peripheral immune cells for development and recovery of chronic pain. Front Immunol (2021) 12:641588. doi: 10.3389/fimmu.2021.641588
38. Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain (2018) 159(3):595–602. doi: 10.1097/j.pain.0000000000001122
39. Teixeira-Santos L, Albino-Teixeira A, Pinho D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and nadph oxidase inhibitors as potential therapeutic strategies. Pharmacol Res (2020) 162:105280. doi: 10.1016/j.phrs.2020.105280
40. Maganin AG, Souza GR, Fonseca MD, Lopes AH, Guimarães RM, Dagostin A, et al. Meningeal dendritic cells drive neuropathic pain through elevation of the kynurenine metabolic pathway in mice. J Clin Invest (2022) 132(23). doi: 10.1172/jci153805
41. Kong E, Li Y, Deng M, Hua T, Yang M, Li J, et al. Glycometabolism reprogramming of glial cells in central nervous system: Novel target for neuropathic pain. Front Immunol (2022) 13:861290. doi: 10.3389/fimmu.2022.861290
42. Kallenborn-Gerhardt W, Hohmann SW, Syhr KM, Schröder K, Sisignano M, Weigert A, et al. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain (2014) 155(10):2161–70. doi: 10.1016/j.pain.2014.08.013
43. Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiology (2011) 24(4):400–7. doi: 10.1097/ACO.0b013e32834871df
44. Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. J Pain (2008) 9(2):122–45. doi: 10.1016/j.jpain.2007.09.006
45. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell (2009) 139(2):267–84. doi: 10.1016/j.cell.2009.09.028
46. Jiang M, Chen X, Zhang L, Liu W, Yu X, Wang Z, et al. Electroacupuncture suppresses glucose metabolism and glut-3 expression in medial prefrontal cortical in rats with neuropathic pain. Biol Res (2021) 54(1):24. doi: 10.1186/s40659-021-00348-0
47. Lin HC, Huang YH, Chao TH, Lin WY, Sun WZ, Yen CT. Gabapentin reverses central hypersensitivity and suppresses medial prefrontal cortical glucose metabolism in rats with neuropathic pain. Mol Pain (2014) 10:63. doi: 10.1186/1744-8069-10-63
48. Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: A mechanistic pet study. Neurorehabilitation Neural Repair (2014) 28(3):250–9. doi: 10.1177/1545968313507632
49. Geng X, Shen J, Li F, Yip J, Guan L, Rajah G, et al. Phosphoenolpyruvate carboxykinase (Pck) in the brain gluconeogenic pathway contributes to oxidative and lactic injury after stroke. Mol Neurobiol (2021) 58(5):2309–21. doi: 10.1007/s12035-020-02251-3
50. Jia J, Zhang H, Liang X, Dai Y, Liu L, Tan K, et al. Application of metabolomics to the discovery of biomarkers for ischemic stroke in the murine model: A comparison with the clinical results. Mol Neurobiol (2021) 58(12):6415–26. doi: 10.1007/s12035-021-02535-2
51. Miyamoto K, Ishikura KI, Kume K, Ohsawa M. Astrocyte-neuron lactate shuttle sensitizes nociceptive transmission in the spinal cord. Glia (2019) 67(1):27–36. doi: 10.1002/glia.23474
52. Kambe Y, Youkai M, Hashiguchi K, Sameshima Y, Takasaki I, Miyata A, et al. Spinal astrocyte-neuron lactate shuttle contributes to the pituitary adenylate cyclase-activating Polypeptide/Pac1 receptor-induced nociceptive behaviors in mice. Biomolecules (2022) 12(12). doi: 10.3390/biom12121859
53. Falnikar A, Hala TJ, Poulsen DJ, Lepore AC. Glt1 overexpression reverses established neuropathic pain-related behavior and attenuates chronic dorsal horn neuron activation following cervical spinal cord injury. Glia (2016) 64(3):396–406. doi: 10.1002/glia.22936
54. Grace PM, Tawfik VL, Svensson CI, Burton MD, Loggia ML, Hutchinson MR. The neuroimmunology of chronic pain: From rodents to humans. J Neurosci Off J Soc Neurosci (2021) 41(5):855–65. doi: 10.1523/jneurosci.1650-20.2020
55. Grace PM, Gaudet AD, Staikopoulos V, Maier SF, Hutchinson MR, Salvemini D, et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci (2016) 39(12):862–79. doi: 10.1016/j.tins.2016.10.003
56. Calcutt NA. Diabetic neuropathy and neuropathic pain: A (Con)Fusion of pathogenic mechanisms? Pain (2020) 161(Suppl 1):S65–s86. doi: 10.1097/j.pain.0000000000001922
57. Rossmann MP, Dubois SM, Agarwal S, Zon LI. Mitochondrial function in development and disease. Dis Models Mech (2021) 14(6). doi: 10.1242/dmm.048912
58. Casas AI, Dao VT, Daiber A, Maghzal GJ, Di Lisa F, Kaludercic N, et al. Reactive oxygen-related diseases: Therapeutic targets and emerging clinical indications. Antioxidants Redox Signaling (2015) 23(14):1171–85. doi: 10.1089/ars.2015.6433
59. Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol (2014) 10(6):326–36. doi: 10.1038/nrneurol.2014.77
60. Peruzzotti-Jametti L, Pluchino S. Targeting mitochondrial metabolism in neuroinflammation: Towards a therapy for progressive multiple sclerosis. Trends Mol Med (2018) 24(10):838–55. doi: 10.1016/j.molmed.2018.07.007
61. Wang Z, Guan D, Wang S, Chai LYA, Xu S, Lam KP. Glycolysis and oxidative phosphorylation play critical roles in natural killer cell receptor-mediated natural killer cell functions. Front Immunol (2020) 11:202. doi: 10.3389/fimmu.2020.00202
62. Andersen JV, Markussen KH, Jakobsen E, Schousboe A, Waagepetersen HS, Rosenberg PA, et al. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology (2021) 196:108719. doi: 10.1016/j.neuropharm.2021.108719
63. Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, et al. A breakdown in metabolic reprogramming causes microglia dysfunction in alzheimer's disease. Cell Metab (2019) 30(3):493–507.e6. doi: 10.1016/j.cmet.2019.06.005
64. Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in t cell activation and requires Cd28-mediated akt-dependent and independent pathways. J Immunol (Baltimore Md 1950) (2008) 180(7):4476–86. doi: 10.4049/jimmunol.180.7.4476
65. Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Sci (New York NY) (2010) 329(5998):1492–9. doi: 10.1126/science.1188015
66. Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y. Lactate boosts Tlr4 signaling and nf-kappab pathway-mediated gene transcription in macrophages Via monocarboxylate transporters and md-2 up-regulation. J Immunol (Baltimore Md 1950) (2009) 182(4):2476–84. doi: 10.4049/jimmunol.0802059
67. Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in t cell activation and lineage differentiation. Semin Immunol (2016) 28(5):514–24. doi: 10.1016/j.smim.2016.10.009
68. MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of t lymphocytes. Annu Rev Immunol (2013) 31:259–83. doi: 10.1146/annurev-immunol-032712-095956
69. Pearce EL. Metabolism in t cell activation and differentiation. Curr Opin Immunol (2010) 22(3):314–20. doi: 10.1016/j.coi.2010.01.018
70. Cluxton D, Petrasca A, Moran B, Fletcher JM. Differential regulation of human treg and Th17 cells by fatty acid synthesis and glycolysis. Front Immunol (2019) 10:115. doi: 10.3389/fimmu.2019.00115
71. Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory Cd4+ t cell subsets. J Immunol (Baltimore Md 1950) (2011) 186(6):3299–303. doi: 10.4049/jimmunol.1003613
72. Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(Reg) balance by hypoxia-inducible factor 1. Cell (2011) 146(5):772–84. doi: 10.1016/j.cell.2011.07.033
73. Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, et al. Metabolic programming and Pdhk1 control Cd4+ t cell subsets and inflammation. J Clin Invest (2015) 125(1):194–207. doi: 10.1172/jci76012
74. Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms t cell metabolism to function in low-glucose, high-lactate environments. Cell Metab (2017) 25(6):1282–93.e7. doi: 10.1016/j.cmet.2016.12.018
75. Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: Insights into metabolism and lymphocyte function. Sci (NY) (2013) 342(6155):1242454. doi: 10.1126/science.1242454
76. van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial respiratory capacity is a critical regulator of Cd8+ t cell memory development. Immunity (2012) 36(1):68–78. doi: 10.1016/j.immuni.2011.12.007
77. Jones RG, Thompson CB. Revving the engine: Signal transduction fuels t cell activation. Immunity (2007) 27(2):173–8. doi: 10.1016/j.immuni.2007.07.008
78. Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (Hif): Implications for cellular physiology. J Physiol (2021) 599(1):23–37. doi: 10.1113/jp280572
79. Hardie DG. Amp-Activated/Snf1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol (2007) 8(10):774–85. doi: 10.1038/nrm2249
80. Qian X, Li X, Shi Z, Xia Y, Cai Q, Xu D, et al. Pten suppresses glycolysis by dephosphorylating and inhibiting autophosphorylated Pgk1. Mol Cell (2019) 76(3):516–27.e7. doi: 10.1016/j.molcel.2019.08.006
81. Kunkl M, Sambucci M, Ruggieri S, Amormino C, Tortorella C, Gasperini C, et al. Cd28 autonomous signaling up-regulates c-myc expression and promotes glycolysis enabling inflammatory t cell responses in multiple sclerosis. Cells (2019) 8(6). doi: 10.3390/cells8060575
82. Jacobs SR, Michalek RD, Rathmell JC. Il-7 is essential for homeostatic control of t cell metabolism in vivo. J Immunol (Baltimore Md 1950) (2010) 184(7):3461–9. doi: 10.4049/jimmunol.0902593
83. Eleftheriadis T, Pissas G, Antoniadi G, Tsogka K, Sounidaki M, Liakopoulos V, et al. Indoleamine 2,3-Dioxygenase downregulates T-Cell receptor complex ζ-Chain and C-Myc, and reduces proliferation, lactate dehydrogenase levels and mitochondrial glutaminase in human T-Cells. Mol Med Rep (2016) 13(1):925–32. doi: 10.3892/mmr.2015.4595
84. Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mtor kinase differentially regulates effector and regulatory t cell lineage commitment. Immunity (2009) 30(6):832–44. doi: 10.1016/j.immuni.2009.04.014
85. Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mtor regulates the differentiation of helper t cells through the selective activation of signaling by Mtorc1 and Mtorc2. Nat Immunol (2011) 12(4):295–303. doi: 10.1038/ni.2005
86. Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, Vogel P, et al. T cell exit from quiescence and differentiation into Th2 cells depend on raptor-Mtorc1-Mediated metabolic reprogramming. Immunity (2013) 39(6):1043–56. doi: 10.1016/j.immuni.2013.09.015
87. Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. Mtorc1 couples immune signals and metabolic programming to establish T(Reg)-cell function. Nature (2013) 499(7459):485–90. doi: 10.1038/nature12297
88. Procaccini C, De Rosa V, Galgani M, Abanni L, Calì G, Porcellini A, et al. An oscillatory switch in mtor kinase activity sets regulatory t cell responsiveness. Immunity (2010) 33(6):929–41. doi: 10.1016/j.immuni.2010.11.024
89. Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. Hif1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of Th17 and treg cells. J Exp Med (2011) 208(7):1367–76. doi: 10.1084/jem.20110278
90. Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor myc controls metabolic reprogramming upon t lymphocyte activation. Immunity (2011) 35(6):871–82. doi: 10.1016/j.immuni.2011.09.021
91. Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. Ampkα1: A glucose sensor that controls Cd8 t-cell memory. Eur J Immunol (2013) 43(4):889–96. doi: 10.1002/eji.201243008
92. Wu S, Le H. Dual roles of Pkm2 in cancer metabolism. Acta Biochim Biophys Sin (2013) 45(1):27–35. doi: 10.1093/abbs/gms106
93. Mazurek S. Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol (2011) 43(7):969–80. doi: 10.1016/j.biocel.2010.02.005
94. Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell (2015) 57(1):95–107. doi: 10.1016/j.molcel.2014.10.027
95. Kono M, Maeda K, Stocton-Gavanescu I, Pan W, Umeda M, Katsuyama E, et al. Pyruvate kinase M2 is requisite for Th1 and Th17 differentiation. JCI Insight (2019) 4(12). doi: 10.1172/jci.insight.127395
96. Angiari S, Runtsch MC, Sutton CE, Palsson-McDermott EM, Kelly B, Rana N, et al. Pharmacological activation of pyruvate kinase M2 inhibits Cd4(+) t cell pathogenicity and suppresses autoimmunity. Cell Metab (2020) 31(2):391–405.e8. doi: 10.1016/j.cmet.2019.10.015
97. Jiang S. Tetrameric Pkm2 activation curbs Cd4(+) t cell overactivation. Trends Endocrinol Metabolism: TEM (2020) 31(6):393–5. doi: 10.1016/j.tem.2020.04.001
98. Liu Z, Xu J, Li H, Shu J, Su G, Zhou C, et al. Pd-1 targeted nanoparticles inhibit activated t cells and alleviate autoimmunity Via suppression of cellular energy metabolism mediated by Pkm2. Int J Nanomed (2022) 17:1711–24. doi: 10.2147/ijn.S349360
99. Lü S, Deng J, Liu H, Liu B, Yang J, Miao Y, et al. Pkm2-dependent metabolic reprogramming in Cd4(+) t cells is crucial for hyperhomocysteinemia-accelerated atherosclerosis. J Mol Med (Berlin Germany) (2018) 96(6):585–600. doi: 10.1007/s00109-018-1645-6
100. Shen MY, Wang X, Di YX, Zhang MF, Tian FX, Qian FY, et al. Triptolide inhibits Th17 differentiation Via controlling Pkm2-mediated glycolysis in rheumatoid arthritis. Immunopharmacol Immunotoxicol (2022) 44(6):838–49. doi: 10.1080/08923973.2022.2086139
101. Wang B, Liu S, Fan B, Xu X, Chen Y, Lu R, et al. Pkm2 is involved in neuropathic pain by regulating erk and Stat3 activation in rat spinal cord. J Headache Pain (2018) 19(1):7. doi: 10.1186/s10194-018-0836-4
102. Liu R, Liu X, Bai X, Xiao C, Dong Y. Different expression of lipid metabolism-related genes in shandong black cattle and luxi cattle based on transcriptome analysis. Sci Rep (2020) 10(1):21915. doi: 10.1038/s41598-020-79086-4
103. Li B, Qiao L, An L, Wang W, Liu J, Ren Y, et al. Transcriptome analysis of adipose tissues from two fat-tailed sheep breeds reveals key genes involved in fat deposition. BMC Genomics (2018) 19(1):338. doi: 10.1186/s12864-018-4747-1
104. Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes (2004) 53(3):585–90. doi: 10.2337/diabetes.53.3.585
105. Sun Y, Zang Z, Zhong L, Wu M, Su Q, Gao X, et al. Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay. PloS One (2013) 8(5):e63354. doi: 10.1371/journal.pone.0063354
106. Sun X, Feng X, Tan W, Lin N, Hua M, Wei Y, et al. Adiponectin exacerbates collagen-induced arthritis Via enhancing Th17 response and prompting rankl expression. Sci Rep (2015) 5:11296. doi: 10.1038/srep11296
107. Zhang Q, Wang L, Jiang J, Lin S, Luo A, Zhao P, et al. Critical role of Adipor1 in regulating Th17 cell differentiation through modulation of hif-1α-Dependent glycolysis. Front Immunol (2020) 11:2040. doi: 10.3389/fimmu.2020.02040
108. Surendar J, Frohberger SJ, Karunakaran I, Schmitt V, Stamminger W, Neumann AL, et al. Adiponectin limits ifn-Γ and il-17 producing Cd4 t cells in obesity by restraining cell intrinsic glycolysis. Front Immunol (2019) 10:2555. doi: 10.3389/fimmu.2019.02555
109. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci (2017) 18(6). doi: 10.3390/ijms18061321
110. Sun L, Li H, Tai LW, Gu P, Cheung CW. Adiponectin regulates thermal nociception in a mouse model of neuropathic pain. Br J Anaesthesia (2018) 120(6):1356–67. doi: 10.1016/j.bja.2018.01.016
111. Geng T, Song ZY, Xing JX, Wang BX, Dai SP, Xu ZS. Exosome derived from coronary serum of patients with myocardial infarction promotes angiogenesis through the mirna-143/Igf-Ir pathway. Int J Nanomed (2020) 15:2647–58. doi: 10.2147/ijn.S242908
112. Xiong Y, Yan C, Chen L, Endo Y, Sun Y, Zhou W, et al. Il-10 induces Mc3t3-E1 cells differentiation towards osteoblastic fate in murine model. J Cell Mol Med (2020) 24(1):1076–86. doi: 10.1111/jcmm.14832
113. DiToro D, Harbour SN, Bando JK, Benavides G, Witte S, Laufer VA, et al. Insulin-like growth factors are key regulators of t helper 17 regulatory t cell balance in autoimmunity. Immunity (2020) 52(4):650–67.e10. doi: 10.1016/j.immuni.2020.03.013
114. Cusick MF, Libbey JE, Trede NS, Fujinami RS. Targeting insulin-like growth factor 1 leads to amelioration of inflammatory demyelinating disease. PloS One (2014) 9(4):e94486. doi: 10.1371/journal.pone.0094486
115. Stemkowski PL, Zamponi GW. The tao of igf-1: Insulin-like growth factor receptor activation increases pain by enhancing t-type calcium channel activity. Sci Signaling (2014) 7(346):pe23. doi: 10.1126/scisignal.2005826
116. Sugawara S, Shinoda M, Hayashi Y, Saito H, Asano S, Kubo A, et al. Increase in igf-1 expression in the injured infraorbital nerve and possible implications for orofacial neuropathic pain. Int J Mol Sci (2019) 20(24). doi: 10.3390/ijms20246360
117. Ratcliffe LE, Vázquez Villaseñor I, Jennings L, Heath PR, Mortiboys H, Schwartzentruber A, et al. Loss of Igf1r in human astrocytes alters complex i activity and support for neurons. Neuroscience (2018) 390:46–59. doi: 10.1016/j.neuroscience.2018.07.029
118. Chen X, Le Y, He WY, He J, Wang YH, Zhang L, et al. Abnormal insulin-like growth factor 1 signaling regulates neuropathic pain by mediating the mechanistic target of rapamycin-related autophagy and neuroinflammation in mice. ACS Chem Neurosci (2021) 12(15):2917–28. doi: 10.1021/acschemneuro.1c00271
119. Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, Lu B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain (2011) 4:11. doi: 10.1186/1756-6606-4-11
120. Rol Á, Todorovski T, Martin-Malpartida P, Escolà A, Gonzalez-Rey E, Aragón E, et al. Structure-based design of a cortistatin analogue with immunomodulatory activity in models of inflammatory bowel disease. Nat Commun (2021) 12(1):1869. doi: 10.1038/s41467-021-22076-5
121. Yue M, Xia Y, Shi C, Guan C, Li Y, Liu R, et al. Berberine ameliorates collagen-induced arthritis in rats by suppressing Th17 cell responses Via inducing cortistatin in the gut. FEBS J (2017) 284(17):2786–801. doi: 10.1111/febs.14147
122. Guo Y, Sun D, Zhang Y, Yu X, Fang Y, Lv C, et al. The neuropeptide cortistatin attenuates Th17 cell response through inhibition of glycolysis Via Ghsr1. Int Immunopharmacol (2022) 108:108843. doi: 10.1016/j.intimp.2022.108843
123. Morell M, Camprubí-Robles M, Culler MD, de Lecea L, Delgado M. Cortistatin attenuates inflammatory pain Via spinal and peripheral actions. Neurobiol Dis (2014) 63:141–54. doi: 10.1016/j.nbd.2013.11.022
124. Falo CP, Benitez R, Caro M, Morell M, Forte-Lago I, Hernandez-Cortes P, et al. The neuropeptide cortistatin alleviates neuropathic pain in experimental models of peripheral nerve injury. Pharmaceutics (2021) 13(7). doi: 10.3390/pharmaceutics13070947
125. Heil M, Land WG. Danger signals - damaged-self recognition across the tree of life. Front Plant Sci (2014) 5:578. doi: 10.3389/fpls.2014.00578
126. Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2z receptor for extracellular atp identified as a P2x receptor (P2x7). Sci (NY) (1996) 272(5262):735–8. doi: 10.1126/science.272.5262.735
127. Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2x7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain (2005) 114(3):386–96. doi: 10.1016/j.pain.2005.01.002
128. Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and atp. Nature (2006) 440(7081):228–32. doi: 10.1038/nature04515
129. Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F. The P2x7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis (2012) 3(8):e370. doi: 10.1038/cddis.2012.105
130. Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, et al. The P2x7 receptor is a key modulator of the Pi3k/Gsk3β/Vegf signaling network: Evidence in experimental neuroblastoma. Oncogene (2015) 34(41):5240–51. doi: 10.1038/onc.2014.444
131. Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, et al. Basal activation of the P2x7 atp receptor elevates mitochondrial calcium and potential, increases cellular atp levels, and promotes serum-independent growth. Mol Biol Cell (2005) 16(7):3260–72. doi: 10.1091/mbc.e04-11-1025
132. Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2x7 receptor in infection and inflammation. Immunity (2017) 47(1):15–31. doi: 10.1016/j.immuni.2017.06.020
133. Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, et al. The purinergic receptor P2rx7 directs metabolic fitness of long-lived memory Cd8(+) t cells. Nature (2018) 559(7713):264–8. doi: 10.1038/s41586-018-0282-0
134. Bartlett R, Stokes L, Sluyter R. The P2x7 receptor channel: Recent developments and the use of P2x7 antagonists in models of disease. Pharmacol Rev (2014) 66(3):638–75. doi: 10.1124/pr.113.008003
135. Luchting B, Heyn J, Woehrle T, Rachinger-Adam B, Kreth S, Hinske LC, et al. Differential expression of P2x7 receptor and il-1β in nociceptive and neuropathic pain. J Neuroinflamm (2016) 13(1):100. doi: 10.1186/s12974-016-0565-z
136. Pandit M, Timilshina M, Chang JH. Lkb1-pten axis controls Th1 and Th17 cell differentiation Via regulating Mtorc1. J Mol Med (Berlin Germany) (2021) 99(8):1139–50. doi: 10.1007/s00109-021-02090-2
137. Kono M, Yoshida N, Maeda K, Skinner NE, Pan W, Kyttaris VC, et al. Pyruvate dehydrogenase phosphatase catalytic subunit 2 limits Th17 differentiation. Proc Natl Acad Sci United States America (2018) 115(37):9288–93. doi: 10.1073/pnas.1805717115
138. Lu Y, Zhao N, Wu Y, Yang S, Wu Q, Dong Q, et al. Inhibition of Pgk1 attenuates autoimmune myocarditis by reprogramming Cd4+ t cells metabolism. Cardiovasc Res (2023). doi: 10.1093/cvr/cvad029
139. Wedel J, Bruneau S, Liu K, Kong SW, Sage PT, Sabatini DM, et al. Deptor modulates activation responses in Cd4(+) t cells and enhances immunoregulation following transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surgeons (2019) 19(1):77–88. doi: 10.1111/ajt.14995
140. Liang Y, Wang X, Wang H, Yang W, Yi P, Soong L, et al. Il-33 activates Mtorc1 and modulates glycolytic metabolism in Cd8(+) t cells. Immunology (2022) 165(1):61–73. doi: 10.1111/imm.13404
141. Zhang E, Ma Z, Li Q, Yan H, Liu J, Wu W, et al. Tlr2 stimulation increases cellular metabolism in Cd8(+) t cells and thereby enhances Cd8(+) t cell activation, function, and antiviral activity. J Immunol (Baltimore Md 1950) (2019) 203(11):2872–86. doi: 10.4049/jimmunol.1900065
142. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: From mechanisms to treatment. Physiol Rev (2021) 101(1):259–301. doi: 10.1152/physrev.00045.2019
143. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers (2017) 3:17002. doi: 10.1038/nrdp.2017.2
144. Burma NE, Leduc-Pessah H, Fan CY, Trang T. Animal models of chronic pain: Advances and challenges for clinical translation. J Neurosci Res (2017) 95(6):1242–56. doi: 10.1002/jnr.23768
145. Coderre TJ, Laferrière A. The emergence of animal models of chronic pain and logistical and methodological issues concerning their use. J Neural Transm (Vienna Austria 1996) (2020) 127(4):393–406. doi: 10.1007/s00702-019-02103-y
146. Murphy D, Lester D, Clay Smither F, Balakhanlou E. Peripheral neuropathic pain. NeuroRehabilitation (2020) 47(3):265–83. doi: 10.3233/nre-208002
147. Kraus FV, Keck S, Klika KD, Graf J, Carvalho RA, Lorenz HM, et al. Reduction of pro-inflammatory effector functions through remodeling of fatty acid metabolism in Cd8(+) t-cells from rheumatoid arthritis patients. Arthritis Rheumatol (Hoboken NJ) (2023). doi: 10.1002/art.42456
148. Shan J, Jin H, Xu Y. T cell metabolism: A new perspective on Th17/Treg cell imbalance in systemic lupus erythematosus. Front Immunol (2020) 11:1027. doi: 10.3389/fimmu.2020.01027
149. Kono M, Yoshida N, Maeda K, Suárez-Fueyo A, Kyttaris VC, Tsokos GC. Glutaminase 1 inhibition reduces glycolysis and ameliorates lupus-like disease in Mrl/Lpr mice and experimental autoimmune encephalomyelitis. Arthritis Rheumatol (Hoboken NJ) (2019) 71(11):1869–78. doi: 10.1002/art.41019
150. Koga T, Sato T, Furukawa K, Morimoto S, Endo Y, Umeda M, et al. Promotion of Calcium/Calmodulin-dependent protein kinase 4 by Glut1-dependent glycolysis in systemic lupus erythematosus. Arthritis Rheumatol (Hoboken NJ) (2019) 71(5):766–72. doi: 10.1002/art.40785
151. Beckermann KE, Dudzinski SO, Rathmell JC. Dysfunctional t cell metabolism in the tumor microenvironment. Cytokine Growth Factor Rev (2017) 35:7–14. doi: 10.1016/j.cytogfr.2017.04.003
152. Hsiao HT, Lin YC, Wang JC, Tsai YC, Liu YC. Hypoxia inducible factor-1α inhibition produced anti-allodynia effect and suppressed inflammatory cytokine production in early stage of mouse complex regional pain syndrome model. Clin Exp Pharmacol Physiol (2016) 43(3):355–9. doi: 10.1111/1440-1681.12536
Keywords: chronic neuropathic pain, T cell, energy metabolism, P2X7R, PKM2, adiponectin
Citation: Dou X, Chen R, Yang J, Dai M, Long J, Sun S and Lin Y (2023) The potential role of T-cell metabolism-related molecules in chronic neuropathic pain after nerve injury: a narrative review. Front. Immunol. 14:1107298. doi: 10.3389/fimmu.2023.1107298
Received: 24 November 2022; Accepted: 27 April 2023;
Published: 17 May 2023.
Edited by:
Javier Aguilera-Lizarraga, University of Cambridge, United KingdomReviewed by:
Shiping Lu, Tulane University, United StatesCopyright © 2023 Dou, Chen, Yang, Dai, Long, Sun and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shujun Sun, c3Vuc2h1anVuX2h1c3RAZm94bWFpbC5jb20=; Yun Lin, ZnJhbmtsaW55dW5AaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.