
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 28 April 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1104180
This article is part of the Research Topic Cardiometabolic diseases and inflammatory responses View all 16 articles
Background: The association between white blood cells (WBCs) and metabolic syndrome (MS) has been consistently reported in previous studies using regional samples. However, it remains unclear whether this relationship has urban–rural differences and is independent of insulin resistance using a large-scale representative sample. Additionally, accurate risk prediction in patients with MS is crucial for developing targeted interventions to enhance the quality of life and prognosis of patients.
Aims: The aims of this study were (1) to examine the cross-sectional association between WBCs and MS among the national population and analyze the urban–rural difference and whether insulin resistance plays a moderator effect in this association and (2) to describe the performance to predict MS using machine learning (ML) models.
Design: A cross-sectional study was performed using 7,014 data from the China Health and Nutrition Survey (CHNS).
Methods: WBCs were analyzed using an automatic hematology analyzer and MS was defined according to the criteria of the American Heart Association scientific statements of 2009. Variables on sociodemographic characteristics (sex, age, and residence), clinical laboratory (BMI and HOMA-IR), and lifestyle characteristics (smoking and drinking status) were used to construct ML models to predict MS [logistic regression (LR) and multilayer perceptron (MLP) neural network].
Results: We found that 21.1% of participants (1,479/7,014) were classified as having MS. In multivariate logistic regression (including insulin resistance), the result revealed a significant positive association between WBCs and MS. The odds ratios (95% CI) for MS with increasing WBC level were 1.00 (reference), 1.65 (1.18, 2.31), and 2.18 (1.36, 3.50) (p for trend: 0.001). For two ML algorithms, two models showed adequate calibration and good discrimination, but the MLP showed better performance (AUC-ROC = 0.862 and 0.867).
Conclusion: With the aim of confirming the association between WBCs and MS, this cross-sectional study is the first to show that maintaining normal WBC count levels is helpful to prevent the development of MS, and this association is independent of insulin resistance. The results also showed that the MPL algorithm represented a more prominent predictive performance to predict MS.
Metabolic syndrome (MS) is a pro-inflammatory disease characterized by a constellation of cardiovascular risk factors, including central obesity, elevated blood pressure (BP), dyslipidemia, and impaired glucose tolerance (1). With socio-economic development and lifestyle changes, the prevalence of MS has shown an increasing trend worldwide. Currently, approximately 25% of the global population suffers from MS (2). Therefore, exploring convenient, effective, and available methods for determining systemic inflammation statuses and diagnosing MS is urgently needed.
Numerous inflammatory markers have been widely used to assess systemic inflammatory statuses in clinical practice and public health practices. These markers included soluble adhesion molecules (such as E-selectin, P-selectin, intracellular adhesion molecule-1, and vascular cell adhesion molecule-1), cytokines (such as interleukin-1β, -6, -8, and -10, and tumor necrosis factor-α), acute-phase reactants [such as fibrinogen, serum amyloid A protein, and high sensitive C-reactive protein (hsCRP)], and white blood cell (WBC) count (3). Among these inflammatory markers, peripheral WBC counts are assayed in routine blood routine examination and used to diagnose systemic infection, tissue damage, and other conditions associated with inflammation (4). Furthermore, prior cross-sectional and cohort studies have shown that peripheral WBC count is associated with MS risk over the past decade (4–7). However, there are conflicting findings in recent studies. For example, two (8, 9) of the three studies (8–10) confirmed the cross-sectional and prospective findings. The association between WBC counts and MS may be different as there are urban–rural differences that have been observed in inflammation (11) and prevalence of MS (2). Additionally, insulin resistance plays an important pathophysiological role in the development of MS (12); however, it is unclear whether insulin resistance plays a moderator effect in this association (7, 13).
The recent progression of computer science has led to the development of machine learning (ML), a powerful tool that has been widely used to identify the risk factors of outcome variables. ML has provided the ability to compare the accuracy of the ML approaches and the traditional logistic regression in predicting the outcome variables (14). Therefore, in this study, we used a large-scale representative sample to comprehensively examine the association between WBC count and MS risk among the general Chinese population, and analyzed the urban–rural difference and whether insulin resistance plays a moderator effect in this association.
The China Health and Nutrition Survey (CHNS), established in 1989, is an ongoing prospective cohort study. A previous study has published a detailed study design (15). In brief, the multi-stage random cluster sampling method was used to select 4,400 households and 19,000 participants, which covers nine provinces (Guizhou, Guangxi, Heilongjiang, Henan, Hubei, Hunan, Liaoning, Jiangsu, and Shandong). So far, the CHNS has completed data collection from 11 waves (1989, 1991, 1993, 1997, 2000, 2004, 2006, 2009, 2011, 2015, and 2018). The CHNS has obtained clinical, dietary, anthropometric, and all other individual data from each household member. Because the collection and analysis of blood samples were only conducted and released in 2009, we therefore used data from the 2009 wave of the CHNS. A detailed study design has been described in a previous study (16).
We analyzed 12,178 individuals who agreed to participate and gave informed consent for analysis of their data. This study included participants aged ≥18 years who participated in the 2009 survey wave. Participants were excluded if they met any of the following criteria: (1) a history of diabetes (n = 131), hypertension (n = 1094), apoplexy (n = 38), or myocardial infarction (n = 28); and (2) incomplete data (n = 1963). After excluding these incomplete data, this study finally included 7,014 individuals (3,253 men and 3,761 women). In order to show that the sample size of this study has sufficient statistical power, sample size was calculated based on a previous study that determined the prevalence rate of MS [control group = 0.14 (514/3,556); experimental group = 0.39 (194/495)] in a Chinese population-based study (7). Statistical power for a two-sample proportion chi-squared test indicated that a total of 156 participants were included in this study, which could achieve 95% statistical power. Ethics approval was obtained from the Institutional Review Board of the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Fasting blood samples were collected in blood collection tubes and tested in a national central lab in Beijing. Fasting blood glucose (FBG) and lipids [triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C)] were analyzed using an automatic biochemical analyzer (Hitachi 7600 automated analyzer, Tokyo, Japan). Insulin was measured by an ELISA Kit (Millipore Corporation, Billerica, MA, USA). The homeostasis model assessment for insulin resistance (HOMA-IR) score was calculated using the following formula: fasting insulin (mU/L) * fasting glucose (mmol/L)/22.5 (17).
In order to determine all the above biochemical markers, FBG was measured by the glucose oxidase method. TG and HDL-C were measured by the enzymatic method.
Fasting blood samples were drawn from the cubital vein for routine blood detection. WBC count was analyzed using an automatic hematology analyzer (Beckman Coulter LH751, Beckman Coulter, USA). Generally, an individual who maintains a very low leukocyte count level is healthier. We grouped the subjects into three categories—low level (subnormal level: ≦3.9), middle level (normal level: 3.91–9.94), and high level (above normal level: ≧10.0). We aimed to examine the association between a subnormal WBC count and risk of MS.
Anthropometric measurements (height, body weight, and waist circumference) were made using standard protocols. Body mass index (BMI) was calculated as the ratio of weight (kg) and height (m) squared. BP was measured three times after participants quietly sat for at least 5 min, and the maximum values of the first two of three readings were considered as the final BP values. All participants were also asked to fill in a standard questionnaire, which included demographic variables [sex, age, residence (city, suburban, town or county, capital city, and rural village)] and lifestyle variables [smoking status (smoker or not) and drinking status (drinker or not)].
According to the criteria of the American Heart Association scientific statements of 2009 (1), if a participant suffered from three or more of the following syndromes, he/she will be defined as having MS:
(1) Central obesity (≥90 cm in men, ≥80 cm in women);
(2) Elevated triglycerides (TG ≥1.7 mmol/L);
(3) Reduced HDL cholesterol (<1.0 mmol/L in men, <1.3 mmol/L in women);
(4) Elevated BP [systolic BP (SBP) ≥ 130 mmHg or diastolic BP (DBP) ≥ 85 mmHg]; and
(5) Elevated fasting glucose (≥5.6 mmol/L).
All continuous variables were expressed as median [interquartile range (IQR)] because of non-normal distribution, and categorical variables were expressed as percentage. To examine differences in participants’ characteristics between WBC count category, Kruskal–Wallis test and chi-square test were applied for continuous variables and categorical variables, respectively. We examine the hypothesis of logistic regression analysis prior to its use. Firstly, to examine the independence of observation variables, interactions were tested by adding the respective multiplicative terms in the models simultaneously. There were significant interactions of WBC with age, residence, apoplexy, and insulin resistance on MS (p for interaction: <0.001, 0.038, <0.001, and 0.015, respectively) (Appendix Table 1). Based on the above findings, we also carried out subgroup analysis based on sex, age (age <65 years, age ≥65 years), residence (city, suburb, town, and village), apoplexy (yes or no), and insulin resistance (yes or no). Secondly, variance inflation factors (VIFs) were used to examine multicollinearity among explanatory variables, and the result shows that the VIFs of explanatory variables range from 1.02 to 1.91, which means that there is no multicollinearity between explanatory variables (Appendix Table 2). Thirdly, Cook’s distance was used to identify extreme outliers, and the results showed that Cook’s distance of each variable is less than or equal to 0.06, showing that no extreme outliers were found. Fourth, to initially assess whether there are linear relationships between continuous independent variables and dependent variable logit conversion values, the Box–Tidwell method was used (transformation of the independent variables). The results of the line test found nonlinear relationships between all continuous independent variables (age, BMI, and insulin resistance) and the dependent variable logit conversion values. To linearize relationships, all continuous variables were converted into ordinal categorical variables. To examine the association between WBC count and MS, multivariate logistic regression analyses were applied to calculate risk of MS in different WBC count categories when several potential confounding factors were adjusted. We adjusted for sex, age (continuous variable), BMI (continuous variable), residence (categorical variable), smoking and drinking status (categorical variable), and HOMA-IR.
To examine the robustness of the above-mentioned results, a sensitivity analyses was further conducted as follows: we excluded participants who was excessively thin and obese [BMI (<18 or >40 kg/m2)]. We also used receiver operating characteristic (ROC) curve analysis, precision, specificity, and sensitivity through ML including multilayer perceptron (MLP) and logistic regression (LR) to compare the predictive performance of the MS risk model. In all two-sided tests, p-values of <0.05 were defined as statistically significant. Stata 16.0 software (Stata Corp LP, College Station, TX, USA) was used for all tests.
Participants’ characteristics according to WBC category are shown in Table 1; 3.4% of participants have a higher WBC level. Participants with a higher WBC level were more likely to be male, to be younger, and to live in a rural village. In addition, BMI and HOMA-IR increased statistically across different WBC categories. The proportion of participants with above-normal WBC count, who were cigarette smokers and alcohol drinkers, was statistically higher. However, there was no significant difference for other participants’ characteristics between WBC categories.
In all 7,014 participants, 1,479 were diagnosed with MS. The prevalence rates of MS in the low level, middle level, and high level were 12.1% (48 of 397), 21.4% (1,368 of 6,380), and 26.6% (63 of 237), respectively. Figure 1 shows a positive association between WBC count and risk of MS and its components. After adjustment for a potential confounder, the adjusted ORs for MS across WBC category were 1.00 (reference) for low level, 1.65 (95%CI: 1.18, 2.31) for middle level, and 2.18 (95%CI: 1.36, 3.50) for high level (p for trend, 0.001). Similar significant relationships were also found when components of MS were analyzed respectively (Figure 1). In addition, a higher WBC level was also associated with increased risk of MS in both male and female (Figure 2).
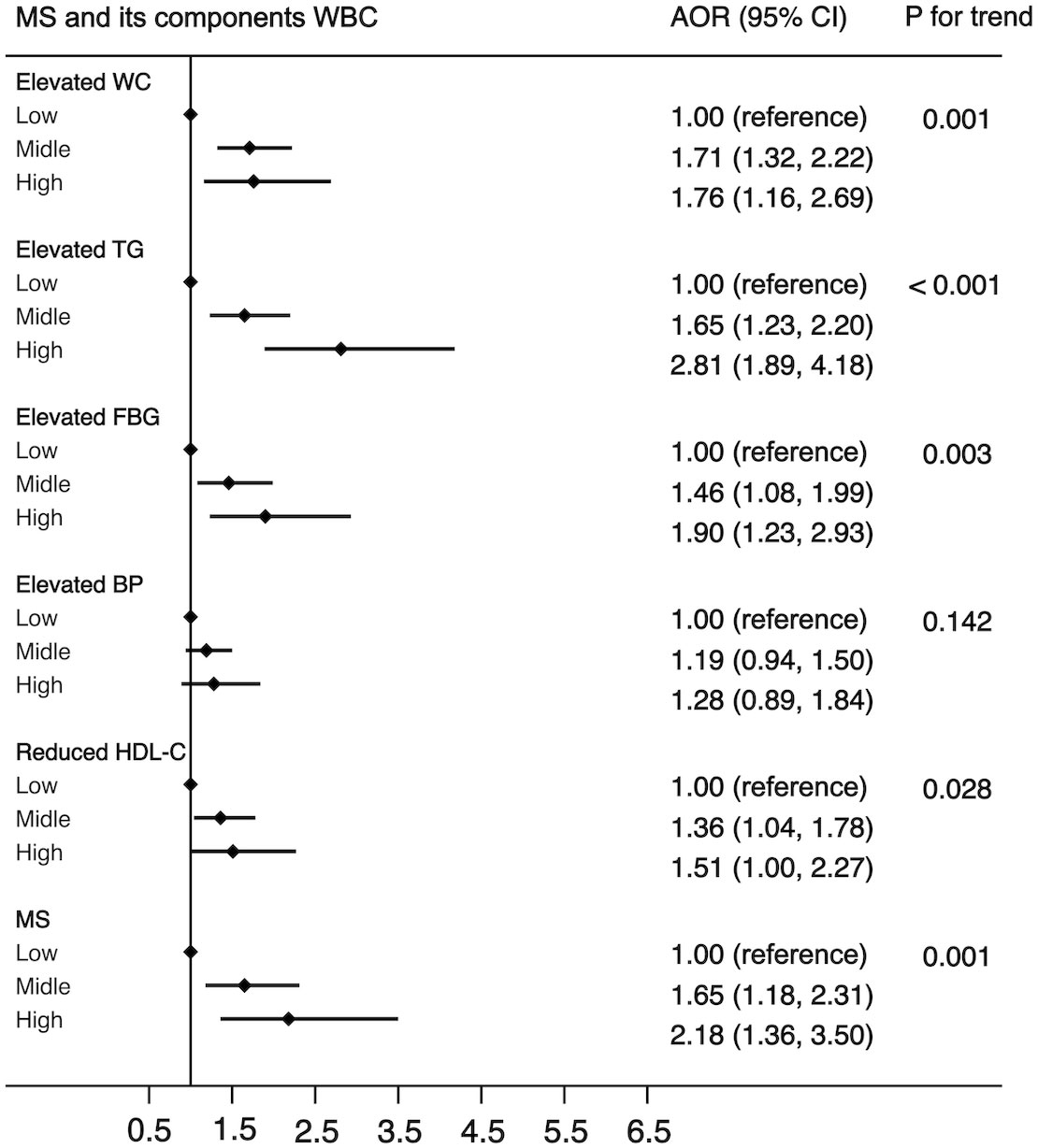
Figure 1 The association between WBC and risk of MS in all population. Adjusted for sex, age (<=39, 40-64, >=65), BMI (<25, >=25 & <30,>=30), residence (city, suburb, town or rural village), smoking (smoker or non-smoker) and drinking status (drinker or non- drinker), HOMA-IR (yes or no). Data are shown as OR and 95% CI.
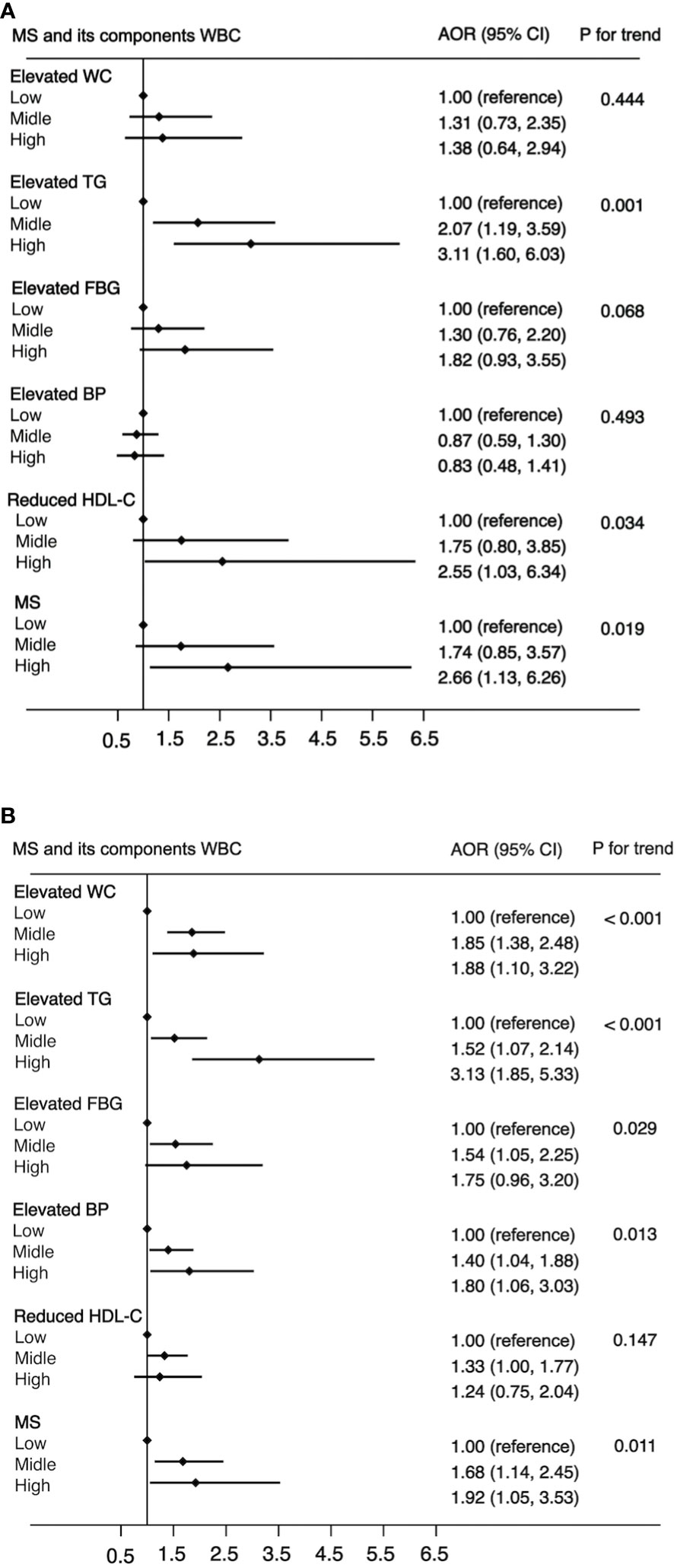
Figure 2 The association between WBC and risk of MS in male (A) and female (B). Adjusted for age (<=39, 40-64, >=65), BMI (<25, >=25 & <30,>=30), residence (city, suburb, town or rural village), smoking (smoker or non-smoker) and drinking status (drinker or non-drinker), HOMA-IR (yes or no). Data are shown as OR and 95% CI.
Another purpose of this study was to examine the associations of WBC category and MS by the following subgroups: age (<65 or ≧65 years), residence (city, suburban, town, or rural village), smoking status (smoker or non-smoker), drinking status (drinker or non-drinker), BMI (<25 kg/m2 or ≧25 kg/m2), and insulin resistance (yes or no). The strength of the association between WBC count and MS increased with age (OR, 1.42 [95%CI, 1.10-1.83] for adults aged <65 years; 1.85 [95%CI, 1.06, 3.21] for those aged ≧65 years). The strength of the association between WBC count and MS was weaker in participants who resided in a rural village (OR, 1.22 [95% CI, 0.90–1.66]) compared with other residences. The association between WBC count and MS was stronger in non-smokers (OR, 1.55 [95% CI, 1.19–2.03]) and non-drinkers (OR, 1.56 [95% CI, 1.19–2.04]) compared with smokers (OR, 1.34 [95%CI, 0.83-2.14))/drinkers (OR, 1.35 [95% CI, 0.86–2.12]). The association between WBC count and MS was stronger in normal/underweight participants compared with overweight/obese participants (OR, 1.71 [95% CI, 1.27–2.30] vs. 1.20 [95% CI, 0.82–1.74]) and was equivalent whether there was insulin resistance or not (OR, 1.54 [95% CI, 1.12–2.10] vs. 1.42 [95% CI, 1.01–1.99]). The detailed results has been showed in Figures 3–5. Distribution of components of MS according to WBC category is also shown in violin plots (Figure S1).
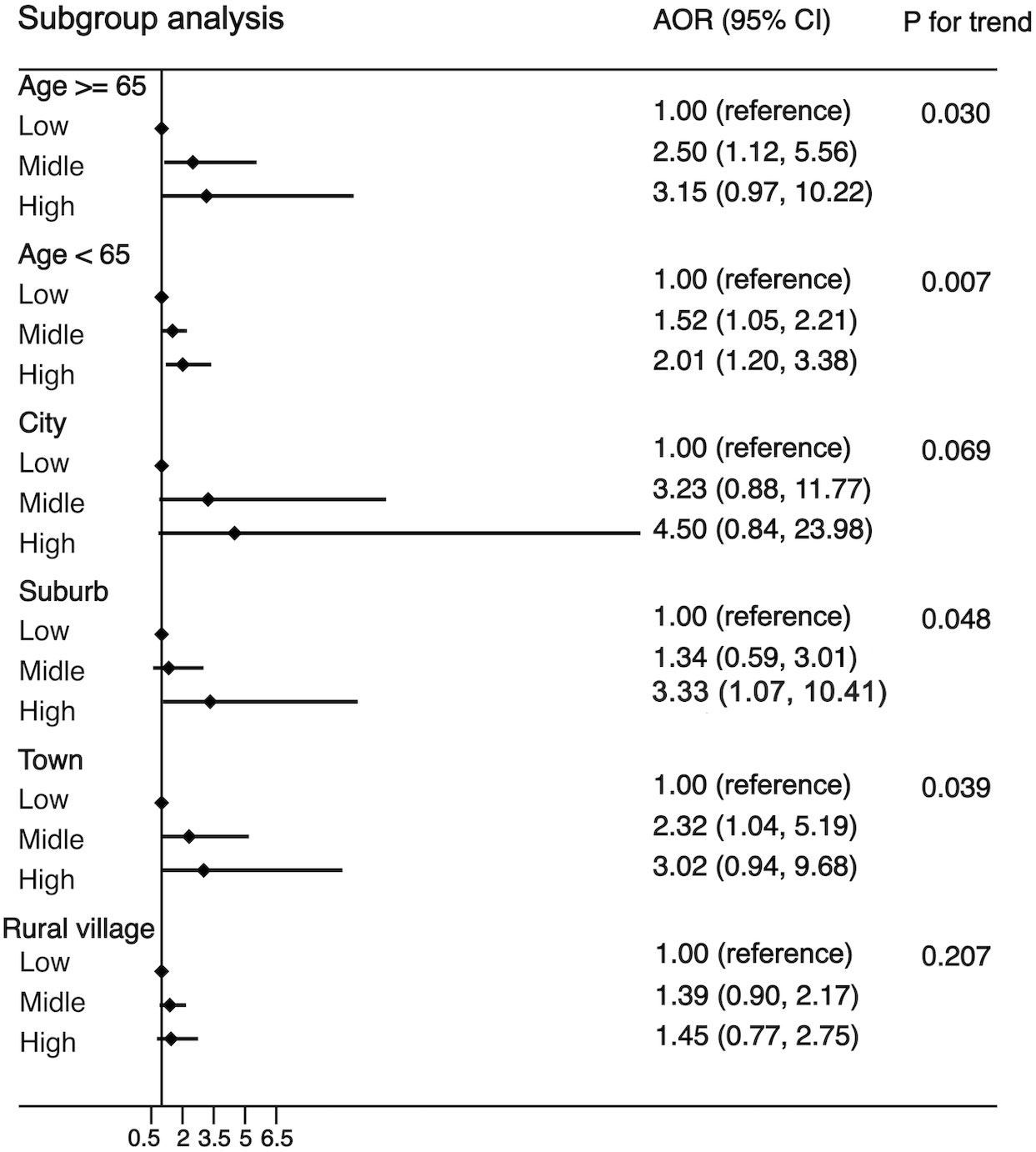
Figure 3 Stratification analysis of the association between WBC and risk of MS. Subgroups based on age (age <65 years, age ≥65 years) or residence (city, suburb, town or rural village). Data are shown as OR and 95% CI.
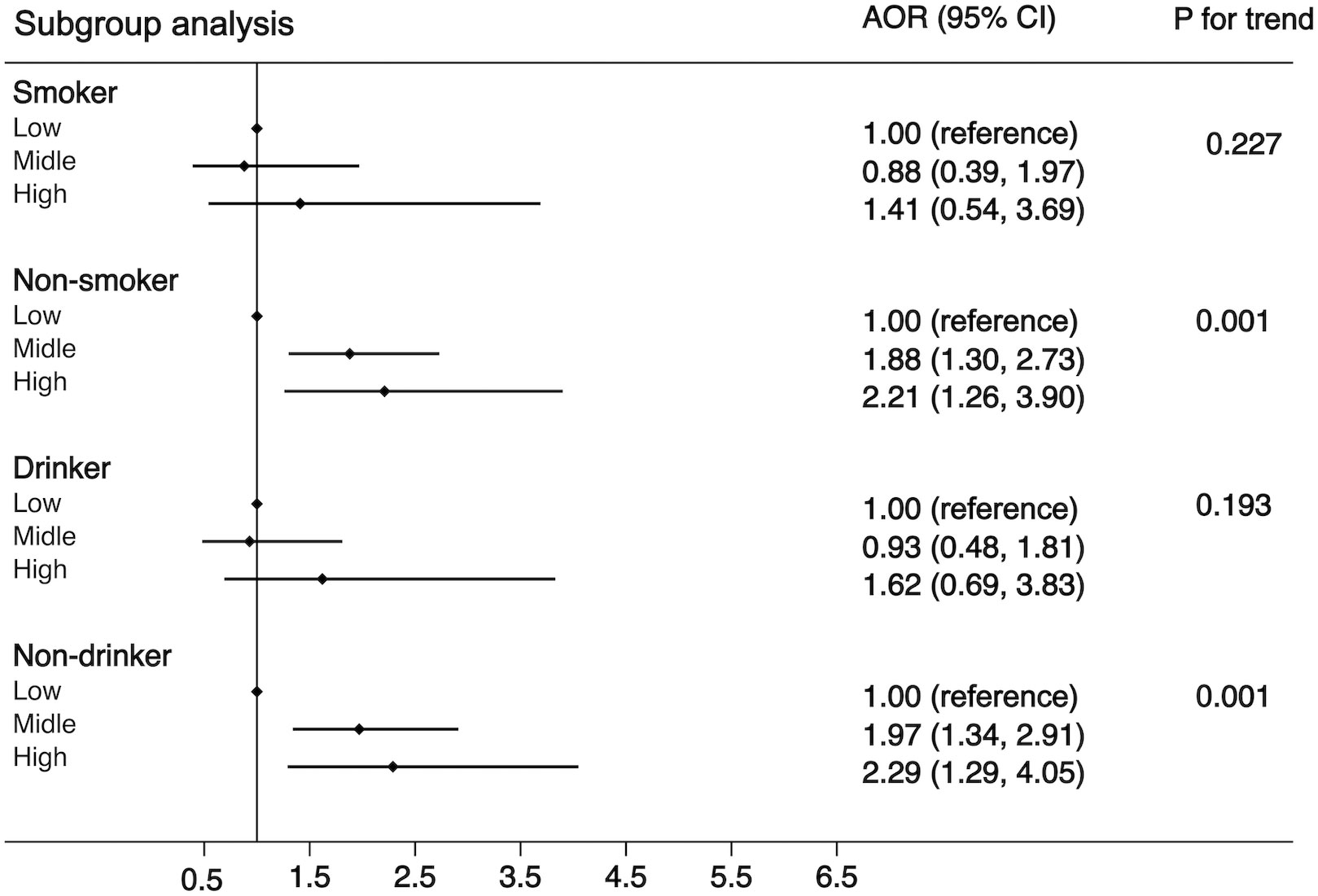
Figure 4 Stratification analysis of the association between WBC and risk of MS. Subgroups based on smoking (smoker or non-smoker) or drinking status (drinker or non-drinker). Data are shown as OR and 95% CI.
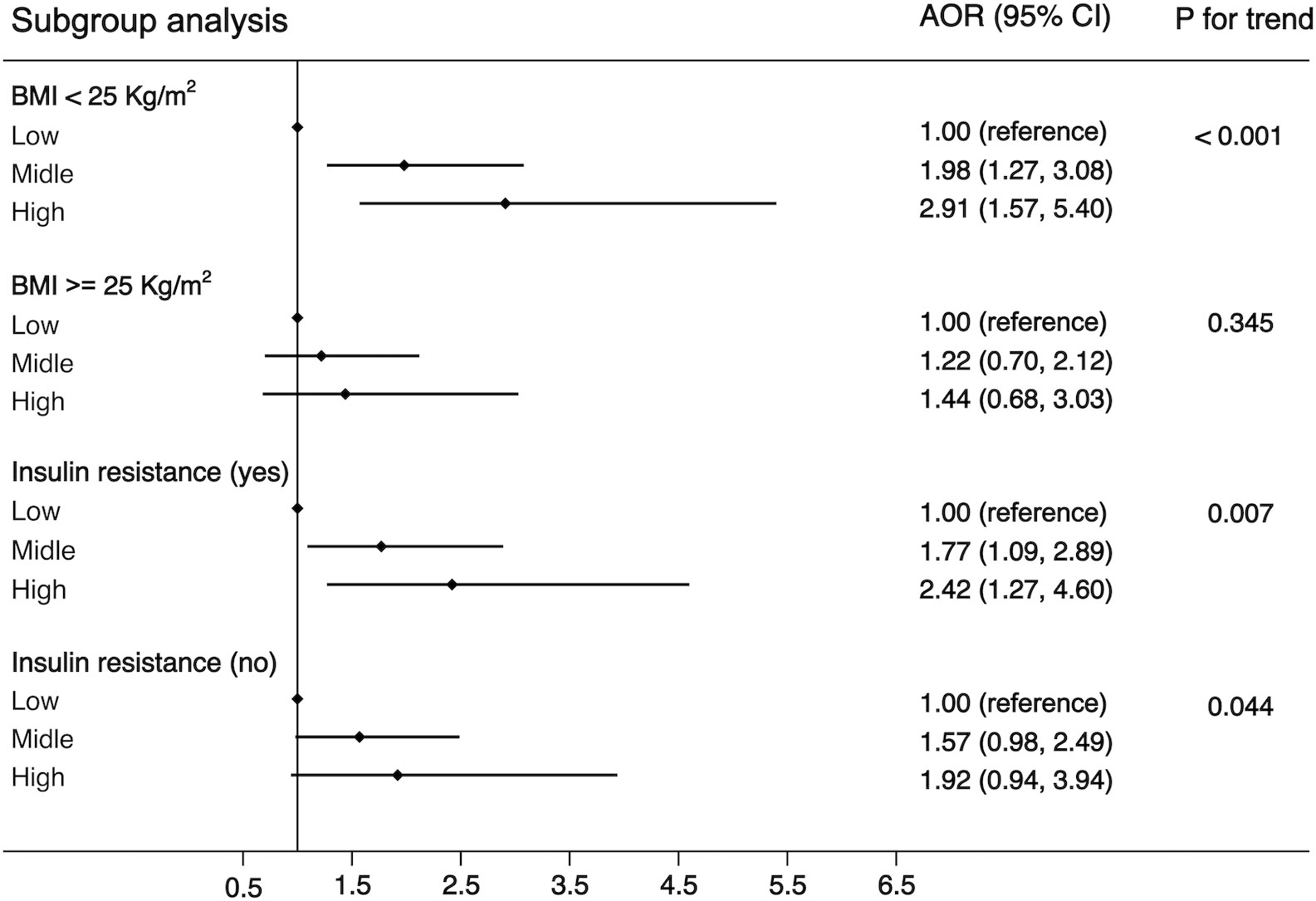
Figure 5 Stratification analysis of the association between WBC and risk of MS. Subgroups based on BMI (<25 or >=25) or HOMA-IR (yes or no). Data are shown as OR and 95% CI.
The aforementioned findings remained stable in a sensitivity analysis (Table S1). The results remained significant with the exclusion of excessively thin and obese participants [BMI (<18 or >40 kg/m2)].
To assess the predictive performance of predictive models, we selected MLP and logistic regression to compare their accuracy. The AUROC was calculated both in the 70% of training set and in the 30% of test set. It is generally accepted that an AUROC curve greater than 0.5 indicated having an adequate predictive performance. The results showed that the MPL algorithm represented a more prominent predictive performance (AUROC in the training set: 0.814; AUROC in the testing set: 0.842) compared to other algorithms (Table S2).
In this study, we comprehensively examined the association between WBC count and the risk of MS in a representative Chinese population, and the results revealed that participants with a higher WBC count had a higher risk of MS and its components than those with a lower WBC count after adjusting for potential confounders. This study obtained the following novel results: (1) the significant associations between WBC count and risk of MS occur in participants living in suburbs or cities but not in towns or rural villages; (2) these significant associations were independent of insulin resistance; and (3) the two models showed adequate calibration and good discrimination, but the MLP showed better performance.
The current cross-sectional study is consistent with the results of previous cross-sectional and cohort studies (4–7), which suggested that a higher WBC count level was related to an increased risk of MS. However, the association with WBC count varied between components of MS. Overall, the previous cross-sectional and prospective studies were heterogeneous, with differences in WBC count category, confounding factor adjustments, and sample selections. Nevertheless, the robust significant positive association between WBC count and MS was retained, despite the challenges introduced by these heterogeneities. This study harmonized fasting blood sample and other data across nine provinces, which largely attenuated these heterogeneities. Insulin resistance is an important marker for MS and its components; therefore, the significant positive associations of WBC count with the risk of MS and its components remained unchanged after adjusting for insulin resistance.
There are several plausible mechanisms that explain how WBC count may increase MS risk. Firstly, it is well known that insulin resistance is considered to be the root cause of MS (12); therefore, insulin resistance may mediate the association between WBC count and MS. Pro-inflammatory cytokines may lead to the activation of protein kinases and subsequently negatively regulate insulin receptor substrate and reduce the expression of glucose transporter 4 (18, 19); this inhibits the efficiency of blood glucose uptake (20). Therefore, compensatory hypersecretion of insulin can induce insulin resistance. However, the results of the current study suggest that elevated WBC count may increase the risk of MS in Chinese adults, even after adjusting for insulin resistance.
Secondly, it has been suggested that pro-inflammatory cytokines can increase hepatic fatty acid synthesis and stimulate lipolysis, which promotes the movement of free fatty acids to the liver (21). The above two mechanisms will subsequently enhance the production and secretion of triglycerides in the liver, resulting in hypertriglyceridemia (21).
Thirdly, the observed association between WBC count and elevated BP may be related to impaired endothelial function. Inflammation causes vasodilation, and the antithrombotic and antiatherosclerotic functions of the vascular endothelium are prevented by limiting the production of nitric oxide and prostacyclin (22). In contrast, the WBCs bind to the vascular endothelium, which may cause an increase in leukocytosis in capillaries, subsequently inducing capillary stenosis and increasing vascular pressure (23, 24), which may eventually lead to elevated BP.
Fourth, accumulated visceral fat can induce elevated fasting glucose levels compared to subcutaneous fat. This is because excessive free fatty acids produced by visceral fat through catabolism will overflow from adipose tissue into islet cells and induce fat ectopic deposition, causing insulin resistance in the muscle, liver, and pancreatic β cells, which subsequently can result in impaired glucose uptake (25). An imbalance between glucose production and uptake generally leads to elevated fasting glucose levels.
Logistic regression is a linear model widely used to analyze the linear association between independent and dependent variables (dichotomous variables), while ignoring the complex relationship between independent variables. MPL is composed of an input layer, one or more hidden layers, and an output layer, and can analyze the complex and potential associations among variables, rather than being limited to the linear relationship between the input and output (26). The results of this study indicated that the MPL algorithm had superior performance in predicting MS compared to logistic regression, indicating that the MPL algorithm is more suitable for predicting MS.
This study has several limitations. Firstly, the cross-sectional design of this study made it difficult to confirm the causal relationship between WBC count and MS and its components. Secondly, because there are fewer participants with a higher WBC level, this study therefore found seemingly inflated OR in Figures 2, 3, indicating that there are some sparse effects that may increase the probability of monotonic likelihood (27). Thirdly, the cross-sectional studies had Neyman bias because we could not identify whether the participants had suffered from MS in the past or only in the present. Therefore, the results of this study may be affected by prevalence–incidence (Neyman’s) bias (28, 29). Further prospective cohort studies, which select the newly identified cases as study participants, will effectively avoid Neyman bias.
This study reveals representative evidence of positive associations between WBC counts and MS as well as its components in a Chinese population. The findings of this study indicate that WBC count, as a convenient and routine examination, could be potentially used as a risk marker in the early identification and prevention of MS.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cpc.unc.edu/projects/china.
Ethics approval was obtained from the Institutional Review Board of the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention. The patients/participants provided their written informed consent to participate in this study.
ZR and LL designed this study, performed the statistical analyses, and wrote the manuscript. ZR and LL revised the manuscript. ZR and SL provided constructive and editorial feedback on drafts of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Fundamental Research Funds for the Central Universities (Grant Nos. SWU1909734) and the Funds for Administration of Sport of Chongqing (Grant No. B2019027).
We thank the CHNS database for providing a platform and the contributors for uploading their representative national datasets. We also thank all participants and the staff who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1104180/full#supplementary-material
Supplementary Figure 1 | Violin plot of components of MS based on different WBC category among all participants.
1. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
2. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep (2018) 20(2):1–8. doi: 10.1007/s11906-018-0812-z
3. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice - a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation (2003) 107(3):499–511. doi: 10.1161/01.CIR.0000052939.59093.45
4. Babio N, Ibarrola-Jurado N, Bullo M, Angel Martinez-Gonzalez M, Waernberg J, Salaverria I, et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the predimed study. PloS One (2013) 8(3):1–11. doi: 10.1371/journal.pone.0058354
5. Oda E, Kawai R. The prevalence of metabolic syndrome and diabetes increases through the quartiles of white blood cell count in Japanese men and women. Internal Med (2009) 48(13):1127–34. doi: 10.2169/internalmedicine.48.2138
6. Meng W, Zhang C, Zhang Q, Song X, Lin H, Zhang D, et al. Association between leukocyte and metabolic syndrome in urban han Chinese: A longitudinal cohort study. PloS One (2012) 7(11):1–7. doi: 10.1371/journal.pone.0049875
7. Sun S, Wu H, Zhang Q, Wang C, Guo Y, Du H, et al. Subnormal peripheral blood leukocyte counts are related to the lowest prevalence and incidence of metabolic syndrome: Tianjin chronic low-grade systemic inflammation and health cohort study. Mediators Inflammation (2014) 2014:1–12. doi: 10.1155/2014/412386
8. Liu C-C, Ko H-J, Liu W-S, Hung C-L, Hu K-C, Yu L-Y, et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine (2019) 98(43):1–12. doi: 10.1097/MD.0000000000017537
9. Yang X-J, Tian S, Ma Q-H, Sun H-P, Xu Y, Pan C-W. Leukocyte-related parameters in older adults with metabolic syndrome. Endocrine (2020) 68(2):312–9. doi: 10.1007/s12020-020-02243-2
10. Lin H-Y, Zhang X-J, Liu Y-M, Geng L-Y, Guan L-Y, Li X-H. Comparison of the triglyceride glucose index and blood leukocyte indices as predictors of metabolic syndrome in healthy Chinese population. Sci Rep (2021) 11(1):1–7. doi: 10.1038/s41598-021-89494-9
11. Thompson AL, Houck KM, Adair L, Gordon-Larsen P, Popkin B. Multilevel examination of the association of urbanization with inflammation in Chinese adults. Health Place (2014) 28:177–86. doi: 10.1016/j.healthplace.2014.05.003
12. Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes Vasc Dis Res (2019) 16(2):118–27. doi: 10.1177/1479164119827611
13. Zhou P, Meng Z, Liu M, Ren X, Zhu M, He Q, et al. The associations between leukocyte, erythrocyte or platelet, and metabolic syndrome in different genders of Chinese. Medicine (2016) 95(44):1–7. doi: 10.1097/MD.0000000000005189
14. Desai RJ, Wang SV, Vaduganathan M, Evers T, Schneeweiss S. Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Netw Open (2020) 3(1):1–15. doi: 10.1001/jamanetworkopen.2019.18962
15. Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: The China health and nutrition survey-monitoring and understanding socio-economic and health change in China, 1989-2011. Int J Epidemiol (2010) 39(6):1435–40. doi: 10.1093/ije/dyp322
16. He T, Wang M, Tian Z, Zhang J, Liu Y, Zhang Y, et al. Sex-dependent difference in the association between frequency of spicy food consumption and risk of hypertension in Chinese adults. Eur J Nutr (2019) 58(6):2449–61. doi: 10.1007/s00394-018-1797-8
17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment - insulin resistance and beta-cell function from fasting plasma-glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883
18. de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett (2008) 582(1):97–105. doi: 10.1016/j.febslet.2007.11.057
19. Kunz HE, Hart CR, Gries KJ, Parvizi M, Laurenti M, Dalla Man C, et al. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am J Physiol-Endocrinol Metab (2021) 321(1):E105–E21. doi: 10.1152/ajpendo.00070.2021
20. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature (2006) 444(7121):840–6. doi: 10.1038/nature05482
21. Feingold KR, Doerrler W, Dinarello CA, Fiers W, Grunfeld C. Stimulation of lipolysis in cultured fat-cells by tumor-necrosis-factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology (1992) 130(1):10–6. doi: 10.1210/endo.130.1.1370149
22. Sinisalo J, Paronen J, Mattila KJ, Syrjala M, Alfthan G, Palosuo T, et al. Relation of inflammation to vascular function in patients with coronary heart disease. Atherosclerosis (2000) 149(2):403–11. doi: 10.1016/S0021-9150(99)00333-0
23. Harlan JM. Leukocyte endothelial interactions. Blood (1985) 65(3):513–25. doi: 10.1182/blood.V65.3.513.513
24. Shankar A, Klein BEK, Klein R. Relationship between white blood cell count and incident hypertension. Am J Hypertens (2004) 17(3):233–9. doi: 10.1016/j.amjhyper.2003.11.005
25. Hajer GR, van Haeften TW, Visseren FLJ. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J (2008) 29(24):2959–71. doi: 10.1093/eurheartj/ehn387
26. Li C-p, Zhi X-y, Ma J, Cui Z, Zhu Z-l, Zhang C, et al. Performance comparison between logistic regression, decision trees, and multilayer perceptron in predicting peripheral neuropathy in type 2 diabetes mellitus. Chin Med J (2012) 125(5):851–7. doi: 10.3760/cma.j.issn.0366-6999.2012.05.022
27. Tzeng IS. To handle the inflation of odds ratios in a retrospective study with a profile penalized log-likelihood approach. J Clin Lab Anal (2021) 35(7):1–2. doi: 10.1002/jcla.23849
28. Neyman J. STATISTICS - SERVANT OF ALL SCIENCES. Science (1955) 122(3166):401–6. doi: 10.1126/science.122.3166.401
29. Anzai T, Grandinetti A, Katz AR, Hurwitz EL, Wu YY, Masaki K. Paradoxical association between atrial fibrillation/flutter and high cholesterol over age 75 years: The kuakini Honolulu heart program and Honolulu-Asia aging study. J Electrocardiol (2021) 65:37–44. doi: 10.1016/j.jelectrocard.2020.12.008
Keywords: cross-sectional study, insulin resistance, white cell counts, metabolic syndrome, Chinese population
Citation: Ren Z, Luo S and Liu L (2023) The positive association between white blood cell count and metabolic syndrome is independent of insulin resistance among a Chinese population: a cross-sectional study. Front. Immunol. 14:1104180. doi: 10.3389/fimmu.2023.1104180
Received: 21 November 2022; Accepted: 06 March 2023;
Published: 28 April 2023.
Edited by:
Wilfried Le Goff, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
I-Shiang Tzeng, National Taipei University, TaiwanCopyright © 2023 Ren, Luo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lian Liu, MDI0MTdAempodS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.