
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 27 January 2023
Sec. Vaccines and Molecular Therapeutics
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1104124
 Maria Rescigno1,2†
Maria Rescigno1,2† Chiara Agrati3,4*†
Chiara Agrati3,4*† Carlo Salvarani5,6
Carlo Salvarani5,6 Diana Giannarelli7
Diana Giannarelli7 Massimo Costantini8
Massimo Costantini8 Alberto Mantovani1,9,10
Alberto Mantovani1,9,10 Raffaella Massafra11
Raffaella Massafra11 Pier Luigi Zinzani12,13
Pier Luigi Zinzani12,13 Aldo Morrone14
Aldo Morrone14 Stefania Notari3
Stefania Notari3 Giulia Matusali15
Giulia Matusali15 Giuseppe Lauria Pinter16,17
Giuseppe Lauria Pinter16,17 Antonio Uccelli18,19
Antonio Uccelli18,19 Gennaro Ciliberto20
Gennaro Ciliberto20 Fausto Baldanti21,22
Fausto Baldanti21,22 Franco Locatelli4,23
Franco Locatelli4,23 Nicola Silvestris24
Nicola Silvestris24 Valentina Sinno25
Valentina Sinno25 Elena Turola26
Elena Turola26 Maria Teresa Lupo-Stanghellini27‡
Maria Teresa Lupo-Stanghellini27‡ Giovanni Apolone8‡ and the VAX4FRAIL study Group§
Giovanni Apolone8‡ and the VAX4FRAIL study Group§Introduction: Immunocompromised patients have been shown to have an impaired immune response to COVID-19 vaccines.
Methods: Here we compared the B-cell, T-cell and neutralizing antibody response to WT and Omicron BA.2 SARS-CoV-2 virus after the fourth dose of mRNA COVID-19 vaccines in patients with hematological malignancies (HM, n=71), solid tumors (ST, n=39) and immune-rheumatological (IR, n=25) diseases. The humoral and T-cell responses to SARS-CoV-2 vaccination were analyzed by quantifying the anti-RBD antibodies, their neutralization activity and the IFN-γ released after spike specific stimulation.
Results: We show that the T-cell response is similarly boosted by the fourth dose across the different subgroups, while the antibody response is improved only in patients not receiving B-cell targeted therapies, independent on the pathology. However, 9% of patients with anti-RBD antibodies did not have neutralizing antibodies to either virus variants, while an additional 5.7% did not have neutralizing antibodies to Omicron BA.2, making these patients particularly vulnerable to SARS-CoV-2 infection. The increment of neutralizing antibodies was very similar towards Omicron BA.2 and WT virus after the third or fourth dose of vaccine, suggesting that there is no preferential skewing towards either virus variant with the booster dose. The only limited step is the amount of antibodies that are elicited after vaccination, thus increasing the probability of developing neutralizing antibodies to both variants of virus.
Discussion: These data support the recommendation of additional booster doses in frail patients to enhance the development of a B-cell response directed against Omicron and/or to enhance the T-cell response in patients treated with anti-CD20.
Vaccination against SARS-CoV-2 has saved millions of lives in populations at risk of developing severe COVID-19 disease (i.e. above 60 years, with comorbidities or immunocompromised patients) (1). It is estimated that in the period from March to December 2021 in Colombia, vaccination avoided 32.4% of expected deaths of COVID-19 in individuals older than 60 (2). A mathematical model has calculated a 60% reduction in mortality in one year thanks to the COVID-19 vaccination. This percentage changes according to vaccination coverage but allows to estimate a number of 14.4 million of avoided deaths globally (3). Vaccination schedules generally include two vaccinations plus a booster dose. Indeed, we have recently described that a third dose of SARS-CoV-2 mRNA vaccination is important to augment anti-spike SARS-CoV-2 neutralizing antibodies and T cell responses, but in some immunocompromised individuals it is still not sufficient to reach antibody titers similar to those of healthy individuals (4). A systematic metanalysis has confirmed this data taking into consideration 82 studies of which 77 were based on mRNA vaccination schedules (5). A fourth dose is thus likely to benefit immunocompromised patients. Despite the recent introduction of new bivalent vaccine formulations (6), a large proportion of immunocompromised patients received the fourth dose with the monovalent vaccine carrying the wild type mRNA. However, in some conditions, such as common variable immunodeficiency (CVID), while the third dose definitely improved their antibody titers, a fourth dose was only slightly improving the response (7). If the study included a higher number of patients (currently n=33) there could have been a statistically significant improvement of the response. However, the possibility remains that in these patients it is difficult to overcome the immunocompromised condition. In another report, although the fourth dose raised the level of neutralizing antibodies from 80 to 96% at one month after the booster in multiple myeloma patients, anti-BCMA (B cell maturation protein) treatment affected the level of neutralizing antibodies after the third and fourth vaccine dose (8). In this group additional vaccine boosters seemed to even reduce the level of neutralizing antibodies (8). Similarly, in patients with lymphoid malignancies, anti-B cell therapies have reduced neutralizing antibodies after the fourth dose (9). A second booster dose seems to increase of seven-fold the level of antibodies in 28 patients with systemic lupus erythematosus or rheumatoid arthritis which were not responding to the third dose, without however improving the level of neutralizing antibodies to Omicron variant (10). Similarly, a second booster dose increased of 9-folds the median antibody level and allowed seroconversion in 60% (3/5) of liver transplanted previously non-responder patients (11) and in 80% of previously low or non-responder hemodialysis patients (12). The advantage of a fourth vaccine dose in protecting against infection has been recently reported in the healthy population. In Israel, for instance, the fourth dose of vaccine led to a reduction of infections (measured as PCR-positive test result) from 20% in health care workers with three doses to 7% in those with 4 doses, suggesting that the fourth dose correlates with SARS-CoV-2 infection protection, even to Omicron (13). An increase in antibody levels and T cell response after the fourth dose (14), was observed without any major adverse events (15). In long term care facility resident populations, vaccine efficacy increased with every received dose reaching a protection of 49% against infection, 69% against symptomatic infection, and 86% against severe disease after the fourth dose in Canada (16); and in Israel, after the fourth dose, there was a reduction of 34% against overall infection, 64% hospitalizations for mild-to- moderate illness, 67% against severe illness, and 72% against related deaths during the Omicron variant surge (17). When individuals older than 60 were analyzed, the fourth dose reduced hospitalizations by 32% and COVID-19 related deaths by 22% (18). A real-world study on the protection offered by 2, 3 or 4 doses of vaccines confirmed that the booster doses protect against COVID-19 associated hospitalization particularly in individuals older than 50 years (19). This is in line with an increase of antibody titers after the second booster dose in older individuals (20). However, this population lacked immunocompromised individuals. Thus, it remains important to understand on a large population whether the fourth dose of mRNA vaccine is beneficial to immunocompromised individuals particularly with respect to associated therapies and whether it induces neutralizing antibodies to the Omicron VOC.
Here we report the follow-up results of VAX4FRAIL, a longitudinal study on COVID-19 vaccination in immunocompromised patients, on the effect of a fourth booster dose of mRNA vaccine on the titer of neutralizing antibodies to both WT and Omicron BA.2 and T cell responses.
The study was approved by the Italian Medicines Agency (AIFA) and by ethics committee (code 304, 2021) and written informed consent was obtained from all the participants. The inclusion criteria were age ≥18 years, SARS-CoV2 mRNA-based vaccination (fourth dose) and a life expectancy of at least 12 months at the time of vaccine administration. The main exclusion criterion was the presence of a previous laboratory-confirmed SARS-CoV-2 infection (documented by serology and/or molecular test). Moreover, patients experiencing a molecularly confirmed SARS-CoV-2 breakthrough infection (RT-PCR assay) or seroconversion to anti-Nucleocapsid antibody during follow-up were also excluded (n=93).
Anti-spike SARS-CoV-2 humoral (binding and neutralizing antibodies) and T-cell response were monitored before 4th dose vaccination (pre-4) and 10-20 days after vaccination (post-4) in patients with hematological malignancies (HM, n=71), solid tumors (ST, n=39) and immune-rheumatological (IR, n=25) diseases. Moreover, the immune response was also compared in treatment-specific subgroups.
The response to vaccination was assessed by quantifying the anti-Nucleoprotein-IgG (anti-N) and the anti-RBD-IgG by commercial available diagnostic kit, according to the manufacturer’s protocol (Architect® i2000sr Abbott Diagnostics, Chicago, IL). As reported in the leaflet, anti-N IgG were expressed as a ratio (S/CO) and values were considered positive when ≥ 1.4. Anti-RBD-IgG were expressed as binding arbitrary units (BAU)/mL and values were considered positive when ≥ 7.1. The anti-N quantification was aimed to identify asymptomatic SARS-CoV-2 infections during the follow-up and to exclude these patients from the analysis. The median level of anti-RBD was calculated using values from all patients (both non-responder and responder patients). To evaluate the functional activity of vaccination-induced antibodies against Omicron, a neutralization assay against BA.2 variant was performed on anti-RBD positive samples according to our previous publication (21). Briefly, heat-inactivated and two-fold serial diluted sera were incubated at 37 °C 5% CO2 for 30 min with equal volumes of 100 TCID50 SARS-CoV-2. Next, 96-well tissue culture plates with sub-confluent Vero E6 cell monolayers were infected with 100 µL/well of virus-serum mixture and incubated at 37 °C and 5% CO2. After 48 h, microplates were observed using a light microscope for the presence of the cytopathic effect (CPE). The highest serum dilution inhibiting at least 90% of the CPE was indicated as the neutralization titer and was expressed as the reciprocal of serum dilution (MNA90). In a subgroup of patients (n=71) whose sample was available after the third dose, the neutralization titer against the Wild Type SARS-CoV-2 strain was also evaluated (22 HM, 10 IR and 39 ST).
The cell-mediated immune response to vaccination was assessed through a standardized whole blood assay (21), shared between clinical centers. Peripheral blood was collected in heparin tubes and stimulated with a pool of peptides spanning the Spike protein (Miltenyi Biotech, Germany) at 37°C (5% CO2). A pool of S protein peptides was generated by combining three peptide pools (all by Miltenyi Biotech): i) PepTivator® SARS-CoV-2 Prot_S (sequence domains aa 304-338, 421-475, 492-519, 683-707, 741-770, 785-802, and 885–1273 of the Spike protein); ii) the PepTivator® SARS-CoV-2 Prot_S1 (sequence domain aa 1–692 of the Spike protein) and iii) the PepTivator® SARS-CoV-2 Prot_S+ (sequence domain aa 689–895 of the Spike protein). The peptide pool was used for cell stimulation at the final concentration of 0.1 ug/ml. The spontaneous cytokines release was calculated in unstimulated culture (background) and a superantigen (SEB) was used as positive control. Plasma was harvested after 16-20 h of stimulation and stored at -80°C. IFN-γ (detection limit 0.17 pg/ml) was quantified in the plasma samples using an automatic ELISA (ELLA, Protein Simple). The results are expressed as the amount of each cytokines after subtracting the background. A concentration equal to or greater than 12 pg/mL of IFN-γ was considered as positive (the cut-off was defined as the mean +/- 2 SEM of 50 anti-S and anti-N negative HCW before vaccination as reported in 4).
Quantitative variables were summarized as median and interquartile range (IQR), while categorical variables were reported as absolute count and percentage. Differences in seroconversion rates across subgroups were analyzed using the chi-square test, and from a multivariable logistic regression model we obtained the odds ratios with their 95% confidence intervals (CI). The model outcome was the seroconversion status after the fourth dose (yes vs no) and independent variables were identified on the basis of availability as required by the study protocol (disease, age, gender, comorbidities and therapy) and used to adjust the vaccination effect on outcome. Current therapy was stratified as follows: for HM patients: Rituximab (RTX), hematopoietic stem cell transplantation (HSCT), chemotherapy (CT), JAK inhibitors (JAK); IR patients: anti-CD20 (Rituximab, RTX), and others (methotrexate, mycophenolate mofetil, and azathioprine); ST patients: chemotherapy (CT), Immunotherapy (ImmTx) and targeted therapy (TarTx).
The Wilcoxon test was used to assess differences between pre-4 and post-4, while Mann-Whitney test was used to assess differences in antibody titers across groups.
The SPSS v.20.0 (IBM) statistical software was used for the analysis.
Between October 2021 and June 2022, 228 patients participating to the VAX4FRAIL study (NCT04848493); received the fourth dose of vaccine. 179 patients received the BNT162b2, 46 the mRNA-1273 vaccine, while for 3 the type of vaccine used was not reported. After having excluded patients resulting positive for antibodies directed to the SARS-CoV-2 nucleocapsid (N) antigen and that presumably had a breakthrough infection after the third or fourth dose, we analyzed the immune response before and after the 4th dose of mRNA vaccine in the remaining 135 patients.
The breakdown of patients receiving the fourth dose of mRNA COVID-19 vaccine was: hematological malignancies (HM, n=71 – 52.6%), solid tumors (ST, n=39 – 28.9%) and immunorheumatological (IR, n=25 – 18.5%) diseases (Table 1). The median age was 63 years (interquartile range 56-71) and 74 patients (54.8%) were women (Table 1).
We first evaluated whether the fourth dose of vaccine was well tolerated. We found that the fourth dose was in general well tolerated and the safety profile in line with previous doses (22) (Table 2). The most common moderate adverse events (AE) were pain at the site of injection (55%), fatigue (42.2%), pain in the bones (36.7%) and headache (26.6%). Very few patients had severe AE which were mostly pain in the bones (9.2%), fatigue (7.3%) and pain at the site of injection (6.4%) as shown in Table 2.
All patient groups displayed a statistically significant improvement of the humoral response after the fourth dose of vaccine (Figure 1A, P<0.0001 for all the groups). However, the antibody response to the spike RBD protein differed in the disease groups with patients with solid tumors responding better (p<0.001 vs IR and p<0.05 vs HM) followed by hematologic malignancies (p<0.005 vs IR and p<0.05 vs ST) and immunorheumatological diseases (p<0.001 vs ST and p<0.005 vs HM) without major discrepancies when analyzing the patients as a group (Figure 1A) or individually (Supplementary Figure 1A). In the HM group only 2 out of 21 (9%) negative patients and in the IR group 3 out of 12 (25%) patients who had not responded to the third dose responded to the fourth dose.”. Altogether, 17.6% of the patients not responding to the third dose became positive after the fourth dose.
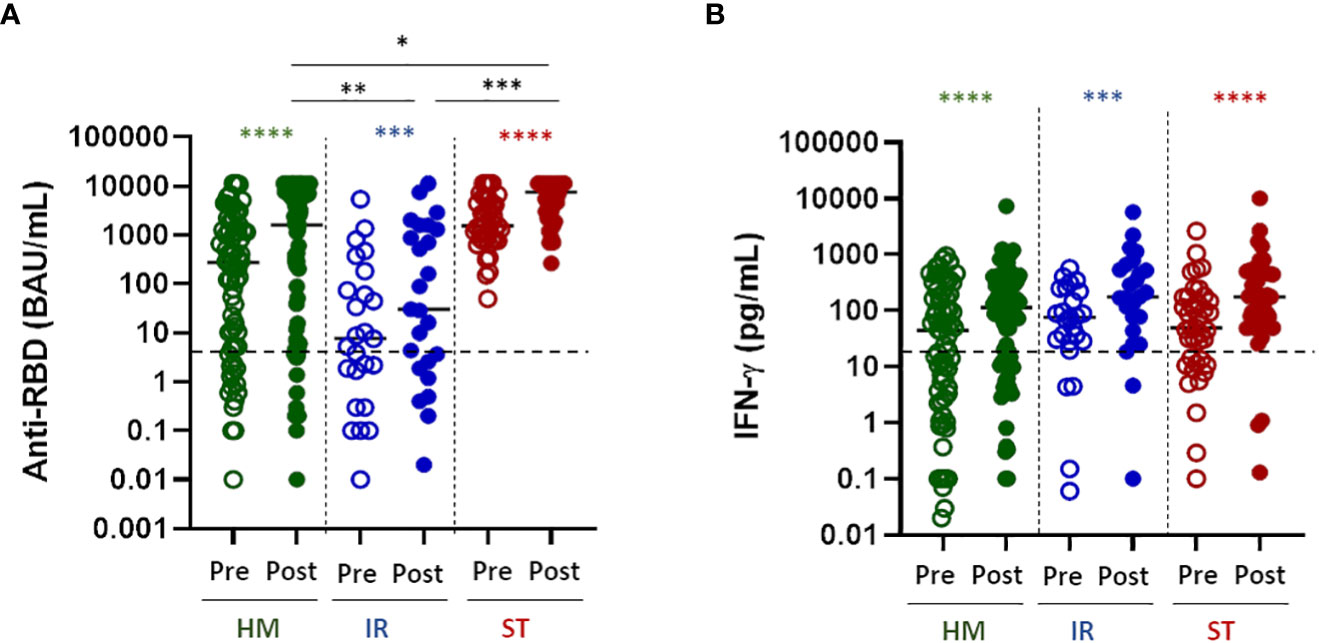
Figure 1 Humoral and T-cell response to the fourth dose of mRNA vaccine in fragile patients. (A) SARS-CoV-2 specific anti-RBD antibodies (Abs) were measured in sera samples of HM (green dots), IR (blue dots) and ST (red dots) patients before (pre) and after (post) the fourth dose of mRNA vaccine. The level of anti-RBD Abs was expressed as BAU/mL. HM-pre median= 274.8 BAU/mL (IQR 5.2–1357 BAU/mL); HM-post median=1624.0 BAU/mL (IQR 10.7-7269). IR-pre median= 7.6 BAU/mL (IQR 1.0-128.3 BAU/mL); IR-post median= 30.5 (IQR 2.2-1458 BAU/mL). ST-pre median= 1546.0 BAU/ml (810.4-4389 BAU/mL); ST-post median= 7632 (3023-11360 BAU/ml). The dashed line indicates the cut-off value (7.1 BAU/mL). Differences between anti-RBD Abs titre before and after the fourth dose within the same group were evaluated by Wilcoxon paired test, while differences across groups were evaluated by Kruskal-Wallis. ****P<0.0001; ***P<0.001. (B) T-cell response were measured in HM (green dots), IR (blue dots) and ST (red dots) patients before (pre) and after (post) the fourth dose of mRNA vaccine. Spike-specific T-cell response was measured after stimulation of whole blood with specific peptides and was expressed as pg/mL of IFN-γ and values 0.12 pg/mL are considered positive. HM-pre median= 44.0 pg/mL (IQR 3.1-185.3 pg/mL); HM-post median=112.6 pg/mL (IQR 14.4-350.9 pg/mL). IR-pre median= 74.8 pg/mL (IQR 28.1-233.0 pg/mL); IR-post median= 175.5 pg/mL (IQR 60.1-595.2 pg/mL). ST-pre median= 48.9 pg/mL (11.9-167.3 pg/mL); ST-post median= 176.0 pg/mL (60.5-495 pg/mL). The dashed line indicates the cut-off value (12 pg/mL). Differences between IFN-γ level before and after the fourth dose within the same group were evaluated by Wilcoxon paired test, while differences across groups were evaluated by Kruskal-Wallis. ****P<0.0001; ***P<0.001. HM, hematological malignancies; IR, immune-rheumatological diseases; ST, solid tumors. **P<0.01; *P<0.05.
Similarly, the spike-specific T-cell response (defined as IFN-γ levels ≥12 pg/mL) was statistically significantly improved in all of the disease groups after the fourth dose (Figure 1B P<0.0001 for all the groups). Notably, differently from the B-cell response, the T-cell response was very similar across disease groups (no statistically significant differences among groups, Figure 1B), suggesting that the T-cell response is less dependent on disease or treatment characteristics.
Patients with fragility have been the first to receive a fourth dose of vaccine. The new version of the vaccine targeting omicron VOC was not available at the time of vaccination. Thus, an important question that arises is whether these patients should undergo a booster dose with the adapted versions of the vaccines to the omicron VOC. We therefore evaluated the neutralizing activity of anti-RBD positive sera against SARS-CoV-2 omicron (BA.2) infectivity in a BSL-3 facility. Sera from all anti-RBD positive patients demonstrated a significantly increased neutralizing activity after the fourth dose of vaccine towards the omicron VOC BA.2 (Figure 2A, P<0.0001 for HM and ST, P= 0.008 for IR). Of note, the neutralization titer after the fourth dose reached higher level in HM and ST than in IR patients (Figure 2A, HM vs IR, P<0.05; ST vs IR, P<0.001). Similarly, to the antibody response, the percentage of anti-RBD positive patients showing a detectable neutralizing activity after the fourth dose of vaccine was higher within ST (97.4%) and HM (80.7%) and lower within IR (66.6%) patients. Interestingly, regarding IR patients, only a minority of them displayed a level of neutralization above baseline for the omicron BA.2 VOC. In order to evaluate whether the low level of neutralization titer was evident only towards the omicron VOC or also against the WT SARS-CoV-2, for a reduced number of patients (67 HM, 24 IR and 39 ST), we compared the difference in neutralizing abilities towards the WT and Omicron variant before and after the fourth dose. As shown in Figure 2B mirroring the increased titer of antibodies induced after vaccination in ST patients, there was a very high level of neutralizing antibodies towards both Omicron BA.2 and WT, with a statistically significantly higher level towards the WT virus (p<0.001). By contrast in HM and even more so in IR patients, the level of neutralization towards both the Omicron BA.2 and WT virus was very low (Figure 2B). 9% of patients with anti-RBD antibodies did not have neutralizing antibodies to either forms of the virus, while an additional 5.7% did not have neutralizing antibodies only to Omicron BA.2 variant (Supplementary Figure 2A), making these patients particularly vulnerable to SARS-CoV-2 infection. We supposed that the probability of finding neutralizing antibodies to both forms of the virus depended on the amount of anti-RBD antibodies elicited during the vaccination. Thus, we analyzed the correlation between the level of anti-RBD antibody response and neutralizing antibodies to WT or Omicron BA.2. As shown in Supplementary Figure 2B there was a positive correlation between anti-RBD antibodies and neutralizing antibodies to WT (p<0.0001, R2 = 0.85) or Omicron (p<0.0001, R2 = 0.69). In particular, anti-RBD levels below 100 BAU/mL correlated with no neutralizing antibodies to WT, while the level of anti-RBD correlating with the presence of neutralizing antibodies towards Omicron BA.2 was more difficult to identify. Nevertheless, a level of antibodies above 350 BAU/mL increased the chances of finding neutralizing anti Omicron BA.2 antibodies (86/89, 96.6%), while at levels above 1000 BAU/mL we found only one patient with still no neutralizing antibodies to Omicron BA.2 (Supplementary Figure 2B). There was also a clear correlation between neutralizing antibodies to WT and Omicron BA.2, suggesting that they may broadly recognize common moieties of the viruses (Supplementary Figure 2B). Thus, even though patients may have detectable anti-RBD antibodies the probability of finding neutralizing antibodies to Omicron BA.2 is low and correlates on the amount of anti-RBD antibodies.
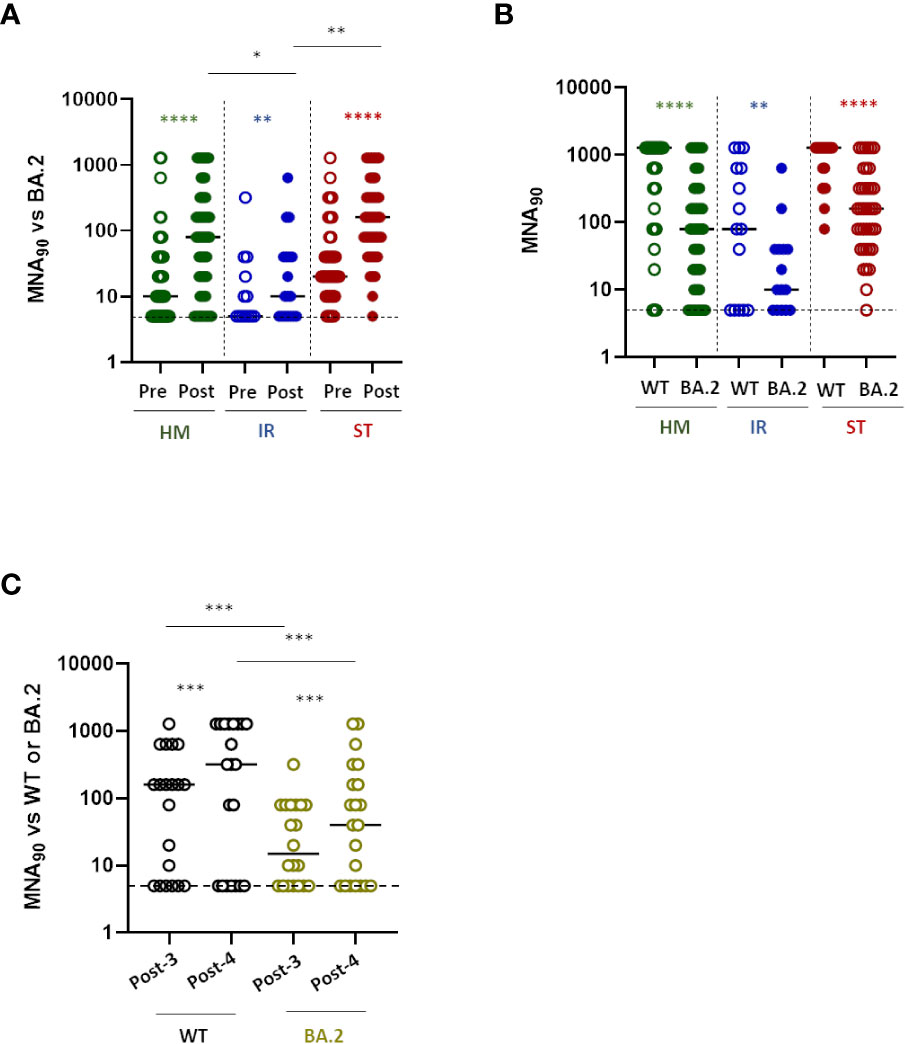
Figure 2 Neutralization response to the fourth dose of mRNA vaccine in fragile patients (A) The levels of neutralizing antibodies were quantified by microneutralization assay (MNA90) against Omicron BA.2 in HM (green dots), IR (blue dots) and ST (red dots) before (pre) and after (post) the fourth dose of mRNA vaccine. The MNA90 was measured only in patients showing a positive anti-RBD response. The results were expressed as reciprocal of dilution and values >5 are considered positive. HM-pre median= 10 reciprocal of dilution (IQR 5-40); HM-post median=80 reciprocal of dilution (IQR 10-320). IR-pre median= 5 reciprocal of dilution (IQR 5-30); IR-post median= 15 reciprocal of dilution (IQR 5-40). ST-pre median= 20 reciprocal of dilution (IQR 10-80); ST-post median= 160 reciprocal of dilution (IQR 50-320). Differences between MNA90 titre before and after the fourth dose within the same group were evaluated by Wilcoxon paired test, while differences across groups were evaluated by Kruskal-Wallis. ****P<0.0001; **P<0.01. (B) The levels of neutralizing antibodies against wild-type (WT) and BA-2 Omicron (BA.2) strains after the fourth dose of mRNA vaccine were compared in HM (green dots), IR (blue dots) and ST (red dots). The results were expressed as reciprocal of dilution and values >5 are considered positive. HM-WT median=1280 reciprocal of dilution (IQR 80-1280); HM-BA.2 median=80 reciprocal of dilution (IQR 10-320). IR-WT median= 80 reciprocal of dilution (IQR 5-640); IR-BA.2 median= 10 reciprocal of dilution (IQR 5-40). ST-WT median= 1280 reciprocal of dilution (IQR 640-1280); ST-BA.2 median= 160 reciprocal of dilution (IQR 40-320). Differences were evaluated by Kruskal-Wallis test. ****P<0.0001; ** P<0.01. (C) The levels of neutralizing antibodies against wild-type (WT) and BA-2 Omicron (BA.2) strains after 10-20 days from the third (post-3) and fourth (post-4) dose of mRNA vaccine were compared in a subgroup of patients (n=21). The results were expressed as reciprocal of dilution and values >5 are considered positive. WT-post-3 median=160 reciprocal of dilution (IQR 5-520); WT-post-4 median= 320 reciprocal of dilution (IQR: 5-1280). BA.2-post-3 median= 15 reciprocal of dilution (IQR 5-80); BA.2-post-4 median= 40 (IQR 5-240). Differences between neutralization titre against WT and BA.2 after the third and fourth dose evaluated by Wilcoxon paired test ***P<0.001. HM, hematological malignancies; IR, immune-rheumatological diseases; ST, solid tumors.
We then asked if the fourth dose may equally boost the response to the WT and Omicron BA.2 VOC and evaluated the increment of neutralizing antibodies after the 3rd and 4th dose of vaccine towards the two viruses. As shown in Figure 2C, we found that the fourth dose was inducing a statistically significant (p<0.005) similar increment of neutralizing antibodies towards both viruses (fold increase: 3.29 ± 3.776 for WT and 3.053 ± 3.61 for BA.2). Thus, patients with ID and HM have a reduced neutralizing antibody response to SARS-CoV-2 independent on the variant. Whenever present, this neutralizing response is similarly boosted towards both forms of the virus across different booster doses.
HM and IR patients often undergo anti-B cell treatment (Rituximab) which can have a major impact on the humoral B cell response to COVID-19 vaccines, as we have shown in this (4) and other cohorts of patients (23). Thus, humoral and T-cell responses were evaluated in relation to the received or ongoing treatment and its presumed immunosuppression (Table 3). It is clear that B-cell directed treatment has an impact on the anti-RBD response (Supplementary Figure 3A) but not on the T-cell response (Supplementary Figure 3B). More in details, in Figure 3, we analyzed the B- and T-cell response to the fourth dose in relation to underlying disease and treatment. It is clear that both in HM (Figure 3A, left panel) and IR (Figure 3B, left panel) the only treatment which strongly affected the B-cell humoral response was anti-CD20 treatment (Rituximab). Patients with HM undergoing anti-CD20 treatment had a reduced anti-RBD response as compared with HSCT (p<0.001), CT (p<0.05) and JAK inhibitors (p<0.01) (Figure 3A). Patients with IR had a reduced anti-RBD response as compared with the other treatments (p<0.001) (Figure 3B). By contrast, the different treatments used in solid tumors (ST) had no effect on the induction of the humoral response. As expected, the T cell response to the Spike protein evaluated as IFN-γ production was not affected by anti-CD20 or any other treatment, independent on the pathology of the patients (Figure 3 right panels) Hence, the antibody immune response to the vaccine is dependent mostly on the patients treatment rather than their pathology.
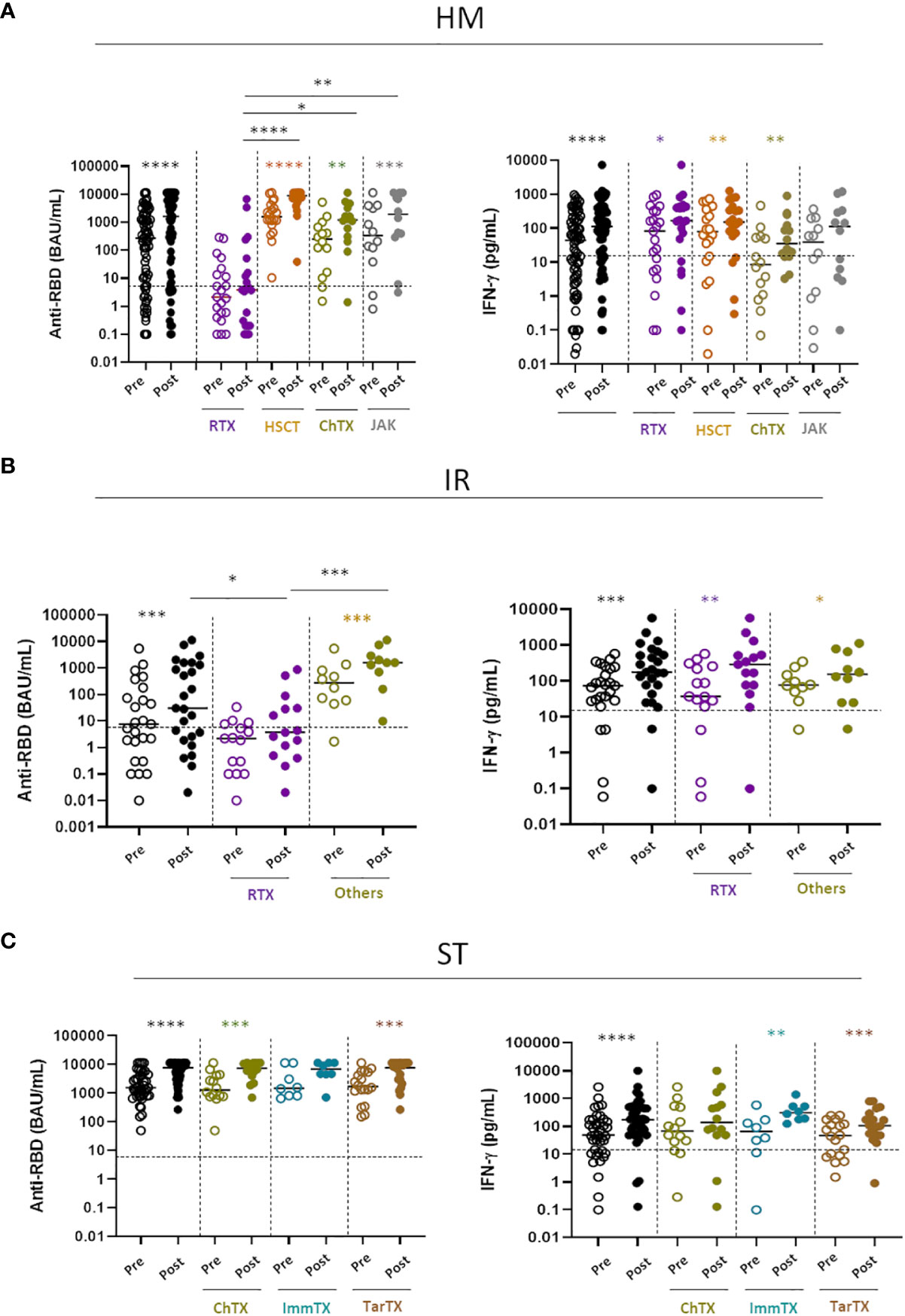
Figure 3 Impact of different treatments on humoral and T-cell response (A) SARS-CoV-2 specific anti-RBD Abs and T cell response before (pre) and after (post) the fourth dose of mRNA vaccine were compared in HM patients receiving different treatments: Rituximab (RTX), hematopoietic stem cell transplantation (HSCT), Chemotherapy (ChTX), JAK inhibitors (JAK) or others. (B) SARS-CoV-2 specific anti-RBD Abs and T cell response before (pre) and after (post) the fourth dose were compared in IR patients receiving different treatments: Rituximab (RTX) and other (methotrexate, mycophenolate mofetil, and azathioprine). (C) SARS-CoV-2 specific anti-RBD Abs and T cell response before (pre) and after (post) the fourth dose of mRNA vaccine were compared in ST patients receiving different treatments: Chemotherapy (ChTX), Immunotherapy (ImmTX) and Target therapy (TarTX). The median and IQR are described in Supplementary material. Differences between anti-RBD or T cells response level before and after vaccination within the same group was evaluated by Wilcoxon paired test, while differences across groups were evaluated by Kruskal-Wallis. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05. HM, hematological malignancies; IR, immune-rheumatological diseases; ST, solid tumors.
In this study we show that the fourth dose of SARS-CoV-2 mRNA vaccine is well tolerated and increases the immune response to both WT and Omicron BA.2 VOC. As already reported for the third dose by us and others (4, 5, 23), the humoral response to the fourth dose was compromised in patients with HM and IR that underwent rituximab (anti-CD20) treatment. However, the fourth dose, induced a similar level of T cell response to the Spike protein in all of the analyzed groups, confirming that fragile patients are capable of mounting a cellular immune response to the vaccine. Interestingly, even if directed to the WT strain of the virus, the vaccine was capable of increasing the level of neutralizing antibodies also to the Omicron BA.2 VOC. The level of neutralization was, as expected, lower than that for the WT version of the virus, however, the increment of neutralizing antibodies after the third and fourth doses was very similar towards both the WT and omicron VOC, suggesting that there is no preferential skewing of the humoral response towards the WT in the booster dose. Interestingly, we observed many patients (9%), particularly those with rheumatologic disorders, whose antibodies were not neutralizing towards both WT and Omicron BA.2, and an additional 5.7% without neutralizing antibodies to Omicron BA.2, and this correlated with the amount of anti-RBD antibodies elicited after the booster dose. This may explain the results of another report on patients with systemic lupus erythematosus or rheumatoid arthritis, where it was observed no neutralizing antibodies to Omicron in patients with an already low response to the third dose (10). However, it is still remarkable that, even in immunocompromised individuals, the mRNA vaccines elicit antibodies that can recognize very different variants of the virus. This is a characteristic that is less evident during the natural infection (24). Indeed, the germinal centers reaction after viral infection is limited and thus the immune response is affected in breadth and this may explain why patients that have been infected are easily re-infected by a different VOC and that the immune response to different variants may be compromised (25–27). By contrast, the mRNA vaccines induce a strong germinal center reaction also due to the persistence of the spike protein which is retained in the lymph nodes for up to 2 months after vaccination thus continuously boosting the immune response (24). We confirm that also in fragile individuals the breadth of the immune response induced by the vaccine is large and includes neutralizing antibodies to the Omicron. We recently demonstrated that anti-Spike IgG can permeate the saliva and thus may protect mucosal sites in the first 3 months after vaccination (28); this correlated with the amount of antibodies present in the blood. Given the low level of neutralizing antibodies against Omicron present in blood, it is likely that an even lower amount is present in the saliva and this may explain why the Omicron was so infectious also in vaccinated people. Consistently, three doses of vaccine are more protective against Omicron than two doses (29). Patients with cancer are also at higher risk of Omicron than Delta infection, especially patients with HM (30) and this may be explained by the low level of neutralizing antibodies to the Omicron VOC. In addition, cancer patients who underwent breakthrough infection with Omicron and had no detectable antibodies to Omicron before, developed these antibodies after infection (9). This may indicate that, even if not detectable, some antibodies to the Omicron variant were present and the B-cells producing them probably expanded after infection, or that the T-cell response, which is still induced in patients without a detectable B-cell response, may promote a fast induction of the antibody response to Omicron. Either way, these results are encouraging and indicate that vaccination booster doses may indirectly protect against variants of the virus that are not included in the vaccine, even in immunocompromised individuals. Further, it is important to note that anti-CD20 treatment, as well as other B-cell depleting therapies such as CAR T cells, has the most detrimental effect on vaccination while other treatments used in patients with solid tumors or hemodialysis (31) do not affect the fourth dose of vaccination. Finally, the evidence that, regardless of frailties and therapies, the T-cell compartment can respond effectively to vaccination represents an important observation, as antigen specific T cells are actively involved in the protection against the severe disease (32). This also prompts patients undergoing anti-CD20 therapies to proceed to vaccination even if their B-cell response will not be detectable.
In conclusion, we show that the fourth dose of vaccine increases the level of circulating anti-RBD antibodies in all of the patients. These levels however remain low in patients undergoing B cell targeted therapies. Nevertheless, the T-cell response is boosted also in immunocompromised patients undergoing anti-CD20 treatment, thus providing a reinforced line of protection against the severe disease. Similarly to healthy individuals, in fragile patients the breadth of antibodies is ample and includes antibodies recognizing WT and to a much lower extent the Omicron BA.2 VOC. The recent introduction of new bivalent vaccine formulations that include mRNA for both wild type and Omicron strains (6) may further improve the overall response. Thus, it is advisable for immunocompromised patients to undergo a 5th dose of bivalent vaccine to further boost the development of an Omicron-directed B-cell response and/or to boost the T-cell response in anti-CD20 treated patients. Future studies should examine how these same patients respond to the new vaccine.
During the course of the study, the policy of vaccination has changed in Italy and only some of the centers of the VAX4FRAIL study protocol could administer the fourth dose. Thus, we could not follow-up with the population of patients with neurological disorders and some of the patients with the other pathologies. Still we had enough patients to draw conclusions from the fourth dose. We have tested the neutralization to BA.2 and not BA.4 or BA.5 Omicron VOC. Hence, the conclusions are not generalizable to the most recent VOC which seem to escape the immune response elicited by the WT vaccine (33, 34).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Italian Medicines Agency (AIFA) and by ethics committee of the National Institute for Infectious Diseases L Spallanzani (code 304, 2021). The patients/participants provided their written informed consent to participate in this study.
GA, AMa and MC conceived the study. MR, CA, CS, RM, PZ, FB, FL, GP, AU, NS, VS, GC, AMo and MS designed the study. CS, MS, FB and NS managed the patients. DG performed the statistical analysis. ET and VS monitored the study. CA, SN and GM performed the immunological analysis. CA, MR and CS analyzed the immunological data. MR and CA drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Italian Ministry of Health within Ricerca Corrente 2021-Special Projects-VAX4FRAIL.
We are indebted to Research Director Dr. Giuseppe Ippolito, and the Deputy General Director for Health Research and Innovation, Dr. Gaetano Guglielmi, of the Italian Ministry of Health for their support to this study. We would also like to thank the patients who were enrolled in the VAX4FRAIL study and their families, the research nurses, the administrative staff, and all those who will be actively involved in their continuous care, study data collection and analysis, and ultimately in the scientific production that will result from this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1104124/full#supplementary-material
Supplementary Figure 1 | SARS-CoV-2 specific anti-RBD Abs per individual patient. Anti-RBD were measured in sera samples of HM (green dots), IR (blue dots) and ST (red dots) patients before (pre) and after (post) the fourth dose of mRNA vaccine. The level of anti-RBD Abs was expressed as BAU/mL. Differences between anti-RBD titre before and after vaccination were evaluated by Wilcoxon paired test. ****P<0.0001, *** P<0.001. HM, hematological malignancies; IR, immune-rheumatological diseases; ST, solid tumors.
Supplementary Figure 2 | Cross reactivity of anti-RBD Abs induced by vaccination. (A) The number (percentage) of patients showing a negative or positive anti-RBD response (cut-off 7.2 BAU/mL) after the fourth dose of mRNA vaccine is shown. Patients with a positive anti-RBD response were further divided in MNA negative (white bars) and positive (grey bars) on the basis of their neutralization capability against WT and BA.2 viral strains (cut-off 5 MNA90). Results are shown as the percentage of MNA90 negative and positive patients and the absolute number of patients are shown within the bars. (B) The correlation between the levels of anti-RBD Abs and neutralization titre (WT or BA.2) after the fourth dose as well as the correlation between the neutralization titre against WT and BA.2 viral strains are shown. Each black dot represents one sample. The analysis was performed by using the Spearman test and Rho and p values are indicated in the figure.
Supplementary Figure 3 | Impact of Rituximab therapy on humoral and T cell response (A) SARS-CoV-2 specific anti-RBD Abs before (pre) and after (post) the fourth dose of vaccine were compared in all enrolled patients receiving Rituximab (RTX) or other treatments. All-pre median= 462.5 BAU/mL (IQR: 10.4-1913 BAU/mL); all-post median= 2212 BAU/mL (IQR: 51.6-8391 BAU/mL). RTX-pre median= 2.1 BAU/mL (IQR: 0.3-10.0 BAU/mL); RTX-post median= 3.7 BAU/mL (IQR: 0.3-30.1). Other-pre median=1155 BAU/mL (IQR: 316.4-3145 BAU/mL); Other-post median=5446 BAU/mL (IQR: 1537-11360 BAU/mL) (B) SARS-CoV-2 specific T cell response before (pre) and after (post) the fourth dose of vaccine were compared in all enrolled patients receiving Rituximab (RTX) or other treatments. All-pre median= 49.5.0 pg/mL (IQR: 8.96-177.1 pg/mL); all-post median= 147.0 pg/mL (IQR: 46.7-439.1 pg/mL). RTX-pre median= 63.0 pg/mL (IQR: 7.9-298.7 pg/mL); RTX-post median= 171 pg/mL (IQR: 53.9-475.0 pg/mL). Other-pre median= 49.0 pg/mL (IQR: 8.9-148 pg/mL); Other-post median= 124.5 pg/mL (IQR: 32.6-345.0 pg/mL).
1. Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med (2022) 387:1011–20. doi: 10.1056/NEJMra2206573
2. Rojas-Botero ML, Fernandez-Nino JA, Arregoces-Castillo L, Ruiz-Gomez F. Estimated number of deaths directly avoided because of COVID-19 vaccination among older adults in Colombia in 2021: An ecological, longitudinal observational study. F1000Res (2022) 11:198. doi: 10.12688/f1000research.109331.2
3. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis (2022) 22(9):1293–1302. doi: 10.1016/S1473-3099(22)00320-6
4. Corradini P, Agrati C, Apolone G, Mantovani A, Giannarelli D, Marasco V, et al. Humoral and T-cell immune response after three doses of mRNA SARS-CoV-2 vaccines in fragile patients: The Italian VAX4FRAIL study. Clin Infect Dis (2022) ciac 404. doi: 10.1101/2022.01.12.22269133
5. Lee A, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ (2022) 376:e068632. doi: 10.1136/bmj-2021-068632
6. Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, et al. A bivalent omicron-containing booster vaccine against covid-19. N Engl J Med (2022) 387(14):1279–91. doi: 10.1056/NEJMoa2208343
7. Nielsen BU, Drabe CH, Barnkob MB, Johansen IS, Hansen AKK, Nilsson AC, et al. Antibody response following the third and fourth SARS-CoV-2 vaccine dose in individuals with common variable immunodeficiency. Front Immunol (2022) 13:934476. doi: 10.3389/fimmu.2022.934476
8. Ntanasis-Stathopoulos I, Karalis V, Gavriatopoulou M, Malandrakis P, Sklirou AD, Eleutherakis-Papaiakovou E, et al. Second booster BNT162b2 restores SARS-CoV-2 humoral response in patients with multiple myeloma, excluding those under anti-BCMA therapy. Hemasphere (2022) 6:e764. doi: 10.1097/HS9.0000000000000764
9. Fendler A, Shepherd STC, Au L, Wu M, Harvey R, Wilkinson KA, et al. Functional immune responses against SARS-CoV-2 variants of concern after fourth COVID-19 vaccine dose or infection in patients with blood cancer. Cell Rep Med (2022) 3:100781. doi: 10.1016/j.xcrm.2022.100781
10. Assawasaksakul T, Sathitratanacheewin S, Vichaiwattana P, Wanlapakorn N, Poovorawan Y, Avihingsanon Y, et al. Immunogenicity of the third and fourth BNT162b2 mRNA COVID-19 boosters and factors associated with immune response in patients with SLE and rheumatoid arthritis. Lupus Sci Med (2022) 9:e000726. doi: 10.1101/2022.03.15.22272350
11. Harberts A, Schaub GM, Ruether DF, Duengelhoef PM, Brehm TT, Karsten H, et al. Humoral and cellular immune response after third and fourth SARS-CoV-2 mRNA vaccination in liver transplant recipients. Clin Gastroenterol Hepatol (2022) 20(11):2558–2566. doi: 10.1016/j.cgh.2022.06.028
12. Tillmann FP, Figiel L, Ricken J, Still H, Korte C, Plassmann G, et al. Effect of third and fourth mRNA-based booster vaccinations on SARS-CoV-2 neutralizing antibody titer formation, risk factors for non-response, and outcome after SARS-CoV-2 omicron breakthrough infections in patients on chronic hemodialysis: A prospective multicenter cohort study. J Clin Med (2022) 11:3187. doi: 10.3390/jcm11113187
13. Cohen MJ, Oster Y, Moses AE, Spitzer A, Benenson S, Israeli-Hospitals 4th Vaccine Working. Association of receiving a fourth dose of the BNT162b vaccine with SARS-CoV-2 infection among health care workers in Israel. JAMA Netw Open (2022) 5:e2224657. doi: 10.1001/jamanetworkopen.2022.24657
14. Bar-Haim E, Eliakim-Raz N, Stemmer A, Cohen H, Elia U, Ness A, et al. Humoral and T-cell response before and after a fourth BNT162b2 vaccine dose in adults >/=60 years. J Clin Med (2022) 11:2649. doi: 10.3390/jcm11092649
15. Romero-Ibarguengoitia ME, Gonzalez-Cantu A, Rivera-Salinas D, Hernandez-Ruiz YG, Armendariz-Vazquez AG, Barco-Flores IA, et al. Analysis of immunization, adverse events, and efficacy of a fourth dose of BNT162b2 vaccine in health workers in Mexico, a pilot study. Vaccines (Basel) (2022) 10:1139. doi: 10.3390/vaccines10071139
16. Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ (2022) 378:e071502. doi: 10.1136/bmj-2022-071502
17. Muhsen K, Maimon N, Mizrahi AY, Boltyansky B, Bodenheimer O, Diamant ZH, et al. Association of receipt of the fourth BNT162b2 dose with omicron infection and COVID-19 hospitalizations among residents of long-term care facilities. JAMA Intern Med (2022) 182:859–67. doi: 10.1001/jamainternmed.2022.2658
18. Arbel R, Sergienko R, Friger M, Peretz A, Beckenstein T, Yaron S, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med (2022) 28:1486–90. doi: 10.1038/s41591-022-01832-0
19. Link-Gelles R, Levy ME, Gaglani M, Irving SA, Stockwell M, Dascomb K, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 omicron BA.1 and BA.2/BA.2.12.1 sublineages predominated - VISION network, 10 states, December 2021-June 2022. MMWR Morb Mortal Wkly Rep (2022) 71:931–9. doi: 10.15585/mmwr.mm7129e1
20. Eliakim-Raz N, Stemmer A, Ghantous N, Ness A, Awwad M, Leibovici-Weisman Y, et al. Antibody titers after a third and fourth SARS-CoV-2 BNT162b2 vaccine dose in older adults. JAMA Netw Open (2022) 5:e2223090. doi: 10.1001/jamanetworkopen.2022.23090
21. Agrati C, Castilletti C, Goletti D, Meschi S, Sacchi A, Matusali G, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms (2021) 9:1315. doi: 10.3390/microorganisms9061315
22. Lupo-Stanghellini MT, Di Cosimo S, Costantini M, Monti S, Mantegazza R, Mantovani A, et al. mRNA-COVID19 vaccination can be considered safe and tolerable for frail patients. Front Oncol (2022) 12:855723. doi: 10.3389/fonc.2022.855723
23. Azzolini E, Pozzi C, Germagnoli L, Oresta B, Carriglio N, Calleri M, et al. mRNA COVID-19 vaccine booster fosters b- and T-cell responses in immunocompromised patients. Life Sci Alliance (2022) 5:e202201381. doi: 10.1101/2022.01.21.22269633
24. Roltgen K, Nielsen SCA, Silva O, Younes SF, Zaslavsky M, Costales C, et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell (2022) 185:1025–1040.e1014. doi: 10.1016/j.cell.2022.01.018
25. Reynolds CJ, Pade C, Gibbons JM, Otter AD, Lin KM, Munoz Sandoval D, et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science (2022) 377:eabq1841. doi: 10.1126/science.abq1841
26. Richardson SI, Madzorera VS, Spencer H, Manamela NP, van der Mescht MA, Lambson BE, et al. SARS-CoV-2 omicron triggers cross-reactive neutralization and fc effector functions in previously vaccinated, but not unvaccinated, individuals. Cell Host Microbe (2022) 30:880–886.e884. doi: 10.1016/j.chom.2022.03.029
27. Rossler A, Knabl L, von Laer D, Kimpel J. Neutralization profile after recovery from SARS-CoV-2 omicron infection. N Engl J Med (2022) 386:1764–6. doi: 10.1056/NEJMc2201607
28. Darwich A, Pozzi C, Fornasa G, Lizier M, Azzolini E, Spadoni I, et al. BNT162b2 vaccine induces antibody release in saliva: a possible role for mucosal viral protection? EMBO Mol Med (2022) 14:e15326. doi: 10.15252/emmm.202115326
29. Chaguza C, Coppi A, Earnest R, Ferguson D, Kerantzas N, Warner F, et al. Rapid emergence of SARS-CoV-2 omicron variant is associated with an infection advantage over delta in vaccinated persons. Med (N Y) (2022) 3:325–334.e324. doi: 10.1016/j.medj.2022.03.010
30. Mair MJ, Mitterer M, Gattinger P, Berger JM, Trutschnig W, Bathke AC, et al. Enhanced SARS-CoV-2 breakthrough infections in patients with hematologic and solid cancers due to omicron. Cancer Cell (2022) 40:444–6. doi: 10.1016/j.ccell.2022.04.003
31. Becker M, Cossmann A, Lurken K, Junker D, Gruber J, Juengling J, et al. Longitudinal cellular and humoral immune responses after triple BNT162b2 and fourth full-dose mRNA-1273 vaccination in haemodialysis patients. Front Immunol (2022) 13:1004045. doi: 10.3389/fimmu.2022.1004045
32. Brasu N, Elia I, Russo V, Montacchiesi G, Stabile SA, De Intinis C, et al. Memory CD8(+) T cell diversity and b cell responses correlate with protection against SARS-CoV-2 following mRNA vaccination. Nat Immunol (2022) 23:1445–56. doi: 10.1038/s41590-022-01313-z
33. Bowen JE, Addetia A, Dang HV, Stewart C, Brown JT, Sharkey WK, et al. Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines. Science (2022) 377:890–4. doi: 10.1126/science.abq0203
Keywords: SARS-CoV-2 mRNA vaccine, humoral response, T cell response, immunocompromised patients, Omicron neutralization, cross immunity
Citation: Rescigno M, Agrati C, Salvarani C, Giannarelli D, Costantini M, Mantovani A, Massafra R, Zinzani PL, Morrone A, Notari S, Matusali G, Pinter GL, Uccelli A, Ciliberto G, Baldanti F, Locatelli F, Silvestris N, Sinno V, Turola E, Lupo-Stanghellini MT, Apolone G and the VAX4FRAIL study Group (2023) Neutralizing antibodies to Omicron after the fourth SARS-CoV-2 mRNA vaccine dose in immunocompromised patients highlight the need of additional boosters. Front. Immunol. 14:1104124. doi: 10.3389/fimmu.2023.1104124
Received: 21 November 2022; Accepted: 09 January 2023;
Published: 27 January 2023.
Edited by:
Ingo Drexler, Heinrich Heine University, GermanyReviewed by:
Chao Zhang, Fifth Medical Center of the PLA General Hospital, ChinaCopyright © 2023 Rescigno, Agrati, Salvarani, Giannarelli, Costantini, Mantovani, Massafra, Zinzani, Morrone, Notari, Matusali, Pinter, Uccelli, Ciliberto, Baldanti, Locatelli, Silvestris, Sinno, Turola, Lupo-Stanghellini, Apolone and the VAX4FRAIL study Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Agrati, Y2hpYXJhLmFncmF0aUBvcGJnLm5ldA==
†These authors share first authorship
‡These authors share last authorship
§VAX4FRAIL study group: see supplementary material
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.