
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 25 January 2023
Sec. Autoimmune and Autoinflammatory Disorders: Autoinflammatory Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1103053
Parkinson’s disease (PD) is a neurodegenerative disorder that frequently occurs in the older population. Previous epidemiological studies have suggested an association between PD and autoimmune diseases (AIDs). However, some studies have shown conflicting results. This study aimed to summarize existing epidemiological studies on the association between PD with AIDs and to conduct a meta-analysis of combinable results. Four electronic databases (PubMed, Embase, Web of Science Core Collection, and MEDLINE) were searched from each database’s inception date until December 12, 2022. All studies that explored the relationship between PD and AIDs were included for quantitative analysis and qualitative review. The pooled relative risk with 95% confidence intervals (CIs) was calculated using a random or fixed effects model. A total of 46 observational studies involving 873,643 patients and 13,402,821 controls were included; ultimately, 38 studies were included in the meta-analysis. The risk of PD combined with AIDs was significantly higher (odds ratio [OR]=1.55, 95% CI: 1.33–1.81), and subgroup analysis found no significant differences in risk by study type, gender, age, and race. Regarding the AID types, the results showed an increased risk of PD combined with bullous pemphigoid (OR=2.67, 95% CI: 2.15–3.31), inflammatory bowel disease (OR=1.30, 95% CI: 1.18–1.45), Crohn’s disease (OR=1.30, 95% CI: 1.20–1.42), ulcerative colitis (OR=1.31, 95% CI: 1.14–1.50), Sjögren’s syndrome (OR=1.61, 95% CI: 1.24–2.09), and Graves’ disease (OR=1.45, 95% CI: 1.24–1.70) than controls. However, there appeared to be no significant association between PD and systemic lupus erythematosus (OR=0.82, 95% CI: 0.66–1.03), multiple sclerosis (OR=2.02, 95% CI: 0.87–4.70), rheumatoid arthritis (OR=0.79, 95% CI: 0.61–1.03), or celiac disease (OR=1.16, 95% CI: 0.79–1.69). This study supports the existence of a strong link between AIDs and PD. When PD and AIDs are identified, clinicians need to be aware of the possibility of coexistence. However, there are some limitations of this study, such as the apparent heterogeneity of some of the results and the fact that most of the included study types were retrospective. Therefore, future larger prospective cohort studies are needed to further explore the interaction between PD and AIDs.
Systematic review registration: INPLASY, identifier INPLASY202280088.
Parkinson’s disease (PD) is a common neurodegenerative movement disorder with symptoms including resting tremor, bradykinesia, rigidity, and postural disturbances, as well as many non-motor symptoms (1, 2). PD affects 1–2/1,000 people, and its prevalence increases with age (3–5). The etiology and pathogenesis of PD are complex and are currently thought to be due to a combination of genetic, environmental, and aging factors (4, 6). There is growing evidence implicating immune dysfunction in the etiology of PD, and it has even been proposed that PD may be an autoimmune disease (AID) (7–9).
Genetic and epidemiological studies have linked AIDs to PD. AIDs and PD were foaund to share a common genetic pathway, suggesting that the immune system influences the pathogenesis of PD (10, 11). Moreover, several epidemiological studies have observed an association between PD and AIDs (12–16). However, two of these large population-based studies reached different conclusions, with Rugbjerg et al. (13) reporting a risk association between 32 AIDs and PD, but not associations between AIDs and subsequent PD risk. Li et al. (12) reported a risk association between 33 AIDs and PD, which suggested an increased risk of combined PD in patients with AIDs. However, conclusions drawn for a single AID type are also inconsistent; for example, Chang et al. (14) Chen et al. (16), and Wu et al. (17) suggest an increased risk of PD in combination with Sjögren’s syndrome (SS), while Rugbjerg et al. (13) and Li et al. (12) suggest no significance.
In summary, there are shared mechanisms between PD and many AIDs, with PD often occurring in conjunction with at least one AID. However, epidemiological studies involving PD and AIDs have yielded inconsistent results, with contradictory and controversial findings. Therefore, To address these issues, we carefully searched four commonly used databases and performed a systematic review and meta-analysis of published clinical studies investigating the relationship between PD and some common AIDs. This will help us to clarify the relationship between PD and AIDs and to provide a reference for research on the pathogenesis of PD.
This study was performed in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) program. This protocol was registered in INPLASY (registration number: INPLASY202280088).
The PubMed, Embase, Web of Science Core Collection, and MEDLINE databases were searched for literature describing a link between PD and AIDs. From the day that each database was created until December 12, 2022, each database was searched. PD and AID-related free-text phrases were combined with restricted vocabulary terms that were particular to the database. To capture other potentially relevant articles, we also combined search terms representing PD with search terms for 34 AIDs (i.e., chronic inflammatory demyelinating polyneuropathy, Guillain–Barre syndrome, multiple sclerosis [MS], myasthenia gravis [MG], Addison’s disease, type 1 diabetes mellitus [T1D], Graves’ disease [GD], Hashimoto disease, autoimmune hepatitis, inflammatory bowel disease [IBD], Crohn’s disease [CD], ulcerative colitis [UC], coeliac disease [CLD], pernicious anemia [PA], primary biliary cirrhosis [PBC], alopecia areata, primary sclerosing cholangitis, antiphospholipid syndrome, autoimmune hemolytic anemia [AIHA], immune thrombocytopenic purpura, polymyositis [PM], rheumatoid arthritis [RA], polyarteritis nodosa, giant cell arteritis, bullous pemphigoid [BP], discoid lupus erythematosus, vitiligo, Behcet’s disease [BD], scleroderma, SS, systemic lupus erythematosus [SLE], dermatitis herpetiformis, narcolepsy, and rheumatic fever) that were reported to be most prevalent (i.e., with a worldwide prevalence rate of ≥10/100,000 people based on a review that included a comprehensive list of AIDs) (18). To be as comprehensive as possible, the search was not restricted to any study type. An example search strategy for the PubMed database is described in Supplementary Table 1.
Peer-reviewed publications that presented population-based research showing a link between PD and any form of AIDs were required for the study’s inclusion. Case reports and case series were not included since it was unclear how well they sampled from among those groups. To prevent duplication and incorrect weighting toward more frequently referenced or discussed articles, review papers, meta-analyses, organizational recommendations, editorial letters, and professional views were eliminated. Additionally, conference abstracts were disregarded since their full study reports could not be obtained for evaluation and their scientific rigor had not undergone peer assessment. Additionally, only research that appeared in English-language publications was considered.
The four databases’ search results yielded articles that were all imported into Endnote for review. For study inclusion, there were two rounds of screening. Two reviewers (ML and JW) independently performed screening in the first round by examining titles, abstracts, and key terms for relevance to both AIDs and PD. The entire contents of the articles found during the first round of screening were collected and carefully read to determine eligibility in the second round of screening. To establish agreement, potential disagreements throughout the study selection process were discussed with a third reviewer (ZX).
Data from included studies were extracted into a standard table, detailing authors, year of publication, country, study period, study design, selection of control and comparison groups, number of study participants, reported risk estimates for PD associated with AIDs, and any adjustments for confounding factors in producing effect estimates.
Study quality was evaluated on the Newcastle–Ottawa Scale (NOS). Studies that achieve seven or more stars on the NOS are considered high quality, while four to six stars indicate moderate quality, and less than four stars indicate poor quality.
Meta-analysis was performed for each study reporting the correlation between PD and AID. Statistical heterogeneity between studies was assessed for each outcome by examining study-specific effect size and heterogeneity (I2) statistics. I2 values of <25%, 25–50%, 51–75%, and >75% were considered to denote no, mild, moderate, and large heterogeneity, respectively (19). In meta-analyses of multiple studies for a specific outcome, a fixed-effect estimate was calculated if the I2 value was <50%; a random-effect estimate was calculated if the I2 value was ≥50%. Forest plots were constructed to present risk estimates and meta-analysis results for each AID reported by at least three studies to be associated with PD. In meta-analyses of cohort studies, the hazard ratio (HR) and standardized incidence ratio (SIR) were treated as being equal to the odds ratio (OR). Publication bias was assessed by observing the symmetry of the funnel plot and performing Egger’s and Begg’s tests.
Statistical analysis was performed using STATA software (Version 16.0, StataCorp, College Station, TX, USA). Statistical significance was set at P<0.05.
In the original search, 4,535 results from MEDLINE, 4,237 from PubMed, 5,676 from the Web of Science Core Collection, and 6,296 from Embase were found. The four databases’ search results yielded articles that were all imported into Endnote for screening. There were 10,379 papers uploaded to Endnote for first-stage screening following the removal of duplicates. Following the initial screening of publications by looking at their titles, abstracts, and key words relating to PD or AIDs, 10,058 publications were disqualified for not addressing both PD and AIDs, and 321 papers were found to be potentially relevant and subsequently evaluated for eligibility. After the first round, 275 articles were excluded for being a review article or meta-analysis (n=100), not having a control group (n=7), being a comment (n=12), a case report (n=129), or a meeting (n=27). Finally, 46 articles met all the inclusion criteria and were included in this review, 38 of which were included for quantitative synthesis for having calculable risk estimates (Figure 1).
Table 1 shows a detailed description of the key characteristics of the 46 included studies. In brief, there were 16 national studies; 18 (39.1%) were conducted in European populations, 5 (10.9%) were conducted in South America, and 23 (50.0%) were conducted in Asia. In terms of study design, 4 (8.5%) were cross-sectional studies, 18 (38.3%) were case-control studies, and 25 (53.2%) were cohort (including 8 prospective cohort and 17 retrospective cohort) studies, with the study by Brick et al. (29) reporting both case-control and retrospective cohort studies.
The search identified clinical studies with 17 AIDs and PD. A total of 14,276,464 individuals, 873,643 patients, and 13,402,821 controls were included in the studies, including 752,488 patients with AIDs and 121,155 with PD. For the reported AIDs, Rugbjerg et al. (13) conducted a national case-control study in Denmark and reported 32 AIDs before diagnosis in 13,695 patients with PD. Li et al. (12) conducted a national epidemiological study in Sweden and reported a total of 33 AIDs in 310,522 patients, with 932 subsequently presenting with PD. In addition, 17 (37.0%) studies reported on BP, 12 (26.1%) on IBDs (including UC and CD), 7 (15.2%) on SS, 7 (15.2%) on RA, 4 (8.7%) on SLE, 5 (10.9%) on MS, 3 (6.5%) on CLD, 3 (6.5%) on GD, 2 (4.3%) on T1D, 2 (4.3%) on BD, 2 (4.3%) on MG, 2 (4.3%) on PM, 2 (4.3%) on scleroderma, 2 (4.3%) on Addison’s disease, 2 (4.3%) on AIHA, 2 (4.3%) on PA, and 2 (4.3%) on PBC. Six studies included patients with PD as the test group, and 39 used patients with AIDs as the test group. Most studies matched 1–10 controls per case based on age and sex. Table 2 lists the studies included in the meta-analysis that included the correlation between PD and AIDs.
The number of clinical studies on T1D, BD, MG, PM, scleroderma, Addison’s disease, AIHA, PA, and PBC with PD was <3; therefore, those diseases were not included in the meta-analysis. Supplementary Table 2 lists the studies that were not included in the meta-analysis. Among them, results from Klimek et al.’s study (32) suggested a significantly higher risk of PD combined with T1D (relative risk [RR]=2.30, 95% CI: 1.90–2.70). Results from Park et al.’s study (49) suggested a significantly higher risk of PD combined with BD of 2.47 (1.65–3.68). Rugbjerg et al. (13) and Li et al. (12) included studies of multiple AIDs and risk of PD; however, a meta-analysis was not performed for some of these diseases due to the small number of studies that could corroborate any associated findings.
Both Hsu et al.’s (20) and Lin et al.’s (34) studies are reports on IBD with patients originating from the same database; therefore, the study with the larger sample size (i.e., Lin et al.’s study) was selected, excluding that by Hsu et al. (20). Studies by Ju et al. (46), Hsu et al. (51), and Chang et al. (14) all originate from a study on SS, with patients from the same database. The study with the larger sample size (i.e., Chang et al.’s study) was selected synthetically, excluding those by Ju et al. (46) and Hsu et al. (51). Studies by Nielsen et al. (30) and Thormann et al. (36) are Danish reports on MS with patients derived from the same database; because of which, Thormann et al.’s study (36) was selected as it included more complete information, excluding Nielsen et al.’s report (30). A retrospective cohort study in South Korea by Noh et al. (52) compared conventional treatment and combination treatment for IBD and found a reduced risk of PD in the combined treatment group without normal controls, because of which it was excluded. Paakinaho et al.’s study (23) examined the association between RA and PD by improving anti-rheumatic drugs, which did not match the data of this study; thus, it was excluded from the meta-analysis. Thirty-seven studies were eventually included in the meta-analysis.
The NOS scores of the included studies ranged from 6 to 9, indicating a high level of overall quality. The studies had clear definitions of exposure and outcome, appropriate adjustment for confounders, and sufficiently long follow-up (Supplementary Table 3).
The results of the meta-analysis of 17 case-control, 4 cross-sectional, and 18 cohort studies showed a significantly higher overall risk of PD with AIDs (OR=1.55, 95% CI: 1.33–1.81, P=0.000, I2 = 95.1%, large heterogeneity), with case-control (OR=1.83, 95% CI: 1.24–2.69, P=0.000, I2 = 96.5%, large heterogeneity), cohort (OR=1.42, 95% CI: 1.22–1.65, P=0.000, I2 = 92.7%, large heterogeneity) and cross-sectional studies (OR= 1.72, 95% CI: 1.06–2.79, P=0.000, I2 = 90.2%, large heterogeneity) all suggesting an increased risk of PD with AIDs (Figure 2). The findings suggested high heterogeneity, and a subgroup analysis was performed to find the source of heterogeneity. However, we found no significant differences in risk by study type, gender, age, race, and study design (Table 3). Therefore, we performed separate analyses to determine the relationship between different types of AID and PD.
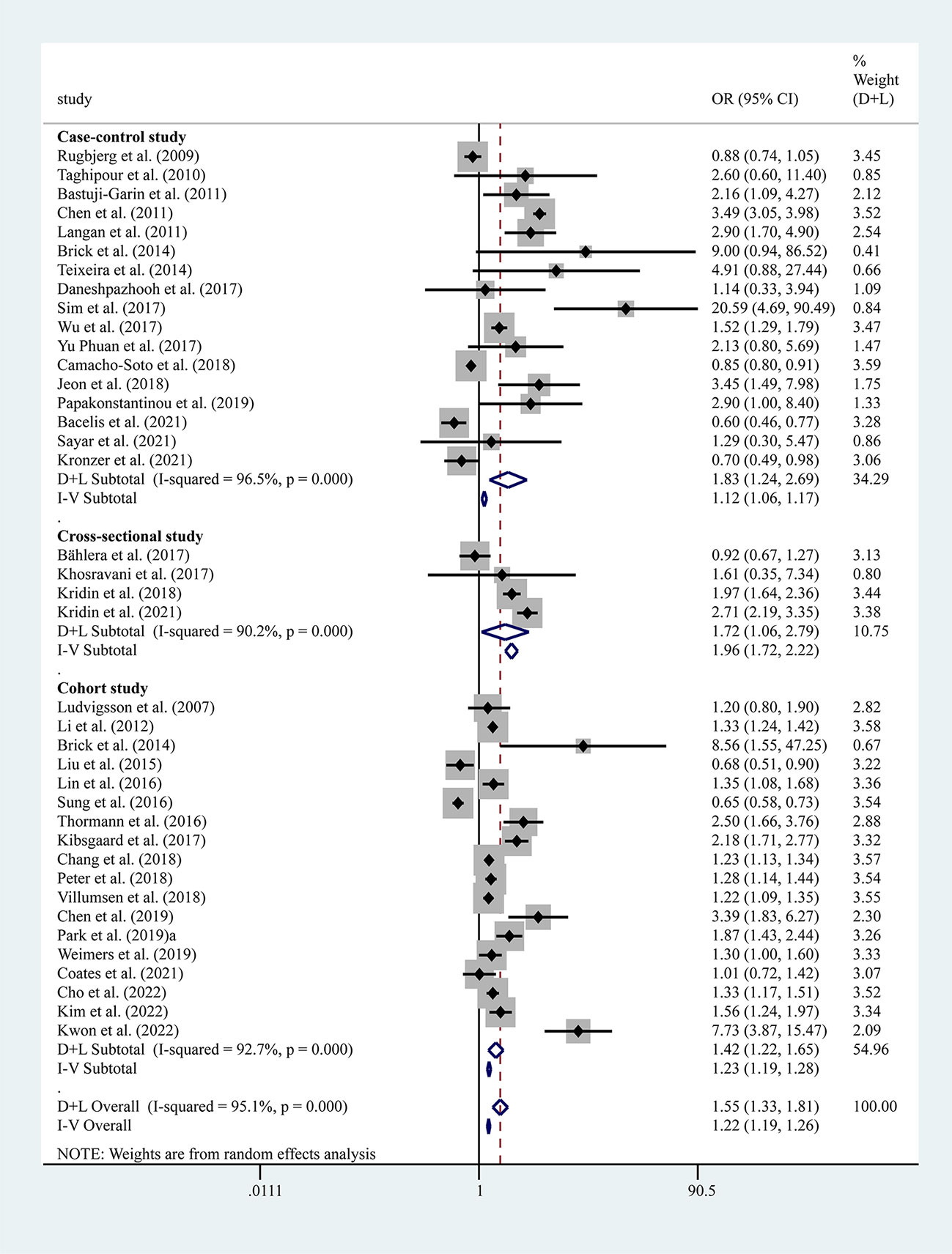
Figure 2 Forest plots of studies association between PD and AIDs. The size of the square is proportional to study-specific statistical weights, horizontal lines represent 95% confidence interval and diamonds represent summary measures of association.
Seventeen studies reported the risk between BP and PD, and the results showed a significantly higher risk for PD combined with BP (OR=2.67, 95% CI: 2.15–3.31, P=0.000, I2 = 63.3%, moderate heterogeneity). According to the grouping of study types, the results showed no heterogeneity in the effect values of the 12 case-control studies combined (OR=3.36, 95% CI: 2.98–3.79, P=0.206, I2 = 24.4%) (Figure 3).
Ten studies reported the risk between IBD and PD and showed a significantly higher risk of PD combined with IBD (OR=1.24, 95% CI: 1.04–1.47, P=0.001, I2 = 91.0%), with large heterogeneity. However, when Camacho-Soto et al.’s study (21) was removed from this analysis, heterogeneity was significantly lower after combining effect values and the association between PD and IBD was stronger. We therefore included nine studies, excluding Camacho-Soto et al.’s report (21), which showed a significantly higher risk of PD with IBD (OR=1.30, 95% CI: 1.18–1.45, P=0.024, I2 = 54.6, moderate heterogeneity). Subtypes of IBD, UC (OR=1.31, 95% CI: 1.14–1.50, P=0.035, I2 = 51.7%, moderate heterogeneity), and CD (OR=1.30, 95% CI: 1.20–1.42, P=0.220, I2 = 25.2%, mild heterogeneity) were also associated with a significantly higher risk of PD (Figure 4).
Five studies reported the risk between SS and PD, and the results showed an increased risk of PD combined with SS (OR=1.61, 95% CI: 1.24–2.09, P=0.033, I2 = 61.9%, moderate heterogeneity) (Figure 5). However, sensitivity analysis showed fewer stable results, and two studies, i.e., those by Chang et al. (14) and Wu et al. (17), would have a greater impact on the results (Supplementary Table 4, Supplementary Figure 1F). Three studies reported the risk between GD and PD, and the results showed an increased risk of PD combined with GD (OR=1.45, 95% CI: 1.24–1.70, P=0.136, I2 = 50.0%, mild heterogeneity) (Figure 5).
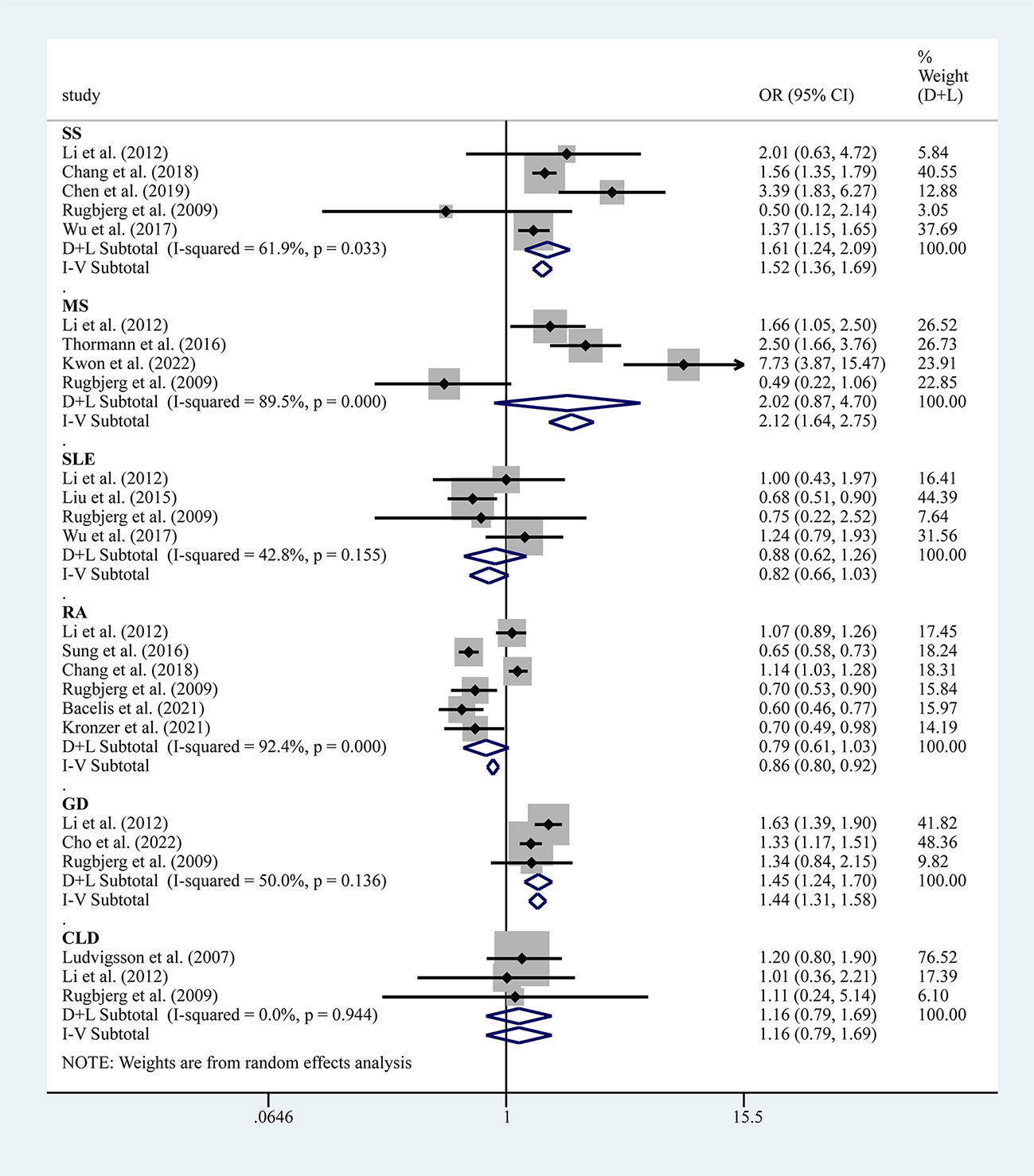
Figure 5 Forest plots of studies association between PD and AIDs (including SS, SLE, MS, RA, GD and CLD).
Six studies reported the risk between RA and PD, and the results showed that the risk of PD combined with RA was not significant (OR=0.79, 95% CI: 0.61–1.03, P=0.000, I2 = 92.4%, large heterogeneity) (Figure 5). Notably, after removing this study by Chang et al. (14) from the sensitivity analysis, the combined effect values became meaningful, showing a negative association between PD and RA (Supplementary Figure 1I).
Four studies reported the risk between SLE and PD, and the results showed that the risk for PD combined with SLE was not significant (OR=0.82, 95% CI: 0.66–1.03, P=0.155, I2 = 42.8%, large heterogeneity) (Figure 5). Four studies reported the risk between MS and PD, and the results showed that the risk of PD combined with MS was not significant (OR=2.02, 95% CI: 0.87–4.70, P=0.001, I2 = 89.5%, large heterogeneity) (Figure 5). Three studies reported the risk between CLD and PD and showed that the risk for PD combined with CLD was not significant (OR=1.16, 95% CI: 0.79–1.69, P=0.944, I2 = 0.0%, no heterogeneity) (Figure 5).
The P-values for both Begg’s and Egger’s tests were >0.05, indicating a low likelihood of potential publication bias (Supplementary Table 5).
Sensitivity analyses removed each included study individually and performed a pooled analysis of the remaining studies to assess whether the individual included reports had a greater impact on the results of the overall meta-analysis. Of the several analyses with positive results, the remaining studies analyzed did not have a disproportionate effect on the results of the meta-analysis, except for the less stable results of SS, indicating that the results of the remaining studies were stable and reliable (Supplementary Table 4, Supplementary Figure 1).
In this meta-analysis of 38 population-based cohort, case-control, and cross-sectional studies, PD may be associated with multiple AIDs, including BP, IBD, CD, UC, SS, and GD, but may not be associated with MS, SLE, RA, and CLD. To the best of our knowledge, this study is the first to comprehensively synthesize the available population-based research evidence on the relationship between PD and AIDs.
Our study benefited from a comprehensive search strategy that included 34 common AIDs, essentially encompassing the majority of reported clinical studies on the relationship between AIDs and PD. The final pooled inclusion of more than 10 million subjects from different geographic regions, including clinical studies in 16 countries with populations in Asia, Europe, and North America, provides reliable evidence of the relationship between AIDs and PD from large-scale subject data. However, it is difficult to distinguish the sequence of development of PD and AIDs in multiple studies, and this study only analyzed the risk of PD combined with AIDs to demonstrate whether there is a correlation between the two, not to determine the causal relationship.
The increased risk of PD combined with AIDs may have a similar pathogenesis. Indeed, there is growing evidence that immune dysfunction is involved in the pathogenesis of PD (7, 60). Some studies have found that an aberrant immune response may start years before the diagnosis of PD (61). Sustained inflammatory response, T-cell infiltration, and glial cell activation play a crucial role in the degeneration of dopaminergic neurons (62, 63). Current experimental and genetic studies linking AIDs to PD have found a role for intestinal microflora, immune response, and genetic variants, although the mechanisms between PD and AIDs remain unclear (9, 10, 60, 63). In the following paragraphs we will discuss each of the several AIDs associated with PD.
A meta-analysis summarizing the association of BP with neurological disorders included eight studies on PD with BP, and the results suggested that patients with BP were more than three times more likely to have PD (RR=3.42, 95% CI: 3.01-3.87) (64). In contrast, our study included 17 papers on BP and PD, and the results suggested a 2.67-fold risk for BP combined with PD. Some studies have shown that BP is also associated with other neurological disorders, such as dementia, MS, epilepsy, stroke, and schizophrenia (47, 54, 64). Studies have shown that human skin and the brain contain BP180 antigen and BP230 antigen, and the mechanism may be that neurological disorders expose antigens such as BP180 and BP230 to the immune system and trigger a subsequent immune response that leads to the manifestation of BP (65, 66). Studies have shown that circulating IgG autoantibodies against BP180 are found in patients with Parkinson’s disease, but their significance for the development of BP is currently unknown, as these anti-BP180 antibodies neither bind to the basement membrane of the skin nor cause BP-like symptoms (67)..
Several clinical studies have shown that IBD, including CD and UC, is associated with an increased risk of PD (34, 45, 48), and a meta-analysis also showed an increased risk of IBD combined with PD (RR=1.24, 95% CI: 1.15-1.34) (63), which is consistent with our results. Gastrointestinal inflammation and neuroinflammation may be important causes of PD due to disorders of the gut-brain axis (68). It is believed that gastrointestinal inflammation promotes misfolding of alpha-synuclein, leading to its aggregation and prion-like propagation in the brain (69, 70). While IBD is a typical gastrointestinal disorder, pro-inflammatory immune response and homeostatic imbalance in the gut have an important role in the pathogenic process of IBD. Therefore, IBD may be involved in the disruption of the gut-brain axis through mechanisms, such as intestinal inflammation. The gut-brain axis may be an important link between PD and IBD. It has also been demonstrated that IBD and PD share common genetic risk profiles, such as CARD15, LRRK2, HLA, and MAPT genes (71, 72). Notably, a two-sample Mendelian randomization study on IBD and PD genetically predicted that neither IBD nor its subtypes CD and UC were associated with an increased risk of PD (73), although another Mendelian randomization study confirmed a causal relationship between PD and IBD (74).
The increased risk of SS combined with PD may be due to the role played by autoantibodies. We know that SS is caused by an immune-mediated mechanism, and autoantibodies (e.g., anti-SSA and anti-SSB), which are associated with central nervous system disorders, are commonly detected in patients with SS (75). The immune-mediated mechanism between SS and PD is postulated, and antibodies in patients with SS may damage the basal ganglia and cause PD. In patients with SS combined with PD, serum anti-β2 glycoprotein antibodies are strongly positive, and it is postulated that this antibody binds to antigen to form an immune complex that is deposited in the vessel wall, causing vasculitis and the clinical manifestations of PD (76).
Studies have shown that thyroid dysfunction (e.g., hyperthyroidism and hypothyroidism) can increase the risk of PD (77). Thyroid dysfunction affects oxidative stress, which contributes significantly to the loss of dopamine neurons and the progression of PD (78, 79). And it is possible that GD combined with PD may also be a result of the presence of common risk factors, such as vitamin D deficiency (80, 81).
In our study, the risk of PD combined with RA was not statistically significant. In contrast, another meta-analysis suggested a negative correlation between RA and PD (RR=0.74, 95% CI: 0.56–0.98) (82). A Mendelian randomization study also supported the protective effect of RA on PD (83). Several clinical studies have suggested a protective mechanism of RA against PD (22, 35, 55). Drugs commonly used by patients with RA are non-steroidal anti-inflammatory drugs (NSAIDs) and immunosuppressive drugs, and epidemiological studies suggest that regular use of anti-inflammatory drugs may be associated with a reduced risk of PD (84, 85). Therefore, it is also necessary to exclude the interference of NSAIDs when studying the correlation between PD and RA.
We did not find an association between PD and SLE, MS, or CLD. Notably, all three clinical studies of CLD included suggested no statistically significant association between PD and CLD. Nine AIDs, T1D, BD, MG, PM, scleroderma, Addison’s disease, AIHA, PA, and PBC, were also retrieved in this study for their association with PD but were not included in the meta-analysis due to an insufficient number of clinical studies. Clinical studies on these diseases also suggest that most are not statistically significantly associated with PD.
The sequential order of disease progression in PD and AIDs and potential causal relationship were difficult to determine in this study. In cross-sectional and case-control studies, the temporal relationship between the two conditions could not be resolved because the timing of diagnosis of the included study diseases was unclear. Moreover, alpha-synuclein can be detected in the gut and olfactory epithelium of PD patients many years ago, and this underlying pathological process takes place over about 20 years, with a significant loss of dopamine neurons in the brain by the time overt motor symptoms appear (86, 87). In addition, some AIDs such as SLE and RA may also go undetected for many years (88, 89), making it challenging to determine the exact time of disease onset to determine the sequence between the two. Most of the studies included in this study were retrospective observational studies with information derived from questionnaires, disease databases, or data from inpatient registries, and differences in diagnostic criteria for PD or AIDs may have biased the results of this meta-analysis. Most of the included studies were adjusted only for age and sex confounders, although they ignored other confounders such as potential risk factors for PD (tobacco, coffee, NSAIDs, and comorbidities, among others), which may limit the prevalence of AIDs with PD. Another limitation of this study is the moderate to high heterogeneity in most of the results, which may be attributed to differences in the study area, sample source, and study design, and we attempted to address the large heterogeneity by using a random effects model. In addition, some of the AIDs included in this study have fewer reported clinical studies and the association of these disorders with PD cannot be well assessed. In conclusion, due to some of the aforementioned limitations of this study, the final results obtained may not be very adequate.
To assess the relationship more accurately between PD and AIDs, we suggest that a prospective cohort study approach be used in the future. More accurate evidence of the relationship between PD and AIDs can be obtained by following up long enough to include a large sample size of the study population, using standardized hospital-based records of disease diagnosis, carefully selecting normal controls, and adjusting for potential confounders. Mechanistic studies on PD and AID comorbidity can improve our understanding of the pathogenesis of both diseases and, if a common pathogenic origin can be observed, help identify new therapeutic and diagnostic targets.
This meta-analysis provides evidence that patients with PD have a significantly increased risk for comorbid AIDs. However, for different AIDs, the OR varied widely, with BP showing the strongest association. Clinicians need to be aware of the potential coexistence of PD and AIDs when they are diagnosed. Further studies are needed to explore the potential molecular mechanisms underlying the interaction between PD and AIDs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
ML and BT are responsible for the study concept and design; ML, JW, and ZX are responsible for the data collection, data analysis, and interpretation; ML drafted the paper; BT supervised the study. All authors contributed to the article and approved the submitted version.
This work was supported by the Hunan Innovative Province Construction Project [2019SK2335]; Hunan Provincial Health and Health Commission 2022 annual research project topics [202203074319]; Clinical Research 4310 Program of the First Affiliated Hospital of The University of South China [20214310NHYCG08].
We would like to thank the authors of the literature included in this meta-analysis; the data you contributed are the basis of this paper. We would also like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1103053/full#supplementary-material
AID, autoimmune disease; AIHA, autoimmune hemolytic anemia; BD, Behcet’s disease; BP, bullous pemphigoid; CD, Crohn’s disease; CI, confidence interval; CLD, coeliac disease; GD, Graves disease; HR, hazard ratio; IBD, inflammatory bowel disease; MG, myasthenia gravis; MS, multiple sclerosis; NOS, Newcastle-Ottawa scale; NSAIDs, non-steroidal anti-inflammatory drugs; OR, odds ratio; PA, pernicious anemia; PBC, primary biliary cirrhosis; PD, Parkinson’s disease; PM, polymyositis; RA, rheumatoid arthritis; SIR, standardized incidence ratio; SLE, systemic lupus erythematosus; SS, Sjögren’s syndrome; T1D, type 1 diabetes mellitus; UC, ulcerative colitis.
1. Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet (2021) 397:2284–303. doi: 10.1016/S0140-6736(21)00218-X
2. Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of parkinson's disease. Lancet Neurol (2021) 20:385–97. doi: 10.1016/S1474-4422(21)00030-2
3. Simon DK, Tanner CM, Brundin P. Parkinson Disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med (2020) 36:1–12. doi: 10.1016/j.cger.2019.08.002
4. Ascherio A, Schwarzschild MA. The epidemiology of parkinson's disease: risk factors and prevention. Lancet Neurol (2016) 15:1255–70. doi: 10.1016/S1474-4422(16)30230-7
5. Tysnes OB, Storstein A. Epidemiology of parkinson's disease. J Neural Transm (2017) 124:901–5. doi: 10.1007/s00702-017-1686-y
6. Jankovic J, Tan EK. Parkinson's disease: etiopathogenesis and treatment. J Neurol Neurosur Ps (2020) 91:795–808. doi: 10.1136/jnnp-2019-322338
7. Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol (2022) 22:657–73. doi: 10.1038/s41577-022-00684-6
8. Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, et al. T Cells from patients with parkinson's disease recognize alpha-synuclein peptides. Nature (2017) 546:656–61. doi: 10.1038/nature22815
9. Bonam SR, Muller S. Parkinson's disease is an autoimmune disease: A reappraisal. Autoimmun Rev (2020) 19:102684. doi: 10.1016/j.autrev.2020.102684
10. Witoelar A, Jansen IE, Wang YP, Desikan RS, Gibbs JR, Blauwendraat C, et al. Genome-wide pleiotropy between Parkinson disease and autoimmune diseases. JAMA Neurol (2017) 74:780–92. doi: 10.1001/jamaneurol.2017.0469
11. Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, Buchel F, et al. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to parkinson's disease. Hum Mol Genet (2014) 23:562. doi: 10.1093/hmg/ddt554
12. Li XJ, Sundquist J, Sundquist K. Subsequent risks of Parkinson disease in patients with autoimmune and related disorders: a nationwide epidemiological study from Sweden. Neurodegener Dis (2012) 10:277–84. doi: 10.1159/000333222
13. Rugbjerg K, Friis S, Ritz B, Schernhammer ES, Korbo L, Olsen JH. Autoimmune disease and risk for Parkinson disease a population-based case-control study. Neurology (2009) 73:1462–8. doi: 10.1212/WNL.0b013e3181c06635
14. Chang CC, Lin TM, Chang YS, Chen WS, Sheu JJ, Chen YH, et al. Autoimmune rheumatic diseases and the risk of Parkinson disease: a nationwide population-based cohort study in Taiwan. Ann Med (2018) 50:83–90. doi: 10.1080/07853890.2017.1412088
15. Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of parkinson's disease: a Danish nationwide cohort study 1977-2014. Gut (2019) 68:18–24. doi: 10.1136/gutjnl-2017-315666
16. Chen HH, Perng WT, Chiou JY, Wang YH, Huang JY, Wei JCC. Risk of dementia among patients with sjogren's syndrome: A nationwide population-based cohort study in Taiwan. Semin Arthritis Rheu (2019) 48:895–9. doi: 10.1016/j.semarthrit.2018.06.007
17. Wu MC, Xu X, Chen SM, Tyan YS, Chiou JY, Wang YH, et al. Impact of sjogren's syndrome on parkinson's disease: A nationwide case-control study. PloS One (2017) 12(7):e0175836. doi: 10.1371/journal.pone.0175836
18. Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev (2012) 11:754–65. doi: 10.1016/j.autrev.2012.02.001
19. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Brit Med J (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20. Hsu YT, Liao CC, Chang SN, Yang YW, Tsai CH, Chen TL, et al. Increased risk of depression in patients with Parkinson disease: a nationwide cohort study. Am J Geriat Psychiat (2015) 23:934–40. doi: 10.1016/j.jagp.2014.10.011
21. Camacho-Soto A, Nielsen SS, Racette BA. Inflammatory bowel disease and risk of parkinson's disease in medicare beneficiaries. Parkinsonism Relat D (2018) 50:23–8. doi: 10.1016/j.parkreldis.2018.02.008
22. Bacelis J, Compagno M, George S, Pospisilik JA, Brundin P, Naluai AT, et al. Decreased risk of parkinson's disease after rheumatoid arthritis diagnosis: a nested case-control study with matched cases and controls. J Parkinson Dis (2021) 11:821–32. doi: 10.3233/JPD-202418
23. Paakinaho A, Koponen M, Tiihonen M, Kauppi M, Hartikainen S, Tolppanen AM. Disease-modifying antirheumatic drugs and risk of Parkinson disease nested case-control study of people with rheumatoid arthritis. Neurology (2022) 98:E1273–81. doi: 10.1212/WNL.0000000000013303
24. Ludvigsson JF, Olsson T, Ekbom A, Montgomery SM. A population-based study of coeliac disease, neurodegenerative and neuroinflammatory diseases. Aliment Pharm Therap (2007) 25:1317–27. doi: 10.1111/j.1365-2036.2007.03329.x
25. Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia a case-control study. Arch Dermatol (2010) 146:1251–4. doi: 10.1001/archdermatol.2010.322
26. Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol (2011) 131:637–43. doi: 10.1038/jid.2010.301
27. Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: a nationwide population-based study. Brit J Dermatol (2011) 165:593–9. doi: 10.1111/j.1365-2133.2011.10386.x
28. Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case-control study. J Invest Dermatol (2011) 131:631–6. doi: 10.1038/jid.2010.357
29. Brick KE, Weaver CH, Savica R, Lohse CM, Pittelkow MR, Boeve BF, et al. A population-based study of the association between bullous pemphigoid and neurologic disorders. J Am Acad Dermatol (2014) 71:1191–7. doi: 10.1016/j.jaad.2014.07.052
30. Nielsen NM, Pasternak B, Stenager E, Koch-Henriksen N, Frisch M. Multiple sclerosis and risk of parkinson's disease: a Danish nationwide cohort study. Eur J Neurol (2014) 21:107–11. doi: 10.1111/ene.12255
31. Teixeira VB, Cabral R, Brites MM, Vieira R, Figueiredo A. Bullous pemphigoid and comorbidities: a case-control study in Portuguese patients. Bras Dermatol (2014) 89:274–8. doi: 10.1590/abd1806-4841.20142516
32. Klimek P, Kautzky-Willer A, Chmiel A, Schiller-Fruhwirth I, Thurner S. Quantification of diabetes comorbidity risks across life using nation-wide big claims data. PloS Comput Biol (2015) 11:e1004125. doi: 10.1371/journal.pcbi.1004125
33. Liu FC, Huang WY, Lin TY, Shen CH, Chou YC, Lin CL, et al. Inverse association of Parkinson disease with systemic lupus erythematosus a nationwide population-based study. Medicine (2015) 94:e2097. doi: 10.1097/MD.0000000000002097
34. Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH. Association between parkinson's disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflammation Bowel Dis (2016) 22:1049–55. doi: 10.1097/MIB.0000000000000735
35. Sung YF, Liu FC, Lin CC, Lee JT, Yang FC, Chou YC, et al. Reduced risk of Parkinson disease in patients with rheumatoid arthritis: a nationwide population-based study. Mayo Clin Proc (2016) 91:1346–53. doi: 10.1016/j.mayocp.2016.06.023
36. Thormann A, Koch-Henriksen N, Laursen B, Sorensen PS, Magyari M. Inverse comorbidity in multiple sclerosis: Findings in a complete nationwide cohort. Mult Scler Relat Dis (2016) 10:181–6. doi: 10.1016/j.msard.2016.10.008
37. Bahler C, Schoepfer AM, Vavricka SR, Brungger B, Reich O. Chronic comorbidities associated with inflammatory bowel disease: prevalence and impact on healthcare costs in Switzerland. Eur J Gastroen Hepat (2017) 29:916–25. doi: 10.1097/MEG.0000000000000891
38. Daneshpazhooh M, Khorassani J, Balighi K, Ghandi N, Mahmoudi H, Tohidinik HR. Neurological diseases and bullous pemphigoid: A case-control study in Iranian patients. Indian J Dermatol Ve (2017) 83:195–9. doi: 10.4103/0378-6323.191132
39. Khosravani S, Handjani F, Alimohammadi R, Saki N. Frequency of neurological disorders in bullous pemphigoid patients: a cross-sectional study. Int Sch Res Notices (2017) 2017:6053267. doi: 10.1155/2017/6053267
40. Kibsgaard L, Rasmussen M, Lamberg A, Deleuran M, Olesen AB, Vestergaard C. Increased frequency of multiple sclerosis among patients with bullous pemphigoid: a population-based cohort study on comorbidities anchored around the diagnosis of bullous pemphigoid. Brit J Dermatol (2017) 176:1486–91. doi: 10.1111/bjd.15405
41. Sim B, Fook-Chong S, Phoon YW, Koh HY, Thirumoorthy T, Pang SM, et al. Multimorbidity in bullous pemphigoid: a case-control analysis of bullous pemphigoid patients with age- and gender-matched controls. J Eur Acad Dermatol (2017) 31:1709–14. doi: 10.1111/jdv.14312
42. Phuan CZY, Yew YW, Tey HL. Bullous pemphigoid and antecedent neurological diseases: An association with dementia. Indian J Dermatol Ve (2017) 83:457–61. doi: 10.4103/0378-6323.198451
43. Jeon HW, Yun SJ, Lee SC, Won YH, Lee JB. Mortality and comorbidity profiles of patients with bullous pemphigoid in Korea. Ann Dermatol (2018) 30:13–9. doi: 10.5021/ad.2018.30.1.13
44. Kridin K, Zelber-Sagi S, Comaneshter D, Cohen AD. Association between pemphigus and neurologic diseases. JAMA Dermatol (2018) 154:281–5. doi: 10.1001/jamadermatol.2017.5799
45. Peter I, Dubinsky M, Bressman S, Park A, Lu CY, Chen NJ, et al. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol (2018) 75:939–46. doi: 10.1001/jamaneurol.2018.0605
46. Ju UH, Liu FC, Lin CS, Huang WY, Lin TY, Shen CH, et al. Risk of Parkinson disease in sjogren syndrome administered ineffective immunosuppressant therapies a nationwide population-based study. Medicine (2019) 98(14):e14984. doi: 10.1097/MD.0000000000014984
47. Papakonstantinou E, Limberg MM, Gehring M, Kotnik N, Kapp A, Gibbs BF, et al. Neurological disorders are associated with bullous pemphigoid. J Eur Acad Dermatol (2019) 33:925–9. doi: 10.1111/jdv.15444
48. Park S, Kim J, Chun J, Han K, Soh H, Kang EA, et al. Patients with inflammatory bowel disease are at an increased risk of parkinson's disease: a south Korean nationwide population-based study. J Clin Med (2019) 8:1191. doi: 10.3390/jcm8081191
49. Park HY, Lee JH, Lee SY, Yu DS, Han KD, Park YG, et al. Risk for parkinson's disease in patients with behcet's disease: a nationwide population-based dynamic cohort study in Korea. J Parkinson Dis (2019) 9:583–9. doi: 10.3233/JPD-191622
50. Weimers P, Halfvarson J, Sachs MC, Saunders-Pullman R, Ludvigsson JF, Peter I, et al. Inflammatory bowel disease and parkinson's disease: a nationwide swedish cohort study. Inflammation Bowel Dis (2019) 25:111–23. doi: 10.1093/ibd/izy190
51. Hsu HC, Hou TY, Lin TM, Chang YS, Chen WS, Kuo PI, et al. Higher risk of Parkinson disease in patients with primary sjogren's syndrome. Clin Rheumatol (2020) 39:2999–3007. doi: 10.1007/s10067-020-05053-z
52. Noh H, Jang J, Kwon S, Cho SY, Jung WS, Moon SK, et al. The impact of Korean medicine treatment on the incidence of parkinson's disease in patients with inflammatory bowel disease: a nationwide population-based cohort study in south Korea. J Clin Med (2020) 9:2422. doi: 10.3390/jcm9082422
53. Coates MD, Ba DM, Liu GD, Dalessio S, Leslie DL, Huang XM. Revisiting the association between inflammatory bowel disease and parkinson's disease. Inflammation Bowel Dis (2022) 28:850–4. doi: 10.1093/ibd/izab175
54. Kridin K, Hubner F, Recke A, Linder R, Schmidt E. The burden of neurological comorbidities in six autoimmune bullous diseases: a population-based study. J Eur Acad Dermatol (2021) 35:2074–8. doi: 10.1111/jdv.17465
55. Kronzer VL, Crowson CS, Sparks JA, Myasoedova E, Davis J. Family history of rheumatic, autoimmune, and nonautoimmune diseases and risk of rheumatoid arthritis. Arthrit Care Res (2021) 73:180–7. doi: 10.1002/acr.24115
56. Sayar SK, Sun GP, Kucukoglu R. Comorbidities of bullous pemphigoid: A single-center retrospective case-control study from Turkey. Dermatol Ther (2021) 34:e15031. doi: 10.1111/dth.15031
57. Cho YY, Kim B, Shin DW, Youn J, Mok JO, Kim CH, et al. Graves' disease and the risk of parkinson's disease: a Korean population-based study. Brain Commun (2022) 4:fcac014. doi: 10.1093/braincomms/fcac014
58. Kim GH, Lee YC, Kim TJ, Kim ER, Hong SN, Chang DK, et al. Risk of neurodegenerative diseases in patients with inflammatory bowel disease: A nationwide population-based cohort study. J Crohns Colitis (2022) 16:436–43. doi: 10.1093/ecco-jcc/jjab162
59. Kwon S, Jung SY, Han KD, Jung JH, Yeo Y, Cho EB, et al. Risk of parkinson's disease in multiple sclerosis and neuromyelitis optica spectrum disorder: a nationwide cohort study in south Korea. J Neurol Neurosurg Psychiatry (2022) jnnp-2022-329389. doi: 10.1136/jnnp-2022-329389
60. Tan EK, Chao YX, West A, Chan LL, Poewe W, Jankovic J. Parkinson Disease and the immune system - associations, mechanisms and therapeutics. Nat Rev Neurol (2020) 16:303–18. doi: 10.1038/s41582-020-0344-4
61. Arlehamn CSL, Dhanwani R, Pham J, Kuan R, Frazier A, Dutra JR, et al. Alpha-synuclein-specific T cell reactivity is associated with preclinical and early parkinson's disease. Nat Commun (2020) 11:1–11. doi: 10.1038/s41467-020-15626-w
62. Wang QQ, Liu YJ, Zhou JW. Neuroinflammation in parkinson's disease and its potential as therapeutic target. Transl Neurodegener (2015) 4:1–9. doi: 10.1186/s40035-015-0042-0
63. Zhu Y, Yuan M, Liu Y, Yang F, Chen WZ, Xu ZZ, et al. Association between inflammatory bowel diseases and parkinson's disease: systematic review and meta-analysis. Neural Regener Res (2022) 17:344–53. doi: 10.4103/1673-5374.317981
64. Lai YC, Yew YW, Lambert WC. Bullous pemphigoid and its association with neurological diseases: a systematic review and meta-analysis. J Eur Acad Dermatol (2016) 30:2007–15. doi: 10.1111/jdv.13660
65. Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid. Front Immunol (2019) 10:1238. doi: 10.3389/fimmu.2019.01238
66. Forsti AK, Huilaja L, Schmidt E, Tasanen K. Neurological and psychiatric associations in bullous pemphigoid-more than skin deep? Exp Dermatol (2017) 26:1228–34. doi: 10.1111/exd.13401
67. Messingham KAN, Aust S, Helfenberger J, Parker KL, Schultz S, McKillip J, et al. Autoantibodies to collagen XVII are present in parkinson's disease and localize to tyrosine-hydroxylase positive neurons. J Invest Dermatol (2016) 136:721–3. doi: 10.1016/j.jid.2015.12.005
68. Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson's disease. Cell (2016) 167:1469–80 e12. doi: 10.1016/j.cell.2016.11.018
69. Dogra N, Mani RJ, Katare DP. The gut-brain axis: Two ways signaling in parkinson's disease. Cell Mol Neurobiol (2022) 42:315–32. doi: 10.1007/s10571-021-01066-7
70. Jan A, Goncalves NP, Vaegter CB, Jensen PH, Ferreira N. The prion-like spreading of alpha-synuclein in parkinson's disease: Update on models and hypotheses. Int J Mol Sci (2021) 22(15):8338. doi: 10.3390/ijms22158338
71. Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for crohn's disease. Nat Genet (2008) 40:955–62. doi: 10.1038/ng.175
72. Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed crohn's disease susceptibility loci. Nat Genet (2010) 42:1118–25. doi: 10.1038/ng.717
73. Freuer D, Meisinger C. Association between inflammatory bowel disease and parkinson's disease: A mendelian randomization study. NPJ Parkinsons Dis (2022) 8:55. doi: 10.1038/s41531-022-00318-7
74. Cui GH, Li SJ, Ye H, Yang Y, Huang QY, Chu YM, et al. Are neurodegenerative diseases associated with an increased risk of inflammatory bowel disease? a two-sample mendelian randomization study. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.956005
75. Alunno A, Carubbi F, Bartoloni E, Cipriani P, Giacomelli R, Gerli R. The kaleidoscope of neurological manifestations in primary sjogren's syndrome. Clin Exp Rheumatol (2019) 37 Suppl 118:192–8.
76. Hassin-Baer S, Levy Y, Langevitz P, Nakar S, Ehrenfeld M. Anti-beta(2)-glycoprotein I in sjogren's syndrome is associated with parkinsonism. Clin Rheumatol (2007) 26:743–7. doi: 10.1007/s10067-006-0398-8
77. Charoenngam N, Rittiphairoj T, Ponvilawan B, Prasongdee K. Thyroid dysfunction and risk of parkinson's disease: A systematic review and meta-analysis. Front Endocrinol (2022) 13. doi: 10.3389/fendo.2022.863281
78. Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and parkinson's disease. Front Neuroanat (2015) 9. doi: 10.3389/fnana.2015.00091
79. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid hormones, oxidative stress, and inflammation. Mediat Inflammation (2016) 2016:6757154. doi: 10.1155/2016/6757154
80. Taheriniya S, Arab A, Hadi A, Fadel A, Askari G. Vitamin d and thyroid disorders: a systematic review and meta-analysis of observational studies. BMC Endocr Disord (2021) 21:171. doi: 10.1186/s12902-021-00831-5
81. Pignolo A, Mastrilli S, Davi C, Arnao V, Aridon P, Dos Santos Mendes FA, et al. Vitamin d and parkinson's disease. Nutrients (2022) 14:1220. doi: 10.3390/nu14061220
82. Li DX, Hong X, Chen TY. Association between rheumatoid arthritis and risk of parkinson's disease: A meta-analysis and systematic review. Front Neurol (2022) 13. doi: 10.3389/fneur.2022.885179
83. Li CY, Ou RW, Shang HF. Rheumatoid arthritis decreases risk for parkinson's disease: a mendelian randomization study. NPJ Parkinsons Dis (2021) 7:17. doi: 10.1038/s41531-021-00166-x
84. Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease a meta-analysis. Neurology (2010) 74:995–1002. doi: 10.1212/WNL.0b013e3181d5a4a3
85. Poly TN, Islam MM, Yang HC, Li YCJ. Non-steroidal anti-inflammatory drugs and risk of parkinson's disease in the elderly population: a meta-analysis. Eur J Clin Pharmacol (2019) 75:99–108. doi: 10.1007/s00228-018-2561-y
86. Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: A review. JAMA (2020) 323:548–60. doi: 10.1001/jama.2019.22360
87. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic parkinson's disease. Neurobiol Aging (2003) 24:197–211. doi: 10.1016/S0197-4580(02)00065-9
88. Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med (2020) 172:ITC81–96. doi: 10.7326/AITC202006020
Keywords: Parkinson’s disease, autoimmune disease, bullous pemphigoid, inflammatory bowel disease, comorbidity, Crohn ‘s disease, ulcerative colitis
Citation: Li M, Wan J, Xu Z and Tang B (2023) The association between Parkinson’s disease and autoimmune diseases: A systematic review and meta-analysis. Front. Immunol. 14:1103053. doi: 10.3389/fimmu.2023.1103053
Received: 19 November 2022; Accepted: 06 January 2023;
Published: 25 January 2023.
Edited by:
Giuseppe Murdaca, University of Genoa, ItalyReviewed by:
Lucio Marinelli, University of Genoa, ItalyCopyright © 2023 Li, Wan, Xu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beisha Tang, YnN0YW5nNzM5OEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.