
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 02 February 2023
Sec. Alloimmunity and Transplantation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1100306
This article is part of the Research TopicNew biomarkers in hematological disease and allogeneic cell transplant complicationsView all 14 articles
 Balaji Balakrishnan1
Balaji Balakrishnan1 Uday Prakash Kulkarni2
Uday Prakash Kulkarni2 Aswin Anand Pai2
Aswin Anand Pai2 Raveen Stephen Stallon Illangeswaran2
Raveen Stephen Stallon Illangeswaran2 Ezhilpavai Mohanan2
Ezhilpavai Mohanan2 Vikram Mathews2
Vikram Mathews2 Biju George2
Biju George2 Poonkuzhali Balasubramanian2*
Poonkuzhali Balasubramanian2*Hematopoietic cell transplantation is an established curative treatment option for various hematological malignant, and non-malignant diseases. However, the success of HCT is still limited by life-threatening early complications post-HCT, such as Graft Versus Host Disease (GVHD), Sinusoidal Obstruction Syndrome (SOS), and transplant-associated microangiopathy, to name a few. A decade of research in the discovery and validation of novel blood-based biomarkers aims to manage these early complications by using them for diagnosis or prognosis. Advances in this field have also led to predictive biomarkers to identify patients’ likelihood of response to therapy. Although biomarkers have been extensively evaluated for different complications, these are yet to be used in routine clinical practice. This review provides a detailed summary of various biomarkers for individual early complications post-HCT, their discovery, validation, ongoing clinical trials, and their limitations. Furthermore, this review also provides insights into the biology of biomarkers and the challenge of obtaining a universal cut-off value for biomarkers.
Hematopoietic cell transplantation (HCT) from matched related or unrelated donors to recipients with various hematological disease conditions has become a widely accepted curative treatment of choice. Especially with malignant hematological diseases, the graft versus tumor/leukemia effect (GVT) is a beneficial phenomenon expected to improve the outcome of the procedure. However, a similar effect where graft acting against the recipient’s cells, such as graft versus host disease (GVHD), leads to an undesirable outcome. Graft versus host disease (GVHD) still remains a predominant cause of morbidity and mortality in patients following HCT. Clinically GVHD may present as acute (aGVHD) or chronic (cGVHD) based on the symptoms and time of their presentation. The classical pathway of occurrence of GVHD includes damage of the target organs such as skin, eye, gastrointestinal (GI) tract, liver, or lung, followed by the release of a storm of cytokines, which increases the chance of the donor’s immunocompetent cells to recognize the host’s alloantigens (1). More than half of HCT patients develop GVHD. Although GVHD is treated by several immunosuppressive agents, responsiveness to these agents, GVHD related morbidity and mortality are still concerns that affect HCT outcomes greatly. In addition to GVHD, other serious complications include hepatic or pulmonary sinusoidal obstruction syndrome (SOS), opportunistic infections (bacterial, viral & fungal), and multiorgan damage. Attempts to improve HCT outcomes include predicting patients who are at high risk of developing post HCT complications, predicting their responsiveness to treatment and early diagnosis of these complications. Composite biomarkers of prognostic values have been recently used in confirming the diagnosis of some of these complications (2, 3).
Excluding the known likely causal factors for some of the adverse effects (such as the donor status, age, comorbidity, sex mismatch between donor and recipient, conditioning, and post-HCT immunosuppressive drug levels), various centers performing allogeneic HCT are concentrating on finding efficient, reliable and robust markers from biological fluids for informative, early detection or differential diagnosis of these complications to optimize the treatment as well as improving the outcome (4–6). Many have successfully reported a variety of blood plasma, serum, and fecal biomarkers, while only a handful of these is repeatedly tested and validated and likely to be used as a biomarker routinely (7). The biomarkers from these sources may be soluble factors, cellular markers, or genetic markers. While many candidate biomarkers from plasma were evaluated and verified in independent cohorts, multi-center clinical trials are still needed to validate their clinical applicability. Similarly cell-free DNA have also been recently evaluated for identifying an array of post-HCT complications including aGVHD, relapse, infection, engraftment failure and chimerism status with an objective of employing a single test/technique for elucidating a comprehensive panel of post-HCT complications (8).
However, one of the major limitations of these biomarker studies is the varying cut-off value as a reference to predict or diagnose these complications between centers. Moreover, and not all biomarkers are referenced across the normal cut-off values between healthy individuals and patients undergoing transplantation. Often these biomarkers are tested between HCT patients with and without complications. This review provides insights into the biological significance of biomarkers, their discovery and validation for HCT complications, challenges in quantification or techniques, and lack of universal target cut-offs.
Plasma biomarkers have been extensively evaluated for complications post HCT, since classic clinical risk scores such as HCT related co-morbidity index often fail to predict, diagnose or prognose such complications. The ultimate aim of plasma biomarker evaluation is its clinical translatability in predicting HCT complications, their severity and their response to treatment. On the other hand understanding the biology of these biomarkers would also pave way for developing more rational and effective treatment strategies for HCT complications. However, literature on biology of the biomarkers for HCT complications appear scanty. While increasing evidences suggest endothelial injury as a common cause for most HCT complications, a complete understanding is still lacking. Here we review the biology of a few biomarkers which are extensively evaluated for multiple overlapping complications.
The suppression of tumorigenicity 2 (ST2) is a receptor belonging to the interleukin (IL)-1 family and binds specifically to IL-33. ST2 is present in two isoforms: a transmembrane form and a soluble form. The membrane-bound ST2 receptor is expressed on various hematopoietic cells such as T helper 2 (Th2) cells, natural killer (NK) cells, mast cells, antigen-presenting cells, and regulatory T cells (Tregs) (9, 10). The IL33/ST2 complex signaling in these cell types has been observed to have proinflammatory and anti-inflammatory responses depending on the disease type (11–13). During acute GVHD, a surge in IL33 has been observed both in the clinical scenario and in mice models of alloHCT. The mucosal barrier tissues, such as the skin, gastrointestinal tract, and liver, have been significant sources of IL33. During the alloHCT conditioning regimen, damage to these tissues increases IL33 production/release that drives donor Th1 cells expansion leading to inflammatory phenotype and further tissue injury. Recently, it was demonstrated that IL-33 acts directly on donor T cells and increases Tbet expression leading to enhanced Th1 cell polarization and expansion. However, despite these observations of elevated IL-33, this could not be used as a specific biomarker for aGVHD due to its pleiotropic effects.
The soluble ST2 (sST2) receptors are expressed in endothelial cells, epithelial cells, fibroblasts, and T cells (14). The soluble ST2 act as decoy receptors, sequestering free IL-33, thereby preventing IL-33-mediated proinflammatory actions (15, 16). Thus, sST2 was generally considered to negatively regulate IL-33 function (Figure 1). However, this contradicts the association of elevated sST2 with GVHD severity in patients. A possible explanation given by earlier studies was that the release of sST2 in the serum occurs very late in the inflammatory response resulting in the inability of sST2 to sequester circulating IL33 (17).
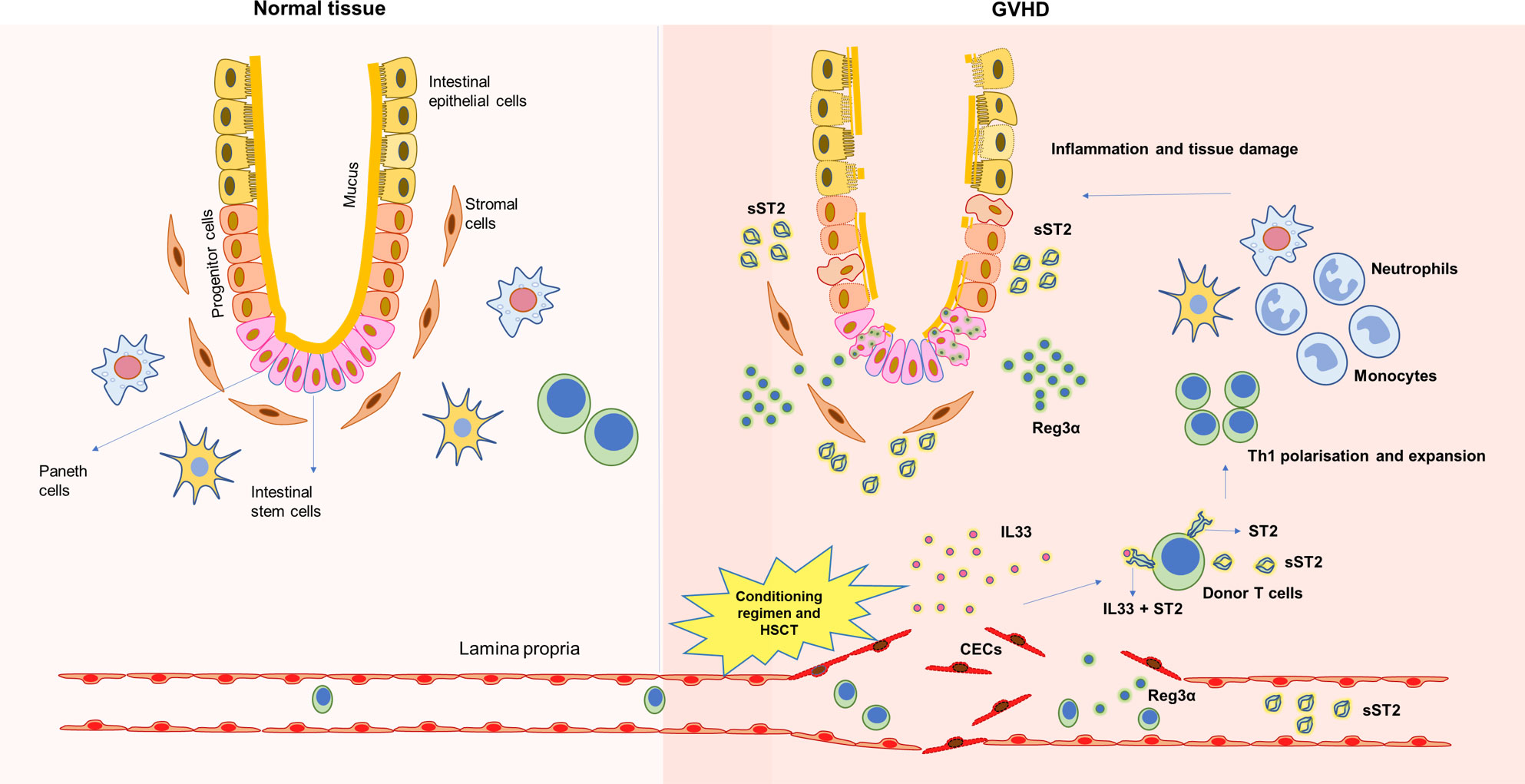
Figure 1 Underlying tissue damage during GVHD and release of soluble biomarkers. HSCT transplant procedures, including conditioning regimen, damage underlying endothelium, inflames the tissue and releases soluble factors that could be used as biomarkers during GVHD.
Zhang J et al. demonstrated in a minor mismatch GVHD model and xenograft GVHD model that sST2 was secreted by intestinal stromal cells, endothelial cells, and alloreactive T cells. More importantly, as GVHD progresses, it was shown that pathogenic T cells (Th17 and Tc17) secrete more sST2 and express less mST2, thereby correlating elevated plasma sST2 levels during alloreactivity. Transient blockade of sST2 during GVHD increased Th2 transcription factor GATA3 and cytokine IL-4, improving Th2 phenotype, which protects against severe GVHD (18).
An overall picture of the ST2/IL33 axis in a severe GVHD context remains elusive. Whether sST2 is involved in the pathophysiology of GVHD or it is just a circulating biomarker indicating GVHD severity remains to be clarified.
Regenerating islet-derived -3 α (Reg3α) alpha is one of the antimicrobial peptides secreted by Paneth cells of the gastrointestinal tract and is a C-type lectin having bactericidal actions on most gram-positive bacteria (19). The crypts’ innate lymphoid cells 3 (ILC3) secrete IL22, which induces Paneth cells to secrete Reg3α (Figure 1) (20). During HSCT, the crypt cells, including the Paneth cells, are damaged; hence, their numbers are inversely associated with GVHD severity (21). GVHD-induced damage to the gastrointestinal crypt and intestinal mucosa decreases IL22 production and releases antimicrobial peptides stored in these cryptic cells into the bloodstream. Thus, the increased plasma level of Reg3α was strongly associated with GI-GVHD enabling their use as a biomarker (22). It was also observed in-vivo in mice models of GVHD that the progression of GVHD suppresses Reg3γ (mouse homolog of human Reg3α) in the GI tract, further worsening GVHD. However, administration of IL22 has been shown to protect the crypt from damage, thereby preventing Reg3γ from being released into circulation. Mechanistically it was demonstrated that Reg3γ functions as an anti-apoptotic protein for intestinal stem cells (ISCs) and Paneth cells (23). Thus, Reg3α has the dual role of being an antimicrobial peptide as well as a survival signal preventing apoptosis of ISCs and Paneth cells.
While Il22 from host cells was recognized to promote intestinal stem cell survival and suppress GI-GVHD (24), a few studies have also shown that IL22 from donor cells augments GI-GVHD (25, 26). In a mouse model of steroid-refractory GVHD, by Song Q. et al. demonstrated that IL22 was produced by donor Th/Tc22 cells, leading to excess production of Reg3γ. However, such excess Reg3γ was shown to result in dysbiosis and worsening of GVHD. Thus, REG3γ could be a therapeutic target for treating steroid-refractory GVHD (27). Hence, whether Reg3alpha is a therapeutic target or a biomarker remains an enigma.
T cell immunoglobulin and mucin domain 3 (TIM3) is a transmembrane receptor protein expressed on interferon γ producing T cells, Tregs, myeloid cells, natural killer cells, and mast cells (28). The primary function of TIM3 is to inhibit Th1 responses and cytokine expressions. Hence, its dysregulation correlates with most autoimmune diseases, such as multiple sclerosis (29) and type I diabetes (30). Increased expression of TIM3 has been observed in solid tumors such as lung cancer, gastric cancer, colon cancer, etc., and their high expression levels were associated with low overall survival (31).
Elevated levels of TIM3 in the plasma of patients with GVHD (32) and osteosarcoma (33) have been observed, facilitating their use as potential biomarkers. However, the mechanism of soluble TIM3 release remains an enigma. It could be a splice variant, a metalloproteinase-dependent cleaved product, or a soluble fragment from apoptotic cells. While soluble TIM3 was found to express as a splice variant in mice splenocytes (34), their existence in humans is still debatable.
The mechanistic understanding of TIM3’s action in aGVHD remains incomplete and is not explored much. Oikawa et al. demonstrated in a murine model of GVHD that TIM3 plays a crucial role in the activation of CD8+ T cells, which are the primary effectors in target organ destruction in aGVHD. Two weeks post-transplantation, the CD8+ T cells in the spleen and liver of GVHD mice showed enhanced TIM3 and interferon γ (IFNγ) expression. Moreover, the CD8+T cell infiltration was dominant in the liver of GVHD mice (35). However, the exact mechanism of TIM3 induction in these cells and their shedding into peripheral circulation remains unclear.
Elafin is a biomarker associated with the diagnosis and prognosis of skin GVHD. It is an epithelial protein secreted by keratinocytes in response to inflammatory cytokines. Hence elafin’s expression is higher in the inflamed epidermis and absent/low in the normal epidermis. It is a peptidase inhibitor-3 or skin-derived anti-leukoprotease (SKALP) with antimicrobial activity and priming innate immune responses (36). It was observed that when induced by GVHD mediating inflammatory cytokines, human keratinocytes express elafin significantly (37, 38). However, the mechanism by which elafin from keratinocytes is released into circulation during GVHD remains unclear.
Biomarkers for acute GVHD have been extensively evaluated over the past decade in multiple HCT centers worldwide. These range from plasma, cellular, genetic and in a few cases, a combination. Biomarkers for aGVHD have been measured pre-HCT to personalize GVHD prophylaxis, post-HCT before or at the onset of GVHD, to confirm the diagnosis of GVHD or after treatment to predict treatment response.
CD86 is the ligand for costimulatory (CD28) and coinhibitory (CTLA-4) molecules. Karaban et al. reported that the recipients’ CTLA-4 CT60GA[GG] genotype, myeloablative conditioning regimen, and use of an unrelated donor were independent predictors of acute GVHD (39). Also, the same group has shown that donor and recipient CTLA-4 mRNA and recipient membrane protein expression measured before transplantation are prognostic for acute GVHD (40). Later they also reported a lack of association of CD86 gene polymorphisms with GVHD. However, they noted a gene-gene interaction wherein patients with a specific CD86 genotype and a CTLA-4 genotype was associated with an increased risk of aGVHD. With a combination of specific donor CD86 genotype and recipient CTLA-4 genotype there was an elevated GVHD risk (41).
In a study exploring the role of donor genetic variations in glucocorticoid pathway on steroid responsiveness of GVHD, although donor SNPs in ZAP70 and DUSP1 genes were associated with response, these were not statistically significant on adjustment for multiple testing (42). Cytokine biomarkers – TLR4 and TNFR1 are significantly increased in steroid-refractory acute GVHD compared to those with steroid-responsive GVHD (43).
DNAX accessory molecule-1 (DNAM-1, or CD226) is a leukocyte adhesion molecule constitutively expressed on most CD4+ T cells, CD8+ T cells, natural killer (NK) cells, and monocytes. A retrospective study from Japan showed that higher soluble DNAM-1 measured between day -7 to day 0 of an allotransplant was predictive of a higher risk of acute GVHD. Using a cut-off of 30pM for soluble DNAM-1, the sensitivity for predicting acute GVHD was 43.8%, while the specificity was 82.6% (44). Serum IL-6 levels measured pre-conditioning and one week after transplant were predictive of acute GVHD and transplant-related mortality (45), although, the pleiotropic nature of IL-6 may be a concern. Specific donor graft characteristics like an elevated proportion of T cells with low CD127 and high PD-1 expression have been associated with subsequent acute GVHD (46).
Specific patterns of immune reconstitution following transplantation, such as increased CD8+ T cells (both naïve and memory) in the early post-transplant period (on day 15), have been associated with development of subsequent acute GVHD (47).
Elevated plasma REG3α measured at the onset of GVHD predicted non-response to treatment at 4 weeks and also 1 year non-relapse mortality (22). Elevated plasma elafin at the onset of skin GVHD is associated with higher maximum grade of GVHD and also non-relapse mortality (48). Also, elevated ST2 predicts steroid resistance in acute GVHD and non-relapse mortality (4). In non-myeloablative transplants, elevated plasma ST2, REG3α, and elafin measured early post-transplant were predictive of acute GVHD (49). Similarly, in T-replete haplotransplants, elevated plasma ST2 and REG3α measured early post-transplant were predictive of acute GVHD and non-relapse mortality (50). In patients undergoing matched donor T deplete transplantation using anti-thymocyte globulin or alemtuzumab, a biomarker panel including HGF, elafin, sIL-2Rα, sTNFR1, and REG3α was predictive of GVHD and its severity (51).
Lower serum sIL-27Rα at the time of neutrophil engraftment is predictive of acute GVHD and has been shown to correlate with other serum GVHD biomarkers (52). Elevated plasma levels of sIL2-Rα and TIM-3 in the early post-transplant period predicted increased transplant-related mortality and acute GVHD (53).
Similarly, expression patterns of genes and a few microRNAs have also been evaluated as biomarkers post HCT. Transcripts levels of FOXP3, ICOS, CD52, and CASP1 genes involved in alloreactive immune responses and immune cell interactions were predictive of acute GVHD using a personalized modeling-based gene selection (PMGS) method (54). A risk score developed using metabolite and transcriptome analysis incorporating 5 metabolite markers from glycerophospholipid metabolism was predictive of acute GVHD (55). miR-155 and miR-146a measured in target tissues at the time of GVHD onset and measured in extracellular vesicles in serum and urine in the early post-transplant period before GVHD onset have been predictive of acute GVHD (56).
Fecal, and not serum calprotectin is a biomarker for acute gut GVHD and can potentially help diagnose gut GVHD (57). Also, low tissue amphiregulin expression on immunohistochemistry has been reported in 74% of patients with acute gut GVHD and might aid in diagnosis without classic apoptotic changes (58). Similarly, tissue, and not plasma, elafin on immunohistochemistry can aid in diagnosing acute GVHD involving the skin (59, 60).
Analysis of exosomal miRNA expression using quantitative RT-PCR on plasma samples showed that miR-128 was elevated in late-onset GVHD and is a promising diagnostic marker of late-onset GVHD (61). However, the turnaround times for these biomarkers may limit their practical utility.
The Mount Sinai Acute GVHD International Consortium (MAGIC) algorithm probability score (MAP score) based on plasma ST2 and REG3α is a response biomarker for acute GVHD. After four weeks of therapy, it was shown to predict non-relapse mortality better than the change in clinical symptoms. The MAP score was predictive of non-relapse mortality within every clinical grade of acute GVHD (62). The MAP score has been shown to be helpful when measured at day 28 along with the disease risk index could also identify patients at high relapse risk and low non-relapse mortality risk who can potentially benefit from strategies to enhance the graft versus leukemia effect for relapse prevention (63). Rising REG3α following treatment for GVHD using a novel combination of upfront steroids+ruxolitinib was shown to be a predictor of refractory GVHD (64). However, there is no prospective clinical study on biomarker-based intervention for adding second-line therapy for acute GVHD. A list of various biomarkers measured at different stages during HCT procedure and/or at GVHD onset, with potential clinical values that could help in prediction, diagnosis or prognosis for acute GVHD is summarized in Table 1.

Table 1 List of various types of biomarkers with clinical values that could help in the prediction, diagnosis, or prognosis for Acute GVHD.
Initial discovery and validation of ST2 as a biomarker for aGVHD also led to studies investigating inhibitors for ST2 in animal models (65). While this is still in progress, biomarker evaluation has progressed towards guided therapy/intervention for aGVHD with already existing anti-GVHD strategies. Gergoudis et al. have recruited patients at high risk for developing steroid-refractory GVHD (SR-GVHD) based on the MAGIC algorithm probability (MAP) scores on days 7 and 14 post-HSCT. These patients were then treated with alpha-1 anti-trypsin (AAT), a serine protease inhibitor with proven activity against GVHD. Although AAT treatment was well tolerated, the incidence of SR-GVHD was not lowered (66). Nevertheless, the power of biomarker-based SR-GVHD prediction could not be undermined. Instead, such studies pave the way for investigating more treatment options. A recent study involving a prospective phase 2 trial stratified patients based on sIL-2Rα and IL-15 levels. High-risk patients (sIL-2Rα 4500 ng/L or IL-15 31 ng/L) were treated with rabbit anti-thymocyte globulin (ATG) 3 mg/kg on day 8 post-transplant and were compared with controls who had the biomarkers measured but did not participate in this interventional trial. A reduction in GVHD was observed in these patients compared to high-risk controls who did not receive ATG (Hazards ratio of 0.48), signifying the feasibility and effectiveness of such an approach (67).
Sinusoidal Obstruction Syndrome (SOS), previously, known as veno-occlusive disease, is a severe complication post HSCT affecting liver sinusoidal endothelial cells. About 13 to 20% of allogeneic HSCT recipients develop SOS and the severe form of SOS is associated with multiorgan failure and mortality (68).
Typically, SOS has been observed between one to three weeks post-HSCT. Often clinically indistinguishable from other causes of weight gain, ascites, abdominal pain, and jaundice.
Factors such as conditioning regimen drugs or radiation, releasing cytokines from injured tissues, and the endogenous microbial substances that cross the compromised mucosal barriers lead to the activation of sinusoidal endothelial cells. Sustained activation can progress to endothelium damage (69). The sinusoidal endothelial cells swell and round, forming gaps in the sinusoidal barrier. These alterations facilitate the egress of leucocytes, RBCs, and cellular debris into the perisinusoidal space beneath the endothelial cells and disrupt the endothelial lining leading to sinusoidal embolisms and obstruction of the sinusoidal flow, liver dysfunction, ascites ultimately leading to multiorgan failure (70, 71).
Some reliable markers of endothelial activation and damage are soluble cellular adhesion molecules (sVCAM1, sICAM1, and sP-selectin), coagulation factors (Von Willebrand factor (VWF), thrombomodulin (TM) and plasminogen activator type-1 (PAI-1)) (Table 2).
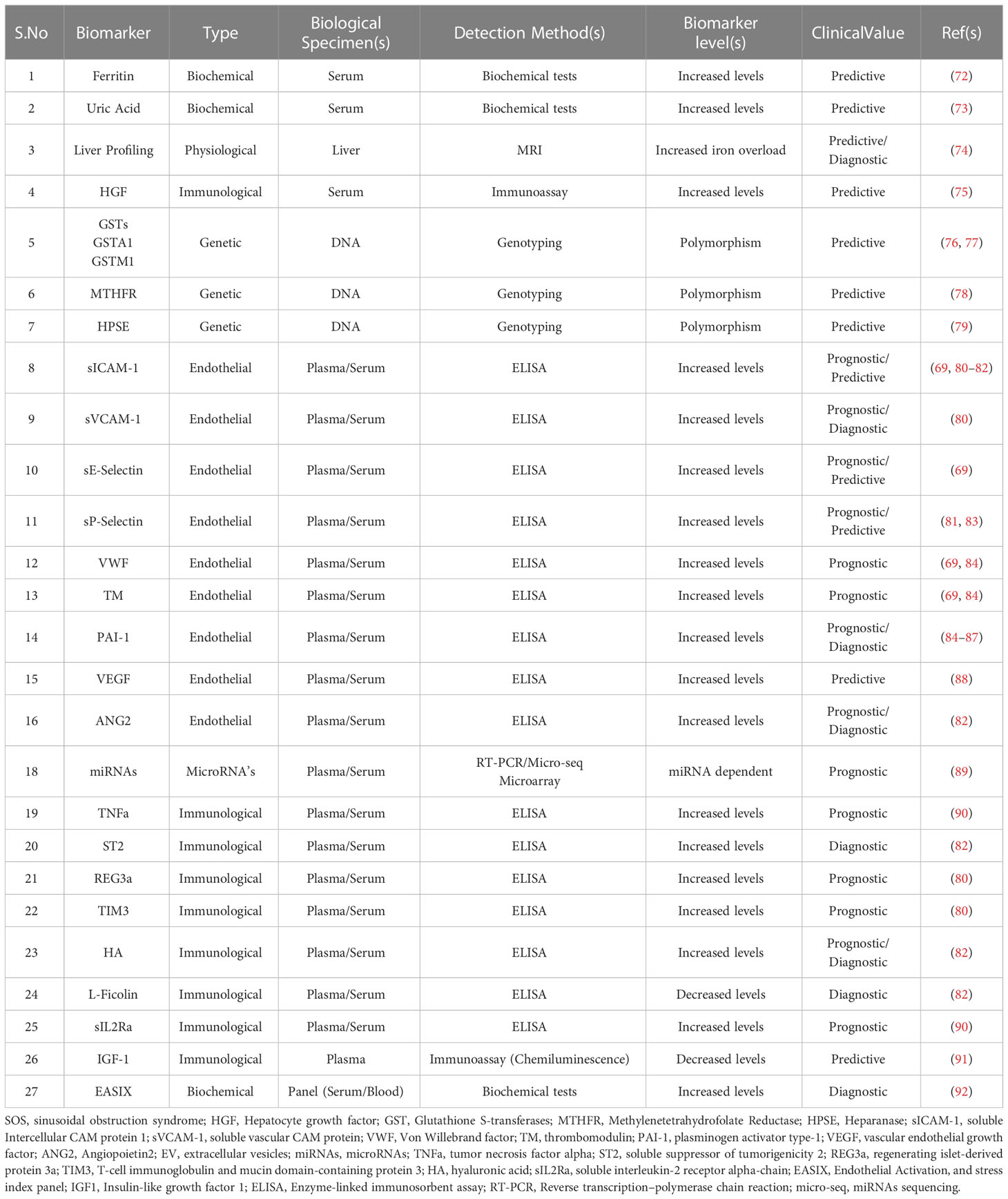
Table 2 List of various types of biomarkers with clinical values that could help in the prediction, diagnosis, or prognosis for SOS.
The microenvironment of the endothelium is significantly altered in patients who undergo allo-HCT. Allo-HCT patients who develop SOS have a significant increase in both VWF and TM levels (69, 84). Furthermore, in patients receiving both tacrolimus and sirolimus as GVHD prophylaxis, levels of VWF and TM (together with ICAM-1 and E-selectin level) serve as SOS predictive biomarkers one-week post HCT (69). Two weeks post- HCT, plasma levels of REG3α, sVCAM1, sICAM1, and TIM3 are shown to be consistently elevated in patients who developed SOS (80). P-selectin levels are shown to be selectively higher in patients who develop severe SOS and elevated circulating levels of PAI-1 allow differential diagnosis between SOS and GVHD, as patients with SOS show elevated PAI-1 but not those with GVHD (81, 85, 86).
In a recent study, a composite diagnostic panel of three biomarkers: L-Ficolin, hyaluronic acid (HA), and VCAM-1, was reported to detect patients at high risk of SOS as early as the first day after HCT, even before clinical manifestation of SOS (82). Additionally, it was proposed that the biomarker panel ST2, ANG2, L-Ficolin, HA, and VCAM-1 could be helpful in the diagnosis of SOS (82). Inflammatory cytokines such as IL2, IL6, IL33, IFNγ, and TNFα are mediators of EC activation and damage. Both TNFα and soluble IL2 receptor α (sIL2Rα) are shown to be elevated during GVHD and SOS (90, 93, 94).
Promising results from the studies evaluating biomarkers for GVHD and SOS have also led to the identification of similar plasma biomarkers for other early HSCT complications, such as transplant-associated- thrombotic microangiopathy (TA-TMA) and engraftment syndrome (ES). TA-TMA is characterized by occlusion and disruption of microcirculation as a result of micro-thrombi deposition. It is believed that the disruption of microcirculation results from endothelial dysfunction. Lia G et al. reviewed that the endothelial dysfunction could be due to persistent insult to the endothelium caused throughout the HSCT procedure, starting from the conditioning regimen and subsequently through calcineurin inhibitors (95).
While there are multiple causes for endothelial injury, neutrophil extrusion traps (NETs) also appear to be one component evaluated in the TA-TMA context. A significantly elevated level of NETS, within the first 4 weeks post-HSCT, has been reported to be associated with an increased risk of TA-TMA (96). In contrast, the same study could not find a possible association of thrombomodulin (expressed by endothelial cells and serves as a cofactor for thrombin) with the occurrence of TA-TMA, indicating challenges in understanding the pathophysiology of TA-TMA. Interestingly, elevated ST2 levels on day 14 post-HSCT was also reported to be associated with TA-TMA. The clinical overlap between GVHD and TA-TMA occurrence and endothelial injury as a common factor for both conditions, indicates that ST2 could also be a possible biomarker for TA-TMA (97). A recent study by Okamura H et al. has shown that elevated levels of complement factor Ba on day 7 post-HSCT significantly predicted TA-TMA (98).
Similarly, the symptoms of engraftment syndrome (ES) post HCT appears overlapping with that of either GVHD or with infections. Biomarkers that could help in the early differential diagnosis of ES from other conditions with overlapping symptoms could improve HCT outcomes. Procalcitonin (PCT), a hormokine has been reported to be elevated in ES patients that could possibly be used as biomarker (99). Knoll et al., reported procalcitonin levels 2ng/ml could possibly distinguish patients with ES from patients with bacterimia (100). However, since PCT is also a FDA approved biomarker for sepsis and febrile neutropenic pateints with infections (101, 102), the use of PCT for ES needs to be evaluated in multiple cohorts.
Most of the complications post HCT appears to be as a result of persistent insult to the damaged endothelium throughout HCT procedures. Hence many markers of endothelium damage have been extensively evaluated as potential biomarkers for most HCT complications as well (103, 104).
More than a decade of progress in discovering and validating biomarkers for HSCT complications led to incorporating them in clinical trials to verify their impact as a diagnostic, prognostic, or tool for pre-emptive therapy/intervention. For example. Reg3α was shown to distinguish diarrhea due to GI-GVHD from diarrhea due to non-GVHD causes (22). In contrast, Elafin could distinguish skin GVHD from drug hypersensitivity rashes (DHR) (ref). Also, REG3α and elafin were shown to distinguish diarrhea and rashes due to a more systemic disease than GI-GVHD alone (105). Similarly, ST2, TIM3, and IL6 were shown to be diagnostic biomarkers for aGVHD (106). However, these biomarkers’ prospective utilization has not yet been achieved. There are more challenges in translating biomarker concentrations toward a possible clinical decision in terms of diagnosis or intervention:
Plasma biomarker concentrations need a range of cut-off values to make clinical decisions. Different HCT centers have evaluated various biomarkers either singly or as a panel. However, there appear to be no universal cut-off valuesfor different biomarkers, probably due to the methods employed to derive cut-off values. For instance, Hartwell et al. developed an algorithm using logistic regression analysis of biomarker concentrations to derive cut-off values (6). Other groups have used individual biomarker concentrations in respective cohorts to derive cut-off values (4).
Similarly, the association of biomarkers towards specific HSCT outcomes that could not be verified in different cohorts precludes deriving a universal cut-off values. For instance, it was shown that high ST2 levels correlated with steroid-refractory GVHD (4) but was subsequently shown in different cohorts to be associated with six months of non-relapse mortality and not with GVHD (107, 108). Various groups have reported different cut-off values for the same biomarker [For example, ST2: 33.9 ng/ml (107); 740 pg/ml (4); 3230 ng/ml (50), REG3α: 151 ng/ml (22); 1989 pg/ml (50)]. Finally, there always appears an overlap in biomarker concentrations in cohorts with and without HSCT complications impedes a universal cut-off values derivation. Thus, establishing a universal reproducible cut-off values remains a challenge.
Due to the overlap in concentrations of biomarkers in patients with and without HSCT complications, many groups have reported that a single biomarker could be of little value correlating with HSCT outcomes as opposed to a biomarker panel. Elafin was initially discovered to be associated with skin GVHD (48). However, its utility appears very limited owing to the lack of reproducibility (60). The inclusion of elafin to REG3α and ST2 was also shown to be of little value in improving the accuracy of assessing HSCT outcomes (109). On the other hand, biomarkers such as ST2 and REG3α are potentially promising as single biomarkers correlating with therapy-resistant GVHD and GI-GVHD and as panels predicting six months of non-relapse mortality (NRM) (4). A special consideration towards the sensitivity and specificity of biomarkers, either as single or panel, needs to be given to biomarkers’ clinical translatability.
Beyond these challenges, the time points for biomarker testing and the frequency of such testing are also not standardized. There is considerable variation in these parameters in the reported literature so far.
Non-invasive biomarkers have been comprehensively evaluated for detection, diagnosis, and/or prognosis of early complications post-HCT in multiple centers. Various studies have evaluated individual biomarkers alone or as a panel towards GVHD. The past decade of voluminous data has shown that biomarker panels, as opposed to individual biomarkers, are more valuable in diagnosing GVHD or predicting GVHD severity. In this context, MAGIC, a GVHD biomarker panel employing an algorithm using logistic regression, appears to be so promising in terms of its clinical translatability since multiple centers have verified this. On the other hand, biomarkers for other complications, such as SOS, TA-TMA, etc., still need to be confirmed in multiple clinical settings. The association of endothelial damage with post-transplant complications has been a promising addition to the arsenal of biomarkers. However, biomarkers based on endothelial damage are greatly influenced by many factors, such as underlying disease, conditioning regimen, and post-transplant conditions. Nevertheless, multiple studies have progressed well in evaluating endothelial damage biomarkers toward post-HCT complications. EASIX panel to predict SOS severity is the best example.
Prospective studies and clinical trials incorporating biomarker based interventions with clinical endpoints are required to further evaluate the clinical translatability of these biomarkers. In absence of such studies being reported, the clinical translation of biomarkers in HCT is not ready for prime time. The longer turn-around times, variable cut-offs, and assay variabilities also remain as barriers towards practical utility of such biomarkers in HCT for clinical decision making and strategies to circumvent these are needed.
Equally important is understanding the biology of the hitherto validated biomarkers, which will have advantages such as guided pre-emptive therapy, finding novel therapeutic targets for HCT complications, and, more importantly, allowing us to validate if the biomarkers are sensitive and specific.
Progress in biomarker evaluation towards HCT complications is accompanied by challenges such as the derivation of a universal cut-off point, evaluation of individual or panel of biomarkers, and prospective biomarkers assessment. These challenges could be due to differences in techniques used to analyze biomarkers, and serum/plasma sample processing, including dilutions, conditioning regimen intensity, and the source of graft. Nevertheless, recently many studies are moving towards using biomarkers as a guide for preemptive therapy. Thus our knowledge of biomarkers for early complications is ever-expanding, leading to more significant progress in its clinical translatability.
BB, UK, PB, designed the review structure and wrote the manuscript. EM, AAP and SI wrote the manuscript. BG and VM contributed to analysis and review of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by a grant from Wellcome DBT India Alliance (IA/S/21/2/505932) to PB.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Justiz Vaillant AA, Modi P, Mohammadi O. Graft versus host disease. StatPearls. Treasure Island (FL: StatPearls Publishing LLC (2022).
2. Ruutu T, Carreras E. Hepatic complications. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT handbook: Hematopoietic stem cell transplantation and cellular therapies. Cham: Springer International Publishing (2019). p. 373–9.
3. Holler E, Greinix H, Zeiser R. Acute graft-Versus-Host disease. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT handbook: Hematopoietic stem cell transplantation and cellular therapies. Cham: Springer International Publishing (2019). p. 323–30.
4. Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. New Engl J Med (2013) 369(6):529–39. doi: 10.1056/NEJMoa1213299
5. Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematology. (2015) 2(1):e21–9. doi: 10.1016/S2352-3026(14)00035-0
6. Hartwell MJ, Ozbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight (2017) 2(3):e89798. doi: 10.1172/jci.insight.89798
7. Milosevic E, Babic A, Iovino L, Markovic M, Grce M, Greinix H. Use of the NIH consensus criteria in cellular and soluble biomarker research in chronic graft-versus-host disease: A systematic review. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.1033263
8. Cheng AP, Cheng MP, Loy CJ, Lenz JS, Chen K, Smalling S, et al. Cell-free DNA profiling informs all major complications of hematopoietic cell transplantation. Proc Natl Acad Sci United States America (2022) 119(4):e2113476118. doi: 10.1073/pnas.2113476118
9. Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol (2009) 183(10):6469–77. doi: 10.4049/jimmunol.0901575
10. Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS letters. (1993) 318(1):83–7. doi: 10.1016/0014-5793(93)81333-U
11. Liu Q, Turnquist HR. Implications for interleukin-33 in solid organ transplantation. Cytokine. (2013) 62(2):183–94. doi: 10.1016/j.cyto.2013.02.026
12. Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol (2010) 10(2):103–10. doi: 10.1038/nri2692
13. Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol (2008) 20(8):1019–30. doi: 10.1093/intimm/dxn060
14. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. (2011) 117(14):3720–32. doi: 10.1182/blood-2010-07-273417
15. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. (2005) 23(5):479–90. doi: 10.1016/j.immuni.2005.09.015
16. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. (2013) 39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010
17. Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. (2015) 125(20):3183–92. doi: 10.1182/blood-2014-10-606830
18. Zhang J, Ramadan AM, Griesenauer B, Li W, Turner MJ, Liu C, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Trans Med (2015) 7(308):308ra160. doi: 10.1126/scitranslmed.aab0166
19. Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. (2006) 313(5790):1126–30. doi: 10.1126/science.1127119
20. Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med (2008) 14(3):282–9. doi: 10.1038/nm1720
21. Levine JE, Huber E, Hammer ST, Harris AC, Greenson JK, Braun TM, et al. Low paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood. (2013) 122(8):1505–9. doi: 10.1182/blood-2013-02-485813
22. Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. (2011) 118(25):6702–8. doi: 10.1182/blood-2011-08-375006
23. Zhao D, Kim YH, Jeong S, Greenson JK, Chaudhry MS, Hoepting M, et al. Survival signal REG3alpha prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest (2018) 128(11):4970–9. doi: 10.1172/JCI99261
24. Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. (2012) 37(2):339–50. doi: 10.1016/j.immuni.2012.05.028
25. Lamarthee B, Malard F, Gamonet C, Bossard C, Couturier M, Renauld JC, et al. Donor interleukin-22 and host type I interferon signaling pathway participate in intestinal graft-versus-host disease via STAT1 activation and CXCL10. Mucosal Immunol (2016) 9(2):309–21. doi: 10.1038/mi.2015.61
26. Couturier M, Lamarthee B, Arbez J, Renauld JC, Bossard C, Malard F, et al. IL-22 deficiency in donor T cells attenuates murine acute graft-versus-host disease mortality while sparing the graft-versus-leukemia effect. Leukemia. (2013) 27(7):1527–37. doi: 10.1038/leu.2013.39
27. Song Q, Wang X, Wu X, Kang TH, Qin H, Zhao D, et al. IL-22-dependent dysbiosis and mononuclear phagocyte depletion contribute to steroid-resistant gut graft-versus-host disease in mice. Nat Commun (2021) 12(1):805. doi: 10.1038/s41467-021-21133-3
28. Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol (2020) 20(3):173–85. doi: 10.1038/s41577-019-0224-6
29. Yang L, Anderson DE, Kuchroo J, Hafler DA. Lack of TIM-3 immunoregulation in multiple sclerosis. J Immunol (2008) 180(7):4409–14. doi: 10.4049/jimmunol.180.7.4409
30. Kanzaki M, Wada J, Sugiyama K, Nakatsuka A, Teshigawara S, Murakami K, et al. Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes. Endocrinology. (2012) 153(2):612–20. doi: 10.1210/en.2011-1579
31. Zhang Y, Cai P, Liang T, Wang L, Hu L. TIM-3 is a potential prognostic marker for patients with solid tumors: A systematic review and meta-analysis. Oncotarget. (2017) 8(19):31705–13. doi: 10.18632/oncotarget.15954
32. Hansen JA, Hanash SM, Tabellini L, Baik C, Lawler RL, Grogan BM, et al. A novel soluble form of Tim-3 associated with severe graft-versus-host disease. Biol Blood marrow Transplant (2013) 19(9):1323–30. doi: 10.1016/j.bbmt.2013.06.011
33. Ge W, Li J, Fan W, Xu D, Sun S. Tim-3 as a diagnostic and prognostic biomarker of osteosarcoma. Tumour Biol (2017) 39(7):1010428317715643. doi: 10.1177/1010428317715643
34. Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, et al. Soluble form of T cell ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol (2006) 176(3):1411–20. doi: 10.4049/jimmunol.176.3.1411
35. Oikawa T, Kamimura Y, Akiba H, Yagita H, Okumura K, Takahashi H, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol (2006) 177(7):4281–7. doi: 10.4049/jimmunol.177.7.4281
36. Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin science. (2006) 110(1):21–35. doi: 10.1042/CS20050115
37. Pfundt R, Wingens M, Bergers M, Zweers M, Frenken M, Schalkwijk J. TNF-alpha and serum induce SKALP/elafin gene expression in human keratinocytes by a p38 MAP kinase-dependent pathway. Arch Dermatol Res (2000) 292(4):180–7. doi: 10.1007/s004030050475
38. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. (2009) 373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3
39. Karabon L, Markiewicz M, Partyka A, Pawlak-Adamska E, Tomkiewicz A, Dzierzak-Mietla M, et al. A CT60GA polymorphism in the CTLA-4 gene of the recipient may confer susceptibility to acute graft versus host disease after allogeneic hematopoietic stem cell transplantation. Immunogenetics (2015) 67(5-6):295–304. doi: 10.1007/s00251-015-0840-7
40. Karabon L, Markiewicz M, Kosmaczewska A, Partyka A, Pawlak-Adamska E, Tomkiewicz A, et al. Pretransplant donor and recipient CTLA-4 mRNA and protein levels as a prognostic marker for aGvHD in allogeneic hematopoietic stem cell transplantation. Immunol letters. (2015) 165(1):52–9. doi: 10.1016/j.imlet.2015.03.011
41. Karabon L, Markiewicz M, Chrobot K, Dzierzak-Mietla M, Pawlak-Adamska E, Partyka A, et al. The influence of genetic variations in the CD86 gene on the outcome after allogeneic hematopoietic stem cell transplantation. J Immunol Res (2018) 2018:3826989. doi: 10.1155/2018/3826989
42. Arora M, Weisdorf DJ, Shanley RM, Thyagarajan B. Pharmacogenetics of steroid-responsive acute graft-versus-host disease. Clin Transplant (2017) 31(5). doi: 10.1111/ctr.12949
43. Li X, Chen T, Gao Q, Zhang W, Xiao Y, Zhu W, et al. A panel of 4 biomarkers for the early diagnosis and therapeutic efficacy of aGVHD. JCI Insight (2019) 4(16):e130413. doi: 10.1172/jci.insight.130413
44. Kanaya M, Shibuya K, Hirochika R, Kanemoto M, Ohashi K, Okada M, et al. Soluble DNAM-1, as a predictive biomarker for acute graft-Versus-Host disease. PLoS One (2016) 11(6):e0154173. doi: 10.1371/journal.pone.0154173
45. Greco R, Lorentino F, Nitti R, Lupo Stanghellini MT, Giglio F, Clerici D, et al. Interleukin-6 as biomarker for acute GvHD and survival after allogeneic transplant with post-transplant cyclophosphamide. Front Immunol (2019) 10:2319. doi: 10.3389/fimmu.2019.02319
46. Stikvoort A, Gaballa A, Solders M, Nederlof I, Onfelt B, Sundberg B, et al. Risk factors for severe acute graft-versus-Host disease in donor graft composition. Biol Blood marrow Transplant (2018) 24(3):467–77. doi: 10.1016/j.bbmt.2017.11.026
47. Rajasekar R, Mathews V, Lakshmi KM, George B, Viswabandya A, Chandy M, et al. Cellular immune reconstitution and its impact on clinical outcome in children with beta thalassemia major undergoing a matched related myeloablative allogeneic bone marrow transplant. Biol Blood marrow Transplant (2009) 15(5):597–609. doi: 10.1016/j.bbmt.2009.01.016
48. Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Trans Med (2010) 2(13):13ra2. doi: 10.1126/scitranslmed.3000406
49. Nelson RP Jr., Khawaja MR, Perkins SM, Elmore L, Mumaw CL, Orschell C, et al. Prognostic biomarkers for acute graft-versus-host disease risk after cyclophosphamide-fludarabine nonmyeloablative allotransplantation. Biol Blood marrow Transplant (2014) 20(11):1861–4. doi: 10.1016/j.bbmt.2014.06.039
50. Solan L, Kwon M, Carbonell D, Dorado N, Balsalobre P, Serrano D, et al. ST2 and REG3alpha as predictive biomarkers after haploidentical stem cell transplantation using post-transplantation high-dose cyclophosphamide. Front Immunol (2019) 10:2338. doi: 10.3389/fimmu.2019.02338
51. Min SS, Mehra V, Clay J, Cross GF, Douiri A, Dew T, et al. Composite biomarker panel for prediction of severity and diagnosis of acute GVHD with T-cell-depleted allogeneic stem cell transplants-single centre pilot study. J Clin pathology. (2017) 70(10):886–90. doi: 10.1136/jclinpath-2017-204399
52. Liu S, Han J, Gong H, Li Y, Bao X, Qi J, et al. Soluble interleukin-27 receptor alpha is a valuable prognostic biomarker for acute graft-versus-host disease after allogeneic haematopoietic stem cell transplantation. Sci Rep (2018) 8(1):10328. doi: 10.1038/s41598-018-28614-4
53. Leotta S, Sapienza G, Camuglia MG, Avola G, Marco AD, Moschetti G, et al. Preliminary results of a combined score based on sIL2-ralpha and TIM-3 levels assayed early after hematopoietic transplantation. Front Immunol (2019) 10:3158. doi: 10.3389/fimmu.2019.03158
54. Cuzzola M, Fiasche M, Iacopino P, Messina G, Martino M, Console G, et al. A molecular and computational diagnostic approach identifies FOXP3, ICOS, CD52 and CASP1 as the most informative biomarkers in acute graft-versus-host disease. Haematologica. (2012) 97(10):1532–8. doi: 10.3324/haematol.2011.059980
55. Liu Y, Huang A, Chen Q, Chen X, Fei Y, Zhao X, et al. A distinct glycerophospholipid metabolism signature of acute graft versus host disease with predictive value. JCI Insight (2019) 5(16):e129494. doi: 10.1172/jci.insight.129494
56. Crossland RE, Norden J, Ghimire S, Juric MK, Pearce KF, Lendrem C, et al. Profiling tissue and biofluid miR-155-5p, miR-155(*), and miR-146a-5p expression in graft vs. host disease. Front Immunol (2021) 12:639171. doi: 10.3389/fimmu.2021.639171
57. Metafuni E, Giammarco S, De Ritis DG, Rossi M, De Michele T, Zuppi C, et al. Fecal but not serum calprotectin is a potential marker of GVHD after stem cell transplantation. Ann hematology. (2017) 96(6):929–33. doi: 10.1007/s00277-017-2974-1
58. Amin K, Yaqoob U, Schultz B, Vaughn BP, Khoruts A, Howard JR, et al. Amphiregulin in intestinal acute graft-versus-host disease: a possible diagnostic and prognostic aid. Modern Pathol (2019) 32(4):560–7. doi: 10.1038/s41379-018-0170-z
59. Mahabal GD, George L, Peter D, Bindra M, Thomas M, Srivastava A, et al. Utility of tissue elafin as an immunohistochemical marker for diagnosis of acute skin graft-versus-host disease: a pilot study. Clin Exp Dermatol (2019) 44(2):161–8. doi: 10.1111/ced.13678
60. George L, Mahabal G, Mohanan E, Balasubramanian P, Peter D, Pulimood S, et al. Limited utility of plasma elafin as a biomarker for skin graft-versus-host disease following allogeneic stem cell transplantation. Clin Exp Dermatol (2021) 46(8):1482–7. doi: 10.1111/ced.14785
61. Yoshizawa S, Umezu T, Saitoh Y, Gotoh M, Akahane D, Kobayashi C, et al. Exosomal miRNA signatures for late-onset acute graft-Versus-Host disease in allogenic hematopoietic stem cell transplantation. Int J Mol Sci (2018) 19(9):2493. doi: 10.3390/ijms19092493
62. Srinagesh HK, Ozbek U, Kapoor U, Ayuk F, Aziz M, Ben-David K, et al. The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood advances. (2019) 3(23):4034–42. doi: 10.1182/bloodadvances.2019000791
63. Aziz MD, Shah J, Kapoor U, Dimopoulos C, Anand S, Augustine A, et al. Disease risk and GVHD biomarkers can stratify patients for risk of relapse and nonrelapse mortality post hematopoietic cell transplant. Leukemia. (2020) 34(7):1898–906. doi: 10.1038/s41375-020-0726-z
64. Yang J, Peng B, Wang L, Li X, Li F, Jin X, et al. Elevated REG3alpha predicts refractory aGVHD in patients who received steroids-ruxolitinib as first-line therapy. Ann hematology. (2022) 101(3):621–30. doi: 10.1007/s00277-021-04727-1
65. Ramadan AM, Daguindau E, Rech JC, Chinnaswamy K, Zhang J, Hura GL, et al. From proteomics to discovery of first-in-class ST2 inhibitors active in vivo. JCI Insight (2018) 3(14):e99208. doi: 10.1172/jci.insight.99208
66. Gergoudis SC, DeFilipp Z, Ozbek U, Sandhu KS, Etra AM, Choe HK, et al. Biomarker-guided preemption of steroid-refractory graft-versus-host disease with alpha-1-antitrypsin. Blood advances. (2020) 4(24):6098–105. doi: 10.1182/bloodadvances.2020003336
67. Khanolkar RA, Kalra A, Kinzel M, Pratt LM, Dharmani-Khan P, Chaudhry A, et al. A biomarker-guided, prospective, phase 2 trial of pre-emptive graft-versus-host disease therapy using anti-thymocyte globulin. Cytotherapy. (2021) 23(11):1007–16. doi: 10.1016/j.jcyt.2021.06.003
68. Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood marrow Transplant (2010) 16(2):157–68. doi: 10.1016/j.bbmt.2009.08.024
69. Cutler C, Kim HT, Ayanian S, Bradwin G, Revta C, Aldridge J, et al. Prediction of veno-occlusive disease using biomarkers of endothelial injury. Biol Blood marrow Transplant (2010) 16(8):1180–5. doi: 10.1016/j.bbmt.2010.02.016
70. McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. (1984) 4(1):116–22. doi: 10.1002/hep.1840040121
71. Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European society for blood and marrow transplantation (EBMT). Bone marrow transplantation. (2015) 50(6):781–9. doi: 10.1038/bmt.2015.52
72. Lee JH. Biomarkers for hepatic sinusoidal obstruction syndrome after hematopoietic cell transplantation. Blood Res (2015) 50(3):123–5. doi: 10.5045/br.2015.50.3.123
73. Visal Okur F, Karapapak M, Warasnhe K, Aslan UE, Kuskonmaz B, Cetinkaya D. Pre-conditioning serum uric acid as a risk factor for sinusoidal obstruction syndrome of the liver in children undergoing hematopoietic stem cell transplantation. Turkish J haematology (2021) 38(4):286–93. doi: 10.4274/tjh.galenos.2021.2021.0174
74. Chan SS, Colecchia A, Duarte RF, Bonifazi F, Ravaioli F, Bourhis JH. Imaging in hepatic veno-occlusive Disease/Sinusoidal obstruction syndrome. Biol Blood marrow Transplant (2020) 26(10):1770–9. doi: 10.1016/j.bbmt.2020.06.016
75. DiCarlo J, Agarwal-Hashmi R, Shah A, Kim P, Craveiro L, Killen R, et al. Cytokine and chemokine patterns across 100 days after hematopoietic stem cell transplantation in children. Biol Blood marrow Transplant (2014) 20(3):361–9. doi: 10.1016/j.bbmt.2013.11.026
76. Ansari M, Curtis PH, Uppugunduri CRS, Rezgui MA, Nava T, Mlakar V, et al. GSTA1 diplotypes affect busulfan clearance and toxicity in children undergoing allogeneic hematopoietic stem cell transplantation: a multicenter study. Oncotarget. (2017) 8(53):90852–67. doi: 10.18632/oncotarget.20310
77. Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, et al. Glutathione s-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. (2004) 104(5):1574–7. doi: 10.1182/blood-2003-11-3778
78. Elmaagacli AH, Koldehoff M, Steckel NK, Trenschel R, Ottinger H, Beelen DW. Cytochrome P450 2C19 loss-of-function polymorphism is associated with an increased treatment-related mortality in patients undergoing allogeneic transplantation. Bone marrow transplantation. (2007) 40(7):659–64. doi: 10.1038/sj.bmt.1705786
79. Seifert C, Wittig S, Arndt C, Gruhn B. Heparanase polymorphisms: influence on incidence of hepatic sinusoidal obstruction syndrome in children undergoing allogeneic hematopoietic stem cell transplantation. J Cancer Res Clin Oncol (2015) 141(5):877–85. doi: 10.1007/s00432-014-1857-2
80. Balakrishnan B, Illangeswaran RSS, Rajamani BM, Pai AA, Raj IX, Paul DZ, et al. Prognostic plasma biomarkers of early complications and graft-versus-host disease in patients undergoing allogeneic hematopoietic stem cell transplantation. EJHaem. (2020) 1(1):219–29. doi: 10.1002/jha2.26
81. Matsuda Y, Hara J, Osugi Y, Tokimasa S, Fujisaki H, Takai K, et al. Serum levels of soluble adhesion molecules in stem cell transplantation-related complications. Bone marrow transplantation. (2001) 27(9):977–82. doi: 10.1038/sj.bmt.1703026
82. Akil A, Zhang Q, Mumaw CL, Raiker N, Yu J, Velez de Mendizabal N, et al. Biomarkers for diagnosis and prognosis of sinusoidal obstruction syndrome after hematopoietic cell transplantation. Biol Blood marrow Transplant (2015) 21(10):1739–45. doi: 10.1016/j.bbmt.2015.07.004
83. Catani L, Gugliotta L, Vianelli N, Nocentini F, Baravelli S, Bandini G, et al. Endothelium and bone marrow transplantation. Bone marrow transplantation. (1996) 17(2):277–80.
84. Nakamura K, Hatano E, Miyagawa-Hayashino A, Okuno M, Koyama Y, Narita M, et al. Soluble thrombomodulin attenuates sinusoidal obstruction syndrome in rat through suppression of high mobility group box 1. Liver Int (2014) 34(10):1473–87. doi: 10.1111/liv.12420
85. Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martine C, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biol Blood marrow Transplant (2010) 16(7):985–93. doi: 10.1016/j.bbmt.2010.02.008
86. DeLeve LD, McCuskey RS, Wang X, Hu L, McCuskey MK, Epstein RB, et al. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. (1999) 29(6):1779–91. doi: 10.1002/hep.510290615
87. Nurnberger W, Michelmann I, Burdach S, Gobel U. Endothelial dysfunction after bone marrow transplantation: increase of soluble thrombomodulin and PAI-1 in patients with multiple transplant-related complications. Ann hematology. (1998) 76(2):61–5. doi: 10.1007/s002770050364
88. Moiseev IS, Lapin SV, Surkova EA, Lerner MY, Vavilov VN, Afanasyev BV. Level of vascular endothelial growth factor predicts both relapse and nonrelapse mortality after allogeneic hematopoietic stem cell transplantation. Biol Blood marrow (2013) 19(12):1677–82. doi: 10.1016/j.bbmt.2013.08.015
89. Takeuchi M, Oda S, Tsuneyama K, Yokoi T. Comprehensive analysis of serum microRNAs in hepatic sinusoidal obstruction syndrome (SOS) in rats: implication as early phase biomarkers for SOS. Arch toxicology. (2018) 92(9):2947–62. doi: 10.1007/s00204-018-2269-x
90. Foley R, Couban S, Walker I, Greene K, Chen CS, Messner H, et al. Monitoring soluble interleukin-2 receptor levels in related and unrelated donor allogenic bone marrow transplantation. Bone marrow transplantation. (1998) 21(8):769–73. doi: 10.1038/sj.bmt.1701163
91. Weischendorff S, Kielsen K, Sengelov H, Jordan K, Nielsen CH, Pedersen AE, et al. Associations between levels of insulin-like growth factor 1 and sinusoidal obstruction syndrome after allogeneic haematopoietic stem cell transplantation. Bone marrow transplantation. (2017) 52(6):863–9. doi: 10.1038/bmt.2017.43
92. Jiang S, Penack O, Terzer T, Schult D, Majer-Lauterbach J, Radujkovic A, et al. Predicting sinusoidal obstruction syndrome after allogeneic stem cell transplantation with the EASIX biomarker panel. Haematologica (2021) 106(2):446–53. doi: 10.3324/haematol.2019.238790
93. Choi SW, Kitko CL, Braun T, Paczesny S, Yanik G, Mineishi S, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. (2008) 112(4):1539–42. doi: 10.1182/blood-2008-02-138867
94. Hill GR, Teshima T, Rebel VI, Krijanovski OI, Cooke KR, Brinson YS, et al. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J Immunol (2000) 164(2):656–63. doi: 10.4049/jimmunol.164.2.656
95. Lia G, Giaccone L, Leone S, Bruno B. Biomarkers for early complications of endothelial origin after allogeneic hematopoietic stem cell transplantation: Do they have a potential clinical role? Front Immunol (2021) 12:641427. doi: 10.3389/fimmu.2021.641427
96. Arai Y, Yamashita K, Mizugishi K, Watanabe T, Sakamoto S, Kitano T, et al. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood marrow Transplant (2013) 19(12):1683–9. doi: 10.1016/j.bbmt.2013.09.005
97. Rotz SJ, Dandoy CE, Davies SM. ST2 and endothelial injury as a link between GVHD and microangiopathy. New Engl J Med (2017) 376(12):1189–90. doi: 10.1056/NEJMc1700185
98. Okamura H, Nakamae H, Shindo T, Ohtani K, Hidaka Y, Ohtsuka Y, et al. Early elevation of complement factor ba is a predictive biomarker for transplant-associated thrombotic microangiopathy. Front Immunol (2021) 12:695037. doi: 10.3389/fimmu.2021.695037
99. Shah NN, Watson TM, Yates B, Liewehr DJ, Steinberg SM, Jacobsohn D, et al. Procalcitonin and cytokine profiles in engraftment syndrome in pediatric stem cell transplantation. Pediatr Blood Cancer (2017) 64(3):10.1002/pbc.26273. doi: 10.1002/pbc.26273
100. Knoll BM, Ahmed J, Karass M, Aujla A, McHale P, Kretschmer P, et al. Procalcitonin as a biomarker to differentiate bacterial infections from engraftment syndrome following autologous hematopoietic stem cell transplantation for multiple myeloma. Am J hematology (2019) 94(3):E74–E6. doi: 10.1002/ajh.25378
101. Haddad HE, Chaftari AM, Hachem R, Michael M, Jiang Y, Yousif A, et al. Procalcitonin guiding antimicrobial therapy duration in febrile cancer patients with documented infection or neutropenia. Sci Rep (2018) 8(1):1099. doi: 10.1038/s41598-018-19616-3
102. Ebihara Y, Kobayashi K, Ishida A, Maeda T, Takahashi N, Taji Y, et al. Diagnostic performance of procalcitonin, presepsin, and c-reactive protein in patients with hematological malignancies. J Clin Lab Anal (2017) 31(6):e22147. doi: 10.1002/jcla.22147
103. Milone G, Bellofiore C, Leotta S, Milone GA, Cupri A, Duminuco A, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: A review based on physiopathology. J Clin Med (2022) 11(3):623. doi: 10.3390/jcm11030623
104. Hildebrandt GC, Chao N. Endothelial cell function and endothelial-related disorders following haematopoietic cell transplantation. Br J haematology. (2020) 190(4):508–19. doi: 10.1111/bjh.16621
105. Harris AC, Ferrara JL, Braun TM, Holler E, Teshima T, Levine JE, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. (2012) 119(12):2960–3. doi: 10.1182/blood-2011-10-387357
106. McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood. (2015) 126(1):113–20. doi: 10.1182/blood-2015-03-636753
107. Abu Zaid M, Wu J, Wu C, Logan BR, Yu J, Cutler C, et al. Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood. (2017) 129(2):162–70. doi: 10.1182/blood-2016-08-735324
108. Kanakry CG, Bakoyannis G, Perkins SM, McCurdy SR, Vulic A, Warren EH, et al. Plasma-derived proteomic biomarkers in human leukocyte antigen-haploidentical or human leukocyte antigen-matched bone marrow transplantation using post-transplantation cyclophosphamide. Haematologica. (2017) 102(5):932–40. doi: 10.3324/haematol.2016.152322
Keywords: biomarker, GVHD, endothelial, SOS, HSCT
Citation: Balakrishnan B, Kulkarni UP, Pai AA, Illangeswaran RSS, Mohanan E, Mathews V, George B and Balasubramanian P (2023) Biomarkers for early complications post hematopoietic cell transplantation: Insights and challenges. Front. Immunol. 14:1100306. doi: 10.3389/fimmu.2023.1100306
Received: 16 November 2022; Accepted: 23 January 2023;
Published: 02 February 2023.
Edited by:
Luisa Giaccone, University of Turin, ItalyReviewed by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaCopyright © 2023 Balakrishnan, Kulkarni, Pai, Illangeswaran, Mohanan, Mathews, George and Balasubramanian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Poonkuzhali Balasubramanian, YnBvb25rdXpoYWxpQGNtY3ZlbGxvcmUuYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.