
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 07 February 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1099468
This article is part of the Research Topic Multiple organ dysfunctions in perioperative critical illness and the prognosis after anesthesia View all 23 articles
 Xuewu Zhang1,2,3†
Xuewu Zhang1,2,3† Jingxia Wang4†
Jingxia Wang4† Xiaohan Huang5†
Xiaohan Huang5† Yue Zhu1
Yue Zhu1 Yijing Zhu2
Yijing Zhu2 Lingling Tang6
Lingling Tang6 Hongliu Cai1,7*
Hongliu Cai1,7* Xueling Fang1*
Xueling Fang1* Lingtong Huang1*
Lingtong Huang1*Immunosuppressed patients can contract parvovirus B19, and some may experience hemophagocytic lymphohistiocytosis (HLH). Herein, we describe the first report of hemophagocytic lymphohistiocytosis in a heart-lung transplant patient with concomitant parvovirus B19 infection. The patient was treated with intravenous immune globulin (IVIG) and the features of HLH were remission. This instance emphasizes the significance of parvovirus B19 monitoring in transplant patients with anemia; if HLH complicates the situation, IVIG may be an adequate remedy. Finally, a summary of the development in diagnosing and managing parvovirus B19 infection complicated by HLH is provided.
● Parvovirus B19 infection complicated by HLH is uncommon in transplant patients
● IVIG is an effective treatment for parvovirus B19 infection complicated by HLH
Parvovirus B19 is an ancient and conserved virus that circulated 100 million years ago or earlier (1). It is associated with pure red cell aplasia (PRCA) (2–4), viral myocarditis (5–8), erythema infectiosum (9), and other clinical manifestations. At the same time, evidence of the presence of parvovirus B19 has also been found in bone marrow transplant recipients (10) and diseases such as systemic lupus erythematosus (11, 12), miscarriage (13), systemic sclerosis (14), hereditary hemolytic anemias (15). Infectious erythema is one of the most common clinical manifestations of parvovirus B19 infection, which often occurs in children (4). Parvovirus B19 infection induced PRCA may present severe anemia and reticulocytopenia (4). Viral reactivation can occur in proerythrocytes and myocardial cells, and could be the cause of multi-organ damage (4–8). The pathogenic effects of parvovirus appear to be immune-mediated (5–8, 11, 12, 14, 15). Besides, the expansion of viral inclusion bodies in proerythroblasts mediating erythroid maturation arrest has also been observed in PRCA patients suggesting the direct pathogenic effect of the virus (2, 16). Intravenous immune globulin (IVIG) may be effective for PRCA (3, 4), intrauterine anemia (17), mantle cell lymphoma (18) due to the presence of IgG-neutralizing antibodies against parvovirus B19. However, the efficacy of IVIG is still unclear for viral myocarditis (19) and chronic fatigue syndrome (20) associated with parvovirus B19.
HLH is a group of rare but life-threatening disorders characterized by hyperinflammatory responses and dysregulated immune cells. There are many causes of HLH, including inborn errors of immunity, inborn errors of metabolism, and many kinds of tumors, including lymphoma (21). A variety of viral infections can trigger HLH (22), including human herpesvirus and human immunodeficiency virus (23). Less commonly, parvovirus B19 is associated with the life-threatening HLH; hence, early identification of triggers and treatment of the primary disease is key to a good prognosis.
There are few case reports of parvovirus B19 infection complicated by HLH in transplant patients (24–26). Herein, we describe a case of HLH in a heart-lung transplant patient due to parvovirus B19 infection. Through IVIG treatment alone, the maturity of the erythroid was recovered, and the features of HLH were in remission. Finally, we summarize the reported cases of parvovirus B19 infection complicated by HLH.
A 59-year-old female suffering from heart and lung failure due to long-term pulmonary hypertension underwent cardiorespiratory combined transplantation and was given tacrolimus and methylprednisolone for anti-rejection after transplantation. She had no other medical history, and no hereditary illnesses ran in her family. The patient had no bleeding from the wound and no acute rejection after the operation. She received two months of rehabilitation. Two months later, her condition changed, and she experienced repeated reductions in hemoglobin (60 g/L, reference range 130-170 g/L) and reticulocytes (0.001×1012/L, 0.1%) (Figure 1).
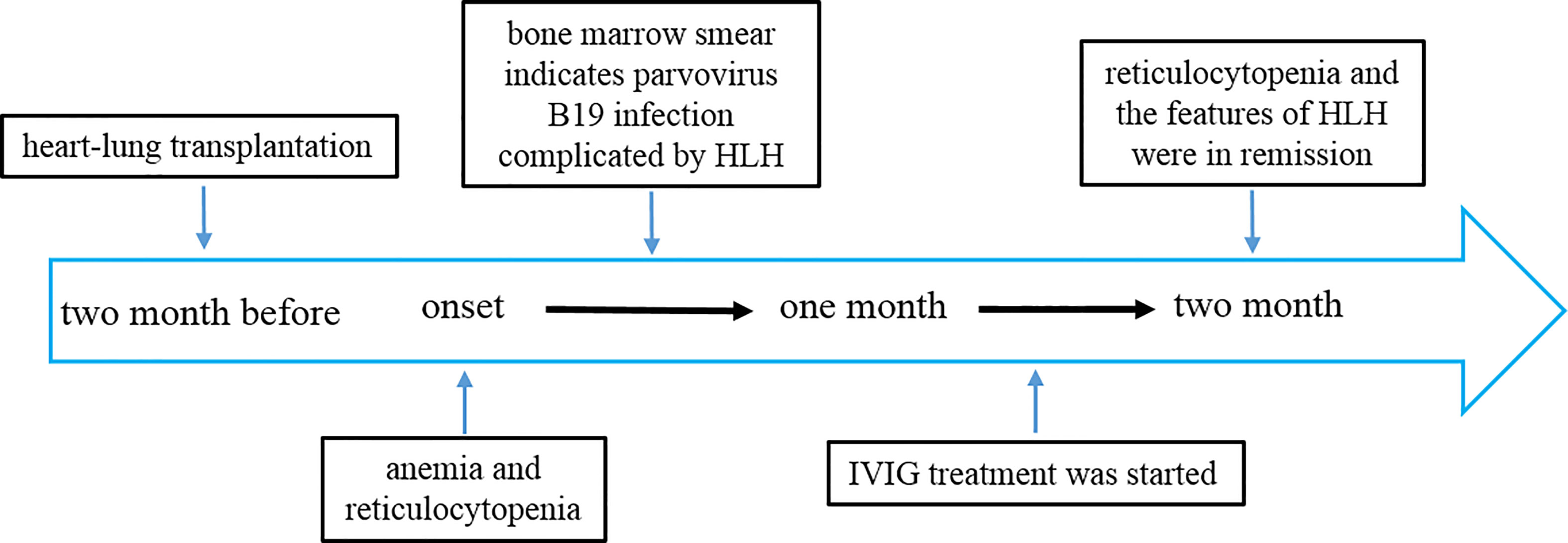
Figure 1 Timeline depicting the disease course of the patient. The timeline illustrates the different events in the course of the patient’s treatment and disease progression.
Anemia was not improved after symptomatic support treatment. The monitoring of biochemical showed alanine aminotransferase, glutamic oxaloacetic aminotransferase, bilirubin, creatinine, and myocardial enzyme were within the normal range which indicated that there was no organ dysfunction of liver and kidney. During this period, although the patient had repeated fever, pathogenic tests of blood culture, sputum culture, urine culture and pleural effusion culture were all negative. Her C-reactive protein was 1.4 mg/L (reference range 0-8 ng/mL) and procalcitonin was 0.08 ng/mL (reference range 0-0.5 ng/mL) which suggested common pathogens were unlikely to be the cause of anemia.
In this condition, bone marrow puncture was performed. The bone marrow smear revealed many giant proerythroblasts (Figure 2A) and erythroid maturation arrest. Basophilic, vacuolar cytoplasm and purple-colored virus inclusion bodies in the nucleus were observed in giant proerythroblasts suggestive of B19 infection. Next-generation sequencing of her peripheral blood confirmed that the only pathogen was parvovirus B19 (Figure 3), and quantitative PCR revealed that the viral load was 1.4×1010 copy/mL (range, 0-103 copy/mL). Patient found to have increased ferritin (3865 ng/mL, reference range 7-323 ng/mL), triglycerides (4.6 mmol/L, reference range 0.3-1.7 mmol/L), reduced fibrinogen (0.83 g/L, reference range 2.0-4.0 g/L), elevated body temperature (38.5°C) for ten days, hemophagocytic cells in the bone marrow smears (Figures 2A, B), enlarged spleen, and cytopenia. Except for the unexecuted assay of serum soluble IL-2R and NK cell activity, the patient’s clinical manifestations met the diagnostic criteria of HLH as described (23).
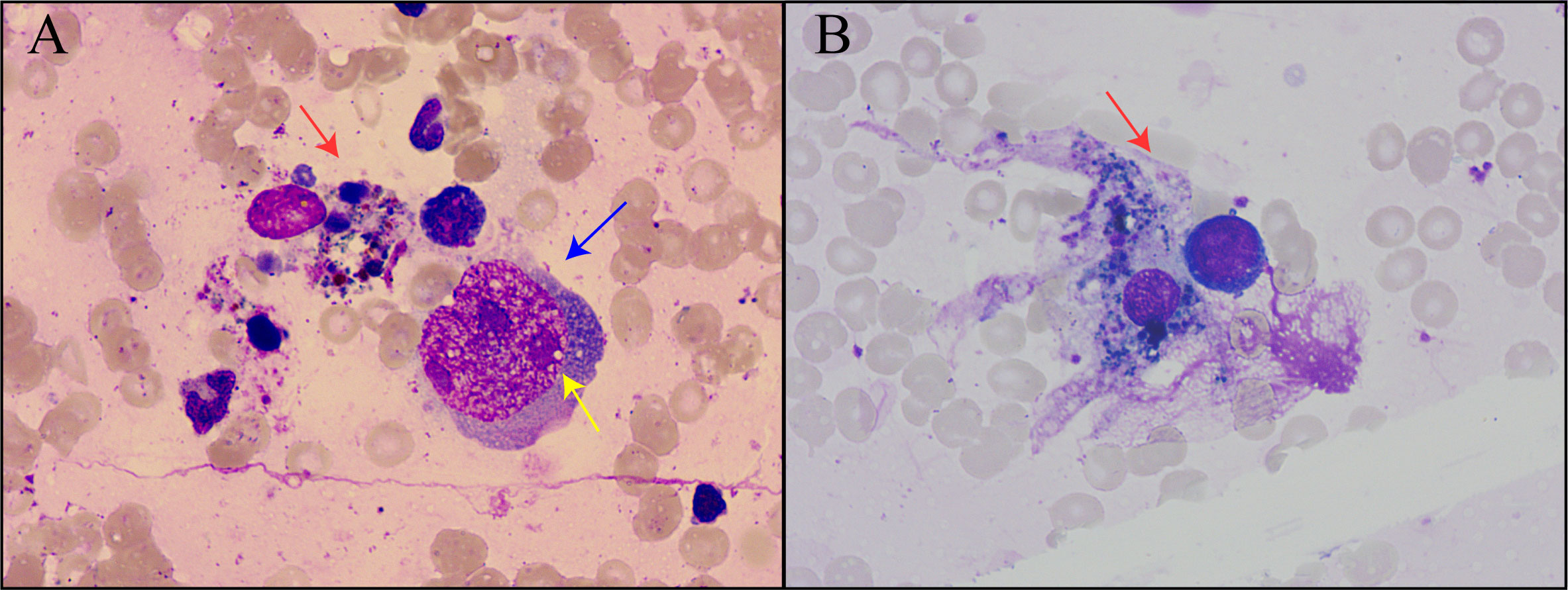
Figure 2 Bone marrow smear of the patient. (A) Hemophagocytic cells and proerythrocytes infected by parvovirus B19. Red arrows indicated hemophagocytic cells, blue arrows indicated proerythroblasts, and yellow arrows indicated viral inclusion bodies. (B) Hemophagocytic cells underwent phagocytosis.
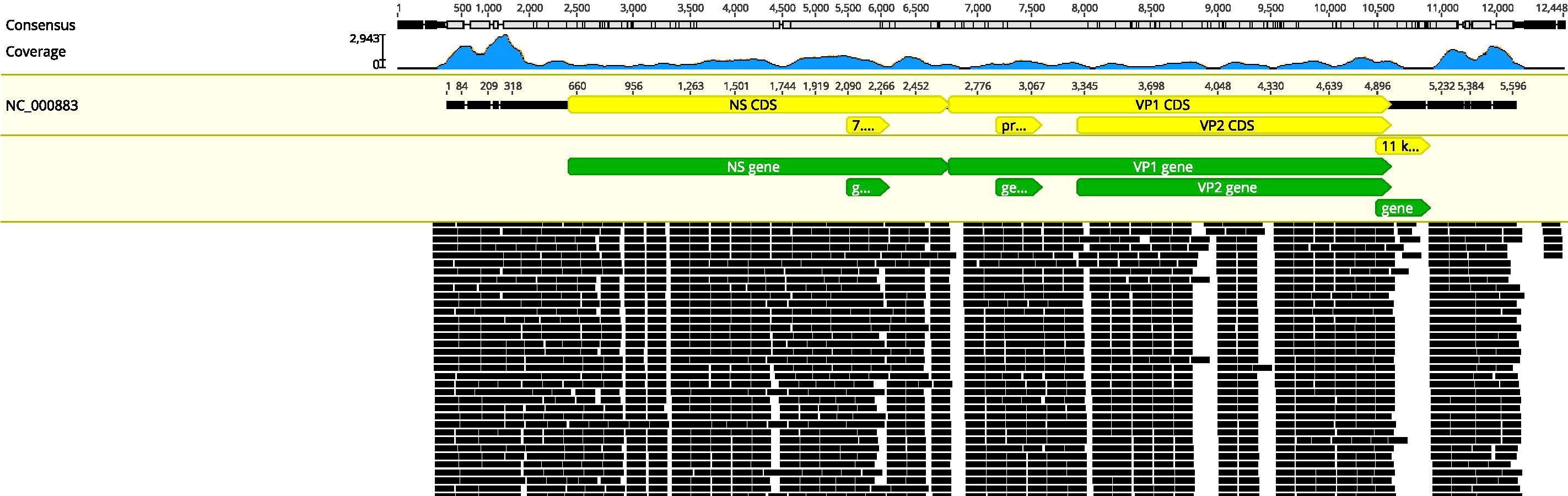
Figure 3 Results of next-generation sequencing in the peripheral blood. Mapping results of nucleotide sequences distributed along the genome of parvovirus B19 in the peripheral blood to parvovirus B19 reference genome NC_000883.
The patient’s peripheral blood did not reveal pathogens other than parvovirus B19 detected by metagenomic next-generation sequencing as described before (27, 28). Also, whole exome sequencing did not identify any HLH-associated mutations. Other possible causes for HLH, including immune disorder and tumor were ruled out, and the patient was eventually diagnosed with parvovirus B19 infection complicated by HLH. After intravenous immunoglobulin (20g/d) for ten days, the patient’s serum IgG increased from 670mg/dL (reference range 860-1740 mg/dL) at the beginning to normal, reticulocytes increased to 3%, and the viral load of parvovirus B19 was reduced to 9.2×104 copies/mL. Another bone marrow smear demonstrated that erythroid maturation was recovered, and the features of HLH were in remission.
Parvovirus B19 infection is common, and the prevalence of IgG antibodies in the population increases with age (29). In most cases, the infection can be asymptomatic and self-limited. Erythema infectiosum or arthropathy occurs in healthy children or adults (29). In immunocompromised patients, bone marrow transplant recipients (10) or patients with hemopathy, the infection can lead to autoimmune hemolytic anemia, neutropenia, thrombocytopenia, acute pure red cell aplasia (PRCA), transient aplastic crisis (AC), and rarely HLH (30).
Published cases of parvovirus B19 complicated by HLH are summarized in Table 1. Hemolytic diseases such as hereditary spherocytosis (42–45), sickle cell disease (40), alpha thalassemia (47), glucose-6-phosphate dehydrogenase deficiency (46), and autoimmune hemolytic anemia (41) were the most frequently reported primary disease. Also, a third of patients were immunocompromised, including patients with acquired immune deficiency syndrome (50), autoimmune diseases (37–39), undergoing chemotherapy (48), and post-transplantation patients (24–26), which can lead to persistent parvovirus B19 infection and may cause pure red cell aplasia. Besides, parvovirus B19 infection complicated with HLH has been reported in otherwise healthy patients (34–36) or patients with pregnancy (31), alcoholic hepatitis (32), myocarditis (33), or Melkersson-Rosenthal syndrome (49). Of note, parvovirus B19-associated reactivation may occur in post-transplantation patients, and some patients will develop pure red cell aplasia and HLH (51). Thus, parvovirus B19 reactivation should be considered in transplant patients with decreased hemoglobin and reticulocytes without a clear cause. Giant proerythroblasts and purple inclusions in the nucleus on bone marrow smears are typical changes in pure red cell aplasia caused by parvovirus B19. If HLH occurs in such patients, it is necessary to rule out the possibility of other pathogens, such as Cytomegalovirus and Epstein-Barr virus (23).
Treatment for parvovirus B19 infection is primarily symptomatic with IVIG used in chronic infection with anemia. A five-day continuous IVIG at 400 mg/kg/day is suggested for patients with solid organ transplantation or other immunosuppression (52), and in this case, parvovirus B19 infection and HLH features were remissions after the treatment of IVIG at 20 g/day. Most patients with parvovirus B19 infection complicated by HLH can achieve remission via IVIG and/or steroids. In addition, 20 out of 24 patients survived, indicating a better prognosis of parvovirus B19-associated HLH compared to other types of HLH (Table 1).
The condition and treatment of transplant patients are complex, and the clinical manifestations of the disease can be very confusing. When these patients present with chronic anemia and cytopenia, clinicians need to be alert to parvovirus B19 infection complicated by HLH, which requires hematologists, infectious disease specialists, critical care medicine specialists, and immunologists to work together to develop a clinical diagnosis and treatment plan to avoid misdiagnosis and inappropriate treatment. IVIG can alleviate or cure parvovirus B19 infection complicated by HLH, and at the same time, patients can be protected from the side effects of HLH-2004 treatment (23). Parvovirus B19 infection easily recurs in transplant patients due to long-term immunosuppression (53), but patients in this condition can avoid death caused by HLH.
In transplant patients receiving long-term immunosuppressive therapy, clinicians need to be aware of parvovirus B19 infection and associated risk for HLH. IVIG treatment can alleviate features of parvovirus B19-associated HLH without the need for more toxic or immunosuppressive therapies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The ethics committees of the First Affiliated Hospital of Zhejiang University School of Medicine, approved the study protocol. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained for the publication of this case report.
All authors drafted the manuscript, prepared the figures and critically reviewed the final manuscript. All authors contributed to the article and approved the submitted version.
The work was supported by the National Natural Science Foundation of China (grant # 82202356).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Simmonds P, Aiewsakun P, Katzourakis A. Prisoners of war - host adaptation and its constraints on virus evolution. Nat Rev Microbiol (2019) 17:321–8. doi: 10.1038/s41579-018-0120-2
3. Mouthon L, Guillevin L, Tellier Z. Intravenous immunoglobulins in autoimmune- or parvovirus b19-mediated pure red-cell aplasia. Autoimmun Rev (2005) 4:264–9. doi: 10.1016/j.autrev.2004.10.004
4. Crabol Y, Terrier B, Rozenberg F, Pestre V, Legendre C, Hermine O, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: A retrospective study of 10 patients and review of the literature. Clin Infect (2013) 56:968–77. doi: 10.1093/cid/cis1046
5. Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner-La Rocca HP, et al. Relevance of cardiac parvovirus b19 in myocarditis and dilated cardiomyopathy: Review of the literature. Eur J Heart failure (2016) 18:1430–41. doi: 10.1002/ejhf.665
6. Ackermann M, Wagner WL, Rellecke P, Akhyari P, Boeken U, Reinecke P. Parvovirus b19-induced angiogenesis in fulminant myocarditis. Eur Heart J (2020) 41:1309. doi: 10.1093/eurheartj/ehaa092
7. Bock CT, Klingel K, Kandolf R. Human parvovirus b19-associated myocarditis. New Engl J Med (2010) 362:1248–9. doi: 10.1056/NEJMc0911362
8. Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J (2008) 29:2073–82. doi: 10.1093/eurheartj/ehn296
9. Rajendran A, Trehan A. Fever and rash in an immunocompromised child. Lancet Infect Dis (2014) 14:256. doi: 10.1016/S1473-3099(13)70215-9
10. Rattani N, Matheny C, Eckrich MJ, Madden LM, Quigg TC. Parvovirus b19-associated graft failure after allogeneic hematopoietic stem cell transplantation. Cancer Rep (2022) 5:e1403. doi: 10.1002/cnr2.1403
11. Rigante D, Mazzoni MB, Esposito S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun Rev (2014) 13:96–102. doi: 10.1016/j.autrev.2013.09.004
12. Illescas-Montes R, Corona-Castro CC, Melguizo-Rodriguez L, Ruiz C, Costela-Ruiz VJ. Infectious processes and systemic lupus erythematosus. Immunology (2019) 158:153–60. doi: 10.1111/imm.13103
13. Keighley CL, Skrzypek HJ, Wilson A, Bonning MA, Gilbert GL. Infections in pregnancy. Med J Aust (2019) 211:134–41. doi: 10.5694/mja2.50261
14. Ferri C, Arcangeletti MC, Caselli E, Zakrzewska K, Maccari C, Calderaro A, et al. Insights into the knowledge of complex diseases: Environmental infectious/toxic agents as potential etiopathogenetic factors of systemic sclerosis. J Autoimmun (2021) 124:102727. doi: 10.1016/j.jaut.2021.102727
15. Elbadry MI, Khaled SAA, Ahmed NM, Abudeif A, Abdelkareem RM, Ezeldin M, et al. Acute human parvovirus b19 infection triggers immune-mediated transient bone marrow failure syndrome, extreme direct hyperbilirubinaemia and acute hepatitis in patients with hereditary haemolytic anaemias: Multicentre prospective pathophysiological study. Br J haematology (2021) 193:827–40. doi: 10.1111/bjh.17484
16. Mende M, Sockel K. Parvovirus b19 infection. New Engl J Med (2018) 379:2361. doi: 10.1056/NEJMicm1807156
17. Rugolotto S, Padovani EM, Sanna A, Chiaffoni GP, Marradi PL, Borgna-Pignatti C. Intrauterine anemia due to parvovirus b19: Successful treatment with intravenous immunoglobulins. Haematologica (1999) 84:668–9.
18. Tang JW, Lau JS, Wong SY, Cheung JL, Chan CH, Wong KF, et al. Dose-by-dose virological and hematological responses to intravenous immunoglobulin in an immunocompromised patient with persistent parvovirus b19 infection. J Med Virol (2007) 79:1401–5. doi: 10.1002/jmv.20870
19. Hazebroek MR, Henkens M, Raafs AG, Verdonschot JAJ, Merken JJ, Dennert RM, et al. Intravenous immunoglobulin therapy in adult patients with idiopathic chronic cardiomyopathy and cardiac parvovirus b19 persistence: A prospective, double-blind, randomized, placebo-controlled clinical trial. Eur J Heart failure (2021) 23:302–9. doi: 10.1002/ejhf.2082
20. Attard L, Bonvicini F, Gelsomino F, Manfredi R, Cascavilla A, Viale P, et al. Paradoxical response to intravenous immunoglobulin in a case of parvovirus b19-associated chronic fatigue syndrome. J Clin Virol (2015) 62:54–7. doi: 10.1016/j.jcv.2014.11.021
21. Huang L, Wu W, Zhu Y, Yu H, Tang L, Fang X. Case report: Hemophagocytic lymphocytosis in a patient with glutaric aciduria type iic. Front Immunol (2021) 12:810677. doi: 10.3389/fimmu.2021.810677
22. Janka GE, Lehmberg K. Hemophagocytic syndromes–an update. Blood Rev (2014) 28:135–42. doi: 10.1016/j.blre.2014.03.002
23. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis (2007) 7:814–22. doi: 10.1016/S1473-3099(07)70290-6
24. Ardalan MR, Shoja MM, Tubbs RS, Esmaili H, Keyvani H. Postrenal transplant hemophagocytic lymphohistiocytosis and thrombotic microangiopathy associated with parvovirus b19 infection. Am J Transplant (2008) 8:1340–4. doi: 10.1111/j.1600-6143.2008.02244.x
25. Steffen CJ, Koch N, Eckardt KU, Amann K, Seelow E, Schreiber A. Hemophagocytic lymphohistiocytosis and thrombotic microangiopathy after parvovirus b19 infection and renal transplantation: A case report. BMC Nephrol (2021) 22:337. doi: 10.1186/s12882-021-02538-0
26. Tavera M, Petroni J, Leon L, Minue E, Casadei D. Reactive haemophagocytic syndrome associated with parvovirus b19 in a kidney-pancreas transplant patient. Nefrologia publicacion oficial la Sociedad Espanola Nefrologia (2012) 32:125–6. doi: 10.3265/Nefrologia.pre2011.Oct.11179
27. Huang L, Zhang X, Pang L, Sheng P, Wang Y, Yang F, et al. Viral reactivation in the lungs of patients with severe pneumonia is associated with increased mortality, a multicenter, retrospective study. J Med Virol (2023) 95:e28337. doi: 10.1002/jmv.28337
28. Huang L, Zhang X, Fang X. Case report: Epstein-barr virus encephalitis complicated with brain stem hemorrhage in an immune-competent adult. Front Immunol (2021) 12:618830. doi: 10.3389/fimmu.2021.618830
29. Qiu J, Soderlund-Venermo M, Young NS. Human parvoviruses. Clin Microbiol Rev (2017) 30:43–113. doi: 10.1128/CMR.00040-16
30. Kerr JR. A review of blood diseases and cytopenias associated with human parvovirus b19 infection. Rev Med Virol (2015) 25:224–40. doi: 10.1002/rmv.1839
31. Mayama M, Yoshihara M, Kokabu T, Oguchi H. Hemophagocytic lymphohistiocytosis associated with a parvovirus b19 infection during pregnancy. Obstet Gynecol (2014) 124:438–41. doi: 10.1097/AOG.0000000000000385
32. Luo J, Zhang J, Lai W, Wang S, Zhou L, Shi Y, et al. Disseminated human parvovirus b19 infection induced multiple organ dysfunction syndrome in an adult patient with alcoholic hepatitis complicated by hemolytic anemia: A case report and literature review. Front Immunol (2021) 12:742990. doi: 10.3389/fimmu.2021.742990
33. Bal A, Mishra B, Singh N, Das A, Jindal SK. Fulminant parvovirus b19-associated pancarditis with haemophagocytic lympho-histiocytosis in an immunocompetent adult. APMIS (2009) 117:773–7. doi: 10.1111/j.1600-0463.2009.02528.x
34. Syruckova Z, Stary J, Sedlacek P, Smisek P, Vavrinec J, Komrska V, et al. Infection-associated hemophagocytic syndrome complicated by infectious lymphoproliferation: A case report. Pediatr Hematol Oncol (1996) 13:143–50. doi: 10.3109/08880019609030804
35. Kaya Z, Ozturk G, Gursel T, Bozdayi G. Spontaneous resolution of hemophagocytic syndrome and disseminated intravascular coagulation associated with parvovirus b19 infection in a previously healthy child. Jpn J Infect Dis (2005) 58:149–51.
36. Pedrosa AF, Mota A, Morais P, Nogueira A, Brochado M, Fonseca E, et al. Haemophagocytic syndrome with a fatal outcome triggered by parvovirus b19 infection in the skin. Clin Exp Dermatol (2014) 39:222–3. doi: 10.1111/ced.12208
37. Sakai H, Otsubo S, Miura T, Iizuka H. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol (2007) 57:S111–114. doi: 10.1016/j.jaad.2006.11.024
38. Kalmuk J, Matar S, Feng G, Kilb E, Lim MY. Parvovirus b19-induced hemophagocytic lymphohistiocytosis: Case report and review of the literature. Clin Case Rep (2019) 7:2076–81. doi: 10.1002/ccr3.2401
39. Jeong JY, Park JY, Ham JY, Kwon KT, Han S. Molecular evidence of parvovirus b19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: Case report. Med (Baltimore) (2020) 99:e22079. doi: 10.1097/MD.0000000000022079
40. Sahu SK, Agrawal A, Das P. The dilemma of diagnosing hemophagocytic lymphohistiocytosis in sickle cell disease. Cureus (2020) 12:e12255. doi: 10.7759/cureus.12255
41. Sekiguchi Y, Shimada A, Imai H, Wakabayashi M, Sugimoto K, Nakamura N, et al. A case of recurrent autoimmune hemolytic anemia during remission associated with acute pure red cell aplasia and hemophagocytic syndrome due to human parvovirus b19 infection successfully treated by steroid pulse therapy with a review of the literature. Int J Clin Exp Pathol (2014) 7:2624–35.
42. Hermann J, Steinbach D, Lengemann J, Zintl F. [parvovirus b 19 associated hemophagocytic syndrome in a patient with hereditary sperocytosis]. Klin Padiatr (2003) 215:270–4. doi: 10.1055/s-2003-42662
43. Yilmaz S, Oren H, Demircioglu F, Firinci F, Korkmaz A, Irken G. Parvovirus b19: A cause for aplastic crisis and hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer (2006) 47:861. doi: 10.1002/pbc.20807
44. Gowarty J, Oda J, Cable C. Hemophagocytic lymphohistiocytosis. Proc (Bayl Univ Med Cent) (2018) 31:350–1. doi: 10.1080/08998280.2018.1446877
45. Kim KT, Hong KT, Kim BK, An HY, Choi JY, Chang YH, et al. Hemophagocytic lymphohistiocytosis associated with parvovirus b19-induced aplastic crisis in a hereditary spherocytosis patient: A case report and literature review. Pediatr Hematol Oncol (2022) 39:158–65. doi: 10.1080/08880018.2021.1949082
46. Zeckanovic A, Perovnik M, Jazbec J, Petrovec M, Pokorn M, Kavcic M. Parvovirus b19-associated hemophagocytic lymphohistiocytosis in a patient with glucose-6-phosphate dehydrogenase deficiency. J Pediatr Hematol Oncol (2018) 40:e550–2. doi: 10.1097/MPH.0000000000001109
47. Aravamudan VM, Er C, Hussain I, Cheong NWW, Chern Hao C, Kuthah N, et al. A case of parvovirus-related haemophagocytic lymphohistiocytosis in a patient with hbh disease. Case Rep Med (2018) 2018:8057045. doi: 10.1155/2018/8057045
48. Strenger V, Merth G, Lackner H, Aberle SW, Kessler HH, Seidel MG, et al. Malignancy and chemotherapy induced haemophagocytic lymphohistiocytosis in children and adolescents-a single centre experience of 20 years. Ann Hematol (2018) 97:989–98. doi: 10.1007/s00277-018-3254-4
49. De Maria A, Zolezzi A, Passalacqua G, Leva M, Tarchino F, Spaggiari P, et al. Melkersson-rosenthal syndrome associated with parvovirus b19 viraemia and haemophagocytic lymphohistiocytosis. Clin Exp Dermatol (2009) 34:e623–625. doi: 10.1111/j.1365-2230.2009.03337.x
50. Macauley P, Abu-Hishmeh M, Dumancas C, Alexander-Rajan V, Piedra-Chavez F, Nada K, et al. Hemophagocytic lymphohistiocytosis associated with parvovirus b19 in a patient with acquired immunodeficiency syndrome. J Investig Med High Impact Case Rep (2019) 7:2324709619883698. doi: 10.1177/2324709619883698
51. Porignaux R, Vuiblet V, Barbe C, Nguyen Y, Lavaud S, Toupance O, et al. Frequent occurrence of parvovirus b19 dnaemia in the first year after kidney transplantation. J Med Virol (2013) 85:1115–21. doi: 10.1002/jmv.23557
52. Eid AJ, Ardura MI, Practice ASTIDCo. Human parvovirus b19 in solid organ transplantation: Guidelines from the american society of transplantation infectious diseases community of practice. Clin Transplant (2019) 33:e13535. doi: 10.1111/ctr.13535
Keywords: parvovirus B19, transplantation, HLH, hemophagocyticsyndrome, hemophagocytic lymphohistiocytosis
Citation: Zhang X, Wang J, Huang X, Zhu Y, Zhu Y, Tang L, Cai H, Fang X and Huang L (2023) Case Report: Parvovirus B19 infection complicated by hemophagocytic lymphohistiocytosis in a heart-lung transplant patient. Front. Immunol. 14:1099468. doi: 10.3389/fimmu.2023.1099468
Received: 15 November 2022; Accepted: 26 January 2023;
Published: 07 February 2023.
Edited by:
Chenyang Duan, Chongqing Medical University, ChinaReviewed by:
Hongping Qu, Shanghai Jiao Tong University, ChinaCopyright © 2023 Zhang, Wang, Huang, Zhu, Zhu, Tang, Cai, Fang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingtong Huang, bGluZ3RvbmdodWFuZ0B6anUuZWR1LmNu; Xueling Fang, MTE5MTAxMkB6anUuZWR1LmNu; Hongliu Cai, MTE5MzAwMUB6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.