- 1Department of Hematology, Central Hospital of Wuhan, Wuhan, China
- 2Department of Rheumatology and Immunology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
There is an increased risk of malignancies in patients with many systemic rheumatic diseases, which negatively impact on their quality of life. The risk and types of malignancies can differ by the type of rheumatic diseases. Possible mechanisms linking them are dynamic and complicated, including chronic inflammation and damage in rheumatic disease, inability to clear oncogenic infections, shared etiology and some anti-rheumatic therapies. Although certain disease-modifying anti-rheumatic drugs (DMARDs) have been proved to be potentially carcinogenic, the majority of them were not associated with increased risk of most malignancies in patients with systemic rheumatic diseases.
1 Introduction
It has been noted that the risk for the development of malignancies increases in patients with many systemic rheumatic diseases compared with general population (1). This may be related to the immunodeficiency and increased inflammatory burden caused by the pathobiology of the underlying rheumatic diseases, as well as other factors, such as environmental exposure and lifestyle. Meanwhile, although progression in anti-rheumatic therapies has been remarkable in the past two decades, favorably affecting quality of life for patients, these treatments such as conventional synthetic disease-modifying anti-rheumatic drugs(csDMARDs), biologic or targeted synthetic DMARDs (b/tsDMARDs) could also be carcinogenic. Here we summarized the risk of malignancies in systemic rheumatic diseases and the carcinogenic possibility of antirheumatic therapies, especially the newly developed b/tsDMARDs.
2 Systemic lupus erythematosus (SLE)
SLE is a systemic autoimmune disease, characterized by aberrant activity of the immune system, leading to varying clinical manifestations in the affected organs (2). SLE is proved to be linked with a slight increase in the overall risk of malignancy. According to previous reports, the standardized incidence ratio (SIR) for overall cancer risk in SLE population is 1.14 (95% CI 1.05-1.23) to 4.13 (95% CI 2.26-6.93) (3, 4). The risk of many malignancies, such as vulva, lung, thyroid, and hematologic cancers, particularly non-Hodgkin lymphoma (NHL), increased in varying degrees (4). However, prostate cancer, cutaneous melanoma, breast and endometrial cancer seem to be associated with a decreased risk in patients with SLE (5, 6).
Many potential risk factors have been related to the increase of certain malignancies in SLE patients. It is found in a large multi-center cohort of SLE patients that male sex and older age of SLE onset were risk factors for most cancer types (7). Another important risk factor for cancer, especially lung cancer in SLE population is smoking (7). Although cyclophosphamide was considered as a risk factor for nonmelanoma skin cancer (NMSC), other immunosuppressive medications for treating SLE were not clearly associated with higher risk of malignancies in these patients (7).
Both genetic predisposition to impaired DNA repair and chronic inflammation may facilitates the accumulation of DNA damage in patients with SLE, and thus partly contribute to the increased risk of certain malignancies (8). Besides, higher risk for virus-associated cancers (e.g., vaginal, cervical, anal cancers associated with HPV) in SLE suggests that inability to clear oncogenic infections may be one of the potential mechanisms linking cancer with this disease (9). As for the trend of decreased risk in other malignancies such as melanoma and breast cancer, particularly oestrogen-receptor-negative breast cancers, one possible explanation is that some DNA-damaging lupus autoantibodies are selectively toxic to cancer cells and tumors with pre-existing defects in DNA repair. Thus, these autoantibodies might contribute to a reduced incidence of DNA-repair-deficient malignancies in SLE (8).
3 Primary Sjogren’s syndrome(pSS)
Sjögren’s syndrome(SS) is a systemic autoimmune disease in which immune cells attack and destroy exocrine glands, resulting in classic symptoms of dry eye and mouth (10). It is divided into two categories: secondary SS which develops in addition to other autoimmune diseases and pSS which we focus here in this review. Numerous previous studies have confirmed that the risk of lymphoma in patients with pSS was increased (11, 12). It has been reported that these patients have a 5–15 times higher risk of lymphoma than the general population (13). Moreover, the risk of lymphoma development in pSS is the highest among all the autoimmune diseases (14).
Many predictive markers for the development of lymphoma in SS have been suggested. Hypocomplementemia and lymphocytopenia at pSS diagnosis are the strongest predictors (15). Parotidomegaly, purpura, splenomegaly, lymphadenopathy, anemia, rheumatoid factor(RF), cryoglobulinemia, presence of ectopic germinal centers, focus score of >3, score of >5 on the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI), and germinal mutations in TNFAIP3 have also been found as significant predictors for the development of lymphoma in patients with SS (10, 15).
Abnormal lymphoproliferation in SS is considered to be associated with lymphoma development. It is a multistep and chronic process during which a benign polyreactive B cell population is finally driven into a malignant monoclonal B cell component (16). Accumulated evidence have shown that chronic antigenic stimulation by exoantigen or autoantigens is of critical importance in this process (17).
Besides lymphoma, a recent meta-analysis showed that pSS was significantly associated with increased risks of overall malignancy(pooled SIR 2.17, 95% CI 1.57-3.00), hematological malignancy(pooled SIR 11.55, 95%CI 4.32-30.90) including NHL, Hodgkin lymphoma, multiple myeloma(pooled SIR 8.27, 95%CI 3.08-22.24), leukemia(pooled SIR 2.56, 95%CI 1.78-3.69) and solid tumors(pooled SIR 1.39, 95%CI 0.90-2.13) including lung cancer(pooled SIR 1.55, 95%CI 1.29-1.85), thyroid cancer(pooled SIR 2.05, 95%CI 1.20-3.48), NMSC (pooled SIR 1.71, 95%CI 1.08-2.72), kidney/urinary tract cancer(pooled SIR 1.36, 95%CI 1.02-1.81), liver cancer(pooled SIR 1.70, 95%CI 1.13-2.57) and prostate cancer(pooled SIR 1.50, 95%CI 1.02-2.22) (18). Data from the French health insurance database reported similar results, but found bladder and breast cancer incidences were lower in hospitalized pSS patients in France (19).
4 Idiopathic inflammatory myopathies (IIM)
IIM are a heterogeneous group of autoimmune disorders usually characterized by chronic inflammation of the muscle with variable clinical symptoms, treatment responses and prognosis (20). The subgroups of IIM include polymyositis (PM), dermatomyositis (DM), inclusion body myositis (IBM), antisynthetase syndrome (ASyS), and immune-mediated necrotizing myopathy (IMNM) (20). IIM has an increased risk of cancer, generally in the subgroups of PM and DM, especially the later (21). Approximately one in four IIM patients are diagnosed with cancer within 3 years before or after IIM onset (22).
The risk factors of malignancies in patients with IIM have been investigated in many studies. A recent meta-analysis showed that among IIM, DM subtype, older age, male sex, dysphagia, cutaneous ulceration and anti-transcriptional intermediary factor-1 gamma(anti-TIF1-γ) positivity were all associated with significantly increased risk of cancer, while PM and clinically amyopathic DM(ADM) subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and anti-Jo1 or anti-EJ positivity were associated with reduced risk (23).
Although DM has been proved to be the subgroup most strongly associated with cancer in IMM, it is, actually, also comprised of a heterogeneous group with different cancer risk. ADM is a subtype within the DM spectrum which lacks the clinical as well as laboratory evidence of muscle involvement. The most commonly reported malignancies in ADM are breast, lung, and ovarian cancer, while nasopharyngeal cancer is among the three most common ones in “classic” DM besides lung and ovarian cancer (24). Moreover, the discovery of myositis specific antibodies(MSA) has revealed that those subtypes can be distinguished from one another based on serology (25). Numerous studies have suggested the presence of anti-TIF1-γ confers a great risk of cancer development in DM (26–28).And data from a large UK-based adult DM cohort found that cancer types differ according to anti-TIF1-Ab status, with female anti-TIF1-Ab-positive patients at increased risk of ovarian cancer (26). Among anti-TIF1-γ-positive DM patients, coexisting autoantibodies against transcription factor Sp4 and cell division cycle and apoptosis regulator protein 1 (CCAR1) are associated with decreased cancer risk (29, 30).
The mechanisms linking IIM to the onset of tumor have not yet been fully understood, yet studies have proposed that cancer-DM relationship is a continuum that can be explained within the framework of cancer immunoediting and a cancer triggered autoimmune mechanism is involved in the pathogenesis of IMM, especially DM (30, 31). One possible mechanism is that antitumor response targeting self-proteins mutated in tumors could be directed toward skeletal muscle cells. Another possible mechanism is that autoantigen overexpression by tumor cells could initiate the process of autoimmunity targeting muscle and other related tissues (31, 32). A recent study demonstrated an increased number of genetic alterations in TIF1 genes of tumors from paraneoplastic anti-TIF1-γ-positive patients, as well as a high expression of TIF1g in the tumor, muscle and skin of these patients. This study suggested that these two facts would be important to understand the genesis of paraneoplastic myositis (33).
5 Systemic sclerosis (SSc)
SSc is a relative rare rheumatic disease with vascular injury and fibrosis as its main clinical features (34). According to a study from the European Alliance of Associations for Rheumatology (EULAR) Scleroderma Trials and Research (EUSTAR) database, malignancy has become one of the leading causes of death in this disease (13%) (35). An Scleroderma Cohort Study showed that SSc patients have more than two fold higher risk of cancer occurrence than age- and sex- matched population peers in Australian (36). And the most common cancers in SSc patients are lung cancer and hematological malignancies (37, 38).
A study by Igusa et al. suggested that cancer risk was different in different subgroups according to SSc phenotype, autoantibodies, and temporal clustering (39). In this study, seronegative patients and anti-RNA-Pol-III-positive patients were found to have a higher risk for malignancy, while anticentromere-positive patients had a lower risk. Another research showed that PM/Scl antibodies seem to be associated with a higher risk of cancer in SSc (40).
The association between SSc and malignancy is complex. On one side, chronic inflammation, as well as several environmental factors and occupational exposures such as crystalline silica, organic solvents, pollutants, pesticides, etc., have been regarded inducers of tumor in SSc patients (41, 42). On the other side, it has been suggested that the “foreign” antigen triggering the autoimmune response in SSc patients might actually be a tumor antigen (43).
6 Rheumatoid arthritis (RA)
RA is a chronic autoimmune disease characterized by inflammation and destruction of cartilage and bone in affected joints (44). The association between RA and malignancy was first reported in 1978 (45). Since then, many studies have investigated the association. A meta-analysis by Simon and colleagues confirmed that compared with general population, patients with RA has a modest increased risk (10%) in overall malignancy (46). Also, site-specific malignancy risks were reported. The risk of lung cancer as well as lymphoma was greatly increased. In contrast, the risk of colorectal cancer and breast cancer was decreased (46).
One theory which might partly explain the increased incidence of certain cancer in RA patients is that both these cancers and RA share similar risk factors (47). For example, smoking is a risk factor for both RA and lung cancer (48, 49). It is also suggested that chronic immune stimulation/chronic inflammation and damage in RA may lead to the increased risk of cancer, which supported by the facts that elevated inflammatory biomarkers such as ESR and CRP were associated with increased cancer risk; whereas longer duration corticosteroid therapy were found to be linked with lower lymphoma risk (9, 50).
With the increasing use of immunosuppressive DMARDs and newly developed biologic agents in the management of RA, the clinical outcomes have been greatly improved (51). However, whether or not the treatment for RA can increase the risk of cancers has been of great interest (47).
7 Anti-rheumatic therapies
7.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids(GCs)
Many studies have demonstrated that inflammation can predispose to tumors, thus people have been interested in whether targeting inflammation and the molecules involved in the inflammatory process could represent a good strategy for cancer prevention and treatment. NSAIDs, as well as some other anti-inflammatory agents, have been demonstrated to be able to reducing cell migration and increasing apoptosis and chemo-sensitivity in the tumor microenvironment in many clinical studies (52). Moreover, several researches also suggested that NSAIDs have the potential to act as chemoprevention therapy or anticancer agents in certain malignancies (52–54).
Treatment with glucocorticoid does not appear to be associated with an increased incidence of some malignancies like lymphomas (55–57). However, studies have demonstrated that GCs have complicated influence on pathophysiological processes related to malignancy which needs further investigation (58).
7.2 csDMARDs
Among csDMARDs, cyclophosphamide(CTX) is the most well-known carcinogenic agent which is associated with the risk of bladder cancer, as well as secondary acute leukemia and skin cancer (59).
Methotrexate (MTX) entered clinical medicine as an innovative anti-neoplastic drug in 1948, and later it became favored over cyclophosphamide by rheumatologist in treating progressive RA because of its clinical benefit and lack of carcinogenicity (60). There is no evidence to confirm any oncogenicity effects of MTX, although some case reports have linked it to lymphomas and pseudo lymphomas (61). Of note, Solomon and colleagues observed in their study that non-biologic DMARDs other than MTX (such as leflunomide, sulfasalazine, or hydroxychloroquine) were associated with a reduced overall cancer risk compared with MTX (Hazard Ratio 0.17, 95% CI 0.05-0.65), as well as for several specific cancers (62).
Mycophenolate is an immunosuppressive drug approved for the prophylaxis of allograft rejection in transplant recipients. It is now widely used in many autoimmune disorders. Generally, it does not show a discernable cancer trend (63), and may even have an antitumor effect for several human cancers, including leukemia and some solid tumors (64).
Azathioprine is also a frequently used csDMARDs. Azathioprine treatment seems to be safe with regards to the risk of malignancy in the SLE population (65), but is likely to be linked with lymphoma development in RA patients (66). A 20 year follow-up study showed that although there was a fivefold increase in the lymphoma rates in azathioprine treated RA patients compared with the general population, this increased risk was still small in relation to the relative increase in “background” risk in the RA population (66).
Low dose of cyclosporine has been used in the treatment of autoimmune diseases, including RA and SLE. Several studies have evaluated its oncogenic possibility in nontransplant patients and the results are conflicting (67, 68). However, the majority of them did not find it related with a higher risk of malignancy, especially compared with other csDMARDs (67–69).
7.3 bDMARDs
Many studies have showed that there was no increased risk of malignancies in RA patients with bDMARDs compared with the general population, except for melanoma and NMSC (70–72).And the risk of most malignancies was not increased in patients treated with bDMARDs compared with those on csDMARDs (73). However, the association between certain bDMARD with urinary tract cancer still needs further investigation, as a recent report from the anti-Rheumatic Treatment in Sweden Register(ARTIS) group reported a statistically significantly increased risk of urinary tract cancer with Tumor necrosis factor inhibitors (TNFi), rituximab(RTX) as well as abatacept(ABA) when comparing site-specific relative risks for cancer with b/tsDMARDs vs. b/tsDMARD naïve RA (74).
7.3.1 Tumor necrosis factor inhibitors (TNFi)
TNFi (such as adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab) are widely used to treat chronic inflammatory conditions, including RA, juvenile idiopathic arthritis, psoriasis, psoriatic arthritis, spondylarthropathies, inflammatory bowel disease and uveitis (75). However, historically, whether anti-TNFα therapies predispose people to cancer has been deeply concerned (76, 77), as the relationship of tumor necrosis factor (TNF), a multifunctional cytokine, with tumor is complex. On one hand, TNF has undisputed tumor-destructive function under certain circumstances; on the other hand, it is a major mediator of cancer-related inflammation and may exert tumor-promoting effects (77). As a result, TNFi was often avoided in patients with a history of malignancy.
In a large observational study by ARTIS group, Wadström and colleague found that the overall cancer risk among RA patients initiating treatment with TNFi was similar compared with that among patients with bDMARD-naïve RA (70). Moreover, according to a nationwide, population-based cohort study, treatment with TNFi was not associated with recurrent or new primary cancer development in patients with previous cancer (78). With respect to site-specific malignant neoplasms, the risk of skin cancer, particularly NMSC, in patients who recieved TNFi is of greatest concern and is still controversial (79–81).
7.3.2 Rituximab(RTX)
RTX is a CD20 specific murine/human chimeric monoclonal antibody agents. Since registered by the Food and Drug Administration in 1997 for treatment of lymphomas, it has improved the prognosis of all B-cell derived lymphoproliferative diseases (82). It has also been used in the treatment of many rheumatic diseases, such as ANCA-associated vasculitis (83). A systematic literature review showed that RTX did not increase the risk of cancer in patients who received this therapy (73).
7.3.3 Tocilizumab(TCZ)
TCZ, an IL-6 receptor antagonist, has been approved for the treatment of RA in many countries throughout the world (84).Compared with csDMARDs, TCZ does not increase the risk of overall cancer, as well as solid cancer (excluding NMSC), NMSC, melanoma, and haematological cancer (70).
7.3.4 Abatacept(ABA)
CTLA-4 agonist ABA is a fusion protein approved for the treatment of RA. It binds to CD80 and CD86 receptors on antigen presenting cells, and then inhibits T-cell proliferation and B-cell stimulation (85). A world observational post-marketing study investigated the risk of overall malignancy and specific cancers in RA patients who received ABA. It was found that compared with other bDMARDs, exposure to ABA was only significantly associated with an increased risk of melanoma. It is not surprising since ABA has an opposite action than ipilimumab, an approved drug which treats malignant melanoma by blocking CTLA-4 (86).
7.4 tsDMARDs
tsDMARDs are made from synthetic chemical compounds. However, unlike csDMARDs which have a dampening effect on the whole immune system, tsDMARDs target specific parts of the immune system, like bDMARDs. In RA and many other rheumatic diseases, the only approved tsDMARDs are Janus kinase (JAK) inhibitors (87). Currently licensed JAK inhibitors include tofacitinib (Pfizer, New York, NY, USA), ruxolitinib (Novartis, Basel,Switzerland), fedratinib (Celgene, Summit, NJ, USA), upadacitinib (AbbVie, North Chicago, IL, USA), peficitinib (Astellas Pharma, Tokyo, Japan), and baricitinib (Eli Lilly, Indianapolis, IN, USA) (88). Among them, tofacitinib, baricitinib, upadacitinib and fedratinib have been approved for treating rheumatic diseases, and peficitinib has only been approved for RA treatment in some Asian countries.
Tofacitinib is a type of JAK inhibitor that preferentially inhibits JAK1 and JAK3. Whether or not tofacitinib increases the risk of developing cancer has been controversial, and the results of studies may be affected by factors such as follow-up time, the definition of the study population, exposure and outcome definition. Maneiro and colleagues showed in a meta-analysis that in RCTs, tofacitinib does not increase the risk for malignancy in RA patients (89). Another meta-analysis also confirmed that there was no increased risk of developing cancer in general or specific cancer types in RA patients receiving treatment with tofacitinib, compared with those receiving csDMARDs or TNFi (90). Recently, however, an open-label, randomised controlled trial (ORAL Surveillance; NCT02092467) which assessed the safety of tofacitinib in comparison with TNFi in patients 50 years of age and older, with at least one cardiovascular risk factor, and with background MTX treatment showed that incidence rates (IRs) for malignancies excluding NMSC and NMSC were higher with tofacitinib versus TNFi (91). Besides, Khosrow-Khavar and colleagues also reported that compared with TNFi, tofacitinib was associated with a numerically increased risk of malignancies(pooled weighted HR: 1.17, 95% CI: 0.85, 1.62) in a subgroup of “RCT-duplicate” RA cohort which emulated the inclusion and exclusion criteria of the ORAL surveillance trial, although it was not associated with the risk in the whole population-based real-world cohort of 83,295 RA patients (92).
Baricitinib is a reversible and selective JAK1/JAK2 inhibitor for treating RA. According to the result from an integrated database of patients who received any baricitinib dose (All-bari-RA), the IR for malignancy (excluding NMSC) during the first 48 weeks was 0.6 (95% CI 0.34-0.91) and remained approximately 1.0 (overall IR 0.9, 95% CI 0.77-1.09) thereafter. The overall age-adjusted SIR was 1.07 (95% CI 0.90-1.26), suggesting comparable incidence of malignancies with the general US population (93).
Upadacitinib is a JAKi engineered for greater JAK1 selectivity over other JAK family members. Integrated analysis from the SELECT phase III clinical programme revealed that rates of malignancies in RA patients receiving upadacitinib were similar among those receiving upadacitinib, MTX or adalimumab (94).
8 Mechanisms linking malignancies and rheumatic diseases
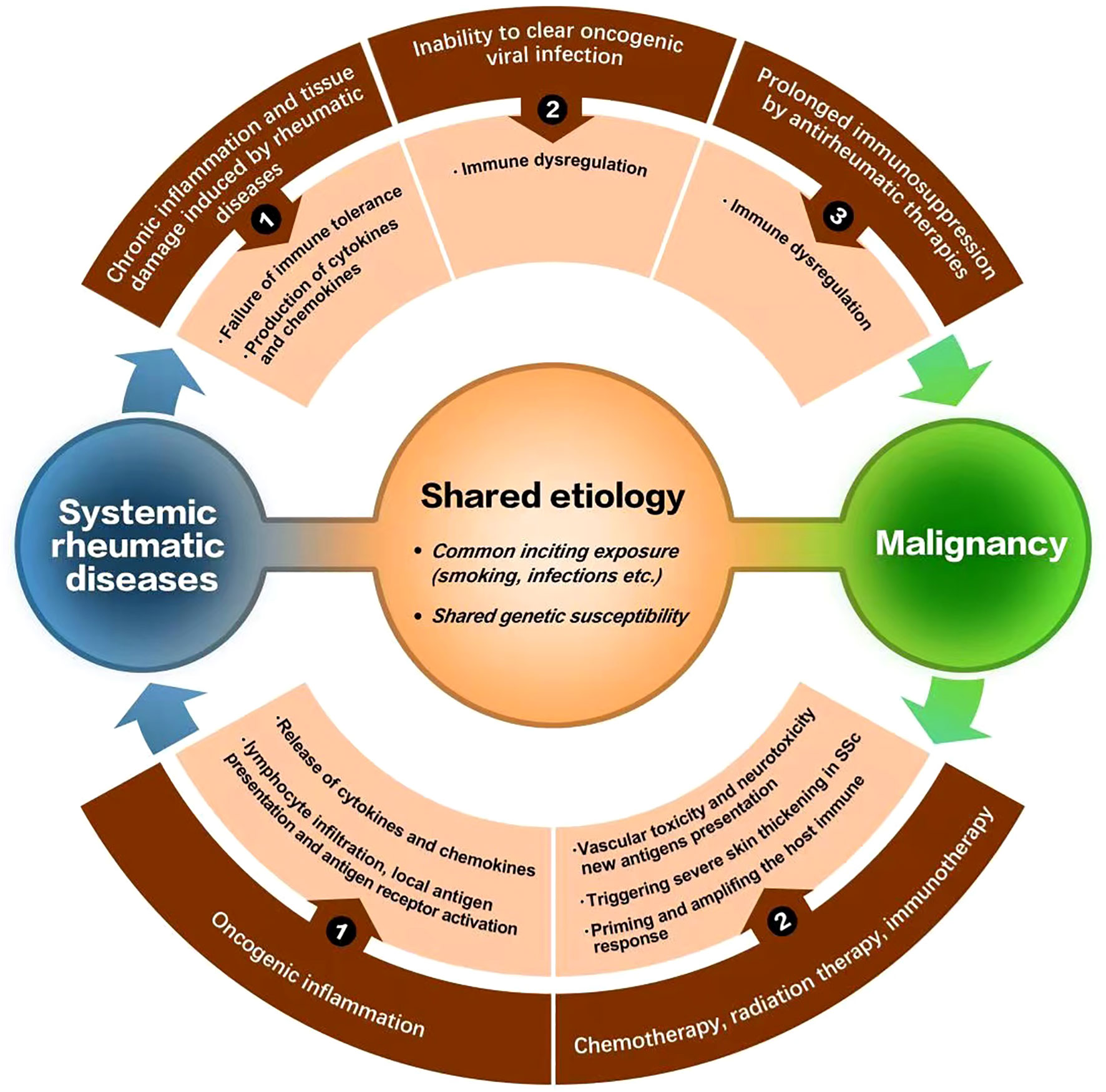
Although possible mechanisms for tumor incidence in systemic rheumatic diseases have been discussed in each section above, several potential mechanisms linking malignancies and rheumatic disease have been generally suggested up to now (9, 95, 96). Chronic inflammation and tissue damage induced by rheumatic disease, inability to clear viral oncogenic infections and certain therapies discussed above are related to malignancies secondary to rheumatic disease; while cancer-induced autoimmunity, treatments for cancer (immunotherapy, chemotherapy or radiation therapy) may contribute to the occurrence of rheumatic disease secondary to malignancies. Besides, shared etiology such as common inciting exposure or shared genetic susceptibility is also a potential mechanism (Figure 1) (9, 95, 96).
9 Discussion
Questions relating to malignancy and rheumatic disease arise commonly in clinical practice. The occurrence of tumor in patients with systemic rheumatic diseases is not rare and negatively impact on their quality of life. The risk and types of malignancies differ in different systemic rheumatic diseases, and even in different subgroups of one particular rheumatic disease. Predictive markers for the development of malignancy in different rheumatic diseases and possible mechanisms linking them have been suggested, which still need further investigation in the coming years.
Generally, the majority of DMARDs was not associated with increased risk of most malignancies in patients with systemic rheumatic diseases. Since most of these patients need to receive DMARDs, and some even need b/tsDMARDs in order to attain and maintain adequate control of the disease, our work may help clinical decisions when regarding malignancy risk of these therapies for them. However, it is of note that some of the above results come from RCTs, especially for newly developed therapies such as JAK inhibitors, and RCTs are not appropriate for assessing the malignancy risk of long-term drug exposure. Therefore, further studies with long-term follow-up and large sample size are still needed for these drugs. And in clinical practice, the risk-benefit ratio should always be fully considered when selecting therapeutic regimen for these patients, so as to improve the long-term prognosis.
Author contributions
Writing - original draft, ZG and CY; Writing - review & editing, and funding acquisition, XZ. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China [82270183 to XZ] and Key R&D plan [2021YFF0703704 to XZ].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shah AA, Casciola-Rosen L, Rosen A. Review: Cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol (2015) 67(2):317–26. doi: 10.1002/art.38928
2. Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med (2020) 172(11):C81–96. doi: 10.7326/AITC202006020
3. Bernatsky S, Clarke AE, Zahedi NO, Labrecque J, Schanberg LE, Silverman ED, et al. Malignancy in pediatric-onset systemic lupus erythematosus. J Rheumatol (2017) 44(10):1484–6. doi: 10.3899/jrheum.170179
4. Bernatsky S, Ramsey-Goldman R, Labrecque J, Joseph L, Boivin JF, Petri M, et al. Cancer risk in systemic lupus: An updated international multi-centre cohort study. J Autoimmun (2013) 42:130–5. doi: 10.1016/j.jaut.2012.12.009
5. Ladouceur A, Tessier-Cloutier B, Clarke AE, Ramsey-Goldman R, Gordon C, Hansen JE, et al. Cancer and systemic lupus erythematosus. Rheum Dis Clin North Am (2020) 46(3):533–50. doi: 10.1016/j.rdc.2020.05.005
6. Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: A systematic review and meta-analysis. Arthritis Res Ther (2018) 20(1):270. doi: 10.1186/s13075-018-1760-3
7. Bernatsky S, Ramsey-Goldman R, Urowitz MB, Hanly JG, Gordon C, Petri MA, et al. Cancer risk in a Large inception systemic lupus erythematosus cohort: Effects of demographic characteristics, smoking, and medications. Arthritis Care Res (Hoboken) (2021) 73(12):1789–95. doi: 10.1002/acr.24425
8. Noble PW, Bernatsky S, Clarke AE, Isenberg DA, Ramsey-Goldman R, Hansen JE. DNA-Damaging autoantibodies and cancer: The lupus butterfly theory. Nat Rev Rheumatol (2016) 12(7):429–34. doi: 10.1038/nrrheum.2016.23
9. Egiziano G, Bernatsky S, Shah AA. Cancer and autoimmunity: Harnessing longitudinal cohorts to probe the link. Best Pract Res Clin Rheumatol (2016) 30(1):53–62. doi: 10.1016/j.berh.2016.03.001
10. Mariette X, Criswell LA. Primary sjogren's syndrome. N Engl J Med (2018) 378(10):931–9. doi: 10.1056/NEJMcp1702514
11. Nocturne G, Virone A, Ng WF, Le Guern V, Hachulla E, Cornec D, et al. Rheumatoid factor and disease activity are independent predictors of lymphoma in primary sjogren's syndrome. Arthritis Rheumatol (2016) 68(4):977–85. doi: 10.1002/art.39518
12. Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: A meta-analysis. Arch Intern Med (2005) 165(20):2337–44. doi: 10.1001/archinte.165.20.2337
13. Nocturne G, Mariette X. Sjogren syndrome-associated lymphomas: An update on pathogenesis and management. Br J Haematol (2015) 168(3):317–27. doi: 10.1111/bjh.13192
14. Tarella C, Gueli A, Ruella M, Cignetti A. Lymphocyte transformation and autoimmune disorders. Autoimmun Rev (2013) 12(8):802–13. doi: 10.1016/j.autrev.2012.11.004
15. Solans-Laque R, Lopez-Hernandez A, Bosch-Gil JA, Palacios A, Campillo M, Vilardell-Tarres M. Risk, predictors, and clinical characteristics of lymphoma development in primary sjogren's syndrome. Semin Arthritis Rheum (2011) 41(3):415–23. doi: 10.1016/j.semarthrit.2011.04.006
16. Goules AV, Tzioufas AG. Lymphomagenesis in sjogren's syndrome: Predictive biomarkers towards precision medicine. Autoimmun Rev (2019) 18(2):137–43. doi: 10.1016/j.autrev.2018.08.007
17. Routsias JG, Goules JD, Charalampakis G, Tzima S, Papageorgiou A, Voulgarelis M. Malignant lymphoma in primary sjogren's syndrome: An update on the pathogenesis and treatment. Semin Arthritis Rheum (2013) 43(2):178–86. doi: 10.1016/j.semarthrit.2013.04.004
18. Zhong H, Liu S, Wang Y, Xu D, Li M, Zhao Y, et al. Primary sjogren's syndrome is associated with increased risk of malignancies besides lymphoma: A systematic review and meta-analysis. Autoimmun Rev (2022) 21(5):103084. doi: 10.1016/j.autrev.2022.103084
19. Goulabchand R, Malafaye N, Jacot W, Witkowski DVP, Morel J, Lukas C, et al. Cancer incidence in primary sjogren's syndrome: Data from the French hospitalization database. Autoimmun Rev (2021) 20(12):102987. doi: 10.1016/j.autrev.2021.102987
20. Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers (2021) 7(1):86. doi: 10.1038/s41572-021-00321-x
21. Opinc AH, Makowska JS. Update on malignancy in myositis-Well-Established association with unmet needs. Biomolecules (2022) 12(1). doi: 10.3390/biom12010111
22. Dobloug GC, Garen T, Brunborg C, Gran JT, Molberg O. Survival and cancer risk in an unselected and complete Norwegian idiopathic inflammatory myopathy cohort. Semin Arthritis Rheum (2015) 45(3):301–8. doi: 10.1016/j.semarthrit.2015.06.005
23. Oldroyd A, Allard AB, Callen JP, Chinoy H, Chung L, Fiorentino D, et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatol (Oxford) (2021) 60(6):2615–28. doi: 10.1093/rheumatology/keab166
24. Udkoff J, Cohen PR. Amyopathic dermatomyositis: A concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol (2016) 17(5):509–18. doi: 10.1007/s40257-016-0199-z
25. DeWane ME, Waldman R, Lu J. Dermatomyositis: Clinical features and pathogenesis. J Am Acad Dermatol (2020) 82(2):267–81. doi: 10.1016/j.jaad.2019.06.1309
26. Oldroyd A, Sergeant JC, New P, McHugh NJ, Betteridge Z, Lamb JA, et al. The temporal relationship between cancer and adult onset anti-transcriptional intermediary factor 1 antibody-positive dermatomyositis. Rheumatol (Oxford) (2019) 58(4):650–5. doi: 10.1093/rheumatology/key357
27. Zhang L, Yang H, Yang H, Liu H, Tian X, Jiang W, et al. Serum levels of anti-transcriptional intermediary factor 1-gamma autoantibody associated with the clinical, pathological characteristics and outcomes of patients with dermatomyositis. Semin Arthritis Rheum (2022) 55:152011. doi: 10.1016/j.semarthrit.2022.152011
28. Ly N, Ma N, Ueda-Hayakawa I, Nguyen C, Anada R, Okamoto H, et al. Clinical and laboratory parameters predicting cancer in dermatomyositis patients with anti-TIF1gamma antibodies. J Dermatol Sci (2021) 104(3):177–84. doi: 10.1016/j.jdermsci.2021.10.003
29. Hosono Y, Sie B, Pinal-Fernandez I, Pak K, Mecoli CA, Casal-Dominguez M, et al. Coexisting autoantibodies against transcription factor Sp4 are associated with decreased cancer risk in patients with dermatomyositis with anti-TIF1gamma autoantibodies. Ann Rheum Dis (2023) 82(2):246–252. doi: 10.1136/ard-2022-222441
30. Fiorentino DF, Mecoli CA, Rosen MC, Chung LS, Christopher-Stine L, Rosen A, et al. Immune responses to CCAR1 and other dermatomyositis autoantigens are associated with attenuated cancer emergence. J Clin Invest (2022) 132(2). doi: 10.1172/JCI150201
31. Tiniakou E, Mammen AL. Idiopathic inflammatory myopathies and malignancy: A comprehensive review. Clin Rev Allergy Immunol (2017) 52(1):20–33. doi: 10.1007/s12016-015-8511-x
32. Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med (2005) 201(4):591–601. doi: 10.1084/jem.20041367
33. Pinal-Fernandez I, Ferrer-Fabregas B, Trallero-Araguas E, Balada E, Martinez MA, Milisenda JC, et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatol (Oxford) (2018) 57(2):388–96. doi: 10.1093/rheumatology/kex413
34. Lafyatis R, Valenzi E. Assessment of disease outcome measures in systemic sclerosis. Nat Rev Rheumatol (2022) 18(9):527–41. doi: 10.1038/s41584-022-00803-6
35. Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis (2010) 69(10):1809–15. doi: 10.1136/ard.2009.114264
36. Morrisroe K, Hansen D, Huq M, Stevens W, Sahhar J, Ngian GS, et al. Incidence, risk factors, and outcomes of cancer in systemic sclerosis. Arthritis Care Res (Hoboken) (2020) 72(11):1625–35. doi: 10.1002/acr.24076
37. Dolcino M, Pelosi A, Fiore PF, Patuzzo G, Tinazzi E, Lunardi C, et al. Gene profiling in patients with systemic sclerosis reveals the presence of oncogenic gene signatures. Front Immunol (2018) 9:449. doi: 10.3389/fimmu.2018.00449
38. Bonifazi M, Tramacere I, Pomponio G, Gabrielli B, Avvedimento EV, La Vecchia C, et al. Systemic sclerosis (scleroderma) and cancer risk: Systematic review and meta-analysis of observational studies. Rheumatol (Oxford) (2013) 52(1):143–54. doi: 10.1093/rheumatology/kes303
39. Igusa T, Hummers LK, Visvanathan K, Richardson C, Wigley FM, Casciola-Rosen L, et al. Autoantibodies and scleroderma phenotype define subgroups at high-risk and low-risk for cancer. Ann Rheum Dis (2018) 77(8):1179–86. doi: 10.1136/annrheumdis-2018-212999
40. Bernal-Bello D, de Tena JG, Guillen-Del CA, Selva-O'Callaghan A, Callejas-Moraga EL, Marin-Sanchez AM, et al. Novel risk factors related to cancer in scleroderma. Autoimmun Rev (2017) 16(5):461–8. doi: 10.1016/j.autrev.2017.03.012
41. Maria A, Partouche L, Goulabchand R, Riviere S, Rozier P, Bourgier C, et al. Intriguing relationships between cancer and systemic sclerosis: Role of the immune system and other contributors. Front Immunol (2018) 9:3112. doi: 10.3389/fimmu.2018.03112
42. Hussain SP, Harris CC. Inflammation and cancer: An ancient link with novel potentials. Int J Cancer (2007) 121(11):2373–80. doi: 10.1002/ijc.23173
43. Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, Wigley FM, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science (2014) 343(6167):152–7. doi: 10.1126/science.1246886
44. Komatsu N, Takayanagi H. Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactions. Nat Rev Rheumatol (2022) 18(7):415–29. doi: 10.1038/s41584-022-00793-5
45. Isomaki HA, Hakulinen T, Joutsenlahti U. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis (1978) 31(11):691–6. doi: 10.1016/0021-9681(78)90071-1
46. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: A meta-analysis. Arthritis Res Ther (2015) 17:212. doi: 10.1186/s13075-015-0728-9
47. De Cock D, Hyrich K. Malignancy and rheumatoid arthritis: Epidemiology, risk factors and management. Best Pract Res Clin Rheumatol (2018) 32(6):869–86. doi: 10.1016/j.berh.2019.03.011
48. Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: A dose-response meta-analysis. Arthritis Res Ther (2014) 16(2):R61. doi: 10.1186/ar4498
49. Jeon J, Holford TR, Levy DT, Feuer EJ, Cao P, Tam J, et al. Smoking and lung cancer mortality in the united states from 2015 to 2065: A comparative modeling approach. Ann Intern Med (2018) 169(10):684–93. doi: 10.7326/M18-1250
50. Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum (2006) 54(3):692–701. doi: 10.1002/art.21675
51. Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet (2017) 389(10086):2338–48. doi: 10.1016/S0140-6736(17)31491-5
52. Zappavigna S, Cossu AM, Grimaldi A, Bocchetti M, Ferraro GA, Nicoletti GF, et al. Anti-inflammatory drugs as anticancer agents. Int J Mol Sci (2020) 21(7). doi: 10.3390/ijms21072605
53. Jankowski J, de Caestecker J, Love SB, Reilly G, Watson P, Sanders S, et al. Esomeprazole and aspirin in barrett's oesophagus (AspECT): A randomised factorial trial. Lancet (2018) 392(10145):400–8. doi: 10.1016/S0140-6736(18)31388-6
54. Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet (2020) 395(10240):1855–63. doi: 10.1016/S0140-6736(20)30366-4
55. Bernatsky S, Lee JL, Rahme E. Non-hodgkin's lymphoma–meta-analyses of the effects of corticosteroids and non-steroidal anti-inflammatories. Rheumatol (Oxford) (2007) 46(4):690–4. doi: 10.1093/rheumatology/kel396
56. Askling J, Klareskog L, Hjalgrim H, Baecklund E, Bjorkholm M, Ekbom A. Do steroids increase lymphoma risk? A case-control study of lymphoma risk in polymyalgia rheumatica/giant cell arteritis. Ann Rheum Dis (2005) 64(12):1765–8. doi: 10.1136/ard.2005.036459
57. Hellgren K, Iliadou A, Rosenquist R, Feltelius N, Backlin C, Enblad G, et al. Rheumatoid arthritis, treatment with corticosteroids and risk of malignant lymphomas: Results from a case-control study. Ann Rheum Dis (2010) 69(4):654–9. doi: 10.1136/ard.2008.096925
58. Azher S, Azami O, Amato C, McCullough M, Celentano A, Cirillo N. The non-conventional effects of glucocorticoids in cancer. J Cell Physiol (2016) 231(11):2368–73. doi: 10.1002/jcp.25408
59. Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: Golden anniversary. Nat Rev Clin Oncol (2009) 6(11):638–47. doi: 10.1038/nrclinonc.2009.146
60. Benedek TG. Methotrexate: From its introduction to non-oncologic therapeutics to anti-TNF-alpha. Clin Exp Rheumatol (2010) 28(5 Suppl 61):S3–8.
61. Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur J Med Chem (2018) 158:502–16. doi: 10.1016/j.ejmech.2018.09.027
62. Solomon DH, Kremer JM, Fisher M, Curtis JR, Furer V, Harrold LR, et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum (2014) 43(4):489–97. doi: 10.1016/j.semarthrit.2013.08.003
63. Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant (2005) 5(12):2954–60. doi: 10.1111/j.1600-6143.2005.01125.x
64. Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation (2005) 80(2 Suppl):S254–64. doi: 10.1097/01.tp.0000186382.81130.ba
65. Nero P, Rahman A, Isenberg DA. Does long term treatment with azathioprine predispose to malignancy and death in patients with systemic lupus erythematosus? Ann Rheum Dis (2004) 63(3):325–6. doi: 10.1136/ard.2002.005371
66. Silman AJ, Petrie J, Hazleman B, Evans SJ. Lymphoproliferative cancer and other malignancy in patients with rheumatoid arthritis treated with azathioprine: a 20 year follow up study. Ann Rheum Dis (1988) 47(12):988–92. doi: 10.1136/ard.47.12.988
67. Beauparlant P, Papp K, Haraoui B. The incidence of cancer associated with the treatment of rheumatoid arthritis. Semin Arthritis Rheum (1999) 29(3):148–58. doi: 10.1016/s0049-0172(99)80026-2
68. Vial T, Descotes J. Immunosuppressive drugs and cancer. Toxicology (2003) 185(3):229–40. doi: 10.1016/s0300-483x(02)00612-1
69. van den Borne BE, Landewe RB, Houkes I, Schild F, van der Heyden PC, Hazes JM, et al. No increased risk of malignancies and mortality in cyclosporin a-treated patients with rheumatoid arthritis. Arthritis Rheum (1998) 41(11):1930–7. doi: 10.1002/1529-0131(199811)41:11<1930::AID-ART6>3.0.CO;2-N
70. Wadstrom H, Frisell T, Askling J. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: A nationwide cohort study from Sweden. JAMA Intern Med (2017) 177(11):1605–12. doi: 10.1001/jamainternmed.2017.4332
71. Mercer LK, Askling J, Raaschou P, Dixon WG, Dreyer L, Hetland ML, et al. Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: Results from a collaborative project of 11 European biologic registers. Ann Rheum Dis (2017) 76(2):386–91. doi: 10.1136/annrheumdis-2016-209285
72. Mercer LK, Green AC, Galloway JB, Davies R, Lunt M, Dixon WG, et al. The influence of anti-TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: Longitudinal results from the British society for rheumatology biologics register. Ann Rheum Dis (2012) 71(6):869–74. doi: 10.1136/annrheumdis-2011-200622
73. Sepriano A, Kerschbaumer A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis (2020) 79(6):760–70. doi: 10.1136/annrheumdis-2019-216653
74. Huss V, Bower H, Wadstrom H, Frisell T, Askling J. Short- and longer-term cancer risks with biologic and targeted synthetic disease-modifying antirheumatic drugs as used against rheumatoid arthritis in clinical practice. Rheumatol (Oxford) (2022) 61(5):1810–8. doi: 10.1093/rheumatology/keab570
75. Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol (2017) 13(4):217–33. doi: 10.1038/nrrheum.2017.22
76. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA (2006) 295(19):2275–85. doi: 10.1001/jama.295.19.2275
77. Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin (2008) 29(11):1275–88. doi: 10.1111/j.1745-7254.2008.00889.x
78. Waljee AK, Higgins P, Jensen CB, Villumsen M, Cohen-Mekelburg SA, Wallace BI, et al. Anti-tumour necrosis factor-alpha therapy and recurrent or new primary cancers in patients with inflammatory bowel disease, rheumatoid arthritis, or psoriasis and previous cancer in Denmark: A nationwide, population-based cohort study. Lancet Gastroenterol Hepatol (2020) 5(3):276–84. doi: 10.1016/S2468-1253(19)30362-0
79. Raaschou P, Simard JF, Asker HC, Askling J. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ (2016) 352:i262. doi: 10.1136/bmj.i262
80. Raaschou P, Simard JF, Holmqvist M, Askling J. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: Nationwide population based prospective cohort study from Sweden. BMJ (2013) 346:f1939. doi: 10.1136/bmj.f1939
81. Peleva E, Exton LS, Kelley K, Kleyn CE, Mason KJ, Smith CH. Risk of cancer in patients with psoriasis on biological therapies: A systematic review. Br J Dermatol (2018) 178(1):103–13. doi: 10.1111/bjd.15830
82. Pavanello F, Zucca E, Ghielmini M. Rituximab: 13 open questions after 20years of clinical use. Cancer Treat Rev (2017) 53:38–46. doi: 10.1016/j.ctrv.2016.11.015
83. Smith RM, Jones RB, Specks U, Bond S, Nodale M, Aljayyousi R, et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis (2020) 79(9):1243–9. doi: 10.1136/annrheumdis-2019-216863
84. Scott LJ. Tocilizumab: A review in rheumatoid arthritis. Drugs (2017) 77(17):1865–79. doi: 10.1007/s40265-017-0829-7
85. Rubbert-Roth A, Enejosa J, Pangan AL, Haraoui B, Rischmueller M, Khan N, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med (2020) 383(16):1511–21. doi: 10.1056/NEJMoa2008250
86. de Germay S, Bagheri H, Despas F, Rousseau V, Montastruc F. Abatacept in rheumatoid arthritis and the risk of cancer: a world observational post-marketing study. Rheumatol (Oxford) (2020) 59(9):2360–7. doi: 10.1093/rheumatology/kez604
87. Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewe R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis (2014) 73(1):3–5. doi: 10.1136/annrheumdis-2013-204317
88. McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet (2021) 398(10302):803–16. doi: 10.1016/S0140-6736(21)00438-4
89. Maneiro JR, Souto A, Gomez-Reino JJ. Risks of malignancies related to tofacitinib and biological drugs in rheumatoid arthritis: Systematic review, meta-analysis, and network meta-analysis. Semin Arthritis Rheum (2017) 47(2):149–56. doi: 10.1016/j.semarthrit.2017.02.007
90. Xie W, Yang X, Huang H, Gao D, Ji L, Zhang Z. Risk of malignancy with non-TNFi biologic or tofacitinib therapy in rheumatoid arthritis: A meta-analysis of observational studies. Semin Arthritis Rheum (2020) 50(5):930–7. doi: 10.1016/j.semarthrit.2020.08.007
91. Curtis JR, Yamaoka K, Chen YH, Bhatt DL, Gunay LM, Sugiyama N, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: Results from the open-label, randomised controlled ORAL surveillance trial. Ann Rheum Dis (2023) 82(3):331–343. doi: 10.1136/ard-2022-222543
92. Khosrow-Khavar F, Desai RJ, Lee H, Lee SB, Kim SC. Tofacitinib and risk of malignancy: Results from the safety of tofacitinib in routine care patients with rheumatoid arthritis (STAR-RA) study. Arthritis Rheumatol (2022) 74(10):1648–59. doi: 10.1002/art.42250
93. Taylor PC, Takeuchi T, Burmester GR, Durez P, Smolen JS, Deberdt W, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis (2022) 81(3):335–43. doi: 10.1136/annrheumdis-2021-221276
94. Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol (2018) 2:23. doi: 10.1186/s41927-018-0031-x
95. Cappelli LC, Shah AA. The relationships between cancer and autoimmune rheumatic diseases. Best Pract Res Clin Rheumatol (2020) 34(1):101472. doi: 10.1016/j.berh.2019.101472
Keywords: malignancy, rheumatic disease, risk, disease-modifying anti-rheumatic drugs, mechanism
Citation: Geng Z, Ye C and Zhu X (2023) Malignancies in systemic rheumatic diseases: A mini review. Front. Immunol. 14:1095526. doi: 10.3389/fimmu.2023.1095526
Received: 24 November 2022; Accepted: 13 February 2023;
Published: 28 February 2023.
Edited by:
Wesley H. Brooks, University of South Florida, United StatesReviewed by:
Albert Selva-O’Callaghan, Vall d’Hebron University Hospital, SpainCopyright © 2023 Geng, Ye and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojian Zhu, emh1eGlhb2ppYW5AaHVzdC5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Zhe Geng1†
Zhe Geng1† Cong Ye
Cong Ye Xiaojian Zhu
Xiaojian Zhu