- 1Department of Immunology, National Institute for Research in Tuberculosis, Indian Council of Medical Research (ICMR), Chennai, India
- 2International Center for Excellence in Research, National Institutes of Health, National Institute for Research in Tuberculosis (NIRT), International Center for Excellence in Research, Chennai, India
- 3Diabetology, Prof. M. Viswanathan Diabetes Research Center, Chennai, India
- 4Department of Bacteriology, National Institute for Research in Tuberculosis, Indian Council of Medical Research (ICMR), Chennai, India
- 5Epidemiology Statistics, National Institute for Research in Tuberculosis, Indian Council of Medical Research (ICMR), Chennai, India
- 6Clinical Research, National Institute for Research in Tuberculosis, Indian Council of Medical Research (ICMR), Chennai, India
- 7Department of Medicine, University of Massachusetts Chan Medical School, Worcester, MA, United States
- 8Laboratory of Parasitic Diseases (LPD), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, MD, United States
Introduction: Chitinase, Indoleamine 2,3-dioxygenesae-1 (IDO-1) and heme oxygenase-1 (HO-1) are candidate diagnostic biomarkers for tuberculosis (TB). Whether these immune markers could also serve as predictive biomarkers of unfavorable treatment outcomes in pulmonary TB (PTB) is not known.
Methods: A cohort of newly diagnosed, sputum culture-positive adults with drug-sensitive PTB were recruited. Plasma chitinase protein, IDO protein and HO-1 levels measured before treatment initiation were compared between 68 cases with unfavorable outcomes (treatment failure, death, or recurrence) and 108 control individuals who had recurrence-free cure.
Results: Plasma chitinase and IDO protein levels but not HO-1 levels were lower in cases compared to controls. The low chitinase and IDO protein levels were associated with increased risk of unfavourable outcomes in unadjusted and adjusted analyses. Receiver operating characteristic analysis revealed that chitinase and IDO proteins exhibited high sensitivity and specificity in differentiating cases vs controls as well as in differentiating treatment failure vs controls and recurrence vs controls, respectively. Classification and regression trees (CART) were used to determine threshold values for these two immune markers.
Discussion: Our study revealed a plasma chitinase and IDO protein signature that may be used as a tool for predicting adverse treatment outcomes in PTB.
Introduction
Tuberculosis (TB) is a communicable disease that is a primary reason for ill health worldwide and is the one of the common infectious causes of death (1, 2). Globally it has been reported that approximately 1.5 million deaths occurred in the year 2021 due to TB. TB is completely treatable and curable, and around 85% of people who develop TB disease can be effectively treated with shortened drug regimens [22]. However there are a subset of individuals who will experience unfavorable treatment outcomes involving TB recurrence, bacteriological failure and death, which are major hurdles to global TB elimination (3). TB treatment monitoring is primarily very important to clinical decision-making and the host biomarkers might play a vital role as point-of-care biomarkers of treatment response for individual monitoring of TB treatment outcomes (4, 5). Biomarkers that aim to identify such individuals with nonsputum-based assays, can be used for TB treatment monitoring (6, 7).
Chitinases are glycosylated hydrolytic enzymes that catalyse the degradation of chitin into N-acetylglucosamine, which is essential for the carbon and nitrogen source (8). Chitinases are considered to play an important role in the innate host defence mechanism against the bacterial infections, especially TB and other pulmonary disorders (9, 10). Indoleamine 2,3-dioxygenase (IDO) is the rate-limiting enzyme in the conversion of tryptophan to kynurenines. IDO mediates immune suppression through depletion of tryptophan which is essential for T cell function and other immune mechanisms (11). A published study has reported that IDO activity and the kynurenine/tryptophan ratio are important biomarkers for diagnosing TB (11). Heme oxygenase-1 (HO-1) is an antioxidant which is vastly expressed in the lungs, and is triggered by a range of stress signals such as ROS and inflammatory mediators (12). Both chitinases and HO-1 have also been described to possess diagnostic biomarker activity and pulmonary and extra-pulmonary forms of TB (13, 14). The main aim of this study was to elucidate whether plasma protein levels of Chitinase, IDO and HO-1 could also aid as prognostic immune biomarkers for unfavorable pulmonary TB treatment outcomes.
Materials and methods
Study population
As shown in Figure 1, the study cohort of n=446 individuals comprised all participants, who were enrolled in the Effect of Diabetes on Tuberculosis Severity (EDOTS) study, a prospective cohort study (2014-2019) conducted in Chennai, India (15) A total of 68 had unfavorable treatment outcomes, comprising treatment failure, relapse, or death. Inclusion criterion was new smear and culture-positive adults (age 18-75 years). Exclusion criteria were previous treatment for TB, drug resistant TB, HIV positivity or taking immunosuppressive drugs, and >7 days of anti-TB treatment prior to enrolment. As part of our study protocol HIV screening was done for all the study participants. The diagnosis of PTB was established by positive sputum culture on solid media with compatible chest x-ray. Anti-TB treatment (ATT) was provided by government TB clinics according the Revised National Tuberculosis Control Programme standards in effect at that time. Participants were followed up monthly through the six-month course of treatment and every three months thereafter till one year after treatment completion. We conducted a nested case-control study with cases having adverse treatment outcomes matched in a 1:1.6 ratio to controls (the ratio was determined by the availability of samples in each group), who were defined as having a recurrence free cure till the end of study. Cure was defined as negative sputum cultures at months 5 and 6 of treatment. Adverse treatment outcomes included treatment failure defined as positive sputum culture at months 5 or 6, all-cause mortality over the duration of treatment, or recurrent TB demonstrated by positive sputum smear and culture with compatible symptoms or chest x-ray within twelve months after initial cure. There were a total of 18 TB treatment failures, 16 deaths and 34 TB recurrences. Case-control matching was based on age, gender, body mass index (BMI), and diabetic status. Peripheral blood was collected in heparinized tubes. Following centrifugation, plasma was collected and stored at -800C till further analysis. Baseline sample collection was performed at enrolment. The demographic and epidemiological data for this cohort have been previously reported (16, 17).
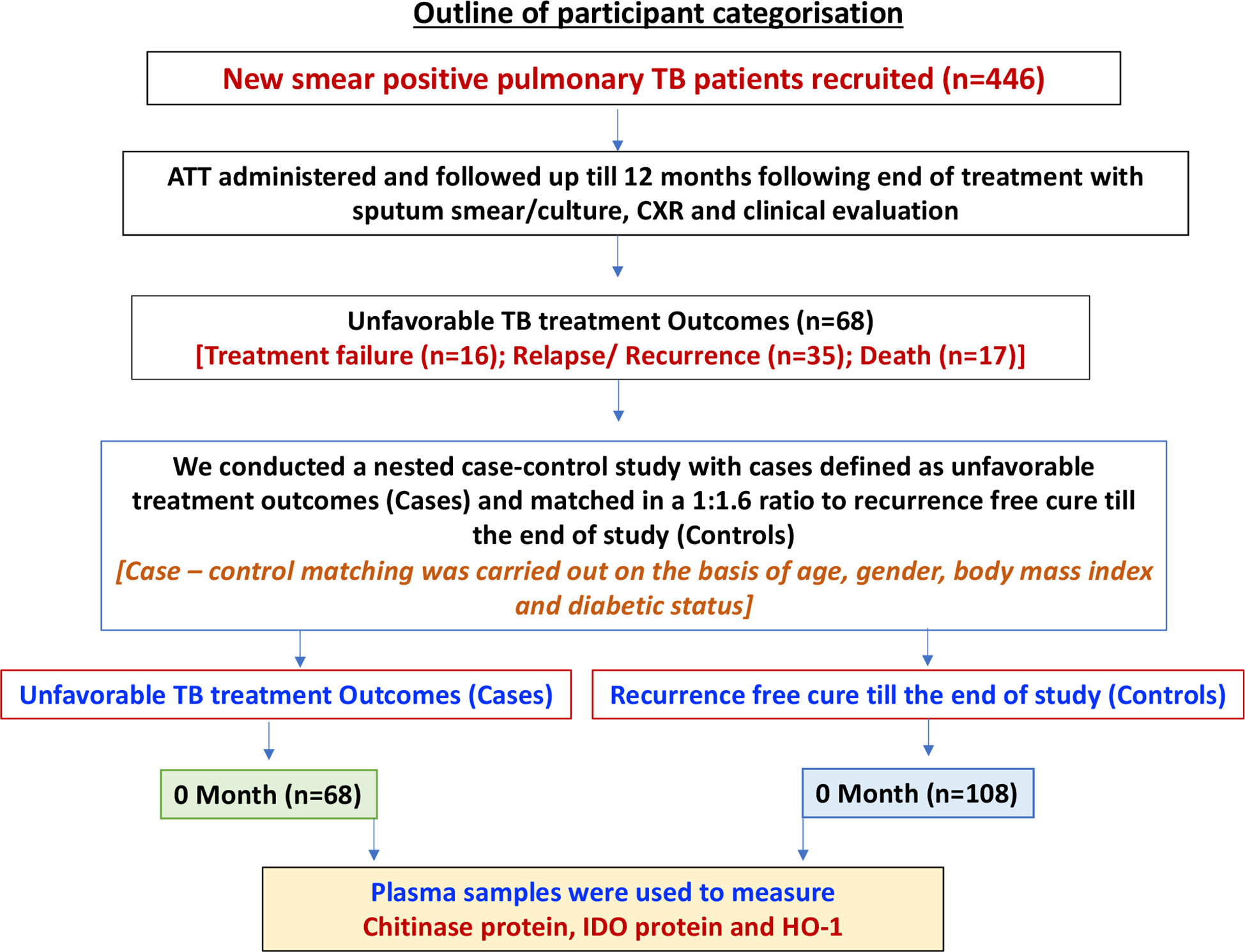
Figure 1 Outline of participant categorization. A schematic of our study cohort showing new smear positive pulmonary TB patients with poor treatment outcome [Cases (n=68)] and recurrence free cure till the end of study [Control (n=108)]. Plasma samples were isolated from whole blood of both the study groups.
Enzyme-linked immunosorbent assay
Venous blood was collected in heparin tubes. Plasma was separated by centrifugation at 3,000 x g for 10 min at 4°C, aliquoted, and stored at -80°C until required. Plasma levels of immune markers such as chitinase protein and IDO protein were measured using the Duo Set ELISA kit from R & D Systems. The HO-1 protein levels were measured using the ENZO Immunoset ELISA kit according to the manufacturer’s protocol. Samples were run in duplicates. The lowest detection limits were as follows: IDO protein: 0.469ng/mL; chitinase protein: 31.2 pg/mL; and HO-1 protein: 0.195 ng/mL.
Statistical analyses
Geometric means (GMs) were used for measurements of central tendency. The statistical differences between the case and the control group were analyzed using Mann–Whitney U tests. Secondary analysis of TB treatment failures versus controls and TB recurrences versus controls were also performed using the Mann-Whitney U test. The Receiver Operator Characteristics (ROC) curves were designed to test the potential of each candidate marker and distinguish between cases and controls. Analyses were performed using Graph-Pad PRISM, version 9.0. Univariate and multivariate analyses were performed using Stata v.16 (StataCorp, College Station, TX). Classification and regression trees (CART) model were employed to identify the cut-off for the biomarkers which separate the TB cases and controls. The analysis was done using R (A Language and Environment for Statistical Computing) software version 4.1.2.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committees of the National Institute for Research in Tuberculosis (NIRT) and the Prof. M. Viswanathan Diabetes Research Center. The patients/participants provided their written informed consent to participate in this study.
Results
Study population characteristics
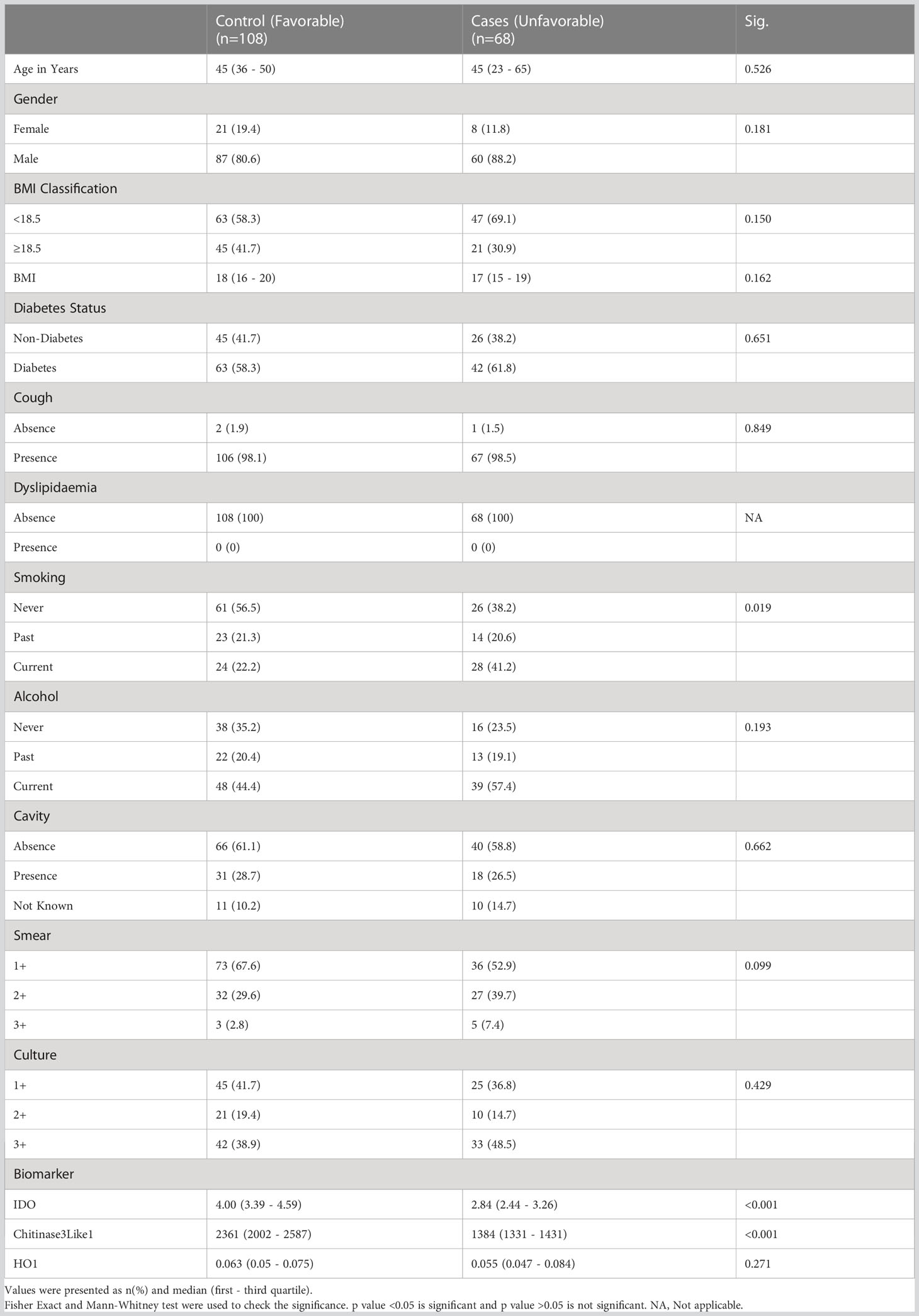
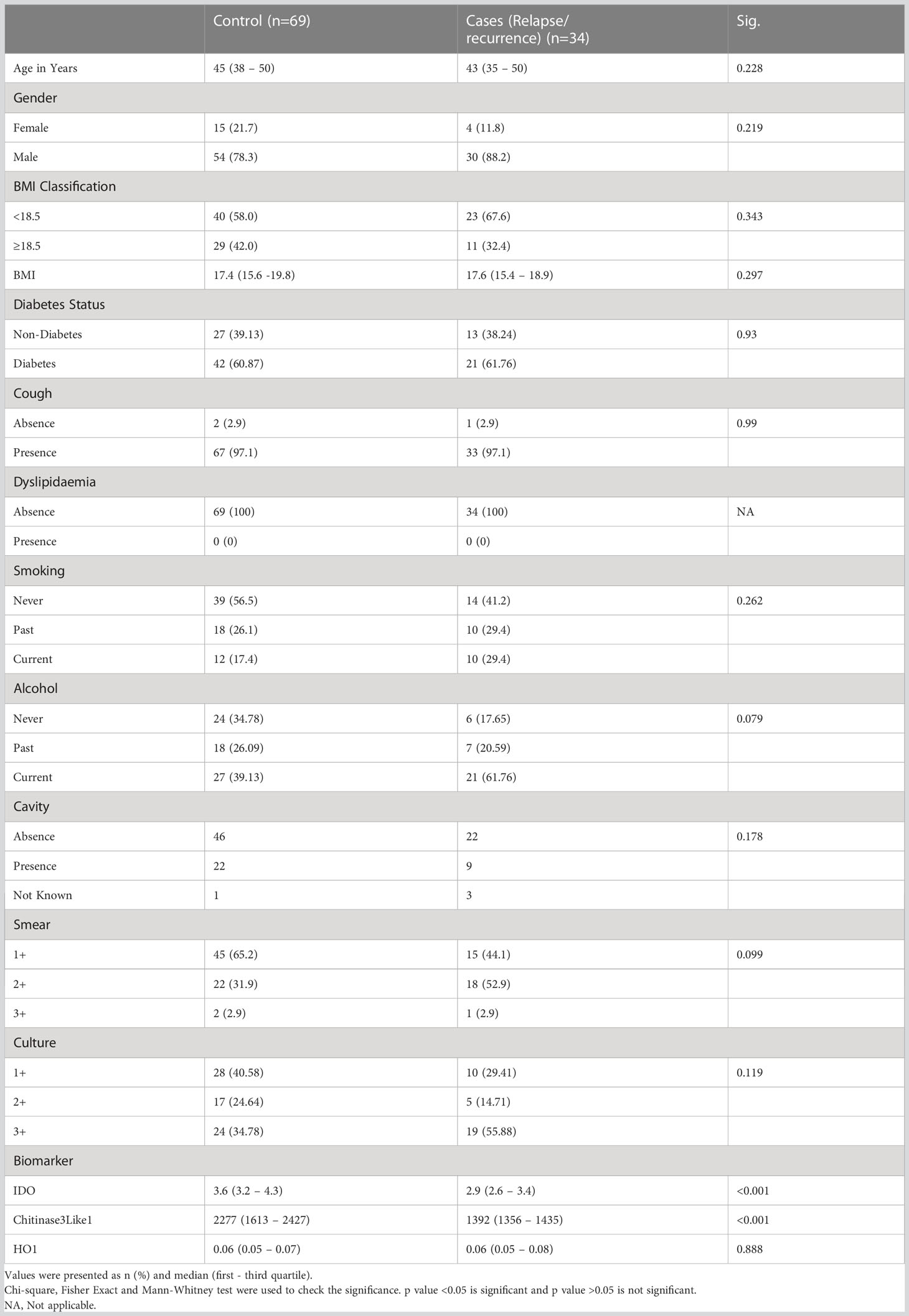
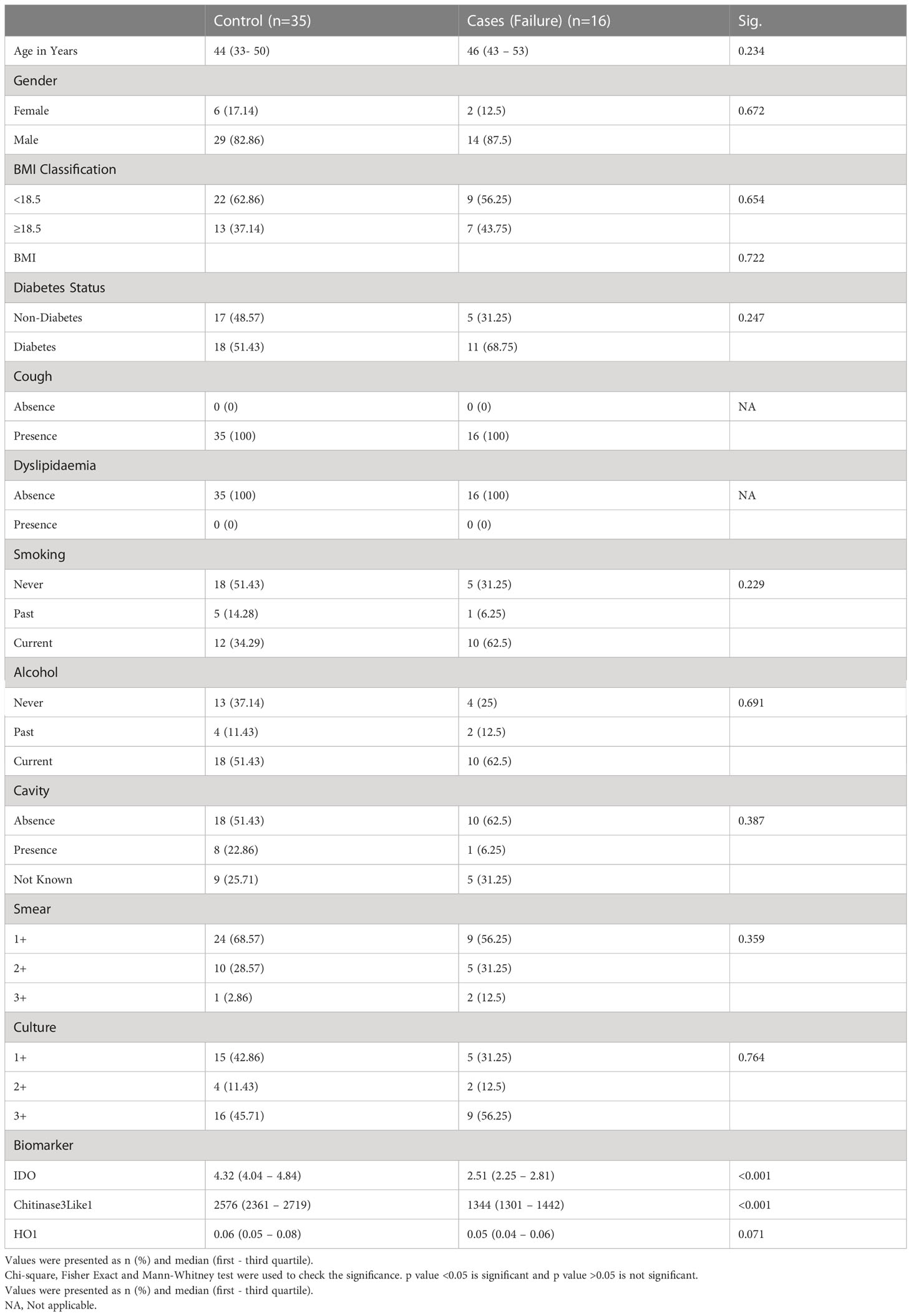
The study population (Figure 1) of this cohort included 68 cases and 108 controls. The median age was 45 years (interquartile range [IQR], 23–65) for cases and 45 years (IQR, 36–50) for controls (p = .526). There were no significant differences in gender, BMI, diabetic status, dyslipidemia, alcohol use, education level, or occupation (Table 1A). There were no differences in culture smear grades or in the presence of cavities at baseline. The control group had a higher number of smokers (either current or former; p= 0.0019). Patient characteristics for TB treatment recurrence/relapse and treatment failure are provided in Tables 1B, 1C.
Baseline plasma levels of chitinase protein and IDO protein are significantly decreased and are correlates of risk for cases
Our aim was to determine the differences in total cases versus controls in the circulating plasma levels of chitinase, IDO protein and HO-1 and we measured the expression of these markers at baseline. As shown in Figure 2A, plasma levels of chitinase protein (Geometric mean (GM) of 1389 pg/mL in cases vs 2213 pg/mL in controls) and IDO protein (Figure 2B) (geometric mean (GM) of 2.2 ng/mL in cases vs 3.9 ng/mL in controls) were significantly lower in cases compared to controls, while the plasma levels of HO-1 (Figure 2C) showed no statistical significant differences between the groups.
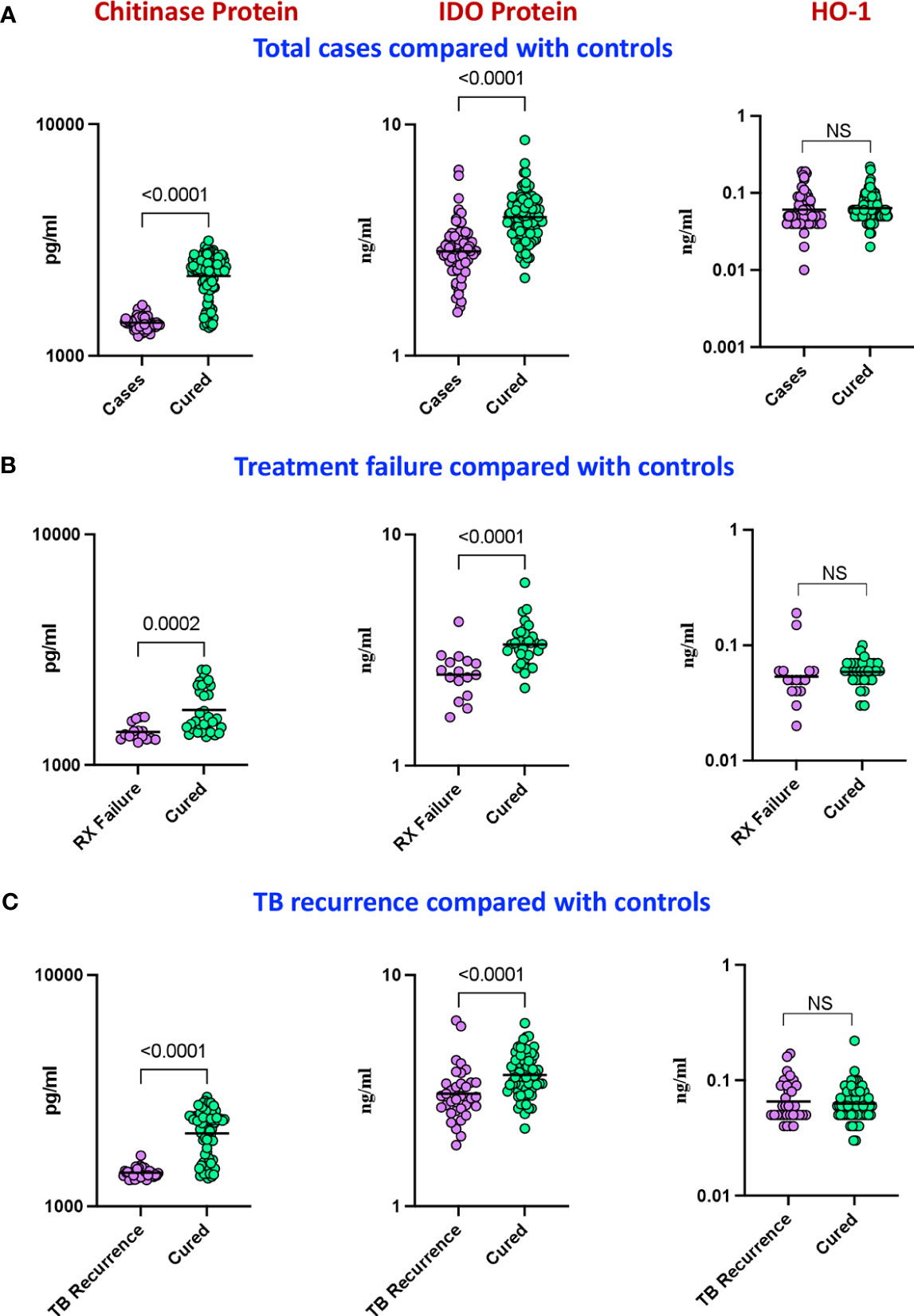
Figure 2 Baseline analysis of plasma levels of chitinase protein and IDO protein in cases. The baseline plasma levels of chitinase protein, IDO protein and HO-1 were measured in (A) cases (n=68) and controls (n=108) (B) TB treatment failure (n=16) and controls (n=35) and (C) TB recurrence (n=34) and controls (n=69). The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann-Whitney U test with Holm’s correction for multiple comparisons. NS : No significance.
In a secondary analysis of differences in TB treatment failure versus controls, we measured the expression of these markers in the patients at baseline. As shown in Figure 2D, the plasma levels of chitinase protein (GM of 1391 pg/mL in treatment failure versus 1731 pg/mL in controls) and IDO protein (Figure 2E) (GM of 2.4 ng/mL in treatment failure versus 3.4 ng/mL in controls) were significantly lower in treatment failure individuals compared to controls, while the plasma levels of HO-1 (Figure 2F) showed no statistically significant differences between the groups. To perform a secondary analysis of differences in TB recurrence vs controls, we measured the expression of these markers in the patients at baseline. As shown in Figure 2G, plasma levels of chitinase protein (GM of 1397 pg/ml in recurrence versus 2066 pg/mL in controls) and IDO protein (Figure 2H) (GM of 3 ng/mL in recurrence versus 3.9 ng/mL in controls) were significantly lower in recurrence compared to controls, while the plasma levels of HO-1 (Figure 2I) showed no statistically significant differences between the groups.
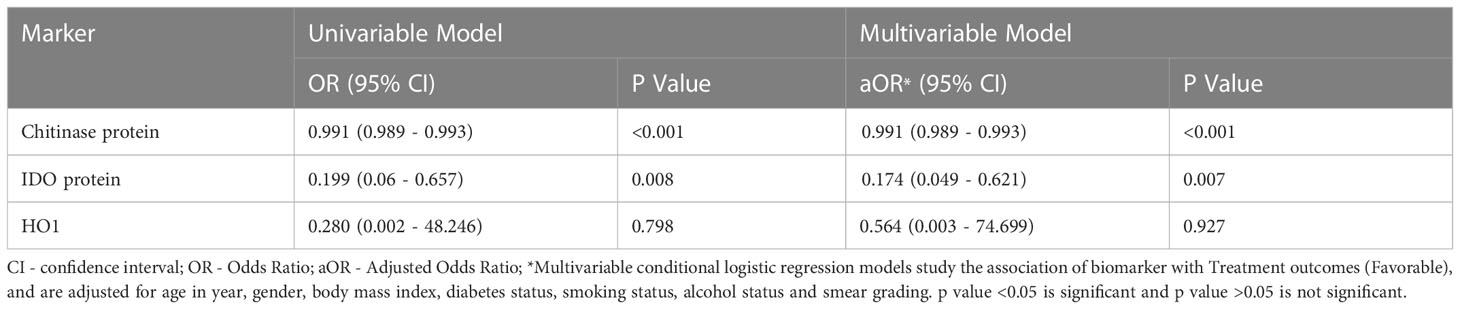
Univariate analysis showed that chitinase protein (odds ratio (OR) [95% CI] 0.991 [0.989 – 0.993]; p<0.001) and IDO protein (OR) [95% CI] 0.199 [0.06 – 0.657]; p=0.008) were associated with decreased risk of the composite adverse treatment outcomes, while HO-1 (OR [95% CI] 3.57 [0.021 – 613.5]; p=0.798) was not associated with unfavorable outcomes (Table 2). Multivariate analysis showed that chitinase protein (adjusted odd ratio (aOR) [95% CI] 0.991 [0.989 – 0.993]; p<0.001) and IDO protein (aOR [95% CI] 0.174 [0.013 – 0.621]; p=0.007) were associated with decreased risk of adverse treatment outcomes, while HO-1 (OR [95% CI] 1.773 [0.013 – 400]; p=0.927) was not associated with adverse treatment outcomes Thus, low baseline plasma levels of IDO protein and chitinase protein are correlates of risk for adverse treatment outcomes in active pulmonary TB patients (Table 2).
Low chitinase protein and IDO protein levels are predictive biomarkers of adverse treatment outcomes in PTB
To determine if we could derive a predictive signature of plasma chitinase, IDO or HO-1 that could be used to identify individuals at risk for unfavorable treatment outcomes (failure, recurrence, and mortality), we performed ROC analysis. As shown in Figure 3A, ROC analysis showed that chitinase protein (AUC = 0.949; sensitivity 100%, specificity 83%) and IDO protein (Figure 3B) (AUC = 0.842; sensitivity 78%, specificity 81%) effectively differentiated cases from controls. However HO-1 (Figure 3C) (AUC = 0.549; sensitivity 52%, specificity 64%) could not significantly differentiate cases from controls. Similarly, as shown in Figure 3D, ROC analysis of chitinase protein (AUC = 0.816; sensitivity 75%, specificity 78%)and IDO protein (Figure 3E) (AUC = 0.968; sensitivity 93%, specificity 97%) exhibited increased sensitivity and specificity in differentiating TB treatment failure from controls. Conversely, HO-1 (Figure 3F) (AUC = 0.660; sensitivity 87%, specificity 60%) could not differentiate cases from controls well. Finally, as shown in Figure 3G, ROC analysis of chitinase protein (AUC = 0.919; sensitivity 97%, specificity 83%) and IDO protein (Figure 3H) (AUC = 0.735; sensitivity 68%, specificity 74%) showed that chitinase protein had excellent and IDO protein had acceptable sensitivity and specificity in differentiating TB recurrence from controls. On the other hand HO-1, (Figure 3I) (AUC = 0.509; sensitivity 32%, specificity 85%) could not differentiate cases from controls well. Thus, plasma chitinase protein and IDO protein measured at the initiation of anti-TB treatment showed potential to serve as predictive biomarkers of individual treatment outcomes.
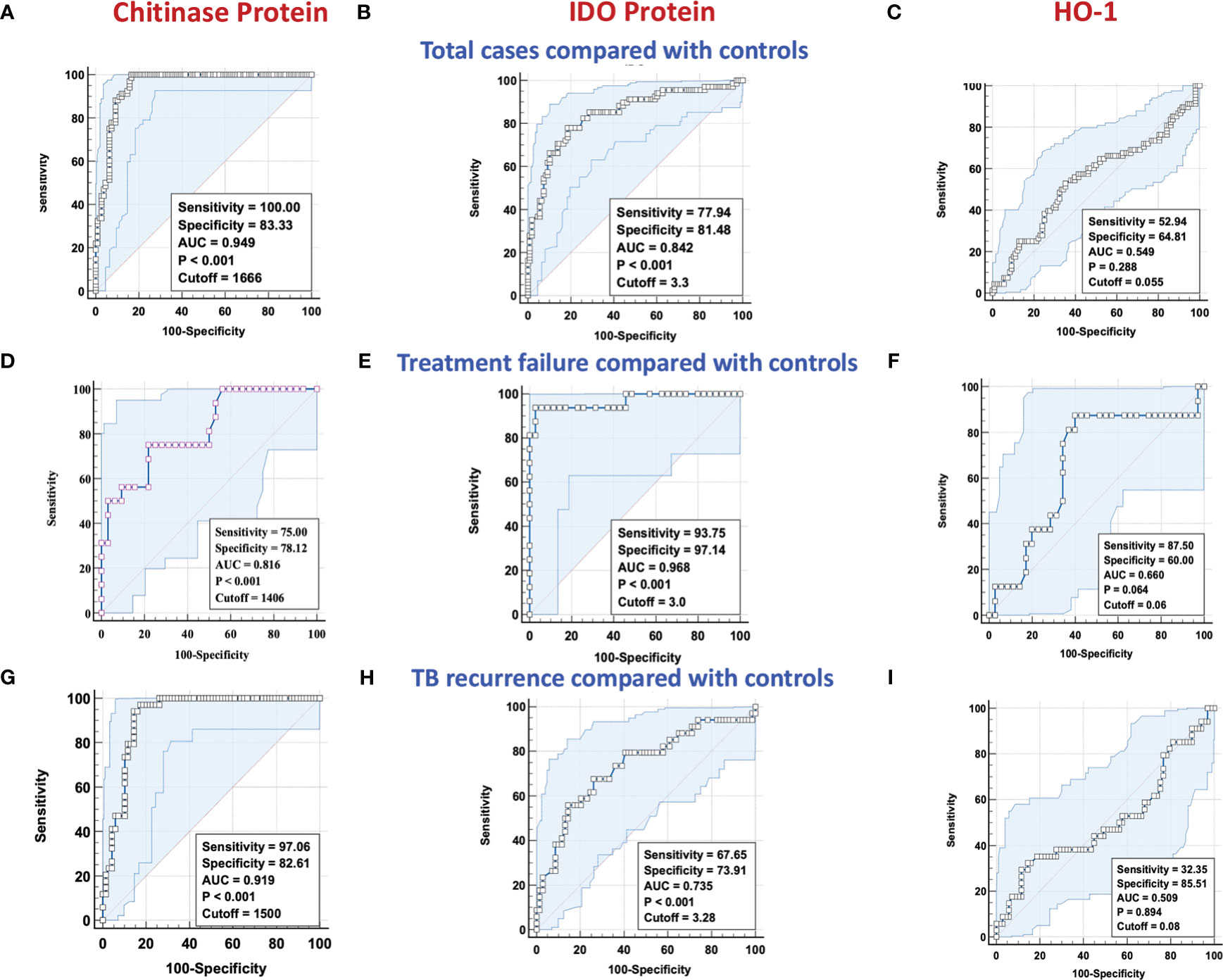
Figure 3 ROC analysis of chitinase protein, IDO protein and HO-1 in discriminating treatment failure and disease recurrence versus controls. Receiver operator characteristic analysis to estimate the sensitivity, specificity, and AUC was performed using chitinase protein, IDO protein and HO-1 to estimate the capacity of these factors to distinguish cases vs controls. (A–C) Total cases (n=68) and controls (n=108) (A) Chitinase protein (B) IDO Protein (C) HO-1. (D–F) TB Treatment failure (n=16) and controls (n=35) (D) Chitinase protein (E) IDO Protein (F) HO-1. (G–I) TB recurrence (n=34) and controls (n=69) (G) Chitinase protein (H) IDO Protein (I) HO-1. NS : No significance.
Threshold values for biomarkers discriminating adverse treatment outcome from cure
Classification and regression trees (CART) models were employed to identify the cut-off points for chitinase protein and IDO protein which best separated cases from controls (Figure 4). Briefly, the data set formed a parent node, which contains the whole population. The best peak to separate the data set was selected. As shown in Figure 4, chitinase protein with a cut off value of <1666 pg/mL with AUC: 0.92, and IDO protein with a cut off value of <3.3 ng/mL with AUC: 0.80, was able to discriminate individuals who experienced an adverse TB treatment outcome from those who had recurrence free cure.
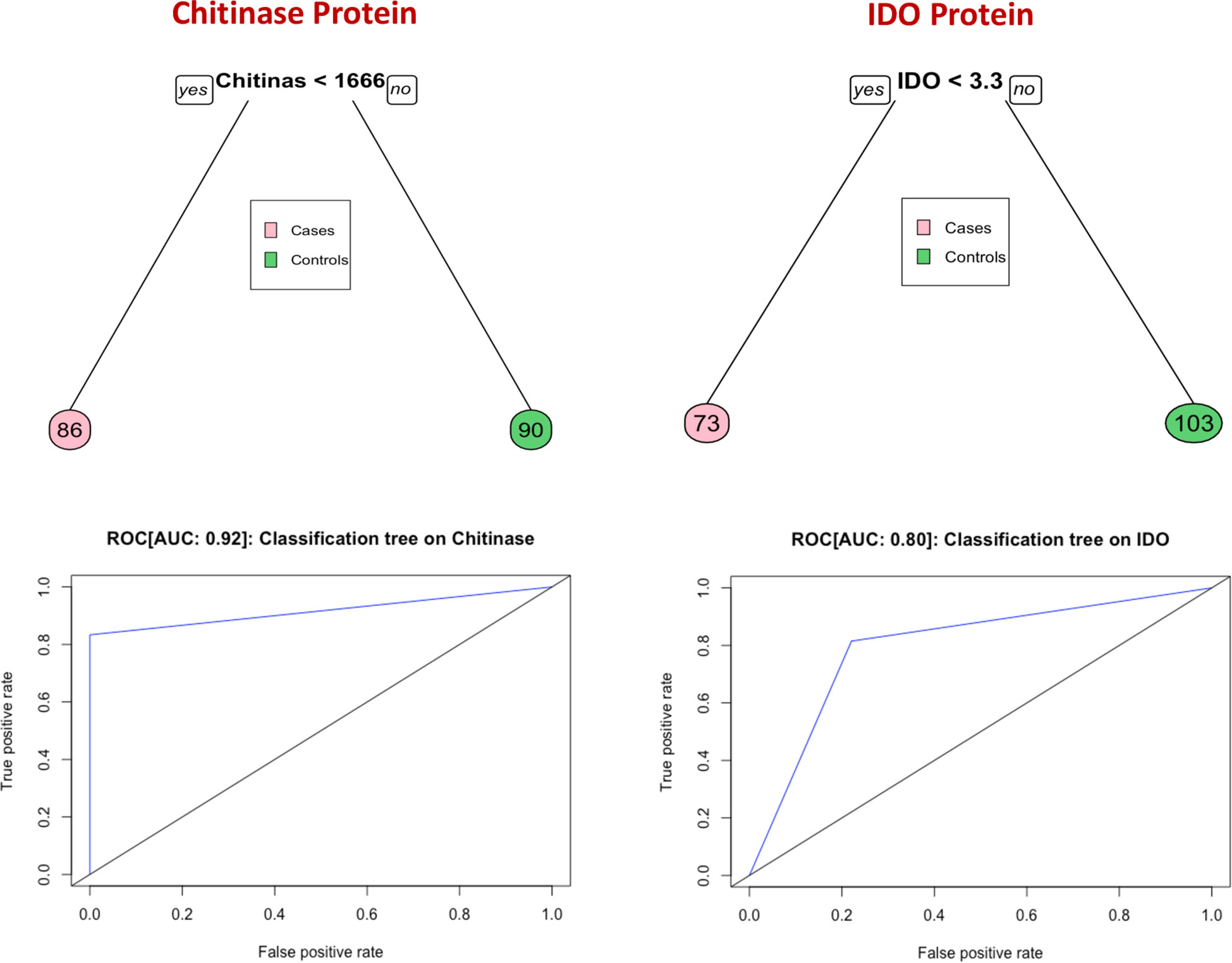
Figure 4 Identification of biomarkers showing the strongest associations with bad treatment outcome in TB disease. CART model analysis shows that chitinase protein and IDO protein exhibited the highest accuracy in discriminating cases from controls and ROC curves were employed to quantify the accuracy of biomarkers Cut-off value of chitinase protein and IDO protein is determined by this model.
Discussion
TB treatment confers a great burden on patient care, compliance, and on public and private health systems due to the necessity of completing a minimum of 6 months of therapy. Since most individuals are cured by 4 months, the justification for the standard 6-month course of treatment is the propensity for a minority of patients to relapse. TB treatment failure results in significant individual and programmatic burdens, with the need for further extended treatment and elevated risk for acquired antibiotic resistance. Mortality during treatment of drug-sensitive TB could be prevented in some cases if caregivers and programs was alerted to at-risk individuals who might benefit from intensified monitoring and investigation (18–21) The availability of one or more biomarkers that could accurately identify the subset of individuals who are at high risk for adverse treatment outcomes could guide management decisions (22–24). Several trials have shown that shortened regimens for drug-susceptible TB are curative for a majority of participants, but hampered by the currently unpredictable risk of recurrence. A predictive test performed at the time of TB diagnosis could be used to assign individuals to a shortened course of antibiotics or to an extended and otherwise intensified course with additional evaluations to identify modifiable mortality risks. Our present study attempted to identify a biomarker of unfavorable treatment outcomes to classify baseline predictors of such outcomes. The results indicate that IDO and chitinase proteins are promising candidates, with lower levels of both factors associating with increased risk for treatment failure, recurrence, or death. Chitinase protein in particular demonstrated very high sensitivity and specificity in this cohort.
IDO is a potent immunoregulatory molecule which catalyzes the rate-limiting step of tryptophan catabolism (25). It was previously reported that IDO activity estimated by the ratio of kynurenines (Kyn) to tryptophan (Trp) was significantly higher in TB patients than controls, and higher in TB patients who died compared to TB survivors (25). IDO is also an intracellular enzyme that can be measured in plasma and a published study has reported that IDO levels were increased in infectious lung diseases such COVID-19 and pulmonary tuberculosis (26). Another study, also using the Kyn-Trp ratio, reported that increased IDO enzyme activity is associated with the progression to TB in HIV co-infected patients and diminishes after TB treatment (27). In addition, there was also a study which reported that IDO activity was significantly higher in the multi drug resistant TB patients than in the single drug resistant TB patients (28). Our data, based on measurement of IDO protein levels in plasma, corroborate its association with TB but with the finding that lower plasma IDO protein levels was associated with adverse TB treatment outcomes. Other published studies have also reported that IDO mediated tryptophan catabolism is increased in Mycobacterium tuberculosis and acts as an immune mediator and surrogate marker for TB diagnosis or treatment response (29, 30). Moreover, while most of the previously reported studies measured the enzymatic activity of kynurenine-to-tryptophan (K/T) ratio, our study measured IDO protein levels (31, 32). Our data indicate that IDO protein levels are decreased in unfavorable treatment outcomes. The possible reasons for the differences between IDO protein findings in our study and IDO enzyme activity in other studies are possibly due to the fact high IDO enzyme activity may downregulate production of IDO protein, the presence of IDO protein in cells/tissues is not reflected in peripheral blood but may influence circulating amino acid levels, and/or the influence of commensal flora on circulating amino acid concentrations (29, 33). The immunoregulatory effect of IDO protein is not limited to T cells but extends to other immune cells, leading to dampened immune response (34).
A previous study reported elevated plasma chitinase enzymatic activity in 42 PTB patients compared with 30 healthy control participants (13). In that study, chitinase activity was positively correlated with radiographic TB severity and sputum smear positivity. Our data add to this evidence by demonstrating the utility of plasma chitinase protein levels as a biomarker of risk of adverse treatment outcomes during and after TB treatment. Like results with IDO protein, lower chitinase protein levels were associated with increased risk for unfavorable outcomes. Finally, we also measured the potent immune activation marker HO-1, which is one of the main antioxidants expressed in the lungs (35). In previous studies, we found that HO-1 precisely differentiates active TB disease from latent TB infection or heathy adult individuals (36) and pediatric cohorts (14). However, results from our present study indicate that HO-1 is not a suitable as a predictive biomarker for TB treatment outcomes.
Unfavorable treatment outcomes are influenced by many factors including treatment adherence, smoking, use of alcohol, malnutrition, occupation, HIV co-infection, and comorbidities such as diabetes mellitus (23, 37). In our study, the participants was matched for age, gender, BMI and diabetes status, and we performed multivariate conditional regression analysis to account for the other factors. Additionally, we also did analysis to account for any differences in the X-ray scores, presence of cavity, culture and smear grades between the cases and controls. Some of the limitations of our study include the use of a moderate sample size, and not including patients with lung diseases other than TB. Nevertheless, our study demonstrates that plasma chitinase and IDO are candidate biomarkers for TB treatment outcome prediction, worthy of advancement to validation in adequately sized, prospective multi-center cohort studies. The ultimate goal of such studies would be a clinical trial using affordable and technically feasible predictive biomarkers to assign newly diagnosed PTB patients to shortened treatment course vs prolonged or intensified treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committees of the National Institute for Research in Tuberculosis (NIRT) and the Prof. M. Viswanathan Diabetes Research Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Designed the study (SB, NK); conducted experiments (NK, AN); acquired data (NK, AN, SS); analyzed data (NK, KT, SA); contributed reagents and also revised subsequent drafts of the manuscript (HK, SB); responsible for the enrolment of participants and also contributed to acquisition and interpretation of clinical data (VV, SH); wrote the manuscript (SB, NK). All authors contributed to the article and approved the submitted version.
Funding
This project has been funded in whole or in part with Federal funds from the Government of India’s (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR), and distributed in part by CRDF Global [grant USB1-31149-XX-13]. This work is also funded by CRDF Global RePORT India Consortium Supplemental Funding [grant OISE-17-62911-1]. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the ICMR, the NIH, or CRDF Global. This work was also funded in part by the Division of Intramural Research, NIAID, NIH.
Acknowledgments
We thank the staff of Department of Clinical Research and the Department of Bacteriology, NIRT for valuable assistance in bacterial cultures and radiology and the staff of MVDRC, RNTCP and Chennai Corporation for valuable assistance in recruiting the patients for this study. Data in this manuscript were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis (2021) 113 Suppl 1:S7–S12. doi: 10.1016/j.ijid.2021.02.107
2. Choi H. Nosocomial exposure to tuberculosis: a snapshot of south Korea. Korean J Intern Med (2021) 36:1061–2. doi: 10.3904/kjim.2021.367
3. Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers (2016) 2:16076. doi: 10.1038/nrdp.2016.76
4. Yong YK, Tan HY, Saeidi A, Wong WF, Vignesh R, Velu V, et al. Immune biomarkers for diagnosis and treatment monitoring of tuberculosis: Current developments and future prospects. Front Microbiol (2019) 10. 2789 doi: 10.3389/fmicb.2019.02789
5. Zimmer AJ, Lainati F, Aguilera Vasquez N, Chedid C, Mcgrath S, Benedetti A, et al. Biomarkers that correlate with active pulmonary tuberculosis treatment response: a systematic review and meta-analysis. J Clin Microbiol (2022) 60:e0185921. doi: 10.1128/jcm.01859-21
6. Wallis RS, Kim P, Cole S, Hanna D, Andrade BB, Maeurer M, et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis (2013) 13:362–72. doi: 10.1016/S1473-3099(13)70034-3
7. Wallis RS, Maeurer M, Mwaba P, Chakaya J, Rustomjee R, Migliori GB, et al. Tuberculosis–advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis (2016) 16:e34–46. doi: 10.1016/S1473-3099(16)00070-0
8. Rathore AS, Gupta RD. Chitinases from bacteria to human: Properties, applications, and future perspectives. Enzyme Res (2015) 2015:791907. doi: 10.1155/2015/791907
9. Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics (2007) 177:959–70. doi: 10.1534/genetics.107.075846
10. Chang SL, Dela Cruz CS. Chitotriosidase: a marker and modulator of lung disease. Eur Respir Rev (2020) 29(156):190143. doi: 10.1183/16000617.0143-2019
11. Suchard MS, Adu-Gyamfi CG, Cumming BM, Savulescu DM. Evolutionary views of tuberculosis: Indoleamine 2,3-dioxygenase catalyzed nicotinamide synthesis reflects shifts in macrophage metabolism: Indoleamine 2,3-dioxygenase reflects altered macrophage metabolism during tuberculosis pathogenesis. Bioessays (2020) 42:e1900220. doi: 10.1002/bies.201900220
12. Kassovska-Bratinova S, Yang G, Igarashi K, Dennery PA. Bach1 modulates heme oxygenase-1 expression in the neonatal mouse lung. Pediatr Res (2009) 65:145–9. doi: 10.1203/PDR.0b013e318191eedc
13. Cakir G, Gumus S, Ucar E, Kaya H, Tozkoparan E, Akgul EO, et al. Serum chitotriosidase activity in pulmonary tuberculosis: response to treatment and correlations with clinical parameters. Ann Lab Med (2012) 32:184–9. doi: 10.3343/alm.2012.32.3.184
14. Pavan Kumar N, Anuradha R, Andrade BB, Suresh N, Ganesh R, Shankar J, et al. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol (2013) 20:704–11. doi: 10.1128/CVI.00038-13
15. Kornfeld H, West K, Kane K, Kumpatla S, Zacharias RR, Martinez-Balzano C, et al. High prevalence and heterogeneity of diabetes in patients with TB in south India: A report from the effects of diabetes on tuberculosis severity (EDOTS) study. Chest (2016) 149:1501–8. doi: 10.1016/j.chest.2016.02.675
16. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Sivakumar S, et al. Association of plasma matrix metalloproteinase and tissue inhibitors of matrix metalloproteinase levels with adverse treatment outcomes among patients with pulmonary tuberculosis. JAMA Netw Open (2020) 3:e2027754. doi: 10.1001/jamanetworkopen.2020.27754
17. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Nair D, et al. Plasma chemokines are baseline predictors of unfavorable treatment outcomes in pulmonary tuberculosis. Clin Infect Dis (2021) 73:e3419–27. doi: 10.1093/cid/ciaa1104
18. Gillespie SH, Crook AM, Mchugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med (2014) 371:1577–87. doi: 10.1056/NEJMoa1407426
19. Lin CH, Lin CJ, Kuo YW, Wang JY, Hsu CL, Chen JM, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis (2014) 14:5. doi: 10.1186/1471-2334-14-5
20. Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med (2014) 371:1588–98. doi: 10.1056/NEJMoa1315817
21. Bhargava A, Bhargava M. Tuberculosis deaths are predictable and preventable: Comprehensive assessment and clinical care is the key. J Clin Tuberc. Other Mycobact. Dis (2020) 19:100155. doi: 10.1016/j.jctube.2020.100155
22. Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis (2017) 17:39–49. doi: 10.1016/S1473-3099(16)30274-2
23. Goletti D, Lindestam Arlehamn CS, Scriba TJ, Anthony R, Cirillo DM, Alonzi T, et al. Can we predict tuberculosis cure? what tools are available? Eur Respir J (2018) 52(5):1801089. doi: 10.1183/13993003.01089-2018
24. Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med (2021) 384:1705–18. doi: 10.1056/NEJMoa2033400
25. Suzuki Y, Suda T, Asada K, Miwa S, Suzuki M, Fujie M, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol (2012) 19:436–42. doi: 10.1128/CVI.05402-11
26. Arinola GO, Abdullahi I, Rahamon SK, Fasasi O. O., Adedeji A. Kehinde, et al. Activities of plasma indoleamine-2, 3-dioxygenase (IDO) enzyme in Nigerian patients with lung diseases: basis for tryptophan supplementation or IDO inhibitor use. Egypt J Bronchol. (2023) 17:2. doi: 10.1186/s43168-022-00174-2
27. Adu-Gyamfi CG, Snyman T, Hoffmann CJ, Martinson NA, Chaisson RE, George JA, et al. Plasma indoleamine 2, 3-dioxygenase, a biomarker for tuberculosis in human immunodeficiency virus-infected patients. Clin Infect Dis (2017) 65:1356–8. doi: 10.1093/cid/cix550
28. Shi W, Wu J, Tan Q, Hu CM, Zhang X, Pan HQ, et al. Plasma indoleamine 2,3-dioxygenase activity as a potential biomarker for early diagnosis of multidrug-resistant tuberculosis in tuberculosis patients. Infect Drug Resist (2019) 12:1265–76. doi: 10.2147/IDR.S202369
29. Almeida AS, Lago PM, Boechat N, Huard RC, Lazzarini LC, Santos AR, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol (2009) 183:718–31. doi: 10.4049/jimmunol.0801212
30. Collins JM, Siddiqa A, Jones DP, Liu K, Kempker RR, Nizam A, et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight (2020) 5(10):e137131. doi: 10.1172/jci.insight.137131
31. Adu-Gyamfi CG, Snyman T, Makhathini L, Otwombe K, Darboe F, Penn-Nicholson A, et al. Diagnostic accuracy of plasma kynurenine/tryptophan ratio, measured by enzyme-linked immunosorbent assay, for pulmonary tuberculosis. Int J Infect Dis (2020) 99:441–8. doi: 10.1016/j.ijid.2020.08.028
32. Adu-Gyamfi C, Savulescu D, Mikhathani L, Otwombe K, Salazar-Austin N, Chaisson R, et al. Plasma kynurenine-to-Tryptophan ratio, a highly sensitive blood-based diagnostic tool for tuberculosis in pregnant women living with human immunodeficiency virus (HIV). Clin Infect Dis (2021) 73:1027–36. doi: 10.1093/cid/ciab232
33. Murray MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis (2003) 3:644–52. doi: 10.1016/S1473-3099(03)00773-4
34. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol (2010) 185:3190–8. doi: 10.4049/jimmunol.0903670
35. Rockwood N, Costa DL, Amaral EP, Du Bruyn E, Kubler A, Gil-Santana L, et al. Mycobacterium tuberculosis induction of heme oxygenase-1 expression is dependent on oxidative stress and reflects treatment outcomes. Front Immunol (2017) 8:542. doi: 10.3389/fimmu.2017.00542
36. Andrade BB, Pavan Kumar N, Mayer-Barber KD, Barber DL, Sridhar R, Rekha VV, et al. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PloS One (2013) 8:e62618. doi: 10.1371/journal.pone.0062618
Keywords: chitinase, indoleamine 2, 3-dioxygenesae-1, heme oxygenase-1, unfavourable TB treatment, pulmonary tuberculosis (PTB)
Citation: Kumar NP, Nancy A, Viswanathan V, Sivakumar S, Thiruvengadam K, Ahamed SF, Hissar S, Kornfeld H and Babu S (2023) Chitinase and indoleamine 2, 3-dioxygenase are prognostic biomarkers for unfavorable treatment outcomes in pulmonary tuberculosis. Front. Immunol. 14:1093640. doi: 10.3389/fimmu.2023.1093640
Received: 09 November 2022; Accepted: 17 January 2023;
Published: 06 February 2023.
Edited by:
Fabian Salazar, University of Exeter, United KingdomReviewed by:
Melinda Shelley Suchard, National Institute of Communicable Diseases (NICD), South AfricaBhavna Gordhan, National Health Laboratory Service (NHLS), South Africa
Copyright © 2023 Kumar, Nancy, Viswanathan, Sivakumar, Thiruvengadam, Ahamed, Hissar, Kornfeld and Babu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathella Pavan Kumar, bmF0aGVsbGEucGtAaWNtci5nb3YuaW47; bmF0aGVsbGFwYXZhbkBnbWFpbC5jb20=
 Nathella Pavan Kumar
Nathella Pavan Kumar Arul Nancy
Arul Nancy Vijay Viswanathan
Vijay Viswanathan Shanmugam Sivakumar
Shanmugam Sivakumar Kannan Thiruvengadam5
Kannan Thiruvengadam5 Shaik Fayaz Ahamed
Shaik Fayaz Ahamed Hardy Kornfeld
Hardy Kornfeld Subash Babu
Subash Babu