
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 22 February 2023
Sec. Nutritional Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1092077
This article is part of the Research TopicNutrition and Cytokine Storm in Immune Disorders and Viral InfectionsView all 4 articles
 Samira Rastgoo1,2
Samira Rastgoo1,2 Ghazaleh Shimi1
Ghazaleh Shimi1 Farideh Shiraseb3
Farideh Shiraseb3 Ashkan Karbasi4
Ashkan Karbasi4 Damoon Ashtary-Larky5
Damoon Ashtary-Larky5 Mohsen Yousefi6
Mohsen Yousefi6 Elnaz Golalipour6
Elnaz Golalipour6 Omid Asbaghi2,7*
Omid Asbaghi2,7* Mohammad Zamani8*
Mohammad Zamani8*Background and aims: Many studies have investigated the effect of conjugated linoleic acid (CLA) supplementation on inflammatory cytokines and adipokines. However, the results of these studies are not consistent. Therefore, this systematic review and meta-analysis were designed to comprehensively evaluate the effect of CLA supplementation on inflammatory cytokines and adipokines.
Methods: Randomized controlled trials (RCTs) examining the effects of CLA supplementation on C-reactive protein (CRP), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), adiponectin, and leptin, published up to March 2022, were identified through PubMed, SCOPUS, and ISI Web of Science databases. A random-effects model was used to calculate weighted mean differences (WMDs) with 95% confidence intervals (CI) for 42 studies that included 1,109 participants.
Results: Findings from 42 studies with 58 arms indicated that CLA supplementation significantly decreased IL-6 and TNF-α levels and also slightly increased CRP levels. However, adiponectin and leptin levels did not change after CLA supplementation. A subgroup analysis found that CLA supplementation reduced adiponectin and leptin in women.
Conclusion: Our results demonstrated that CLA supplementation increased CRP levels and decreased TNF-α and IL-6 levels. Therefore, it seems that CLA can have both proinflammatory and anti-inflammatory roles.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier (CRD42022331110).
Inflammation is a protective reaction by an organism in response to injury, irritation, or infection that eliminates harmful stimuli and initiates the healing process (1). However, uncontrolled or unresolved inflammation can lead to tissue damage as well as the development of chronic inflammatory diseases, including type 2 diabetes, cardiovascular disease, cancer, etc. (2, 3). Therefore, the control and prevention of chronic inflammation should be considered in order to prevent some chronic diseases and even improve health.
Weight loss strategies such as dietary interventions and physical activity are commonly used as nonpharmacological interventions to combat and/or prevent proinflammatory conditions (4, 5). Despite the considerable benefits of pharmacological therapies, these may exert undesirable side effects and may not be tolerated by some individuals. On the other hand, they may cause drug resistance (reduction of efficiency) and even toxicity (6). Several studies have suggested that dietary strategies, such as certain supplements, can modulate inflammation. For example, a clinical trial has reported that magnesium and vitamin E co-supplementation led to a significant decrease in C-reactive protein (CRP) and a significant increase in total antioxidant capacity levels (7–9). Another study has suggested that l-glutamine supplementation in the early period of COVID-19 infection may reduce inflammatory responses and boost the immune system (10). Therefore, it is practical to find nutraceuticals and natural compounds with anti-inflammatory effects that may serve as alternative therapies to pharmacological interventions.
Conjugated linoleic acid (CLA) is a collective term for geometric isomers of linoleic acid (C18:2, n-6). This polyunsaturated fatty acid has two double bonds separated by a methylene group. This conjugation of the double bond is generally in positions 9 and 11 or 10 and 12 and may be a cis or trans configuration. Depending on the position and geometry of the double bonds, several isomers of CLA have been identified, such as 9cis,11trans-CLA and 10trans,12cis-CLA (11, 12). Most of the beneficial properties of CLA are elicited by these two main isomers. For example, 10trans,12cis-CLA is involved in catabolic processes such as lipolysis and fat oxidation, whereas 9cis,11trans-CLA seems to be the active anabolic agent. In addition, based on the evidence, 10trans,12cis-CLA is thought to be anticarcinogenic, antiobesity, and antidiabetic, whereas 9cis,11trans-CLA is mainly anti-inflammatory (13).
CLA is formed via biohydrogenation by bacterial enzymes known as catalyst, present in the intestine microbiota of ruminant mammals; hence, CLA is mainly found in their flesh and milk (14). Nonetheless, it is present in foods only in finite amounts; therefore, commercialized CLA supplements have been provided to offer potential benefits, such as reduction of body weight and total fat mass, anticancer effects including reducing tumor growth (15, 16), reducing insulin resistance, lipid disorders, and oxidative stress, improving liver function in nonalcoholic fatty liver (17), and immunomodulatory effects (18–20).
A large number of studies have investigated the effect of CLA supplementation on inflammatory cytokines and adipokines. However, the results of these studies are contradictory. For instance, some studies have reported that CLA supplementation had no significant effect on leptin levels (21, 22), whereas others found a significant decrease in serum leptin during CLA supplementation (23, 24). CLA supplementation has even been shown to significantly increase serum leptin levels (25). Also, some studies have reported that CLA supplements lead to a decrease in adiponectin levels (26), while another study has reported that CLA supplements have little or no effect on adiponectin levels (27). However, Sneddon et al. (28) have reported that CLA supplementation was associated with a significant increase in serum adiponectin levels (12%) in young obese subjects. Furthermore, while one study has found that CLA supplementation may increase CRP and tumor necrosis factor-alpha (TNF-α) and marginally decrease interleukin-6 (IL-6), indicating a proinflammatory state (29, 30), a recent review article suggests that CLA may have an anti-inflammatory effect by reducing inflammatory mediators such as cytokines, particularly IL-6, TNF-α, IFN-γ, and IL-1β (30). Therefore, due to the inconsistencies in previous studies, the present study sought to update previous meta-analyses in light of the plethora of new studies. Thus, the current meta-analysis sought to investigate the effects of CLA on inflammatory cytokines and adipokines in adults.
This systematic review has been conducted according to the PRISMA statement (31). The present study was registered at PROSPERO CRD42022331110.
We conducted a comprehensive literature search in scientific databases such as PubMed/Medline, Scopus, Web of Science, EMBASE, the Cochrane databases, and Google Scholar to find relevant prospective studies on the effects of CLA supplementation on inflammatory cytokines, adiponectin, and leptin published up to March 2022. There were no restrictions on the length of time or language of publications. The Participant, Intervention, Comparison/Control, Outcome (PICOS) search framework was used (32), which stands for population (both healthy and unhealthy adults), intervention (CLA supplementation), comparison (placebo/control group), outcome (changes in CRP, IL-6, TNF, adiponectin, and leptin), and research design (parallel and crossover randomized clinical trials).
The combination of MESH and non-MESH terms were used for the search, as follows: (“conjugated linoleic acid” OR “conjugated fatty acid” OR “boric acid” OR “rumenic acid” OR “CLA”) AND (intervention OR “intervention study” OR “intervention studies” OR “controlled trial” OR randomized OR random OR randomly OR placebo OR “randomized controlled trial” OR “randomized clinical trial” OR RCT OR blinded OR “double blind” OR “double blinded” OR trial OR “clinical trial” OR trials OR “Pragmatic Clinical Trial” OR “Cross-Over Studies” OR “Cross-Over” OR “cross-over study” OR parallel OR “parallel study” OR “parallel trial”).
By reading the titles, abstracts, and full text of the papers as needed, two researchers independently (FS and OA) chose the appropriate articles. The effects of CLA supplementation on inflammatory cytokines and adipokines variables in adults with different health statuses in all human randomized clinical trials)RCTs(.
We incorporated studies that satisfied the following criteria: (1) RCTs (parallel or crossover); (2) used oral intake of CLA; (3) examined the effects of CLA supplementation on CRP, IL-6, TNF-α, adiponectin, and leptin; (4) had an intervention duration of at least 2 weeks (RCTs with two or more eligible arms were considered as separate studies); (5) were performed on adults (≥18 years old); (6) provided means and standard deviations (SDs) for all outcome variables, or any other effect sizes from which the calculation of mean and SD was possible. The searches were limited to human studies with no language restrictions. Animal studies, reviews, in vitro research, research on kids and teenagers, grey literature, conference abstracts, opinion pieces, books, and RCTs without a placebo or control group were excluded. Studies that used CLA in combination with vitamins or minerals were also excluded.
In the present study, we searched for studies that assessed the effects of CLA supplementation on all inflammatory cytokines. After screening and finding eligible studies, we found that most studies evaluated CRP, IL-6, and TNF. In addition, a limited number of studies have evaluated other inflammatory factors such as IL-1 and IL-8. Therefore, we included studies that evaluated the effects of CLA on CRP, IL-6, and TNF.
After quickly skimming the titles, abstracts, and full text to choose the most pertinent research following a separate review of each qualifying RCT, the following data were gleaned by two independent researchers (OA and FS). Name of the first author, country of origin, year of publication, type of clinical trial, participant characteristics (mean age, body mass index (BMI), sex), randomization, blinding, sample size, number of participants in the intervention and control groups, type and dosage of supplemented CLA, duration of the study, and related data were extracted for additional measurements. The CLA dosages were converted to milligrams per day, whether they were given in grams per day or another unit.
To rate the quality of the studies, the Cochrane Collaboration technique was utilized (33). Two researchers (SR and GS) independently assessed the methods, and any disagreements between their assessments were resolved through discussion. Each study was evaluated for any bias, including those caused by randomized sequence generation, allocation concealment, participant and staff blindness, outcome assessor blindness, insufficient outcome data, selective reporting, and other biases. Thus, three categories were established: high risk of bias (general risk of bias > 2 high risks), low risk of bias (general risk of bias < 2 high risks), and undetermined risk of bias (general risk of bias = 2 high risk).
Stata 11.0 was used to conduct the statistical analysis (Stata Corp, College Station, TX, USA). p-values <0.05 were deemed statistically significant for all two-tailed tests. The pooled weighted mean difference (WMD) was calculated using a random-effects model to take into account any existing heterogeneity (due to the different intervention doses, duration, participant health, sample sizes, and length of intervention) developed by Der Simonian and Laird (34). We computed the mean differences in CRP, IL-6, TNF-α, adiponectin, and leptin between the CLA supplementation and control groups from the preintervention to the postintervention. Formula: SD = square root [(SD at baseline)2 + (SD at end of study)2 (2rSD at baseline, SD at end of study)] was used to get the SD of the mean difference (35). For RTCs that only provided standard error of the mean (SEM) information, SD was determined using the following formula: Standard Error of Mean (SEM): SD =, where n is the number of subjects in each group (36). After visual inspection of forest plots or Cochrane’s Q test, heterogeneity was evaluated using the I square (I2) statistic (p < 0.05 and I2 > 40%), with I2 > 40% considered clinically significant (37). The baseline CRP, IL-6, TNF-α, adiponectin, and leptin levels were analyzed, while the intervention duration (< 12 weeks, ≥ 12 weeks) and dosage of CLA (≥ 3 and < 3 g/day) were based on the median values of the included studies and health status (healthy: people without any disease; unhealthy: people suffering from a disease such as atherosclerosis, rheumatoid arthritis, diabetes, etc.). Other subgroup analyses were performed according to gender (man, woman), baseline BMI (normal (18.5–24.9 kg/m²), overweight (25–29.9 kg/m²), and obese (>30 kg/m²)) and isomer’s type (CLA-c9t11, CLA-t10c12, both). Studies examining the effect of CLA supplementation on inflammatory cytokines and adipokines used the Begg’s test and the funnel plot test to evaluate publication bias (38). We used the leave-one-out approach to do a sensitivity analysis to identify how many inferences were dependent on a single sample to examine the influence of each study on the pooled effect size (39). The possible linear association between CLA (mg/day) dose and duration on outcome variables was evaluated using meta-regression, whereas a nonlinear dose–response analysis was used to assess the effect of CLA supplementation dose and duration (40, 41).
The flow chart presented in Figure 1 describes the selection process and the references retrieved from the database.
A total of 8,351 studies were identified in the first step of the literature search of electronic databases, PubMed (n = 3,582), ISI Web of Science (n = 1,991), Scopus (n = 2,310), and the Cochrane library (n = 468). After excluding duplicated (n = 2,419) and irrelevant studies based on titles and abstracts (n = 2,804), 104 potentially relevant articles were considered for full-text review. Out of 104 studies, 62 did not have the desired data. Finally, 42 studies (11, 12, 16, 18, 19, 27, 28, 42–76) were included in the present meta-analysis, and their characteristics are illustrated in Table 1. The risk of bias assessment is summarized in Table 2.
All of these studies were RCTs published between 2000 and 2020. Study design characteristics are presented in Table 1. The WMD and 95% CI of CRP (mg/dl), IL-6 (pg/ml), TNF-α (ng/ml), adiponectin (µg/ml), and leptin (ng/ml) and their changes are presented in Figures 2A–E, respectively. The two studies were conducted in the USA (42, 67), three in Sweden (43, 45, 49), three in the Netherlands (44, 52, 65), four in Canada (12, 14, 46, 68), two in Norway (27, 47), four in the UK (28, 48, 51, 56), two in France (50, 53), two in Turkey (11, 72), one in Japan (55), one in Ireland (57), one in Korean (58), one in Finland (59), one in Denmark (61), one in China (62), three in Germany (62, 69, 70), one in Italy (64), one in Spain (16), one in Poland (75), and the others in Iran (18, 19, 60, 66, 71, 73, 74, 76). Thirteen studies included only men and seven women, and 22 included both sexes. There were 37 parallel (11, 12, 16, 18, 19, 27, 42–45, 47–63, 65–67, 69, 71–76) and five crossover (28, 46, 64, 68, 70) studies. A total of 1,192 subjects (intervention: 597/control: 595) in terms of CRP, 817 subjects (intervention: 418/control: 399) for IL-6, 779 subjects (intervention: 402/control: 377) for TNF-α, 845 subjects (intervention: 424/control: 421) for adiponectin, and 1,274 subjects (intervention: 649/control: 625) for leptin were enrolled. The duration of the intervention varied from 4 to 104 weeks. The CLA supplements were used in doses of 1.6 to 10.8 mg/day. The mean age and baseline BMI ranged from 20 to 61.55 years and 22.2 to 45.71 kg/m2, respectively. Participant’s health conditions were as follows: obese men with metabolic syndrome (43), overweight subjects with LDL phenotype B (52), both older obese and young lean, older lean, young obese (28), birch pollen allergy (59), obesity-related hypertension (62), postmenopausal women with type 2 diabetes mellitus (63), overweight mild asthmatics (12), postmenopausal women (66), overweight and hyperlipidemic (68), overweight type 2 diabetes (71), healthy overweight people (16, 47, 56), healthy subjects (11, 42, 44, 48, 49, 51, 53, 54, 57, 58, 61, 64, 67, 70, 72), atherosclerotic patients (73), rheumatoid arthritis (19, 60), athletic (74), overweight/obese (27, 45, 46, 50, 55, 65, 69, 75), nonalcoholic fatty liver disease (76), and chronic obstructive pulmonary disease (COPD) (18). Iwata et al. (55), Albers et al. (44), and Steck et al. used two different CLA doses in their studies. Out of the 42 studies, CRP, IL-6, TNF-α, adiponectin, and leptin were reported in 20, 15, 16, 12, and 20, respectively. For supplementation, 47 study arms used mixed isomers (9cis,11trans-CLA and 10trans,12cis-CLA), four study arms used 9cis,11trans-CLA isomer, and five study arms used 10trans,12cis-CLA isomer. In these studies, detection methods of CRP were Behring latex-enhanced high-sensitivity assays, Eckman Synchron CX7 System, enzyme-linked immunosorbent assay, enhanced turbidimetric immunoassay, highly sensitive immunoassay with a monoclonal antibody coated with polystyrene particles, immunoturbidimetric assay, and rabbit antihuman.

Figure 2 Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of CLA consumption on (A) CRP (mg/L), (B) IL-6 (pg/ml), (C) TNF-α (ng/ml), (D) adiponectin (µg/ml), and (E) leptin (ng/ml). Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference; CI, confidence interval; CLA, conjugated linoleic acid; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
Overall, 24 effect sizes from 20 studies for CRP were included in the analysis. The results of the analysis showed that CLA supplementation significantly increased CRP compared to placebo [(WMD = 0.26 mg/dl; 95% CI, 0.00 to 0.52; p = 0.048 (I2 = 56.3%, <0.001)] (Figure 2A). The effect of CLA on CRP was significant in studies with baseline CRP less than 3 mg/dl (WMD = 0.31 mg/dl; 95% CI, 0.05 to 0.57; p = 0.016), supplementation dose ≥3 (g/day) (WMD = 0.49 mg/dl; 95% CI, 0.09 to 0.90; p = 0.017), and healthy subgroup (WMD = 0.39 mg/dl; 95% CI, 0.10 to 0.68; p = 0.008). Heterogeneity disappeared in supplementation subgroups of less than 3 (g/day) (I2 = 36.9%, p = 0.104), overweight (25–29.9) (I2 = 15.9%, p = 0.288), and unhealthy subjects (I2 = 52.4%, p = 0.078). In the other subgroups, the effect of CLA supplementation on serum concentrations of CRP was not significant (Table 3).
Pooling effect sizes from 15 studies showed that CLA supplementation significantly decreased IL-6 compared to placebo [(WMD = −0.66 mg/dl; 95% CI, −1.14 to −0.19; p = 0.006 (I2 = 86.3%; p < 0.001)] (Figure 2B). The effect of CLA on IL-6 was significant in trials lasting <12 weeks (WMD = −1.16 pg/ml; 95% CI, −1.82 to −0.50; p = 0.001), male subjects (WMD = −0.31 mg/dl; 95% CI, −0.51 to −0.12; p = 0.002), unhealthy subgroup (WMD = −0.61 mg/dl; 95% CI, −1.09 to −0.14; p = 0.010), and when a mixture of two isomers was used (WMD = −0.68 mg/dl; 95% CI, −1.15 to −0.21; p = 0.005). Heterogeneity disappeared in the trial duration of ≥12 week (I2 = 11.7%; p = 0.335) and the male subgroup (I2 = 15.5%; p = 0.335). In the other subgroups, the effect of CLA supplementation on serum concentrations of IL-6 was not significant (Table 3).
Results of pooled 19 random-effects size of CLA supplementation on TNF-α found a significant reduction in TNF-α compared to placebo [(WMD = −0.99 mg/dl; 95% CI, −1.76 to −0.22; p = 0.011; (I2 = 90.2%; p < 0.001)] (Figure 2C). Subgroup analysis showed a more beneficial effect of CLA supplementation in studies with an intervention dose of <3 (g/day) (WMD = −3.08 mg/dl; 95% CI, −4.91 to −1.25; p = 0.001), healthy people (WMD = −2.01 mg/dl; 95% CI, −3.31 to −0.71; p = 0.002), male subjects (WMD = −1.01 mg/dl; 95% CI, −1.95 to −0.08; p = 0.033), subjects with normal range of BMI (18.5–24.9) (WMD = −9.29 mg/dl; 95% CI, −18.51 to −0.08; p = 0.048), and when a mixture of two isomers was used (WMD = −1.41 mg/dl; 95% CI, −2.31 to −0.50; p = 0.002). Interestingly, when the 10trans12cis-CLA isomer was administered, TNF-α was significantly increased (WMD = 1.29 mg/dl; 95% CI, 0.64 to 1.93; p < 0.001). Between-study heterogeneity was out of sight in studies with unhealthy subjects (I2 = 36.8%; p = 0.191). In the other subgroups, the effect of CLA supplementation on serum concentrations of TNF-α was not significant (Table 3).
Combining 18 effect sizes from 12 studies showed that CLA supplementation had no significant effect on adiponectin level in the intervention group compared to placebo [(WMD = −0.01 mg/dl; 95% CI, −0.68 to 0.71; p = 0.976 (I2 = 98.0%, p < 0.001)] (Figure 2D). Subgroup analyses showed a significant decrease in adiponectin levels in the female subjects (WMD = −1.05 mg/dl; 95% CI, −1.58 to −0.52; p < 0.001). Our subgroup analyses indicated no significant between-study heterogeneity in studies conducted on the female subjects (I2 = 2.8%; p = 0.311) and overweight (I2 = 0.0%; p = 0.943) (Table 3).
Combining 28 effect sizes from 20 studies showed that CLA supplementation had no significant effect on leptin level in the intervention compared to the placebo group [(WMD = −0.30 mg/dl; 95% CI, −0.79 to 0.17; p = 0.214 (I2 = 95.9%; p < 0.001)] (Figure 2E). Subgroup analyses showed a significant decrease in leptin levels in unhealthy subjects (WMD = −1.69 mg/dl; 95% CI, −1.80 to −1.58; p < 0.001), female subjects (WMD = −0.95 mg/dl; 95% CI, −1.79 to −0.12; p = 0.025), and a significant increase in leptin levels in the subjects with normal BMI (18.5–24.9) (WMD = 0.50 mg/dl; 95% CI, 0.28 to 0.72; p < 0.001). Subgroup analyses indicated no significant between-study heterogeneity in studies conducted on unhealthy subjects (I2 = 0.0%; p=0.451), female subjects (I2 = 0.0%; p = 0.696), overweight subjects (I2 = 0.0%; p = 0.943), and subjects with normal range of BMI (I2 = 0.0%; p = 0.703) (Table 3).
Although the visual inspection of funnel plots showed slight asymmetries for all of the outcome variables, no significant publication bias was detected according to Begg’s test for most of the variables, such as CRP (p = 0.189), IL-6 (p = 0.441), adiponectin (p = 0.198), and leptin (p = 0.050). Publication bias was considerable just for TNF-α (p = 0.042) (Figures 3A–E).
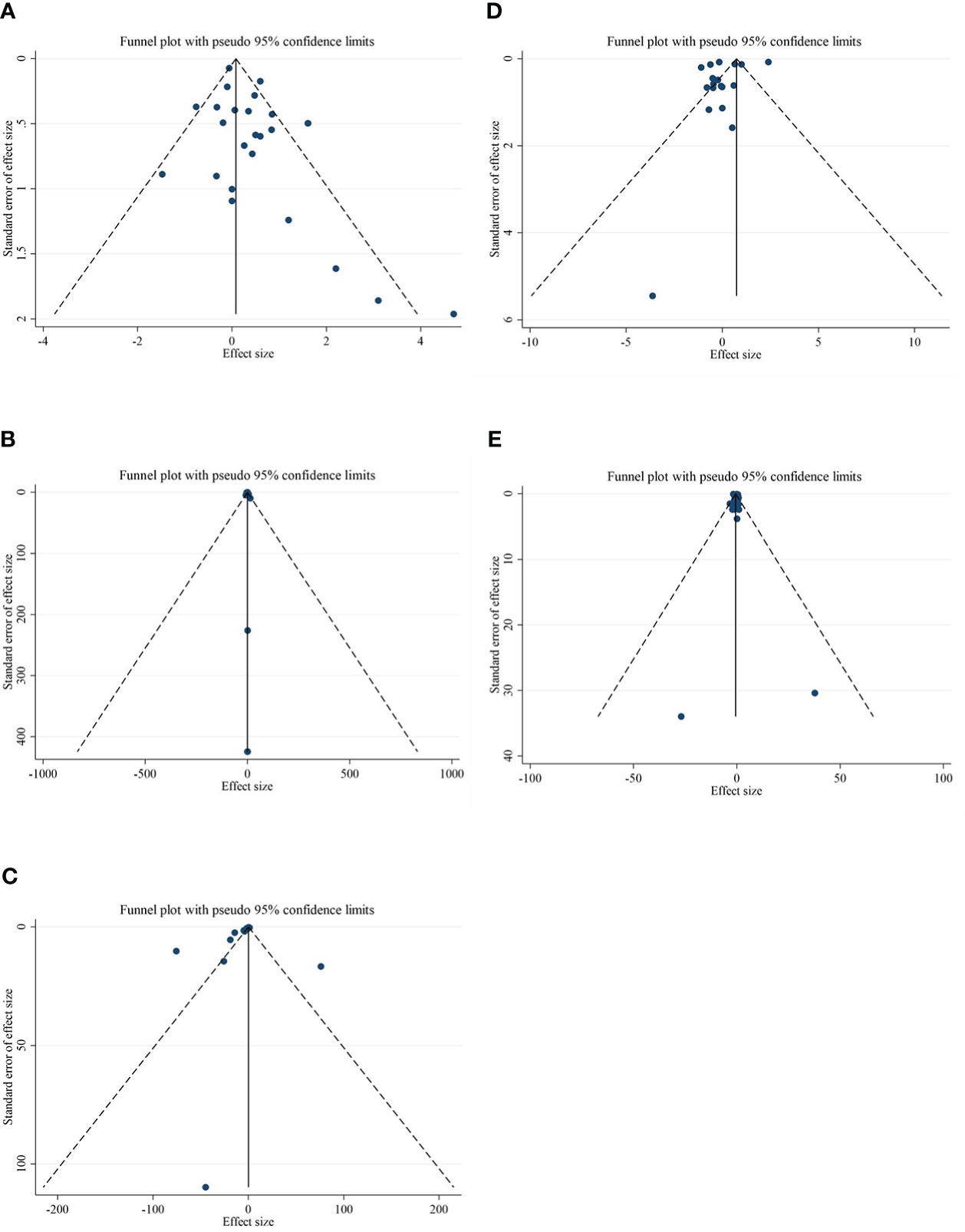
Figure 3 Funnel plots for the effect of CLA consumption on (A) CRP (mg/L), (B) IL-6 (pg/ml), (C) TNF-α (ng/ml), (D) adiponectin (µg/ml), and (E) leptin (ng/ml). Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference; CI, confidence interval; CLA, conjugated linoleic acid; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
The present study conducted a nonlinear dose–response regression to analyze the dose–response relationship between CLA supplementation and inflammatory cytokines and adipokines. We did not find a significant nonlinear relationship between dose (g/day) and changes in CRP [(coefficients = −0.059; pnonlinearity = 0.353)] (Figure 4A), IL-6 [(coefficients = −10.437; pnonlinearity = 0.244)] (Figure 4B), TNF-α [(coefficients = −791.208; pnonlinearity = 0.081)] (Figure 4C), adiponectin [(coefficients = 1.337; pnonlinearity = 0.522)] (Figure 4D), and leptin [(coefficients = −77.607; pnonlinearity = 0.841)] (Figure 4E). Moreover, there was a nonlinear association between the duration of intervention and CRP [(coefficients = −0.798; pnonlinearity = 0.164)] (Figure 5A), IL-6 [(coefficients = 2.635; pnonlinearity = 0.247)] (Figure 5B), TNF-α 9[(coefficients = −8.156; pnonlinearity = 0.622)] (Figure 5C), adiponectin [(coefficients = −0.343; pnonlinearity = 0.752)] (Figure 5D), and leptin [(coefficients = 0.056; pnonlinearity = 0.748)] (Figure 5E).
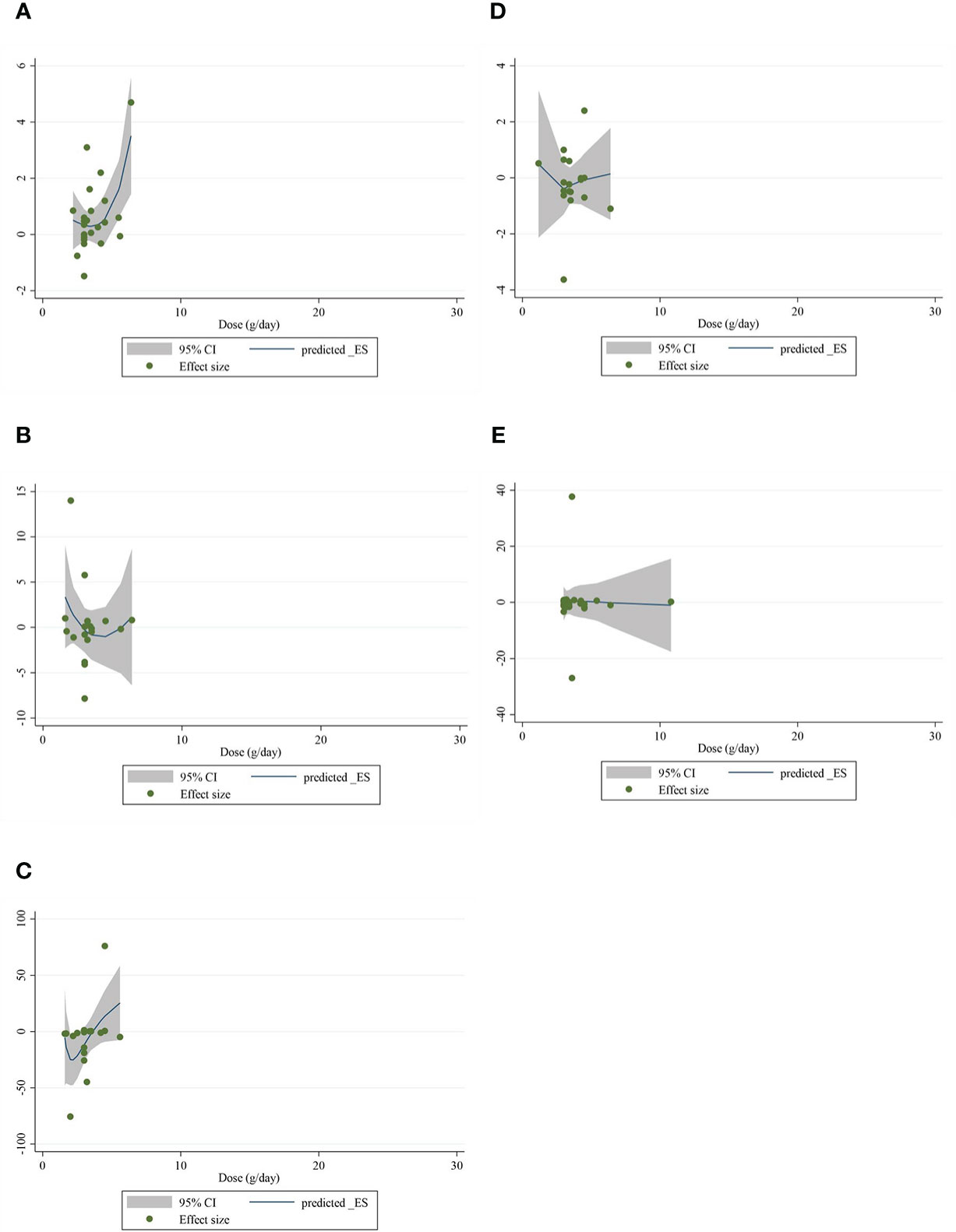
Figure 4 Nonlinear dose–response analysis on the effects of the CLA dosage (mg/day) on (A) CRP (mg/L), (B) IL-6 (pg/ml), (C) TNF-α (ng/ml), (D) adiponectin (µg/ml), and (E) leptin (ng/ml). Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference; CI, confidence interval; CLA, conjugated linoleic acid; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
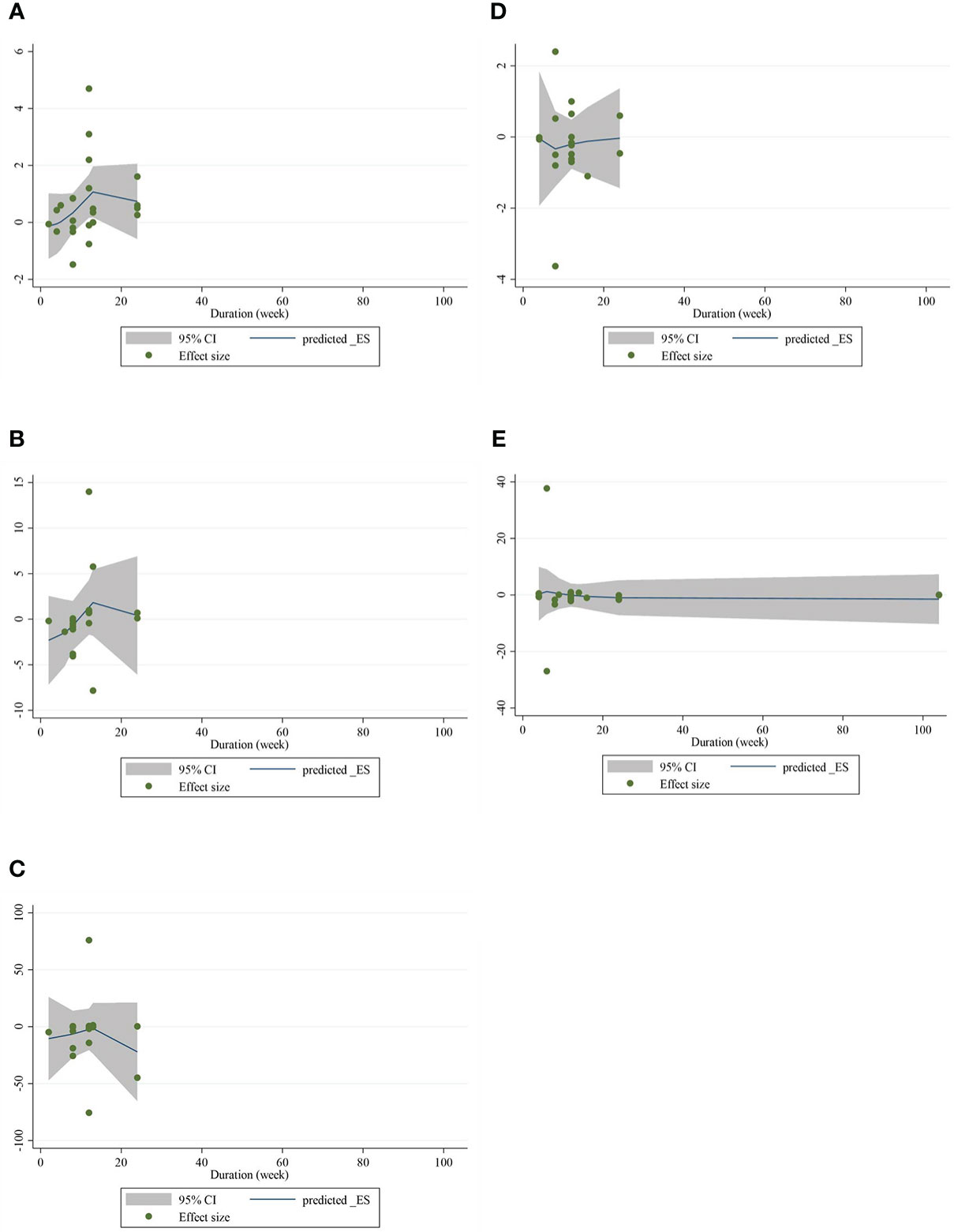
Figure 5 Nonlinear dose–response analysis on the effects of the duration of the intervention (week) of CLA on (A) CRP (mg/L), (B) IL-6 (pg/ml), (C) TNF-α (ng/ml), (D) adiponectin (µg/ml), and (E) leptin (ng/ml). Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference; CI, confidence interval; CLA, conjugated linoleic acid; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
Meta-regression analyses were performed to assess whether inflammatory cytokines and adipokines were affected by CLA supplementation doses and intervention duration. We found no significant linear association between intervention dose (g/day) and changes in CRP [(coefficients = 0.279; plinearity = 0.304)] (Figure 6A), IL-6 [(coefficients = 0.162; plinearity = 0.384)] (Figure 6B), TNF-α [(coefficients = 0.051; plinearity = 0.538)] (Figure 6C), adiponectin [(coefficients = −0.152; plinearity = 0.616)] (Figure 6D), leptin [(coefficients = 0.138; plinearity = 0.693)] (Figure 6E), nor duration of supplementation (week) and changes in CRP [(coefficients = 1.001; plinearity = 0.365)] (Figure 7A), IL-6 [(coefficients = 0.327; plinearity = 0.528)] (Figure 7B), TNF-α [(coefficients = 0.038; plinearity = 0.737)] (Figure 7C), adiponectin [(coefficients = −0.094; plinearity = 0.944)] (Figure 7D), and leptin [(coefficients = −0.006; plinearity = 0.994)] (Figure 7E).
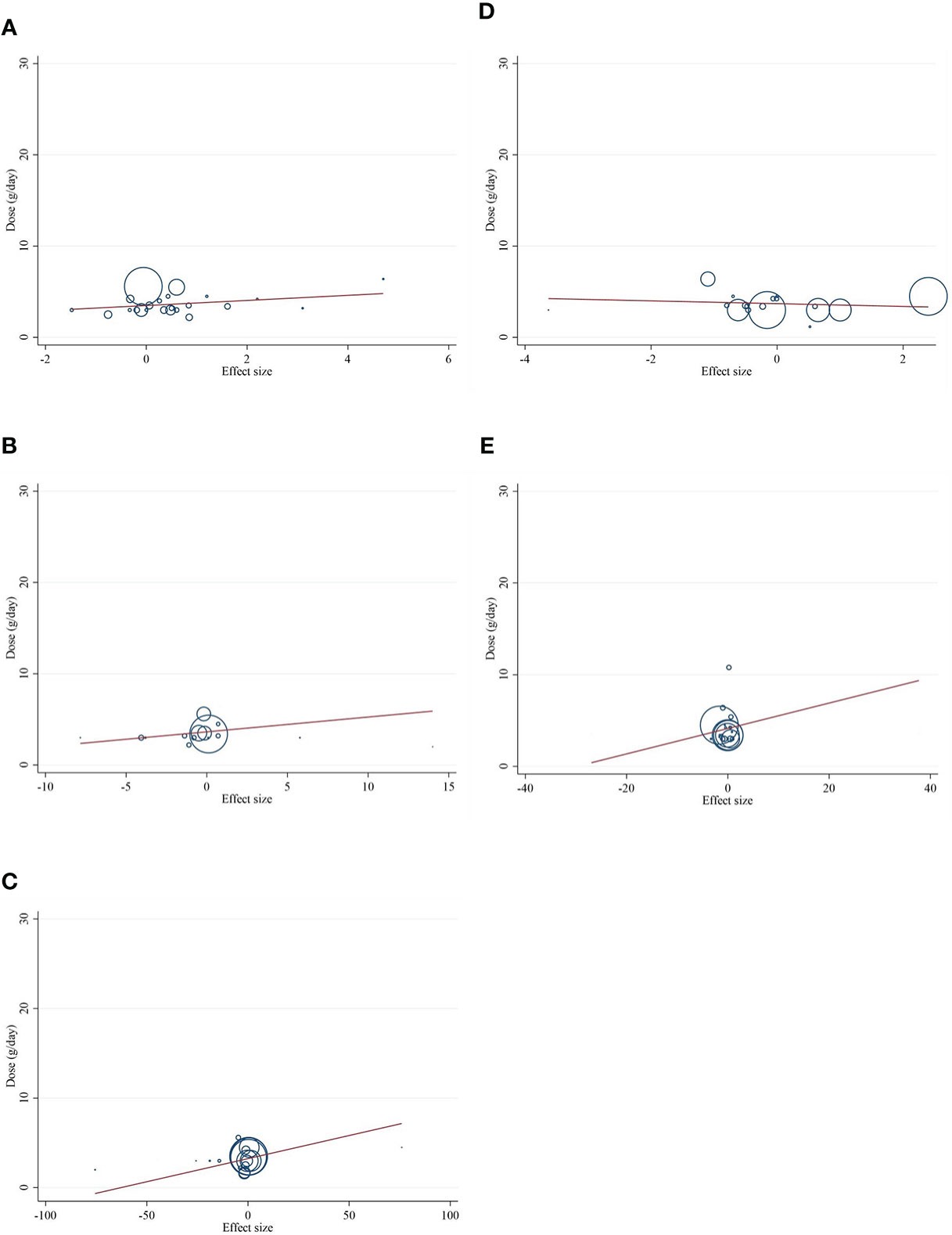
Figure 6 Random-effects meta-regression plots of the association between dose of CLA (mg/day) and weighted mean difference of (A) CRP (mg/L), (B) IL-6 (pg/ml), (C) TNF-α (ng/ml), (D) adiponectin (µg/ml), and (E) leptin (ng/ml). Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference; CI, confidence interval; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
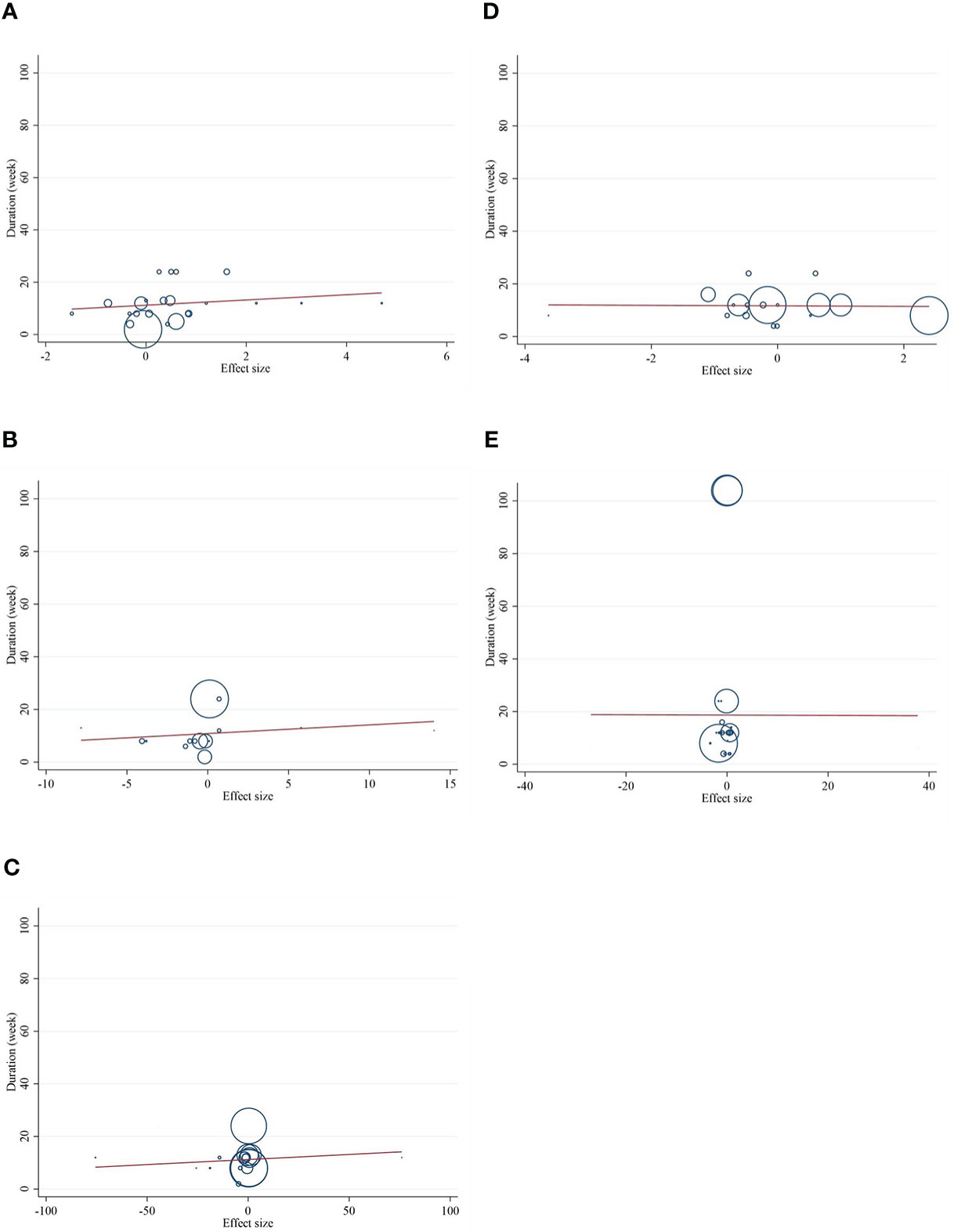
Figure 7 Random-effects meta-regression plots of the association between duration of intervention of CLA and weighted mean difference of (A) CRP (mg/L), (B) IL-6 (pg/ml), (C) TNF-α (ng/ml), (D) adiponectin (µg/ml), and (E) leptin (ng/ml). Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference; CI, confidence interval; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
The sensitivity analysis demonstrated that no study had a significant impact on the overall findings. Although the overall result of studies reporting data on TNF-α was sensitive to the study by Song et al. (48) (WMD = −0.644; 95% CI, −1.353, 0.065), and IL-6 was sensitive to the study by Sofi et al. (64) (WMD = −0.284; 95% CI, −0.601, 0.033), by omitting these studies, the significance of the correlation between CLA and TNF-α and IL-6 was lost.
In the case of CRP, some studies had an impact on the overall effect size, including that of Whigham et al. (54) (WMD = 0.253; 95% CI, −0.013, 0.520), Smedman et al. (49) (WMD = 0.247; 95% CI, −0.010, 0.506), and Pfeuffer et al. (69) (WMD = 0.257; 95% CI, −0.007, 0.523); the arm “a” and “b” of the study of Ramakers et al. (50) (WMD = 0.258; 95% CI, −0.013, 0.529; WMD = 0.245; 95% CI, −0.028, 0.518), Taylor et al. (51) (WMD = 0.251; 95% CI, −0.009, 0.512), Gaullier et al. (27) (WMD = 0.190; 95% CI, −0.053, 0.434), and Mullen et al. (57) (WMD = 0.226; 95% CI, −0.036, 0.490); the arm “a” and “b” of the study of Steck et al. (56) (WMD = 0.245; 95% CI, −0.010, 0.501; WMD = 0.237; 95% CI = −0.011, 0.485), Raff et al. (61) (WMD = 0.219; 95% CI, −0.046, 0.484), and Sluijs et al. (65) (WMD = 0.262; 95% CI, −0.003, 0.529); and the arm “b” of the study of Joseph et al. (68) (WMD = 0.237; 95% CI, 0.026, 0.501) and Lopez-Plaza et al. (16) (WMD = 0.249; 95% CI, −0.016, 0.515). It was shown that the exclusion of every individual study by the sensitivity analysis could not change the direction of the correlation but eliminated the significant effect of CLA on CRP.
Table 4 displays the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) profile of CLA supplementation on inflammatory cytokine and adipokine variables together with the certainty in outcomes. For CRP, because of serious limitations in inconsistency, the quality of evidence was moderate. In the case of IL-6, because of very serious limitations in inconsistency and for TNF-α because of very serious limitations in inconsistency ad serious limitations for publication bias, the quality of evidence was low. For both outcomes, including leptin and adiponectin, because of very serious limitations in inconsistency and serious limitations in imprecision, the quality of evidence was very low.
Using the GRADE methodology, which was previously outlined, the total degree of evidence certainty across the studies was evaluated and summarized (77).
In this review, an analysis of pooling 42 studies indicated that CLA supplementation increased CRP concentrations, decreased IL-6 and TNF-α values, and had no effect on adiponectin and leptin levels. Findings of subgroup analyses showed that CLA consumption increased CRP in subjects with normal CRP levels (less than 3 mg/dl) and those who were healthy or consumed CLA in doses of ≥3 g/day. Taking CLA decreased IL-6 in male individuals and unhealthy subjects if the trial duration was less than 12 weeks and when a mixture of two isomers was used. TNF-α was reduced by CLA consumption in studies with an intervention dose of <3 g/day, male subjects, healthy persons, or those having normal baseline BMI, and when a mixture of two isomers was used. However, in subgroup analyses based on isomer type, 10trans12cis- CLA isomer significantly increased TNF-α. Supplementation with CLA reduced adiponectin in women. It also decreased leptin in women and unhealthy adults but increased in those with normal baseline BMI. Moreover, the results of the meta-regression analysis showed that there was no significant linear association between intervention doses and duration of supplementation with inflammatory cytokines and adipokines.
The majority of studies included in this meta-analysis used supplements consisting of mixed amounts of CLA isomers. Therefore, since CLA isomers have shown different effects on inflammation (30, 78), it is suggested that this issue be considered in research. It is also important to consider the contents of the placebo used in studies. Some included RCTs have used vegetable oils as a placebo, such as safflower and olive oils. One study has indicated the reduced effect of safflower oil on CRP levels compared to CLA (79). Another study has elucidated the anti-inflammatory effect of olive oils through the prevention of lipooxygenase and enzymes that synthesize leukotriene (80). In other words, prescribing these vegetable oils as a placebo might misinterpret results.
Diet and physical activity can play an important role in inflammatory conditions (81). It is better to consider the effects of these two factors in the result of the present meta-analysis. Although, in some studies, the confounding variables of physical activity and diet were controlled, in some trials, these factors were not considered.
Diet is an important regulatory factor in the immune response. Malnutrition leads to suppression of the immune system, whereas overnutrition leads to immune activation. Some foods have anti-inflammatory effects, and there are still controversies about others (81). For example, several studies have reported a positive association between the glycemic index/glycemic load of dietary carbohydrates and inflammatory cytokines (81). Also, a high-fat diet causes excessive body fat accumulation and impairs the immune system. On the other hand, it has been reported that trans and saturated fatty acids are significantly associated with the inflammatory state (82). Although ruminant trans fatty acids have different effects than industrial trans fatty acids, several studies show that industrial trans fatty acids promote inflammation, whereas ruminant trans fatty acids have been reported to be harmless or even beneficial to health, as well as also having anti-inflammatory properties (83).
In relation to physical activity, it has been reported that although exercise produces a short-term inflammatory response, regular exercise in the long term has an “anti-inflammatory” effect (84). One study reported that sedentary subjects had higher levels of inflammatory factors such as IL-6 and TNF-α compared to subjects with higher physical activity (85).
Other confounding factors that can affect inflammatory markers are sleep duration and sleep quality. Recently, studies have reported a relationship between longer sleep duration (>8 h) and increased levels of inflammatory markers such as CRP and IL-6 (86, 87). In addition, a study on healthy subjects reported that higher IL-6 levels were associated with lower sleep quality (88).
Here, we showed CLA consumption increased CRP concentrations, decreased IL-6 and TNF-α, and did not change adiponectin and leptin levels. There are some meta-analyses that have investigated the effect of CLA on the abovementioned markers. For example, Derakhshandeh-Rishehri et al. (2019) (89), working on 15 RCTs, revealed that long-term (24 weeks) CLA intake significantly increased CRP levels (89). A meta-analysis conducted by Haghighatdoost et al. (28) showed a significant increase in CRP (n = 8) and TNF-α levels (n = 8) along with a slight decrease in blood IL-6 (n = 9) following CLA supplementation (29). Another meta-analysis (2018) conducted on 19 RCTs demonstrated that CLA intake had a small but not significant decline in plasma leptin. However, it significantly decreased leptin in obese individuals, with trials lasting for less than 24 weeks (21). A study carried out by Mazidi et al. (89) on 14 RCTs indicated that treatment with CLA had no effect on IL-6 but significantly reduced serum TNF-α and adiponectin, besides enhancing serum CRP concentrations (90). By combining previous and current results, it can be acknowledged that except for the effect of CLA in increasing CRP levels, there is no consensus on other markers (i.e., IL-6, TNF-α, adiponectin, and leptin). Moreover, it is noteworthy to state that exercise, dietary intake, sleep duration, and sleep quality can alter the effects of CLA. Therefore, further well-designed studies may clarify the role of CLA on the abovementioned markers.
In the present study, the gender-dependent impact of CLA has also been shown in the reduction of IL-6, TNF-α, adiponectin, and leptin. Based on evidence, CLA can induce testosterone biosynthesis (91), and subsequently, testosterone is able to decrease IL-6 expression (92). It has also been stated that circulating CLA concentrations are greater in women than in men (93). Therefore, the aforementioned evidence can justify the different effects seen between men (IL-6 and TNF-α reduction) and women (adiponectin and leptin reduction) after taking CLA.
The effect of CLA on inflammatory responses has generated inconsistent results. This may be due to isomers and tissue-specific responses of CLA. Moreover, across the included RCTs, administration doses of CLA varied from 1.6 to 10.8 mg/day, and the duration of the treatment differed from 4 to 104 weeks. Health characteristics, inflammation status, and even gut microbiome composition of the recruited individuals were also different (78). These variations in the studies can influence the results and interpretations.
For example, in relation to isomer and tissue-specific response, 9cis,11trans-CLA isomer exerts its anti-inflammatory effect by activating the peroxisome proliferator-activated receptor-γ (PPAR-γ)-dependent pathway and ultimately reducing the production of proinflammatory cytokines such as TNF-α and IL-6 (30). However, the 10trans,12cis-CLA isomer has been reported to have a proinflammatory effect in adipose tissue, in contrast to its effects on innate immunity and vascular cells. In addition, in adipose tissue, activation of PPARγ is contrary to the physiological effects of 10trans,12cis-CLA in vitro and in vivo (78). This isomer downregulates PPAR-γ gene expression and increases TNF-α, IL-6, and CRP production in adipose tissue (78, 94). It seems that the reason for this inconsistency is cytokines secreted from adipocytes, which in turn alter PPARγ expression and activity in the fat cell. Therefore, inconclusive results regarding the anti-inflammatory properties of CLA are likely due to the subtle proinflammatory effects of the 10trans,12cis-CLA isomers in specific tissues (30, 78). Overall, it appears that CLA can elicit both anti-inflammatory and proinflammatory features.
Some proposed mechanisms for the effects of CLA isomers on inflammation include the following: (1) Modulation of eicosanoid signaling. This means that CLA reduces the production of inflammatory eicosanoids derived from arachidonic acid (AA) through the inhibition of several steps in the AA cascade. (2) Modulating the transcription of some genes, such as the PPAR gene, plays an important role in the regulation of inflammation. Also, it suppresses the expression of the inducible NO synthase (iNOS) gene, which leads to a decrease in IL-6 production. (3) Inhibition of NF-kB activity, which is a transcription factor involved in cytokine gene expression, cellular adhesion, cell cycle activation, apoptosis, and carcinogenesis and is integrally related to inflammatory responses (78, 95).
This meta-analysis has some limitations that should be addressed. Most of the included studies had a relatively small sample size, which can cause an overestimation of the results. Observation of publication bias for TNF-α findings suggests overestimation of CLA efficiency on TNF-α. Moreover, TNF-α, IL-6, and CRP results were sensitive to some studies.
In conclusion, it is suggested that CLA supplementation can have both proinflammatory and anti-inflammatory roles. It can enhance CRP concentrations while reducing TNF-α and IL-6 levels. Furthermore, CLA is able to decrease adiponectin and leptin in women. It can also decrease leptin in unhealthy adults and increase it in subjects with a normal baseline BMI. Finally, in order to improve the quality of studies, future clinical trials are encouraged to carefully consider CLA-isomer-specific regulation of inflammatory markers and take notice of the contents of the placebo used in their control groups. It is also important to keep in mind the gender-dependent impact of CLA.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
OA and SR contributed to the conception and design of the study. DA-L, MZ, and GS contributed to data extraction. FS and AK screened articles for inclusion criteria. OA contributed to the data analysis. SR, MY, and EG contributed to manuscript drafting, OA and MZ supervised the study. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Asbaghi O, Ashtary-Larky D, Bagheri R, Moosavian SP, Nazarian B, Afrisham R, et al. Effects of folic acid supplementation on inflammatory markers: A grade-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Nutrients (2021) 13(7):2327. doi: 10.3390/nu13072327
2. Ghafourian M, Ashtary-Larky D, Chinipardaz R, Eskandary N, Mehavaran M. Inflammatory biomarkers’ response to two different intensities of a single bout exercise among soccer players. Iran Red Crescent Med J (2016) 18(2):e21498. doi: 10.5812/ircmj.21498
3. Zouhal H, Bagheri R, Ashtary-Larky D, Wong A, Triki R, Hackney AC, et al. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Phys Behavior (2020) 225:113090. doi: 10.1016/j.physbeh.2020.113090
4. Bagheri R, Rashidlamir A, Ashtary-Larky D, Wong A, Alipour M, Motevalli MS, et al. Does green tea extract enhance the anti-inflammatory effects of exercise on fat loss? Br J Clin Pharmacol (2020) 86(4):753–62. doi: 10.1111/bcp.14176
5. Bagheri R, Rashidlamir A, Ashtary-Larky D, Wong A, Grubbs B, Motevalli MS, et al. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur J Appl Physiol (2020) 120(4):915–23. doi: 10.1007/s00421-020-04332-6
6. Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electronic Physician (2016) 8(1):1832. doi: 10.19082/1832
7. Shokrpour M, Asemi Z. The effects of magnesium and vitamin e Co-supplementation on hormonal status and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. Biol Trace Elem Res (2019) 191(1):54–60. doi: 10.1007/s12011-018-1602-9
8. Kalmarzi RN, Naleini SN, Ashtary-Larky D, Peluso I, Jouybari L, Rafi A, et al. Anti-inflammatory and immunomodulatory effects of barberry (Berberis vulgaris) and its main compounds. Oxid Med Cell Longev (2019) 2019:6183965. doi: 10.1155/2019/6183965
9. Dehbalaei MG, Ashtary-Larky D, Mesrkanlou HA, Talebi S, Asbaghi O. The effects of magnesium and vitamin e co-supplementation on some cardiovascular risk factors: A meta-analysis. Clin Nutr ESPEN (2021) 41:110–7. doi: 10.1016/j.clnesp.2020.10.021
10. Cengiz M, Uysal BB, Ikitimur H, Ozcan E, Islamoğlu MS, Aktepe E, et al. Effect of oral l-glutamine supplementation on covid-19 treatment. Clin Nutr experimental (2020) 33:24–31. doi: 10.1016/j.yclnex.2020.07.003
11. Colakoglu S, Colakoglu M, Taneli F, Cetinoz F, Turkmen M. Cumulative effects of conjugated linoleic acid and exercise on endurance development. J sports Med Phys fitness. (2006) 46:4.
12. MacRedmond R, Singhera G, Attridge S, Bahzad M, Fava C, Lai Y, et al. Conjugated linoleic acid improves airway hyper-reactivity in overweight mild asthmatics. Clin Exp Allergy (2010) 40(7):1071–8. doi: 10.1111/j.1365-2222.2010.03531.x
13. Wang T, Lee HG. Advances in research on cis-9, trans-11 conjugated linoleic acid: A major functional conjugated linoleic acid isomer. Crit Rev Food Sci Nutr (2015) 55(5):720–31. doi: 10.1080/10408398.2012.674071
14. Wallace RJ, McKain N, Shingfield KJ, Devillard E. Isomers of conjugated linoleic acids are synthesized via different mechanisms in ruminal digesta and bacteria. J Lipid Res (2007) 48(10):2247–54. doi: 10.1194/jlr.M700271-JLR200
15. Yamasaki M, Nagatomo T, Matsuyama T, Ikeho Y, Kato E, Nishiyama K, et al. Conjugated linoleic acids inhibit hypoxia inducible factor-1α stabilization under hypoxic condition in human hepatocellular carcinoma cells. J Oleo Science. (2012) 61(9):491–6. doi: 10.5650/jos.61.491
16. López-Plaza B, Bermejo LM, Weber TK, Parra P, Serra F, Hernández M, et al. Effects of milk supplementation with conjugated linoleic acid on weight control and body composition in healthy overweight people. Nutricion Hospitalaria. (2013) 28(6):2090–8. doi: 10.3305/nh.2013.28.6.7013
17. Ebrahimi-Mameghani M, Jamali H, Mahdavi R, Kakaei F, Abedi R, Kabir-Mamdooh B. Conjugated linoleic acid improves glycemic response, lipid profile, and oxidative stress in obese patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Croatian Med J (2016) 57(4):331–42. doi: 10.3325/cmj.2016.57.331
18. Aslani MR, Matin S, Nemati A, Mesgari-Abbasi M, Ghorbani S, Ghobadi H. Effects of conjugated linoleic acid supplementation on serum levels of interleukin-6 and sirtuin 1 in COPD patients. Avicenna J Phytomedicine (2020) 10(3):305.
19. Aryaeian N, Djalali M, Shahram F, Djazayery A, Eshragian MR, et al. Effect of conjugated linoleic acid Effect of conjugated linoleic Acid, vitamin E, alone or combined on immunity and inflammatory parameters in adults with active rheumatoid arthritis: A randomized controlled trial Alone or combined on immunity and inflammatory parameters in adults with active rheumatoid arthritis: A randomized controlled trial. Int J Prev Med (2014) 5(12):1567.
20. Villacorta L, Minarrieta L, Salvatore SR, Khoo NK, Rom O, Gao Z, et al. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol (2018) 15:522–31. doi: 10.1016/j.redox.2018.01.005
21. Mohammadi-Sartang M, Sohrabi Z, Esmaeilinezhad Z, Jalilpiran Y. Effect of conjugated linoleic acid on leptin level: A systematic review and meta-analysis of randomized controlled trials. Hormone Metab Res (2018) 50(02):106–16. doi: 10.1055/s-0044-100041
22. Syvertsen C, Halse J, Høivik HO, Gaullier J, Nurminiemi M, Kristiansen K, et al. The effect of 6 months supplementation with conjugated linoleic acid on insulin resistance in overweight and obese. Int J Obes (2007) 31(7):1148–54. doi: 10.1038/sj.ijo.0803482
23. Shahmirzadi FE, Ghavamzadeh S, Zamani T. The effect of conjugated linoleic acid supplementation on body composition, serum insulin and leptin in obese adults. Arch Iranian Med (2019) 22(5):255.
24. Ghobadi H, Matin S, Nemati A, Javadi H, Alipanah-Moghadam R, Saeidi-Nir M. The effect of conjugated linoleic acid supplementation on the serum leptin level, pulmonary function and quality of life in COPD patients. J Ardabil Univ Med Sci (2019) 19(1):53–60. doi: 10.29252/jarums.19.1.53
25. Faramarzi E, Mohammadzadeh M, Sanaie S, Andersen V, Mahdavi R. Effects of conjugated linoleic acid supplementation on serum leptin levels, oxidative stress factors and tumor marker in rectal cancer patients undergoing preoperative chemoradiotherapy. Mediterr J Nutr Metab (2021) 14(3):245–53. doi: 10.3233/MNM-200507
26. von Frankenberg AD, Silva FM, de Almeida JC, Piccoli V, do Nascimento FV, Sost MM, et al. Effect of dietary lipids on circulating adiponectin: A systematic review with meta-analysis of randomised controlled trials. Br J Nutr (2014) 112(8):1235–50. doi: 10.1017/S0007114514002013
27. Gaullier J-M, Halse J, Høivik HO, Høye K, Syvertsen C, Nurminiemi M, et al. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr (2007) 97(3):550–60. doi: 10.1017/S0007114507381324
28. Sneddon AA, Tsofliou F, Fyfe CL, Matheson I, Jackson DM, Horgan G, et al. Effect of a conjugated linoleic acid and ω-3 fatty acid mixture on body composition and adiponectin. Obesity (2008) 16(5):1019–24. doi: 10.1038/oby.2008.41
29. Haghighatdoost F, Gh NM, Fatemeh B. Effect of conjugated linoleic acid on blood inflammatory markers: A systematic review and meta-analysis on randomized controlled trials. Eur J Clin Nutr (2018) 72(8):1071–82. doi: 10.1038/s41430-017-0048-z
30. Viladomiu M, Hontecillas R, Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur J Pharmacol (2016) 785:87–95. doi: 10.1016/j.ejphar.2015.03.095
31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol (2009) 62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
32. Nang C, Piano B, Lewis A, Lycett K, Woodhouse M. Using the PICOS model to design and conduct a systematic search: A speech pathology case study. (2015).
33. Higgins JGS. Cochrane handbook for systematic reviews of interventions version 5.1. 0 Vol. 2011. The Cochrane Collaboration (2011).
34. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials. (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
35. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. (UK: John Wiley & Sons) (2021).
36. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res methodology. (2005) 5(1):1–10. doi: 10.1186/1471-2288-5-13
37. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
38. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
39. Duval S. The trim and fill method. publication bias in meta-analysis: Prevention, assessment and adjustments. (London: Elsevier Ltd) (2005) 127–44. doi: 10.1002/0470870168.ch8
40. Xu C, Doi SA. The robust error meta-regression method for dose–response meta-analysis. JBI Evidence Implementation (2018) 16(3):138–44. doi: 10.1097/XEB.0000000000000132
41. Xie Y, Gou L, Peng M, Zheng J, Chen L. Effects of soluble fiber supplementation on glycemic control in adults with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr (2021) 40(4):1800–10. doi: 10.1016/j.clnu.2020.10.032
42. Medina EA, Horn WF, Keim NL, Havel PJ, Benito P, Kelley DS, et al. Conjugated linoleic acid supplementation in humans: effects on circulating leptin concentrations and appetite. Lipids. (2000) 35(7):783–8. doi: 10.1007/s11745-000-0586-y
43. Riserus U, Berglund L, Vessby B. Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in obese middle-aged men with signs of the metabolic syndrome: A randomised controlled trial. Int J Obes (2001) 25(8):1129–35. doi: 10.1038/sj.ijo.0801659
44. Albers R, van der Wielen R, Brink E, Hendriks H, Dorovska-Taran V, Mohede I. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers on immune function in healthy men. Eur J Clin Nutr (2003) 57(4):595–603. doi: 10.1038/sj.ejcn.1601585
45. Risérus U, Vessby B, Ärnlöv J, Basu S. Effects of cis-9, trans-11 conjugated linoleic acid supplementation on insulin sensitivity, lipid peroxidation, and proinflammatory markers in obese men. Am J Clin Nutr (2004) 80(2):279–83. doi: 10.1093/ajcn/80.2.279
46. Desroches S, Chouinard PY, Galibois I, Corneau L, Delisle J, Lamarche BT, et al. Lack of effect of dietary conjugated linoleic acids naturally incorporated into butter on the lipid profile and body composition of overweight and obese men–. Am J Clin Nutr (2005) 82(2):309–19. doi: 10.1093/ajcn/82.2.309
47. Gaullier J-M, Halse J, Høye K, Kristiansen K, Fagertun H, Vik H, et al. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr (2005) 135(4):778–84. doi: 10.1093/jn/135.4.778
48. Song H, Grant I, Rotondo D, Mohede I, Sattar N, Heys S, et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr (2005) 59(4):508–17. doi: 10.1038/sj.ejcn.1602102
49. Smedman A, Basu S, Jovinge S, Fredrikson GN, Vessby B. Conjugated linoleic acid increased c-reactive protein in human subjects. Br J Nutr (2005) 94(5):791–5. doi: 10.1079/BJN20041419
50. Ramakers JD, Plat J, Sébédio J-L, Mensink RP. Effects of the individual isomers cis-9, trans-11 vs. trans-10, cis-12 of conjugated linoleic acid (CLA) on inflammation parameters in moderately overweight subjects with LDL-phenotype b. Lipids (2005) 40(9):909–18. doi: 10.1007/s11745-005-1451-8
51. Taylor JS, Williams SR, Rhys R, James P, Frenneaux MP. Conjugated linoleic acid impairs endothelial function. Arteriosclerosis thrombosis Vasc Biol (2006) 26(2):307–12. doi: 10.1161/01.ATV.0000199679.40501.ac
52. Naumann E, Carpentier YA, Saebo A, Lassel TS, Chardigny J-M, Sébédio J-L, et al. Cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) do not affect the plasma lipoprotein profile in moderately overweight subjects with LDL phenotype b. Atherosclerosis (2006) 188(1):167–74. doi: 10.1016/j.atherosclerosis.2005.10.019
53. Nazare J-A, de la Perriere AB, Bonnet F, Desage M, Peyrat J, Maitrepierre C, et al. Daily intake of conjugated linoleic acid-enriched yoghurts: effects on energy metabolism and adipose tissue gene expression in healthy subjects. Br J Nutr (2007) 97(2):273–80. doi: 10.1017/S0007114507191911
54. Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: A meta-analysis in humans. Am J Clin Nutr (2007) 85(5):1203–11. doi: 10.1093/ajcn/85.5.1203
55. Iwata T, Kamegai T, Yamauchi-Sato Y, Ogawa A, Kasai M, Aoyama T, et al. Safety of dietary conjugated linoleic acid (CLA) in a 12-weeks trial in healthy overweight Japanese male volunteers. J Oleo Science (2007) 56(10):517–25. doi: 10.5650/jos.56.517
56. Steck SE, Chalecki AM, Miller P, Conway J, Austin GL, Hardin JW, et al. Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J Nutr (2007) 137(5):1188–93. doi: 10.1093/jn/137.5.1188
57. Mullen A, Moloney F, Nugent AP, Doyle L, Cashman KD, Roche HM. Conjugated linoleic acid supplementation reduces peripheral blood mononuclear cell interleukin-2 production in healthy middle-aged males. J Nutr Biochem (2007) 18(10):658–66. doi: 10.1016/j.jnutbio.2006.12.008
58. Kim J-H, Kim O-H, Ha Y-L, Kim J-O. Supplementation of conjugated linoleic acid with γ-oryzanol for 12 weeks effectively reduces body fat in healthy overweight Korean women. Prev Nutr Food Science (2008) 13(3):146–56. doi: 10.3746/jfn.2008.13.3.146
59. Turpeinen AM, Ylönen N, von Willebrand E, Basu S, Aro A. Immunological and metabolic effects of cis-9, trans-11-conjugated linoleic acid in subjects with birch pollen allergy. Br J Nutr (2008) 100(1):112–9. doi: 10.1017/S0007114507886326
60. Aryaeian N, Shahram F, Djalali M, Eshragian MR, Djazayeri A, Sarrafnejad A, et al. Effect of conjugated linoleic acid, vitamin e and their combination on lipid profiles and blood pressure of Iranian adults with active rheumatoid arthritis. Vasc Health Risk Management (2008) 4(6):1423. doi: 10.2147/VHRM.S3822
61. Raff M, Tholstrup T, Basu S, Nonboe P, Sørensen MT, Straarup EM. A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. J Nutr (2008) 138(3):509–14. doi: 10.1093/jn/138.3.509
62. Zhao W-S, Zhai J-J, Wang Y-H, Xie P-S, Yin X-J, Li L-X, et al. Conjugated linoleic acid supplementation enhances antihypertensive effect of ramipril in Chinese patients with obesity-related hypertension. Am J Hypertension. (2009) 22(6):680–6. doi: 10.1038/ajh.2009.56
63. Norris LE, Collene AL, Asp ML, Hsu JC, Liu L-F, Richardson JR, et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am J Clin Nutr (2009) 90(3):468–76. doi: 10.3945/ajcn.2008.27371
64. Sofi F, Buccioni A, Cesari F, Gori AM, Minieri S, Mannini L, et al. Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: A dietary intervention study. Nutrition Metab Cardiovasc Diseases (2010) 20(2):117–24. doi: 10.1016/j.numecd.2009.03.004
65. Sluijs I, Plantinga Y, De Roos B, Mennen LI, Bots ML. Dietary supplementation with cis-9, trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr (2010) 91(1):175–83. doi: 10.3945/ajcn.2009.28192
66. Tavakkoli Darestani A, Hosseinpanah F, Tahbaz F, Amiri Z, Tavakkoli Darestani R, Hedayati M. Effects of conjugated linoleic acid supplementation on body composition and leptin concentration in post-menopausal women. Iranian J Endocrinol Metab (2010) 12(1):48–59.
67. Brown AW, Trenkle AH, Beitz DC. Diets high in conjugated linoleic acid from pasture-fed cattle did not alter markers of health in young women. Nutr Res (2011) 31(1):33–41. doi: 10.1016/j.nutres.2010.12.003
68. Joseph SV, Jacques H, Plourde M, Mitchell PL, McLeod RS, Jones PJ. Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J Nutr (2011) 141(7):1286–91. doi: 10.3945/jn.110.135087
69. Pfeuffer M, Fielitz K, Laue C, Winkler P, Rubin D, Helwig U, et al. CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J Am Coll Nutr (2011) 30(1):19–28. doi: 10.1080/07315724.2011.10719940
70. Rubin D, Herrmann J, Much D, Pfeuffer M, Laue C, Winkler P, et al. Influence of different CLA isomers on insulin resistance and adipocytokines in pre-diabetic, middle-aged men with PPARγ2 Pro12Ala polymorphism. Genes Nutr (2012) 7(4):499–509. doi: 10.1007/s12263-012-0289-3
71. Shadman Z, Taleban FA, Saadat N, Hedayati M. Effect of conjugated linoleic acid and vitamin e on glycemic control, body composition, and inflammatory markers in overweight type2 diabetics. J Diabetes Metab Disord (2013) 12(1):1–9. doi: 10.1186/2251-6581-12-42
72. Bulut S, Bodur E, Colak R, Turnagol H. Effects of conjugated linoleic acid supplementation and exercise on post-heparin lipoprotein lipase, butyrylcholinesterase, blood lipid profile and glucose metabolism in young men. Chemico-biological Interactions (2013) 203(1):323–9. doi: 10.1016/j.cbi.2012.09.022
73. Eftekhari MH, Aliasghari F, Babaei-Beigi MA, Hasanzadeh J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA atherosclerosis. (2013) 9(6):311.
74. Baghi AN, Mazani M, Nemati A, Amani M, Alamolhoda S, Mogadam RA. Anti-inflammatory effects of conjugated linoleic acid on young athletic males. J Pak Med Assoc (2016) 66(3):280–4.
75. Dus-Zuchowska M, Madry E, Krzyzanowska P, Bogdanski P, Walkowiak J. Twelve-week-conjugated linoleic acid supplementation has no effects on the selected markers of atherosclerosis in obese and overweight women. Food Nutr Res (2016) 60(1):32776. doi: 10.3402/fnr.v60.32776
76. Abedi R, Aref-Hosseini S-R, Khoshbaten M, Ebrahimi-Mameghani M, Laleh HJ, Jalalypour F, et al. The effect of conjugated linoleic acid (CLA) on inflammatory factors in non-alcoholic fatty liver disease (NAFLD): A randomized controlled clinical trial. Prog Nutr (2018) 20:173–81. doi: 10.23751/pn.v20i2-S.5593
77. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Bmj (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
78. Belda BJ, Lee Y, Heuvel JPV. Conjugated linoleic acids and inflammation: isomer-and tissue-specific responses. Clin Lipidology (2010) 5(5):699–717. doi: 10.2217/clp.10.54
79. Asp ML, Collene AL, Norris LE, Cole RM, Stout MB, Tang S-Y, et al. Time-dependent effects of safflower oil to improve glycemia, inflammation and blood lipids in obese, post-menopausal women with type 2 diabetes: A randomized, double-masked, crossover study. Clin Nutr (2011) 30(4):443–9. doi: 10.1016/j.clnu.2011.01.001
80. Escrich E, Moral R, Grau L, Costa I, Solanas M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol Nutr Food Res (2007) 51(10):1279–92. doi: 10.1002/mnfr.200700213
81. Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr gastroenterology Hepatol Nutr (2013) 16(3):143–52. doi: 10.5223/pghn.2013.16.3.143
82. Fritsche KL. The science of fatty acids and inflammation. Adv Nutr (2015) 6(3):293S–301S. doi: 10.3945/an.114.006940
83. Oteng A-B, Kersten S. Mechanisms of action of trans fatty acids. Adv Nutr (2020) 11(3):697–708. doi: 10.1093/advances/nmz125
84. Kasapis C, Thompson PD. The effects of physical activity on serum c-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol (2005) 45(10):1563–9. doi: 10.1016/j.jacc.2004.12.077
85. Cho SMJ, Lee H, Shim J-S, Jeon JY, Kim HC. Association between physical activity and inflammatory markers in community-dwelling, middle-aged adults. Appl Physiology Nutrition Metab (2021) 46(7):828–36. doi: 10.1139/apnm-2020-1069
86. Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep (2009) 32(2):200–4. doi: 10.1093/sleep/32.2.200
87. Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol (2011) 21(11):799–806. doi: 10.1016/j.annepidem.2011.07.004
88. Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behavior Immunity (2005) 19(2):165–72. doi: 10.1016/j.bbi.2004.07.008
89. Derakhshandeh-Rishehri S-M, Rahbar AR, Ostovar A. Effects of conjugated linoleic acid intake in the form of dietary supplement or enriched food on c-reactive protein and lipoprotein (a) levels in humans: A literature review and meta-analysis. Iranian J Med Sci (2019) 44(5):359.
90. Mazidi M, Karimi E, Rezaie P, Ferns GA. Effects of conjugated linoleic acid supplementation on serum c-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Cardiovasc Ther (2017) 35(6):e12275. doi: 10.1111/1755-5922.12275
91. Barone R, Macaluso F, Catanese P, Marino Gammazza A, Rizzuto L, Marozzi P, et al. Endurance exercise and conjugated linoleic acid (CLA) supplementation up-regulate CYP17A1 and stimulate testosterone biosynthesis. PLoS One (2013) 8(11):e79686. doi: 10.1371/journal.pone.0079686
92. Bellido T, Jilka R, Boyce B, Girasole G, Broxmeyer H, Dalrymple S, et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest (1995) 95(6):2886–95. doi: 10.1172/JCI117995
93. Abdelmagid SA, Clarke SE, Wong J, Roke K, Nielsen D, Badawi A, et al. Plasma concentration of cis 9trans 11 CLA in males and females is influenced by SCD1 genetic variations and hormonal contraceptives: A cross-sectional study. Nutr Metab (2013) 10(1):50. doi: 10.1186/1743-7075-10-50
94. Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10, cis-12–conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes (2006) 55(6):1634–41. doi: 10.2337/db06-0036
Keywords: inflammation, cytokines, adipokines, conjugated linoleic acid, meta-analysis
Citation: Rastgoo S, Shimi G, Shiraseb F, Karbasi A, Ashtary-Larky D, Yousefi M, Golalipour E, Asbaghi O and Zamani M (2023) The effects of conjugated linoleic acid supplementation on inflammatory cytokines and adipokines in adults: A GRADE-assessed systematic review and dose–response meta-analysis. Front. Immunol. 14:1092077. doi: 10.3389/fimmu.2023.1092077
Received: 07 November 2022; Accepted: 02 February 2023;
Published: 22 February 2023.
Edited by:
Reinaldo B. Oria, Federal University of Ceara, BrazilCopyright © 2023 Rastgoo, Shimi, Shiraseb, Karbasi, Ashtary-Larky, Yousefi, Golalipour, Asbaghi and Zamani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omid Asbaghi, b21pZC5hc2JhZ2hpQGdtYWlsLmNvbQ==; Mohammad Zamani, bWRfemFtYW55QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.