- 1Ecology and Allergology Lab, Department of Zoology, The University of Burdwan, Burdwan, India
- 2Department of Physiology, Medical College and Hospital, Kolkata, India
- 3Fishery and Eco-toxicology Research Lab, Department of Zoology, The University of Burdwan, Burdwan, India
- 4Post Graduate Department of Zoology, Krishnagar Government College, Krishnagar, India
- 5Apollo Multispecialty Hospitals, Kolkata, India
Introduction: Prevalence of asthma is increasing steadily among general population in developing countries over past two decades. One of the causative agents of broncho-constriction in asthma is thromboxane A2 receptor (TBXA2R). However few studies of TBXA2R polymorphism were performed so far. The present study aimed to assess potential association of TBXA2R rs34377097 polymorphism causing missense substitution of Arginine to Leucine (R60L) among 482 patients diagnosed with pollen-induced asthma and 122 control participants from West Bengal, India. Also we performed in-silico analysis of mutated TBXA2R protein (R60L) using homology modeling.
Methods: Clinical parameters like Forced expiratory volume in 1 second (FEV1), FEV1/Forced vital capacity (FVC) and Peak expiratory flow rate (PEFR) were assessed using spirometry. Patients’ sensitivity was measured by skin prick test (SPT) against 16 pollen allergens. Polymerase chain reaction-based Restriction fragment length polymorphism was done for genotyping. Structural model of wild type and homology model of polymorphic TBXA2R was generated using AlphaFold2 and MODELLER respectively. Electrostatic surface potential was calculated using APBS plugin in PyMol.
Results: Genotype frequencies differed significantly between the study groups (P=0.03). There was no significant deviation from Hardy-Weinberg equilibrium in control population (χ2=1.56). Asthmatic patients have significantly higher frequency of rs34377097TT genotype than control subjects (P=0.03). SPT of patients showed maximum sensitivity in A. indica (87.68%) followed by C. nusifera (83.29%) and C. pulcherima (74.94%). Significant difference existed for pollen sensitivity in adolescent and young adult (P=0.01) and between young and old adult (P=0.0003). Significant negative correlation was found between FEV1/FVC ratio and intensity of SPT reactions (P<0.0001). Significant association of FEV1, FEV1/FVC and PEFR was observed with pollen-induced asthma. Furthermore, risk allele T was found to be clinically correlated with lower FEV1/FVC ratio (P=0.015) in patients. Our data showed R60L polymorphism, which was conserved across mammals, significantly reduced positive electrostatic charge of polymorphic protein in cytoplasmic domain thus altered downstream pathway and induced asthma response.
Discussion: The present in-silico study is the first one to report association of TBXA2R rs34377097 polymorphism in an Indian population. It may be used as prognostic marker of clinical response to asthma in West Bengal and possible target of therapeutics in future.
Introduction
Asthma is correctly termed the 21st century epidemic and modern age disease and represents a substantial public health burden in many countries including India (1–3). The prevalence of asthma has been dramatically increasing in both developed and under developed countries across the world. According to Zvezdin 2015 (4), about 300 million people of all ages suffered from asthma. The disease severity is unevenly distributed, being mild in most cases, while severe asthma forms are registered in the minority of the patients (around 15%). It is assessed that another 100 million people will be affected by the disease until 2025 (5, 6). This lower airway disorder is not a new discovery, as it had been recognized over two hundred years ago. It is therefore amazing that it took centuries to realize the importance of disease diagnosis and adequate treatment. Estimate suggests that India alone reported 6% of children and 2% of adults suffering from asthma (3, 7). According to Global Burden of Disease (GBD, 1990–2019) study, India has 34.3 million asthmatics which accounts for 13.09% of the global burden.Evidence suggests that in Indian population, asthma accounted for 27.9% of disability-adjusted life years (DALYs) (8). One of the most significant causes of asthma was found to be natural exposure to pollens (9) that play a major role in its pathogenesis (10). An estimate suggested that thepollen induced asthma prevalence in India ranges between 10-40% of the total asthmatic population (11–14). Early detection of genetically predisposed individuals to asthma is important for better management of the disease. Bronchial asthma is characterized by several inflammatory mediators including histamine, bradykinin and arachidonic acid metabolites such as prostaglandins, thromboxane A2 (TBXA2), andcysteinyl leukotrienes (LTC4, LTD4, LTE4); responsible for the clinical and pathological events (15, 16). Over the last few decades, there has been a steady growth in interest in exploring the important roles of TBXA2 in the pathogenesis of asthma. TBXA2 binds to its receptor TBXA2R which is primarily expressed in tissues targeted by TBXA2, including bronchial and vascular smoothmuscle and platelets (17). TBXA2R stimulate broncho-constriction inhuman small airways (18) in response to pollen-induced mast cell activation in atopic asthma patients (19). It is a G protein-coupled receptor (GPCR) encoded by the TBXA2R gene located in Chromosome 19 at position p13.3 (20). Very few studies were recorded regarding the genetic association of TBXA2R with bronchial asthma so far.
So, in the present study, attempts have been made to investigate the association of TBXA2R rs34377097 polymorphism with pollen induced bronchial asthma among population of West Bengal, India. rs34377097 is the only non-synonymous polymorphism within TBXA2R gene reported till date (21). In this study, computational algorithms were performed on TBXA2R missense polymorphism (R60L) to examine the changes in protein structure and function. The change of amino acid from Arginine (R) to Leucine (L) may affect the protein conformation, leading to change in downstream signaling pathway. Therefore, the present study performed in-silico analyses of the mutated TBXA2R protein using homology modeling to explore and confirm the above outcome. Our study also attempted to determine the signaling molecules of TBXA2R pathway which are responsible for elevated asthmatic response in individuals carrying the risk allele.
Materials and methods
Ethics approval and consent to participate
The Clinical Research Ethics Committee of Allergy and Asthma Research Center, West Bengal, India approved the present study protocols vide CREC-AARC Ref: 62/21. Written informed consent was taken from each patient and control subjects of this study and preserved for future reference.
Participants
A total of 632 individuals (both male and female) were selected for this case-control study during March to December, 2021. Pregnant and lactating women (n=5), patients suffering from illness other than asthma (n=11) and who did not provide the written informed consent (n=12) were excluded from this study. Finally, 482 patients and 122 control subjects were included in this present study. Details regarding epidemiology viz. age, gender, habitat, age of onset of symptoms, life-style, family history (Paternal or maternal), clinical features, aggravating factors and non-specific stimuli such as cold, exercise and other irritant factors, etc. were recorded in a self-prepared questionnaire. 482 participants diagnosed of having aero-allergen induced bronchial asthma and reported to be suffering from different asthmatic manifestations like airway hyper responsiveness, recurring episodes of airway obstruction, wheezing, dyspnoea and cough either alone or in different combination were considered as cases. Both patients and controls were classified according to their age like children (age 5–12 years), adolescents (age>12–19 years), young adults (age >19–40 years) and old adults (>40-70 years). Peripheral blood samples were collected from all the participants and centrifuged for separation of serum and kept in −20°C refrigerator for further analysis.
Spirometry
The recruited subjects were screened for the presence of asthma by spirometry test following Global Initiative for Asthma (GINA) guidelines 2017 (22). The following parameters were measured: Forced expiratory volume at time interval of 1.0 second (FEV1), Forced vital capacity (FVC), FEV1/FVC ratio and Peak expiratory flow rate (PEFR). 20% or more reduction of FEV1 and PEFR value than predicted is considered as asthmatic (23). FEV1/FVC ratio less than 0.75-0.80 is considered as asthmatic (22).
Skin prick test
Asthmatic patients those were positive in Spirometry test were undergone skin prick test (SPT) using 16 common aero allergen extracts (Credisol, Mumbai, India) viz. Azadirachta indica, Cocos nucifera, Caesalpinia pulcherrima, Brassica nigra, Pinus sylvestris, Carica papaya, Zea mays, Triticum aestivum, Lantana camara, Poa pratensis, Chenopodium album, Peltophorum pterocarpum, Areca catechu, Parthenium hysterophorus, Lolium perenne and Plantago lanceolata. Histamine phosphate and normal saline were used as positive and negative controls respectively. Wheel diameter ≥3mm was considered as positive response to that particular allergen.
DNA extraction and genotyping
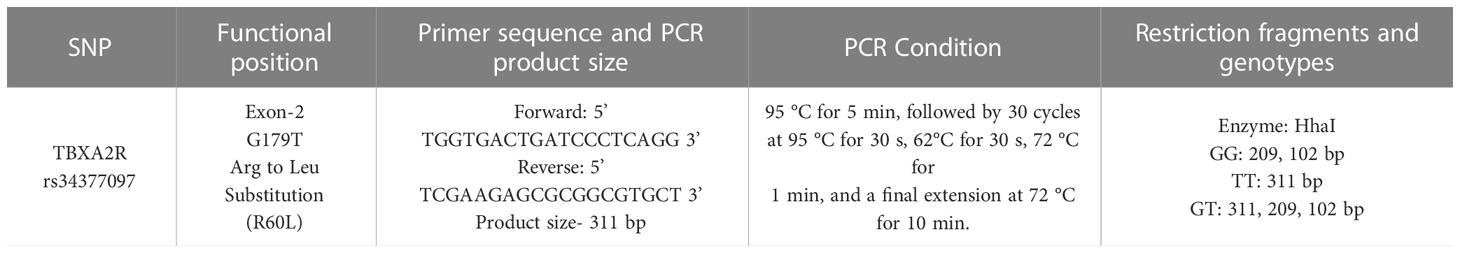
Extraction of genomic DNA was performed from blood samples of 482 patients and 122 control subjects using QIAamp DNA blood mini kit (Qiagen, Hilden, Germany). TBXA2R rs34377097 polymorphism was identified using polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) technique (Figure 1). The details of both the primers, PCR conditions, PCR product, RFLP fragments are given in Table 1. Each reaction mixture (20µl) contains 50ng of genomic DNA, 200nM of each primer (Sigma), 200mM of each deoxyribonucleotide triphosphate (dNTP) (ThermoFisher Scientific, USA) and 2.5 units of Taq polymerase (Applied Biological Materials, Canada).

Figure 1 TBXA2R rs34377097 polymorphism. (A) PCR Product (311 bp). The last lane shows the 100 bp ladder. (B) RFLP fragments. GG genotype in Lane 4 and 7; GT genotype in Lane 1, 2, 3 and 6; TT genotype in Lane 5 (C) Representative chromatograms of GG, GT and TT genotype. The position of the genotypes is shown with an arrow.
DNA sequencing
Representative PCR products for each of the three polymorphic genotypes of the selected polymorphism were undergone Sanger sequencing to validate the RFLP results (Figure 1).
In silico analysis
Structural model of wild type TBXA2R was generated using AlphaFold2. Homology model of TBXA2R R60L was generated using MODELLER (24, 25). Figures were designed using PyMol (26). The electrostatic surface potential was evaluated using APBS plugin in PyMol.T-Coffee server was used for multiple sequence alignment and data were prepared using ESPript (27).
Statistical analysis
Continuous data are described by mean ± standard error (SE) and categorical data by number with percentages. Patients and controls were assessed by independent t test for continuous variables or Pearson chi-square test for categorical variables. Genotypic and allele distributionin patient and control group was evaluated using contingency chi-square or Fisher’s exact test, where applicable. Risk analysis was performed by odds ratio (OR) under additive, recessive and dominant models. Hardy-Weinberg equilibrium (HWE) was checked for the genotypes by goodness-of-fit chi-square test with one degree of freedom (df). SPT sensitivity for pollens among different age groups was analyzed using One way ANOVA followed by Tukey’s Multiple Comparison test. The association between FEV1/FVC ratio and atopy was analyzed using linear regression. FEV1/FVC ratio of both patient and control individuals residing in different habitats was analyzed using one way ANOVA test. The association of the TBXA2R polymorphic allele with FEV1/FVC ratio was analyzed using Independent t test. The association of the TBXA2R polymorphism with atopy was analyzed using one way ANOVA test. All statistical calculations were performed using GraphPad Prism ver. 7 (San Diego, CA). Significance level was taken as P<0.05.
Results
Characteristics of the subjects
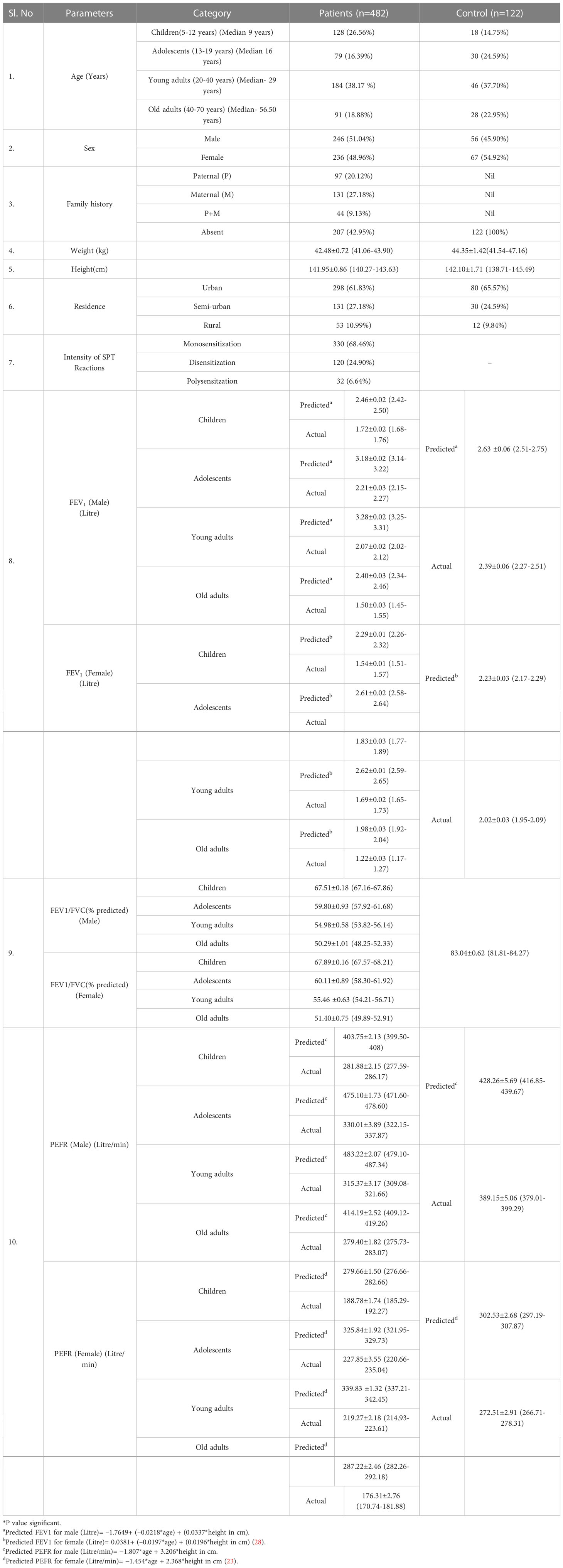
The present study comprises of 482 asthmatic patients and 122 control individuals. Demographic and clinical characteristics are described in Table 2. Majority of the study population including patients and controls reside in urban area compared to sub-urban and rural ones. In case of FEV1, FEV1/FVC ratio and PEFR, a significant deviation was observed from the predicted value both in male and female patients in all age groups as compared to control (Table 2).
Sensitization of patients against pollens by SPT
The highest prevalence of sensitization was obtained with Azadirachta indica (87.68%), followed by Cocos nucifera (83.30%), Caesalpinia pulcherrima (74.95%), Brassica nigra (72.23%), etc. Sensitivity of patients to different pollen allergens categorized according to age is depicted in Figure 2. One-way ANOVA test showed significant difference in sensitivity between the different age groups (F=7.156 and P=0.0003). A pairwise Tukey's Multiple Comparison Test also showed significant difference in number of sensitive patients between adolescent and young adult (P= 0.01) and between young and old adult (P= 0.0003).
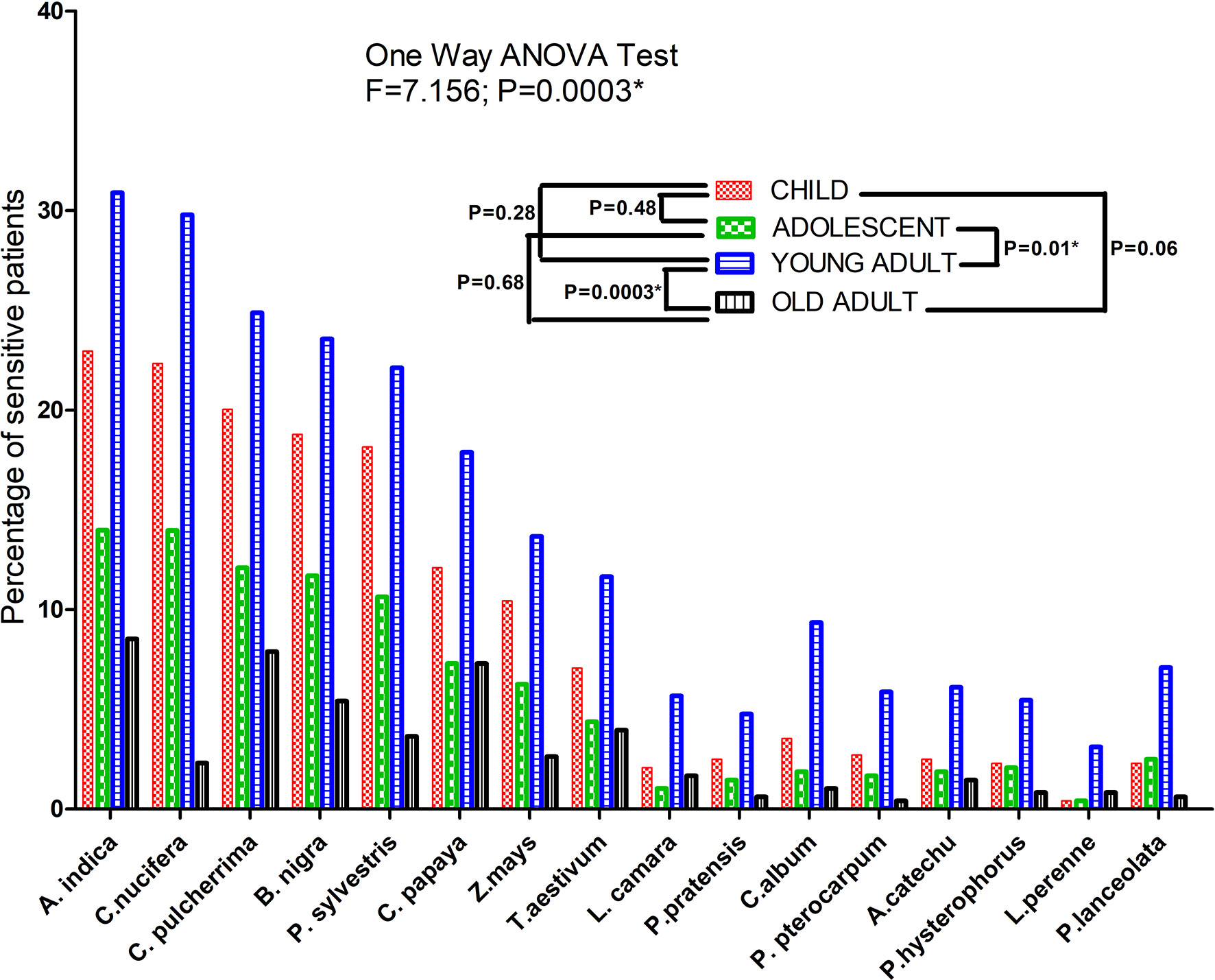
Figure 2 SPT sensitization profile of bronchial asthma patients against different pollen allergen sources. Each bar represents percentage of sensitive patients which is divided according to three age groups. One Way ANOVA revealed a significant difference of SPT sensitivity among different age groups. For pairwise comparison, Post hoc Tukey’s Multiple Comparison test applied which showed significant difference of sensitivity between adolescent and adult. (*P value significant).
FEV1/FVC association with atopy and habitat
The association of FEV1/FVC ratio with atopy is depicted in Figure 3. Significant negative correlation was found between FEV1/FVC ratio and intensity of SPT reactions which is denoted by wheel diameter in all age groups and sexes (P<0.0001).The association of FEV1/FVC ratio with habitat of both patients and controls is depicted in Figure 4. One-way ANOVA test revealed that significant difference existed in FEV1/FVC ratio of both male and female patients residing in different habitats (Urban, semi-urban and rural) (F=40.63 and P<0.0001 and F=24.28 and P<0.0001 respectively); while that in control males and females, it remained non-significant (F=0.21 and P=0.81 and F=0.31 and P=0.73 respectively). Urban patients showed the lowest FEV1/FVC value than others.
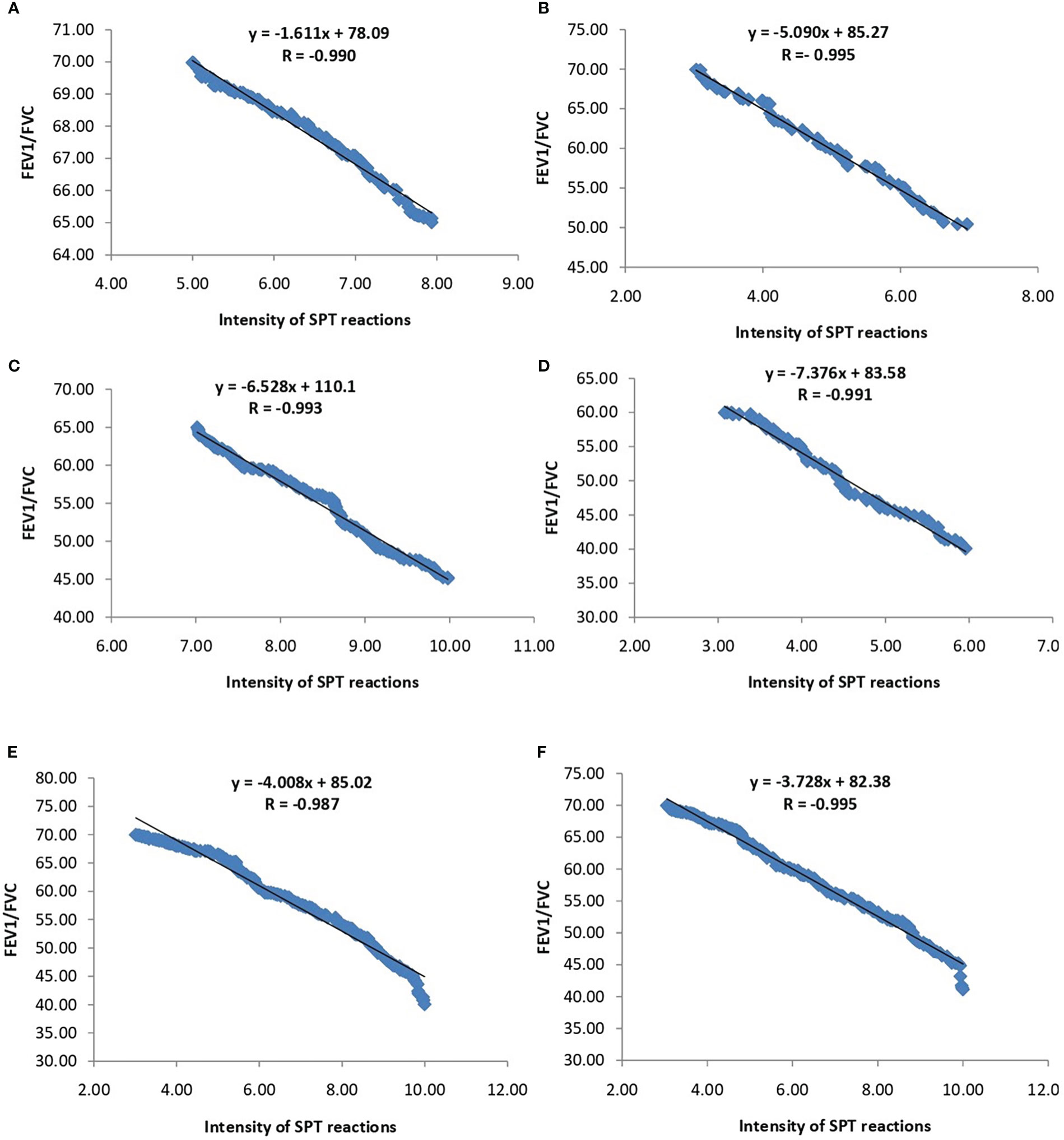
Figure 3 Regression analysis of FEV1/FVC ratio and intensity of SPT reactions (in terms of wheel diameter). (A) Children (B) Adolescents (C) Young adults (D) Old adults (E) Male (F) Female.
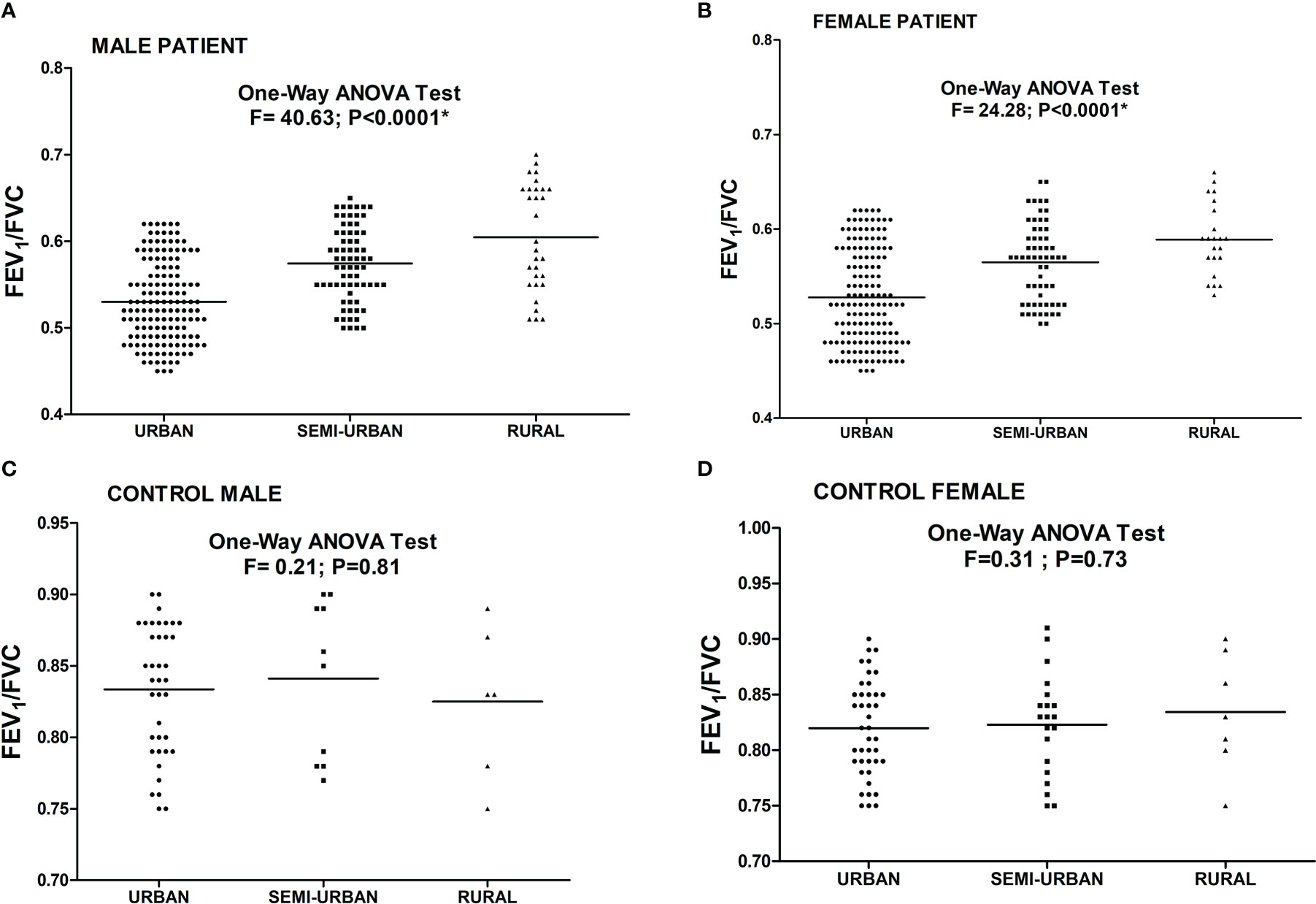
Figure 4 Level of FEV1/FVC ratio in asthmatic patients [(A) Male (B) Female] and controls [(C) Male (D) Female] residing in different habitats. One Way ANOVA revealed a significant difference in FEV1/FVC ratio in male and female patients residing in different habitats. (*P value significant).
Genotype distribution in patients and controls
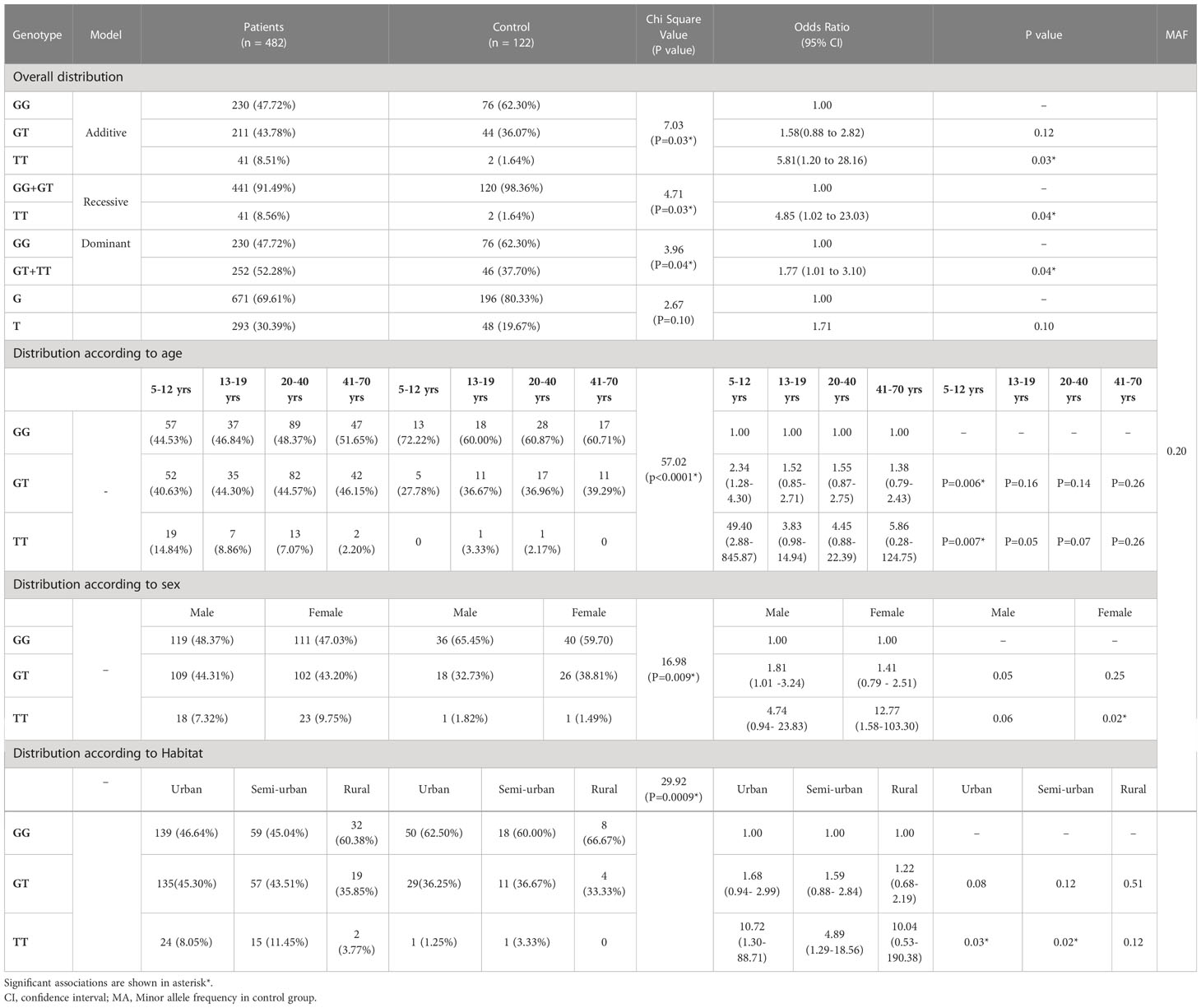
The genotype frequencies differ significantly between the study groups (χ2 =7.03; P=0.03) (Table 3A). The allele frequencies showed no significant deviation between case and control group (χ2 =2.67; P=0.10).There was no significant deviation from HWE in the control population (χ2 =1.56 at df=1). The frequency of the rs34377097 TT genotype was significantly higher in asthma patients than in controls (OR=5.81, P=0.03). Both recessive and dominant models showed significant difference of rs34377097 TT genotype frequency between the study groups (P=0.04). Table 3B shows genotypic frequency distribution of both patients and controls according to age groups (5-12 yrs, 13-19 yrs, 20-40 yrs and 41-70 yrs). The genotype frequencies differed significantly among all the age groups of patients and controls (χ2 =57.02; P<0.0001).The rs34377097 GT and TT genotype bears significant risk of asthma in children of age 5-12 years (OR=2.34, P=0.006 and OR=49.40, P=0.007 respectively). Table 3C depicts frequency distribution of both patients and controls according to sex. The genotype frequencies differed significantly between both sexes of patients and controls (χ2 =16.98; P=0.009). The rs34377097 TT genotype showed significant odds ratio in females (OR=12.77, P=0.02). Table 3D explains frequency distribution of both patients and controls residing in different habitats (Urban, Semi-urban and rural). The genotype frequencies differed significantly between patients and controls of all habitats (χ2 =29.92; P=0.0009). The rs34377097 TT genotype showed significant risk of asthma in urban and semi-urban dwelling subjects (OR=10.72, P=0.03 and OR=4.89, P=0.02 respectively).
Correlation of polymorphic genotypes with FEV1/FVC ratio and atopy
Asthma patients bearing rs34377097 T allele had significantly lower FEV1/FVC ratio (t=8.004, P=0.015) than T allele bearing controls; while that in G allele bearing patient and control individuals remained non-significant (t=2.425, P=0.136) (Figure 5). Furthermore, FEV1/FVC ratio differ significantly between G and T bearing asthma patients (P=0.04) while no significant difference is observed in controls (P=0.91) (Figure 5). One-way ANOVA test revealed that individuals bearing TT genotype showed highest intensity of SPT reactions in terms of wheel diameter than GG and GT bearing individuals (F=56.25 and P<0.0001) (Figure 6).
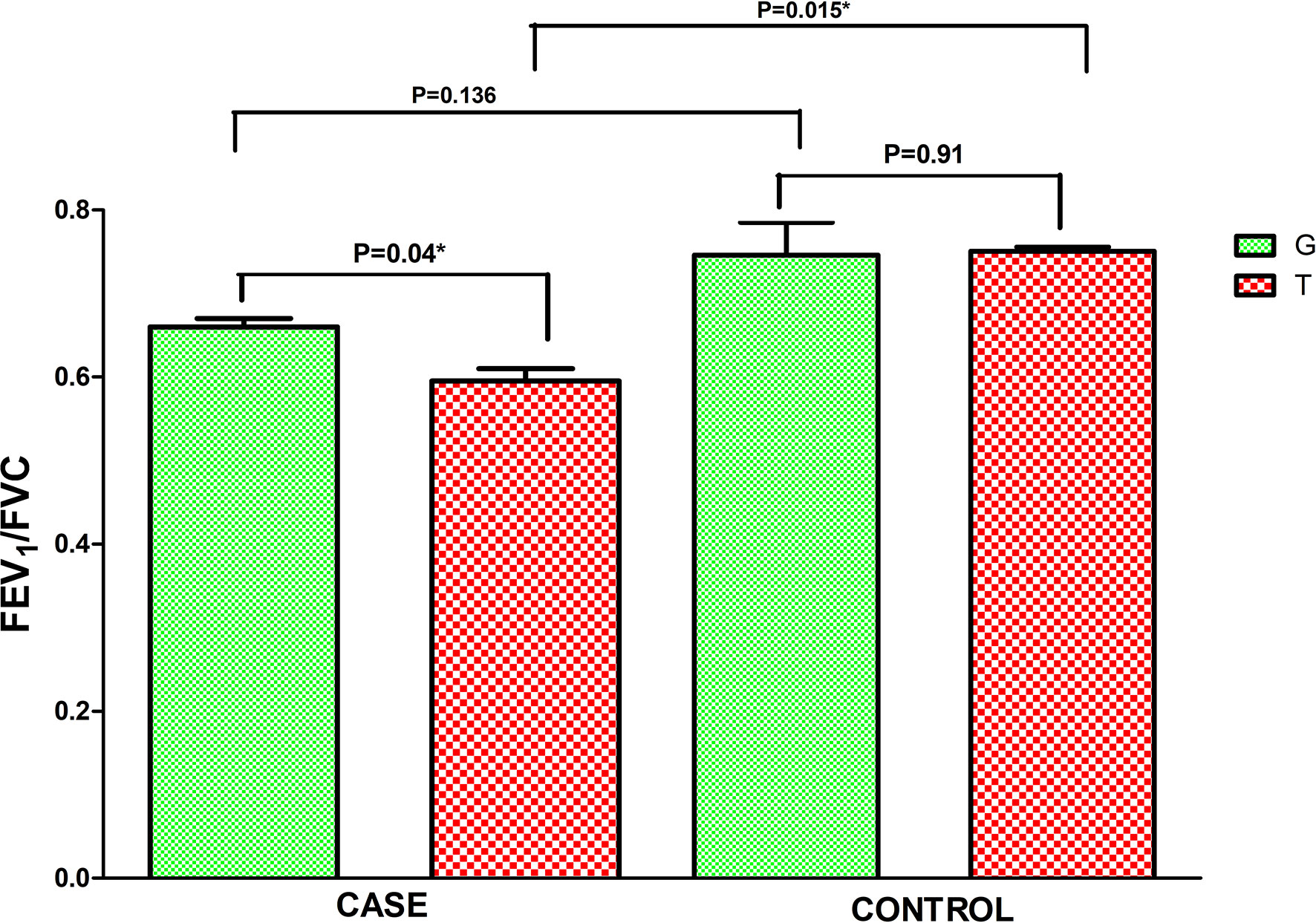
Figure 5 Level of FEV1/FVC ratio in asthmatic patients bearing different alleles of TBXA2R rs34377097 polymorphism. Comparison between cases and controls was performed by Independent t test. (*P value significant).
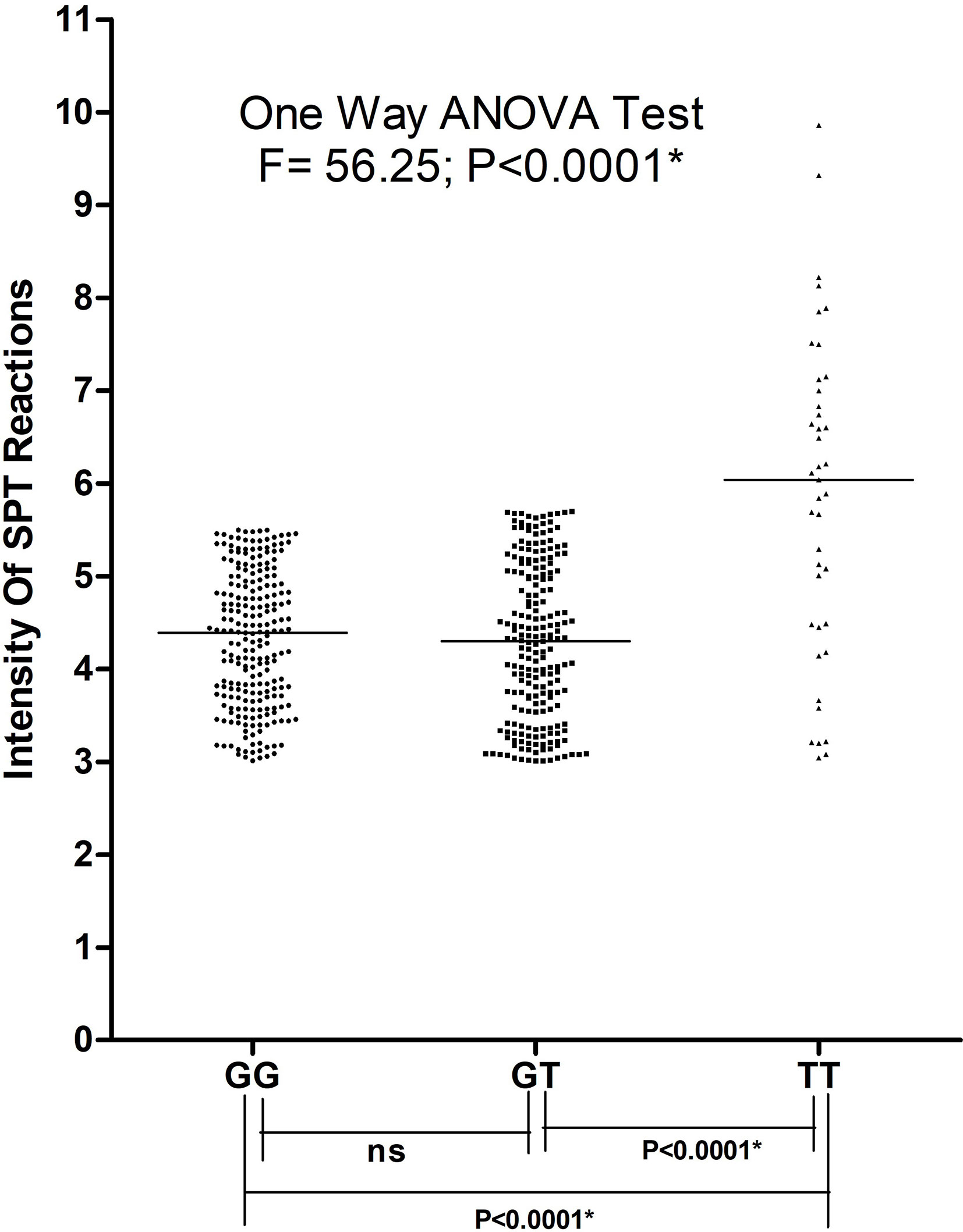
Figure 6 Intensity of SPT reactions in asthmatic patients bearing different genotypes of TBXA2R rs34377097 polymorphism. One-way ANOVA test revealed that individuals bearing TT genotype showed highest intensity of SPT reactions in terms of wheel diameter than GG and GTbearing individuals. (*P value significant).
Mechanistic and structural analysis of asthmatic response induced by rs34377097
Multiple sequence alignment of TBXA2R gene among the orthologs in mammals (Mus musculus, Rattus norvegicus, Bos taurus, Mesocricetus auratus, Macaca mulatta, Pan troglodytes, Felis catus, Chlorocebus aethiops) denoted that the R60 amino acid is conserved across the species (Figure 7A). The crystal structure of human TBXA2R (PDB: 6IIU) revealed that the structures share a canonical seven-transmembrane helical bundle similar to other known GPCR structures (Figure 7B) (29). The N-terminal extracellular domain functions as a receiver domain, whereas the C-terminal cytoplasmic domain functions as a signal transmitter domain. The Guanine nucleotide-binding Gq protein binds at the C-terminal cytoplasmic domain of TBXA2R and relays the signal. In the crystal structure of human TBXA2R, a number of important cytoplasmic loops, including the first cytoplasmic loop that contains the R60 residue, were lacking their electron densities (shown by dotted lines in Figure 7C). In order to model the cytoplasmic loops, we have prepared a structural model of TBXA2R using the artificial intelligence (AI) based tool AlphaFold2 (30). Superimposition of the model and the crystal structure (PDB: 6IIU) yielded root-mean-square deviations of 0.049Å. In order to understand the effect of R60L, we have prepared a homology model of the mutated TBXA2R protein. Figure 7D shows presence of L60 amino acid at the first cytoplasmic loop. We have generated the electrostatic potential surface of wild type TBXA2R (Figure 7E) and TBXA2R R60L protein (Figure 7F). It is interesting to note that the TBXA2R protein in its wild type exhibits strong positive charge potential (depicted in blue) on its cytoplasmic surface (Figure 7E). Hence, it can be assumed that proteins that interact with the cytoplasmic domain of TBXA2R must possess negative charge potentiality. Further, the cytoplasmic domain of TBXA2R R60L displays much less positive charge potentiality (location is marked with red arrow) as compared to the wild type protein (Figure 7F). This is because Arg is a positively charged amino acid whereas Leu is a hydrophobic amino acid. The interaction between TBXA2R and the Gq protein may be inhibited if the positive charge potential at the cytoplasmic domain of TBXA2R is reduced.
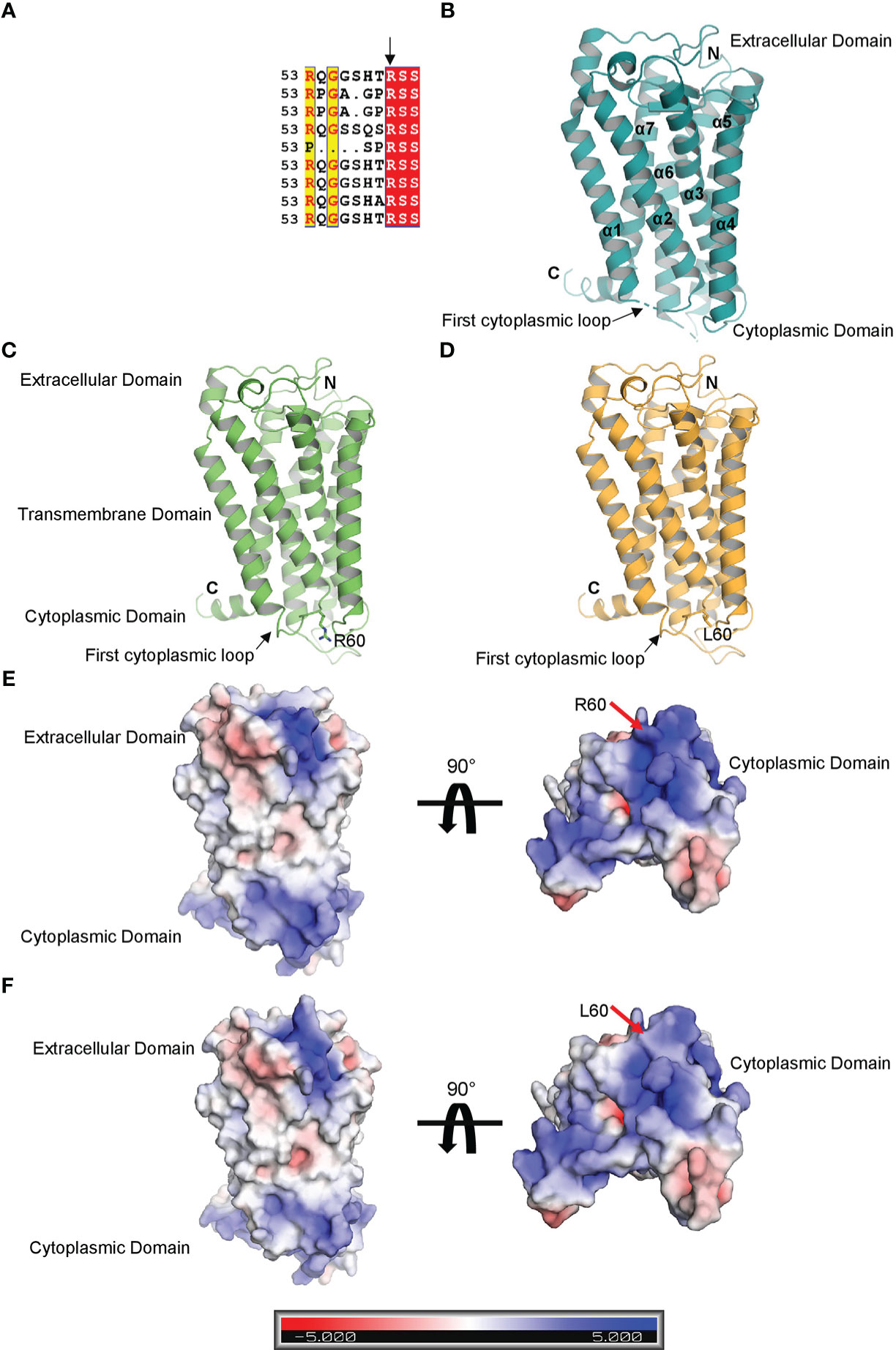
Figure 7 Structural analysis of TBXA2R and TBXA2R R60L. (A) Multiple sequence alignment of TBXA2R mammalian orthologs. (B) Cartoon representation of the crystal structure of human TBXA2R(PDB: 6IIU). Electron density deficient loops are shown with dotted lines. (C) Cartoon representation of 3-D Structural model of human TBXA2R depicted in smudge. R60 residue is depicted in stick. (D) Homology model of TBXA2R R60L is depicted in bright orange. L60 residue is shown in stick.Electrostatic surface potential of Wild type TBXA2R (E) and TBXA2R R60L (F) are coloured according to the bar underneath.The scale ranges from −5 kT/e (red) to +5 kT/e (blue).
Discussion
Asthma is a heterogeneous and chronic airways disease and estimated to affect an average of 4.5% individuals throughout the globe, with substantial variation in different countries (31). Bronchial asthma is influenced by both environmental and genetic components (32). There is a substantial curiosity in upgrading of our understanding regarding biological mechanism of bronchial asthma by expanding genomic approaches. Current investigation is based on the association of TBXA2R rs34377097 polymorphism in an Indian population.
Earlier reports indicated that TBXA2 was a negative regulator of LTC4 synthase, which produces leukotriene (33). Evidence suggested that in absence of TBXA2, LTC4 synthase activity is enhanced through its receptor (TBXA2R), resulting in increased leukotriene biosynthesis and asthma symptoms (34). These results clearly signify that TBXA2R polymorphism might play an important role in bronchial asthma pathogenesis. TBXA2 is synthesized from arachidonic acid by the enzyme, cyclooxygenase (COX) (35). It causes constriction of bronchial smooth muscles in asthma (36–38). Pollen grains are important aeroallergens therefore it is essential to havethe knowledge of locally prevalent pollen allergens for better diagnosis and therapy of pollen induced asthma (11, 39). Allergenic potentials of common pollens are investigated throughout India viz. Cynodon in northern region, Fusarium solani and Curvularia from southern region, Azaridacta in central region, Cocos and Phoenix from eastern region (40). In our study, more than 87% of patients were sensitive to Azadirachta indica, one of the major pollens found in West Bengal. This study also reported Cocos nucifera, Caesalpinia pulcherrima and Brassica nigraas top sensitizer as depicted in Figure 2 which is in accordance to reports from Dey et al. (2019) (14). Podder et al. 2009, 2010 (41, 42) reported that the occurrence of allergic symptoms to various inhalants is similar for both sexes. We also found significant difference in SPT sensitivity between adolescent & young adult and between young & old adult. Dey et al. (2019) (14) also reported similar observations among atopic population of West Bengal, India. This difference in sensitivity may be due to the increased exposure to various pollen allergens in the residential/occupational area encountered by young adults compared to adolescents and old adults. Figure 3 showed significant negative correlation of FEV1/FVC ratio and intensity of SPT reactions (in terms of wheel diameter) in all age groups and in both sexes which clearly indicated a role of atopy in lung function impairment. Various studies were conducted previously showing association of allergen sensitization and asthma severity in terms of spirometric parameters (43, 44). Jaen et al. 2002 (45) reported that asthmatics sensitized to various allergens including pollens have significantly lower FEV1/FVC ratio which is in accordance with our result. Allergens are thought to initiate acute episodes of asthma through inflammatory processes and bronchial hyper-responsiveness that might result into airway obstruction (46). Figure 4 depicted existence of significant difference in FEV1/FVC ratio of both male and female patients residing in different habitats (Urban, semi-urban and rural) while that in control subjects remained non-significant. Some previous studies reported that asthma patients residing in urban and semi urban habitat with high indoor and atmospheric pollution show reduced FEV1/FVC ratio than patients from rural counterparts (47, 48) which supported our observation.
Table 2 showed that 56.43% of our study population inherited asthma from their family (either maternal or paternal or both), which supports the role of genetic predisposition in asthma. Literature suggested that TBXA2R pathway play a significant role in asthma (21, 33). In our study, we found significant association of rs34377097 polymorphic TT genotype in the exon-2 region of the TBXA2R gene with elevated pollen induced asthma response such as acute broncho and tracheal constriction in West Bengal population, India. Table 3A clearly demonstrated that TBXA2R rs34377097 TT genotype was significantly associated with pollen induced asthma (OR=5.81, P=0.03). Table 3B depicted that children of age 5-12 years bearing GT and TT genotype have higher risk of asthma than adolescent, young adult and old adult. Prashanth et al. 2022 (49) reported mean urinary Leukotriene level in asthmatic children as 378.47 Pg/Mg and Green et al. 2004 (50) observed mean urinary Leukotriene level in asthmatic adult as 111.70 Pg/Mg which may be a probable cause of higher asthma risk in children. Besides this, Kim 2004 (51) reported that children spend more time outdoors than adults, mostly in summer during late afternoon. This long-term exposure to various outdoor air pollutants significantly contributes in developing asthma since their immune system and lungs are still immature. Table 3C showed that females bearing TT genotype have significant higher risk of asthma. Pignataro et al. 2017 (52) reported that females have higher risk of developing asthma than males which is in accordance to our result. This difference in asthma risk between female and male may be due to expression of female sex hormone receptors on mast cells (53). In Table 3D, we observed that urban and semi-urban dwelling patients bearing TT genotype have higher risk of asthma. Priftis et al. 2009 (54) reported that children living in urban areas have significantly higher risk of asthma than their rural counterparts which confirmed our result. This may be due to increased exposure to indoor and outdoor air pollutants in urban and semi-urban habitat. Emissions of harmful gases from motor traffic contribute significantly to the development of asthma in urban and semi-urban dwelling patients (55).
As per our knowledge is concerned, our study which is based on both structural and functional aspects, is the first one to report the association of TBXA2R rs34377097 polymorphism in an Indian population. It is also the first extensive in silico analysis that perform both polymorphism study and modeling approach to assess TBXA2R rs34377097 polymorphism clinically. Another study conducted in Japanese population reported lack of association of rs34377097 polymorphism with bronchial asthma (21). The present study also depicted significant associations of several clinical parameters like FEV1, FEV1/FVC ratio and PEFR with pollen induced asthma both in male and female patients in all age groups. Furthermore, in patients the risk allele T was found to be clinically correlated with FEV1/FVC ratio (P=0.015). Figure 6 demonstrated that TBXA2R rs34377097TT genotype bearing individuals showed highest intensity of SPT reactions in terms of wheel diameter than GG and GT bearing individuals which indicate association of atopic status with asthma risk.
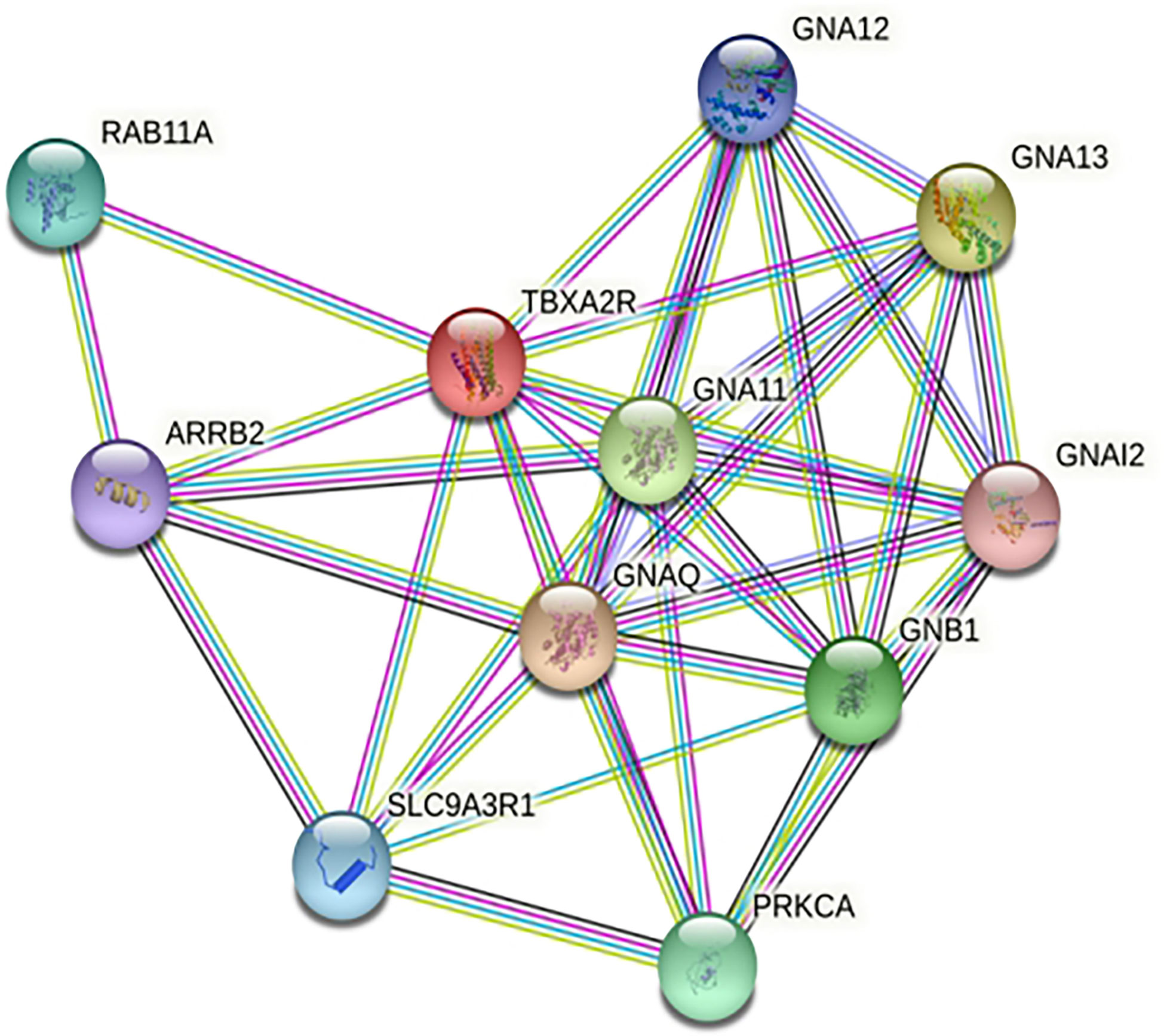
STRING analysis showed that TBXA2R are involved in interaction with different known and predicted Gq proteins (guanine nucleotide-binding G protein, subunits alpha, group q) (Figure 8). Out of all Gq proteins, GNA11 acts as an activator of phospholipase C (PLC). PLC hydrolyses phosphoinositides into the two stimulatory second messengers - inositol 1,4,5-triphosphate (IP3) and diacylglycerol (56). IP3enhances cytoplasmic free calcium level and diacylglycerol (DAG) activates protein kinase C (PKC). Activated protein kinase C either directly phosphorylates LTC4 synthase enzyme and inactivates it or this regulation may involve another regulatory protein which is yet to be discovered. Inactivation of LTC4 synthase leads to reduction of Leukotriene C4 (LTC4) biosynthesis in platelets. However, in the case of risk allele rs34377097 T-bearing individuals, the non-synonymous mutation (R60L) in TBXA2R protein inhibits the interaction between GNA11 and TBXA2R due to the change in the positive charge potentiality at the cytoplasmic domain. This is in line with Hirata et al. 1996 (57), who demonstrated that the R60L mutation significantly reduces PLC activity. That leads to simultaneous inactivation of PKC which ultimately results in LTC4 synthase enzyme activation. Activation of this enzyme leads to LTA4 to LTC4 conversion which results in acute asthmatic response including broncho-constriction, tracheolar constriction and increased mucus secretion as shown in Figure 9.
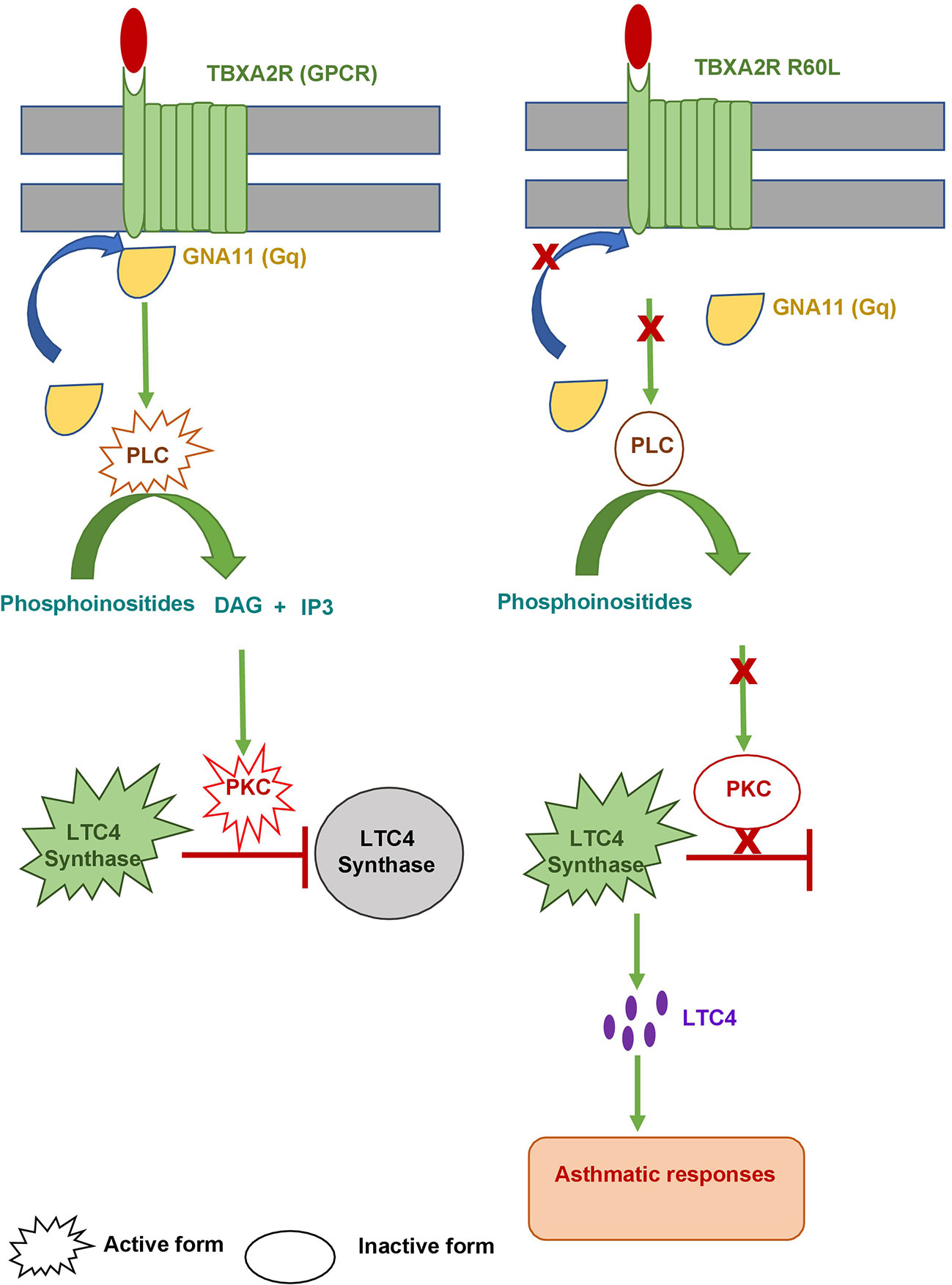
Figure 9 Model for asthmatic regulation via. TBXA2R. The left panel shows wild type TBXA2R prevents asthmatic responses by converting active LTC4 Synthase into an inactive one. The right panel shows how a mutation (R60L) in TBXA2R causes an asthmatic response.
Conclusion
The present study provided several lines of evidence to support that TBXA2R rs34377097 polymorphism act as a potent risk factor for asthma in West Bengal population, India. More studies are warranted in diverse ethnic groups to validate the above findings. The present in-silico study will be useful for the advancement of gene based therapy for better management and treatment of asthma in near future.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: SCV003761534 (ClinVar).
Ethics statement
The studies involving human participants were reviewed and approved by Clinical Research Ethics Committee, Allergy and Asthma Research Center, West Bengal, India (CREC-AARC Ref: 62/21). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
Collection of samples was done by IG, SS and NS. IG and AL contributed to data compilation and wrote first draft of the manuscript. PB did the structural modeling. SM diagnosed the disease and provided the samples. Statistical analysis was done by IS, HB and AL. SP conceived the study design, supervised the work, critically revised the manuscript and made the final draft. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by Science and Engineering Research Board, Govt. of India (Sanction no. EEQ/2021/000042 dated 24th February 2022).
Acknowledgments
The authors convey thanks to the patients who voluntarily participated in our study. We are also grateful to the lab professionals of the Allergy and Asthma Research Center, Kolkata, for their technical help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fahy JV. Type 2 inflammation in asthma-present in most, absent in many. Nat Rev Immunol (2015) 15(1):57–65. doi: 10.1038/nri3786
2. Elsaid A, Shoaib RMS, Badr SS, Wahba Y, Ayyad SN. Polymorphisms of interleukin 4 and interleukin 4 receptor genes and bronchial asthma risk among Egyptian children. Clin Biochem (2021) 93:66–72. doi: 10.1016/j.clinbiochem.2021.04.006
3. Ghosh A, Dutta S, Podder S, Mondal P, Laha A, Saha NC, et al. Sensitivity to house dustmites allergens with atopic asthma and its relationship with CD14 c (-159T) polymorphism in patients of West Bengal, India. J Med Entomol (2018) 55(1):14–9. doi: 10.1093/jme/tjx178
4. Zvezdin B, Hromis S, Kolarov V, Milutinov S, Zarić B, Jovancević L, et al. Allergic asthma and rhinitis comorbidity. Vojnosanit Pregl (2015) 72(11):1024–31. doi: 10.2298/VSP140605099Z
5. Masoli M, Fabian D, Holt S, Beasley R. Global initiative for asthma (GINA) program. the global burden of asthma: Executive summary of the GINA dissemination committee report. Allergy (2004) 59(5):469–78. doi: 10.1111/j.1398-9995.2004.00526.x
6. Braman SS. The global burden of asthma. Chest (2006) 130(1 Suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S
7. Rashmi R, Kumar P, Srivastava S, Muhammad T. Understanding socio-economic inequalities in the prevalence of asthma in India: An evidence from national sample survey 2017-18. BMC Pulm Med (2021) 21(1):372. doi: 10.1186/s12890-021-01742-w
8. Salvi S, Kumar GA, Dhaliwal RS, Paulson K, Agrawal A, Koul PA, et al. The burden of chronic respiratory diseases and their heterogeneity across the states of India: The global burden of disease study 1990–2016. Lancet Glob Heal (2018) 6(12):e1363–74. doi: 10.1016/S2214-109X(18)30409-1
9. Baxi SN, Phipatanakul W. The role of allergen exposure and avoidance in asthma. Adolesc Med State Art Rev (2010) 21(1):57–71.
10. Singh AB, Shahi S. Aeroallergens in clinical practice of allergy in India- ARIA Asia pacific workshop report. Asian Pac J Allergy Immunol (2008) 26(4):245–56.
11. Podder S, Chowdhury I, Das A, Gupta S, Saha G. Immediate hypersensitivity to common inhalants - an investigation of nasobronchial allergy patients in kolkata, India. Allergy ClinImmunol Int (2006) 18(3):114–9.
12. Dutta S, Mondal P, Saha NC, Moitra S, Podder S, Ghosh A, et al. Role of offending out-door aero-allergen and CD14 c(-159)T polymorphism in development and severity of asthma in a kolkata patient population. Afr Health Sci (2017) 17(4):1101–9. doi: 10.4314/ahs.v17i4.18
13. Bhattacharya K, Sircar G, Dasgupta A, Bhattacharya SG. Spectrum of allergens and allergen biology in India. Int Arch Allergy Immunol (2018) 177(3):219–37. doi: 10.1159/000490805
14. Dey D, Mondal P, Laha A, Sarkar T, Moitra S, Bhattacharyya S, et al. Sensitization to common aeroallergens in the atopic population of West Bengal, India: An investigation by skin prick test. Int Arch Allergy Immunol (2019) 178(1):60–5. doi: 10.1159/000492584
15. Rolin S, Masereel B, Dogné JM. Prostanoids as pharmacological targets in COPD and asthma. Eur J Pharmacol (2006) 533(1-3):89–100. doi: 10.1016/j.ejphar.2005.12.058
16. Peebles RS Jr. Prostaglandins in asthma and allergic diseases. PharmacolTher (2019) 193:1–19. doi: 10.1016/j.pharmthera.2018.08.001
17. Pan Y, Li S, Xie X, Li M. Association between thromboxane A2 receptor polymorphisms and asthma risk: A meta-analysis. J Asthma (2016) 53(6):576–82. doi: 10.3109/02770903.2015.1126849
18. Säfholm J, Manson ML, Bood J, Delin I, Orre AC, Bergman P, et al. Prostaglandin E2 inhibits mast cell-dependent bronchoconstriction in human small airways through the e prostanoid subtype 2 receptor. J Allergy Clin Immunol (2015) 136(5):1232–9. doi: 10.1016/j.jaci.2015.04.002
19. Daham K, James A, Balgoma D, Kupczyk M, Billing B, Lindeberg A, et al. Effects of selective COX-2 inhibition on allergen-induced bronchoconstriction and airway inflammation in asthma. J Allergy Clin Immunol (2014) 134(2):306–13. doi: 10.1016/j.jaci.2013.12.002
20. Mundell SJ, Mumford A. TBXA2R gene variants associated with bleeding. Platelets (2018) 9(7):739–42. doi: 10.1080/09537104.2018.1499888
21. Unoki M, Furuta S, Onouchi Y, Watanabe O, Doi S, Fujiwara H, et al. Association studies of 33 single nucleotide polymorphisms (SNPs) in 29 candidate genes for bronchial asthma: Positive association a T924C polymorphism in the thromboxane A2 receptor gene. Hum Genet (2000) 106(4):440–6. doi: 10.1007/s004390000267
22. Gallucci M, Carbonara P, Pacilli AMG, di Palmo E, Ricci G, Nava S. Use of symptoms scores, spirometry, and other pulmonary function testing for asthma monitoring. Front Pediatr (2019) 7:54. doi: 10.3389/fped.2019.00054
23. Kodgule RR, Singh V, Dhar R, Saicharan BG, Madas SJ, Gogtay JA, et al. Reference values for peak expiratory flow in Indian adult population using a European union scale peak flow meter. J Postgrad Med (2014) 60(2):123–9. doi: 10.4103/0022-3859.132311
24. Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol (1993) 234(3):779–815. doi: 10.1006/jmbi.1993.1626
25. Sali A, Overington JP. Derivation of rules for comparative protein modeling from a database of protein structure alignments. Protein Sci (1994) 3(9):1582–96. doi: 10.1002/pro.5560030923
26. DeLano WL. An open-source molecular graphics tool CCP4 newsletter on protein crystallography. Pymol (2002) 40:82–92.
27. Robert X, Gouet P. Deciphering key features in protein structures with the new END script server. Nucleic Acids Res (2014) 42:W320–4. doi: 10.1093/nar/gku316
28. Dasgupta A, Ghoshal AG, Mukhopadhyay A, Kundu S, Mukherjee S, Roychowdhury S, et al. Reference equation for spirometry interpretation for Eastern India. Lung India (2015) 32(1):34–9. doi: 10.4103/0970-2113.148443
29. Fan H, Chen S, Yuan X, Han S, Zhang H, Xia W, et al. Structural basis for ligand recognition of the human thromboxane A2 receptor. Nat Chem Biol (2019) 15(1):27–33. doi: 10.1038/s41589-018-0170-9
30. Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature (2021) 596(7873):583–9. doi: 10.1038/s41586-021-03819-2
31. Lundbäck B, Backman H, Lötvall J, Rönmark E. Is asthma prevalence still increasing? Expert Rev Respir Med (2016) 10(1):39–51. doi: 10.1586/17476348.2016.1114417
32. Bijanzadeh M, Mahesh PA, Ramachandra NB. An understanding of the genetic basis of asthma. Indian J Med Res (2011) 134(2):149–61.
33. Kim JH, Lee SY, Kim HB, Jin HS, Yu JH, Kim BJ, et al. TBXA2R gene polymorphism and responsiveness to leukotriene receptor antagonist in children with asthma. Clin Exp Allergy (2008) 38(1):51–9. doi: 10.1111/j.1365-2222.2007.02931.x
34. Zhao Y, Zhang X, Han C, Cai Y, Li S, Hu X, et al. Pharmacogenomics of leukotriene modifiers: A systematic review and meta-analysis. J Pers Med (2022) 12(7):1068. doi: 10.3390/jpm12071068
35. Wang Q, Morris RJ, Bode AM, Zhang T. Prostaglandin pathways: Opportunities for cancer prevention and therapy. Cancer Res (2022) 82(6):949–65. doi: 10.1158/0008-5472.CAN-21-2297
36. Suzuki R, Miyazaki Y, Takagi K, Torii K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: Implications for therapy. Treat Respir Med (2004) 3(1):17–27. doi: 10.2165/00151829-200403010-00003
37. Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, Snouwaert JN, et al. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol Lung Cell Mol Physiol (2006) 291(5):L1005–17. doi: 10.1152/ajplung.00174.2006
38. Cyphert JM, Allen IC, Church RJ, Latour AM, Snouwaert JN, Coffman TM, et al. Allergic inflammation induces a persistent mechanistic switch in thromboxane-mediated airway constriction in the mouse. Am J Physiol Lung Cell Mol Physiol (2012) 302(1):L140–51. doi: 10.1152/ajplung.00152.2011
39. Mondal P, Dey D, Sarkar T, Laha A, Moitra S, Bhattacharyya S, et al. Evaluation of sensitivity toward storage mites and house dust mites among nasobronchial allergic patients of kolkata, India. J Med Entomol (2019) 56(2):347–52. doi: 10.1093/jme/tjy206
40. Singh AB, Dahiya P. Aerobiological researches on pollen and fungi in India during the last fifty years: An overview. Ind J Allergy Asthma Immunol (2008) 22(1):27–38.
41. Podder S, Gupta S, Saha G. Seasonal prevalence of allergenic mites in house dust of kolkata metropolis, India. Aerobiologia (2009) 25(1):39–47. doi: 10.1007/s10453-008-9107-1
42. Podder S, Gupta SK, Saha GK. Incrimination of blomia tropicalis as a potent allergen in house dust and its role in allergic asthma in kolkata metropolis, India. World Allergy Organ J (2010) 3(5):182–7. doi: 10.1097/WOX.0b013e3181df4d4f
43. Tunnicliffe WS, Fletcher TJ, Hammond K, Roberts K, Custovic A, Simpson A, et al. Sensitivity and exposure to indoor allergens in adults with differing asthma severity. Eur Respir J (1999) 13(3):654–9. doi: 10.1183/09031936.99.13365499
44. Ezeamuzie CI, Al-Ali S, Khan M, Hijazi Z, Dowaisan A, Thomson MS, et al. IgE-mediated sensitization to mould allergens among patients with allergic respiratory diseases in a desert environment. Int Arch Allergy Immunol (2000) 121(4):300–7. doi: 10.1159/000024343
45. Jaén A, Sunyer J, Basagaña X, Chinn S, Zock JP, Antó JM, et al. Specific sensitization to common allergens and pulmonary function in the European community respiratory health survey. Clin Exp Allergy (2002) 32(12):1713–9. doi: 10.1046/j.1365-2222.2002.01539.x
46. Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res (2010) 690(1-2):24–39. doi: 10.1016/j.mrfmmm.2009.09.005
47. Tam E, Miike R, Labrenz S, Sutton AJ, Elias T, Davis J, et al. Volcanic air pollution over the island of hawai'i: Emissions, dispersal, and composition. association with respiratory symptoms and lung function in hawai'i island school children. Environ Int (2016) 92-93:543–52. doi: 10.1016/j.envint.2016.03.025
48. Yu S, Park S, Park CS, Kim S. Association between the ratio of FEV1 to FVC and the exposure level to air pollution in neversmoking adult refractory asthmatics using data clustered by patient in the soonchunhyang asthma cohort database. Int J Environ Res Public Health (2018) 15(11):2349. doi: 10.3390/ijerph15112349
49. Prashanth SN, Raveendra R, Kumar KJ, Ragavendra R. Study of urinary leukotriene E4 levels and total serum IgE levels in children with acute exacerbations of asthma. Sri Lanka J Child Health (2022) 51(1):52–7. doi: 10.4038/sljch.v51i1.9992
50. Green SA, Malice MP, Tanaka W, Tozzi CA, Reiss TF. Increase in urinary leukotriene LTE4 levels in acute asthma: Correlation with airflow limitation. Thorax (2004) 59(2):100–4. doi: 10.1136/thorax.2003.006825
51. Kim JJ, American Academy of Pediatrics Committee on Environmental Health. Ambient air pollution: Health hazards to children. Pediatrics (2004) 114(6):1699–707. doi: 10.1542/peds.2004-2166
52. Pignataro FS, Bonini M, Forgione A, Melandri S, Usmani OS. Asthma and gender: The female lung. Pharmacol Res (2017) 119:384–90. doi: 10.1016/j.phrs.2017.02.017
53. Zierau O, Zenclussen AC, Jensen F. Role of female sex hormones, estradiol and progesterone, in mast cell behavior. Front Immunol (2012) 19(3):169. doi: 10.3389/fimmu.2012.00169
54. Priftis KN, Mantzouranis EC, Anthracopoulos MB. Asthma symptoms and airway narrowing in children growing up in an urban versus rural environment. J Asthma (2009) 46(3):244–51. doi: 10.1080/02770900802647516
55. Zhang J, Smith KR. Indoor air pollution: A global health concern. Br Med Bull (2003) 68:209–25. doi: 10.1093/bmb/ldg029
56. Lyon AM, Tesmer JJ. Structural insights into phospholipase c-β function. MolPharmacol (2013) 84(4):488–500. doi: 10.1124/mol.113.087403
Keywords: pollen sensitivity, asthma, thromboxane A2 receptor gene, SNP, FEV1/FVC ratio, homology modeling, West Bengal (India)
Citation: Ganai I, Saha I, Banerjee P, Laha A, Sultana S, Sultana N, Biswas H, Moitra S and Podder S (2023) In silico analysis of single nucleotide polymorphism (rs34377097) of TBXA2R gene and pollen induced bronchial asthma susceptibility in West Bengal population, India. Front. Immunol. 14:1089514. doi: 10.3389/fimmu.2023.1089514
Received: 04 November 2022; Accepted: 14 February 2023;
Published: 01 March 2023.
Edited by:
Loredana Bury, University of Perugia, ItalyReviewed by:
Jean-Francois Lauzon-Joset, Laval University, CanadaTaushif Khan, Jackson Laboratory for Genomic Medicine, United States
Copyright © 2023 Ganai, Saha, Banerjee, Laha, Sultana, Sultana, Biswas, Moitra and Podder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanjoy Podder, c2twem9vMkByZWRpZmZtYWlsLmNvbQ==
 Indranil Ganai1
Indranil Ganai1 Priyajit Banerjee
Priyajit Banerjee Sanjoy Podder
Sanjoy Podder