
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 08 February 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1084558
Objective: To present the pooled quantitative evidence of baseline characteristics and clinical outcomes of tocilizumab (TCZ) in patients with refractory Takayasu arteritis (TAK).
Methods: A comprehensive systematic review and meta-analysis was performed on all available studies retrieved from the MEDLINE, Embase, and Cochrane databases, using TCZ in patients with refractory TAK. We applied the commands metan and metaprop_one in Stata Software to pool overall estimates of continuous data and binomial data, respectively. A random-effects model was recruited for analysis.
Results: Nineteen studies with 466 patients were included in this meta-analysis. The mean age at implementation of TCZ was 34.32 years. Female sex and Numano Type V were the most prominent baseline characteristics. During the 12-month follow-up when receiving TCZ treatment, pooled CRP was 1.17 mg/L (95% confidence interval [CI] -0.18-2.52), pooled ESR was 3.54 mm/h (95% CI 0.51-6.58), and pooled glucocorticoid dose was 6.26 mg/d (95% CI 4.24-8.27). Approximately 76% (95% CI 58-87%) of patients achieved a decrease in glucocorticoid dosage. Meanwhile, patients with TAK had a remission rate of 79% (95% CI 69-86%), a relapse rate of 17% (95% CI 5-45%), an imaging progress rate of 16% (95% CI 9-27%), and a retention rate of 68% (95% CI 50-82%). Adverse events occurred in 16% (95% CI 5-39%) of patients, and infection was the most common adverse event, with a rate of 12% (95% CI 5-28%).
Conclusion: TCZ treatment can provide favorable outcomes in terms of inflammatory markers, steroid-sparing effects, clinical response, drug retention and minimizing adverse effects for patients with refractory TAK.
Takayasu arteritis (TAK), a type of large-vessel vasculitis, is defined as an immune arteritis involving granulomatous inflammation of the aorta and its major branches (1). It is predominantly a disease of young Asian women, with variable clinical manifestations ranging from asymptomatic incidental discovery to fever, myalgias, hypertension, limb claudication and absent pulses due to arterial stenosis and/or aneurysms (1). The standard first-line treatment option for active TAK patients is high-dose glucocorticoids (GCs) plus adjunctive conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), as recommended by both EULAR and ACR guidelines (2, 3). However, approximately 40% of TAK patients can’t control the disease despite high-dose GC therapy plus csDMARDs (4, 5). Meanwhile, patients frequently relapse during GC tapering, with reported rates ranging from 50–80% in the literature (6, 7). How to treat these refractory TAK patients remains an unresolved issue.
Inflammation plays a crucial role in the pathophysiology of TAK (1). Inflammatory cells, including T-helper 1 and T-helper 17 cells, and proinflammatory cytokines, including interferon-γ, tumor necrosis factor-α, and interleukin-6 (IL-6), are involved in the initiation and propagation of inflammation in TAK (8–10). Hence, biologic targeted therapies should be considered as an alternate choice. To date, tumor necrosis factor-α inhibitors (TNFIs), such as infliximab and its biosimilar, have been shown to improve remission and relapse in several observational studies (11–15), and are recommended to refractory TAK patients by ACR guidelines (3). However, tocilizumab (TCZ), an anti-IL-6 receptor that is efficacious for active giant cell arteritis (GCA) (16, 17), is not recommended for refractory TAK patients due to a lack of forceful clinical evidence (2, 3). In the only randomized controlled trial (RCT) of TCZ in refractory TAK patients, the results from both intent-to-treat and per-protocol set populations showed no statistically significant difference in terms of relapse-free survival between TCZ and placebo (18). Data from other case series or retrospective cohorts presented some conflicting results, with a wide range of relapse rates from 0% to 66.7% during 12-month follow-up (18–21). Most studies had a small patient sample size, limiting the application of findings to the actual population and clinical practice.
Recently, a series of studies concerning efficacy and safety of TCZ in refractory TAK patients have been published (19–29). This systematic review and meta-analysis presents pooled quantitative evidence of baseline characteristics and clinical outcomes of TCZ in refractory TAK patients.
This systematic review and meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions (version 6.3) (30) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (31).
The MEDLINE, Embase, and Cochrane databases were searched through Ovid access (https://ovidsp.ovid.com). The following keywords or Medical Subject Headings terms were used: “tocilizumab [Mesh]” or “IL-6 [Mesh]” or “tocilizumab [All Fields]” or “IL-6 [All Fields]” or “inerleukin-6 [All Fields]”; “arteritis [Mesh]” or “vasculitis [Mesh]” or “arteritis [All Fields]” or “vasculitis [All Fields]”; and “takayasu [All Fields]”. There was no restriction on publication dates, but publications were limited to the English language. Potential related studies in the reference lists of included studies were manually searched one at a time. The latest search was updated on July 18, 2022. The detailed search strategies are listed in Supplementary Table 1.
A preestablished selection criteria was used to select candidate studies by two independent authors (LK and YL), and any discrepancies were discussed and solved by a third author (ZL). Inclusion criteria were as follows: clinical studies that used TCZ in patients with refractory TAK; reported baseline characteristics or clinical outcomes; and used a sample size of ≥ 5 patients. Exclusion criteria were as follows: reviews, letters, or conference abstracts; efficacy and safety studies of TCZ in newly diagnosed or treatment-naive TAK; studies that reported other biological DMARDs, such as infliximab, etanercept, adalimumab, etc.
Two independent authors (LK and YL) extracted available data from all included studies. A third author (ZL) would intervene if there were any disagreements. Extracted data were listed as follows when available: author, year, country, study design, patient source, trial period, sample size, gender, age, diagnosis criteria, Numano classification, onset age, disease duration, previous DMARDs, TCZ usage, TCZ duration, follow-up, and clinical outcomes (including serological response, clinical response, imaging response, drug retention, and adverse events). In addition, some necessary data were retrieved by emailing the authors if data were inadequate to pool effect estimates.
Definitions of the following terms were based on the recommendations of EULAR and ACR guidelines (2, 3). Specifically, refractory TAK was defined as a persistent active disease associated with an inability to induce remission despite an appropriate course of standard care therapy. Remission was defined as absence of all clinical signs and symptoms attributed to active TAK and normalization of the C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), with or without immunosuppressive therapy. Relapse was defined as the recurrence of active disease following a period of remission. Imaging progress was defined as the progress of vessel wall thickness, stenosis, occlusions, aneurysms, or new vascular lesions at the follow-up imaging (28). Drug retention was defined as the duration from the time of administration to the time of definitive TCZ interruption (22).
Quality assessments of included studies were performed using the Newcastle‒Ottawa Scale (www.ohri.ca/programs/clinical_epidemiology/oxford.asp) for cohort studies, and the 20-criterion quality appraisal checklist with the modified Delphi technique (32) for case reports and case series by two independent authors (LK and YL). Details of the quality assessment are presented in Supplementary Tables 2–4.
All statistical analyses were performed according to the Cochrane Handbook for Systematic Reviews of Interventions (version 6.3) (30).
For continuous variables, reported means and standard deviations were directly extracted from individual studies; if they were unavailable, means and standard deviations were obtained using the methods introduced by Wan and Luo et al. (33, 34) Overall estimates were pooled in Stata Software (version 15.1, Stata Corporation, College Station, TX, USA) by applying the command metan. For binomial data, numbers of events and totals for all studies were extracted, and study-specific proportions with 95% confidence intervals (CIs) were computed using the exact method (35). Considering the significant between-study heterogeneity and specific estimates with cure rates at or close to 0% or 100% in some studies, the logistic-normal random-effect model was employed to calculate the pooled estimates with the updated command metaprop_one in Stata Software (35). With this model, overall pooled proportions with 95% CIs were obtained by logit transformation and back transformation. The Chi2 statistic of the likelihood ratio (LR) test that compares the random-effect and fixed-effect models was presented to explore potential heterogeneity across studies, which was analogous to the Q-statistic. In this review, p < 0.05 was considered significant.
In the latest update, a total of 766 potentially relevant records were identified in a MEDLINE, Embase, and Cochrane database search. After removing duplicates and screening titles and abstracts, 127 candidate publications were retrieved for further eligibility assessment and were read in full. Of these, 108 studies were excluded due to a sample size of less than five patients, conference abstracts, letters, non-refractory TAK, duplicate patients, or unavailable data. Ultimately, 19 studies fulfilled our inclusion criteria and were included in this meta-analysis (13, 18–29, 36–41). A flow diagram of the literature search and selection is shown in Figure 1. Among the included studies, most came from Asia and Europe. Twelve studies were case series (19–21, 23, 25, 26, 29, 36, 37, 39–41) and seven were cohort studies or RCTs (13, 18, 22, 24, 27, 28, 38). Detailed characteristics for these individual studies are summarized in Table 1.
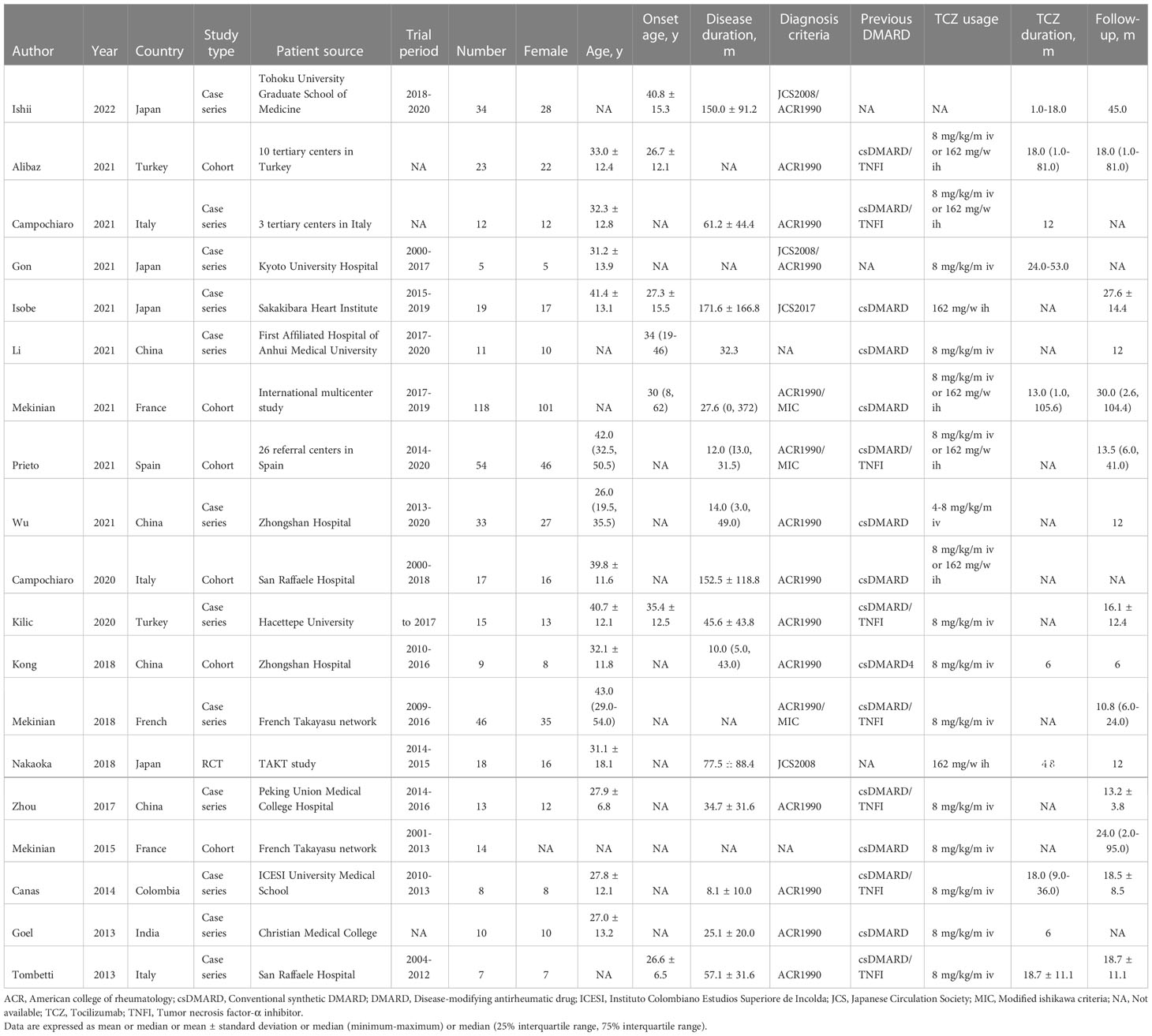
Table 1 Detailed characteristics of 19 included studies on tocilizumab in patients with refractory Takayasu arteritis.
A total of 466 patients with refractory TAK using TCZ were analyzed across 19 studies. Diagnosis of TAK was mainly based on the criteria of the American College of Rheumatology (ACR 1990) or the Japanese Circulation Society (JCS 2008). All patients had persistent active disease despite the use of standard care therapy. Sex distribution was predominantly female (88%, 95% CI 82-92%). The mean age at implementation of TCZ had a pooled estimate of 34.32 years (95% CI 30.39-38.23), and the mean age at symptom onset had a pooled estimate of 31.94 years (95% CI 27.68-35.19). The mean disease duration between onset and implementation was 64.68 months (95% CI 46.21-83.15). Type V (43%, 95% CI 34-53%) and type II (26%, 95% CI 17-37%) were the most common disease types according to the Numano classification, which meant that the involvement of the ascending aorta and aortic arch was the most prominent lesion characteristic. Before TCZ began, the mean NIH score and ITAS 2010 score were 2.64 (95% CI 2.21-3.06) and 6.68 (95% CI 5.30-8.05), respectively. Conventional synthetic DMARDs (csDMARDs) were used previously in 76% of patients (95% CI 55-89%), and tumor necrosis factor inhibitors (TNFIs) were used in 34% of patients (95% CI 16-59%). TCZ was usually administered 8 mg/kg every month intravenously or 162 mg every week subcutaneously, and combined csDMARDs were administered in 45% of patients (95% CI 34-58%). The pooled TCZ duration was 24.25 months (95% CI 16.92-31.57), and the follow-up period was 20.47 months (95% CI 16.42-24.53). A summary of patient-level information in the pooled results is presented in Table 2.
Fourteen studies with 384 patients provided the baseline CRP when giving TCZ. The pooled baseline CRP was 29.04 mg/L (95% CI 23.16-34.92) with high heterogeneity (I2 = 85.60%). During the follow-ups of 3 months, 6 months, and 12 months, pooled CRP levels were 3.06 mg/L (95% CI 0.83-5.29), 1.66 mg/L (95% CI 0.97-2.35), and 1.17 mg/L (95% CI -0.18-2.52), respectively. During the last follow-up, however, the pooled CRP reached up to 10.67 mg/L (95% CI 3.51-17.84) (Figure 2).
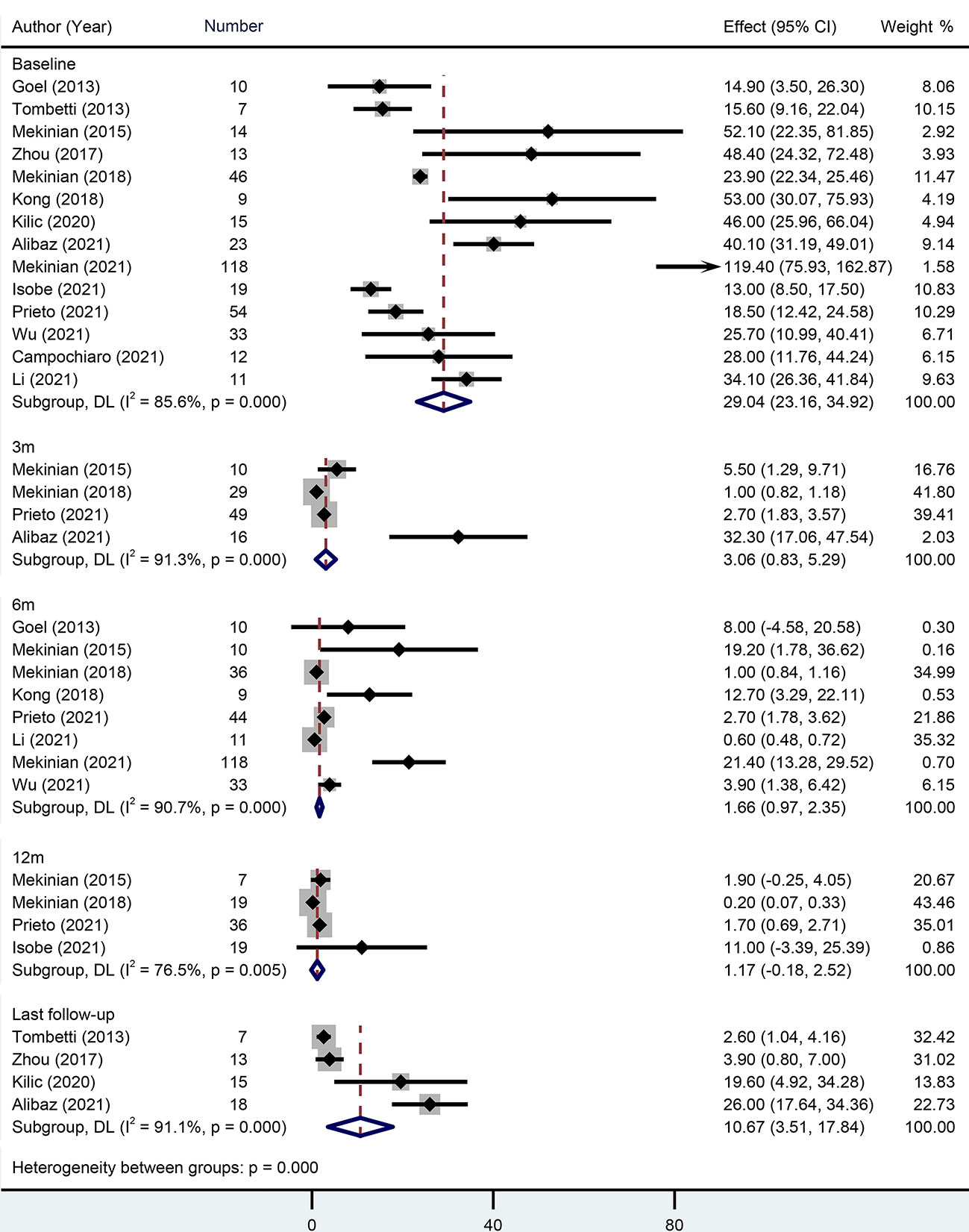
Figure 2 Forest plot showing the pooled results of CRP level at the baseline, 3-month, 6-month, 12-month and last follow-up.
Twelve studies with 209 patients reported baseline ESR. Pooled baseline ESR was 40.92 mm/h (95% CI 35.36-46.47). During 6-month and 12-month follow-ups, pooled ESR levels were 7.48 mm/h (95% CI 4.08-10.88) and 3.54 mm/h (95% CI 0.51-6.58), respectively. At the last follow-up, however, the pooled ESR reached up to 10.2 mm/h (95% CI 5.26-15.14) (Supplementary Figure 1).
In terms of clinical response, GC dose, remission rate, and relapse rate were analyzed.
When TCZ was initiated, baseline GC dose was reported in 16 studies with 409 patients. The pooled estimate was 23.92 mg/d (95% CI 19.16-28.67) with high heterogeneity (I2 = 94.20%). At the 3-month, 6-month, 12-month, and last follow-ups, the pooled GC doses were 11.79 mg/d (95% CI 8.12-15.47), 8.41 mg/d (95% CI 6.40-10.43), 6.26 mg/d (95% CI 4.24-8.27), and 6.71 mg/d (95% CI 5.19-8.23), respectively. Approximately 76% (95% CI 58-87%) of patients achieved a GC dose decrease (Figure 3).
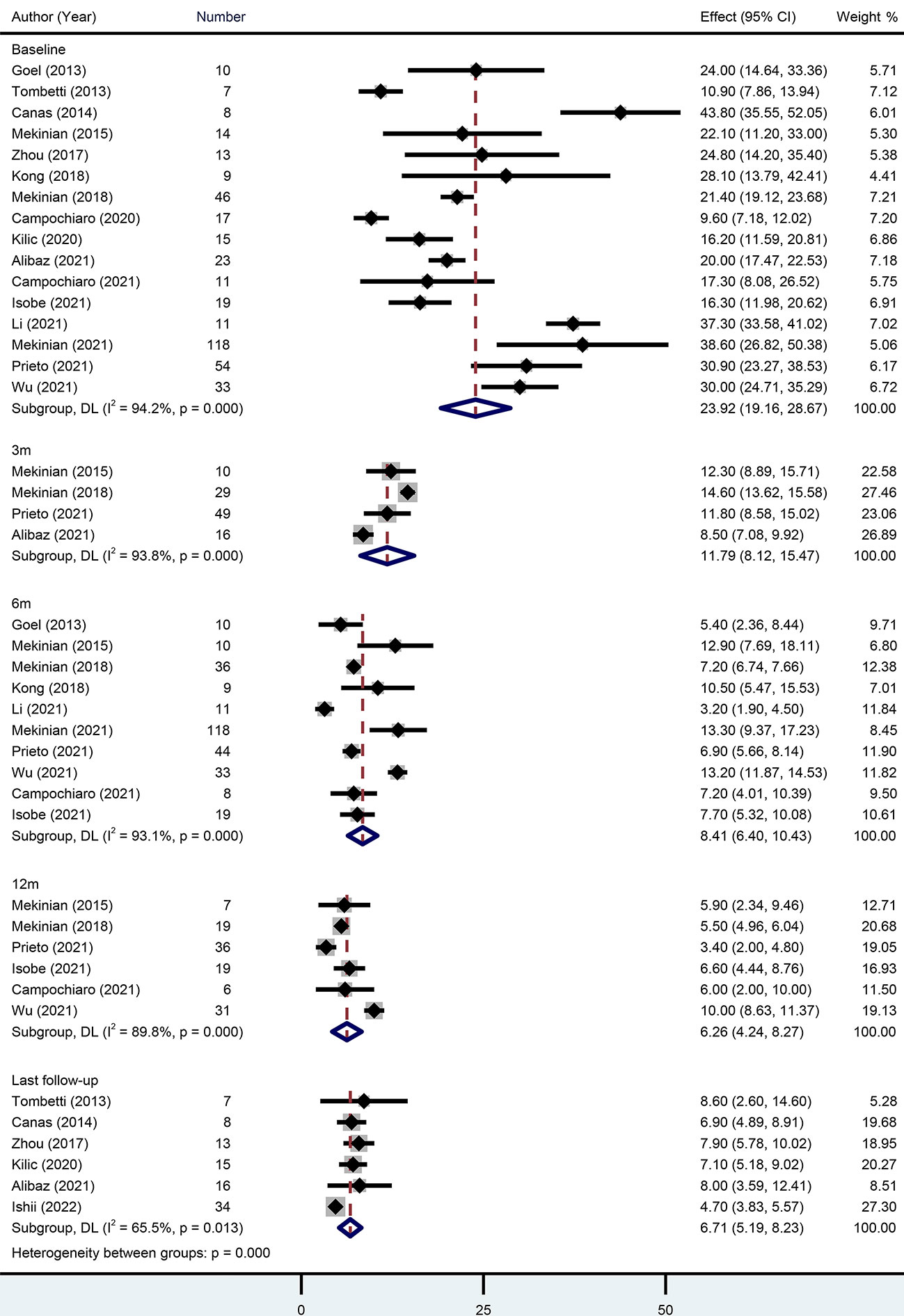
Figure 3 Forest plot showing the pooled results of glucocorticoid level at the baseline, 3-month, 6-month, 12-month and last follow-up.
Several individual studies calculated remission rates during follow-up. At the 3-month, 6-month, 12-month, and last follow-ups, the rates were 45% (95% CI 36-54%), 80% (95% CI 63-90%), 79% (95% CI 69-86%), and 73% (95% CI 43-91%), respectively (Figure 4).
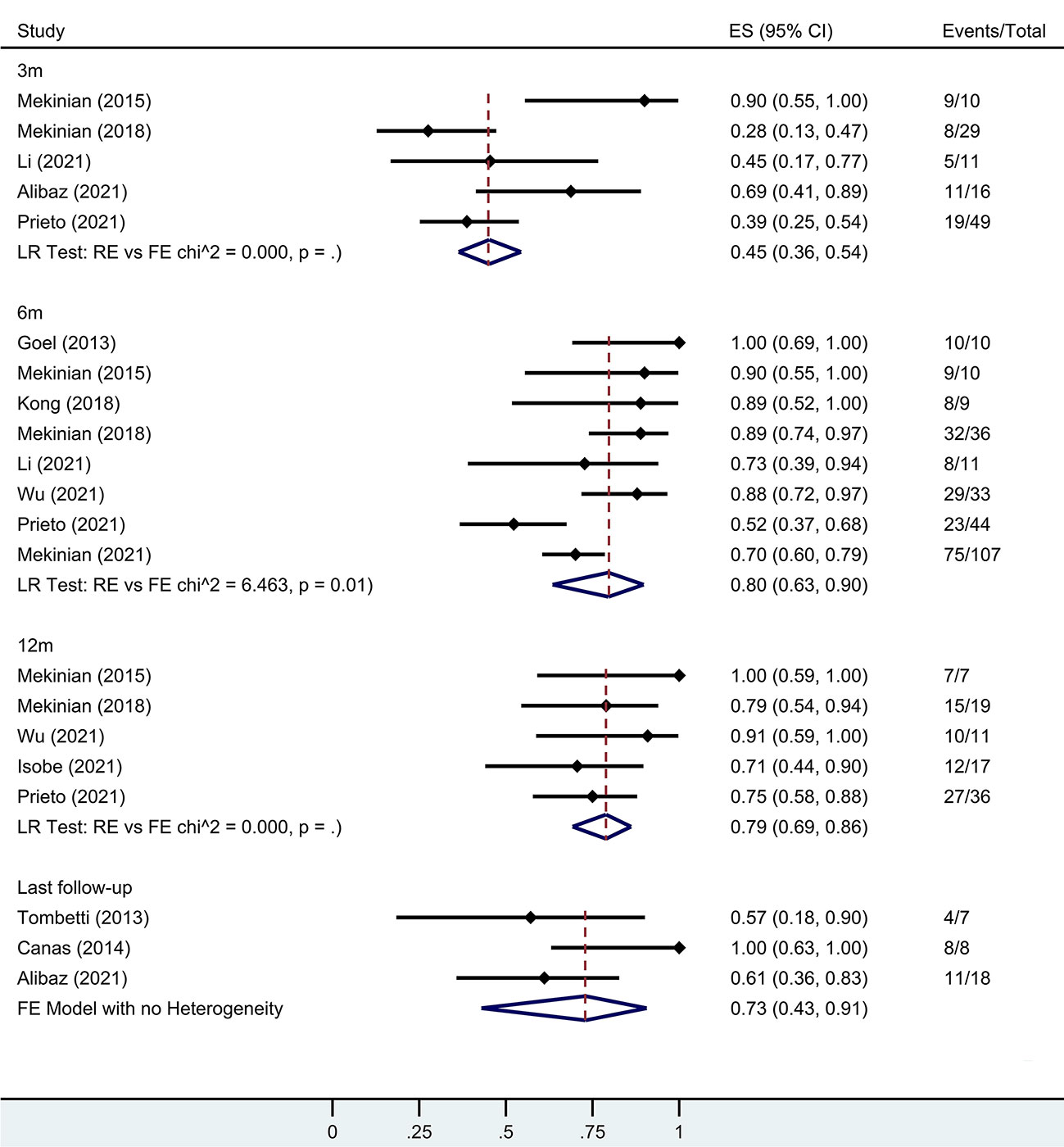
Figure 4 Forest plot showing the pooled results of remission rate at the 3-month, 6-month, 12-month and last follow-up. LR, Likelihood Ratio. RE, Random-Effect model. FE, Fixed-Effect model.
Seven studies with 117 patients reported 12-month relapse rates during TCZ. Pooled results showed that TAK patients under TCZ treatment had a relapse rate of 17% (95% CI 5-45%) during the 12-month follow-up. Four studies calculated relapse rates after TCZ discontinuation. The pooled estimate was 18% (95% CI 2-70%) when the TCZ discontinuation time was 7.35 months (95% CI 5.34-9.35).
Computed tomography angiography (CTA), magnetic resonance angiography (MRA), 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET), and ultrasonography (US) were the most common imaging techniques for assessing the efficacy of TAK therapy. Most patients were evaluated as “improved” or “stable” on imaging; however, the rates of imaging progress were pooled as 28% (95% CI 15-46%), 16% (95% CI 9-27%), and 16% (95% CI 4-42%) at the 6-month, 12-month, and last follow-ups, respectively.
Two studies had a 6-month trial period and TCZ was stopped after the trial ended (37, 38). For patients taking medicine for a long time, TCZ retention rates were pooled as 89% (95% CI 74-96%) at 3 months, 75% (95% CI 46-92%) at 6 months, 68% (95% CI 50-82%) at 12 months, and 69% (95% CI 51-82%) at the last follow-up. Main reasons for TCZ discontinuation included inefficacy, adverse events, remission, and cost.
Regarding adverse events, data from 10 studies with 314 patients were analyzed. Adverse events occurred in 16% (95% CI 5-39%) of patients on the basis of pooled results. Specifically, infection was the most common adverse event, with a rate of 12% (95% CI 5-28%). Severe adverse events had a rate of 4% (95% CI 2-8%), including serious infection, major adverse cardiovascular events, and other adverse events leading to TCZ discontinuation.
The treatment option of refractory TAK is challenging. Most refractory TAK patients suffer GC-resistant events and frequent relapses after GC tapering, even when combined with csDMARDs (4, 5). TCZ, as a novel biologic agent of the anti-IL-6 receptor, selectively blocks the IL-6 signaling cascade, as a way to induce remission, decrease relapse, and reduce GC toxicity (42, 43). However, due to limited clinical data and experience, TCZ is not recommended for routine use in refractory TAK patients by either the EULAR or ACR guidelines (2, 3). Therefore, the evidence of all current literature on TCZ in refractory TAK patients urgently needs to be summarized.
Compared with previous systematic reviews focusing on the efficacy of biological agents in TAK (5, 44–46), the current study pays special attention to the baseline characteristics and clinical outcomes of TCZ in refractory TAK patients, with the largest sample size and the most included studies. It was found that TCZ treatment provided favorable outcomes in terms of steroid-sparing effects, clinical response, drug retention, and minimizing adverse effects for patients with refractory TAK.
Prior to TCZ usage, CRP and ESR were up to 29.04 mg/L and 40.92 mm/h, respectively, indicating active disease status in refractory TAK patients despite high-dose GC with adjunctive DMARD therapy. When receiving TCZ treatment, most patients presented significant decreases in inflammatory marker levels and GC doses. At the end of the 12-month follow-up, pooled CRP, ESR, and GC dose fell back to 1.17 mg/L, 3.54 mm/h, and 6.26 mg/d, respectively, achieving the target GC dose of ≤10 mg/day as recommended by the EULAR guideline (2). Interestingly, at the last follow-up (at approximately 20 months), CRP, ESR, and GC dose were slightly raised (Supplementary Figure 2), indicating new potential risk of the disease becoming active again. The above phenomenon was further confirmed by the remission rate: from 80% and 79% at the 6- and 12-month follow-ups, to 73% at the last follow-up. Two main reasons might contribute to these results. First, to reduce GC-related adverse events, GC exposure was limited when patients achieved remission, resulting in a fluctuation of disease activity. Second, some patients might develop resistance to TCZ treatment.
Disease remission and relapse were the two major concerns during TAK treatment. We found that TCZ treatment could provide favorable clinical outcomes, with a remission rate of 79% and a relapse rate of 17% during the 12-month follow-up. High-dose GCs, csDMARDs, and TNFIs, as the most common agents for achieving disease control, were compared with TCZ in a series of studies. For GCs, the TAKT study is the only RCT comparing the efficacy of TCZ vs. GC in patients with refractory TAK. The results showed that relapse occurred in 44.4% of TCZ-treated patients and 61.1% of GC-treated patients; however, no statistically significant difference between the two groups was found owing to limited sample size (18). In the open-label extension to 96 weeks, the final results indicated that TCZ provided a steroid-sparing effect and improvements on imaging evaluation (47). For csDMARDs, a retrospective cohort study compared 46 TCZ-treated patients and 46 age- and sex-matched csDMARDs-treated patients, and found that TCZ had a significantly lower cumulative incidence of relapse in refractory TAK patients (6.3% vs. 34.6%) (13). The results from another meta-analysis revealed remission and relapse rates of csDMARDs were 57.9% and 53.9% (5), inferior to the pooled results of TCZ in our review (79% and 17%). Although TCZ showed some signs, there was insufficient high-quality evidence to prove it superiority over csDMARDs. For TNFIs, several observational studies compared the efficacy of TCZ and TNFIs in patients with refractory TAK. Mekinian et al. found that the proportions of complete and partial remission rates and relapse-free survival were comparable for TCZ and TNFIs (13). In their latest multicenter retrospective study with 209 patients, the results still showed an equivalent relapse rate (27). Another multicenter comparative study also observed the similar results in clinical outcomes (24). However, more RCTs are warranted to investigate the efficacy and safety of TCZ and TNFIs in the future.
The pooled TCZ retention rate was favorable, up to 69% at the last follow-up. Inefficacy was the main reason for TCZ discontinuation, followed by adverse events, remission, and cost. However, the indication for discontinuation of TCZ due to remission is unclear. Several studies reported inconsistent results comparing the retention rates of TCZ and TNFIs. One study showed a comparable drug retention rate between TCZ and TNFIs (57% vs. 56%) (24), but another reported a significantly lower retention rate under TCZ treatment (41% vs. 67%) (22). The observed difference may come from the physician’s preference and a bias about TNFIs as the first-choice of biologic therapy in most TAK patients.
In this review, approximal 18% of patients experienced disease relapse after TCZ discontinuation, with a pooled discontinuation duration of 7.35 months. Wu et al. (21) reported that six out of fourteen cases experienced relapse after TCZ withdrawal, but no patient suffered from disease flare in another study (28). It seemed that prolonged TCZ treatment might help prevent disease relapse. At present, no guidelines make any recommendations on the best duration of TCZ treatment (2, 3). Further comparative studies concerning the effectiveness and duration of TCZ are needed.
It is worth noting that adverse events occur during TCZ treatment. A series of high-quality RCTs presented incidence rates of adverse events in other autoimmune diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic sclerosis (48–50). The reported rates of adverse events and severe adverse events were 86-94% and 7-13%, respectively. In our pooled results, adverse events occurred in 16% of TAK patients. Infection proved the most common adverse event and a rate of 12%. Therefore, when administering TCZ therapy to refractory TAK patients, physicians should pay more attention to monitoring vital signs and observing potential symptoms of infection, such as fever, asthenia, rash, and elevated leucocyte count.
Because of accurate disease monitoring during GC tapering and a high risk of relapse during TCZ treatment, regular follow-up should be considered in all patients. A comprehensive disease activity assessment is needed based on a combination of clinical symptoms, laboratory investigation, and imaging examination. Imaging surveillance is regarded as mandatory (2, 3), because of the better ability to detect signs of vessel wall thickening, stenosis, or other active inflammation performance. CTA, MRA, and FDG-PET are the most popular imaging modalities used to distinguish persisting vascular inflammation and identify luminal abnormalities (51, 52). Nevertheless, there is no current consensus on the optimal frequency interval between imaging examinations. According to our experience, imaging surveillance might start every 6–12 months in the quiescent course and every 1–3 months (or more frequent) early in the active course.
Some studies have also aimed to evaluate the benefit of TCZ in non-refractory TAK patients (53, 54). The TOCITAKA study was the first prospective multicenter open-label trial to assess the long-term efficacy of TCZ in treatment-naive TAK patients (54). Thirteen patients were included, eleven (85%) of whom achieved TAK remission, and six of whom discontinued GCs after 6 months of TCZ therapy. During the 12-month follow-up after TCZ discontinuation, relapse occurred among five of the patients (45%). Another study from Yoshida et al. (53) comprised 14 active TAK patients with GC+TCZ, and 18 patients with GC or GC+csDMARDs. All patients achieved remission after initial therapy, however, GC+TCZ therapy had a significantly lower relapse rate during GC tapering (14.3% vs. 55.6%). The above findings revealed that TCZ seemed to be an effective alternative induction regimen for non-refractory TAK patients, but disease relapse after TCZ discontinuation and GC tapering were still an issue.
Several limitations were taken into account in this review. First, our findings were mainly based on case series or cohorts, which may limit the application of our findings to the actual population. Therefore, we only selected studies with more than five patients in an attempt to strengthen the robustness and representativeness of the results as much as possible. Second, individual participant data were not available from the included studies, which may bring some potential publication bias and insufficient evidence. Nevertheless, this study is the largest systematic review and meta-analysis for refractory TAK patients treated with TCZ and can provide better clinical guidance. Third, there were seven multicenter studies covering a long period of time from France, Japan, Turkey, Italy, Spain, and international cooperation (13, 18, 19, 24, 27, 28, 39), some of which had overlapping patient sources and trial periods with the included individual cohort. For example, data retrieved from San Raffaele Hospital in Italy by Campochiaro et al. (22) from 2000 to 2018 had potential duplications when compared with multicenter studies by Mekinian et al. from 2017 to 2019 (27). We made an effort to find and remove the overlapping data, however, we could not guarantee the removal of all duplicate data.
In conclusion, this systematic review and meta-analysis supports the favorable outcomes of TCZ treatment in terms of inflammatory markers, steroid-sparing effects, clinical response, drug retention and minimizing adverse effects for patients with refractory TAK. More high-quality comparative studies are needed to explore the efficacy and safety of TCZ in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conception and design: LK, YL, GY, QX. Data collection: LK, YL, ZL. Statistical analysis: LK, YL, BC. Writing the article: LK, YL, ZL, YZ. Critical revision of the article: BC, GY, QX. Final approval of the article: LK, YL, ZL, YZ, BC, GY, QX. Obtained funding: YL, GY, QX. Overall responsibility: GY, QX. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Department of Sichuan Province (Grant No. 2021JDRC0045 and 2021YFS0164); Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 2021HXBH012); Clinical Research Incubation Project of West China Hospital, Sichuan University (Grant No. 2019HXFH038 and 2021HXFH018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1084558/full#supplementary-material
1. Pugh D, Karabayas M, Basu N, Cid MC, Goel R, Goodyear CS, et al. Large-Vessel vasculitis. Nat Rev Dis Primers (2022) 7(1):93. doi: 10.1038/s41572-021-00327-5
2. Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheumat Dis (2020) 79(1):19–30. doi: 10.1136/annrheumdis-2019-215672
3. Maz M, Chung SA, Abril A, Langford CA, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis foundation guideline for the management of giant cell arteritis and takayasu arteritis. Arthritis Rheumatol (2021) 73(8):1349–65. doi: 10.1002/art.41774
4. Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med (1994) 120(11):919–29. doi: 10.7326/0003-4819-120-11-199406010-00004
5. Barra L, Yang G, Pagnoux C, Canadian Vasculitis N. Non-glucocorticoid drugs for the treatment of takayasu's arteritis: A systematic review and meta-analysis. Autoimmun Rev (2018) 17(7):683–93. doi: 10.1016/j.autrev.2018.01.019
6. Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of takayasu arteritis patients. Arthritis Rheumatol (2007) 56(3):1000–9. doi: 10.1002/art.22404
7. Comarmond C, Biard L, Lambert M, Mekinian A, Ferfar Y, Kahn JE, et al. Long-term outcomes and prognostic factors of complications in takayasu arteritis: A multicenter study of 318 patients. Circulation. (2017) 136(12):1114–22. doi: 10.1161/CIRCULATIONAHA.116.027094
8. Alibaz-Oner F, Yentür SP, Saruhan-Direskeneli G, Direskeneli H. Serum cytokine profiles in takayasu's arteritis: Search for biomarkers. Clin Exp Rheumatol (2015) 33(2 Suppl 89):S–32-35.
9. Kong X, Sun Y, Ma L, Chen H, Wei L, Wu W, et al. The critical role of IL-6 in the pathogenesis of takayasu arteritis. Clin Exp Rheumatol (2016) 34(3 Suppl 97):S21–27.
10. Saadoun D, Garrido M, Comarmond C, Desbois AC, Domont F, Savey L, et al. Th1 and Th17 cytokines drive inflammation in takayasu arteritis. Arthritis Rheumatol (2015) 67(5):1353–60. doi: 10.1002/art.39037
11. Molloy ES, Langford CA, Clark TM, Gota CE, Hoffman GS. Anti-tumour necrosis factor therapy in patients with refractory takayasu arteritis: Long-term follow-up. Ann Rheum Dis (2008) 67(11):1567–9. doi: 10.1136/ard.2008.093260
12. Schmidt J, Kermani TA, Bacani AK, Crowson CS, Matteson EL, Warrington KJ. Tumor necrosis factor inhibitors in patients with takayasu arteritis: Experience from a referral center with long-term followup. Arthritis Care Res (2012) 64(7):1079–83. doi: 10.1002/acr.21636
13. Mekinian A, Comarmond C, Resche-Rigon M, Mirault T, Kahn JE, Lambert M, et al. Efficacy of biological-targeted treatments in takayasu arteritis: Multicenter, retrospective study of 49 patients. Circulation. (2015) 132(18):1693–700. doi: 10.1161/CIRCULATIONAHA.114.014321
14. Campochiaro C, Tomelleri A, Sartorelli S, Sembenini C, Papa M, Fallanca F, et al. A prospective observational study on the efficacy and safety of infliximab-biosimilar (CT-P13) in patients with takayasu arteritis (TAKASIM). Front Med (2021) 8:723506. doi: 10.3389/fmed.2021.723506
15. Tomelleri A, Campochiaro C, Sartorelli S, Baldassi F, Fallanca F, Picchio M, et al. Effectiveness and safety of infliximab dose escalation in patients with refractory takayasu arteritis: A real-life experience from a monocentric cohort. Mod Rheumatol (2022) 32(2):406–12. doi: 10.1093/mr/roab012
16. Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med (2017) 377(4):317–28. doi: 10.1056/NEJMoa1613849
17. Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet. (2016) 387(10031):1921–7. doi: 10.1016/S0140-6736(16)00560-2
18. Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, et al. Efficacy and safety of tocilizumab in patients with refractory takayasu arteritis: Results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheumat Diseases (2018) 77(3):348–54. doi: 10.1136/annrheumdis-2017-211878
19. Campochiaro C, Tomelleri A, Galli E, Cocchiara E, Sartorelli S, Muratore F, et al. Failure of first anti-TNF agent in takayasu's arteritis: To switch or to swap? Clin Exp Rheumatol (2021) 39 Suppl 129(2):129–34. doi: 10.55563/clinexprheumatol/1xi8ag
20. Li H, Shuai Z. Efficacy of tocilizumab for refractory takayasu arteritis: A retrospective study and literature review. Heart Vessels (2022) 08:08. doi: 10.1007/s00380-021-01981-1
21. Wu S, Kong X, Cui X, Chen H, Ma L, Dai X, et al. Effectiveness and safety of tocilizumab in patients with refractory or severe takayasu's arteritis: A prospective cohort study in a Chinese population. Joint Bone Spine: Rev du Rhumat (2021) 88(5):105186. doi: 10.1016/j.jbspin.2021.105186
22. Campochiaro C, Tomelleri A, Sartorelli S, Cavalli G, De Luca G, Baldissera E, et al. Drug retention and discontinuation reasons between seven biologics in patients with takayasu arteritis. Semin Arthritis Rheum (2020) 50(3):509–14. doi: 10.1016/j.semarthrit.2020.01.005
23. Kiliç L, Karadağ Ö, Erden A, Sari A, Armağan B, Yardimci GK, et al. Anti-interleukin-6 (tocilizumab) therapy in takayasu’s arteritis: A real life experience. Turk J Med Sci (2020) 50(1):31–6. doi: 10.3906/sag-1906-39
24. Alibaz-Oner F, Kaymaz-Tahra S, Bayindir O, Yazici A, Ince B, Kalkan K, et al. Biologic treatments in takayasu's arteritis: A comparative study of tumor necrosis factor inhibitors and tocilizumab. Semin Arthritis Rheum (2021) 51(6):1224–9. doi: 10.1016/j.semarthrit.2021.09.010
25. Gon Y, Yoshifuji H, Nakajima T, Murakami K, Nakashima R, Ohmura K, et al. Long-term outcomes of refractory takayasu arteritis patients treated with biologics including ustekinumab. Modern Rheumatol (2021) 31(3):678–83. doi: 10.1080/14397595.2020.1800560
26. Isobe M, Maejima Y, Saji M, Tateishi U. Evaluation of tocilizumab for intractable takayasu arteritis and 18F-fluorodeoxyglucose-positron emission tomography for detecting inflammation under tocilizumab treatment. J Cardiol (2021) 77(5):539–44. doi: 10.1016/j.jjcc.2020.12.011
27. Mekinian A, Biard L, Dagna L, Novikov P, Salvarani C, Espita O, et al. Efficacy and safety of TNF-alpha antagonists and tocilizumab in takayasu arteritis: Multicenter retrospective study of 209 patients. Rheumatology (Oxford) (2022) 61(4):1376–84. doi: 10.1093/rheumatology/keab635
28. Prieto-Peña D, Bernabeu P, Vela P, Narváez J, Fernández-López JC, Freire-González M, et al. Tocilizumab in refractory Caucasian takayasu's arteritis: A multicenter study of 54 patients and literature review. Ther Adv Musculoskelet Dis (2021) 13:1759720x211020917. doi: 10.1177/1759720X211020917
29. Ishii K, Shirai T, Kakuta Y, Machiyama T, Sato H, Ishii T, et al. Development of severe colitis in takayasu arteritis treated with tocilizumab. Clin Rheumatol (2022) 21:21. doi: 10.1007/s10067-022-06108-z
30. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane (2022). Available at: www.training.cochrane.org/handbook.
31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Bmj (2009) 339:b2535. doi: 10.1136/bmj.b2535
32. Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol (2016) 69:199–207.e192. doi: 10.1016/j.jclinepi.2015.07.010
33. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
34. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
35. Nyaga VN, Arbyn M, Aerts M. Metaprop: A stata command to perform meta-analysis of binomial data. Arch Public Health (2014) 72(1):39. doi: 10.1186/2049-3258-72-39
36. Canas CA, Canas F, Izquierdo JH, Echeverri AF, Mejia M, Bonilla-Abadia F, et al. Efficacy and safety of anti-interleukin 6 receptor monoclonal antibody (tocilizumab) in Colombian patients with takayasu arteritis. JCR: J Clin Rheumatol (2014) 20(3):125–9. doi: 10.1097/RHU.0000000000000098
37. Goel R, Danda D, Kumar S, Joseph G. Rapid control of disease activity by tocilizumab in 10 'difficult-to-treat' cases of takayasu arteritis. Int J Rheumat Diseases (2013) 16(6):754–61. doi: 10.1111/1756-185X.12220
38. Kong X, Zhang X, Lv P, Cui X, Ma L, Chen H, et al. Treatment of takayasu arteritis with the IL-6R antibody tocilizumab vs. cyclophosphamide. Int J Cardiol (2018) 266:222–8. doi: 10.1016/j.ijcard.2017.12.066
39. Mekinian A, Resche-Rigon M, Comarmond C, Soriano A, Constans J, Alric L, et al. Efficacy of tocilizumab in takayasu arteritis: Multicenter retrospective study of 46 patients. J Autoimmun (2018) 91:55–60. doi: 10.1016/j.jaut.2018.04.002
40. Tombetti E, Franchini S, Papa M, Sabbadini MG, Baldissera E. Treatment of refractory takayasu arteritis with tocilizumab: 7 Italian patients from a single referral center. J Rheumatol (2013) 40(12):2047–51. doi: 10.3899/jrheum.130536
41. Zhou J, Chen Z, Li J, Yang Y, Zhao J, Chen H, et al. The efficacy of tocilizumab for the treatment of Chinese takayasu's arteritis. Clin Exp Rheumatol (2017) 35 Suppl 103(1):171–5.
42. Buttgereit F. Views on glucocorticoid therapy in rheumatology: The age of convergence. Nat Rev Rheumatol (2020) 16(4):239–46. doi: 10.1038/s41584-020-0370-z
43. Miloslavsky EM, Naden RP, Bijlsma JW, Brogan PA, Brown ES, Brunetta P, et al. Development of a glucocorticoid toxicity index (GTI) using multicriteria decision analysis. Ann Rheum Dis (2017) 76(3):543–6. doi: 10.1136/annrheumdis-2016-210002
44. Misra DP, Rathore U, Patro P, Agarwal V, Sharma A. Disease-modifying anti-rheumatic drugs for the management of takayasu arteritis-a systematic review and meta-analysis. Clin Rheumatol (2021) 40(11):4391–416. doi: 10.1007/s10067-021-05743-2
45. Shuai ZQ, Zhang CX, Shuai ZW, Ge SL. Efficacy and safety of biological agents in the treatment of patients with takayasu arteritis: A systematic review and meta-analysis. Eur Rev Med Pharmacol Sci (2021) 25(1):250–62. doi: 10.26355/eurrev_202101_24391
46. Singh A, Danda D, Hussain S, Najmi AK, Mathew A, Goel R, et al. Efficacy and safety of tocilizumab in treatment of takayasu arteritis: A systematic review of randomized controlled trials. Modern Rheumatol (2021) 31(1):197–204. doi: 10.1080/14397595.2020.1724671
47. Nakaoka Y, Isobe M, Tanaka Y, Ishii T, Ooka S, Niiro H, et al. Long-term efficacy and safety of tocilizumab in refractory takayasu arteritis: Final results of the randomized controlled phase 3 TAKT study. Rheumatology. (2020) 59(9):2427–34. doi: 10.1093/rheumatology/kez630
48. Khanna D, Lin CJF, Furst DE, Goldin J, Kim G, Kuwana M, et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med (2020) 8(10):963–74. doi: 10.1016/S2213-2600(20)30318-0
49. Humby F, Durez P, Buch MH, Lewis MJ, Rizvi H, Rivellese F, et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet. (2021) 397(10271):305–17. doi: 10.1016/S0140-6736(20)32341-2
50. Devauchelle-Pensec V, Carvajal-Alegria G, Dernis E, Richez C, Truchetet ME, Wendling D, et al. Effect of tocilizumab on disease activity in patients with active polymyalgia rheumatica receiving glucocorticoid therapy: A randomized clinical trial. Jama. (2022) 328(11):1053–62. doi: 10.1001/jama.2022.15459
51. Bardi M, Diamantopoulos AP. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice summary. Radiol Med (2019) 124(10):965–72. doi: 10.1007/s11547-019-01058-0
52. Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O, et al. Management of large-vessel vasculitis with FDG-PET: A systematic literature review and meta-analysis. Medicine (2015) 94(14):e622. doi: 10.1097/MD.0000000000000622
53. Yoshida S, Suzuki E, Matsumoto H, Yokose K, Fujita Y, Jumpei T, et al. Effectiveness of combination tocilizumab and glucocorticoids as an induction therapy in patients with takayasu arteritis: An observational study. Modern Rheumatol (2022) 19:19. doi: 10.1093/mr/roac033
Keywords: tocilizumab, Takayasu arteritis, refractory, baseline characteristic, clinical outcome
Citation: Kang L, Liu Y, Luo Z, Zhou Y, Chen B, Yin G and Xie Q (2023) Systematic review and meta-analysis of the current literature on tocilizumab in patients with refractory Takayasu arteritis. Front. Immunol. 14:1084558. doi: 10.3389/fimmu.2023.1084558
Received: 30 October 2022; Accepted: 23 January 2023;
Published: 08 February 2023.
Edited by:
Pier Paolo Sainaghi, University of Eastern Piedmont, ItalyReviewed by:
Simone Parisi, University Hospital of the City of Health and Science of Turin, ItalyCopyright © 2023 Kang, Liu, Luo, Zhou, Chen, Yin and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qibing Xie, eGllcWliaW5nMTk3MUAxNjMuY29t; Geng Yin, eWluZ2VuZzE5NzVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.