
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 27 April 2023
Sec. Immunological Tolerance and Regulation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1083755
This article is part of the Research Topic Precision Medicine in Allergy Diagnosis and Treatment View all 4 articles
 Yi Liu1,2†
Yi Liu1,2† Lan Zhao3,4†
Lan Zhao3,4† Jiaofeng Wang2†
Jiaofeng Wang2† Yinshi Guo5†
Yinshi Guo5† Yifei Wang2
Yifei Wang2 Lishan Zhang1
Lishan Zhang1 Zhoujie Wu2
Zhoujie Wu2 Mingzhi Zhu2
Mingzhi Zhu2 Xukai Yang2
Xukai Yang2 Puyang Xu2
Puyang Xu2 Shandong Wu2*
Shandong Wu2* Zhongshan Gao3,4*
Zhongshan Gao3,4* Jin-Lyu Sun1*
Jin-Lyu Sun1*Background: House dust mite (HDM) is the most common airborne source causing complex allergy symptoms. There are geographic differences in the allergen molecule sensitization profiles. Serological testing with allergen components may provide more clues for diagnosis and clinical management.
Objective: This study aims to investigate the sensitization profile of eight HDM allergen components in a large number of patients enrolled in the clinic and to analyze the relation of gender, age, and clinical symptoms in North China.
Methods: The 548 serum samples of HDM-allergic patients (ImmunoCAP® d1 or d2 IgE ≥0.35) were collected in Beijing City and divided in four different age groups and three allergic symptoms. The specific IgE of HDM allergenic components, Der p 1/Der f 1, Der p 2/Der f 2, Der p 7, Der p 10, Der p 21, and Der p 23, was measured using the micro-arrayed allergen test kit developed by Hangzhou Zheda Dixun Biological Gene Engineering Co., Ltd. The new system was validated by comparing to single-component Der p 1, Der p 2, and Der p 23 tests by ImmunoCAP in 39 sera. The epidemiological study of these IgE profiles and the relation to age and clinical phenotypes were analyzed.
Results: A greater proportion of male patients was in the younger age groups, while more female patients were in the adult groups. Both the sIgE levels and the positive rates (approximately 60%) against Der p 1/Der f 1 and Der p 2/Der f 2 were higher than for the Der p 7, Der p 10, and Der p 21 components (below 25%). The Der f 1 and Der p 2 positive rates were higher in 2–12-year-old children. The Der p 2 and Der f 2 IgE levels and positive rates were higher in the allergic rhinitis group. The positive rates of Der p 10 increased significantly with age. Der p 21 is relevant in allergic dermatitis symptom, while Der p 23 contributes to asthma development.
Conclusion: HDM groups 1 and 2 were the major sensitizing allergens, with group 2 being the most important component relevant to respiratory symptoms in North China. The Der p 10 sensitization tends to increase with age. Der p 21 and Der p 23 might be associated with the development of allergic skin disease and asthma, respectively. Multiple allergen sensitizations increased the risk of allergic asthma.
An allergic disease seriously affects the quality of life of patients. High medical costs and social and economic impact have made it a major global public health problem, especially in fast-developing countries like China (1, 2). House dust mite (HDM), the most common source of indoor allergens, induces a variety of allergic diseases, including allergic rhinitis, conjunctivitis, allergic asthma, and atopic dermatitis. HDM is the major cause of allergy in warm and humid areas. The dominant species of HDMs in most areas are Dermatophagoides pteronyssinus and Dermatophagoides farinae, with different proportions depending on the geographic location (3).
More than 30 HDM molecular allergens have been identified from D. pteronyssinus and D. farinae, of which groups 1, 2, 5, 7, 21, and 23 are clinically the most relevant according to the frequency of IgE sensitization and the ability to induce allergic reactions (4, 5). Der p 10 is evolutionarily conserved: it cross-reacts among organisms such as shellfish and parasites (6, 7) and is known to be an important inducer of severe systemic anaphylaxis (8). Significant variations reported in the incidence of IgE-mediated allergy, triggered by major and relevant minor mite allergens, may be caused by differences in geographic areas (9), age, and clinical phenotypes of the study populations (10). There are relatively few clinical studies of HDM components related to China, the biggest developing country with a substantial population and vast territory (11–15). The sensitization profiles for HDM component allergens differ considerably among distinct geographic regions and allergic diseases (12). In China, especially South China, Der p 1 and group 2 (Der p 2/Der f 2) have been shown to be the dominant allergens in patients with HDM allergy (12, 15), with the relative importance of Der p 2 being higher (15). South China has also been found to have the highest sensitization and levels of HDM allergens (12). Allergic rhinitis (AR) patients showed more frequent sensitization to Der p 1 and Der p 2 than asymptomatic subjects sensitized to HDM (14).
Nowadays, the immunotherapy of HDM-allergic diseases still relies on the preparation of allergen extracts. D. pteronyssinus and D. farinae extracts have also been used for allergen immunotherapy (AIT) treatment generally (16). However, there is a problem of large individual differences in the effects of treatment because the sensitization profiles of main allergen molecules and variable allergen extracts contain a variety of true allergenic and non-allergenic components (17). With the difficulty in standardizing raw materials and processing methods, allergen extracts have been shown to vary in allergen content, which can affect the accuracy of the test results. In recent years, the research of allergens has gradually been refined and developed at the molecular level, and now component-resolved diagnostics (CRD), proposed by Rudolf Valenta et al. (17), can identify the specific molecular allergen (18). This approach can help explain the potential molecular basis of cross-reactions (11), evaluate the risk of allergic reactions, identify the main molecules responsible for symptoms, and identify patients suitable for specific immunotherapy (10, 19). According to recent publications on mite CRD, Der p 1, Der p 2, Der p 7, Der p 10, Der p 21, Der p 23, Der f 1, and Der f 2 are the most relevant allergens in HDM-allergic patients (20–25).
In China, multiple allergens have been used in clinical studies on Artemisia pollen allergy (26) by ImmunoCAP and milk allergy with the microarrayed allergen test kit developed by Dixun Company in China (27), but there is a lack of comprehensive research with major and minor mite allergen components.
In our study, 548 HDM-allergic patients were diagnosed with three main symptoms—allergic asthma (asthma), AR, allergic skin disease (AD)—and their serum samples were analyzed with a panel of specific IgE to Der p 1, Der p 2, Der p 7, Der p 10, Der p 21, Der p 23, Der f 1, and Der f 2. The possible associations of the symptoms with age, gender, and clinical phenotypes were evaluated to obtain valuable information to assist treatment and scientific research.
A total of 548 serum samples from patients with confirmed dust mite allergy, identified by professional allergy physicians, were collected at Peking Union Medical College Hospital from May to July 2019. The patients’ clinical information on age, gender, and allergic symptoms were collected. The basic information and clinical profiles of the participants in this study are shown in Table 1. These patients, from Beijing City and neighboring cities and provinces, had typical symptoms of allergic asthma (asthma), AR, and AD. The sIgE levels against D. pteronyssinus extract (D1) and D. farinae extract (D2) were measured using ImmunoCAP® (Phadia, Sweden) (28), and all 548 samples were found positive. No pollen allergenic sources were involved.
The allergic symptom evaluation was based on questionnaires, clinical observations, and tests. We defined three categories: AR in the nose and upper respirational tract; allergic asthma (AS) as a history of dyspnea, wheezing, and/or coughing episodes; and allergic skin disease including urticaria and dermatitis. The protocol of this study had the approval of the Ethics Committee of Peking Union Medical College Hospital (JS-3303). Written informed consent was obtained from all participants.
An HDM allergen component sIgE system from Hangzhou Zheda Dixun Biological Gene Engineering Co. Ltd. (hereinafter referred to as “HDM component sIgE test system”) was used to detect the sIgE level of serum samples and assess the risk of allergy. The HDM component sIgE test system uses a protein chip technology, and sIgE levels were determined with a DX-Autoblot 50 automatic immunoassay analyzer to reduce the measurement error. For serological testing, allergenic component proteins of D. pteronyssinus and D. farinae were used: Der p 1, Der p 2, Der p 7, Der p 10, Der p 21, and Der p 23 expressed in E. coli. Briefly, the coding sequences of corresponding allergens were cloned using specific primers and inserted into pET28a vector. After transformation into E. coli Rosetta (DE3), recombinant allergens were induced by 0.5 mM isopropyl-beta-D-thiogalactopyranoside at OD600 = 0.6, and cells were grown at 16°C for 12 h. Then, cell pellets were harvested and disrupted by sonication. The soluble allergens with 6×His-tag were purified using Ni-NTA agarose (Sangon Biotech, China) following the manufacturer’s instruction, and Der f 1 and Der f 2 were purified from a Pichia pastoris culture by multi-step chromatography. In total, 250 μl of undiluted serum was applied to chips wetted using a washing buffer, diluted to working concentration, and incubated for 45 min. The secondary antibody was applied and incubated for 45 min after washing five times; then, the enzyme solution was added and incubated for 20 min after washing five times. Finally, the substrate was applied, and the chips were incubated for 20 min and washed five times. All steps were carried out using the DX-Autoblot 50 automatic immunoassay analyzer at 22°C. The built-in software (version 1.0) exported test results, and the serum samples with an antibody content of more than 0.35 IU/ml were considered positive according to a typical standard curve for sIgE determination (29). A previous research using this test kit has been published (12–14). The diagnostic performance of the HDM component sIgE test system was compared with three single components using ImmunoCAP for 39 patients’ sera. The agreement of positive and negative rates was 93%–100% in Der p 1, Der p 2, and Der p 23, and a significant linear correlation of sIgE values between the two assays was obtained (Figure 1).
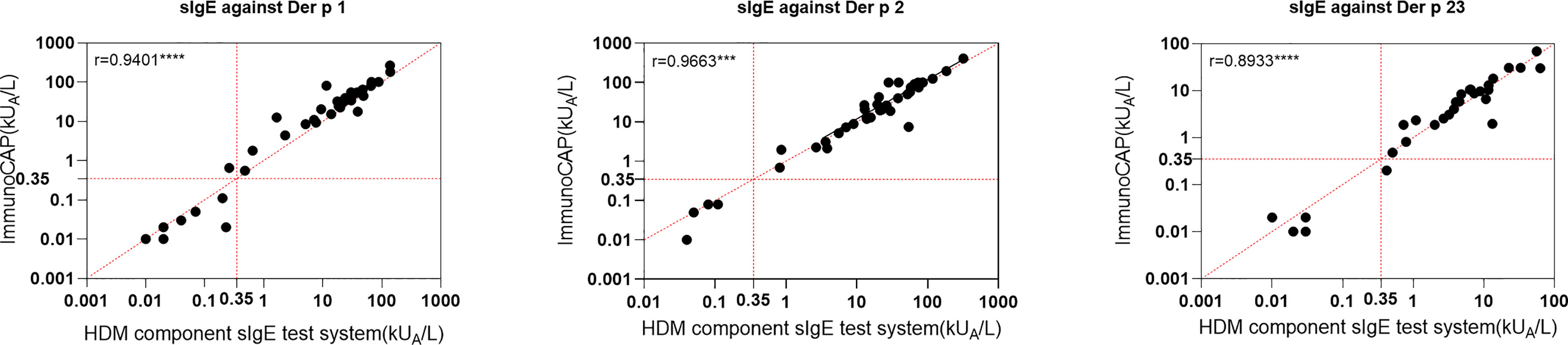
Figure 1 Linear regression between house dust mite component sIgE test system in this study and ImmunoCAP for three components. ***p < 0.001; ****p < 0.0001.
Statistics Package for Social Science (SPSS version R27.0.0.0), an IBM statistical analysis software, and GraphPad Prism software (version 8.2.0, Fay Avenue, CA, USA) were used for statistical data analysis. The results were visualized with GraphPad Prism software.
Pearson chi-square or the Kruskal–Wallis test with 95% confidence intervals were used to compare two or more groups based on positive rates or sIgE levels. The Spearman correlation coefficient was used to assess the correlation between two groups, with a p-value less than 0.05 considered statistically significant.
Of the 548 HDM-allergic patients (Table 1), the average age was 27 years (range, 2–67 years), and 46.9% were male subjects. The majority had allergic rhinitis (79.6%), followed by allergic asthma (40.1%) and allergic skin disease (18.1%) (Table 1; Figure 2A). A component-resolved diagnosis demonstrated that Der p 1 (61.5%)/Der p 2 (52.4%) and Der f 1 (62.6%)/Der f 2 (69.5%) were the most frequently recognized allergen components in these patients from Northern China. Der p 10 (23.7%), Der p 7 (19.3%), Der p 21 (16.2%), and Der p 23 (16.6%) had a low positivity (Figure 2; Table 2).
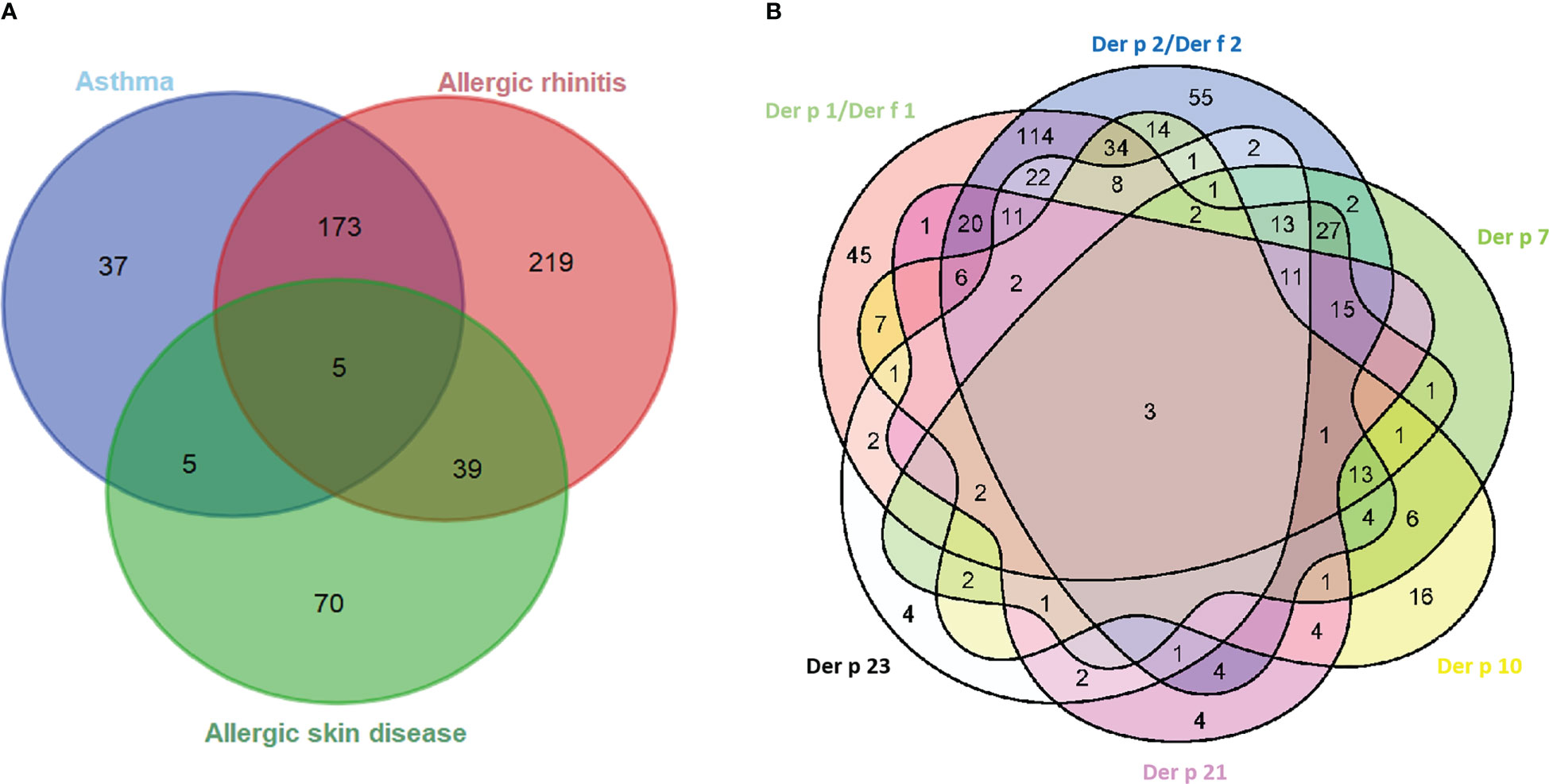
Figure 2 Venn diagrams of the interrelation of allergic symptom groups (A) and sensitized allergens (B). Der p 1/Der f 1 and Der p 2/Der f 2 groups mean one or both of the components are positive.

Table 2 House dust mite component allergen sIgE-positive numbers and constituent ratio of three symptom groups.
There were statistically significantly high sIgE levels of Der p 1, Der f 1, Der p 2, and Der f 2 compared with other components (Figure 3, p < 0.0001). The sIgE levels against Der p 1 (p < 0.01), Der f 1 (p < 0.0001), Der p 2 (p < 0.0001), and Der f 2 (p < 0.0001) were significantly different between age groups (Table 1). However, the incidence of allergic clinical symptoms did not appear to be affected by age (Table 1).
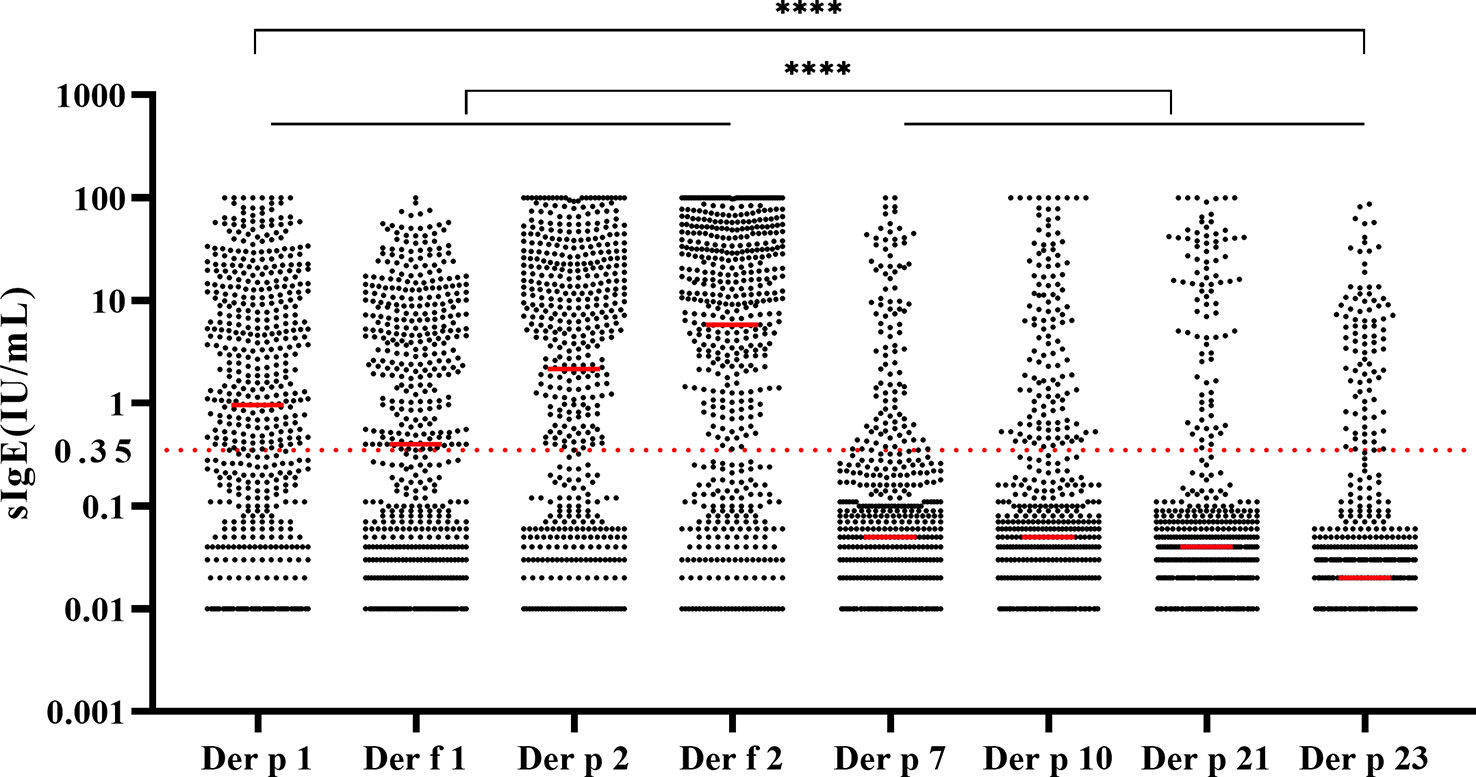
Figure 3 Levels and distribution of house dust mite component-specific IgE in 548 samples. All the groups were compared by Kruskal–Wallis test. ****p < 0.0001.
The relationships between the IgE levels of Der p 1 and Der f 1 were linear, as was that between Der p 2 and Der f 2 (Figure 4). We integrated the positive data from Der p 1 and Der f 1 as group 1 allergen (Der p 1/Der f 1) and from Der p 2 and Der f 2 as group 2 allergen (Der p 2/Der f 2) because of the similar biochemistry, and we investigated co-sensitization among the six HDM allergen groups of Der p 1/Der f 1, Der p 2/Der f 2, Der p 7, Der p 10, Der p 21, and Der p 23 (Figure 2B). This revealed that the highest co-sensitization level was in group 1 and group 2, with co-sensitization in a total of 302 patients (62.1%). Der p 7 was demonstrated and co-sensitive with other components, without any mono-sensitivity. Der p 10 was the third highest mono-sensitizer—3.3% of the subjects were responsive to Der p 10 alone, lower than group 2 (11.3%) and group 1 (9.3%). Der p 21 and Der p 23 had more co-sensitivity with group 1 and group 2 allergens.
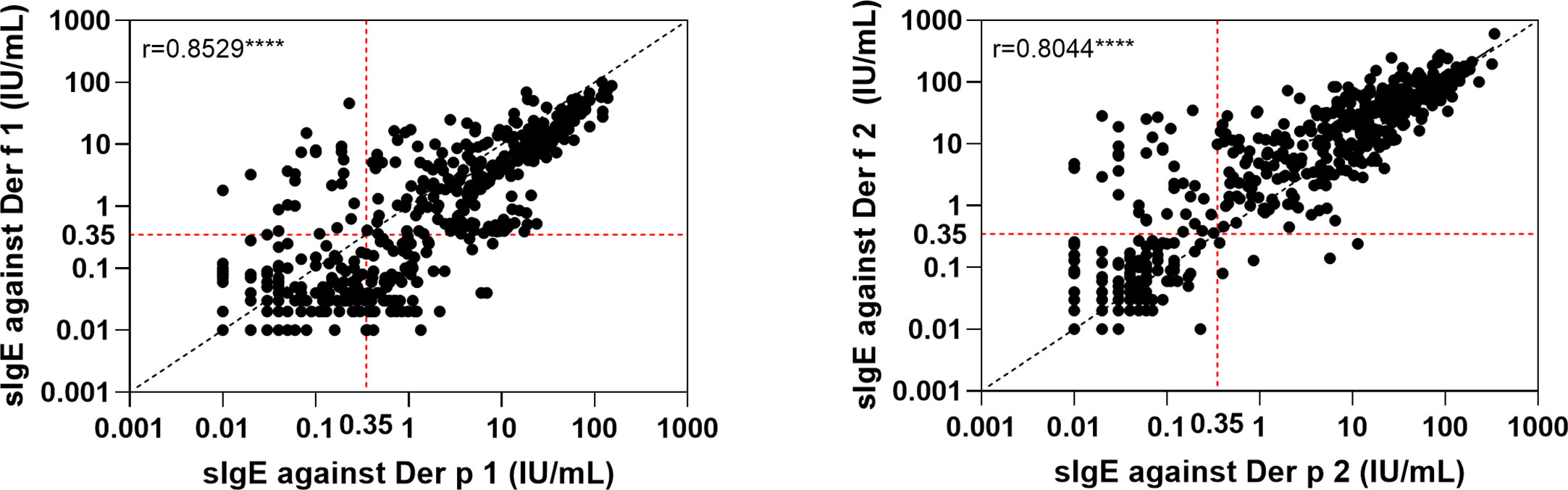
Figure 4 Linear regression of the two major components from two house dust mite species. ****p < 0.0001.
In our study, the positive value in female patients was slightly higher than that in males for most HDM component sIgEs, except for Der f 2 and Der p 10, but none of the disparities was statistically significant (p > 0.05). Analyzing the HDM component sIgE positive rates in different age groups, we found that children (2–12 years old), adolescents (13–18 years old), and the middle adult (46+ years old) had higher positive rates than the young adult age group (19–45 years) (Figure 5). Significant differences in positive rates of Der f 1 (p < 0.001), Der p 2 (p < 0.01), and Der p 10 (p < 0.05) were found between age groups. In addition, there was an increasing trend of a positive value of Der p 10 with age, while, in contrast, both Der f 1 and Der p 2 decreased with age at first and then rebounded after 46 years of age (Table 1; Figure 5).
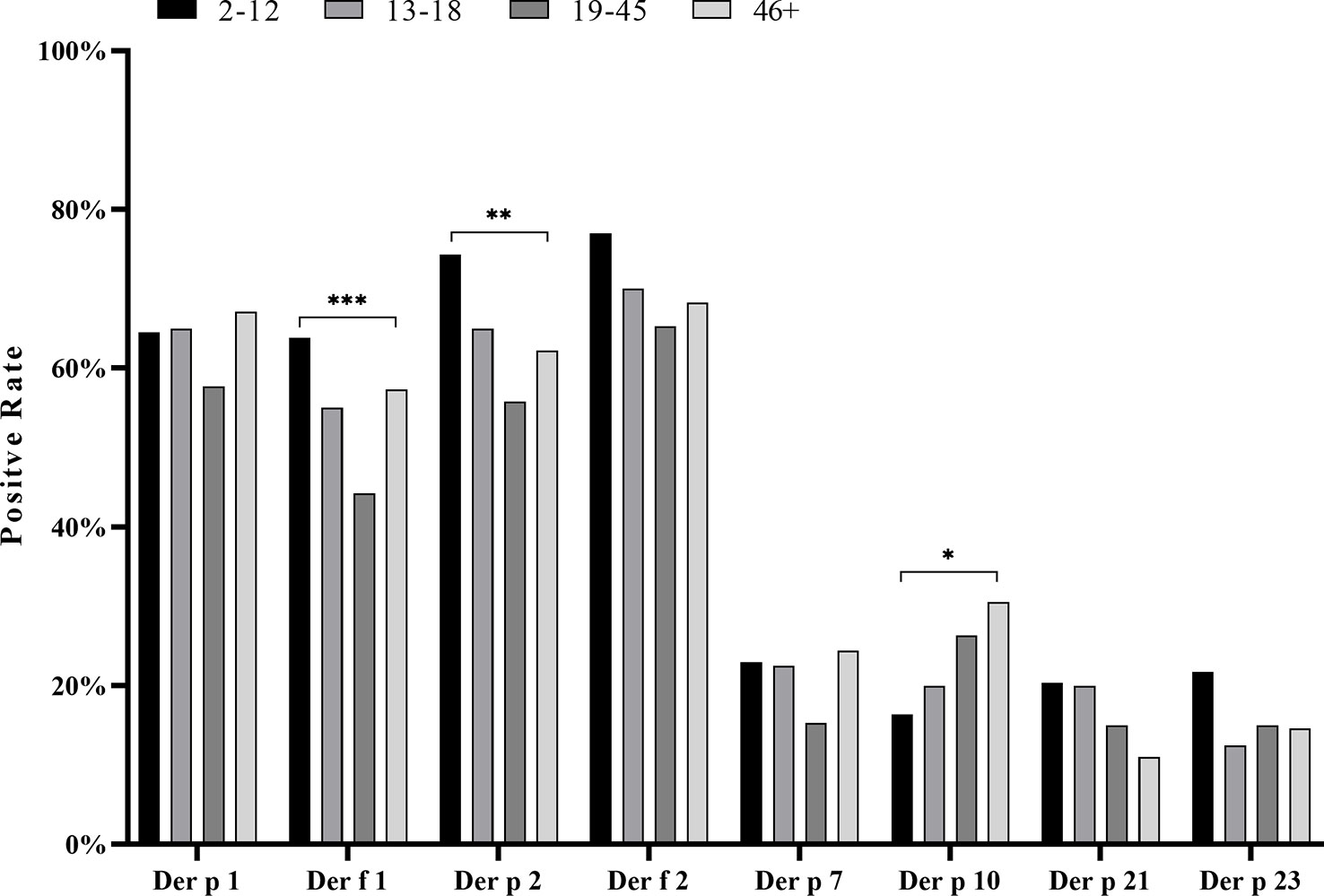
Figure 5 Positive rate of house dust mite component sIgE within four different age groups. Compared by chi-square test, the positive rate of each whole age group was analyzed. *p < 0.05; **p < 0.01; ***p < 0.001.
In the relationship between the complexity of allergic symptoms and the allergenic components of HDM, we observed a strong sensitization of Der p 1, Der f 1, Der p 2, and Der f 2 among patients with a single symptom to multiple symptoms compared with other components (Figure 6A). The positive rate of the sIgE of most components was not significantly different between simple symptomatic and multi-symptomatic patients, except with Der p 23 (p < 0.05). The number of triple symptoms was five (Figure 2A), and this did not conform to statistical principles.
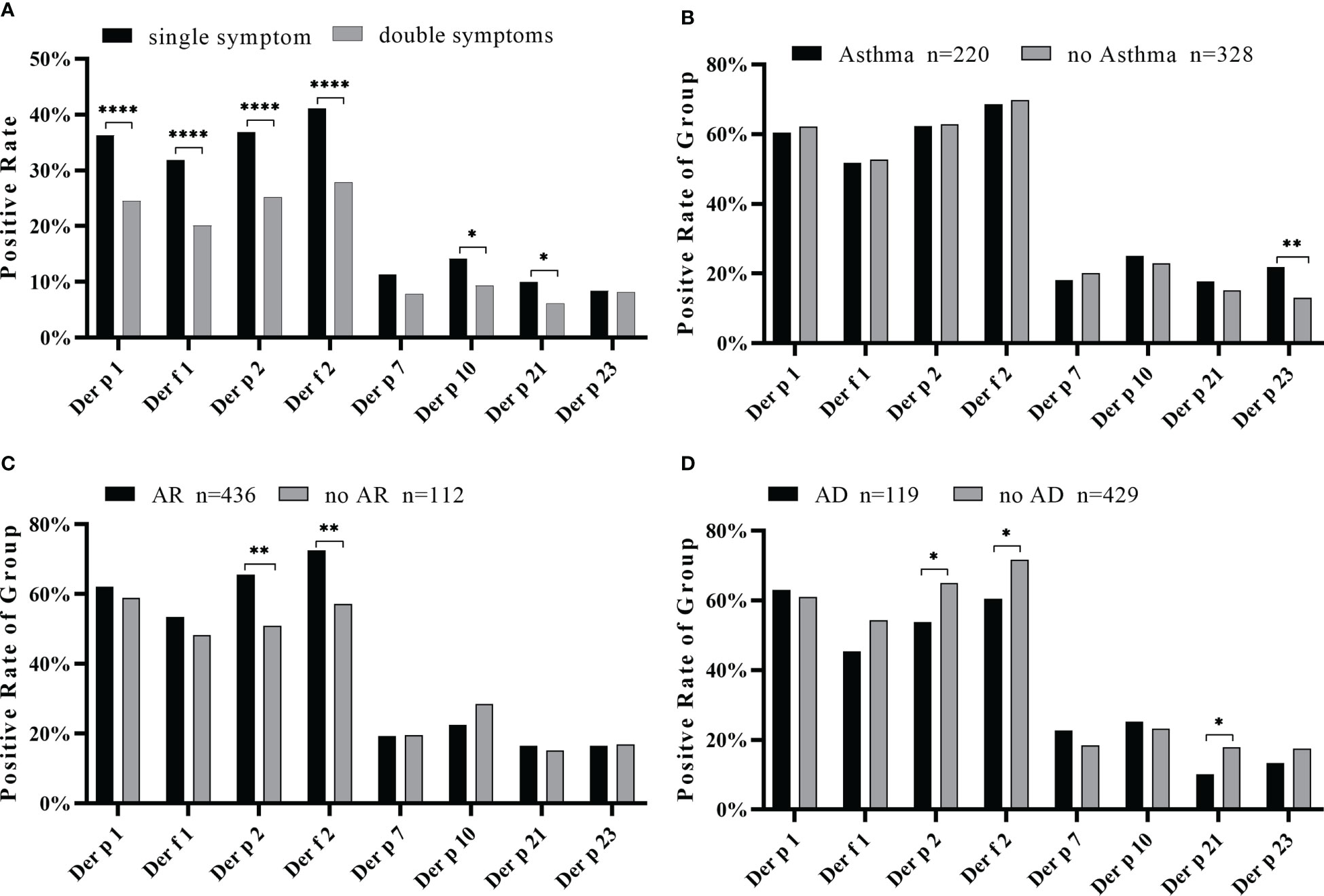
Figure 6 Positive rate of each component in simple or complex symptoms. (A) Positive rates of house dust mite component sIgE in one or two symptoms. The positive rate of component sIgE in the asthma group (B), allergic rhinitis group (C), and allergic skin disease group (D). Compared by chi-square test, all data of positive rate of each group were analyzed. *p < 0.05; **p < 0.01; ****p < 0.0001.
An analysis of the relationship between the symptoms and the positive frequency of HDM components revealed that Der p 23 was significantly higher in asthmatics (p < 0.01) (Figure 6B). Der p 2 and Der f 2 were significantly higher in patients with AR (Figure 6C) while lower in those with AD (Figure 6D). Moreover, the IgE levels of Der p 2 and Der f 2 were generally high in the AS and AR groups (Table 2). As for the impact of the number of positive components, there was an increasing trend in the frequency of asthma in line with the more sensitized allergens, while there was no such trend for allergic rhinitis and allergic skin disease (Figure 7).
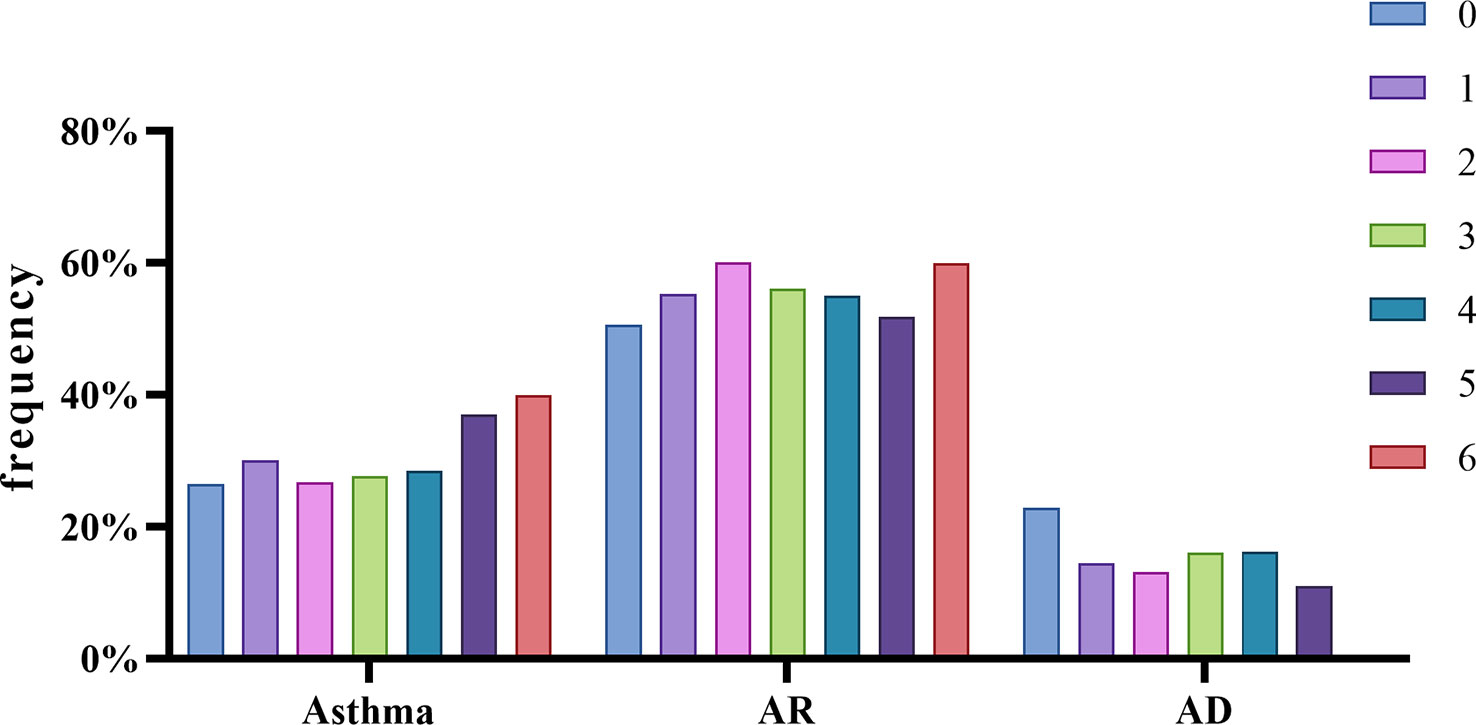
Figure 7 Different allergic phenotypes related to the number of sensitizing allergen component. Compared by chi-square test, all data of positive rate of each whole phenotype group were analyzed.
House dust mites are important allergen sources globally and can be seen almost everywhere in our lives: dust, curtains, bedding, carpets, sofas, towels, and even our food. Therefore, it is harder to avoid or eradicate than food and plant allergens. HDM causes allergic reactions such as allergic rhinitis, asthma, atopic dermatitis, and even severe anaphylaxis, which seriously affect the quality of life (30, 31). The current clinical diagnosis reliant on HDM extract can more accurately screen outpatients with HDM allergy, but it cannot provide more detailed and effective information for subsequent treatment. Nowadays, immune preparations using the allergen component protein of HDM have a more accurate treatment and better effect (32). Appropriate HDM component-specific antibody detection can clarify the molecular allergy map and help develop HDM component-targeted immune preparations. Considering that over 30 groups of HDM allergens have been identified, the six groups that we selected may not be sufficient, with 8% of the subjects not positive to any of the six group allergens tested. To improve the HDM molecular allergen test, more candidate allergens should be included for trial.
The subjects collected in our study were mainly from Northern China, where the prevalence of sIgE against Der p 1and Der p 2 was the lowest at approximately 40% (12). As shown in Table 2, the positive rates of Der p 1 and Der p 2 sIgE were 52.4% to 61.5% in the three symptom groups, similar to the 50%–65% positive rate published elsewhere (33) but lower than the data of Southern China (12, 15), possibly a regional feature related to temperature and humidity. The results showed that Der p 1, Der p 2, Der f 1, and Der f 2 in the sample had higher sIgE levels than the other components (Table 1), which might be determined by the characteristics of the component protein in content and sensitizing capacity (34).
The allergic characteristics of the HDM components in the three symptom groups are given in Table 2 and Figure 2. A high overlap of Der p 1-, Der f 1-, Der p 2-, and Der f 2-positive subjects indicated that a considerable number of samples were positive because of these proteins being highly homologous as well as highly allergenic (35, 36). In the database of WHO/IUIS (http://www.allergen.org/viewallergen.php?aid=289; http://www.allergen.org/viewallergen.php?aid=274) and NCBI, Der p 1 and Der f 1 are both HDM cysteine proteases with 83% sequence homology and similar molecular weights, 24 and 27 kDa, respectively, with sensitization rates as high as 92% and 87%. Both Der p 2 and Der f 2 were 15-kDa NPC2 family protein with 87% sequence homology and sensitization rates of 71% and 90%, respectively. In addition, we also found that some samples were positive for an HDM component but negative for its highly homologous component. The reason may be amino acid sequence polymorphism (37), indicating that the allergic response to HDM is heterogeneous and diverse.
Der p 10 is a kind of tropomyosin, which has some homology with invertebrate tropomyosin (38). The positive rate of Der p 10 was low as shown in WHO/IUIS database (http://www.allergen.org/viewallergen.php?aid=290) but varied in different regions (5%–18%) (38). As a recognized cross-reactive HDM component protein, Der p 10 has been shown to have homology with the tropomyosin of shrimp and crab as a cross-reactive allergen (38). Generally, adults tend to have a more complex diet and consumption of more relevant seafood, so the increasing positivity in Der p 10 with age might be associated with this cross-reactivity. Patients sensitized to Der p 10 should also underwent the sIgE measurement of tropomyosin allergen in the relevant seafood, such as shrimp and crab, to check the possible food allergy, and then their diet is changed accordingly for safety. Der p 10 had a relatively higher mono-sensitization and seemed to play a role in the development of some symptoms in our study population.
The HDM allergic reaction is a complex physiological process involving the respiratory tract, mucous membrane, skin, and other tissues (39, 40). The pathogenesis of allergic diseases caused by distinctive allergens often involves IgE-mediated allergic reactions such as allergic rhinitis, allergic dermatitis, and allergic asthma (41).We attempted to explore the correlation between allergic symptoms and specific IgE antibodies of HDM components. The number of positive components were counted in each sample and grouped according to symptoms, and the result showed that the greater the number of positive components, the greater the probability of asthma (Figure 7). A significantly high positive rate of Der p 23 in the asthma group was observed (Figure 6B). Clinically related to asthma, Der p 23 sensitization had a high prevalence in HDM-sensitive people and was discovered in recent years (9, 22, 42). Walsemann and colleagues have shown that Der p 21 sIgE can affect the development of AD (43). In our study, the Der p 21 sIgE positive rate in the non-AD group was higher than in the AD group (Figure 6D), with the same trend for Der p 2 and Der f 2, indicating that Der p 21, Der p 2, and Der f 2 might play a more important role in respiratory allergic disease. In addition, the results of the comparative analysis showed that the positive rates of Der p 2 and Der f 2 increased in the AR group (Figure 6C), suggesting a possible association between AR and these allergens. Our results indicated that the HDM component detection system has distinct potential for helping with clinical diagnosis and enhancing the effects of treatment.
Gender and age are important factors that affect the AIT treatment of dust mite drops (44). However, in our study, the results for sIgE related to symptoms were not affected by gender, similar to Giorgio’s research (45).
In summary, using HDM component sIgE detection, we found the distribution characteristics of component sensitization in age and allergic diseases and the relationship between Der p 23, Der p 2, Der f 2, and Der p 21 and allergic symptoms. This provides more detailed molecular biological evidence for diagnosis and might technically solve the problem of universal treatment regardless of patient heterogeneity (46, 47). More research on the integration of routine CRD diagnosis and personalized treatment still needs to be done.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
YL, LZ, and JW contributed equally to the work. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of China (grant number 2019YFE0106600), “Pioneer” and “Leading Goose” R&D Program of Zhejiang (grant number 2022C03055), and Clinical and Translational Medicine Research Foundation of Chinese Academy of Medical Sciences (grant number 2020-I2M-C&T-B-001).
Authors YL, JW, YW, ZW, MZ, XY, PX, and SW were employed by the company Hangzhou Zheda Dixun Biological Gene Engineering Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet (2019) 394(10196):407–18. doi: 10.1016/S0140-6736(19)31147-X
2. Wang XD, Zheng M, HF L, CS W, Zhang Y, MY Bo, et al. An increased prevalence of self-reported allergic hinitis in major Chinese cities from 2005 to 2011. Allergy (2016) 71(8):1170–80. doi: 10.1111/all.12874
3. Liu XY, Wu J, Wang B, Li M, Ran PX, Liu ZG. Investigation on house dust mite in different geographical regions of China. Chin J Zoonoses (2010) 26(4):310–4. doi: 10.3969/j.issn.1002-2694.2010.04.004
4. Thomas WR. IgE and T-cell responses to house dust mite allergen components. Mol Immunol (2018) 100:120–5. doi: 10.1016/j.molimm.2018.03.016
5. Chen KW, Zieglmayer P, Zieglmayer R, Lemell P, Horak F, Bunu CP, et al. Selection of house dust mite-allergic patients by molecular diagnosis may enhance success of specific immunotherapy. J Allergy Clin Immunol (2019) 143(3):1248–52.e12. doi: 10.1016/j.jaci.2018.10.048
6. Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol (1999) 119(4):247–58. doi: 10.1159/000024201
7. Asero R, Pravettoni V, Scala E, Villalta D. House dust mite-shrimp allergen interrelationships. Curr Allergy Asthma Rep (2020) 20(4):9. doi: 10.1007/s11882-020-0902-2
8. Farioli L, Losappio LM, Giuffrida MG, Pravettoni V, Micarelli G, Nichelatti M, et al. Mite-induced asthma and IgE levels to shrimp, mite, tropomyosin, arginine kinase, and der p 10 are the most relevant risk factors for challenge-proven shrimp allergy. Int Arch Allergy Immunol (2017) 174(3-4):133–43. doi: 10.1159/000481985
9. Muddaluru V, Valenta R, Vrtala S, Schlederer T, Hindley J, Hickey P, et al. Comparison of house dust mite sensitization profiles in allergic adults from Canada, Europe, south Africa and USA. Allergy (2021) 76(7):2177–88. doi: 10.1111/all.14749
10. Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol (2016) 27 Suppl 23:1–250. doi: 10.1111/pai.12563
11. Huang Z, Zou X, Chen H, Liao C, Hu H, Luo W, et al. Identifying potential Co-sensitization and cross-reactivity patterns based on component-resolved diagnosis. Int Arch Allergy Immunol (2020) 181(2):81–93. doi: 10.1159/000504320
12. Gan H, Luo W, Huang ZF, Zhang T, Hou XQ, Chen YW, et al. House dust mite components sensitization profile in China, a multi-centre study. Clin Exp Allergy (2023) 53(2):226–9. doi: 10.1111/cea.14255
13. Yang L, Yang Y, Xu Q, Zhang W, Jiang Q, Li W, et al. Specific IgE and IgG4 profiles of house dust mite components in allergen-specific immunotherapy. Front Immunol (2022) 12:786738. doi: 10.3389/fimmu.2021.786738
14. Xu QX, Jiang Q, Yang L, Li WJ, Huang N, Yang YQ, et al. IgE and IgG4 repertoire in asymptomatic HDM-sensitized and HDM-induced allergic rhinitis patients. Int Arch Allergy Immunol (2021) 182(12):1200–11. doi: 10.1159/000517824
15. Wang HY, Gao ZS, Zhou X, Dai Y, Yao W, Zhang X-F, et al. Evaluation of the role of IgE responses to der p 1 and der p 2 in Chinese house dust mite-allergic patients. Int Arch Allergy Immunol (2015) 167(3):203–10. doi: 10.1159/000438724
16. Yang L, Zhu R. Immunotherapy of house dust mite allergy. Hum Vaccin Immunother (2017) 13(10):2390–6. doi: 10.1080/21645515.2017.1364823
17. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (Crd and crit). Clin Exp Allergy (1999) 29(7):896–904. doi: 10.1046/j.1365-2222.1999.00653.x
18. Cui Y, Wang Q, Jia H. Consideration of methods for identifying mite allergens. Clin Transl Allergy (2018) 8:14. doi: 10.1186/s13601-018-0200-4
19. Conti A, Burastero GJ, Suli C, Banerjee S, Vrtala S, Alessio M, et al. Identification by serological proteome analysis of paramyosin as prominent allergen in dust mite allergy. J Proteomics (2017) 166:19–26. doi: 10.1016/j.jprot.2017.06.024
20. Curin M, Huang HJ, Garmatiuk T, Gutfreund S, Resch-Marat Y, Chen KW, et al. IgE epitopes of the house dust mite allergen der p 7 are mainly discontinuous and conformational. Front Immunol (2021) 12:687294. doi: 10.3389/fimmu.2021.687294
21. Bronnert M, Mancini J, Birnbaum J, Agabriel C, Liabeuf V, Porri F, et al. Component-resolved diagnosis with commercially available d. pteronyssinus der p 1, der p 2 and der p 10: relevant markers for house dust mite allergy. Clin Exp Allergy (2012) 42(9):1406–15). doi: 10.1111/j.1365-2222.2012.04035.x
22. Banerjee S, Weber M, Blatt K, Swoboda I, Focke-Tejkl M, Valent P, et al. Conversion of der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol (2014) 192(10):4867–75. doi: 10.4049/jimmunol.1400064
23. Pang SL, Matta SA, Sio YY, Ng YT, Say YH, Ng CL, et al. Ige-binding residues analysis of the house dust mite allergen der p 23. Sci Rep (2021) 11(1):921. doi: 10.1038/s41598-020-79820-y
24. Weghofer M, Dall'Antonia Y, Grote M, Stöcklinger A, Kneidinger M, Balic N, et al. Characterization of der p 21, a new important allergen derived from the gut of house dust mites. Allergy (2008) 63(6):758–67. doi: 10.1111/j.1398-9995.2008.01647.x
25. Curin M, Garmatiuk T, Resch-Marat Y, Chen KW, Hofer G, Fauland K, et al. Similar localization of conformational ige epitopes on the house dust mite allergens der p 5 and der p 21 despite limited ige cross-reactivity. Allergy (2018) 73(8):1653–61. doi: 10.1111/all.13398
26. Gao ZS, Fu WY, Sun YM, Gao BY, Wang HY, Liu ML, et al. Artemisia pollen allergy in China: component-resolved diagnosis reveals a allergic asthma patients have significant multiple allergen sensitization. Allergy (2019) 74:284–93. doi: 10.1111/all.13597
27. Tang R, Lyu X, Liu Y, Zhu M, Yang X, Wu Z, et al. Four clinical phenotypes of cow’s milk protein allergy based on dairy product specific IgE antibody types in north China. Front Immunol (2022) 13:949629. doi: 10.3389/fimmu.2022.949629
28. van Hage M, Hamsten C, Valenta R. Immunocap assays: pros and cons in allergology. J Allergy Clin Immunol (2017) 140(4):974–7. doi: 10.1016/j.jaci.2017.05.008
29. Lambert C, Sarrat A, Bienvenu F, Brabant S, Nicaise-Roland P, Alyanakian MA, et al. The importance of en iso 15189 accreditation of allergen-specific ige determination for reliable in vitro allergy diagnosis. Allergy (2015) 70(2):180–6. doi: 10.1111/all.12546
30. Huang FL, Liao EC, Yu SJ. House dust mite allergy: its innate immune response and immunotherapy. Immunobiology (2018) 223(3):300–2. doi: 10.1016/j.imbio.2017.10.035
31. Jacquet A. The role of innate immunity activation in house dust mite allergy. Trends Mol Med (2011) 17(10):604–11. doi: 10.1016/j.molmed.2011.05.014
32. Krzych-Fałta E, Furmańczyk K, Piekarska B, Tomaszewska A, Sybilski A, Samoliński BK. Allergies in urban versus countryside settings in Poland as part of the epidemiology of the allergic diseases in Poland (Ecap) study - challenge the early differential diagnosis. Postepy Dermatol Alergol (2016) 33(5):359–68. doi: 10.5114/pdia.2016.61338
33. Hales BJ, Martin AC, Pearce LJ, Laing IA, Hayden CM, Goldblatt J, et al. IgE and igg anti-house dust mite specificities in allergic disease. J Allergy Clin Immunol (2006) 118(2):361–7. doi: 10.1016/j.jaci.2006.04.001
34. Reithofer M, Jahn-Schmid B. Allergens with protease activity from house dust mites. Int J Mol Sci (2017) 18(7):1368. doi: 10.3390/ijms18071368
35. Osinski T, Pomés A, Majorek KA, Glesner J, Offermann LR, Vailes LD, et al. Structural analysis of der p 1-antibody complexes and comparison with complexes of proteins or peptides with monoclonal antibodies. J Immunol (2015) 195(1):307–16. doi: 10.4049/jimmunol.1402199
36. Mueller GA, Benjamin DC, Rule GS. Tertiary structure of the major house dust mite allergen der p 2: sequential and structural homologies. Biochemistry (1998) 37(37):12707–14. doi: 10.1021/bi980578+
37. Jeong KY, Lee IY, Yong TS, Lee JH, Kim EJ, Lee JS, et al. Sequence polymorphisms of der f 1, der p 1, der f 2 and der p 2 from Korean house dust mite isolates. Exp Appl Acarol (2012) 58(1):35–42. doi: 10.1007/s10493-012-9553-x
38. Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, et al. Molecular characterization of der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy (2011) 41(10):1468–77. doi: 10.1111/j.1365-2222.2011.03798.x
39. Rangkakulnuwat P, Sanit S, Lao-Araya M. Anaphylaxis after ingestion of dust mite (Dermatophagoides farinae)-contaminated food: a case report. Trop BioMed (2020) 37(2):318–23.
40. Miller JD. The role of dust mites in allergy. Clin Rev Allergy Immunol (2019) 57(3):312–29. doi: 10.1007/s12016-018-8693-0
41. Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol (2015) 136(1):38–48. doi: 10.1016/j.jaci.2014.10.012.43
42. Martín-López L, López-Matas MA, Calzada D, Benjumeda-Maira A, Martín E, García E, et al. Environmental exposure of der p 23 in household dust samples. Clin Exp Allergy (2021) 51(12):1645–7. doi: 10.1111/cea.14004
43. Walsemann T, Böttger M, Traidl S, Schwager C, Gülsen A, Freimooser S, et al. Specific ige against the house dust mite allergens der p 5, 20 and 21 influences the phenotype and severity of atopic diseases. Allergy (2023) 78(3):731–42. doi: 10.1111/all.15553
44. Borg M, Løkke A, Hilberg O. Geographical and socioeconomic differences in compliance with and access to allergen immunotherapy in Denmark: a nationwide registry-based study - 1998-2016. Respir Med (2021) 178:106332. doi: 10.1016/j.rmed.2021.106332
45. Celi G, Brusca I, Scala E, Villalta D, Pastorello E, Farioli L, et al. House dust mite allergy in Italy-diagnostic and clinical relevance of der p 23 (and of minor allergens): a real-life, multicenter study. Allergy (2019) 74(9):1787–9. doi: 10.1111/all.13776
46. Pfaar O, Alvaro M, Cardona V, Hamelmann E, Mösges R, Kleine-Tebbe J. Clinical trials in allergen immunotherapy: current concepts and future needs. Allergy (2018) 73(9):1775–83. doi: 10.1111/all.13429
Keywords: allergen component, house dust mite allergy, serological analysis, IgE, clinical epidemiology
Citation: Liu Y, Zhao L, Wang J, Guo Y, Wang Y, Zhang L, Wu Z, Zhu M, Yang X, Xu P, Wu S, Gao Z and Sun J-L (2023) Serological analysis of allergic components of house dust mite provides more insight in epidemiological characteristics and clinical symptom development in North China. Front. Immunol. 14:1083755. doi: 10.3389/fimmu.2023.1083755
Received: 17 November 2022; Accepted: 03 April 2023;
Published: 27 April 2023.
Edited by:
Enza D’Auria, Ospedale dei Bambini Vittore Buzzi, ItalyReviewed by:
Leonardo Puerta, University of Cartagena, ColombiaCopyright © 2023 Liu, Zhao, Wang, Guo, Wang, Zhang, Wu, Zhu, Yang, Xu, Wu, Gao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Lyu Sun, c3VuamlubHZAcHVtY2guY24=; Zhongshan Gao, Z2FvemhvbmdzaGFuQHpqdS5lZHUuY24=; Shandong Wu, d3VzaGFuZG9uZ0B6ZGJpb2dlbmUuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.