- Department of Dermatology, Hunan Key Laboratory of Medical Epigenomics, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
Psoriasis is a recurring inflammatory skin condition characterized by scaly, red patches on the skin. It affects approximately 3% of the US population and is associated with histological changes such as epidermal hyperplasia, increased blood vessel proliferation, and infiltration of leukocytes into the skin’s dermis. T cells, which are classified into various subtypes, have been found to play significant roles in immune-mediated diseases, particularly psoriasis. This paper provides a review of the different T lymphocyte subtypes and their functions in psoriasis, as well as an overview of targeted therapies for treating psoriasis.
1 Introduction
Psoriasis is a long-lasting and recurring inflammatory skin condition that affects nearly 3% of the US population (1). Psoriasis is characterized by raised, red patches on the skin covered by silver scales, and the histological features include an increase in the thickness of the skin’s top layer, proliferation of blood vessels in the skin’s dermis, and the presence of inflammatory leukocytes in the dermis (2). Psoriasis is not only a skin condition but is also associated with several comorbid conditions, including psoriatic arthritis (PsA), metabolic syndrome, cardiovascular diseases, and mental illnesses (3). Psoriasis is a dermatosis with multiple contributing factors. Genetics is one of the most significant factors, as almost 40% of individuals with psoriasis have a family history of the condition (4). Environmental factors may cause or aggravate psoriasis, such as stress, trauma, certain medications, infections, smoking and alcohol consumption (5). Originally, the pathogenesis of psoriasis was considered to be related with aberrant epidermal keratinocyte proliferation (6). There is a growing consensus that activated T lymphocytes play a crucial role in the development of psoriasis, which is supported by the successful treatment by cyclosporine. Cyclosporine has proved to be highly effective in the treatment of psoriasis, but its side effects (including renal toxic effects, hypertension, and an increased risk of malignant neoplasm) limit the use of cyclosporine as an acceptable long-term monotherapy for psoriasis (7). As a result, T cells are now considered to be the primary contributors to the pathology of psoriasis (8). And more precise and safe therapies are needed for patients with psoriasis.
Traditionally, T lymphocytes can be broadly classified into two groups: conventional T cells and innate-like T cells. Conventional T cells, such as CD4+ T helper lymphocytes and CD8+ cytotoxic T lymphocytes, recognize peptide antigens presented by major histocompatibility complex (MHC) molecules. In contrast, innate-like T cells, which include natural killer T cells, gamma delta T cells, and mucosal-associated invariant T cells, are involved in rapid immune responses that are not dependent on MHC expression (9). Here, we will review recent advances and roles of T lymphocytes in psoriasis as well as summarize currently targeted anti-psoriatic therapies.
2 Conventional adaptive T lymphocytes in psoriasis
2.1 CD4+ helper T cells in psoriasis
CD4+ helper T cells are so named because they express the CD4 glycoprotein on their surface. These cells become activated when presented with peptide antigens by MHC class II molecules, which are expressed on the surface of antigen-presenting cells and then produce cytokines that regulate or assist the immune system. Research has shown that CD4+ helper T cells are present in the dermal skin of individuals with psoriasis (10). The effects of CD4+ helper T cells in psoriasis can be observed in grafted skin with injected cells from psoriasis patients into graft sites on severe combined immunodeficiency disease (SCID) mice. These alterations in the skin demonstrate the functions of the CD4+ helper T cells in psoriasis (11).
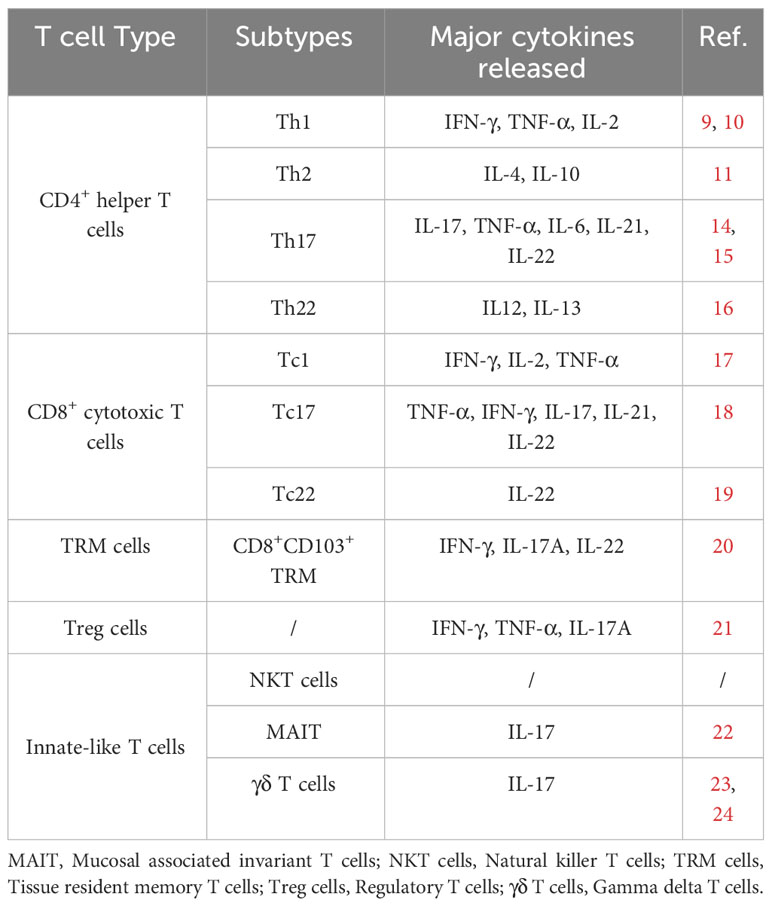
These CD4+ helper T lymphocytes can be classified into Th1, Th2, Th17 and Th22 cells, performing different functions. Th1 cells can increase macrophages and cytotoxic T cells - mediated immune response by releasing interferon-γ (IFN-γ) and TNF-α, which are critical players in the development of psoriasis (12, 13) (Table 1). Studies found that cytokines secreted by T cells associated with psoriasis are almost Th1 related cytokines (IL-2, IFN-γ, and TNF-a), but not Th2 type cytokines (IL-4 and IL-10) (25). The data suggest that psoriasis is primarily influenced by Th1 cells. However, the use of humanized monoclonal antibodies that target IFN-γ for psoriasis therapy was not as successful as expected. This suggests that IFN-γ may have a more complex role in psoriasis than previously thought (26).
T helper 17 cells (Th17 cells) are defined as a group of pro-inflammatory T helper cells secreting IL-17, but not IFN-γ (27). With the stimulus of IL-1 and IL-6, Th17 cells develop from naive CD4+ T cells and maintain on IL-23 produced by keratinocytes, Langerhans cells, Dendritic cells (DCs), and macrophages (14). IL-23 is a cytokine composed of two chains (p19 and p40) that are also shared by IL-12. An increasing amount of evidence suggests that the IL-23/Th17 axis and related cytokines play a significant role in psoriasis. In psoriatic skin, the expression levels of IL-23 are higher than in normal skin. Injecting IL-23 intradermally into murine models results in abnormal epidermal hyperplasia and keratinocyte proliferation (15). Biological agents that target IL-12/-23 and IL-23 have been effective in treating psoriasis patients (28, 29). On the other side, besides secreting IL-17, Th17 cells can also release TNF-α, IL-6, IL-21, and IL-22, participating in the development of psoriasis (30, 31). Clinical randomized trials targeting IL-17A and IL-17F antibodies in psoriasis have proven that these two cytokines can be used for therapeutic targets (16, 32, 33). A newly discovered subtype of T cells named Th22 cells produce IL-22/-13, but not IFN-γ, IL-4 or IL-17 (34). Similar with Th1/17 lymphocytes, the numbers of Th22 cells were upregulated in patients with psoriasis as well (35).
Recently, a newly discovered subtype of CD4+ T lymphocytes was defined as T follicular helper (Tfh) cells, exhibiting different functions compared to other T cell subsets. Tfh cells can express CXCR5, ICOS, PD-1, Bcl-6 and generate IL-21 as well (17). CXCR5 is the surface marker of Tfh cells and critical for Tfh-cell function (36). ICOS is required for Tfh-cell differentiation and PD-1 plays a role in regulating Tfh-cell function (37, 38). Tfh cells can be grouped as two subtypes — conventional and circulating Tfh cells. Conventional Tfh cells mainly contribute to the formation of germinal center, B cells differentiation and antibody production (18). Circulating Tfh cells are grouped as three subtypes by CXCR3 and CCR6, including Tfh 17 (CXCR3-CCR6+), Tfh1 (CXCR3+CCR6-), and Tfh2 (CXCR3-CCR6-) cells. Tfh 17 cells produce the Th17 cytokines (IL-17A and IL-22), while Tfh1 cells produce the Th1 cytokine (IFN-γ) and Tfh2 cells secrete IL-4, IL-5 and IL-13, which are Th2 cytokines (19). Changed in the balance of circulating Tfh cells are found to be related with autoimmune diseases such as systemic lupus erythematosus (SLE) (39), Henoch-Schonlein purpura (HSP) (40), rheumatoid arthritis (RA) (41), ankylosing spondylitis (AS) (42). Tfh cells can also participate in regulating B cell’s activation and function (43). In psoriasis, the components of circulating Tfh cells were also proven to be abnormal and Tfh 17 cell subset was increased and correlated with psoriasis area and severity index (PASI) score (20, 44). In addition, the frequency of Tfh17 cells diminished during the follow-up periods (20).
Generalized pustular psoriasis (GPP) is a subtype of psoriasis that is characterized by the infiltration of neutrophils into the epidermis, resulting in severe symptoms. Haskamp S et al. used single-cell RNA sequencing to analyze the transcriptomes of MPO-deficient patients in a stable disease state. The cell types were identified through multimodal reference mapping of the single-cell RNA sequencing data. The results indicated that the proportion of CD4+ cytotoxic T lymphocytes and other CD4+ effector cells were increased in GPP, while the frequency of naïve CD4+ T cells was significantly lower (45).
2.2 CD8+ cytotoxic T cells in psoriasis
For a long time, psoriasis was regarded as Th1 cell-mediated skin disorder (25). Gradually, growing evidence suggests that the IL-23/Th17 axis and IL-22/Th22 pathway play critical roles in psoriasis (46). In psoriatic skin lesions, CD4+ T cells are concentrated at upper dermis, while CD8+ T cells are mainly found in the epidermis (47). Expect CD4+ T lymphocytes, cytotoxic CD8+ T cells are also able to secret IL-2, IFN-γ, TNF-α, IL-17, and the IL-22 cytokine family, which are consecutively named as Tc1, Tc17, and Tc22 cells (48).
In psoriasis, Tc1 cells release IFN-γ, IL-2, and TNF-α, playing different roles during the development of psoriasis (49). Early in the psoriatic cascade, IFN-γ is capable of activating antigen-presenting cells (APCs) and keratinocytes to generate IL-22 and IL-1β, therefore enhancing cytokine storms in psoriasis (50). TNF-α in psoriasis is able to regulate APCs (51) and stimulate DCs to secret cytokines such as IL-23 (52). Besides, TNF-a can form strong synergies with other cytokines such as IL-17A (50) to amplify the inflammatory cascade and promote proliferation and chemotaxis of T cells to the lesional sites (51).
Through single-cell transcriptomics, researchers discovered two pathogenic cytotoxic type 17 T-cell (Tc17) subsets of CD8+ T cells in the psoriatic skin of 11 psoriasis patients and five healthy control individuals (53). In contrast to Th17 cells, Tc17 cells in psoriatic tissue can release TNF-α, IFN-γ (Th1-related cytokines) and IL-17/-21/-22 (Th17-related cytokines) (54). Moreover, Tc17 cells express CCR6 (the ligand for CCL20), which is necessary for epidermal homing of all CD8+ T cells (54). Except for the secretion of cytokine, CD8+IL-17+ T cells in psoriatic lesions can produce cytotoxic molecules (namely granzyme B) and decrease target cells in a T-cell receptor (TCR)/CD3-dependent way (54). However, the exact mechanism of cytotoxic target cell killing is still unclear.
Tc22 cells are another recently determined CD8+ T cell subtype in psoriasis and are predominately enriched in the psoriatic epidermis (55). Without IL-17 and IFN-γ, Th22 only secrete IL-22 in psoriatic skin (55). And those cells derived from Th17 and Tc17 cells are incapable of expressing IL-17A, and then develop into T-lymphocytes only producing IL-22 (55).
Approximately 30% of individuals with psoriasis may develop PsA, which is characterized by peripheral arthritis, enthesitis, and dactylitis (56). Studies have shown that the expansion of memory CD8+ T cells in the joints of individuals with PsA is significantly higher than in their peripheral blood. Additionally, CD8+ T cells have been observed in the synovial fluid of PsA patients in previous studies (57). The use of single-cell sequencing revealed that there is a greater presence of CD8+T cells in the synovial fluid of PsA patients that express CXCR3, a receptor that aids in tissue homing. Additionally, the ligands of CXCR3 (CXCL9 and CXCL10) were found to be expressed at higher levels, providing a molecular understanding of the cellular immune mechanism involved in PsA (58).
2.3 Tissue resident memory T cells (TRM cells) in psoriasis
TRM cells are defined as long-time survival, memory T cells and are abundant at epithelial and mucosal tissues (skin, mucosa, lung, brain, and gastrointestinal tract), which are different from recirculating central and effector memory T cells in transcriptional and functional aspects (59). CD49, CD69 and CD103 are regarded as the surface hall markers of TRM cells, among which CD69 and CD103 belong to tissue-retention markers (60). Although anti-psoriasis biologic agents inhibiting TNF-a, IL-23/IL-12, IL-17A, and IL-17 receptors have achieved great therapeutic effectiveness compared to traditional treatments, the therapeutic effects are varied between patients and the skin lesions often recur once the biologics will be withdrawn (61). Interestingly, psoriasis plaques are prone to recur at the same anatomical locations (62).
Skin TRM cells are thought to play roles in the pathogenetic process in psoriasis, which acts as a strong factor for lesion recurrence (63). CD8+CD103+ TRM cells release psoriasis-associated cytokines such as IFN-γ, IL-17A, and IL-22, while CD4+CD103+ TRM cells and CD8+CD103- TRM cells don’t secrete these cytokines (64). In addition, CD8+CD103+ TRM cells can be further classified as two subtypes: CD49a-IL-17A+ and CD49a+IFNγ+, assumedly related to psoriasis and vitiligo, respectively (21). Furthermore, skin TRM cells are also related to the clinical changes. For example, CD103+TRM cells can release IL-17A in epidermis of subsided psoriasis and are related to early recurrence (65).
2.4 Regulatory T cells (Treg cells) in psoriasis
Treg cells are T cells that express biomarkers CD4, CD25 and FOXP3, and originated from the same cell lineage as naïve CD4+ T cells (66). Treg cells are immune-suppressive and suppress the induction and proliferation of effector T cells, playing roles in maintaining self-antigens tolerance (67).
Currently, the linkage between Treg frequency and psoriasis severity are ambiguous. Some studies revealed a downregulated trends of Tregs in the peripheral blood cells of psoriatic patients but their correlations with the severity of disease are not in accordance (68–70). However, there wasn’t significantly difference of circulating Treg frequency in other studies (22, 71–73). Compared to healthy skin, many studies have shown that there is an increased frequency of the infiltration of Treg cells into lesional skin (23, 72, 74–76). Furthermore, a higher frequency of Treg cells in dermis compared to epidermis in plaque psoriasis was observed (75) and the opposite results were also reported (23). Compared to non-lesional skin, Foxp3+ Tregs were downregulated in lesional skin of psoriatic patients with acute exacerbation but upregulated in chronic phase (73). Yan et al. reported that compared to normal skin, Foxp3+Tregs were increased in lesional skins of plaque psoriasis but decreased in guttate psoriasis (77).
It is indicated that Treg cells are dysfunctional in most patients with psoriasis. For example, CD4+ CD25high Foxp3+ Treg cells from skin lesions and peripheral blood are unable to inhibit the proliferation of effector T cells (22). Tregs from peripheral blood of psoriatic patients are phosphorylated and responsible for abnormal activation of STAT3 pathway and over-expression of the pro-inflammatory cytokines (e.g. IFN-γ, TNF-α and IL-17A). The phosphorylation of STAT3 and subsequent dysfunction of Tregs are caused by IL-6, IL-21 and IL-23, suggesting the role of pro-inflammatory cytokine milieu in the impaired Treg dysfunction (78).
Besides, abnormal adenosine signaling pathway may weaken the suppressive function of Tregs in psoriasis. Normally, Treg cells express both CD39 and CD73 and use adenosine signaling for immune suppression (24, 79). However, the expression of CD73 expression of Treg cells from psoriatic patients is greatly decreased, and the CD73/AMPK pathway is inactive, therefore impairing the immunosuppressive function of Treg cells (80).
3 Innate-like T cells in psoriasis
3.1 Natural killer T cells (NKT cells)
NKT cells involve the receptor of NK and a TCR with α and β chains. According to the TCR type, NKT cells are sorted into type I and type II (81). The role of NKT cells in psoriasis development is not clearly understood. NKT cells are increased in skin lesions from psoriatic patients (82) and decreased after anti-psoriatic treatment (83). Although the current literature regarding the circulating NKT cells in psoriasis are inconsistent, transplantation of immune cells from patients with psoriasis into SCID mice with human skin xenografts develops psoriasis-like lesions and some of the infiltrating cells express NK receptors (84).
3.2 Mucosal associated invariant T cells (MAIT) in psoriasis
As a subtype of innate-like T cells, MAIT cells have a semi-invariant TCR including Vα7.2 and Jα33, Jα12 or Jα20 and predominately a Vβ13 (TRBV6) and Vβ2 (TRBV20) TCRβ chain (85). MAIT cells are mainly found in the skin of normal people and psoriatic patients (86). Data has shown that MAIT cells are the majority of the IL-17-producing CD8+ T cells in the blood after phorbol myristate acetate + ionomycin stimulation (87). The IL-17+ CD8+ T cells were accumulated in lesional skin of psoriasis and able to release TNFα, IL-17 and IFN-γ, resulting in promoting inflammatory process (54).
3.3 Gamma delta T cells (γδ T cells) in psoriasis
γδ T cells have a γδ TCR on their surface, which was first discovered by using the TCRγ gene sequence (88). Most T cells belong to αβ T cells which contain two glycoprotein chains, named α and β TCR chains. However, γδ T cells are made up of two chains, γ and δ chain, which are mainly distributed in skin, digestive, respiratory, and reproductive tracts (89).
In the skin, nearly 99% of the T cells belong to αβ T cells, while γδ T cells only make up 1% (63). γδ T cells were enriched in both epidermal and dermal skin in both human and mice (90). Humans γδ T cells are grouped by δ chain expression including the Vδ1, Vδ2, and Vδ3 subtypes (91), while murine γδ T cells can be sorted according to γ chain expression named Vγ1–Vγ7 subtypes (92). These γδ T cells in psoriatic skin are also named as skin homing Vγ9 Vδ2 T cells and their distribution varies in peripheral blood and skin tissue (93). Different from epidermal γδ T cells and conventional αβ T cells, derma γδ T cells are known as IL-17-producing γδ T cell and release IL-17 upon IL-23 stimulation in the skin (90, 94). Abnormal T cell activation, especially IFN-γ-producing Th 1 cells, has been known to play a vital part in psoriasis. Besides, IL-23/Th17 axis was confirmed to be impaired in the psoriasis development. In addition, the number of γδT cells in psoriasis is negatively related with the PASI score and neutrophil-lymphocyte ratio which is associated with the risk of cardiovascular event, implying the potential role of γδT cells in the development of psoriasis-related cardiovascular event (93). A Recent study showed that glutamine metabolism is a key factor for the generation of γδ T cells, which could be a possible target for γδ T cell-related disorders, such as psoriasis (95).
4 Targeted anti-psoriasis therapies
Targeted anti-psoriasis therapies namely biological agents have been developed with the recognition of TNF-a, IL-23/Th17 axis and IL-22/Th22 pathway, representing better efficacy as well as relative safety. Besides, there are several newly developed types of small molecule inhibitors (Table 2).
4.1 TNF-a inhibitors
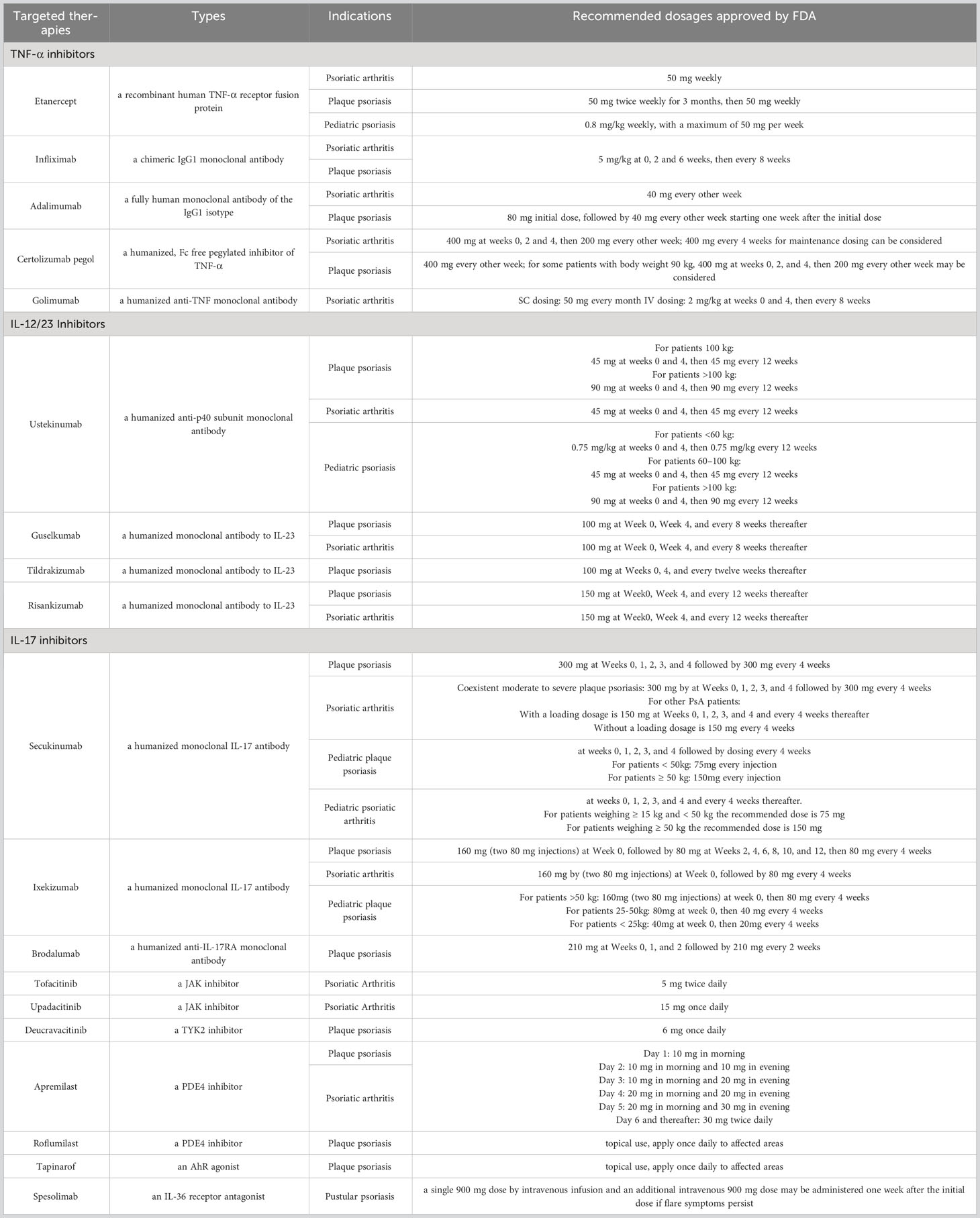
TNF-a is a crucial factor in immune-related conditions such as RA, AS, inflammatory bowel disease (IBD), psoriasis, and hidradenitis suppurativa (HS). It can be produced by various cells, including DCs, T cells, neutrophils, and keratinocytes. TNF-a inhibitors includes two types: circulating receptor fusion protein (such as etanercept) and monoclonal antibodies (e.g. infliximab, adalimumab, certolizumab pegol and golimumab). For psoriasis, these TNF-a inhibitors can be used alone or with methotrexate to decrease the frequency of antidrug antibodies (ADAs) (96). And they are more suitable to treat psoriatic patients together with IBD or PsA. Certolizumab pegol is effective and well tolerated for women of childbearing potential (97). Reactivation of latent tuberculosis or hepatitis, lymphomas, heart failure and lupus are the severe side effects for TNF-a inhibitors (98).
4.2 IL-17 inhibitors
IL-17 is considered as a vital factor during the process of psoriasis because it contributes to the inflammatory response that damages and overturns epidermal keratinocytes (99, 100). Currently, IL-17 inhibitors approved to treat psoriasis include secukinumab, ixekizumab, bimekizumab, and brodalumab. Secukinumab and ixekizumab both act by inhibiting IL-17A, while bimekizumab suppress IL-17A and IL-17F. However, brodalumab selectively binds to the receptor of IL-17. IL-17 inhibitors represent more effective and fast-acting outcomes in patients with psoriasis and have low ADA incidence and low tendency of reactivating tuberculosis. Secukinumab and ixekizumab have also been approved to treat PsA by FDA (101). In a phase 2b trial, significant improvements were achieved by bimekizumab in PsA (102). Since the natural effect of antifungal immunity by IL-17A, long-term use of IL-17 inhibitors may lead to Candida infections (103). Brodalumab was also assumed to be related with suicide, joint pain, and headache (104). Different from secukinumab and ixekizumab only targeting IL-17A, brodalumab inhibits IL-17A, IL-17C, IL-17F, and IL-17E through IL-17RA, representing better efficacy (105).
4.3 IL-12/IL-23 p40 and IL-23 p19 inhibitors
The expression of the p40 subunit and p19 subunit in psoriasis is increased (106). The subunit of p40 is commonly shared by IL-12 and IL-23, while p19 is only owned by IL-23. Ustekinumab is a humanized biological antibody neutralizing the subunit p40 in IL-12 and IL-23, treating Crohn’s disease, ulcerative colitis, psoriasis and PsA (107). The subunit of p35 is another component of IL12 and the expression level the p35 subunit was not upregulated in the skin of patients with psoriasis, implying the direct inhibiting of IL-23 is likely to be more effective. To date, biologic agents targeting the p19 subunit of IL-23 involving guselkumab, tildrakizumab, risankizumab, and mirikizumab are approved for psoriasis treatment (108). Guselkumab is shown to be effective in PsA patients without biotherapy experience or previously treated with TNF-α inhibitors (109, 110). Besides, risankizumab has been proven to have much better effects than adalimumab and ustekinumab (28, 111).
4.4 Small molecule inhibitors
Small molecule inhibitors refer to chemical substances that can attach themselves to enzymes or proteins, thereby hindering their functions. Small molecule inhibitors have a wide range of applications in medicine, including the treatment of psoriasis.
Janus kinase (JAK) inhibitors are compounds that obstruct the intracellular signaling pathway that is facilitated by JAK and STAT proteins. This interference results in the inhibition of the transcription of proinflammatory cytokines (112). The signaling mechanism of the IL-23 receptor depends on a heterodimer of JAK2 and tyrosine kinase (TYK) 2 for transducing signals, which emphasizes the significance of JAKs in the development of psoriasis and the potential of JAK inhibitors for therapeutic purposes (113). Two JAK inhibitors (tofacitinib and upadacitinib) have been authorized by the FDA to manage PsA (114). Moreover, the FDA granted approval for deucravacitinib in September 2022 for the treatment of moderate-to-severe psoriasis, which is a specific type of JAK inhibitor known as a TYK2 inhibitor.
Phosphodiesterase - 4 (PDE-4) is an enzyme that plays a key role in the regulation of intracellular levels of cyclic adenosine monophosphate (cAMP), resulting in activation of NF-kB and inhibition of CRE-binding protein and ATF-1. PDE-4 inhibitors are a class of drugs that block the activity of PDE-4 enzymes. By inhibiting PDE-4, these drugs increase the levels of cAMP in cells, which has anti-inflammatory effects. Apremilast is an oral PDE4 inhibitor approved for treating patients with moderate to severe plaque psoriasis and PsA (115, 116). A topical agent of PDE-4 inhibitor named roflumilast cream is being investigated for the topical treatment of psoriasis (117).
The Aryl hydrocarbon receptor (AhR) is a transcription factor that is dependent on cytosolic ligands and is expressed in various types of skin cells (118), playing a role in the pathogenesis of inflammatory skin diseases, including psoriasis (119). Tapinarof, an AhR agonist, has recently been approved by the FDA as a non-steroidal topical treatment for plaque psoriasis. Studies have shown that it is effective in treating the condition and has a favorable safety profile (118).
5 Conclusions
T lymphocytes are of utmost importance in the pathogenesis of psoriasis. Great progress on T lymphocytes and their functions in the immune-mediated diseases have been made over the past few years, helping us to understand the pathogenesis of psoriasis more clearly and specifically. Psoriasis is no longer regarded as a Th1 type disease and many other T lymphocytes such as Th1, Th17, Treg and Th22 cells also make contributions to the psoriasis development by interacting with each other (Figure 1). Undoubtedly, new types of T lymphocytes may be discovered with the increasingly understandings of the pathogenesis of psoriasis, which will in turn provide novel therapy approaches in the future.
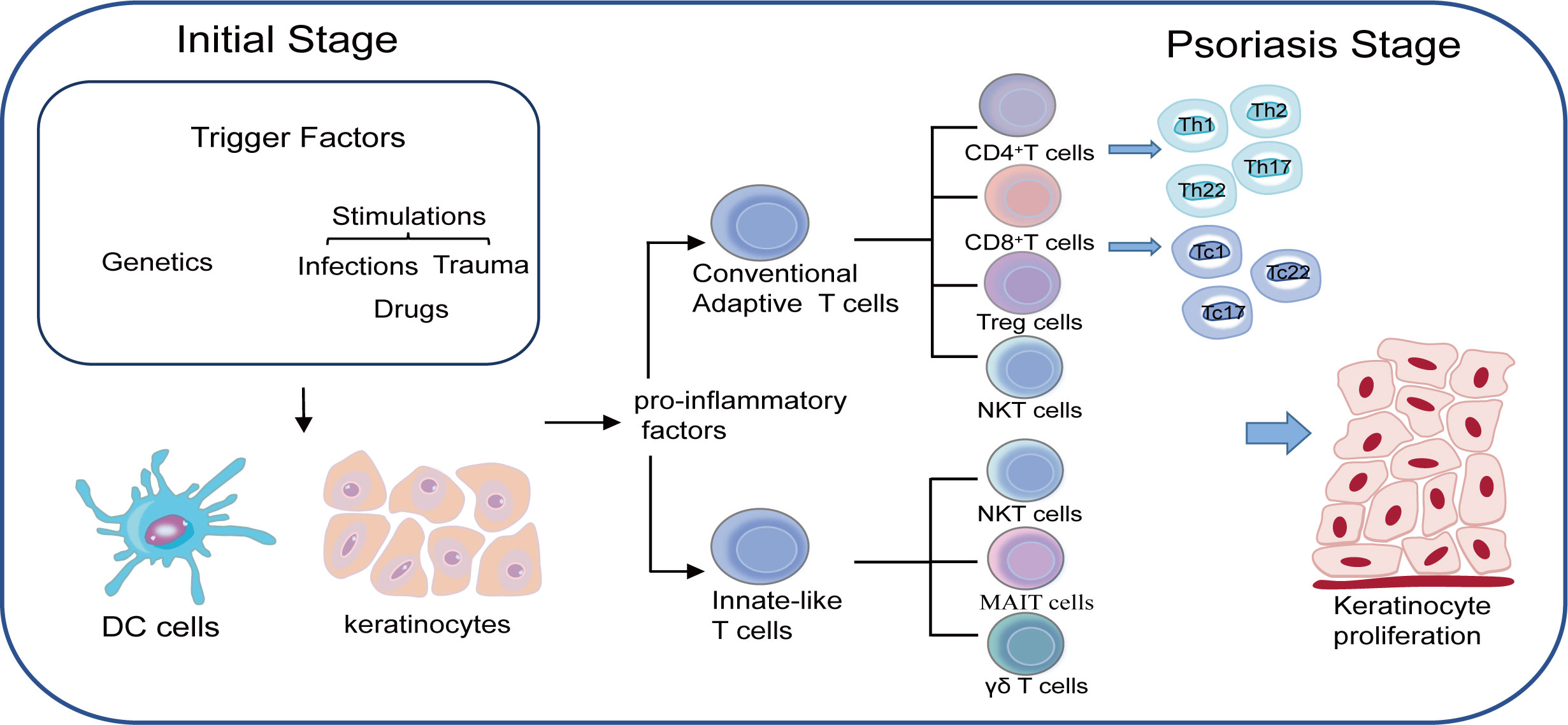
Figure 1 Both genetic susceptibility and environmental triggers such as infections, drugs and trauma play important roles in developing psoriasis. Keratinocytes together with DC cells are responsible for the initial stage through the production of pro-inflammatory factors. T cells are believed to be in the center of accelerating the process of psoriasis as two main subtypes of T cells namely conventional adaptive T cells and innate-like T cells work together to maintain the inflammatory loop during the psoriasis.
Author contributions
HW and RW contributed to conception and design of the study. PZ and SL wrote the first draft of the manuscript. HC performed the chart. All authors contributed to the article and approved the submitted version.
Funding
This article was funded by the National Nature Science Foundation of China (NO.81903223 and NO.82173425), and the Nature Science Foundation of Hunan (NO.2022JJ40716 and NO.2020JJ2055).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol (2021) 157(8):940–6. doi: 10.1001/jamadermatol.2021.2007
2. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet (2007) 370(9583):263–71. doi: 10.1016/S0140-6736(07)61128-3
3. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol (2017) 76(3):377–90. doi: 10.1016/j.jaad.2016.07.064
4. Lopez-Estebaranz JL, Sanchez-Carazo JL, Sulleiro S. Effect of a family history of psoriasis and age on comorbidities and quality of life in patients with moderate to severe psoriasis: Results from the ARIZONA study. J Dermatol (2016) 43(4):395–401. doi: 10.1111/1346-8138.13157
5. Branisteanu DE, Cojocaru C, Diaconu R, Porumb EA, Alexa AI, Nicolescu AC, et al. Update on the etiopathogenesis of psoriasis (Review). Exp Ther Med (2022) 23(3):201. doi: 10.3892/etm.2022.11124
6. Voorhees JJ. Pathophysiology of psoriasis. Annu Rev Med (1977) 28:467–73. doi: 10.1146/annurev.me.28.020177.002343
7. Grossman RM, Chevret S, Abi-Rached J, Blanchet F, Dubertret L. Long-term safety of cyclosporine in the treatment of psoriasis. Arch Dermatol (1996) 132(6):623–9. doi: 10.1001/archderm.1996.03890300039008
8. Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med (1991) 324(5):277–84. doi: 10.1056/NEJM199101313240501
9. Shim CH, Cho S, Shin YM, Choi JM. Emerging role of bystander T cell activation in autoimmune diseases. BMB Rep (2022) 55(2):57–64. doi: 10.5483/BMBRep.2022.55.2.183
10. Nikaein A, Phillips C, Gilbert SC, Savino D, Silverman A, Stone MJ, et al. Characterization of skin-infiltrating lymphocytes in patients with psoriasis. J Invest Dermatol (1991) 96(1):3–9. doi: 10.1111/1523-1747.ep12514646
11. Bochenska K, Smolinska E, Moskot M, Jakobkiewicz-Banecka J, Gabig-Ciminska M. Models in the research process of psoriasis. Int J Mol Sci (2017) 18(12). doi: 10.3390/ijms18122514
12. Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol (2012) 7:385–422. doi: 10.1146/annurev-pathol-011811-132448
13. Belizario JE, Brandao W, Rossato C, Peron JP. Thymic and postthymic regulation of naive CD4(+) T-cell lineage fates in humans and mice models. Mediators Inflamm (2016) 2016:9523628. doi: 10.1155/2016/9523628
14. Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol (2005) 35(2):469–75. doi: 10.1002/eji.200425677
15. Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med (2006) 203(12):2577–87. doi: 10.1084/jem.20060244
16. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol (2016) 175(2):273–86. doi: 10.1111/bjd.14493
17. Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity (2009) 30(3):324–35. doi: 10.1016/j.immuni.2009.03.003
18. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity (2014) 41(4):529–42. doi: 10.1016/j.immuni.2014.10.004
19. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol (2014) 35(9):436–42. doi: 10.1016/j.it.2014.06.002
20. Wang Y, Wang L, Shi Y, Wang F, Yang H, Han S, et al. Altered circulating T follicular helper cell subsets in patients with psoriasis vulgaris. Immunol Lett (2017) 181:101–8. doi: 10.1016/j.imlet.2016.09.008
21. Cheuk S, Schlums H, Gallais Serezal I, Martini E, Chiang SC, Marquardt N, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity (2017) 46(2):287–300. doi: 10.1016/j.immuni.2017.01.009
22. Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol (2005) 174(1):164–73. doi: 10.4049/jimmunol.174.1.164
23. Fujimura T, Okuyama R, Ito Y, Aiba S. Profiles of Foxp3+ regulatory T cells in eczematous dermatitis, psoriasis vulgaris and mycosis fungoides. Br J Dermatol (2008) 158(6):1256–63. doi: 10.1111/j.1365-2133.2008.08504.x
24. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med (2007) 204(6):1257–65. doi: 10.1084/jem.20062512
25. Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol (1994) 102(2):145–9. doi: 10.1111/1523-1747.ep12371752
26. Harden JL, Johnson-Huang LM, Chamian MF, Lee E, Pearce T, Leonardi CL, et al. Humanized anti-IFN-gamma (HuZAF) in the treatment of psoriasis. J Allergy Clin Immunol (2015) 135(2):553–6. doi: 10.1016/j.jaci.2014.05.046
27. Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med (2009) 361(9):888–98. doi: 10.1056/NEJMra0707449
28. Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet (2018) 392(10148):650–61. doi: 10.1016/S0140-6736(18)31713-6
29. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaci D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet (2017) 390(10091):276–88. doi: 10.1016/S0140-6736(17)31279-5
30. Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol (2006) 18(6):670–5. doi: 10.1016/j.coi.2006.09.008
31. Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol (2007) 150(3):407–15. doi: 10.1111/j.1365-2249.2007.03511.x
32. Leonardi C, Maari C, Philipp S, Goldblum O, Zhang L, Burkhardt N, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: Three-year results from the UNCOVER-3 study. J Am Acad Dermatol (2018) 79(5):824–30 e2. doi: 10.1016/j.jaad.2018.05.032
33. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med (2014) 371(4):326–38. doi: 10.1056/NEJMoa1314258
34. Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol (2009) 123(6):1244–52 e2. doi: 10.1016/j.jaci.2009.03.041
35. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol (2010) 130(5):1373–83. doi: 10.1038/jid.2009.399
36. Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol (2010) 32(2):183–96. doi: 10.1007/s00281-009-0194-z
37. Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol (2006) 177(7):4927–32. doi: 10.4049/jimmunol.177.7.4927
38. Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol (2007) 179(8):5099–108. doi: 10.4049/jimmunol.179.8.5099
39. Yang X, Yang J, Chu Y, Xue Y, Xuan D, Zheng S, et al. T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PloS One (2014) 9(2):e88441. doi: 10.1371/journal.pone.0088441
40. Zhang Z, Zhao S, Zhang L, Crew R, Zhang N, Sun X, et al. A higher frequency of CD4(+)CXCR5(+) T follicular helper cells in patients with newly diagnosed Henoch-Schonlein purpura nephritis. Int Immunopharmacol (2016) 32:8–15. doi: 10.1016/j.intimp.2015.12.037
41. Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma L, et al. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Exp Immunol (2013) 174(2):212–20. doi: 10.1111/cei.12162
42. Wu S, Yang T, Pan F, Xia G, Hu Y, Liu L, et al. Increased frequency of circulating follicular helper T cells in patients with ankylosing spondylitis. Mod Rheumatol (2015) 25(1):110–5. doi: 10.3109/14397595.2014.902149
43. Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci USA (2011) 108(33):E488–97. doi: 10.1073/pnas.1100898108
44. Niu J, Song Z, Yang X, Zhai Z, Zhong H, Hao F. Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol (2015) 29(9):1791–6. doi: 10.1111/jdv.13027
45. Haskamp S, Frey B, Becker I, Schulz-Kuhnt A, Atreya I, Berking C, et al. Transcriptomes of MPO-deficient patients with generalized pustular psoriasis reveals expansion of CD4(+) cytotoxic T cells and an involvement of the complement system. J Invest Dermatol (2022) 142(8):2149–58.e10. doi: 10.1016/j.jid.2021.12.021
46. Hu P, Wang M, Gao H, Zheng A, Li J, Mu D, et al. The role of helper T cells in psoriasis. Front Immunol (2021) 12:788940. doi: 10.3389/fimmu.2021.788940
47. Bovenschen HJ, Seyger MM, Van de Kerkhof PC. Plaque psoriasis vs. atopic dermatitis and lichen planus: a comparison for lesional T-cell subsets, epidermal proliferation and differentiation. Br J Dermatol (2005) 153(1):72–8.
48. Nedoszytko B, Sokolowska-Wojdylo M, Ruckemann-Dziurdzinska K, Roszkiewicz J, Nowicki RJ. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol (2014) 31(2):84–91. doi: 10.5114/pdia.2014.40920
49. Ovigne JM, Baker BS, Brown DW, Powles AV, Fry L. Epidermal CD8+ T cells in chronic plaque psoriasis are Tc1 cells producing heterogeneous levels of interferon-gamma. Exp Dermatol (2001) 10(3):168–74. doi: 10.1034/j.1600-0625.2001.010003168.x
50. Di Meglio P, Duarte JH. CD8 T Cells and IFN-gamma emerge as critical players for psoriasis in a novel model of mouse psoriasiform skin inflammation. J Invest Dermatol (2013) 133(4):871–4. doi: 10.1038/jid.2012.426
51. Lima Ede A, Lima Mde A. Reviewing concepts in the immunopathogenesis of psoriasis. Bras Dermatol (2011) 86(6):1151–8.
52. Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol (2013) 34(4):174–81. doi: 10.1016/j.it.2012.11.005
53. Liu J, Chang HW, Huang ZM, Nakamura M, Sekhon S, Ahn R, et al. Single-cell RNA sequencing of psoriatic skin identifies pathogenic Tc17 cell subsets and reveals distinctions between CD8(+) T cells in autoimmunity and cancer. J Allergy Clin Immunol (2021) 147(6):2370–80. doi: 10.1016/j.jaci.2020.11.028
54. Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol (2009) 86(2):435–43. doi: 10.1189/JLB.0109046
55. Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PloS One (2010) 5(11):e14108. doi: 10.1371/journal.pone.0014108
56. FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, et al. Psoriatic arthritis. Nat Rev Dis Primers (2021) 7(1):59. doi: 10.1038/s41572-021-00293-y
57. Costello PJ, Winchester RJ, Curran SA, Peterson KS, Kane DJ, Bresnihan B, et al. Psoriatic arthritis joint fluids are characterized by CD8 and CD4 T cell clonal expansions appear antigen driven. J Immunol (2001) 166(4):2878–86. doi: 10.4049/jimmunol.166.4.2878
58. Penkava F, Velasco-Herrera MDC, Young MD, Yager N, Nwosu LN, Pratt AG, et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat Commun (2020) 11(1):4767. doi: 10.1038/s41467-020-18513-6
59. Brown MC, Butler RG. Proceedings: Evidence for innervation of muscle spindle intrafusal fibres by branches of alpha motoneurones following nerve injury. J Physiol (1974) 238(1):41P–3P.
60. Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol (2015) 36(7):428–35. doi: 10.1016/j.it.2015.05.003
61. van de Kerkhof PC. From empirical to pathogenesis-based treatments for psoriasis. J Invest Dermatol (2022) 142(7):1778–85. doi: 10.1016/j.jid.2022.01.014
62. Puig L, Costanzo A, Munoz-Elias EJ, Jazra M, Wegner S, Paul CF, et al. The biological basis of disease recurrence in psoriasis: a historical perspective and current models. Br J Dermatol (2022) 186(5):773–81. doi: 10.1111/bjd.20963
63. Matos TR, O'Malley JT, Lowry EL, Hamm D, Kirsch IR, Robins HS, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing alphabeta T cell clones. J Clin Invest (2017) 127(11):4031–41. doi: 10.1172/JCI93396
64. Tokura Y, Phadungsaksawasdi P, Kurihara K, Fujiyama T, Honda T. Pathophysiology of skin resident memory T cells. Front Immunol (2020) 11:618897. doi: 10.3389/fimmu.2020.618897
65. Gallais Serezal I, Classon C, Cheuk S, Barrientos-Somarribas M, Wadman E, Martini E, et al. Resident T cells in resolved psoriasis steer tissue responses that stratify clinical outcome. J Invest Dermatol (2018) 138(8):1754–63. doi: 10.1016/j.jid.2018.02.030
66. Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest (2007) 117(5):1167–74. doi: 10.1172/JCI31202
67. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature (2006) 441(7090):235–8. doi: 10.1038/nature04753
68. Quaglino P, Ortoncelli M, Comessatti A, Ponti R, Novelli M, Bergallo M, et al. Circulating CD4+CD25 bright FOXP3+ T cells are up-regulated by biological therapies and correlate with the clinical response in psoriasis patients. Dermatology (2009) 219(3):250–8. doi: 10.1159/000238305
69. Ma L, Xue H, Gao T, Gao M, Zhang Y. Notch1 signaling regulates the Th17/Treg immune imbalance in patients with psoriasis vulgaris. Mediators Inflamm (2018) 2018:3069521. doi: 10.1155/2018/3069521
70. Karamehic J, Zecevic L, Resic H, Jukic M, Jukic T, Ridjic O, et al. Immunophenotype lymphocyte of peripheral blood in patients with psoriasis. Med Arch (2014) 68(4):236–8. doi: 10.5455/medarh.2014.68.236-238
71. Furuhashi T, Saito C, Torii K, Nishida E, Yamazaki S, Morita A. Photo(chemo)therapy reduces circulating Th17 cells and restores circulating regulatory T cells in psoriasis. PloS One (2013) 8(1):e54895. doi: 10.1371/journal.pone.0054895
72. Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol (2010) 135(1):108–17. doi: 10.1016/j.clim.2009.11.008
73. Yun WJ, Lee DW, Chang SE, Yoon GS, Huh JR, Won CH, et al. Role of CD4CD25FOXP3 regulatory T cells in psoriasis. Ann Dermatol (2010) 22(4):397–403. doi: 10.5021/ad.2010.22.4.397
74. Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, et al. Memory regulatory T cells reside in human skin. J Clin Invest (2014) 124(3):1027–36. doi: 10.1172/JCI72932
75. Bovenschen HJ, van Vlijmen-Willems IM, van de Kerkhof PC, van Erp PE. Identification of lesional CD4+ CD25+ Foxp3+ regulatory T cells in Psoriasis. Dermatology (2006) 213(2):111–7. doi: 10.1159/000093849
76. Keijsers RR, van der Velden HM, van Erp PE, de Boer-van Huizen RT, Joosten I, Koenen HJ, et al. Balance of Treg vs. T-helper cells in the transition from symptomless to lesional psoriatic skin. Br J Dermatol (2013) 168(6):1294–302.
77. Yan KX, Fang X, Han L, Zhang ZH, Kang KF, Zheng ZZ, et al. Foxp3+ regulatory T cells and related cytokines differentially expressed in plaque vs. guttate psoriasis vulgaris. Br J Dermatol (2010) 163(1):48–56. doi: 10.1111/j.1365-2133.2010.09742.x
78. Yang L, Li B, Dang E, Jin L, Fan X, Wang G. Impaired function of regulatory T cells in patients with psoriasis is mediated by phosphorylation of STAT3. J Dermatol Sci (2016) 81(2):85–92. doi: 10.1016/j.jdermsci.2015.11.007
79. Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood (2007) 110(4):1225–32. doi: 10.1182/blood-2006-12-064527
80. Yan K, Xu W, Huang Y, Zhang Z, Huang Q, Xin KZ, et al. Methotrexate restores the function of peripheral blood regulatory T cells in psoriasis vulgaris via the CD73/AMPK/mTOR pathway. Br J Dermatol (2018) 179(4):896–905. doi: 10.1111/bjd.16560
81. Slauenwhite D, Johnston B. Regulation of NKT cell localization in homeostasis and infection. Front Immunol (2015) 6:255. doi: 10.3389/fimmu.2015.00255
82. Bonish B, Jullien D, Dutronc Y, Huang BB, Modlin R, Spada FM, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol (2000) 165(7):4076–85. doi: 10.4049/jimmunol.165.7.4076
83. Vissers WH, Berends M, Muys L, van Erp PE, de Jong EM, van de Kerkhof PC. The effect of the combination of calcipotriol and betamethasone dipropionate versus both monotherapies on epidermal proliferation, keratinization and T-cell subsets in chronic plaque psoriasis. Exp Dermatol (2004) 13(2):106–12. doi: 10.1111/j.0906-6705.2004.00151.x
84. Nickoloff BJ, Wrone-Smith T, Bonish B, Porcelli SA. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells, including CD94, CD158, and CD161. Arch Dermatol (1999) 135(5):546–52. doi: 10.1001/archderm.135.5.546
85. Yip KH, Papadopoulos M, Pant H, Tumes DJ. The role of invariant T cells in inflammation of the skin and airways. Semin Immunopathol (2019) 41(3):401–10. doi: 10.1007/s00281-019-00740-9
86. Teunissen MBM, Yeremenko NG, Baeten DLP, Chielie S, Spuls PI, de Rie MA, et al. The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J Invest Dermatol (2014) 134(12):2898–907. doi: 10.1038/jid.2014.261
87. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood (2011) 117(4):1250–9. doi: 10.1182/blood-2010-08-303339
88. Brenner MB, McLean J, Dialynas DP, Strominger JL, Smith JA, Owen FL, et al. Identification of a putative second T-cell receptor. Nature (1986) 322(6075):145–9. doi: 10.1038/322145a0
89. Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol (1993) 11:637–85. doi: 10.1146/annurev.iy.11.040193.003225
90. Cai Y, Fleming C, Yan J. Dermal gammadelta T cells–a new player in the pathogenesis of psoriasis. Int Immunopharmacol (2013) 16(3):388–91. doi: 10.1016/j.intimp.2013.02.018
91. Fichtner AS, Ravens S, Prinz I. Human gammadelta TCR repertoires in health and disease. Cells. (2020) 9(4). doi: 10.3390/cells9040800
92. Munoz-Ruiz M, Sumaria N, Pennington DJ, Silva-Santos B. Thymic determinants of gammadelta T cell differentiation. Trends Immunol (2017) 38(5):336–44. doi: 10.1016/j.it.2017.01.007
93. Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol (2011) 187(5):2783–93. doi: 10.4049/jimmunol.1100804
94. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity (2011) 35(4):596–610. doi: 10.1016/j.immuni.2011.08.001
95. Li G, Liu L, Yin Z, Ye Z, Shen N. Glutamine metabolism is essential for the production of IL-17A in gammadelta T cells and skin inflammation. Tissue Cell (2021) 71:101569. doi: 10.1016/j.tice.2021.101569
96. Magis Q, Jullien D, Gaudy-Marqueste C, Baumstark K, Viguier M, Bachelez H, et al. Predictors of long-term drug survival for infliximab in psoriasis. J Eur Acad Dermatol Venereol (2017) 31(1):96–101. doi: 10.1111/jdv.13747
97. Vender RB, Lynde CW. Certolizumab pegol use in the treatment of moderate-to-severe psoriasis: real-world data from two Canadian centers. J Cutan Med Surg (2022) 26(3):267–73. doi: 10.1177/12034754221078203
98. Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat (2004) 15(5):280–94. doi: 10.1080/09546630410017275
99. Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol (2014) 32:227–55. doi: 10.1146/annurev-immunol-032713-120225
100. Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol (2012) 130(1):145–54 e9. doi: 10.1016/j.jaci.2012.04.024
101. Brownstone ND, Hong J, Mosca M, Hadeler E, Liao W, Bhutani T, et al. Biologic treatments of psoriasis: an update for the clinician. Biologics (2021) 15:39–51.
102. Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet (2020) 395(10222):427–40. doi: 10.1016/S0140-6736(19)33161-7
103. Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med (2009) 206(2):299–311. doi: 10.1084/jem.20081463
104. Iznardo H, Puig L. The safety of brodalumab for the treatment of psoriasis. Expert Opin Drug Saf (2020) 19(4):365–72. doi: 10.1080/14740338.2020.1730326
105. Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol (2013) 133(1):17–26. doi: 10.1038/jid.2012.194
106. Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med (2004) 199(1):125–30. doi: 10.1084/jem.20030451
107. Toussirot E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets (2012) 11(2):159–68. doi: 10.2174/187152812800392805
108. Yu J, Zhao Q, Wang X, Zhou H, Hu J, Gu L, et al. Pathogenesis, multi-omics research, and clinical treatment of psoriasis. J Autoimmun (2022) 133:102916. doi: 10.1016/j.jaut.2022.102916
109. Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1115–25. doi: 10.1016/S0140-6736(20)30265-8
110. Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1126–36. doi: 10.1016/S0140-6736(20)30263-4
111. Reich K, Gooderham M, Thaci D, Crowley JJ, Ryan C, Krueger JG, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet (2019) 394(10198):576–86. doi: 10.1016/S0140-6736(19)30952-3
112. Kvist-Hansen A, Hansen PR, Skov L. Systemic treatment of psoriasis with JAK inhibitors: a review. Dermatol Ther (Heidelb) (2020) 10(1):29–42. doi: 10.1007/s13555-019-00347-w
113. Singh S, Pradhan D, Puri P, Ramesh V, Aggarwal S, Nayek A, et al. Genomic alterations driving psoriasis pathogenesis. Gene (2019) 683:61–71. doi: 10.1016/j.gene.2018.09.042
114. Keeling S, Maksymowych WP. JAK inhibitors, psoriatic arthritis, and axial spondyloarthritis: a critical review of clinical trials. Expert Rev Clin Immunol (2021) 17(7):701–15. doi: 10.1080/1744666X.2021.1925541
115. Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol (2015) 73(1):37–49. doi: 10.1016/j.jaad.2015.03.049
116. Torres T, Puig L. Apremilast: a novel oral treatment for psoriasis and psoriatic arthritis. Am J Clin Dermatol (2018) 19(1):23–32. doi: 10.1007/s40257-017-0302-0
117. Lebwohl MG, Papp KA, Stein Gold L, Gooderham MJ, Kircik LH, Draelos ZD, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med (2020) 383(3):229–39. doi: 10.1056/NEJMoa2000073
118. Nogueira S, Rodrigues MA, Vender R, Torres T. Tapinarof for the treatment of psoriasis. Dermatol Ther (2022) 35(12):e15931. doi: 10.1111/dth.15931
Keywords: psoriasis, T lymphocytes, cytokines, targeted therapies, immunology
Citation: Zhang P, Su Y, Li S, Chen H, Wu R and Wu H (2023) The roles of T cells in psoriasis. Front. Immunol. 14:1081256. doi: 10.3389/fimmu.2023.1081256
Received: 27 October 2022; Accepted: 29 September 2023;
Published: 24 October 2023.
Edited by:
Vito Di Lernia, Azienda USL-IRCCS di Reggio Emilia, ItalyReviewed by:
Yonghu Sun, Shandong Provincial Hospital of Dermatology, ChinaNick David Jones, University of Birmingham, United Kingdom
Rui-qun Qi, First Hospital of China Medical University, China
Copyright © 2023 Zhang, Su, Li, Chen, Wu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruifang Wu, cnVpZmFuZ3d1QGNzdS5lZHUuY24=; Haijing Wu, Y2hyaXN3dTEwMTBAY3N1LmVkdS5jbg==
 Peng Zhang
Peng Zhang Siying Li
Siying Li Haijing Wu
Haijing Wu