
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 30 January 2023
Sec. Vaccines and Molecular Therapeutics
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1078736
This article is part of the Research Topic Insights in Vaccines and Molecular Therapeutics: 2022 View all 13 articles
 Janneke W. Duijster1*
Janneke W. Duijster1* Thomas Lieber1
Thomas Lieber1 Silvia Pacelli1,2
Silvia Pacelli1,2 Leontine Van Balveren1
Leontine Van Balveren1 Loes S. Ruijs1
Loes S. Ruijs1 Monika Raethke1
Monika Raethke1 Agnes Kant1
Agnes Kant1 Florence Van Hunsel1
Florence Van Hunsel1Background: Albeit the need for sex-disaggregated results of adverse events after immunization (AEFIs) is gaining attention since the COVID-19 pandemic, studies with emphasis on sexual dimorphism in response to COVID-19 vaccination are relatively scarce. This prospective cohort study aimed to assess differences in the incidence and course of reported AEFIs after COVID-19 vaccination between males and females in the Netherlands and provides a summary of sex-disaggregated outcomes in published literature.
Methods: Patient reported outcomes of AEFIs over a six month period following the first vaccination with BioNTech-Pfizer, AstraZeneca, Moderna or the Johnson&Johnson vaccine were collected in a Cohort Event Monitoring study. Logistic regression was used to assess differences in incidence of ‘any AEFI’, local reactions and the top ten most reported AEFIs between the sexes. Effects of age, vaccine brand, comorbidities, prior COVID-19 infection and the use of antipyretic drugs were analyzed as well. Also, time-to-onset, time-to-recovery and perceived burden of AEFIs was compared between the sexes. Third, a literature review was done to retrieve sex-disaggregated outcomes of COVID-19 vaccination.
Results: The cohort included 27,540 vaccinees (38.5% males). Females showed around two-fold higher odds of having any AEFI as compared to males with most pronounced differences after the first dose and for nausea and injection site inflammation. Age was inversely associated with AEFI incidence, whereas a prior COVID-19 infection, the use of antipyretic drugs and several comorbidities were positively associated. The perceived burden of AEFIs and time-to-recovery were slightly higher in females.
Discussion: The results of this large cohort study correspond to existing evidence and contribute to the knowledge gain necessary to disentangle the magnitude of the effect sex in response to vaccination. Whilst females have a significant higher probability of experiencing an AEFI than males, we observed that the course and burden is only to a minor extent different between the sexes.
● Differences in response to vaccination between the sexes are generally well known. Yet, large studies specifically focusing on identifying differences between males and females with regard to reported adverse events and the course of adverse events are still scarce.
● In the Netherlands, the odds of experiencing an adverse event after COVID-19 vaccination is around two-fold higher in females compared to males for a range of different adverse events, while time-to-onset did not differ between the sexes and the time-to-recovery and perceived burden only slightly differed.
● Increasing knowledge on sexual dimorphism in response to vaccination can aid in providing more targeted safety information in future vaccination campaigns.
The large scale vaccination of people around the world as preventive measure against COVID-19 infection has put more emphasis on safety and efficacy of vaccines. In the Netherlands, a vaccination campaign has been implemented since January 2021. Depending on age and the availability of vaccine brands during the vaccination schedule, people received one of the four vaccine brands authorized in Europe at that time, including the viral vector vaccines Oxford-AstraZeneca and Johnson&Johnson/Janssen, and the mRNA vaccines Moderna and BioNTech/Pfizer; hereafter referred to as AstraZeneca, Johnson&Johnson, Moderna and Pfizer respectively. Monitoring of vaccine safety (amongst others for the COVID-19 vaccines) allows for early detection of adverse reactions possibly linked to vaccination, thereby providing the first step in minimizing possible negative effects attributable to vaccines and allowing the provision of information about possible adverse reactions to vaccine recipients based on up-to-date data. This is achieved by The Netherlands Pharmacovigilance Centre Lareb through a spontaneous reporting system as well as longitudinal cohort event monitoring (CEM) studies (1, 2). AEFIs are defined as any unintended medical occurrence after immunization which is not necessarily causally related to the application of the vaccine (3). AEFIs that present as physical symptoms of the inflammatory response after vaccination, such as swelling, redness or pain at the injection site, headache, arthralgia or fever, are referred to as reactogenicity and constitute a significant part of all AEFIs reported (4). The types and degree of adverse events after immunization (AEFIs) differ between vaccine brands, with the Pfizer generally being associated with the lowest incidence of reported AEFIs (5–7). Apart from vaccine brand, several recipient-factors influence the probability of experiencing an AEFI. These include age and sex and also the presence of comorbidities such as diabetes, cardiovascular disorders and conditions/medications that suppress the function of the immune system (4, 8).
It is well recognized that most females mount stronger antibody responses against vaccination and experience a higher level of reactogenicity compared to males which is mainly attributable to levels of sex hormones and chromosomal differences (9, 10). In this context, ‘sex’ is referring to differences between individuals based on biological characteristics (i.e. sex organs, chromosomes, endogenous hormone profiles) rather than cultural and social aspects which more reflect a person’s self-representation and is often denoted as ‘gender’ (11). Sex is commonly controlled for in epidemiological studies rather than being the main topic of study. Nonetheless, the importance of reporting sex-disaggregated results of medical conditions and interventions is gaining attention, particularly since the COVID-19 pandemic where differences in responses to infection as well as safety and efficacy of vaccines could be studied in diverse and larger populations.
Several studies report a significant higher incidence of reported AEFIs after COVID-19 vaccination in females than males (12–14). Yet, only a limited number of studies focus specifically on the association between the occurrence of AEFIs and sex. Hence, much is still unknown about differences in the course of the AEFI and the sex-disaggregated effects of aging on AEFI occurrence. In this longitudinal cohort event monitoring study, we investigated the incidence of reported AEFIs between males and females after the first and second dose of vaccination against Covid-19 in a Dutch cohort covering four vaccine brands. Secondly, we aimed to assess whether the latency, duration and perceived burden of the reported AEFIs differed between males and females. Third, we reviewed existing literature assessing the incidences of AEFIs in males and females to allow for comparison with the results from our cohort study.
The design of the study and the process of data collection have been described in detail elsewhere (2, 5). Briefly, Dutch residents aged above 16 years who received the first dose of COVID-19 vaccination between February and August 2021 were eligible to participate in this prospective cohort study. Data were collected through web-based questionnaires using the Lareb Intensive Monitoring (LIM) system. At registration (at a maximum of two days after the first dose of vaccination), vaccinees filled in a baseline questionnaire covering questions about age, sex, body weight and length, presence of comorbidities, concomitant use of medication, the use of antipyretic drugs shortly before vaccination and whether the person had a history of COVID-19 infection before vaccination. Following the baseline questionnaire, participants received six questionnaires over a period of six months in which they could self-report the occurrence of AEFIs possibly attributable to their first or second COVID-19 vaccination. Moreover, the course (burden and time to recovery) of reported AEFIs was retrieved in the questionnaires. People who only completed the baseline questionnaire and none of the six follow-up questionnaires were excluded from analyses as well as people who received a different vaccine brand for their second dose vaccination compared to the first dose.
The AEFIs reported in the study were coded using the Medical Dictionary for Regulatory Activities (MedDRA®) terminology 23.0 and 24.0 (15). The incidence and course of reported AEFIs was compared between males and females for multiple outcomes for the first and second dose of vaccination separately. These outcomes include any reported AEFI (hereafter referred to as ‘any AEFI’), local reactions at the injection site and the 10 most frequently reported AEFIs regardless of sex. The MedDRA preferred terms classified as local reactions are listed in Supplementary Table S1. For all outcomes, the maximal time between vaccination and onset of the AEFI for an AEFI to be included in the statistical analyses was 28 days (≤672 hours). Age and body mass index (BMI) were included in the analyses as categorical variables (age groups <40, 40-54, 55-74, ≥75 years; BMI <18.5, 18.5-24.9, 25.0-29.9, ≥30.0). Underlying medical conditions were categorized in 12 variables comprising the presence/absence of allergies, cardiovascular disorders, diabetes, hepatic diseases, hypertension, renal diseases, respiratory diseases, malignant tumors, neurological disorders, psychological disorders, a suppressed immune function or other comorbidities. In addition, the use of antipyretic drugs in the hours before or after the first dose of vaccination and whether or not an individual had a PCR-confirmed COVID-19 infection before vaccination were included in the analyses as well. Also, participants could report the time to onset (TTO) of an AEFI and the time to recovery (TTR) in date format and/or a number of seconds, minutes, hours, days or weeks. For TTO analysis, we considered a reported TTO in seconds, minutes or hours as more precise than a date of onset or a reported number of days or weeks after vaccination, hence, we restricted the TTO analysis to only those AEFIs which were reported in seconds, minutes or hours. With regard to TTR analysis, we did not restrict on time unit as recovery is presumably more gradual than onset. The perceived burden was retrieved in a question with five answer options ranging from ‘not at all’, ‘slightly’, ‘somewhat’, ‘moderately’ to ‘extremely’ burdensome.
First, we made an overview of the AEFIs which were exclusively reported by one of the sexes. The AEFIs exclusively reported by one of the sexes were included in further analyses as well. Second, we performed univariate logistic regression for the outcome ‘any AEFI’ and local reactions on the whole cohort by dose to assess which variables might predict the occurrence of AEFIs. Subsequently, least absolute shrinkage and selection operator (LASSO) regression was applied to select the combination of variables used in further multivariable logistic regression for the outcomes ‘any AEFI’ and local reactions for the first and second dose separately, using the R glmnet package (16). For the multivariable logistic regression of the 10 most frequently reported AEFIs, a smaller subset of covariates was used, as the number of reported outcomes in these groups were lower. For all outcomes (any AEFI, local reactions and the top 10 most frequent), because the distribution of the time to onset data as well as the time to recovery data did not meet the assumptions of normality, we performed Kruskal-Wallis tests for ‘any AEFI’, local AEFIs and the top 10 most reported AEFIs by sex and vaccine separately. Subsequently, as we tested many correlations, we applied a Bonferroni correction to account for multiple testing to protect from Type I errors. As the proportional odds assumption was violated for the perceived burden data (i.e. indicating that the distance between each of the answer options is presumably not equal), we assessed the association between sex and burden by means of a multivariable logistic regression model using sex as outcome, and age, vaccine brand, and burden level as covariates. All analyses were done with R version 4.1.3. P-values <0.05 were considered significant.
In addition to the data analysis of the longitudinal cohort, we performed a literature review to summarize the main outcomes of studies that previously investigated the difference in incidence of reported AEFIs between males and females after COVID-19 vaccination with one of the vaccine brands covered by our cohort study (i.e. AstraZeneca, Johnson&Johnson, Moderna, Pfizer). We systematically searched the PubMed database for articles reporting sex-disaggregated outcomes of adverse effects in cross-sectional studies, cohort studies, clinical trials and case-control studies, published between 2019 and May 2022. The search was conducted by combining several groups of search terms listed in Supplementary Table S2 related to vaccine brand, study type and adverse effects and including at least one keyword referring to sex or one of the sexes in the title and/or abstract. Additional exclusion criteria comprised articles focusing on one specific AEFI or a selective population such as people with a specific underlying comorbidity (e.g. rheumatoid arthritis), those exclusively focusing on pregnant or lactating women or articles reporting the outcomes of pre-clinical or non-clinical studies (Supplementary Table S3). After removal of duplicates, stepwise exclusion of irrelevant articles was achieved by screening title, abstract and full text. Any disagreements in the selection process were solved through discussion between the reviewers to reach consensus. Subsequently, year of publication, country/countries of study, vaccine brands used, size of the study population and main outcomes were extracted from each of the included articles.
The initial cohort consisted of 31,033 vaccinees who filled in the baseline questionnaire of which 38.6% were males. After exclusion of people who did not complete any of the follow-up questionnaires about the occurrence of AEFIs, 27,540 vaccinees remained for inclusion in the analysis. Table 1 shows the number of male and female vaccinees who completed at least one follow-up questionnaire after the first and the second dose, by vaccine brand. For Johnson & Johnson and Moderna, about two-third of the vaccinees in the cohort were females, whereas for AstraZeneca the proportion of females was higher and for Pfizer the proportion was lower compared to males (Table 1). The average age differed by age and vaccine brand (Table 1), with generally a lower age for those who received the Johnson & Johnson vaccine and a higher age for those who received the Pfizer vaccine. About half of the vaccinees reported presence of one or more comorbidities, mainly hypertension (16.4%), allergies (11.0%) and other comorbidities not classified into one of the other categories (11.7%).
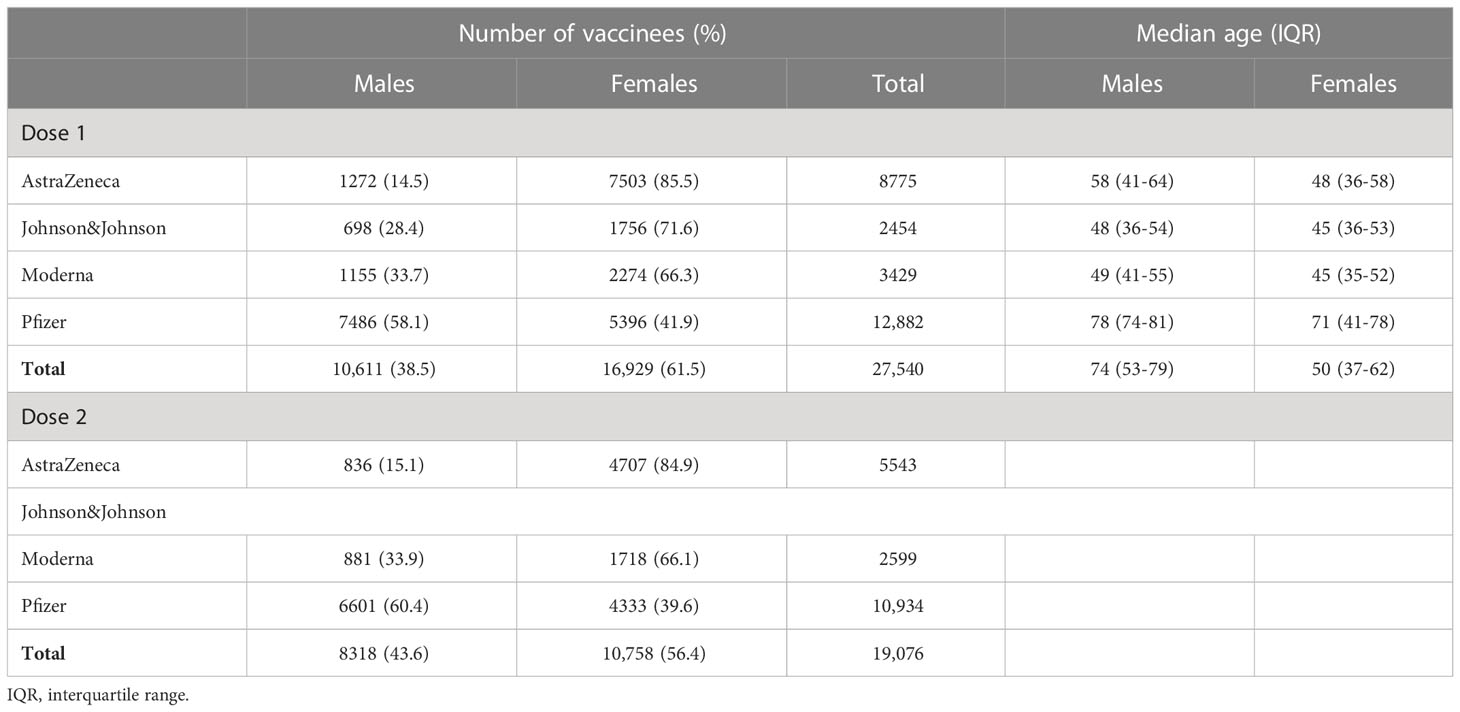
Table 1 Number and median age of vaccinees in the cohort who completed ≥1 questionnaire after the first and second dose.
A total of 4774 males and 13857 females reported at least one AEFI within 4 weeks after the first dose of vaccination, which corresponds to 45.0% and 81.9% of the male and female vaccinees respectively. The higher proportion of females compared to males who reported an AEFI was consistent for both the first and second dose for all four vaccine brands. Three sex-restricted AEFIs (at MedDRA lower level term) were reported by males, including erectile failure, pain in testis and scrotum swelling, whereas in females a variety of sex-restricted AEFIs were reported, mostly associated with the menstrual cycle or menstruation (Supplementary Table S4).
In the univariate regression analysis, most of the variables included in the analysis (n=17/19) appeared significantly associated with the occurrence of ‘any AEFI’ (Supplementary Table S5). In the univariate analyses, the odds of having ‘any AEFI’ was 5.52 times higher in females compared to males (95%CI 5.22-5.83; p<0.001; Supplementary Table S5) after the first dose, whereas the OR for having a local AEFI was 3.18 (95%CI 3.02-3.36; p<0.001; Supplementary Table S6). Both ORs for any AEFI and local reactions were less pronounced after the second dose (any AEFI 2.74; 95%CI 2.58-2.91; local reaction 2.35; 95%CI 2.19-2.52; Supplementary Tables S5, S6). The effect of age and vaccine brand was similar in both sexes. The probability of experiencing an AEFI decreased by age in both males and females (Supplementary Figure S1). Furthermore, the odds of having an AEFI was higher after COVID-19 infection, yet, this association was stronger for males compared to females (ORs first dose 3.46 [95%CI 2.76-4.37] vs. 2.59 [95%CI 2.11-3.21]; ORs second dose 1.93 [95%CI 1.50-2.48] vs. 1.19 [95%CI 1.02-1.39]). Similarly, the use of antipyretic drugs shortly before vaccination was associated with a higher odds of having an AEFI in males than in females (ORs 2.85 [95%CI 2.42-3.38] vs. 1.99 [95%CI 1.77-2.25]).
Lasso regression on ‘any AEFI’ as outcome showed that sex, age group, confirmed COVID-19 infection in the past, the use of antipyretic drugs, and seven comorbidities were eligible variables for inclusion in the multivariable model for the first dose. For the data of the second dose, the same variables appeared eligible for the multivariable model with the exception of confirmed COVID-19 infection in the past, the use of antipyretic drugs, hypertension and a suppressed immune function. In these multivariable models, the odds of having any AEFI in females compared to males was 2.28 (95%CI 2.13-2.43; p<0.001) after the first dose and 2.02 (95%CI 1.87-2.17; p<0.001) after the second dose. While far less vaccinees reported an AEFI after the second dose of vaccination with AstraZeneca or Pfizer compared to the first dose, no difference was observed in the occurrence of an AEFI between the first and second dose of Moderna vaccination for both males and females (Table 2). Six out of the seven comorbidity variables were associated with a significant higher odds of having an AEFI. For the local reactions, the effects of sex, age, prior confirmed COVID-19 infection and comorbidities were somewhat less pronounced compared to ‘any AEFI’ for the for the first dose. Whilst the odds of experiencing ‘any AEFI’ in people who received the first dose of Moderna versus the AstraZeneca vaccine was 0.37, the odds of having a local reaction was higher for those who received the Moderna versus AstraZeneca vaccine (OR 1.22).
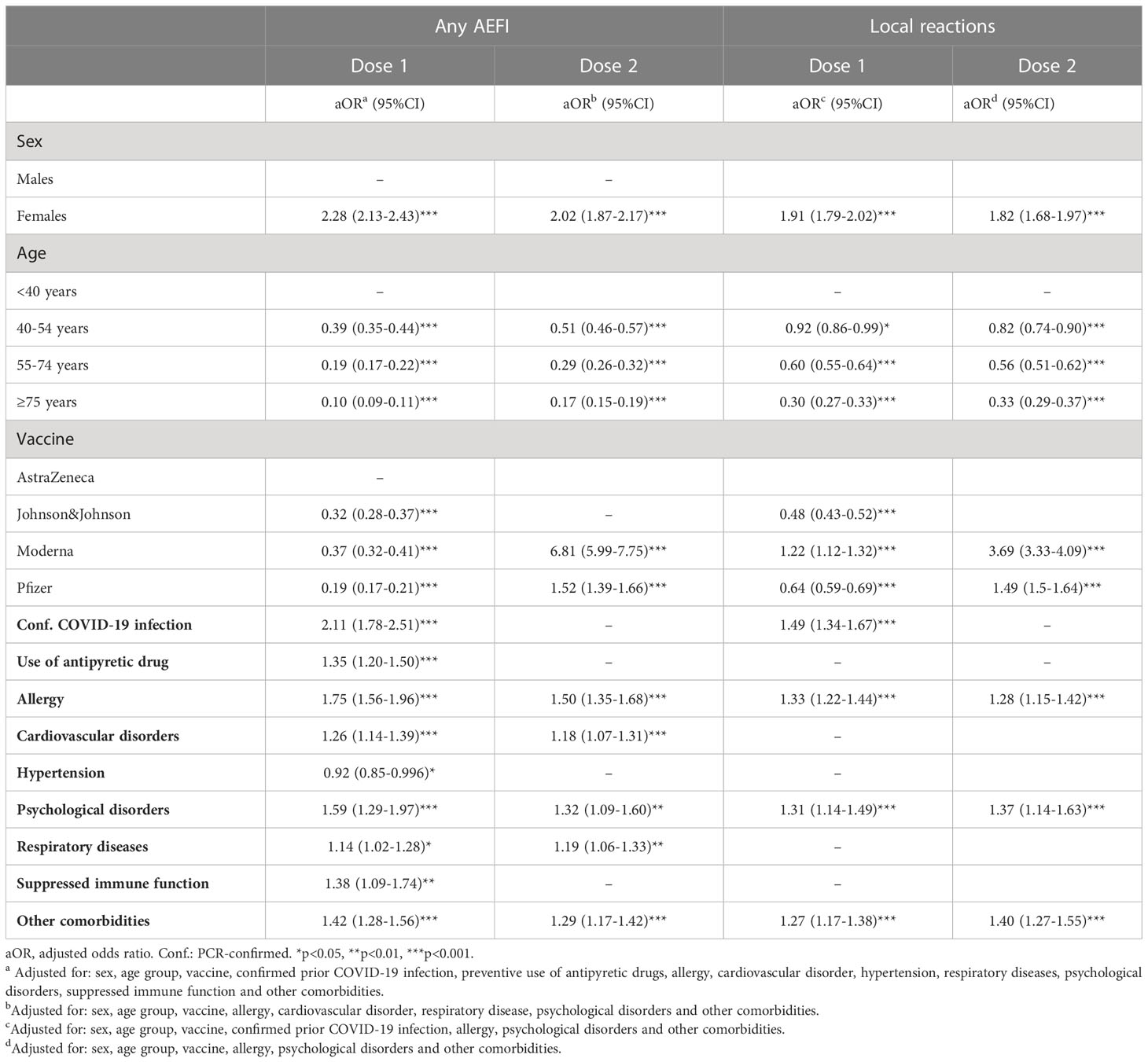
Table 2 Multivariable logistic regression of factors associated with the occurrence of ‘any AEFI’ and local reactions, by dose.
The 10 most frequent reported AEFIs (regardless of sex) consist of two local reactions and eight systemic reactions and all were solicited adverse effects. Figure 1 shows the ORs for the occurrence of the 10 AEFIs in females versus males. Overall, the odds of developing any of the 10 AEFIs was around two-fold higher in females compared to males. The ORs were similar for the first and second dose, with the largest difference between the first and second dose for nausea and injection site inflammation. For both doses of vaccination, the least difference in odds between males and females was found for myalgia, with ORs ranging between the different vaccine brands of 1.16-1.56 and 1.52-1.65 for the first and second dose respectively. The highest differences between males and females were observed for nausea (OR range 2.39-4.05) and injection site inflammation (OR range 2.29-4.00). The difference in odds between males and females was lowest for the Moderna vaccine and highest for the Pfizer vaccine for most AEFIs. Particularly for nausea and chills, the ORs for females versus males who received the Pfizer vaccine deviated substantially from the other vaccine brands (Figure 1).
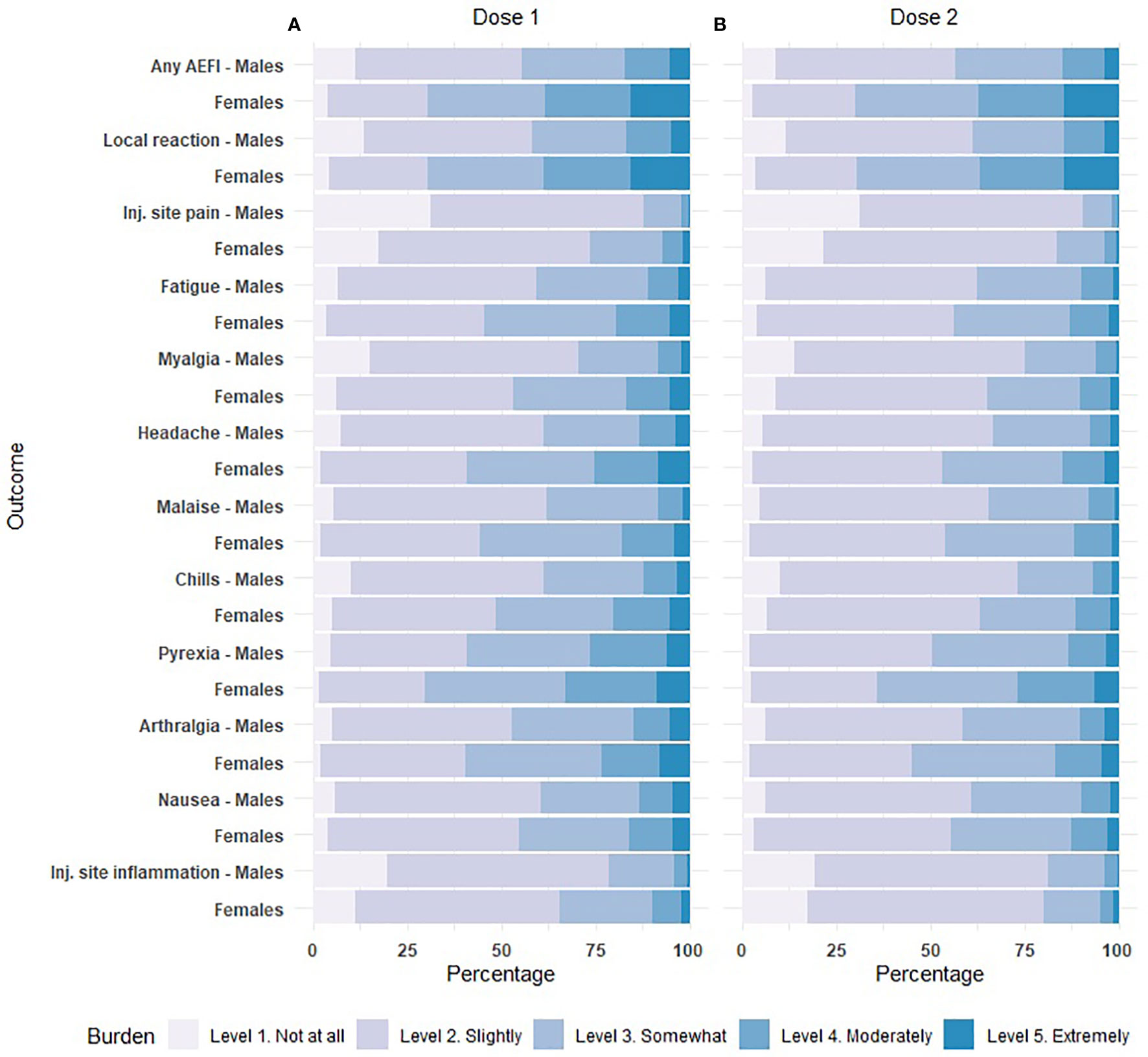
Figure 1 Forest plot of the odds of experiencing an adverse event after immunization (AEFI) in females versus males after dose 1 and dose 2, based on multivariable logistic regression. The size of the square corresponds to the inverse of the standard error. aOR: adjusted odds ratio. Inj.: injection. * p<0.05, **p<0.01, ***p<0.001. (A). Adjusted for: sex, age group, confirmed prior COVID-19 infection, preventive use of antipyretic drugs. (B). Adjusted for: sex, age group.
We compared the TTO of ‘any AEFI’, local reactions and the top 10 most reported AEFIs between males and females only for those AEFIs which included a TTO in seconds, minutes or hours (as this was considered more precise than only a date of onset). Overall, for 27.3% of the AEFIs (reported ≤28 days after vaccination) such detailed latency information was provided, which were slightly more originating from females vaccinees compared to males (28.0% vs. 24.3) and for the first dose as compared to the second dose (29.6% vs. 21.2%). Also, the proportion of AEFIs with detailed latency information was lower for the Moderna (23.1%) and Pfizer vaccines (23.6%) as compared to Johnson&Johnson (32.0%) and AstraZeneca (30.2%). For over 95% of the reported AEFIs the time to recovery (TTR) was reported. Supplementary Table S7 displays the median and interquartile range (IQR) of the reported TTO and TTR of the AEFIs by sex, dose and vaccine brand. Overall, little difference existed between the TTO of the AEFIs reported by males and females. For both sexes, shorter TTOs were observed for local reactions (medians of 3 and 2 hours for males and females respectively) compared to the systemic reactions such as pyrexia, nausea and chills were the median latency times frequently exceeded eight hours. In contrast to TTO, the TTR lasted significantly longer in females than males for several AEFIs (Supplementary Table S7). Females suffered significantly longer from ‘any AEFI’, local reactions, injection site pain and inflammation, fatigue, headache and malaise compared to males when using data from all vaccine brands combined. For most AEFIs the TTR lasted 1-3 days, the longest TTR was reported for injection site inflammation with medians of 72 and 96 hours in males and females respectively. For most AEFIs the median TTRs were equal or lower after the second dose compared the first dose, while no such tendency was observed for TTO.
For over 97% of the reported AEFIs, the perceived burden was reported. Figure 2 shows the proportions for all five burden levels for any AEFI, local reactions and the 10 most reported AEFIs by sex, based on the maximal burden level reported by each person for the respective outcome. For all outcomes, a higher fraction of males compared to females reported the lowest burden level (‘Not at all burdensome’), whereas the opposite was true for the highest burden levels. Injection site pain was considered not very burdensome by both males and females.
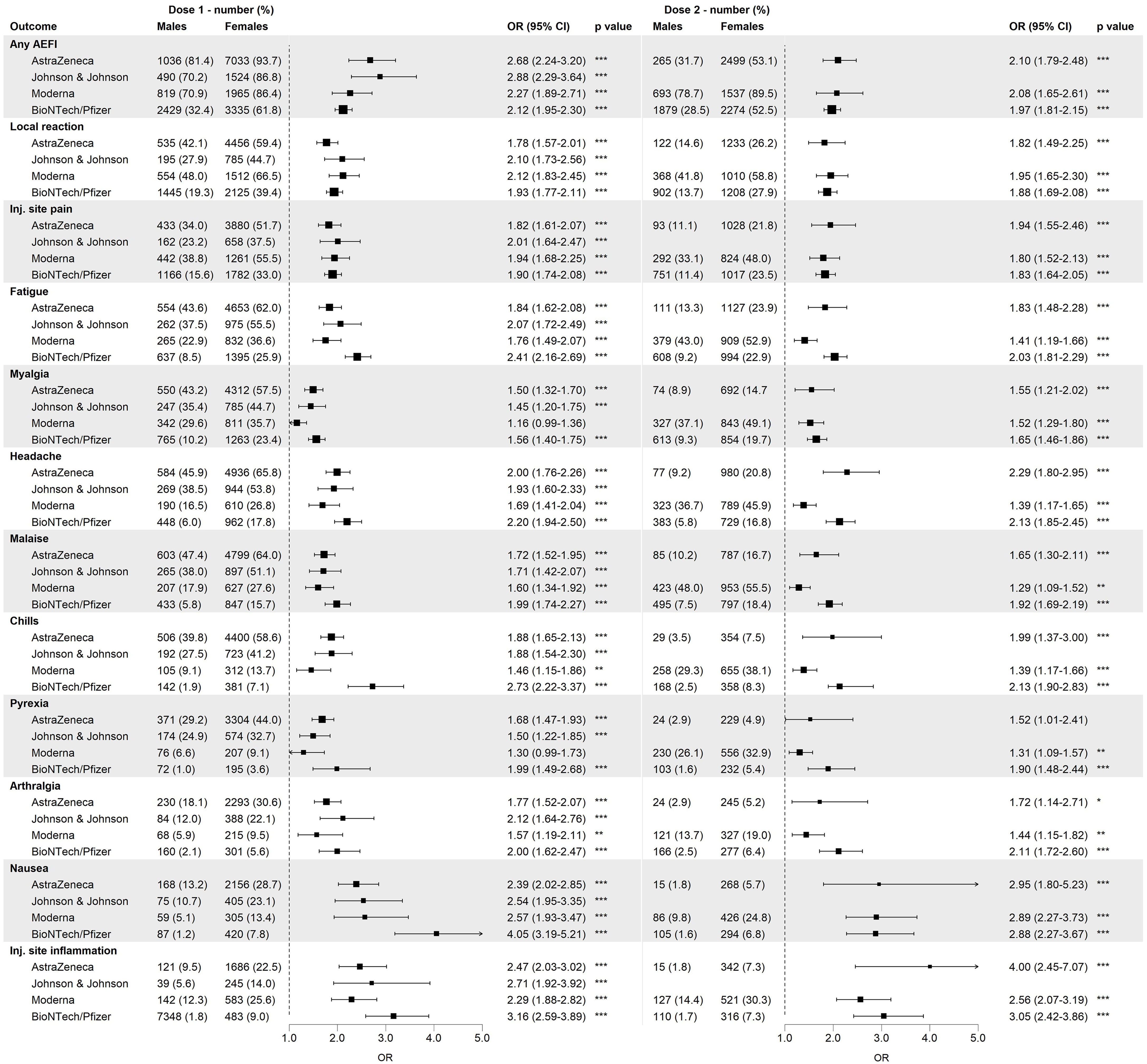
Figure 2 Distribution of the burden classes among males and females among different adverse events following immunization (AEFIs), based on data of the first dose and all vaccine brands combined. Inj.: injection.
The multivariable logistic regression showed that for ‘any AEFI’, vaccinees who reported a burden level of 2, 3, 4 and 5 had respectively 1.40, 2.13, 2.92 and 4.39 times higher odds of being female as compared to vaccinees who reported the lowest burden level (‘Not at all burdensome’) (Supplementary Figure S2), indicating that with each step increase in burden level, the fraction of females compared to males increased as well. This trend was observed for most AEFIs though with a varying magnitude of the association between sex and burden for the studied AEFIs as well as the two doses. The reported burden levels of nausea were not significantly associated with sex, neither was the burden of pyrexia and injection site inflammation after the second dose of vaccination. In contrast, ORs for arthralgia after the second dose were high as a relative small portion of females reported the arthralgia being not at all burdensome as compared to males.
The literature search resulted in 436 unique potentially relevant articles. After exclusion of 340 irrelevant articles based on title or abstract, 96 articles were subjected to full-text screening of which 84 were eligible to be included in the review (Supplementary Figure S3 and Table S8). Forty-three of these articles concerned cross-sectional studies (51.2%) (12, 13, 17–57), 26 were prospective cohort studies (31.0%) (2, 5, 14, 58–80), eight retrospective cohort studies (9.5%) (81–88) and seven were other types of studies (6, 89–94) (8.3%). The majority of the studies were performed in Europe (n=26, 31.0%), in Asia (n=24, 28.6%) and in the Middle East (n=21, 25.0%). With regard to the vaccine brands investigated, the highest percentage of the articles under review assessed the occurrence of AEFIs after Pfizer vaccination (n=71, 84.5%), followed by AstraZeneca (n=34, 40.5%), Moderna (n=24, 28.6%), and Johnson&Johnson (n=9, 10.7%) vaccines (Supplementary Table S8).
Nearly all articles reported a higher incidence of AEFIs in females compared to males with the exception of four articles which found an opposite outcome, for most of which no clear reason could be identified other than a possible skewed distribution of the sexes and age groups in the studies (17, 18, 80, 85). Of the 21 articles which reported a OR of any adverse reaction for sex, the median OR was 1.93 (range: 0.85-3.45, IQR 1.49-2.50) using males as reference group (Supplementary Table S8) (2, 6, 13, 22, 24, 25, 34, 43, 45–47, 52, 54, 56, 61, 63, 66, 68, 79, 81, 85). Similarly, females had a median 1.96 times higher odds of reporting a local reaction compared to males (range: 1.02-2.90, IQR1.85-2.54) (22, 32, 42, 54, 76). With regard to specific common AEFIs, median ORs of 2.42 (range 2.07-4.72), 2.01 (range 1.57-2.61), and 1.77 (range 1.63-1.84) were reported for headache (32, 51, 67, 76), fatigue (22, 32, 51, 67, 76), and fever (32, 68, 76) respectively. TTR was assessed in four articles, all of which reported higher TTR in females compared to males ranging from 1.2-1.9 days in males to 1.4- 2.2 days in females, though the difference was only statistically significant in one article (23, 31, 54, 66). The results in the articles regarding health care seeking behaviour, hosptial admissions and absenteeism due to adverse effects were unambiguous across studies. Females showed a higher rate of health care seeking behavior and absenteeism in two studies (30, 86), whereas two studies reported higher hospital admission rates for males (87, 92).
In this nationwide prospective cohort study, using patient reported outcomes, we assessed the incidences of reported AEFIs after COVID-19 vaccination in males and females and compared the reported TTO, TTR and perceived burden between the sexes. Also, we summarized the literature which reported sex-disaggregated outcomes concerning possible adverse reactions after vaccination with one of the vaccine brands included in our cohort study.
In the past, women were underrepresented in clinical trials and sex-disaggregated results were often not published (95). Current clinical study guidelines recommend that trials include an adequate demographic characterization of the patient/target population, including a representative sex distribution, and that analyses of safety and efficacy data will be stratified on sex, as one cannot assume the absence of sexual dimorphism in the effects of vaccination/drugs (96, 97). Large clinical trials for COVID-19 vaccines have accordingly included a proportionate number of females, however, a literature review showed that 30% of the studies with efficacy data and 34% of the studies with safety data presented sex-disaggregated outcomes (98, 99).
Consistent with the existing body of literature which mainly included post-marketing studies, females showed an around two-fold higher incidence of AEFIs than males in our cohort. The difference was most pronounced after the first dose and for the AEFIs nausea and injection site inflammation. Several factors might contribute to this dimorphism in AEFI incidence including biological differences between both sexes. Compared to males, females show higher humoral and cell-mediated immune responses to vaccination. Binding of sex hormones such as estrogens to specific receptors on immune cells, including dendritic cells, macrophages and lymphocytes, affects signaling pathways involved in the production of chemokines and cytokines ultimately inducing a stronger immune response (9). In contrast, the activity of immune cells is suppressed by testosterone and dihydrotestosterone (100). Beyond sex hormones, the X chromosome expresses 10-fold more genes related to immunity compared to the Y chromosome and polymorphisms in genes on the sex chromosomes can affect immune responses, which explains the presence of differences between the sexes before the reproductive age and after reproductive senescence (9).
Moreover, for some reported AEFIs comprising physical complaints not restricted to vaccination such as headache and nausea, a substantial difference in background incidence (i.e. regardless of vaccination status) exists between both sexes which diluted or fortified the observed effect of vaccination. For instance, over two-fold higher background incidences of headache have been documented in females (101, 102). Similarly, females tend to suffer more from nausea than males in other contexts such as motion sickness or after general anesthesia which is hypothesized to be associated with sexual dimorphisms in availability of neurokinin-1 receptors involved in vomiting and nausea (103).
In both males and females a clear inverse association between age and the probability of experiencing AEFIs was observed owing to the process of immunosenescence (104). Although the effect of aging is stronger, at a higher pace and with an earlier onset in males than females (105, 106), this was not reflected by the incidence of AEFIs in our cohort. The outcomes of multivariable logistic regression showed significant positive associations between occurrence of ‘any AEFI’ and presence of several comorbidities, with ORs varying between 1.14 and 1.75. As for part of these associations a direct causal relationship is lacking, this could be subject of future studies to further elucidate these findings. The use of antipyretic drugs shortly before or after vaccination was associated with a higher odds of having ‘any AEFI’. Although the preventive use of antipyretic drugs would logically assume a lower incidence of AEFIs, the positive association can be the result of the phrasing of the question as people could have taken antipyretic drugs to suppress the symptoms of early AEFI(s) occurring within a few hours after vaccination.
The median TTO and TTR were in line with existing literature although results were not very consistent across studies (23, 24, 31, 54, 66, 83). For the articles which mentioned the TTR, the differences between males and females were generally less than one day (31, 54, 66, 83). The perceived burden of the AEFIs was significantly different between males and females, yet, comparing our results with other studies is difficult as only few studies incorporated a burden or severity indicator and mostly these are based on absenteeism or hospital admission rather than on a perceived scale.
We separately reported on sex-specific AEFIs. Over 300 females reported one or multiple menstrual disorders after the first and/or second dose of vaccination and an additional 13 females reported postmenopausal hemorrhage. The Netherlands Pharmacovigilance Centre Lareb has previously reported on data on menstrual disorders and post-menopausal bleeding, both from the spontaneous reporting system and this cohort (107, 108).
The strengths of this study includes the large study population size and availability of information on immunization status, vaccine brand, comorbidities and COVID-19 infection history. By using patient reported outcomes we ensure that also AEFIs for which no medical attention is needed are captured. Yet, for the interpretation of the outcomes of this study, some limitations should be taken in consideration. We confined the period of an AEFI to be attributed to vaccination to 28 days as most AEFIs occur within the first days to weeks after vaccination. Choosing a narrower time interval could have resulted in AEFIs potentially being missed, whereas a broader time interval would have interfered with the second dose of vaccination. Nonetheless, we might have missed AEFIs with a longer latency time and might have attributed AEFIs with a long latency time incorrectly to the second vaccine dose in case the onset of the AEFI was after the date of second vaccination. Another limitation is the fact that we likely missed several articles in the literature review which reported sex-disaggregated results in the main body of the article but not in the abstract (to which we restricted our search), although this probably does not affect the overall summarized outcomes. Noteworthy, in our analysis, the AEFI pyrexia (at MedDRA PT level) included people with a maximum body temperature of 38-40.5°C or those with fever without measurements. In other studies, the coding or questionnaires might have been different.
In conclusion, this study presents sex-disaggregated outcomes of adverse effects following COVID-19 vaccination of four different vaccine brands. The incidence of any AEFI, local reactions as well as the top 10 most frequent AEFIs was higher in females compared to males for both doses. Also, the results of this cohort study showed that the TTR and the perceived burden differed to a minor extent between both sexes. Our results confirm the outcomes of previous studies and aid in the gain of knowledge with regard to differences in response to vaccination between males and females.
All data relevant to the study are included in the article or uploaded as Supplementary Information.
Dutch regulations oblige all studies in the Netherlands subjected to the Medical Research Involving Human Subjects Act (WMO) to be reviewed by an accredited Medical Ethics Committee (METC) or the central committee on research involving human subjects (CCMO). The board of the METC Brabant reviewed this study and concluded that the rules included in the WMO act did not apply to this study, hence, this study was considered exempt from the requirement for approval.
AK, FH, JD, TL, LB, MR, and TL designed the study part covering analysis of cohort data. TL and LB: performed data cleaning. TL and JD performed data preparation and pre-analysis data coding. JD performed statistical data analysis. FH, JD, and SP designed the literature review. SP performed the literature search. JD, FH, and SP original draft preparation. All authors contributed to the article and approved the submitted version.
The Dutch Ministry of Health, Welfare and Sport granted the funding for the cohort event monitoring of COVID-19 vaccine safety (grant number: 331.880).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1078736/full#supplementary-material
1. Kant A, van Hunsel F, van Puijenbroek E. Numbers of spontaneous reports: How to use and interpret? Br J Clin Pharmacol (2022) 88(3):1365–8. doi: 10.1111/bcp.15024
2. Rolfes L, Härmark L, Kant A, van Balveren L, Hilgersom W, van Hunsel F. COVID-19 vaccine reactogenicity–a cohort event monitoring study in the Netherlands using patient reported outcomes. Vaccine. (2022) 40(7):970–6. doi: 10.1016/j.vaccine.2022.01.013
3. EMA. Guideline on good pharmacovigilance practices (GVP) - annex I - definitions (Rev 4). (2017). EMA/873138/2011 Rev 2, 6/144.
4. Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines (2019) 4(1):1–11. doi: 10.1038/s41541-019-0132-6
5. Kant A, Jansen J, van Balveren L, van Hunsel F. Description of frequencies of reported adverse events following immunization among four different COVID-19 vaccine brands. Drug safety (2022) 45(4):319–31. doi: 10.1007/s40264-022-01151-w
6. Sa S, Lee CW, Shim SR, Yoo H, Choi J, Kim JH, et al. The safety of mRNA-1273, BNT162b2 and JNJ-78436735 COVID-19 vaccines: safety monitoring for adverse events using real-world data. Vaccines. (2022) 10(2):320. doi: 10.3390/vaccines10020320
7. Singh A, Khillan R, Mishra Y, Khurana S. The safety profile of COVID-19 vaccinations in the united states. Am J infection control (2022) 50(1):15–9. doi: 10.1016/j.ajic.2021.10.015
8. Ahamad MM, Aktar S, Uddin MJ, Rashed-Al-Mahfuz M, Azad A, Uddin S, et al. Adverse effects of COVID-19 vaccination: Machine learning and statistical approach to identify and classify incidences of morbidity and post-vaccination reactogenicity. Healthcare (2021) 11(1):31. doi: 10.1101/2021.04.16.21255618
9. Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol (2017) 33:577–99. doi: 10.1146/annurev-cellbio-100616-060718
10. Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin immunopathol (2019) 41(2):239–49. doi: 10.1007/s00281-018-0726-5
11. Hammarström A, Annandale E. A conceptual muddle: an empirical analysis of the use of ‘sex’and ‘gender’in ‘gender-specific medicine’journals. PLosOne (2012) 7(4):e34193. doi: 10.1371/journal.pone.0034193
12. Green MS, Peer V, Magid A, Hagani N, Anis E, Nitzan D. Gender differences in adverse events following the pfizer-BioNTech COVID-19 vaccine. Vaccines. (2022) 10(2):233. doi: 10.3390/vaccines10020233
13. Namiki T, Komine-Aizawa S, Takada K, Takano C, Trinh QD, Hayakawa S. Adverse events after BNT162b2 mRNA COVID-19 vaccination in health care workers and medical students in Japan. J Infection Chemotherapy (2022) 28(8):1220–24. doi: 10.1016/j.jiac.2022.05.002
14. Rivera-Izquierdo M, Soler-Iborte E, de Rojas JP, Pegalajar-García MD, Gil-Villalba A, Ruiz-Villaverde R, et al. Factors associated with adverse reactions to BNT162b2 COVID-19 vaccine in a cohort of 3969 hospital workers. Vaccines. (2021) 10(1):15. doi: 10.3390/vaccines10010015
15. MedDRA. The international conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Welcome to MedDRA (2017).
17. Adam M, Gameraddin M, Alelyani M, Alshahrani MY, Gareeballah A, Ahmad I, et al. Evaluation of post-vaccination symptoms of two common COVID-19 vaccines used in abha, aseer region, kingdom of Saudi Arabia. Patient preference adherence (2021) 15:1963. doi: 10.2147/PPA.S330689
18. Al Bahrani S, Albarrak A, Alghamdi OA, Alghamdi MA, Hakami FH, Al Abaadi AK, et al. Safety and reactogenicity of the ChAdOx1 (AZD1222) COVID-19 vaccine in Saudi Arabia. Int J Infect Dis (2021) 110:359–62. doi: 10.1016/j.ijid.2021.07.052
19. Al Ghafri TS, Al Balushi L, Al Balushi Z, Al Hinai F, Al Hasani S, Anwar H, et al. Reporting at least one adverse effect post-COVID-19 vaccination from primary health care in Muscat. Cureus (2021) 13(8):e17055. doi: 10.7759/cureus.17055
20. Alessa MY, Aledili FJ, Alnasser AA, Aldharman SS, Al Dehailan AM, Abuseer HO, et al. The side effects of COVID-19 vaccines and its association with ABO blood type among the general surgeons in Saudi Arabia. Cureus (2022) 14(3):e23628. doi: 10.7759/cureus.23628
21. Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med (2021), 1796. doi: 10.3389/fmed.2021.760047
22. Aliberti SM, Schiavo L, Boccia G, Santoro E, Franci G, Ruggiero A, et al. Gender and AB0 blood type differences in a unicentric group of university professors in southern Italy who received the vaxzevria COVID-19 vaccine: A cross-sectional survey of vaccine side effects, attitudes, and hesitation. Vaccines. (2022) 10(3):373. doi: 10.3390/vaccines10030373
23. Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, et al. Safety of COVID-19 vaccines. J Med virology (2021) 93(12):6588–94. doi: 10.1002/jmv.27214
24. Almohaya AM, Qari F, Zubaidi GA, Alnajim N, Moustafa K, Alshabi MM, et al. Early solicited adverse events following the BNT162b2 mRNA vaccination, a population survey from Saudi Arabia. Prev Med Rep (2021) 24:101595. doi: 10.1016/j.pmedr.2021.101595
25. Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in iraq; a retrospective cross-sectional study. Diabetes Metab Syndrome: Clin Res Rev (2021) 15(5):102207. doi: 10.1016/j.dsx.2021.102207
26. Bauernfeind S, Salzberger B, Hitzenbichler F, Scigala K, Einhauser S, Wagner R, et al. Association between reactogenicity and immunogenicity after vaccination with BNT162b2. Vaccines. (2021) 9(10):1089. doi: 10.3390/vaccines9101089
27. Borroni E, Consonni D, Cugno M, Lombardi A, Mangioni D, Bono P, et al. Side effects among healthcare workers from a large Milan university hospital after second dose of BNT162b2 mRNA COVID-19 vaccine. La Medicina del lavoro (2021) 112(6):477. doi: 10.23749/mdl.v112i6.12507
28. Bukhari AE, Almutlq MM, Bin Dakhil AA, Alhetheli GI, Alfouzan SK, Alqahtani MA, et al. Cutaneous adverse reactions to coronavirus vaccines: A Saudi nationwide study. Dermatologic Ther (2022) 35(6):e15452. doi: 10.1111/dth.15452
29. Canlas FQ, Nair S, Paat ID. Exploring COVID-19 vaccine side effects: A correlational study using Python. Proc Comput Science (2022) 201:752–7. doi: 10.1016/j.procs.2022.03.102
30. Cohen G, Jungsomsri P, Sangwongwanich J, Tawinprai K, Siripongboonsitti T, Porntharukchareon T, et al. Immunogenicity and reactogenicity after heterologous prime-boost vaccination with CoronaVac and ChAdox1 nCov-19 (AZD1222) vaccines. Hum Vaccines Immunotherapeutics (2022) 18(5):1–7. doi: 10.1080/21645515.2022.2052525
31. Costantino M, Sellitto C, Conti V, Corbi G, Marongiu F, Genovese G, et al. Adverse events associated with BNT162b2 and AZD1222 vaccines in the real world: Surveillance report in a single Italian vaccine center. J Clin Med (2022) 11(5):1408. doi: 10.3390/jcm11051408
32. Cuschieri S, Borg M, Agius S, Souness J, Brincat A, Grech V. Adverse reactions to pfizer-BioNTech vaccination of healthcare workers at malta's state hospital. Int J Clin Practice (2021) 75(10):e14605. doi: 10.1111/ijcp.14605
33. Dar-Odeh N, Abu-Hammad O, Qasem F, Jambi S, Alhodhodi A, Othman A, et al. Long-term adverse events of three COVID-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan and Saudi Arabia. Hum Vaccines Immunotherapeutics. (2022) 18(1):2039017. doi: 10.1080/21645515.2022.2039017
34. Elnaem MH, Mohd Taufek NH, Ab Rahman NS, Mohd Nazar NI, Zin CS, Nuffer W, et al. COVID-19 vaccination attitudes, perceptions, and side effect experiences in Malaysia: Do age, gender, and vaccine type matter? Vaccines (2021) 9(10):1156. doi: 10.3390/vaccines9101156
35. El-Shitany NA, Harakeh S, Badr-Eldin SM, Bagher AM, Eid B, Almukadi H, et al. Minor to moderate side effects of pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med (2021) 14:1389. doi: 10.2147/IJGM.S310497
36. Gan L-L, Kamaluzaman M, Zulkeplee NAB, Amin INBM, Ng DC-E. Adverse events following BNT162b2 mRNA COVID-19 vaccination among healthcare workers: A single-centre experience in Malaysia. MJM. (2022) 77(3):300.
37. Ganesan S, Al Ketbi LMB, Al Kaabi N, Al Mansoori M, Al Maskari NN, Al Shamsi MS, et al. Vaccine side effects following COVID-19 vaccination among the residents of the UAE–an observational study. Front Public Health (2022) 10. doi: 10.3389/fpubh.2022.876336
38. Hatmal M, Al-Hatamleh MA, Olaimat AN, Mohamud R, Fawaz M, Kateeb ET, et al. Reported adverse effects and attitudes among Arab populations following COVID-19 vaccination: a large-scale multinational study implementing machine learning tools in predicting post-vaccination adverse effects based on predisposing factors. Vaccines. (2022) 10(3):366. doi: 10.3390/vaccines10030366
39. Kang YM, Lim J, Choe K-W, Lee K-D, Jo DH, Kim MJ, et al. Reactogenicity after the first and second doses of BNT162b2 mRNA coronavirus disease vaccine: a single-center study. Clin Exp Vaccine Res (2021) 10(3):282. doi: 10.7774/cevr.2021.10.3.282
40. Khan M, Ferdous J, Akhter S, Esha A, Islam M. Tracking side effects of the COVID-19 vaccine in mymensingh district of Bangladesh. Mymensingh Med Journal: MMJ. (2022) 31(1):1–9.
41. Kitagawa H, Kaiki Y, Sugiyama A, Nagashima S, Kurisu A, Nomura T, et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J Infection Chemother (2022) 28(4):576–81. doi: 10.1016/j.jiac.2021.12.034
42. Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt H-P, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology. (2021) 10(8):752. doi: 10.3390/biology10080752
43. Lounis M, Rais MA, Bencherit D, Aouissi HA, Oudjedi A, Klugarová J, et al. Side effects of COVID-19 inactivated virus vs. adenoviral vector vaccines: Experience of Algerian healthcare workers. Front Public Health (2022) 10. doi: 10.3389/fpubh.2022.896343
44. Mahallawi WH, Mumena WA. Reactogenicity and immunogenicity of the pfizer and AstraZeneca COVID-19 vaccines. Front Immunol (2021), 5169. doi: 10.3389/fimmu.2021.794642
45. Mohammed RA, Garout RM, Wahid S, Ayub F, ZinAlddin LMF, Sultan I. A survey on the side effects of Pfizer/BioNTech COVID-19 vaccine among vaccinated adults in Saudi Arabia. Cureus. (2021) 13(11):e19222. doi: 10.7759/cureus.19222
46. Moll ME, Martínez AM, Cisneros BT, Onofre JI, Floriano GN, de León MB. Extension and severity of self-reported side effects of seven COVID-19 vaccines in Mexican population. Front Public Health (2022) 10:834744.
47. Muluneh AG, Merid MW, Gelaye KA, Tilahun SY, Teshager NW, Abereha AY, et al. More than three-fourths of AstraZeneca (ChAdox1 COV-19) COVID-19 vaccinated individuals develop post immunization adverse event in Northwest Ethiopia. Infection Drug Resistance. (2022) 15:2409. doi: 10.2147/IDR.S360605
48. Nguyen HA, Le TTA, Truong TT, Nguyen PT, Nguyen TTH. Factors influencing adverse events following immunization with AZD1222 in Vietnamese adults during first half of 2021. Vaccine. (2021) 39(44):6485–91. doi: 10.1016/j.vaccine.2021.09.060
49. Nishizawa T, Jinta T, Koyamada R, Uehara Y, Taki F, Arioka H. Adverse reactions of BNT162b2 vaccine booster against COVID-19 in Japan. J Gen Family Med (2022) 23(5):360–2. doi: 10.1002/jgf2.545
50. Otani J, Ohta R, Sano C. Association between immunoglobulin G levels and adverse effects following vaccination with the BNT162b2 vaccine among Japanese healthcare workers. Vaccines. (2021) 9(10):1149. doi: 10.3390/vaccines9101149
51. Rahmani A, Dini G, Orsi A, Sticchi L, Bruzzone B, Montecucco A, et al. Reactogenicity of bnt162b2 mrna covid-19 vaccine in a young working age population: A survey among medical school residents, within a mass vaccination campaign, in a regional reference teaching hospital in italy. Vaccines. (2021) 9(11):1269. doi: 10.3390/vaccines9111269
52. Ripabelli G, Tamburro M, Buccieri N, Adesso C, Caggiano V, Cannizzaro F, et al. Active surveillance of adverse events in healthcare workers recipients after vaccination with COVID-19 BNT162b2 vaccine (Pfizer-BioNTech, comirnaty): a cross-sectional study. J Community Health (2022) 47(2):211–25. doi: 10.1007/s10900-021-01039-3
53. Riad A, Hocková B, Kantorová L, Slávik R, Spurná L, Stebel A, et al. Side effects of mRNA-based COVID-19 vaccine: nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals. (2021) 14(9):873. doi: 10.3390/ph14090873
54. Riad A, Pokorná A, Klugarová J, Antalová N, Kantorová L, Koščík M, et al. Side effects of mRNA-based COVID-19 vaccines among young adults (18–30 years old): an independent post-marketing study. Pharmaceuticals. (2021) 14(10):1049. doi: 10.3390/ph14101049
55. Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, et al. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines. (2021) 9(6):673. doi: 10.3390/vaccines9060673
56. Ughi N, Del Gaudio F, Dicuonzo A, Orso M, Micheloni G, Puoti M, et al. Host factors and history of SARS-CoV-2 infection impact the reactogenicity of BNT162b2 mRNA vaccine: results from a cross-sectional survey on 7,014 workers in healthcare. Eur Rev Med Pharmacol Sci (2021) 25(24):7985–96.
57. Vigezzi GP, Lume A, Minerva M, Nizzero P, Biancardi A, Gianfredi V, et al. Safety surveillance after BNT162b2 mRNA COVID-19 vaccination: results from a cross-sectional survey among staff of a large Italian teaching hospital. Acta Bio Medica: Atenei Parmensis (2021) 92(Suppl 6):e2021450. doi: 10.23750/abm.v92iS6.12217
58. Alharbi NK, Al-Tawfiq JA, Alghnam S, Alwehaibe A, Alasmari A, Alsagaby SA, et al. Outcomes of single dose COVID-19 vaccines: Eight month follow-up of a large cohort in Saudi Arabia. J infection Public Health (2022) 15(5):573–7. doi: 10.1016/j.jiph.2022.04.001
59. Amodio E, Minutolo G, Casuccio A, Costantino C, Graziano G, Mazzucco W, et al. Adverse reactions to anti-SARS-CoV-2 vaccine: A prospective cohort study based on an active surveillance system. Vaccines. (2022) 10(3):345. doi: 10.3390/vaccines10030345
60. Anastassopoulou C, Antoni D, Manoussopoulos Y, Stefanou P, Argyropoulou S, Vrioni G, et al. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: A mixed effects model across two vaccination periods. PLos One (2022) 17(4):e0266958. doi: 10.1371/journal.pone.0266958
61. Azzolini E, Canziani LM, Voza A, Desai A, Pepys J, De Santis M, et al. Short-term adverse events and antibody response to the BNT162b2 SARS-CoV-2 vaccine in 4156 health care professionals. Vaccines. (2022) 10(3):439. doi: 10.3390/vaccines10030439
62. Bae S, Lee YW, Lim SY, Lee J-H, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in south Korea. J Korean Med Sci (2021) 36(17):e115. doi: 10.3346/jkms.2021.36.e115
63. Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA network Open (2021) 4(12):e2140364–e. doi: 10.1001/jamanetworkopen.2021.40364
64. Chapin-Bardales J, Myers T, Gee J, Shay DK, Marquez P, Baggs J, et al. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system. Vaccine. (2021) 39(48):7066–73. doi: 10.1016/j.vaccine.2021.10.019
65. Coggins SAA, Laing ED, Olsen CH, Goguet E, Moser M, Jackson-Thompson BM, et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. Open Forum Infect Dis (2022) 9(1):ofab575. doi: 10.1093/ofid/ofab575
66. Darraj MA, Al-Mekhlafi HM. Prospective evaluation of side-effects following the first dose of Oxford/AstraZeneca COVID-19 vaccine among healthcare workers in Saudi Arabia. Vaccines. (2022) 10(2):223. doi: 10.3390/vaccines10020223
67. Filippatos F, Tatsi E-B, Dellis C, Dessypris N, Syriopoulou V, Michos A. Association of clinical and epidemiological characteristics with COVID-19 BNT162b2 mRNA vaccine short-term adverse reactions in healthcare workers. Hum Vaccines Immunotherapeutics. (2021) 17(12):4755–60. doi: 10.1080/21645515.2021.1985356
68. Izumo T, Kuse N, Awano N, Tone M, Sakamoto K, Takada K, et al. Side effects and antibody titer transition of the BNT162b2 messenger ribonucleic acid coronavirus disease 2019 vaccine in Japan. Respir Invest (2021) 59(5):635–42. doi: 10.1016/j.resinv.2021.06.003
69. Jacobson MA, Zakaria A, Maung Z, Hart C, McCalmont TH, Fassett M, et al. Incidence and characteristics of delayed injection site reaction to the mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (Moderna) in a cohort of hospital employees. Clin Infect Dis (2022) 74(4):591–6. doi: 10.1093/cid/ciab518
70. Koh JS, Hoe RHM, Yong MH, Chiew HJ, Goh Y, Yong KP, et al. Hospital-based observational study of neurological disorders in patients recently vaccinated with COVID-19 mRNA vaccines. J Neurological Sci (2021) 430:120030. doi: 10.1016/j.jns.2021.120030
71. Lee SW, Lee H, Lee S-K, Moon J-Y, Moon S, Chung SJ, et al. Risk factors for grade 3 to grade 4 adverse reactions to the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2. Front Med (2021) 8. doi: 10.3389/fmed.2021.738049
72. Lee YW, Lim SY, Lee J-H, Lim JS, Kim M, Kwon S, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci (2021) 36(21):e153. doi: 10.3346/jkms.2021.36.e153
73. Maruyama A, Sawa T, Teramukai S, Katoh N. Adverse reactions to the first and second doses of pfizer-BioNTech COVID-19 vaccine among healthcare workers. J Infection Chemother (2022) 28(7):934–42. doi: 10.1016/j.jiac.2022.03.015
74. Saita M, Yan Y, Ito K, Sasano H, Seyama K, Naito T. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers in Japan. J Infection Chemother (2022) 28(1):116–9. doi: 10.1016/j.jiac.2021.09.009
75. Sauserienė J, Liseckienė I, Neverauskė V, Šepetauskienė E, Serapinas D, Mačinskas Š, et al. Adverse events and immunogenicity of mRNA-based COVID-19 vaccine among healthcare workers: a single-centre experience. Medicina. (2022) 58(3):441. doi: 10.3390/medicina58030441
76. Urakawa R, Isomura ET, Matsunaga K, Kubota K, Ike M. Impact of age, sex and medical history on adverse reactions to the first and second dose of BNT162b2 mRNA COVID-19 vaccine in Japan: a cross-sectional study. BMC Infect Dis (2022) 22(1):1–8. doi: 10.1186/s12879-022-07175-y
77. Undugodage C, Dissanayake U, Kumara H, Samarasekera B, Yapa L, Ganegma R, et al. Reactogenicity to ChAdOx1 nCoV-19 vaccine in health care workers: A multicenter observational study in Sri Lanka. Ceylon Med J (2021) 66(4):177–84. doi: 10.4038/cmj.v66i4.9508
78. Uwamino Y, Kurafuji T, Sato Y, Tomita Y, Shibata A, Tanabe A, et al. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: An observational study of 646 Japanese healthcare workers and university staff. Vaccine. (2022) 40(7):1019–25. doi: 10.1016/j.vaccine.2022.01.002
79. Warkentin L, Zeschick N, Kühlein T, Steininger P, Überla K, Kaiser I, et al. Reactogenicity after heterologous and homologous COVID-19 prime-boost vaccination regimens: descriptive interim results of a comparative observational cohort study. BMC Infect Dis (2022) 22(1):1–15. doi: 10.1186/s12879-022-07443-x
80. Wi Y-M, Kim S-H, Peck K-R. Early adverse events between mRNA and adenovirus-vectored COVID-19 vaccines in healthcare workers. Vaccines. (2021) 9(8):931. doi: 10.3390/vaccines9080931
81. Almohaya AM, Alsubie H, Alqarni B, Alzayad B, Alghar A, Alshahrani K, et al. Acute unsolicited adverse events following BNT162b2 vaccine in Saudi Arabia, a real-world data. Vaccine. (2022) 40(3):477–82. doi: 10.1016/j.vaccine.2021.12.001
82. Hibino M, Ishihara T, Iwata M, Doi Y. Delayed injection site reaction after mRNA-1273 vaccination in Japan: a retrospective, cross-sectional study. Open Forum Infect Dis (2021) 8(10):ofab497. Oxford University Press US. doi: 10.1093/ofid/ofab497
83. Higashino T, Yamazaki Y, Senda S, Satou Y, Yonekura Y, Imai K, et al. Assessment of delayed Large local reactions after the first dose of the SARS-CoV-2 mRNA-1273 vaccine in Japan. JAMA Dermatol (2022) 158(8):923–7. doi: 10.1001/jamadermatol.2022.2088
84. Hoffmann MA, Wieler HJ, Enders P, Buchholz H-G, Plachter B. Age-and sex-graded data evaluation of vaccination reactions after initial injection of the BNT162b2 mRNA vaccine in a local vaccination center in Germany. Vaccines. (2021) 9(8):911. doi: 10.3390/vaccines9080911
85. Loosen SH, Bohlken J, Weber K, Konrad M, Luedde T, Roderburg C, et al. Factors associated with non-severe adverse reactions after vaccination against SARS-CoV-2: A cohort study of 908,869 outpatient vaccinations in Germany. Vaccines. (2022) 10(4):566. doi: 10.3390/vaccines10040566
86. Nachtigall I, Bonsignore M, Hohenstein S, Bollmann A, Günther R, Kodde C, et al. Effect of gender, age and vaccine on reactogenicity and incapacity to work after COVID-19 vaccination: a survey among health care workers. BMC Infect Dis (2022) 22(1):1–13. doi: 10.1186/s12879-022-07284-8
87. Oh TH, Woo SH, Hong S, Lee C, Lee WJ, Jeong SK. Clinical features of patients presenting to the emergency department with cardiovascular adverse reactions after COVID-19 mRNA vaccination. J Korean Med Sci (2022) 37(9):e73. doi: 10.3346/jkms.2022.37.e73
88. Powell AA, Power L, Westrop S, McOwat K, Campbell H, Simmons R, et al. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, march– June 2021, England. Eurosurveillance. (2021) 26(28):2100634. doi: 10.2807/1560-7917.ES.2021.26.28.2100634
89. Kaur RJ, Dutta S, Charan J, Bhardwaj P, Tandon A, Yadav D, et al. Cardiovascular adverse events reported from COVID-19 vaccines: a study based on WHO database. Int J Gen Med (2021) 14:3909. doi: 10.2147/IJGM.S324349
90. Sanyaolu A, Marinkovic A, Prakash S, Desai P, Haider N, Abbasi AF, et al. Reactogenicity to COVID-19 vaccination in the united states of America. Clin Exp Vaccine Res (2022) 11(1):104. doi: 10.7774/cevr.2022.11.1.104
91. Shay DK. Safety monitoring of the janssen (Johnson & Johnson) COVID-19 vaccine–united states, march–April 2021. MMWR Morbidity mortality weekly Rep (2021) 70(18):680–4. doi: 10.15585/mmwr.mm7018e2
92. Xiong X, Yuan J, Li M, Jiang B, Lu ZK. Age and gender disparities in adverse events following COVID-19 vaccination: Real-world evidence based on big data for risk management. Front Med (2021) 8. doi: 10.3389/fmed.2021.700014
93. Zhang R, Leung K-Y, Liu D, Fan Y, Lu L, Chan P-C, et al. Correlation of immunogenicity and reactogenicity of BNT162b2 and CoronaVac SARS-CoV-2 vaccines. Msphere. (2022) 7(2):e00915–21. doi: 10.1128/msphere.00915-21
94. Zhao H, Souders C, Carmel M, Anger JT. Low rates of urologic side effects following coronavirus disease vaccination: an analysis of the food and drug administration vaccine adverse event reporting system. Urology. (2021) 153:11–3. doi: 10.1016/j.urology.2021.04.002
95. Liu KA, Dipietro Mager NA. Women’s involvement in clinical trials: historical perspective and future implications. Pharm Pract (Granada) (2016) 14(1):0–. doi: 10.18549/PharmPract.2016.01.708
96. Agency EM ICH gender considerations in the conduct of clinical trials. (2005). EMEA/CHMP/3916/2005 – ICH, 1–9.
97. Jensen A, Stromme M, Moyassari S, Chadha AS, Tartaglia MC, Szoeke C, et al. COVID-19 vaccines: Considering sex differences in efficacy and safety. Contemp Clin Trials (2022) 115:106700. doi: 10.1016/j.cct.2022.106700
98. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. New Engl J Med (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
99. Vassallo A, Shajahan S, Harris K, Hallam L, Hockham C, Womersley K, et al. Sex and gender in COVID-19 vaccine research: Substantial evidence gaps remain. Front Global women's Health (2021) 2. doi: 10.3389/fgwh.2021.761511
100. Klein SL, Jedlicka A, Pekosz A. The xs and y of immune responses to viral vaccines. Lancet Infect dis (2010) 10(5):338–49. doi: 10.1016/S1473-3099(10)70049-9
101. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the united states: Updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache: J Head Face Pain (2021) 61(1):60–8. doi: 10.1111/head.14024
102. Lyngberg A, Rasmussen B, Jørgensen T, Jensen R. Incidence of primary headache: a Danish epidemiologic follow-up study. Am J Epidemiol (2005) 161(11):1066–73. doi: 10.1093/aje/kwi139
103. Arslanian-Engoren C, Engoren M. Physiological and anatomical bases for sex differences in pain and nausea as presenting symptoms of acute coronary syndromes. Heart Lung (2010) 39(5):386–93. doi: 10.1016/j.hrtlng.2009.10.013
104. Xu W, Wong G, Hwang YY, Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol (2020) 42(5):559–72. doi: 10.1007/s00281-020-00824-x
105. Shapiro JR, Morgan R, Leng SX, Klein SL. Roadmap for sex-responsive influenza and COVID-19 vaccine research in older adults. Front Aging (2022) 7. doi: 10.3389/fragi.2022.836642
106. Márquez EJ, C-h C, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, et al. Sexual-dimorphism in human immune system aging. Nat Commun (2020) 11(1):1–17. doi: 10.1038/s41467-020-14396-9
107. Lareb. Overview menstrual disorders after covid-19 vaccination – update. (2022). Available at: https://www.lareb.nl/media/dxcjbjmv/signals_2022_covid19-vaccines-and-menstrual-disorders_update.pdf.
108. Lareb. Postmenopausal bleeding after administration of COVID-19 vaccines. (2022). Available at: https://www.lareb.nl/media/uc4hen1c/signals_2022_postmenopausal-bleeding_covid-19-vaccines.pdf.
Keywords: COVID-19 vaccine, sex, adverse event after vaccination, patient reported outcome, pharmacovigilance, longitudinal cohort design
Citation: Duijster JW, Lieber T, Pacelli S, Van Balveren L, Ruijs LS, Raethke M, Kant A and Van Hunsel F (2023) Sex-disaggregated outcomes of adverse events after COVID-19 vaccination: A Dutch cohort study and review of the literature. Front. Immunol. 14:1078736. doi: 10.3389/fimmu.2023.1078736
Received: 24 October 2022; Accepted: 11 January 2023;
Published: 30 January 2023.
Edited by:
Tao-Hsin Tung, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, ChinaReviewed by:
Qihan Li, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2023 Duijster, Lieber, Pacelli, Van Balveren, Ruijs, Raethke, Kant and Van Hunsel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janneke W. Duijster, ai5kdWlqc3RlckBsYXJlYi5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.