
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 18 January 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1078705
This article is part of the Research Topic The Immunosuppressive Tumor Microenvironment and Strategies to Revert its Immune Regulatory Milieu for Cancer Immunotherapy View all 15 articles
 Feng Zhou1,2,3,4
Feng Zhou1,2,3,4 Yang Liu1,2,3,4
Yang Liu1,2,3,4 Cong Liu1,2,3,4
Cong Liu1,2,3,4 Fangfei Wang1,2,3,4
Fangfei Wang1,2,3,4 Jianxiang Peng1,2,3,4
Jianxiang Peng1,2,3,4 Yong Xie1,2,3,4*
Yong Xie1,2,3,4* Xiaojiang Zhou1,2,3,4*
Xiaojiang Zhou1,2,3,4*Background and aims: Tumor-associated macrophage (TAM) is a highly abundant immune population in tumor microenvironment, which plays an important role in tumor growth and progression. The aim of our study was to explore the development trends and research hotspots of TAM by bibliometric method.
Methods: The publications related to TAM were obtained from the Web of Science Core Collection database. Bibliometric analysis and visualization were conducted using VOSviewer, CiteSpace and R software.
Results: A total of 6,405 articles published between 2001 and 2021 were included. The United States and China received the most citations, whereas the University of Milan, the university of California San Francisco and Sun Yat-sen University were the main research institutions. Mantovani, Alberto from Humanitas University was the most productive authors with the most citations. Cancer Research published the most articles and received the most co-citations. Activation, angiogenesis, breast cancer, NF-κB and endothelial growth factor were important keywords in TAM research. Among them, PD-1/L1, nanoparticle, PI3Kγ, resistance and immune microenvironment have become the focus of attention in more recent research.
Conclusions: The research on TAM is rapidly evolving with active cooperation worldwide. Anticancer therapy targeting TAM is emerging and promising area of future research, especially in translational application. This may provide guidance and new insights for further research in the field of TAM.
Macrophages has long been considered to be an evolutionarily ancient cell type involved in tissue homeostasis and immune defense. Recently, macrophages were discovered to regulate a variety of diseases depending on the surrounding tissue microenvironment, especially for cancer (1–3). Tumor-associated macrophage (TAM) is a highly abundant immune population in tumors, which plays an important role in cancer progression, metastasis and treatment resistance.
The ability of macrophages to adapt to subtle changes in external stimuli results in the diversity of TAM between different types of cancer or within the same tumor. Macrophages are generally divided into classically activated M1 phenotypes and alternately activated M2 phenotypes to reflect the Th1/Th2 immune response. Although TAM often shows more similar patterns to M2- polarized macrophages that suppresses immune responses and promotes tumor progression, the simplified M1/M2 definition might not be sufficient to cover the full complexity of TAMs (4). In fact, TAM rarely completely follow the true M1 and M2 phenotypes, and even some macrophages can share both M1 and M2 signatures (5–7). In addition, the cell subsets do not exist at a steady stage and changes as the tumor progresses. Each population has a unique landscape based on the type, stage and immune composition of the infiltrated tumors. The plasticity and heterogeneity allow TAM to promote or suppress tumor growth and progression through multiple pathways. Therefore, there is great significance to quantitatively evaluate the research status, focus area and development trend of TAM.
Bibliometrics is an interdisciplinary science that provides a comprehensive and objective assessment of knowledge carriers by mathematics and statistics (8–10). The bibliographic analysis helps scholars understand the development of specific topic and reveals the evolution trend of this field. This study aimed to explore the landscape of tumor-associated macrophages, hoping to provide new clues and ideas for future research in the field of TAM.
Scientific output data was extracted from the Web of Science Core Collection (WoSCC) database, which is one of the most widely used source for academic and bibliometric analysis. The search formula was presented as follows: TS = (“tumor associated macrophage*”) OR (“tumor-associated macrophage*”) OR (“tumour associated macrophage*”) OR (“tumour-associated macrophage*”) OR (“cancer associated macrophage*”) OR (“cancer-associated macrophage*”). The publication period was limited to between 2001 and 2021, and the publication type was limited to original articles written in English. Moreover, we also used broader terms as a benchmark dataset to better evaluate the overall trend of immune cell research in cancer such as “tumor OR tumour OR cancer” and “T cell OR macrophage OR neutrophil*”. The literature search and data collection were performed independently by two researchers to ensure the reliability of the results.
Original data was extracted from selected publications, including titles, abstracts, authors, affiliations, countries/regions, journals, publication years, references and keywords. The H-index of scholars, impact factor (IF) and Journal citation reports (JCR) division of journals were obtained from the Web of Science. Productivity of activities is measured by the number of citations. Overlapping items were merged into a single element and misspelled words were corrected artificially. The cleaned data were exported for further analysis.
Bibliometric indicators are used to quantitatively describe and evaluate the characteristics of literature and its trends. We used R software to conduct Lotka’s Law analysis (11). VOSviewer is a bibliometric tool for developing scientometric network and knowledge visualization (12). The network graph generated by VOSviewer displays the size of nodes according to the number of publications, where closely related nodes are grouped into the same cluster. The connection indicates the association of different nodes, and the thickness of the connection depends on the strength of the association. Centrality is used to measure the importance of a node’s location in the network, and nodes with centrality greater than 0.1 are generally considered as critical nodes. CiteSapce software provides new angle for the bursts of research hotspots in the field of TAM (13).
A total of 9,694 literatures were published in the field between 2001 and 2021. According to the exclusion criteria, we finally included 6,405 eligible original articles in our study. The specific flow diagram was illustrated in Figure 1.
The overall growth trend of immune cell research in cancer were showed in Figure 2A. Although T cell is the most heavily studied immune population, the field of macrophage showed similar increase rate of up to three times. For tumor-associated macrophage research, the number of articles published exhibited a steady increase from 2001 to 2021 (Figure 2B). The output of publications from 2001 to 2008 was low, with less than 100 articles per year. With the fast increase in the number of annual publications, there were 6,028 articles on TAM published between 2009 and 2021, accounting for 94.1% in the past two decades. These findings indicated that TAM has gained great interest and entered the phase of rapid development.
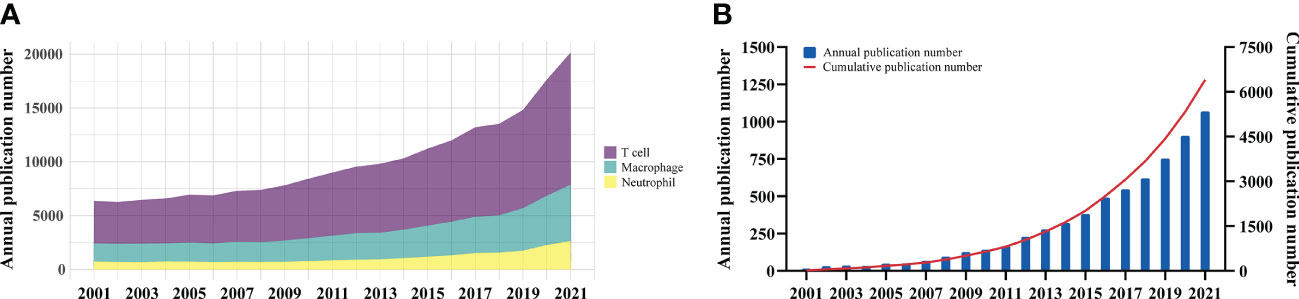
Figure 2 (A) The overall growth trend of annual immune cell research in cancer. (B) Annual and cumulative growth trend of TAM publications.
The publications on TAM were conducted by 5,294 institutions in 99 countries/regions (Table 1). The United States received the highest citations (N=123799), followed by China (N=71126) and Italy (N=28368). Annual citations per publication peaked in the middle of the study period in most countries/regions, especially for Italy (Figure 3A). Although China carried out the most publications, the average number of citations is lower than other countries/regions. The bibliometric map revealed the tight communications between countries/regions (Figure 3B). Intense collaborations between countries/regions resulted in thicker connecting lines between nodes. Of them, the centrality of the United States is as high as 0.37, suggesting that it plays a strong bridge role between the cooperations. In addition, China, Italy, Germany, Japan and United Kingdom are also important nodes among clusters, with centrality greater than 0.1.
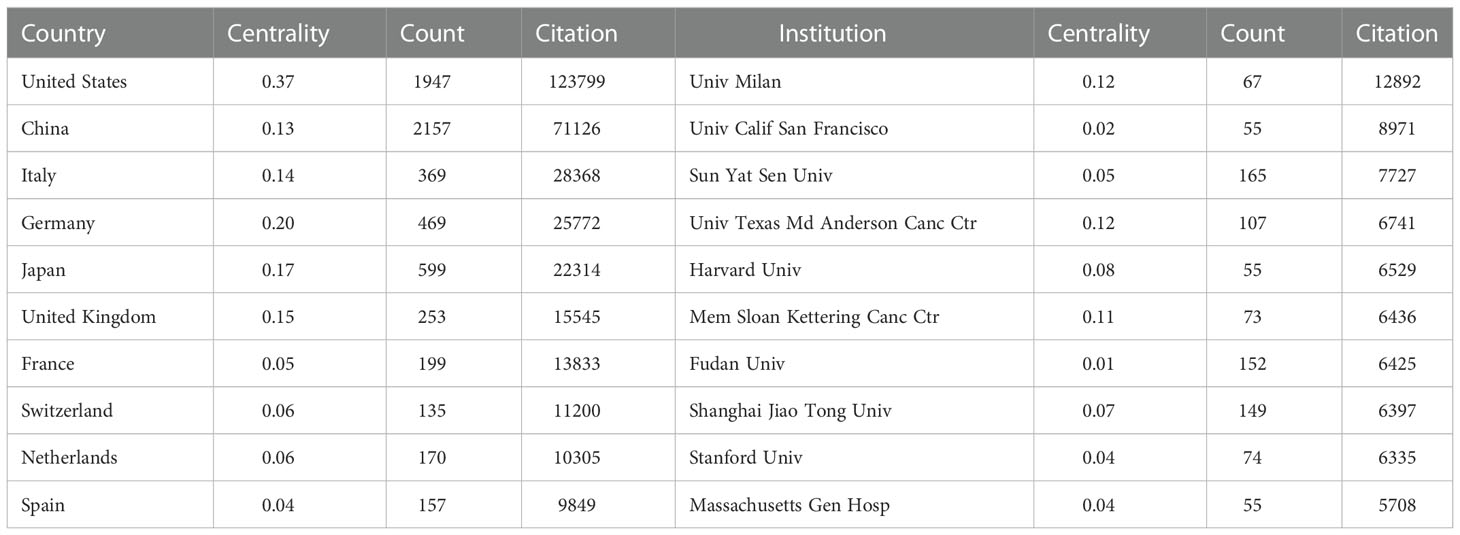
Table 1 The top 10 countries/regions and institutions that have contributed to publications on tumor-associated macrophage research.
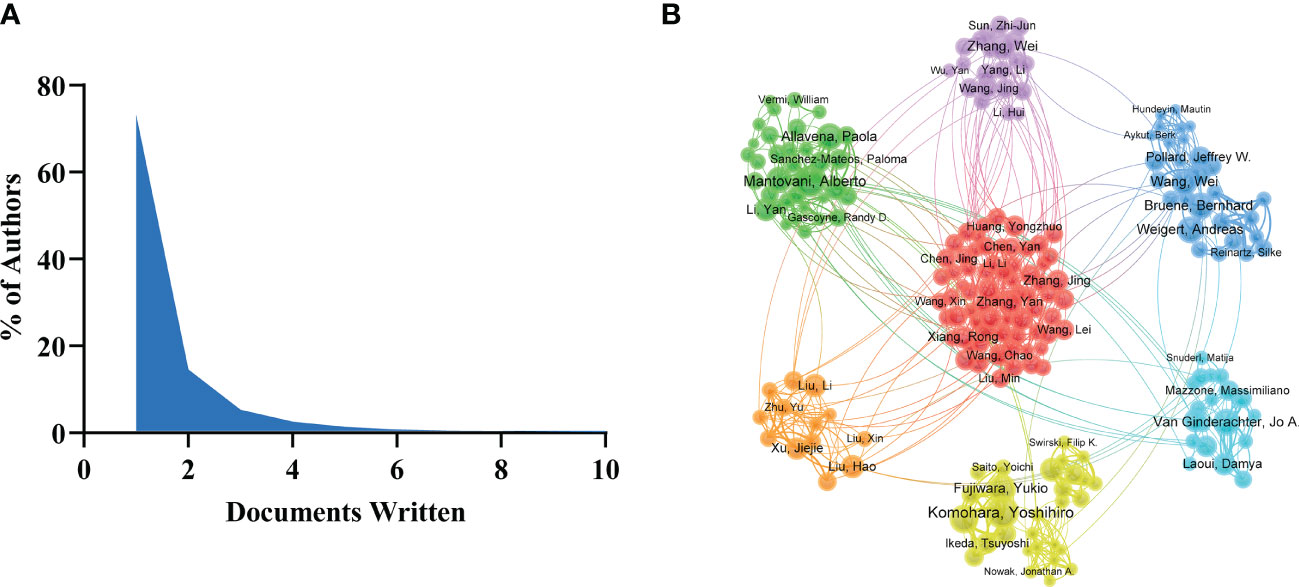
Figure 3 (A) Scientific productivity of authors based on Lotka’s Law. (B) The network map of authors on tumor-associated macrophage research.
The 5,294 institutions constituted seven main clusters (Figure 3C). The University of Milan, the university of California San Francisco and Sun Yat-sen University were the most productive institutions, with centrality ranged from 0.02 to 0.12. The University of Texas MD Anderson Cancer Center and Memorial Sloan-Kettering Cancer Center also had a centrality of more than 0.1 and belonged to a key node of the network.
There were 41,399 authors involved in the study of tumor-associated macrophages. Scientific productivity based on Lotka’s law shows that 73.1% of authors contributed only one publication (Figure 4A). Mantovani, Alberto from Humanitas University received the most citations (N=10675) with the most publications (Table 2). The next productive authors were Sica, Antonio from University of Eastern Piedmont Amedeo Avogadro (N=9344) and Coussens, Lisa M from University of California San Francisco (N=5745). There were active collaborations among the author clusters of seven different colors (Figure 4B). A certain degree of collaborations existed between two linked nodes in different clusters, such as Pollard, Jeffrey W and De Palma, Michele.
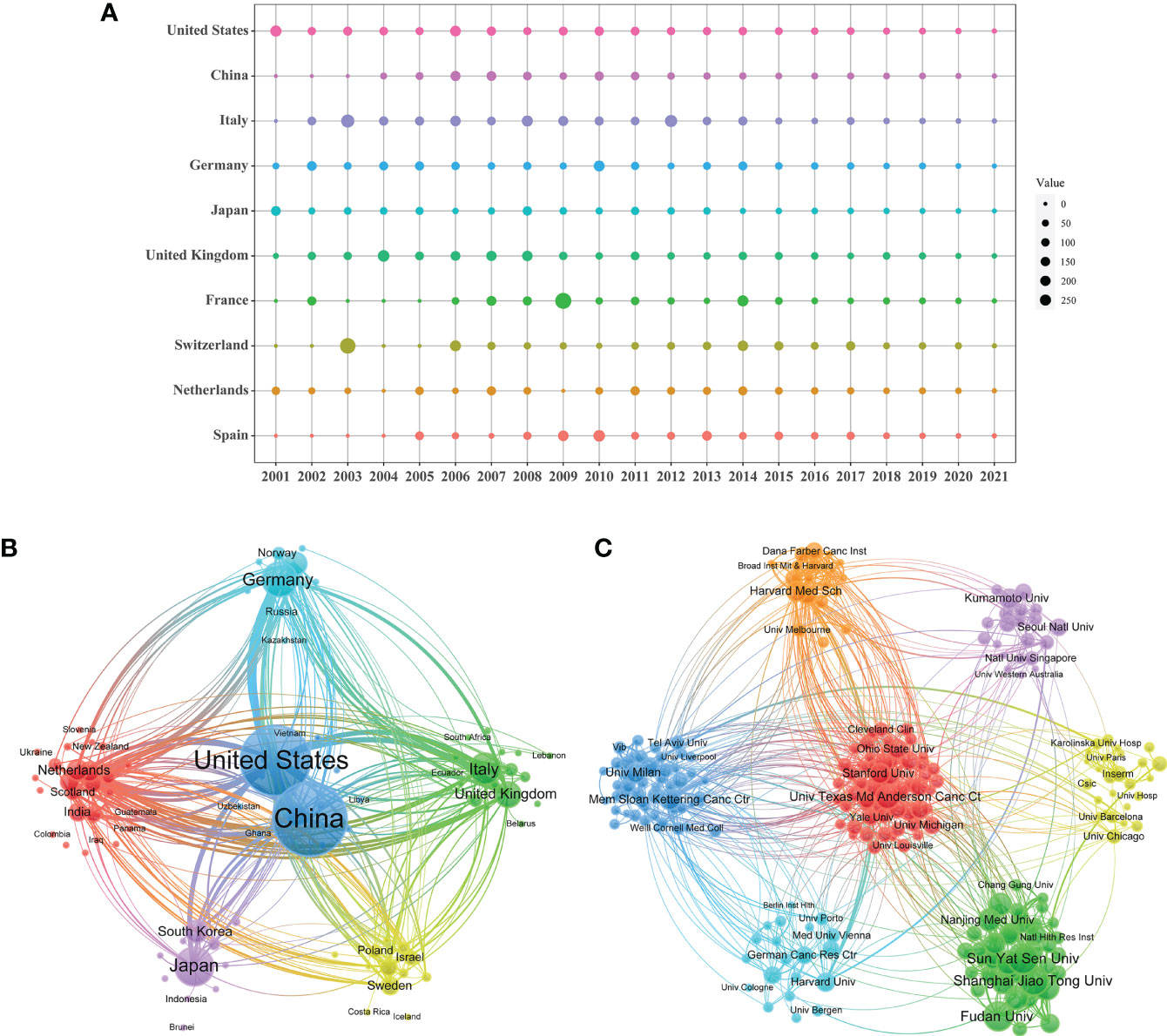
Figure 4 (A) Annual citations per publication for the top 10 countries. The network map of countries/regions (B) and institutions (C) on tumor-associated macrophage research.
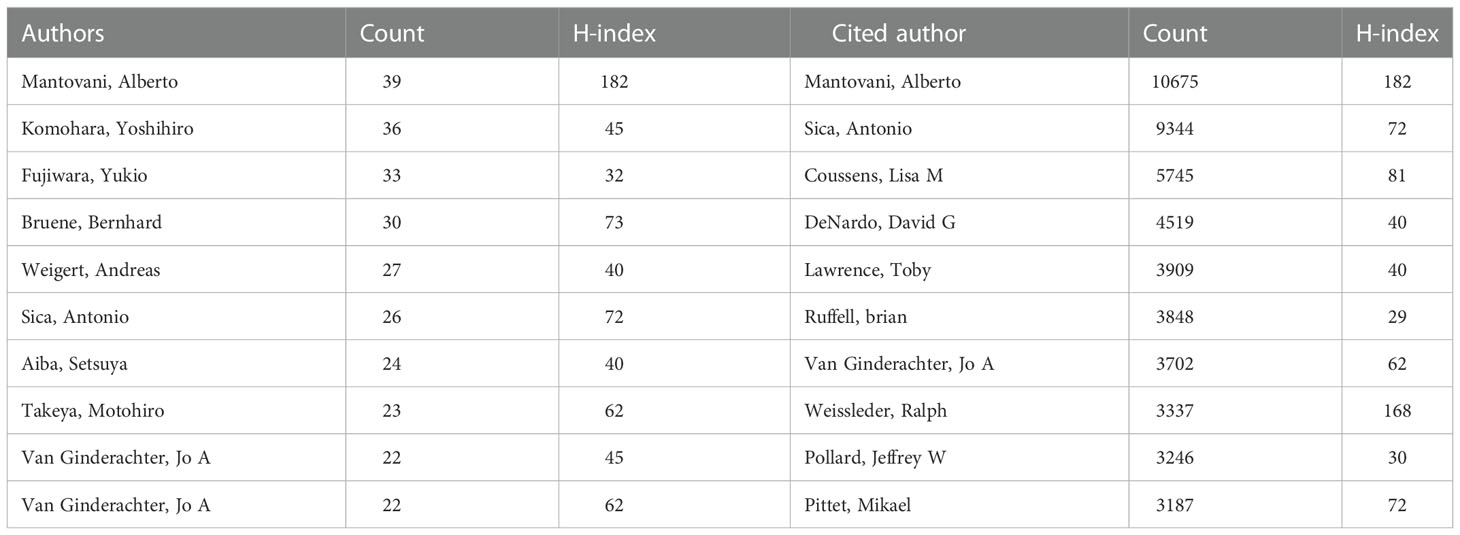
Table 2 The top 10 productive authors and cited authors in the field of tumor-associated macrophages.
A total of 1,201 journals were identified in this research field. The journal with the most publications was Cancer Research (N=173), followed by Plos One (N=152) and Oncotarget (N=148). Among the top ten journals related to TAM, 7 journals have an impact factor greater than 5, and 5 journals were at the Q1 JCR division (Table 3). At the same time, Cancer Research generated the most co-citations (N=18479). Figure 5A showed Scientific Reports, Cancers and Frontiers in Oncology were relatively new to this field, but developed rapidly.
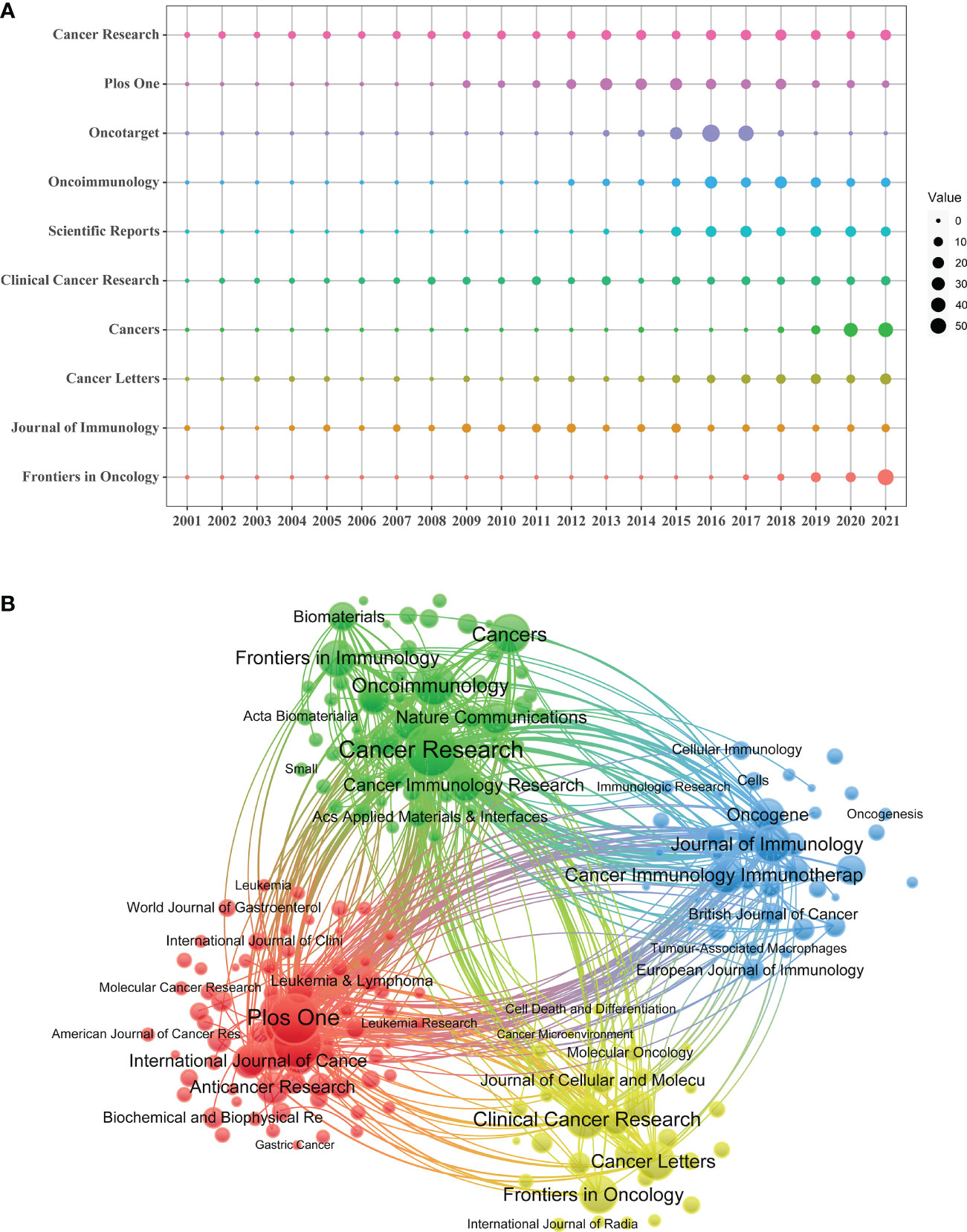
Figure 5 (A) Annual number of publications for the top 10 journals. (B) The network map of journals on tumor-associated macrophage research.
The cited journals network indicated the association between two journals. Journals are divided into four clusters, and the size of nodes represented the number of co-citations (Figure 5B). There was similar theme between journals of the same color, especially for red cluster.
Keywords were extracted from the 6,405 published articles. As shown in Table 4, NF-κB (N=336), endothelial growth factor (N=204) and PD-L1 (N=170) were the most commonly involved molecules. Activation (N=1002), polarization (N=903) and angiogenesis (N=806) appeared more frequently for pathological processes. As for specific diseases, breast cancer (N=840), colorectal cancer (N=287) and lung cancer (N=251) received the most attention.
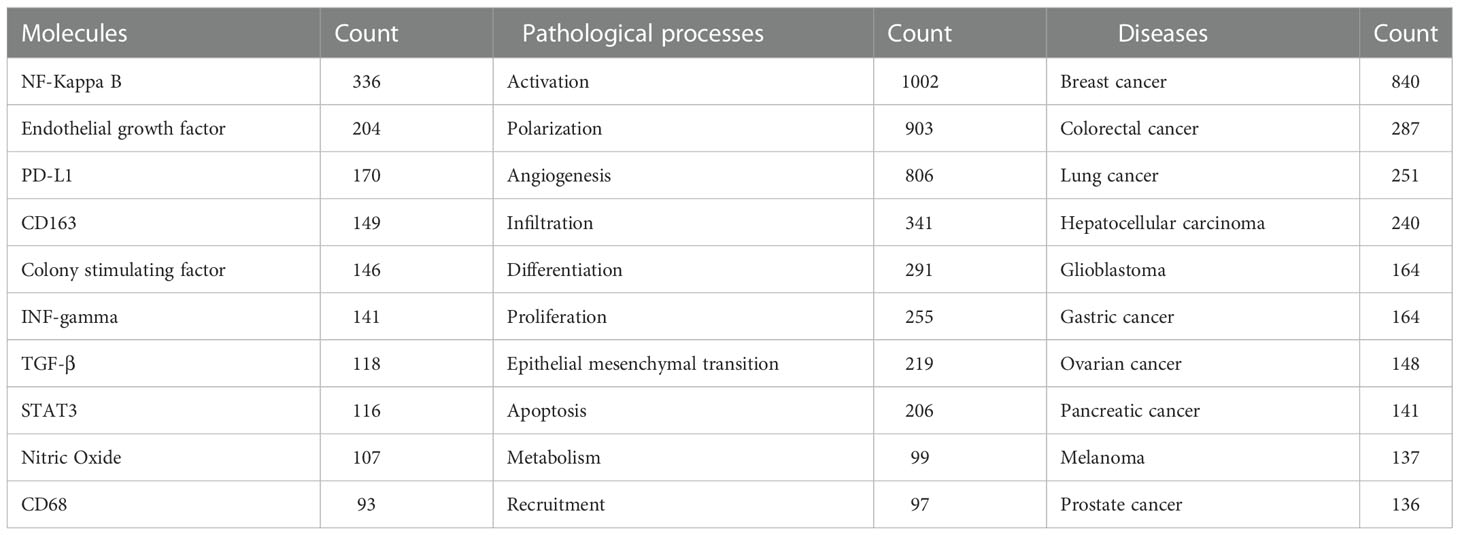
Table 4 The top 10 molecules, pathological process and disease related to tumor associated macrophages research.
Clustering keywords help to identify the distribution of research content on a specific topic (Figure 6). The largest blue cluster consisted of keywords was associated with the pathological processes and molecules of macrophages, including angiogenesis, NF-Kappa B and oxidative stress. Red cluster involved the cancer treatment, including immunotherapy, resistance and nanoparticle. Yellow cluster mainly explored the factors associated with tumor prognosis.
A visual map was constructed to show the trend of keywords bursts, where the red part represented the duration of citation burst (Figure 7). The early burst keywords included angiogenesis, epithelial growth factor, and colony stimulating factor. Citation bursts in the middle period (2011-2016) were significantly attenuated with a decrease in hotspot keywords such as NF-κB, Hodgkin lymphoma and scavenger receptor. In recent years (2018-2020), the treatment of cancer received increasing attention from researchers. PD-1/L1, PI3Kγ, resistance, nanoparticle and immune microenvironment has become the focus of attention of current research.
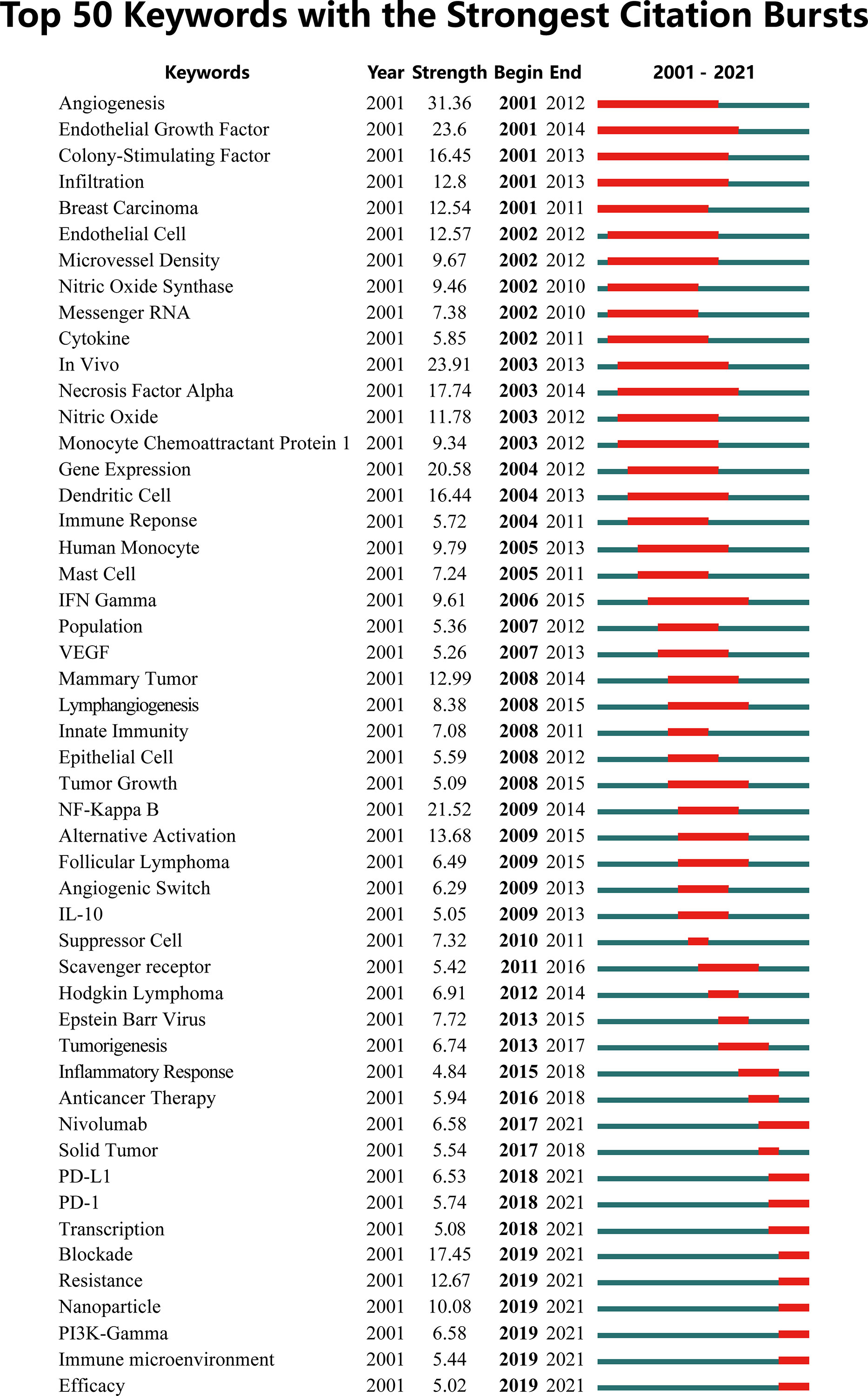
Figure 7 The top 50 keywords with the strongest citation bursts on tumor-associated macrophage research.
Tumor-associated macrophage is an important part of the tumor microenvironment and interacts with cancer cells to maintain the most of characteristics of tumors. The diversity of TAM forms a complex communication network between cancer and immune cells (14). In this study, we extracted TAM studies from public databases for bibliometric analysis to identify its hotspots and development trends. The increasing trend in annual publication volume demonstrated the significant potential of TAM in cancers.
The United States and China were the countries with the most citations. The distribution of institutions is consistent with countries/regions based on geographical location. However, the average citations for most countries/regions and institutions did not correspond well to the number of publications in this field. More robust efforts may be required to deeply clarify the role and mechanism of TAM in tumor. Meanwhile, the United States achieves a maximum level of cooperation in TAM research with a centrality of 0.37. Compared with other countries, it constitutes several cooperative subnetworks to better promote the development of the field, such as the University of Texas MD Anderson Cancer Center, Memorial Sloan-Kettering Cancer Center and University of Chicago.
Regarding the productivity of authors, Alberto Mantovani received the most citations in the TAM field. Mantovani mainly focused on the regulatory effect of chemokines on TAM (15–17) and some related anticancer drugs such as trabectedin (18, 19). Given the limitation of the binary M1-M2 classification of macrophage, Mantovani also attempted to divide macrophages into additional subsets (M2a, M2b and M2c) (20) or used looser terms (M1-like and M2-like) (21). Besides the collaborations with Mantovani, Antonio Sica made efforts to link inflammatory reaction to cancer through NF-κB (22–24). Coussens’s group from University of California San Francisco focused more on the immune cell crosstalk in breast cancer (25–27).
Cancer research published the most articles and received the highest number of co-citations. Scientific Reports, Cancers and Frontiers in Oncology were emerging journals spreading macrophage research. Papers published in highly cited journals such as Nature, Blood and Cell were more likely to be reviewed by scholars and have more access to citations.
The clustering analysis of keywords indicated that TAM research ranged from the biological properties of macrophages to the targeted therapy of cancer. TAM is highly related to specific pathological context, and its complex mechanism in tumors has attracted extensive attention. Angiogenesis is the initial research focus, which provides basic condition for tumor growth and dissemination. Angiogenesis is the initial research focus, which provides basic condition for tumor progression. Studies have shown that TAM can promote angiogenesis through the release of cytokines, growth factors and matrix metalloproteinases or the expression of TIE2 receptors (28–32). NF-κB is considered to be a molecular link between the inflammation and cancer. In the middle period, it gradually presented the highest citation burst strength. NF-κB activation in macrophages is essential for tumor growth. Inhibition of IKKβ leads to a significant reduction in tumor onset and load of several inflammation-induced cancer models (33–35). However, TAM often shows alternative immunosuppressive M2-like phenotype, which is not easily reconciled with the proinflammatory function of NF-κB in TAM. The scavenger receptor MARCO expressed on the surface of macrophages is able to regulate macrophage polarization and enhance tumor killing (36, 37).
Recently, anticancer therapy targeting TAM has generated the most research enthusiasm. Immunosuppression microenvironment limits the efficacy of checkpoint block and adoptive cell therapy, particularly in solid tumors (38). TAMs can suppress immunotherapy efficacy by inhibiting T-cell activity and enhancing the expression of PD-L1 in the TME. In addition to inhibiting T cell activation, a study from Sydney et al. showed that immune checkpoint inhibitor PD-1/L1 also inhibited TAM phagocytosis, which may be associated with M2 polarization (39). In-depth inquiry of PD-1/L1 expanded the knowledge of PD-1/L1 from its role in T cells to many other cell types, including macrophages. PI3K/Akt is also an important signaling pathway participating in macrophages survival, proliferation and cytoskeleton rearrangement. PI3K induces TAMs into M2-like phenotype and is closely correlated with poor clinical outcomes of cancers (40). Inhibition of PI3Kγ make tumors sensitive to immune checkpoint inhibitors by reprogramming TAM, demonstrating the importance of macrophage-mediated immune microenvironment for optimal immunotherapy efficacy (41–43). CSF-1R expressed on TAMs is involved in the activation of PI3K signaling pathway, and regulate the immune inhibition in macrophages. Blockade of CSF1 has been shown to deplete TAM and prevent TAM recruitment to the tumor (44, 45). Targeting TAM can play its unique regulatory function in promoting the antitumor effects of current immunotherapy.
Due to the unique biophysical properties, nanoparticles show greater advantages and potentials in cancer treatment. Compared with traditional drugs, nanoparticles can extend retention time and achieve targeted delivery with a decreased toxicity. Some studies have reported that nanoparticles specifically enhance anticancer immune responses by targeting TAM (46–50). The rich blood circulation and strong phagocytosis ability also make macrophages themselves become the optimal carrier of drug delivery. TAM allows the delivery of nanotherapeutic drugs to tumor cells and alters the spatial diffusion of drugs within the tumor (51, 52). Imaging the response between tumors and nanomaterials provide a reliable basis for the development of highly effective targeted therapies.
The bibliometric study reflected the development trend and research hotspots in this field to a certain extent. At the same time, this study has several limitations. The included literatures were collected from WOSCC database, which caused the omission of some information. Furthermore, there were potential biases in bibliometric method based on natural language processing. Excessive adjustments for inaccurate elements may reduce the credibility of the results.
In conclusion, the research on TAM is rapidly evolving with active cooperation worldwide. And anticancer therapy targeting TAM is emerging and promising area of future research, especially in translational application. This may provide guidance and new insights for further research in the field of TAM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
XZ and YX designed the study. YL, CL and FW conducted data extraction. FZ, YX and JP performed data analysis. FZ drafted the manuscript. XZ interpreted the data and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pittet MJ, Michielin O, Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol (2022) 19(6):402–21. doi: 10.1038/s41571-022-00620-6
2. Mehla K, Singh PK. Metabolic regulation of macrophage polarization in cancer. Trends Cancer (2019) 5(12):822–34. doi: 10.1016/j.trecan.2019.10.007
3. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature (2013) 496(7446):445–55. doi: 10.1038/nature12034
4. Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol (2022) 23(8):1148–56. doi: 10.1038/s41590-022-01267-2
5. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
6. Yang M, McKay D, Pollard JW, Lewis CE. Diverse functions of macrophages in different tumor microenvironments. Cancer Res (2018) 78(19):5492–503. doi: 10.1158/0008-5472.Can-18-1367
7. Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: Different gene signatures in M1(Lps+) vs. classically and M2(Lps-) vs. alternatively activated macrophages. Front Immunol (2019) 10:1084. doi: 10.3389/fimmu.2019.01084
8. Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, et al. Bibliometrics: Tracking research impact by selecting the appropriate metrics. Asian J Androl (2016) 18(2):296–309. doi: 10.4103/1008-682x.171582
9. Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PloS One (2019) 14(10):e0223994. doi: 10.1371/journal.pone.0223994
10. Chen C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc Natl Acad Sci U.S.A. (2004) 101 Suppl 1(Suppl 1):5303–10. doi: 10.1073/pnas.0307513100
11. Aria M, Cuccurullo C. Bibliometrix: An r-tool for comprehensive science mapping analysis. J Informetrics (2017) 11(4):959–75. doi: 10.1016/j.joi.2017.08.007
12. van Eck NJ, Waltman L. Software survey: Vosviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
13. Chen C. Science mapping: A systematic review of the literature. J Data Inf Sci (2017) 2(2):1–40. doi: 10.1515/jdis-2017-0006
14. Li MO, Wolf N, Raulet DH, Akkari L, Pittet MJ, Rodriguez PC, et al. Innate immune cells in the tumor microenvironment. Cancer Cell (2021) 39(6):725–9. doi: 10.1016/j.ccell.2021.05.016
15. Marelli G, Erreni M, Anselmo A, Taverniti V, Guglielmetti S, Mantovani A, et al. Heme-Oxygenase-1 production by intestinal Cx3cr1(+) macrophages helps to resolve inflammation and prevents carcinogenesis. Cancer Res (2017) 77(16):4472–85. doi: 10.1158/0008-5472.Can-16-2501
16. Savino B, Caronni N, Anselmo A, Pasqualini F, Borroni EM, Basso G, et al. Erk-dependent downregulation of the atypical chemokine receptor D6 drives tumor aggressiveness in kaposi sarcoma. Cancer Immunol Res (2014) 2(7):679–89. doi: 10.1158/2326-6066.Cir-13-0202
17. Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of the chemokine receptor Cxcr4 by hypoxia. J Exp Med (2003) 198(9):1391–402. doi: 10.1084/jem.20030267
18. Germano G, Frapolli R, Simone M, Tavecchio M, Erba E, Pesce S, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res (2010) 70(6):2235–44. doi: 10.1158/0008-5472.Can-09-2335
19. Belgiovine C, Bello E, Liguori M, Craparotta I, Mannarino L, Paracchini L, et al. Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br J Cancer (2017) 117(5):628–38. doi: 10.1038/bjc.2017.205
20. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol (2004) 25(12):677–86. doi: 10.1016/j.it.2004.09.015
21. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol (2010) 11(10):889–96. doi: 10.1038/ni.1937
22. Porta C, Ippolito A, Consonni FM, Carraro L, Celesti G, Correale C, et al. Protumor steering of cancer inflammation by P50 nf-κb enhances colorectal cancer progression. Cancer Immunol Res (2018) 6(5):578–93. doi: 10.1158/2326-6066.Cir-17-0036
23. Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (Defective nf-kappab and enhanced irf-3/Stat1 activation). Blood (2006) 107(5):2112–22. doi: 10.1182/blood-2005-01-0428
24. Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, et al. P50 nuclear factor-kappab overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res (2006) 66(23):11432–40. doi: 10.1158/0008-5472.Can-06-1867
25. Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage il-10 blocks Cd8+ t cell-dependent responses to chemotherapy by suppressing il-12 expression in intratumoral dendritic cells. Cancer Cell (2014) 26(5):623–37. doi: 10.1016/j.ccell.2014.09.006
26. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. Cd4(+) t cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell (2009) 16(2):91–102. doi: 10.1016/j.ccr.2009.06.018
27. Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. Th2-polarized Cd4(+) t cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res (2015) 3(5):518–25. doi: 10.1158/2326-6066.Cir-14-0232
28. Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol (2020) 353:104119. doi: 10.1016/j.cellimm.2020.104119
29. Larionova I, Kazakova E, Gerashchenko T, Kzhyshkowska J. New angiogenic regulators produced by tams: Perspective for targeting tumor angiogenesis. Cancers (Basel) (2021) 13(13). doi: 10.3390/cancers13133253
30. Deryugina EI, Quigley JP. Tumor angiogenesis: Mmp-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol (2015) 44-46:94–112. doi: 10.1016/j.matbio.2015.04.004
31. Duran CL, Borriello L, Karagiannis GS, Entenberg D, Oktay MH, Condeelis JS. Targeting Tie2 in the tumor microenvironment: From angiogenesis to dissemination. Cancers (Basel) (2021) 13(22). doi: 10.3390/cancers13225730
32. Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3a/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell (2013) 24(6):695–709. doi: 10.1016/j.ccr.2013.11.007
33. Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. "Re-educating" tumor-associated macrophages by targeting nf-kappab. J Exp Med (2008) 205(6):1261–8. doi: 10.1084/jem.20080108
34. Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. Ikkbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell (2004) 118(3):285–96. doi: 10.1016/j.cell.2004.07.013
35. Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of Nemo/Ikkgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell (2007) 11(2):119–32. doi: 10.1016/j.ccr.2006.12.016
36. La Fleur L, Boura VF, Alexeyenko A, Berglund A, Pontén V, Mattsson JSM, et al. Expression of scavenger receptor marco defines a targetable tumor-associated macrophage subset in non-small cell lung cancer. Int J Cancer (2018) 143(7):1741–52. doi: 10.1002/ijc.31545
37. Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep (2016) 15(9):2000–11. doi: 10.1016/j.celrep.2016.04.084
38. Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol (2018) 11(1):31. doi: 10.1186/s13045-018-0578-4
39. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. Pd-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature (2017) 545(7655):495–9. doi: 10.1038/nature22396
40. Yang D, Yang L, Cai J, Li H, Xing Z, Hou Y. Phosphoinositide 3-Kinase/Akt and its related signaling pathways in the regulation of tumor-associated macrophages polarization. Mol Cell Biochem (2022) 477(10):2469–80. doi: 10.1007/s11010-022-04461-w
41. De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting Pi3kγ in myeloid cells. Nature (2016) 539(7629):443–7. doi: 10.1038/nature20554
42. Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage Pi3kγ drives pancreatic ductal adenocarcinoma progression. Cancer Discovery (2016) 6(8):870–85. doi: 10.1158/2159-8290.Cd-15-1346
43. Han MG, Jang BS, Kang MH, Na D, Kim IA. Pi3kγδ inhibitor plus radiation enhances the antitumour immune effect of pd-1 blockade in syngenic murine breast cancer and humanised patient-derived xenograft model. Eur J Cancer (2021) 157:450–63. doi: 10.1016/j.ejca.2021.08.029
44. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-Csf-1r antibody reveals a strategy for cancer therapy. Cancer Cell (2014) 25(6):846–59. doi: 10.1016/j.ccr.2014.05.016
45. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. Csf-1r inhibition alters macrophage polarization and blocks glioma progression. Nat Med (2013) 19(10):1264–72. doi: 10.1038/nm.3337
46. Trac N, Chen LY, Zhang A, Liao CP, Poon C, Wang J, et al. Ccr2-targeted micelles for anti-cancer peptide delivery and immune stimulation. J Control Release (2021) 329:614–23. doi: 10.1016/j.jconrel.2020.09.054
47. Liu Y, Wang J, Zhang J, Marbach S, Xu W, Zhu L. Targeting tumor-associated macrophages by Mmp2-sensitive apoptotic body-mimicking nanoparticles. ACS Appl Mater Interfaces (2020) 12(47):52402–14. doi: 10.1021/acsami.0c15983
48. Zhang Y, Chen Y, Li J, Zhu X, Liu Y, Wang X, et al. Development of toll-like receptor agonist-loaded nanoparticles as precision immunotherapy for reprogramming tumor-associated macrophages. ACS Appl Mater Interfaces (2021) 13(21):24442–52. doi: 10.1021/acsami.1c01453
49. Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl (2020) 59(5):2018–22. doi: 10.1002/anie.201912524
50. Li H, Somiya M, Kuroda S. Enhancing antibody-dependent cellular phagocytosis by re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials (2021) 268:120601. doi: 10.1016/j.biomaterials.2020.120601
51. Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater (2020) 32(40):e2002054. doi: 10.1002/adma.202002054
Keywords: tumor-associated macrophage, cancer, bibliometrics, visualization, hotspots
Citation: Zhou F, Liu Y, Liu C, Wang F, Peng J, Xie Y and Zhou X (2023) Knowledge landscape of tumor-associated macrophage research: A bibliometric and visual analysis. Front. Immunol. 14:1078705. doi: 10.3389/fimmu.2023.1078705
Received: 24 October 2022; Accepted: 03 January 2023;
Published: 18 January 2023.
Edited by:
Gulderen Yanikkaya Demirel, Yeditepe University, TurkeyReviewed by:
Mario Leonardo Squadrito, Vita-Salute San Raffaele University, ItalyCopyright © 2023 Zhou, Liu, Liu, Wang, Peng, Xie and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojiang Zhou, eWZ5enhqMTk3MEAxNjMuY29t; Yong Xie, eGlleW9uZ190ZmFob25jdUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.