
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 31 January 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1067291
 Zhengge Jin1,2†
Zhengge Jin1,2† Shuqin Li1,2†
Shuqin Li1,2† Ruoyu Li1,2†
Ruoyu Li1,2† Xianbing Song3
Xianbing Song3 Shichen Zhang1,2
Shichen Zhang1,2 Ying Sun1,2
Ying Sun1,2 Fangbiao Tao1,2
Fangbiao Tao1,2 Yuhui Wan1,2*
Yuhui Wan1,2*Background: The impact of childhood maltreatment on multiple inflammatory cytokines among middle school students remains to be elucidated. This study aimed to examine the associations of different types of childhood maltreatment with peripheral serum inflammatory cytokines (interleukin-10, interleukin-1β, interleukin-6, interleukin-8, and tumor necrosis factor-α) in middle school students, and to explore the differences in these associations between boys and girls and between late (≥15 and<20 years) and early (≥11 and <15 years) adolescence.
Methods: A total of 1122 students were recruited from a boarding middle school. Each participant was asked to respond to a detailed questionnaire on childhood maltreatment, from whom one blood sample was drawn via venous blood.
Results: In the overall sample there was no association between childhood maltreatment and peripheral serum inflammatory cytokines; (2) emotional abuse was significantly correlated with IL-1β only in girls (B = -0.16; 95% CI, -0.28~-0.03; p = 0.06); (3) in late adolescence, emotional abuse, emotional neglect, and childhood maltreatment had marked link with IL-8 (B = 0.39; 95%CI, 0.16~0.63; p = 0.01; B =0.20; 95% CI, 0.04~0.37; p = 0.08; B = 0.50; 95% CI, 0.18~0.82; p = 0.01, respectively).
Conclusion: These findings also strengthened an inference regarding the effects of childhood maltreatment on inflammation of students in late adolescence.
Inflammation, the response of body tissues to injury, is protective against various bacterial and viral infections (1). However, low-grade, chronic inflammation can persist for a long time, contributing to the pathogenesis of other human diseases. For example, inflammatory processes have also been prospectively linked to the pathogenesis of depression (2–4). Moreover, evidence suggests that chronic inflammation is a significant risk predictor of cardiovascular diseases (5) as well as a biological marker for development and progression of cancer (6). In addition, chronic inflammation is the leading underlying cause of death (7, 8). Some studies have indicated that the elevated levels of inflammation are linked to experiences of childhood adversities, stress, and trauma (9–11).
Childhood maltreatment has been shown to result in a host of harmful outcomes immediately and throughout the lifespan (12–14), and systemic inflammation is considered a potential biological mechanism mediating this association (15). Based on the situation, some cytokines are involved in both pro-inflammatory and anti-inflammatory activities. Cytokines that promote the inflammatory cascade are called pro-inflammatory mediators, including interleukin (IL)-6, IL-8, IL-1β, tumor necrosis factor-α (TNF-α), etc. The production of anti-inflammatory cytokines such as IL-4, IL-10, IL-11, and IL-13 blocks this process by regulating the pro-inflammatory cytokine response (16). In recent years, relevant studies have shown that IL-1β, IL-6, IL-8, TNF-α and IL-10, as parameters reflecting the level of inflammation in the body, are good indicators of immune dysfunction and changes in the level of inflammatory factors (10, 17–19). Many studies have elucidated the relationship between a single type of childhood maltreatment and inflammation, and it is likely that different types of childhood maltreatment manifest different associations with inflammation. Based on previous research, adults with a documented history of childhood maltreatment, especially physical abuse, were prone to increased risk of higher levels of inflammatory cytokines (C-reactive protein [CRP]) than non-maltreated individuals were (10). Some systematic reviews and meta-analyses also demonstrated that adults exposed to childhood trauma (sexual, physical, or emotional abuse) may have apparently elevated baseline peripheral levels of CRP, IL-6, and TNF-α (17, 20). Childhood trauma (emotional abuse, physical abuse, sexual abuse and emotional neglect before the age of 16) was associated with increased levels of lipopolysaccharide-stimulated cytokines, such as IL-10, IL-6, and IL-8, with evidence for a dose-response relationship (21). According to a study with the application of longitudinal data from the Multidimensional Assessment of Preschoolers Study, substance abuse was correlated to higher levels of pro-inflammatory markers such as IL-1β and IL-6 (18). Focusing on the single adversity of childhood maltreatment may help to sort out the life-course mechanisms involved (19), yet the impact of such adversity may be confounded by the experience of other adversities excluded in the analysis. Therefore, the associations of different types of childhood maltreatment with inflammatory markers call for further exploration.
Furthermore, the potential gender difference remains an interesting focus in the link between childhood maltreatment and inflammatory biomarkers. As indicated in some research, women have greater inflammatory responses to childhood stressors (22, 23), while others document no gender difference in the effect of childhood maltreatment on inflammatory dysregulation (17). Apparently, whether the impacts vary by gender remains unclear. On the other hand, for a better implementation of interventions in schools, it is necessary to determine whether gender difference exists in the relationship between childhood maltreatment and inflammatory cytokines among middle school students under the influence of school settings. Links between child maltreatment and low-grade inflammation in adulthood seemed to be well documented (24, 25); however, only a handful of studies dwelt on the correlation between child maltreatment and low-grade inflammation in adolescents (11, 26). As the immune system advances rapidly in adolescence, it is crucial to characterize the progression of the inflammatory process in adolescents concerning child maltreatment.
Despite the clarification of the relationship between childhood abuse and inflammatory factor levels in former studies (10, 15–21), similar evidence is still lacking in China. Worse still, inconsistency could be observed in the previous evidence found in the association affected by different gender and age groups (11, 17, 22–26). To address this gap in the roles of gender and age in the relationship between childhood maltreatment and inflammation, we made a preliminary investigation on the relationship between childhood maltreatment and inflammatory profiles [i.e., pro-inflammatory (IL-1β, IL-6, IL-8, TNF-α) and anti-inflammatory (IL-10)] in middle school students, with an examination of the discrepancy in the patterns between boys and girls and between students in early and late adolescence.
From October to December, 2018, a survey was conducted on a sample of 1312 students selected from a boarding school in Shenyang City, Liaoning Province, China, where all juniors and seniors were recruited. An anonymous questionnaire was required to be completed, and a blood sample of each participant was provided through a venous blood draw in cooperation with doctors. Besides, the survey was conducted in classrooms by investigators during extracurricular hours. Teachers, though excluded from the survey, were invited to be in charge of maintaining classroom discipline. The objective of the survey was introduced, the questionnaires offered, and the principles of anonymity and confidentiality of participation were emphasized. Investigators were on the spot in case questions from students arose during the survey. The ID number of students was used for the only identification of their questionnaires and blood samples. The sober venous blood draw was performed within two to three days before and after the completion of the questionnaires.
Due to an unwillingness to respond to the questionnaire, absence from school, high levels of missing data (questionnaires with missing values >5% were eliminated), or obviously fictitious responses, some students failed to contribute, and eventually, 1203 questionnaires were recovered out of 1312 students. A total of 1182 blood samples were obtained because of the following reasons (1): some students had anemia, cough, or fever (n = 7); (2) some students who were interviewed declined to have blood drawn (n = 115); and (3) when the inflammatory factor level of a sample exceeded the mean value ±3 times the standard deviation range, the inflammatory factor level was treated as a missing value (n = 8).
The study design and data collection procedures were approved by the Ethics Committee of Anhui Medical University (20170290). Informed consent was obtained from both the students and their parents or guardians.
Childhood maltreatment was evaluated via using the Childhood Trauma Questionnaire (CTQ) (27), a widely used 28-item tool to assess five forms of childhood trauma (physical abuse, sexual abuse, emotional abuse, physical neglect, and emotional neglect). The CTQ was translated and validated in Chinese (28). The participants were interviewed about their abusive childhood experiences before entering middle school. Response scores ranged from 1 to 5 (never true, rarely true, sometimes true, often true, and very often true, respectively). The higher the scores, the more severe childhood maltreatment one received. Cronbach’s α coefficient of the emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect, and childhood maltreatment scale in this study was 0.692, 0.731, 0.767, 0.804, 0.637, and 0.768, respectively.
Pro-inflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α) and anti-inflammatory cytokines (IL-10) were collected from the fasting serum from 7:00 to 9:00 in the morning. The detailed procedure was conducted as follows. The venous blood samples of 1182 participants were placed into tubes. The blood was allowed to clot for at least 30 minutes before centrifugation for 10 minutes at 1000 × g. The serum was immediately removed, and the samples were stored at ≤-20°C. Serum cytokine levels were measured using the Human High Sensitivity T Cell Magnetic Bead Panel Protocol from the Milliplex® MapKit (cat. no. HSTCMAG-28SK, USA), following the manufacturer’s instructions. Briefly, assay plates were washed with wash buffer, sealed and mixed on a plate shaker for 10 min at room temperature. The wash buffer was decanted, and 50 μL of the diluted standards, quality controls, and serum samples were added to the appropriate wells. After the addition of samples or controls, the plates were incubated overnight at 4°C on a plate shaker with fluorescently labeled capture antibody-coated beads. After overnight incubation with capture antibodies to detect IL-1β, IL-6, IL-8, TNF-α, and IL-10, the well content was removed and washed using a handheld magnet. Followed by the addition of 150 μL drive fluid to all wells, the sample was run on MAGPIX® with xPONENT® software (Luminex Corporation, Austin, Texas, USA). Analysis of the cytokine median fluorescence intensity was performed using Milliplex Analyst version 5.1. The intra- and inter-assay coefficients of variation for all tested cytokines were <5% and <15-20%, respectively.
Based on the previous investigation of the research group (29, 30), the covariates in the multivariate analyses were sociodemographic characteristics, including age, gender, urban/rural residence, parental education level (middle school and below, high school and above), and perceived economic status of the family (poor, moderate, or good). Body mass index was also adjusted, which was calculated according to objectively measured height and weight.
Sociodemographic data and childhood maltreatment were described in the total population. Five forms of childhood abuse and serum cytokine values (TNF-α, IL-6, IL-1β) were natural log transformed to better approximate the normality of the residuals. Measurement data were expressed as mean ± standard deviation (mean ± SD), and two independent samples t-tests were used to compare the log-transformed inflammatory cytokine levels in different genders and ages. Linear regression analyses were performed to examine the association between childhood maltreatment and inflammatory cytokine levels. We also examined the associations of specific maltreatment subtypes (emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect). Given the literature on gender and age differences, separate regression analyses were conducted, with the sample split first by gender (boys vs. girls) and then by age [late adolescence (≥15 and<20 years) vs. early adolescence (≥11 and <15 years)] (31). Moderation analyses were used to assess the moderating role of gender and age in the relationships between child maltreatment and inflammatory markers. All analyses were conducted using SPSS version 23.0. The threshold for statistical significance was set at p< 0.05. To reduce the false-positive rate, we used the false discovery rate (FDR) approach with a cut-off of 0.10.
Table 1 showed the descriptive data on the distribution of age, gender, urban/rural residence, parental education level, perceived economic status of the family, BMI, and log-transformed child maltreatment.
Table 2 showed that the serum levels of IL-10 and IL-1β in boys were higher than in girls on average, and serum IL-8 and TNF-α in girls were higher than those in boys with statistical differences (p<0.05). Meanwhile, the serum levels of IL-1β, IL-6 and TNF-α in students in late adolescence were higher than those in early adolescence on average, with statistical differences (p<0.05). The statistical differences were enhanced after FDR adjustment (adjusted p<0.001, Supplementary Table A1). Further analysis of the log-transformed inflammatory cytokine distributions by gender and age was presented in Supplementary Figure 1.
Table 3 showed that in the overall sample, despite the introduction of all control variables, the association between emotional abuse with elevated levels of IL-6 (B = 0.15, 95% CI, 0.01~0.28) disappeared after adjustment for FDR (Table A2).
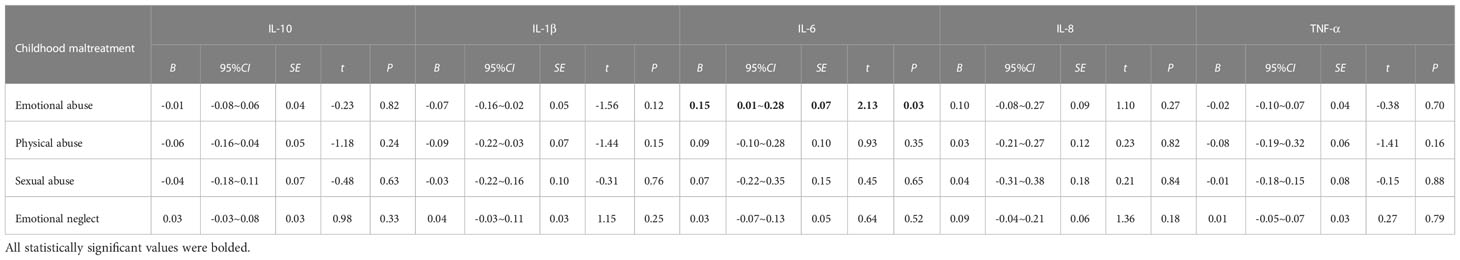
Table 3 Associations of childhood maltreatment with the levels of peripheral serum inflammatory cytokines in the total sample.
In separate gender-specific regressions (Table 4), which were adjusted for age, urban/rural, parental education level, perceived family economic status, and BMI, girls with increased emotional abuse scores showed decreased IL-1β levels (B = -0.16; 95% CI, -0.28~-0.03), but the relationship was attenuated after FDR was adjusted (adjusted P=0.06, Supplementary Table A3). In addition, boys with emotional abuse scores showed elevated inflammatory IL-6 (P=0.05), but the relationship was attenuated after FDR was adjusted (adjusted P=0.28, Supplementary Table A3). The findings indicated that no moderated roles were detected in the relationships between types of child maltreatment and inflammatory cytokine levels by gender.
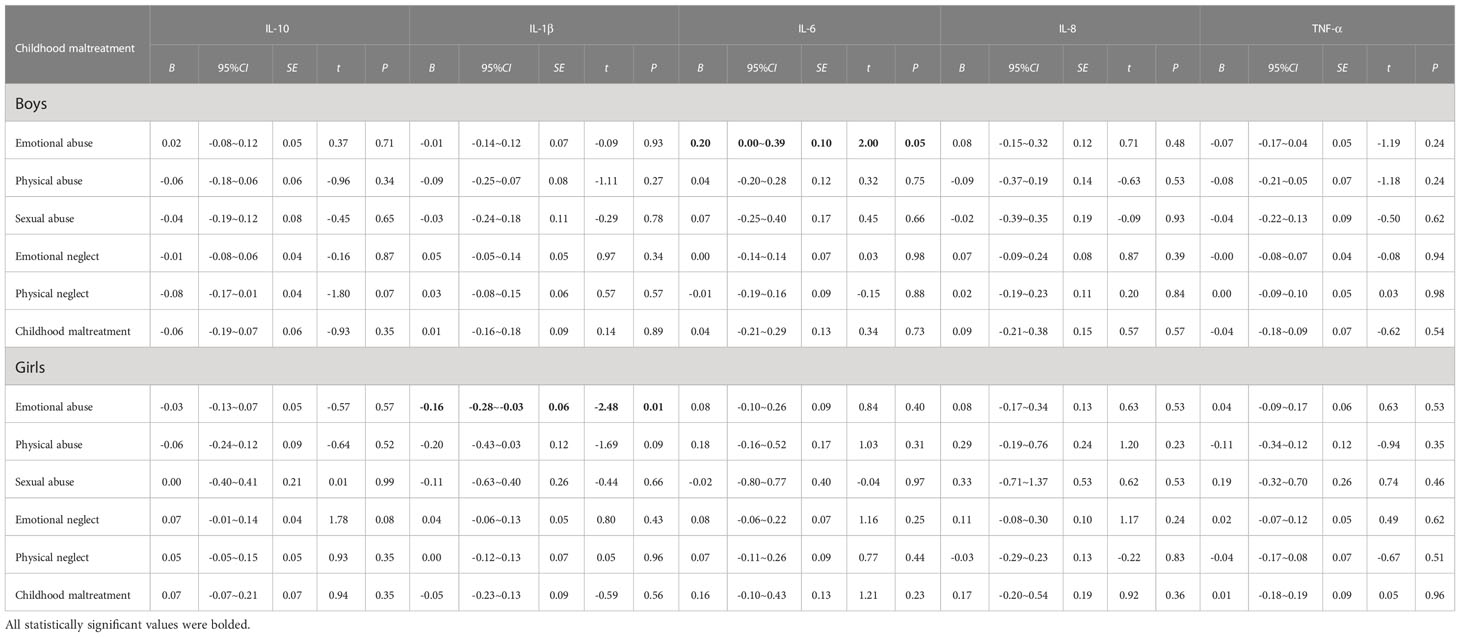
Table 4 Gender-specific associations of childhood maltreatment with the levels of peripheral serum inflammatory cytokines.
In separate regressions for late and early adolescence (Table 5), which were adjusted for gender, urban/rural, parental education level, perceived family economic status, and BMI, emotional abuse was associated with significantly lower odds of elevated IL-1β levels among students in early adolescence (B = -0.14; 95% CI, -0.26~-0.01). However, for those in late adolescence, emotional abuse was positively associated with elevated IL-6 (B = 0.19; 95% CI, 0.00~0.38) and IL-8 (B = 0.39; 95%CI, 0.16~0.63), physical abuse was positively associated with increased IL-6 levels (B = 0.37; 95% CI, 0.04~0.69), emotional neglect was positively associated with uplifted IL-8 levels (B = 0.20; 95% CI, 0.04~0.37), and childhood maltreatment was linked to a significantly higher risk of raised IL-8 levels (B = 0.50; 95% CI, 0.18~0.82). But the correlation between emotional abuse and IL-1β levels in early adolescence presented no statistical significance after FDR adjusted (adjusted P = 0.24). Besides, the relationship between emotional abuse and physical abuse and elevated IL-6 levels was attenuated after FDR adjusted (Supplementary Table A4). These findings indicated that the moderating roles of age were detected in the association between emotional abuse, emotional neglect and child maltreatment and IL-8 (B = 0.39, 0.20 and 0.50 respectively, P < 0.1 for all).
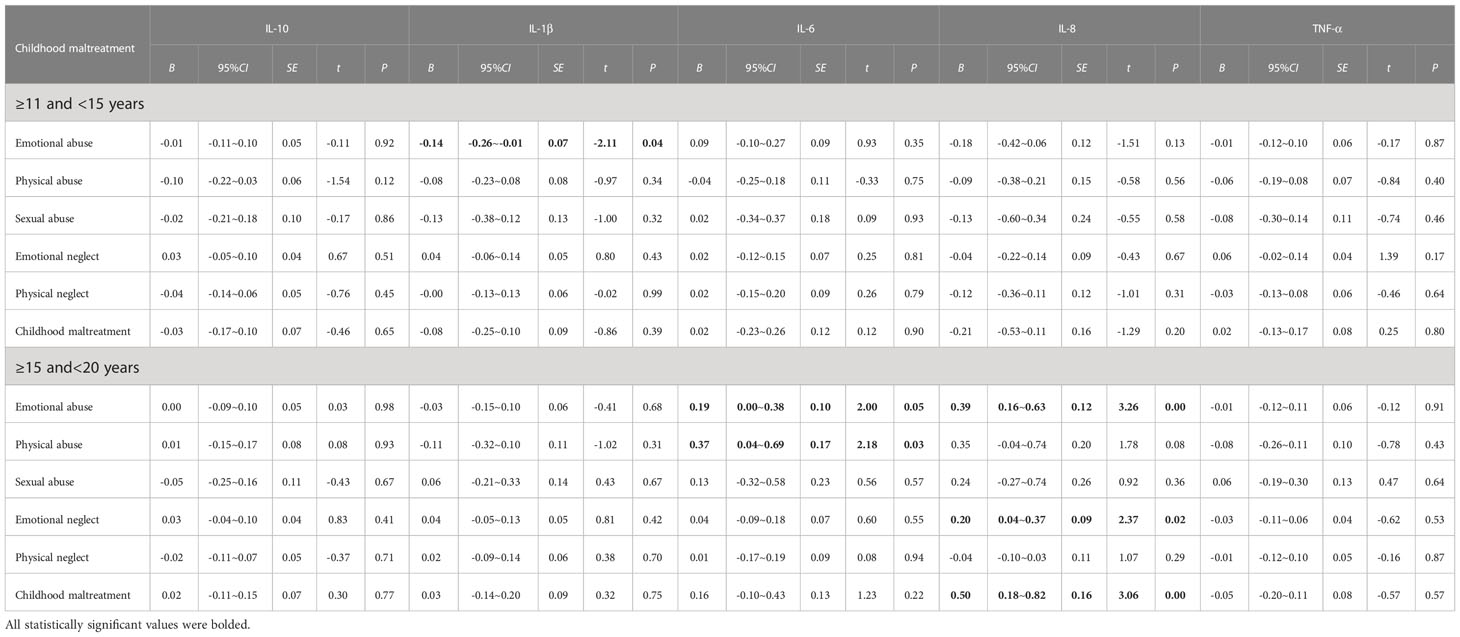
Table 5 Age-specific associations of childhood maltreatment with the levels of peripheral serum inflammatory cytokines.
The present study explored the relationship between childhood maltreatment and inflammatory cytokines. Besides, stratification by gender and age highlighted the relevance of child maltreatment and inflammatory cytokines. This study mainly identified that girls with increased emotional abuse scores showed decreased IL-1β levels. Further, we identified that emotional abuse, emotional neglect, and childhood maltreatment was positively associated with increased IL-8 levels in late adolescence.
However, our study failed to respectively evaluate the associations of child maltreatment across dimensions with inflammatory cytokines in the whole sample, which proved to be conflicting with the findings of some previous literature (32, 33), possibly due to the fact that participants were generally healthy middle school students rather than clinical patients. The results might also have been influenced by notable methodological limitations, such as sample size and the timing of the childhood maltreatment, diet, sleep, and other confounding factors that were not addressed in this study, either. A recent systematic review showed that child trauma (sexual, physical, or emotional abuse) was associated with elevated levels of inflammatory markers like CRP, IL-6, and TNF-α (17). This finding further supported data from an earlier systematic review, in which strong evidence was detected only for associations between child maltreatment and CRP, though the authors noted that no similar association could be found in several studies on IL-6 (24). Also, a positive association was observed in some studies between early adversities and at least one measure of in vitro production of inflammatory cytokines (34, 35), many maltreated individuals avoided these outcomes, and negative associations could be found between early adversities and in vitro production of pro-inflammatory cytokines (36, 37). For example, research by Wright et al. demonstrated that a higher stress level in early childhood was significantly linked with increased production of TNF-α, suggesting a trend between more stress and less interferon-γ production (37). Thus, the correlation between different types of child maltreatment and diverse inflammatory cytokines deserves further exploration.
The present study also revealed a converse association between emotional abuse and lower IL-1β levels in girls, whereas no significant relation was detected in boys. IL-1β, produced by central and peripheral immune cells, is a key proinflammatory cytokine involved in local and systemic inflammation (38). The neural immune network hypothesis pointed out that early stress caused a low inflammatory state of the body by affecting HPA axis activity and sympathetic nervous system response, and IL 1β could cross the blood-brain barrier to induce inflammation of small glial cells in the brain, affecting neuronal plasticity, which in turn predisposed to suicidal behavior, major depression, post-traumatic stress disorder, panic disorder, bipolar disorder, and other psychological and behavioral problems (3, 25, 39–42). Our results contradicted previous data on the association between childhood trauma and IL-1β levels (43, 44). Childhood maltreatment may exert pro-inflammatory effects on immune cells throughout development. This might occur due to the association between increased stress during childhood and parallel increases in activity of the HPA axis and its end-product, cortisol (45). After binding to glucocorticoid receptors in healthy immune cells, cortisol inhibited the production of inflammatory cytokines (46). Therefore, measured by circulating markers, the pro-inflammatory phenotype of children may be masked by the upregulation of the HPA axis. In fact, the HPA axis was often socially adjusted by caregivers during much of childhood, so that the youth may exhibit attenuated HPA-axis activity around supportive caregivers (47). Thus, the students exposed to higher levels of childhood maltreatment may demonstrate lower levels of circulating inflammatory markers. Gender differences in the association between child maltreatment and inflammatory cytokines have been currently controversial. For instance, Osborn and Widom reported an apparent correlation between childhood trauma and inflammation among women instead of among men (10). A recent review examining the gender-specific effects of early life stress on inflammation (22) clarified that the association between childhood maltreatment and inflammation was driven by significant links in females, not in males. The result of this study supported the above evidence. Adolescence is a period of rapid growth and development culminating in full reproductive maturation. Major anabolic regulators induce marked changes in physiology and metabolism, leading to the pubertal transition and subsequent changes in body composition, a process in which many confounding factors affect the growth of adolescents. Teenagers’own hormone levels like reproductive hormones, testosterone and estrogen, and insulin may also take effect, and thereby conceivably influencing the levels of inflammatory cytokines. For example, testosterone in androgens is involved in the negative biofeedback mechanism of HPA, inhibiting the secretion of pro-gonadotropic hormones. Androgens (i.e., testosterone) have immune-modulating properties compared with estrogens, which may suppress the expression of the pro-inflammatory cytokines (48). On the contrary, a prospective study failed to detect the link between interpersonal stressors and circulating levels of CRP or IL-6 in a sample of female adolescents (mean age, 17 years) (49). Similarly, some studies could not find such notable gender differences in the effect of childhood adversity on physical health outcomes (17, 50). Consequently, whether the association between adolescent childhood maltreatment with inflammation is gender specific requires more far-reaching research.
After adjusting for other covariates, we observed positive associations between emotional abuse, emotional neglect, childhood maltreatment and IL-8 in late adolescence. As a proinflammatory cytokine, IL-8, mainly produced by macrophages and microglia (51), played a regulatory role in the immune response of the central nervous system. Studies proved that IL-8 could interfere with neurobiological processes during critical periods of brain development (52). Increased serum levels of 1L-8 led to dysfunctional cytokine signaling, which could induce apparent abnormal behavioral changes and various psychiatric diseases, including the onset of depression, anxiety, and cognitive dysfunction (3). The biological embedding model suggests that adverse experiences suffered by the organism during sensitive periods of growth and development can be “programmed” into macrophages through epigenetic markers, post-translational modifications and tissue remodeling, thus rendering these cells pro-inflammatory (53). Notably, we identified that the association between childhood maltreatment and IL-8 was only in late adolescence. A possible explanation is that the inflammatory system remains resilient during early adolescence, adapting to the environment and maintaining homeostasis in the body (54). This also may be due to unmeasured the timing of the childhood maltreatment on the results may be masked. Research by Hung et al. (55) demonstrated that inflammation accumulated with age, even after adjusted for confounding factors such as education, BMI, physical activity, alcohol use, and smoking status. Puberty may be the time when a pro-inflammatory phenotype appears in the circulating markers of immune function due to increases in risky behaviors, such as smoking, lack of exercise, unhealthy diet, and impulsive behaviors that increase stress exposure (56–58). Thus, as children developed into young adults (e.g., college students), the immune system received more biological and behavioral inputs which induced the production and regulation of inflammation, leading to more reliable elevations in circulating inflammatory markers among at-risk populations (with early life adversity exposure) (26). This observation may remind future researchers of the significance of taking developmental processes into account in studies of inflammatory processes in children. In conclusion, our findings put forward a hint that some other factors like age may be more pertinent in affecting inflammatory cytokine levels.
Significant as the study of childhood maltreatment in relation to inflammatory markers in middle school students was, the main contribution of this study lay in the revelation of the roles of gender and age in the relationship between childhood maltreatment and inflammation. Besides, the large sample size and high response rate of participants were noteworthy. However, several limitations could not be overlooked in the interpretation of our results. First, the study design was cross-sectional, and the temporal ordering of the variables remained unclear, although our findings pertaining to the association between childhood maltreatment and inflammatory markers were similar to those of a previous cohort study (18). Second, due to the same background of participants in one boarding school, apparently, the findings might not be applied to all adolescents. Finally, because of the complexity of the influencing factors of inflammation, many confounding variables, such as the timing of the childhood maltreatment, sleep, diet, and recent stressful life events, may also affect inflammation, which was not included in this study, however. Despite these limitations, our study added to the literature on childhood maltreatment associated with an inflammatory pathway.
This study reveals that girls with elevated scores of childhood maltreatment are more likely to show changes in the levels of inflammatory markers; students in late adolescence are particularly more susceptible to low-grade inflammation. These results strengthen an inference concerning the effects of childhood maltreatment on inflammation in middle school students and have implications for the ways in which childhood maltreatment can be incorporated into health programs in adolescents.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Anhui Medical University (20170290). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
ZJ, SL, and RL contributed to data acquisition, data analysis and the writing of this manuscript. XS contributed to help with sample collection and data analysis. SZ, YS, and FT assisted in revising the manuscript. The present study was conceived and designed by YW. All authors contributed to the article and approved the submitted version.
Funding for the project was provided by the National Natural Science Foundation of China (81773453 & 81202223) and the Natural Science Foundation in Higher Education of Anhui (KJ2019ZD72). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to acknowledge the staff and students from the boarding middle school.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1067291/full#supplementary-material
1. Hänsel A, Hong S, Cámara RJ, von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev (2010) 35(1):115–21. doi: 10.1016/j.neubiorev.2009.12.012
2. Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology (2015) 40(7):1709–16. doi: 10.1038/npp.2015.17
3. Tsai SJ. Role of interleukin 8 in depression and other psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry (2021) 106:110173. doi: 10.1016/j.pnpbp.2020.110173
4. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol (2016) 16(1):22–34. doi: 10.1038/nri.2015.5
5. Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol (2020) 1216:55–64. doi: 10.1007/978-3-030-33330-0_7
6. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med (2019) 18(3):121–6. doi: 10.4103/aam.aam_56_18
7. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation (2016) 133(4):e38–360. doi: 10.1161/CIR.0000000000000350
8. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci (2014) 69 Suppl 1:S4–9. doi: 10.1093/gerona/glu057
9. Kerr DM, McDonald J, Minnis H. The association of child maltreatment and systemic inflammation in adulthood: A systematic review. PloS One (2021) 16(4):e0243685. doi: 10.1371/journal.pone.0243685
10. Osborn M, Widom CS. Do documented records and retrospective reports of childhood maltreatment similarly predict chronic inflammation? Psychol Med (2020) 50(14):2406–15. doi: 10.1017/S0033291719002575
11. Ehrlich KB, Miller GE, Rogosch FA, Cicchetti D. Maltreatment exposure across childhood and low-grade inflammation: Considerations of exposure type, timing, and sex differences. Dev Psychobiol (2021) 63(3):529–37. doi: 10.1002/dev.22031
12. Metzler M, Merrick MT, Klevens J, Ports KA, Ford DC. Adverse childhood experiences and life opportunities: Shifting the narrative. Child Youth Serv Rev (2017) 72:141–9. doi: 10.1016/j.childyouth.2016.10.021
13. Ports KA, Holman DM, Guinn AS, Pampati S, Dyer KE, Merrick MT, et al. Adverse childhood experiences and the presence of cancer risk factors in adulthood: A scoping review of the literature from 2005 to 2015. J Pediatr Nurs. (2019) 44:81–96. doi: 10.1016/j.pedn.2018.10.009
14. Sheikh MA. Child maltreatment, psychopathological symptoms, and onset of diabetes mellitus, hypothyroidism and COPD in adulthood. J Affect Disord (2018) 241:80–5. doi: 10.1016/j.jad.2018.07.085
15. Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: A systematic review. Brain Behav Immun (2012) 26(2):239–50. doi: 10.1016/j.bbi.2011.11.003
16. Boshtam M, Asgary S, Kouhpayeh S, Shariati L, Khanahmad H. Aptamers against pro- and anti-inflammatory cytokines: A review. Inflammation (2017) 40(1):340–9. doi: 10.1007/s10753-016-0477-1
17. Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral c-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry (2016) 21(5):642–9. doi: 10.1038/mp.2015.67
18. Heard-Garris N, Davis MM, Estabrook R, Burns J, Briggs-Gowan M, Allen N, et al. Adverse childhood experiences and biomarkers of inflammation in a diverse cohort of early school-aged children. Brain Behav Immun (2020) 1:100006. doi: 10.1016/j.bbih.2019.100006
19. Lacey RE, Kumari M, McMunn A. Parental separation in childhood and adult inflammation: The importance of material and psychosocial pathways. Psychoneuroendocrinology (2013) 38(11):2476–84. doi: 10.1016/j.psyneuen.2013.05.007
20. Gill H, El-Halabi S, Majeed A, Gill B, Lui LMW, Mansur RB, et al. The association between adverse childhood experiences and inflammation in patients with major depressive disorder: A systematic review. J Affect Disord (2020) 272:1–7. doi: 10.1016/j.jad.2020.03.145
21. de Koning RM, Kuzminskaite E, Vinkers CH, Giltay EJ, Penninx BWJH. Childhood traumaandLPS-stimulated inflammationin adulthood: Results from the Netherlands study of depression and anxiety. Brain Behav Immun (2022) 106:21–9. doi: 10.1016/j.bbi.2022.07.158
22. Baldwin JR, Arseneault L, Caspi A, Fisher HL, Moffitt TE, Odgers CL, et al. Childhood victimization and inflammation in young adulthood: A genetically sensitive cohort study. Brain Behav Immun (2018) 67:211–7. doi: 10.1016/j.bbi.2017.08.025
23. Takizawa R, Danese A, Maughan B, Arseneault L. Bullying victimization in childhood predicts inflammation and obesity at mid-life: A five-decade birth cohort study. Psychol Med (2015) 45(13):2705–15. doi: 10.1017/S0033291715000653
24. Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiatr Scand (2014) 129(3):180–92. doi: 10.1111/acps.12217
25. Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol Psychiatry (2016) 80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017
26. Kuhlman KR, Horn SR, Chiang JJ, Bower JE. Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain Behav Immun (2020) 86:30–42. doi: 10.1016/j.bbi.2019.04.028
27. Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry (1997) 36(3):340–8. doi: 10.1097/00004583-199703000-00012
28. Zhao XF, Zhang YL, Li LF, Zhou YF, Li HZ, Yang SC. Reliability and validity of the Chinese version of childhood trauma questionnaire. Chin J Clin Rehabil. (2005) 20:105–7.
29. Wan Y, Chen R, Wang S, Clifford A, Zhang S, Orton S, et al. Associations of coping styles with nonsuicidal self-injury in adolescents: Do they vary with gender and adverse childhood experiences? Child Abuse Negl (2020) 104:104470. doi: 10.1016/j.chiabu.2020.104470
30. Wang S, Xu H, Li S, Jiang Z, Wan Y. Sex differences in the determinants of suicide attempt among adolescents in China. Asian J Psychiatr (2020) 49:101961. doi: 10.1016/j.ajp.2020.101961
31. Patton GC, Sawyer SM, Santelli JS, Ross DA, Afifi R, Allen NB, et al. Our future: A lancet commission on adolescent health and wellbeing. Lancet (2016) 387(10036):2423–78. doi: 10.1016/S0140-6736(16)00579-1
32. Li Y, Jinxiang T, Shu Y, Yadong P, Ying L, Meng Y, et al. Childhood trauma and the plasma levels of IL-6, TNF-α are risk factors for major depressive disorder and schizophrenia in adolescents: A cross-sectional and case-control study. J Affect Disord (2022) 305:227–32. doi: 10.1016/j.jad.2022.02.020
33. Trotta A, Arseneault L, Danese A, Mondelli V, Rasmussen LJH, Fisher HL. Associations between childhood victimization, inflammatory biomarkers and psychotic phenomena in adolescence: A longitudinal cohort study. Brain Behav Immun (2021) 98:74–85. doi: 10.1016/j.bbi.2021.08.209
34. Azad MB, Lissitsyn Y, Miller GE, Becker AB, HayGlass KT, Kozyrskyj AL. Influence of socioeconomic status trajectories on innate immune responsiveness in children. PloS One (2012) 7(6):e38669. doi: 10.1371/journal.pone.0038669
35. Ehrlich KB, Ross KM, Chen E, Miller GE. Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype? Dev Psychopathol. (2016) 28(4pt2):1273–83. doi: 10.1017/S0954579416000845
36. Ayaydin H, Abali O, Akdeniz NO, Kok BE, Gunes A, Yildirim A, et al. Immune system changes after sexual abuse in adolescents. Pediatr Int (2016) 58(2):105–12. doi: 10.1111/ped.12767
37. Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol (2004) 113(6):1051–7. doi: 10.1016/j.jaci.2004.03.032
38. Menke A, Lehrieder D, Fietz J, Leistner C, Wurst C, Stonawski S, et al. Childhood trauma dependent anxious depression sensitizes HPA axis function. Psychoneuroendocrinology (2018) 98:22–9. doi: 10.1016/j.psyneuen.2018.07.025
39. Ganança L, Oquendo MA, Tyrka AR, Cisneros-Trujillo S, Mann JJ, Sublette ME. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology (2016) 63:296–310. doi: 10.1016/j.psyneuen.2015.10.008
40. Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry (2015) 2(11):1002–12. doi: 10.1016/S2215-0366(15)00309-0
41. Quagliato LA, Nardi AE. Cytokine alterations in panic disorder: A systematic review. J Affect Disord (2018) 228:91–6. doi: 10.1016/j.jad.2017.11.094
42. Wiener CD, Moreira FP, Cardoso TA, Mondin TC, da Silva Magalhães PV, Kapczinski F, et al. Inflammatory cytokines and functional impairment in drug-free subjects with mood disorder. J Neuroimmunol. (2017) 307:33–6. doi: 10.1016/j.jneuroim.2017.03.003
43. Tyrka AR, Parade SH, Valentine TR, Eslinger NM, Seifer R. Adversity in preschool-aged children: Effects on salivary interleukin-1β. Dev Psychopathol. (2015) 27(2):567–76. doi: 10.1017/S0954579415000164
44. Ridout KK, Parade SH, Seifer R, Price LH, Gelernter J, Feliz P, et al. Interleukin 1B gene (IL1B) variation and internalizing symptoms in maltreated preschoolers. Dev Psychopathol. (2014) 26(4 Pt 2):1277–87. doi: 10.1017/S0954579414001023
45. Kuhlman KR, Repetti RL, Reynolds BM, Robles TF. Change in parent-child conflict and the HPA-axis: Where should we be looking and for how long? Psychoneuroendocrinology (2016) 68:74–81. doi: 10.1016/j.psyneuen.2016.02.029
46. Vandewalle J, Luypaert A, De Bosscher K, Libert C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol Metab (2018) 29(1):42–54. doi: 10.1016/j.tem.2017.10.010
47. Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology (2002) 27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2
48. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab (2004) 89(7):3313–8. doi: 10.1210/jc.2003-031069
49. Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med (2009) 71(1):57–62. doi: 10.1097/PSY.0b013e318190d7de
50. Campbell JA, Farmer GC, Nguyen-Rodriguez S, Walker R, Egede L. Relationship between individual categories of adverse childhood experience and diabetes in adulthood in a sample of US adults: Does it differ by gender? J Diabetes Complications. (2018) 32(2):139–43. doi: 10.1016/j.jdiacomp.2017.11.005
51. Remick DG. Interleukin-8. Crit Care Med (2005) 33(12 Suppl):S466–7. doi: 10.1097/01.ccm.0000186783.34908.18
52. Morimoto K, Nakajima K. Role of the immune system in the development of the central nervous system. Front Neurosci (2019) 13:916. doi: 10.3389/fnins.2019.00916
53. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull (2011) 137(6):959–97. doi: 10.1037/a0024768
54. Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci (2010) 21(6):848–56. doi: 10.1177/0956797610370161
55. Hung J, Knuiman MW, Divitini ML, Davis T, Beilby JP. Prevalence and risk factor correlates of elevated c-reactive protein in an adult Australian population. Am J Cardiol (2008) 101(2):193–8. doi: 10.1016/j.amjcard.2007.07.061
56. Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry (2015) 77(4):314–23. doi: 10.1016/j.biopsych.2014.04.020
57. Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res (2013) 37(4):616–23. doi: 10.1111/acer.12016
Keywords: childhood maltreatment, inflammatory cytokines, gender, age, middle school students
Citation: Jin Z, Li S, Li R, Song X, Zhang S, Sun Y, Tao F and Wan Y (2023) Gender- and age-specific associations of childhood maltreatment with peripheral serum inflammatory cytokines in middle school students. Front. Immunol. 14:1067291. doi: 10.3389/fimmu.2023.1067291
Received: 11 October 2022; Accepted: 19 January 2023;
Published: 31 January 2023.
Edited by:
Jason C. O’Connor, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Milica Milovan Borovcanin, University of Kragujevac, SerbiaCopyright © 2023 Jin, Li, Li, Song, Zhang, Sun, Tao and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhui Wan, MjAwNDUwMDAzOUBhaG11LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.