- 1Department of General Surgery, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 2Institute (College) of Integrative Medicine, Dalian Medical University, Dalian, Liaoning, China
- 3Laboratory of Integrative Medicine, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 4Genome and Computational Biology Lab, Mohanlal Sukhadia University, Udaipur, Rajasthan, India
- 5Department of Molecular Diagnostics and Experimental Therapeutics, Beckman Research Institute of City of Hope, Biomedical Research Center, Comprehensive Cancer Center, Monrovia, CA, United States
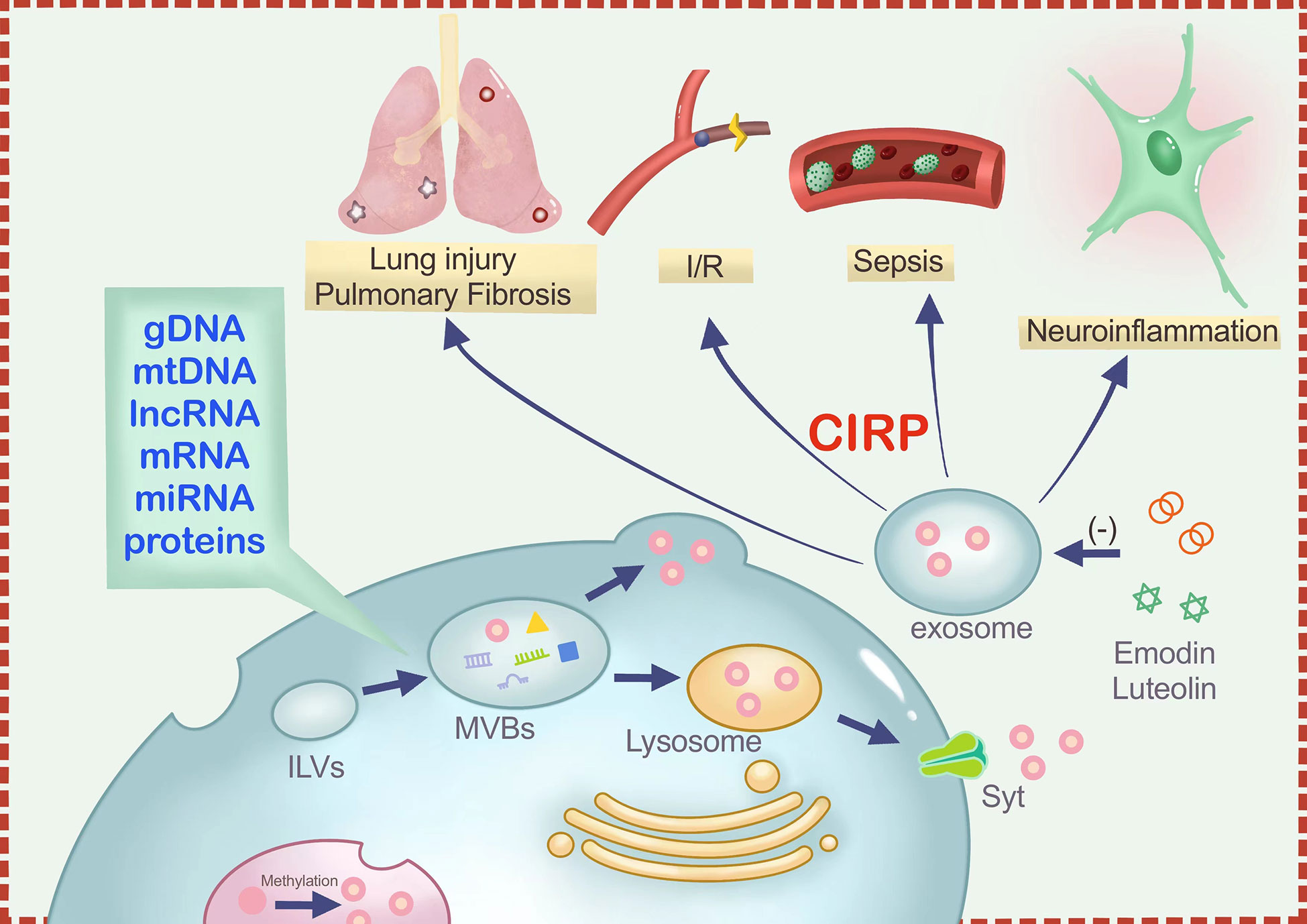
Cold-inducible RNA-binding protein (CIRP) is an intracellular stress-response protein and a type of damage-associated molecular pattern (DAMP) that responds to various stress stimulus by altering its expression and mRNA stability. Upon exposure to ultraviolet (UV) light or low temperature, CIRP get translocated from the nucleus to the cytoplasm through methylation modification and stored in stress granules (SG). During exosome biogenesis, which involves formation of endosomes from the cell membrane through endocytosis, CIRP also gets packaged within the endosomes along with DNA, and RNA and other proteins. Subsequently, intraluminal vesicles (ILVs) are formed following the inward budding of the endosomal membrane, turning the endosomes into multi-vesicle bodies (MVBs). Finally, the MVBs fuse with the cell membrane to form exosomes. As a result, CIRP can also be secreted out of cells through the lysosomal pathway as Extracellular CIRP (eCIRP). Extracellular CIRP (eCIRP) is implicated in various conditions, including sepsis, ischemia-reperfusion damage, lung injury, and neuroinflammation, through the release of exosomes. In addition, CIRP interacts with TLR4, TREM-1, and IL-6R, and therefore are involved in triggering immune and inflammatory responses. Accordingly, eCIRP has been studied as potential novel targets for disease therapy. C23 and M3, polypeptides that oppose eCIRP binding to its receptors, are beneficial in numerous inflammatory illnesses. Some natural molecules such as Luteolin and Emodin can also antagonize CIRP, which play roles similar to C23 in inflammatory responses and inhibit macrophage-mediated inflammation. This review aims to provide a better understanding on CIRP translocation and secretion from the nucleus to the extracellular space and the mechanisms and inhibitory roles of eCIRP in diverse inflammatory illnesses.
1 Introduction
Cold-inducible RNA-binding protein (CIRP) were first discovered in mouse testis more than two decades ago (1). It came to the attention of researchers as a result of its expression during moderate cold stress. The RG/RGG region of CIRP is critical for mediating CIRP phase separation in vitro and stress granules (SGs) association in cells (2). As a RNA-binding protein (RBP), CIRP plays important roles in transcription, pre-mRNA processing/transport, mRNA degradation, translation, and non-coding RNA processing. Studies have shown that the 3`-UTR binding sites of CIRP are enriched within 100 nucleotides upstream of the polyadenylation sites, and UU and UUU are most likely the core recognition sequences of CIRP (3). CIRP consists of an N-terminal RNA-recognition motif (RRM) and a C-terminal arginine-rich region. The X-ray quaternary structure of the CIRP RRM has been resolved recently and four important residues with possible involvement in protein-nucleic acid binding have been identified (4). Expression of CIRP during cold stress modulates RNA stability and translation in hibernating mammals that reduce their body temperature from 37°C to as low as 0~5°C during bouts of dullness (5). CIRP is translocated from the nucleus to the cytoplasm, whereby they stabilize mRNAs. They can regulate mRNA transcripts and protect them from degradation for future protein synthesis. Studies have also shown that the expression of CIRP can also be regulated by several kinds of stress conditions suggesting that CIRP is generally expressed as stress-response proteins. Besides, CIRP is widely expressed in a large variety of tissues and cells.
According to the literature, iCIRP has been implicated in multiple cellular processes such as cell proliferation (6), cell survival (7, 8), telomere maintenance (9), circadian modulation (10), DNA repair (11) and tumor formation and progression (12, 13). Intracellular CIRP (iCIRP) can migrate from the nucleus to the cytoplasm via methylation-dependent mechanism in response to stress and regulate the stability of mRNA through their binding sites on the 3′-UTR of their target mRNAs (3). The translocation of CIRP is dependent on GSK3β and CK2. Both GSK3β and CK2 cause phosphorylation of CIRP and affect its cellular localization and pretreatment of cells with either CK2 inhibitors or GSK3β inhibitors prior to UV treatment significantly reduce CIRP localization to the cytosol (14). These results suggest that both methylation and phosphorylation of CIRP is required for CIRP translocation to the cytosol upon stress stimulus. Some studies have demonstrated that both CK2 sites and GS3Kβ sites on CIRP have overlapping functional role in regulating CIRP translocation to the cytosol, but only GSK3β sites are involved in the RNA-binding activity of CIRP, in response to UV radiation (14, 15).
In contrast to its functions in the intracellular space, extracellular CIRP (eCIRP) has been discovered recently as a damage-associated molecular pattern (DAMP) capable of triggering inflammation in various inflammatory conditions (16), including sepsis (17), neuroinflammation (18)and ischemia-reperfusion injury (19–24). For example, administering exogenous CIRP to healthy mice was shown to induce lung injury through vascular leakage, neutrophil infiltration, local production of pro-inflammatory cytokines, and activating the NLRP3 inflammasome in the vascular endothelial cells of the lung (25). Altogether, a fair number of studies have investigated the potential role of eCIRP in different models of inflammatory diseases, such as sepsis (16, 26–29), acute pancreatitis (30), and Alzheimer’s disease (31). The important pro-inflammatory role of eCIRP suggests that targeting eCIRP may be of potential therapeutic importance in controlling inflammatory diseases. Nevertheless, the translation of such findings into clinical practice still remains nascent field to be explored.
Here, we summarize how eCIRP, especially exosome derived CIRP, amplify inflammation in different inflammatory conditions with the aim of providing new insights for the development of novel targeted therapies.
2 Conventional secretion mechanisms of eCIRP
CIRP release mechanisms include necrosis, lysosome-mediated release, extracellular traps, and exosomes (32).CIRP can be released either via inflammasome activation or passively following cell death (33). Necroptosis, apoptosis, pyroptosis, and ferroptosis might contribute to the passive release of the CIRP (32). Studies have shown that CIRP is among the main factors that induces mitochondrial DNA (mtDNA) fragmentation in damaged tissues (34). However, according to an in-vitro study on macrophages, it has been shown that cell necrosis does not trigger the passive release of CIRP (16).
Active release of CIRP is mediated mainly by lysosomes and exosomes. When cells are stimulated by oxidative stress, hypothermia, ultraviolet (UV) radiation, etc. CIRP are transferred to the extracellular space through the lysosomal pathway (12, 33). CIRP migrates from the nucleus to cytoplasmic SGs via a methylation-dependent mechanism and act as translational repressors. Stressors such as oxidative stress, endoplasmic reticulum (ER) stress, osmotic shock, and heat shock may lead to the methylation of iCIRP (35). Oxidative stress leads to CIRP migration to SGs without altering their expression (36). SGs are dynamic cytoplasmic foci in which stalled translation initiation complexes accumulate in cells that are subjected to environmental stress. The relocation of CIRP to the SG also occurs in osmotic stress, heat shock and in response to endoplasmic reticulum stress (36). CIRP/hnRNP A18 is an RNA-binding factor consisting of an RNA recognition motif (RRM) at the N-terminus and a region at the c-terminus containing multiple repeats of the RGG motif. Methylation is important for the recruitment of CIRP from the nucleus to the SGs. As mentioned earlier, TIA-1 has been shown to be the main mediator of SGs formation (37). In the absence of TIA-1 or TIAR proteins, CIRP is still recruited to SGs. Since CIRP does not contain any signaling peptides, their secretion cannot be mediated by the classical Endoplasmic Reticulum-Golgi-dependent pathway. In one study, biochemical fractionation revealed that CIRP is enriched in the lysosomal compartment of macrophages undergoing hypoxia, suggesting that CIRP is released through lysosomal secretions (16, 35). As a class of DAMPs, eCIRP conforms to the general mechanism of the extracellular release of DAMPs (32). The well-studied carriers of DAMPs during active release are the secreted lysosomes and exosomes, both of which are normally secreted by exocytosis (16, 38).
Lysosomal secretion is a typical feature of stressed cells and has been also demonstrated to be a release pathway for eCIRP (16, 32). The synaptotagmin (Syt) assay found that lysosomal secretion can be initiated by stimulating cell surface receptors as well as through increasing intracellular Ca2+. Syt mobilizes lysosomes to the microtubule organizing center, where lysosomes are associated with kinesin motility (39). The motor proteins further transport the lysosome near the secretion site, where the lysosome travels to the docking site using actin-based movements. The docking and fusion of lysosomes with the plasma membrane are mediated by Rabs and SNARE (Soluble NSF Attachment Protein Receptors) complexes, respectively (39, 40).
3 Regulation of CIRP expression
The overexpression of CIRP has been consistently witnessed in different organs and species (from amphibians to humans) upon mild hypothermia or cold stress (10), which supports the plausible protective role of CIRP in adaptation of these species to cold stress. However, CIRP homologs in different species responded differently to different environmental stresses. Besides this, CIRP is also overexpressed upon UV radiation, mild hypoxia and low glucose conditions, but is under expressed in response to heat stress stimulus and upon treatment with inflammatory cytokines such as TNF-α and TGF-β (12), underscoring that CIRP is general stress responsive protein. While, CIRP is overexpressed upon mild hypoxia, the chronic hypoxia results in completely opposite genetic outcome, that is low CIRP expression (13). Glycogen synthase kinase 3β (GSK3β) is an important serine/threonine kinase, which is involved in various biochemical processes in cells, for instance cell metabolism, cell cycle, transcription, vesicular transport and others (41). Activation of GSK3β has shown to increase the transcription of CIRP (16). CIRP is also upregulated by IGF1 (17). As expected, the transcription of the CIRP homolog in Salmon can be upregulated by osmotic stress, but not by cold stress (18) as expected to be respond differently in a species-specific manner. Similar to this, there exist different homologs of CIRP in Xenopus cells including xCIRP, xCIRP-1 and xCIRP-2. All homologs express and respond differently to different environmental stimulus. For instance, low temperature (cold stress) induces overexpression of xCIRP and xCIRP 2, but not xCIRP-1 (9, 19, 20). This implies that the CIRP is a general stress protein and its expression and response to a particular stress differs significantly between species.
4 Exosomal CIRP
An essential mode of intercellular communication is the transport of information components such as proteins, nucleic acids, and lipids via exosomes (42, 43), extracellular vesicles measuring between 40 and 100 nm in diameter and found in various bodily fluids (44). Exosomes support the interior environment’s balance under normal physiological settings (45). However, these exosomes may have radically altered contents in a state of excessive inflammation. Many studies have shown that miRNAs such as miR-155 (46) and miR-21 (47) transported by exosomes play a crucial role in inflammation. It has been shown that CIRs can be mainly released outside the cell in two ways: passively through necrosis and actively through lysosomes (32). However, Murao et al. (48), discovered that CIRP might stimulate inflammatory cell aggregation and activation through the exosome secretion.
Murao and colleagues found that the serum exosomes of LPS-injected and CLP-operated mice contained significantly higher amounts of eCIRP than those in control animals. Similarly, activation of macrophages with LPS resulted in a significant increase in the release of exosomal CIRP. Based on these findings, it appears that exosome-carried CIRP are a substantial source of eCIRP. Accordingly, the presence of eCIRP in exosomes could be justified by the presence of the lipid bilayer on the surface of exosomes, which can protect proteins or microRNAs from being degraded during transport process. CD63 is a well-known marker used to identify exosomes (49). Studies have revealed that CIRP can stably bind to CD63. Furthermore, they discovered that exosome biogenesis and release inhibitors (50) could not only block exosome release but also simultaneously decrease the expression of eCIRP. With the exposure of inflammatory cells to exosomes, eCIRP present on the surface of the exosomes stimulates the release of pro-inflammatory cytokines and migration of neutrophils by binding to cell surface receptors (48) (Figure 1). However, there is no direct evidence of eCIRP release from the interior of exosome/luminal vesicles to the surface. Through literature review, it was found that there were transmembrane proteins on the exosome surface, such as lysosome-associated membrane protein (LAMP) and transferrin receptor (TfR) (51).Therefore, we hypothesized that proteins carried in exosomes cross the phospholipid bimolecular wind of exosomes through the stimulation of specific signaling molecules to reach the surface, and CIRP is no exception. Taken together, these findings support the notion that exosomes are mediators of CIRP release that targeting exosomal CIRP may represent a novel treatment strategy.
5 Role of eCIRP in inflammatory diseases
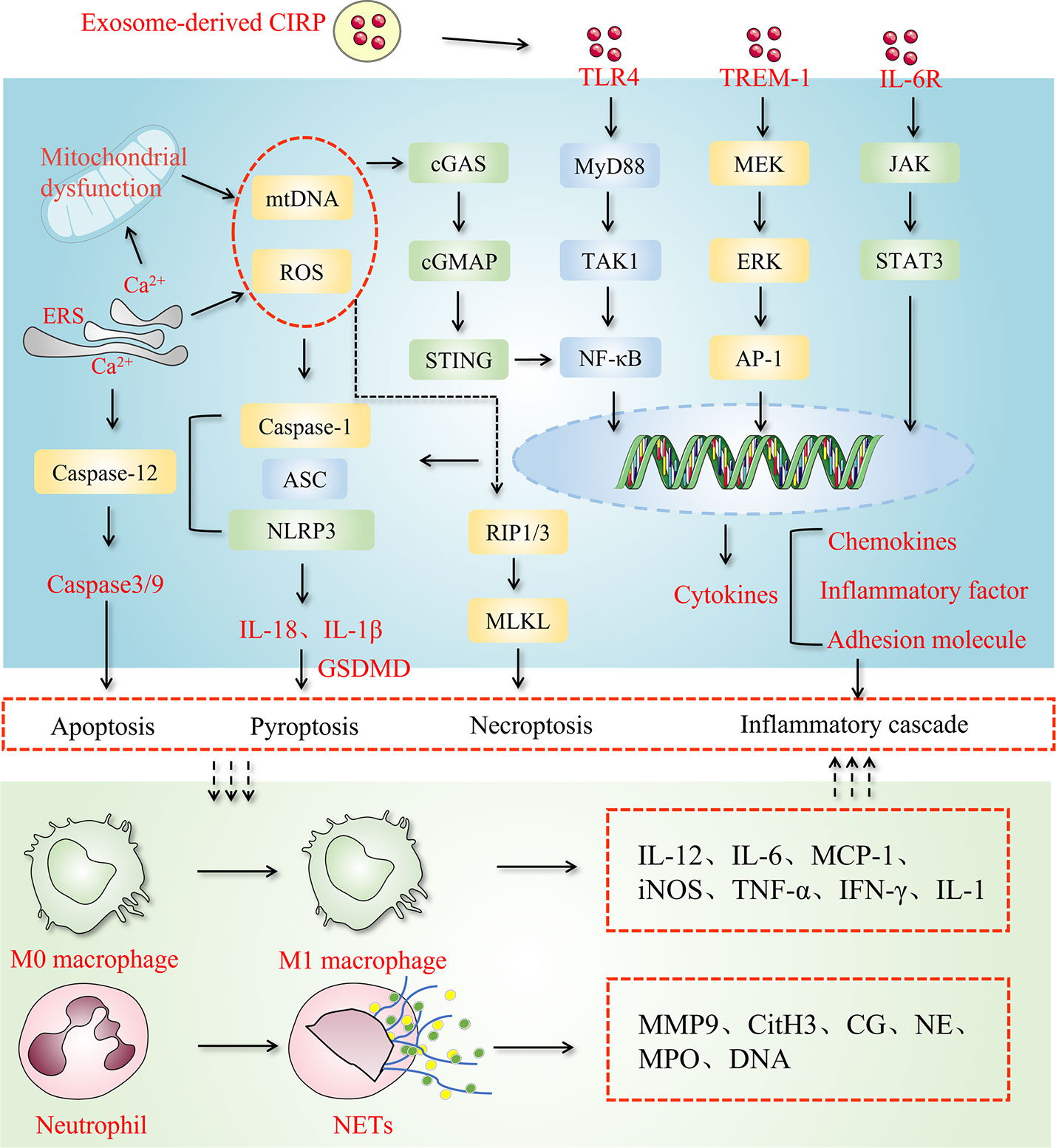
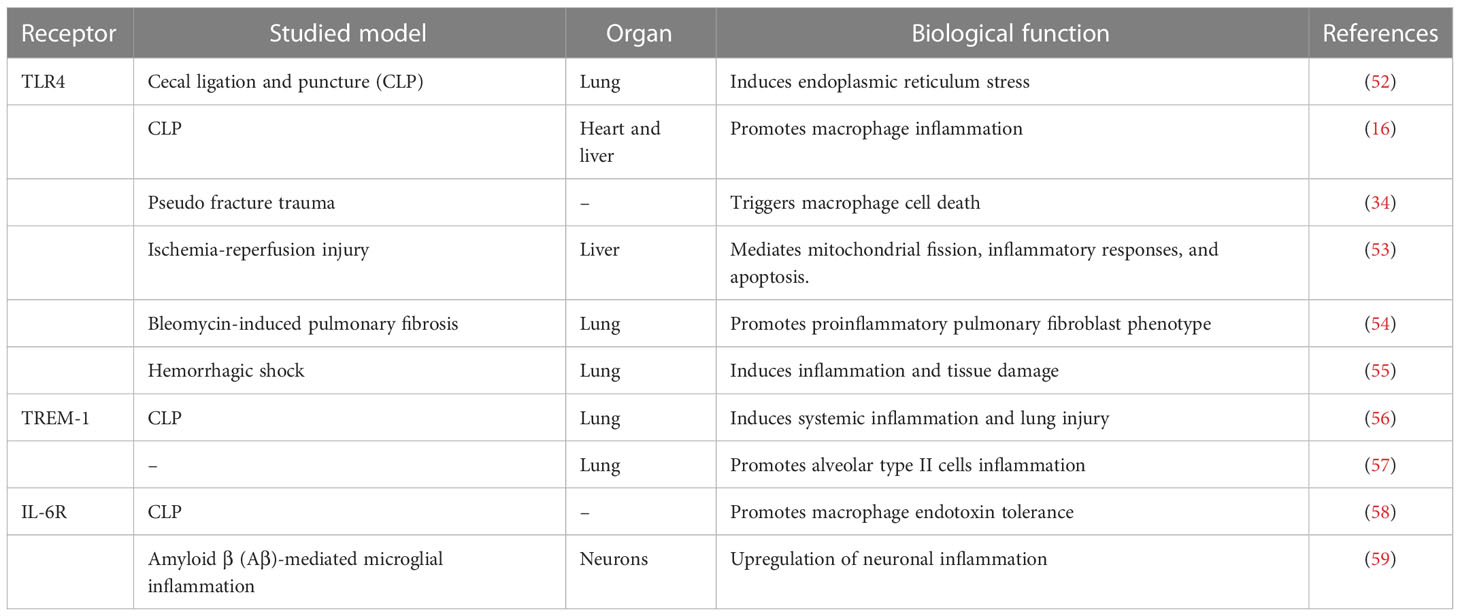
In inflammatory diseases, eCIRP acts as an inflammatory amplifier by binding to its receptors. TLR4, TREM-1, and IL-6R are three receptors to which CIRP binding occurs, as mentioned in the Table 1. TLR4 is a member of an important class of molecules involved in adaptive immunity and acts as a bridge between adaptive and innate immunity. CIRP binds to TLR4-MD2, a complex formed in systemic inflammation induced by hemorrhagic shock and sepsis (16). Hemorrhagic shock increases eCIRP levels and activates STING through the TLR4/MyD88/TRIF pathway to exacerbate inflammation (55). In lung fibroblasts, eCIRP amplifies pro-inflammatory cytokines in a TLR4-dependent manner, triggering pulmonary fibrosis (54). As a receptor for eCIRP, TREM-1 plays a vital role in ischemia-reperfusion in the liver, intestine, and other organs (60), and induces inflammation in macrophages and neutrophils (35), forming NETs (61). In hemorrhagic shock and sepsis, TREM-1 can also be a key target of eCIRP (16, 56, 62). In the alveoli, TREM-1 can act as a target to trigger inflammation in alveolar type II cells (57). One study showed a strong binding affinity between eCIRP and IL-6R (58). Supporting this finding, it is well known that CIRP can skew the macrophages towards the M2 phenotype and that using IL-6R antibodies CIRP can inhibit M2 polarization (58). In neurological diseases, eCIRP activates the IL-6Rα/STAT3/Cdk5 pathway in neurons, inducing neuroinflammation (59). Combining the above two studies, we can find that IL-6R plays a double-edged sword role in inflammation. It can improve the tolerance of macrophages to endotoxin in sepsis, and can also promote microglial inflammation in the nervous system.
5.1 Sepsis
Sepsis is a life-threatening inflammatory disorder caused by a dysregulated host immune response to infection (63). In a recent survey, the annual global incidence of sepsis has been estimated at 31.5 million cases resulting in 5.3 million deaths worldwide every year (64). According to a previous study (16), CIRP translocates from the nucleus to the cytoplasm and are subsequently released into the circulation during sepsis (33). As a well-described member of the DAMP family, there is evidence that eCIRP plays a crucial role in regulating sepsis. eCIRP has long been the subject of great interest in sepsis.
In this context, the regulatory effects of eCIRP on neutrophils has received extensive attention due to the vital roles of neutrophils in sepsis. For instance, ICAM-1-positive neutrophils, via increased production of neutrophil extracellular traps (NETs) and inducible nitric oxide synthase (iNOS), play a major role in the induction of exaggerated inflammation during sepsis (65). Furthermore, eCIRP has been shown to promote the formation of NETs by inducing PAD4 expression in the lungs of a mouse model of sepsis (66). By activating TREM-1 on the surface of neutrophils, eCIRP induces NET-forming ICAM-1-positive neutrophils (61). In addition, ICAM-1-mediated Rho activation further promotes the formation of NETs as a novel pathway (61). Another study showed that eCIRP could induce neutrophil reverse transendothelial migration (rTEM) in sepsis by increasing neutrophil elastase (NE) and decreasing the junctional adhesion molecule-C (JAM-C) (27). The reversely migrated (RM) neutrophils showed a prolonged lifespan and were associated with systemic inflammation in the cremaster muscle ischemia-reperfusion (I/R) injury in a mouse model (67). These studies consistently indicate that RM neutrophils may contribute to the exacerbation of a local inflammation into a systemic inflammatory status. CIRP also triggered endoplasmic reticulum (ER) stress via TLR4 activation in preclinical models of sepsis and promoted inflammation, apoptosis, and histological injury (52). Induction of ER stress due to CIRP release in sepsis can be blocked by creating knockout of TLR4 or CIRP in mice, suggesting that CIRP can modulate ER stress through TLR4 signaling pathway (52). Besides this, the CRIP release in sepsis is also reported to trigger adaptive immune system in the spleen by activating T-lymphocytes such as CD4+ and CD8+ T cells in a TLR4-depentent manner (28). Most importantly, the plasma level of CIRP of patients with sepsis is also correlated with the survival as the non-surviving patients have high level of serum CIRP than the survivors, suggesting that CIRP may act as a potent prognostic marker of sepsis in human (17). Altogether, it can be ruled out that CIRP plays acts as a mediator in organ dysfunction during sepsis by amplifying inflammation in immune cells and damaging vascular EC in all vital organs.
5.2 Lung injury
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is a complex clinical syndrome. eCIRP, as a type of DAMP, triggers inflammation in various inflammatory conditions including inflammatory lung injury (16, 68, 69). eCIRP can promote ALI by activating macrophages (16), neutrophils (57), pneumocytes (57), and pulmonary vascular endothelial cells (25).
A recent study (25) related to CIRP has shown that administering exogenous CIRP to healthy mice causes lung injury through vascular leakage, neutrophil infiltration, local production of pro-inflammatory cytokines, and activating the NLRP3 inflammasome in lung vascular endothelial cells. During the past years, a fair amount of studies have investigated the potential role of eCIRP in different models of inflammatory lung injury such as ALI caused by various diseases (52), chronic obstructive pulmonary disease (COPD) (70, 71), and pulmonary fibrosis (72). Recently, CIRP’s elevated expression was found in the bronchi in patients suffering from chronic obstructive pulmonary diseases and corresponding in-vitro study demonstrated that CIRP induces expression of inflammatory cytokines and mucin in human airway epithelial cells through activation of activating ERK and TLR4/NF-κB signaling pathway (70, 73). The important pro-inflammatory role of eCIRP suggests that targeting eCIRP may have a therapeutic potential in controlling inflammatory lung injury.
Lately, a study (74) has revealed the possible regulation pathway of eCIRP in ALI. It was found that the expression of CIRP increased in lung tissues of the LPS-induced ALI/ARDS mice and inhibited the polarization of M2 macrophages and increased the inflammatory response. eCIRP-neutralizing antibodies attenuated the M1 phenotype and enhanced the M2 phenotype in macrophages. eCIRP controls inflammation by regulating the phenotypic changes in macrophages. Inhibition of eCIRP was able to attenuate local and systemic inflammation like AP-associated acute lung injury (APALI) (68).
5.3 Neuroinflammation
Neuroinflammation is a disorder observed in the central nervous system (CNS) in response to infection, toxic metabolites, trauma, or autoimmune stimuli. Recent research (18) has revealed that CIRP has a considerable effect on neuroinflammation.
Alcohol, as an exogenous stimulant, affects the CNS and causes memory loss.Some researchers believe that eCIRP might be a key player in the relationship between alcohol and Alzheimer’s disease (AD) (31). Besides, both alcohol (31) and cerebral ischemia (19) resulted in the expression of eCIRP in microglial cells as well as its release. CIRP is a potential novel mediator of alcohol-induced brain inflammation, leading to local inflammation and neuronal cell damage via the TLR4 pathway (75). A recent study (59) reported that eCIRP activated neurotoxic cyclin-dependent kinase-5 (Cdk5)/p25 through the induction of the IL-6Rα/STAT3 pathway in neurons, and C23 subsided the eCIRP-induced increase in neuronal STAT3 phosphorylation and p25 level. Additionally, CIRP may activate TLR4 signaling and further induce NF-κB activation (75). In a mouse model of stroke induced by middle cerebral artery occlusion (MCAO)in CIRP deficient mice (19), TNF-α induction and microglial activation were significantly reduced, and the volume of cerebral infarction was attenuated after MCAO.
5.4 Ischemia-reperfusion Injury
Ischemia-reperfusion (I/R) injury is defined as the impaired tissue function after ischemia (76), which can occur in multiple organs causing organ damage and failure. CIRP has been shown to be an important factor involved in the regulation of I/R injury, and that it plays vital roles in the pathways of the liver (20), kidney (21, 22), and intestinal I/R injury (23, 24).
Following ischemic tissue damage and subsequent reperfusion, endogenous DAMPs, which are normally intracellular, are released extracellularly and trigger sterile inflammation (77). M3, an eCIRP-derived peptide (62), can inhibit TREM-1 by protecting mice against intestinal I/R injury and showed good therapeutic effects after reperfusion. The severity of organ injury was mitigated, serum levels of systemic inflammation markers such as IL-6, and TNF-α were reduced and the inflammation in the intestinal tissue itself was also improved.
Interestingly, recent evidence has established a causal link between CIRP and ALI during intestinal I/R injuries. In an adult male CIRP knock-out (CIRP -/-) mouse model, pro-inflammatory cytokine, myeloperoxidase, and apoptotic cells were significantly lower than in C57BL/6J wild-type mice, which led to decreased lung injury (24). In another parallel experiment (23), the intraperitoneal injection of C23, 60 minutes after the ischemic insult, could mitigate the systemic inflammation, extent of injury and the ALI of intestinal I/R mice.
According to the results from an adult male C57BL/6J mouse model of hepatic I/R, circulating levels of CIRP were increased and the treatment of an anti-CIRP antibody reduced the inflammatory storm and cellular damage in the liver and significantly inhibited neutrophil infiltration into the liver (20).
There is also some evidence that CIRP may affect renal injury after I/R. The deficiency of CIRP reduces renal injury after renal I/R by reducing inflammation and oxidative stress (21). A study (22) showed that the expressions of kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin were significantly decreased in C23-treated mice.
5.5 Idiopathic pulmonary fibrosis
Pulmonary fibrosis is a devastating sequela of many chronic inflammatory diseases characterized by a progressive decline in lung volume capacity and high mortality. eCIRP induces pro-inflammatory cytokines and differentially-expressed pathways in lung fibroblasts in a TLR4-dependent manner, and the accessory pathways MD2 and Myd88 are involved in the induction of the inflammatory phenotype (54). Furthermore, CIRP is involved in the regulation of pulmonary fibrosis, as eCIRP can directly activate and induce inflammatory phenotype fibroblasts (IPF) in the lung. In a recent research with bleomycin-induced pulmonary fibrosis in a mouse model, C23 alleviated pulmonary fibrosis and molecular markers of fibrosis were ameliorated in TLR4-/-mice (54), suggesting that eCIRP acts as a key a key promoter of PF, and blockage eCIRP with C23 can significantly attenuate this inflammatory PF.
6 Inhibitors of CIRP
6.1 Polypeptides
Focusing on the previously shown mechanisms of action of eCIRP, there are also some studies on novel therapeutic drugs. C23, an oligopeptide derived from the human CIRP protein, binds to the CIRP receptor with high affinity (16). Studies have shown that treatment with C23 decreased systemic inflammation in adult or neonatal sepsis mouse models (78, 79). Although C23 is the best polypeptide known thus block CIRP from its interaction, there exist many more inhibitors as well. C23 stands out among several short sequences derived from CIRP with a higher affinity for TLR4-MD2 receptor complexes compared to CIRP in terms of binding activity (16). As of today, C23 has been shown to act as a protective agent (22) against tissue damage resulting from hemorrhagic shock (78), sepsis (29), and intestinal ischemia-reperfusion (23) by inhibition of CIRP-induced inflammatory response. Remarkably, C23 has a far greater affinity to the TLR4-MD2 receptor complex than LPS and HMGB1, suggesting that C23 derived from CIRP may be a potential therapeutic approach for tissue and organ damage caused by cytokine storm.
M3 is another interesting CIRP-derived polypeptide with slight differences compared to C23. M3 acts on TREM-1, a receptor for eCIRP. During hepatic I/R, the binding of eCIRP to TREM-1 increases the number of inflammatory cells in the liver leading to increased tissue damage therein. M3 treatment, on the other hand, had an increased effect on the survival rate of mice after hepatic I/R (60). When M3 competes with TREM-1, it also blocks the eCIRP signaling pathway in the heart (80) and kidney (81). M3 exerts protective effects against sepsis-induced myocardial injury and acute kidney injury after renal I/R by inhibiting the eCIRP/TREM-1 interaction (81).
Similarly, pre-treating with M3, a human eCIRP-derived ligand-dependent 7-amino acid (aa) peptide, acts as an antagonist of TREM-1 (56), and LP17, a TREM-1 decoy peptide (82), significantly decreased the production of IL-6 and CXCL2 in the cuboidal type II cells (ATII) (57). MicroRNAs (miRNAs) have already been identified in the extracellular space and are involved in different physiological or pathological processes (83). Recent research also identified a novel interaction between miR-130b-3p and eCIRP via the TLR4 pathway. miRNA 130b-3p (84) serves as a new endogenous inhibitor of eCIRP-mediated inflammatory responses.
6.2 Natural products
Due to their anti-inflammatory properties, some natural products have been shown to be as effective as synthetic drugs in treating various diseases and ailments. There has been a lot of evidence that natural products and their derivatives can help reduce, inflammation, inflammatory cell infiltration, inflammatory signaling, and release of DAMPs in-vitro and in-vivo (85). In addition, because of their low toxicity and high safety profiles, they are usually the subject of a great deal of attention for clinical and therapeutic interventions.
For example, Emodin (EMO), which is an anthraquinone, has numerous pharmacological effects, particularly as a potent anti-inflammatory phytochemical. Pancreatic and lung tissues as well as the serum were shown to have high levels of CIRP in a rat model of acute pancreatitis, which was found to be associated with the formation of the NLRP3 inflammasome in alveolar macrophages and infiltration of neutrophils (25, 68). Intriguingly, EMO reverses these effects in rats thereby minimizes damage to the pancreas and lungs. In addition, EMO had a comparable pharmacological inhibitory action to C23 in rat alveolar macrophages, inhibiting the production of inflammatory mediators in the presence of CIRP. On such a basis, EMO could be a potential CIRP antagonist (68).
Another natural agent, Luteolin (LUT), has been also shown to inhibit the synthesis of CIRP. Lut has significant anti-inflammatory and antioxidant properties (86). Research suggests that Lut can reduce organ damage caused by I/R and endotoxemia by blocking the HMGB1 (a well-known DAMP) signaling pathway, indicating that Lut and DAMPs may have a unique functional relationship (87). Zhang et al. (88) showed that Lut treatment in neonatal mice with sepsis reduces the expression of CIRP mRNA and protein and attenuates lung injury. Lut also decreases CIRP formation in peritoneal macrophages following LPS administration. In contrast to EMO, the protective effect of Lut on neonatal mouse macrophages can be attributed to the reduction in the production of HIF-1 (89) and the NLRP3 (90) inflammasome, as indicated by in vitro experiments.
In the inflammatory response, endogenous CIRP-mediated signaling pathways cause damage to multiple organs. Antagonizing endogenous CIRP can help prevent I/R or sepsis-induced lung, kidney, heart, liver, and intestinal injury. By blocking CIRP-mediated signaling pathways, C23 and M3 effectively prevent organ damage. This is done through their effects on different receptors. Further investigations on whether natural products such as Luteolin (88) and Emodin (68) could act directly on the CIRP protein and its downstream receptor or by affecting the transcription of upstream genes need to be investigated in depth.
7 Exosomes as drug carriers in CIRP targeting-based therapies
Delivery systems use specialized carriers to facilitate the aggregation of drugs in their target tissues or cells in order to increase their efficacy and usage (91). Combining targeted medications with nanocarriers is one of the most important means of increasing the effectiveness of targeted therapeutics (92–94). Exosomes generated from intracellular membranes (95) have many benefits over conventional nanocarriers, including good biocompatibility and targeting (96), easy modification of the membrane surface (97), and the capacity to traverse the blood-brain barrier and placental barrier (98). As a new means of drug delivery, exosomes are gaining attention (99).
Exosome-based targeted drug delivery systems have been summarized in several studies (100). Naturally occurring exosomes can be used as carriers for a wide range of therapeutic agents such as natural products (101–104), synthetic pharmaceuticals (105), nucleic acid drugs (106, 107), and protein-based peptides or proteins (108). Exosomes can penetrate biological barriers that regular nanomaterials cannot, and they exhibit good efficacy. The exosome-carried CIRP brings a more severe inflammatory response to the disease while providing us with a potential therapeutic strategy (48, 109). Using exosomes that have been artificially modified may be a way to reduce the eCIRP-induced inflammatory response. On one hand, adding peptides or proteins to the surface of exosomes inhibits the activation of downstream signaling pathways by targeting inflammatory cell surface receptors (110, 111) and on the other hand, exosomes directly loaded with natural products through incubation (108), electroporation (112), sonication (113), and transfection exert anti-inflammatory effects. In conclusion, these methods may address the low water solubility and bioavailability of natural compounds (114). Moreover, the drug loading and modification (115) of EVs may enhance the targeting ability of the drug and prevent its rapid oxidation.
8 Conclusions
Since the first description of CIRP two decades ago, the physiopathological and biological roles of CIRP have been extensively studied. Although we have gained significant achievements in unraveling the role of CIRP in diseased etiology and pathogenesis; however, there are still many unanswered questions, for instance, what is the role of CIRP in chronic inflammatory diseases such as obesity, diabetes and other kinds of diseases. It has been proved that iCIRP participates in cell proliferation, cell survival, apoptosis, and circadian rhythm, telomere maintenance, and carcinoma progression through regulating mRNA stability as an RNA chaperone (28).
Whereas little is known about the function of exosome-carried CIRP and its role in inflammation. Here we summarized former and recent studies on the roles of eCIRP, especially exosome-derived CIRP, in inflammatory disease. One of the most notable recent developments is that eCIRP has been shown to play an important part in various types of ALI through participating in different inflammatory pathways. The discovery of eCIRP’s role as an important DAMP has led to a new field in inflammatory disease research, while many knowledge gaps still exist and need to be addressed. As a stabilizing RNA-binding protein, eCIRP is pro-inflammatory in most inflammatory conditions but also reduces inflammation by unknown mechanisms and pathways. On one hand, it remains to be investigated in other cell types such as B lymphocytes and NK cells since current studies have only focused on eCIRPs role in macrophages, neutrophils, and T cell biology. On the other hand, only macrophages have been identified as the source of exosomal CIRP, while other cell types as sources of exosomal CIRP remain to be studied.
Both in-vivo and in-vitro evidence support the notion that eCIRP can be considered as a novel target for the research and development of innovative drugs for the prevention or treatment of inflammatory diseases. Future studies should be aimed at clarifying the pivotal roles eCIRP plays in other potential pathways of inflammatory diseases in the hope that more therapeutic targets can be identified. Peptides and natural products targeting exosomal CIRP may facilitate the clinical translation of these new therapeutic strategies against inflammation.
The proinflammatory role and the plausible protective effects of CIRP blockage using neutralizing antibody or proteins/peptides in sepsis and other inflammatory diseases suggested a pivotal role of CIRP in inflammation-related diseases and has offered a strong basis for the exploration of therapeutic potential of neutralizing antibody or peptide in tackling various inflammatory diseases. Therefore, elucidating the role of CIRP in these diseases will have a significant impact on our understanding of physiopathological of diseases and may provide the rationale for the design of novel therapeutics.
Author contributions
Conceptualization, CX, HC; writing—original draft preparation, JH, YZ, PG, TD, HW, ST, YL, QY, BH and GZ. All authors have edited the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82104594 and 82074158) and the National Key R&D Program of China (2019YFE0119300).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1066721/full#supplementary-material
References
1. Nishiyama H, Danno S, Kaneko Y, Itoh K, Yokoi H, Fukumoto M, et al. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol (1998) 152(1):289–96.
2. Zhou Q, Usluer S, Zhang F, Lenard AJ, Bourgeois BMR, Madl T. ATP regulates RNA-driven cold inducible RNA binding protein phase separation. Protein Sci (2021) 30(7):1438–53. doi: 10.1002/pro.4123
3. Xia Z, Zheng X, Zheng H, Liu X, Yang Z, Wang X. Cold-inducible RNA-binding protein (CIRP) regulates target mRNA stabilization in the mouse testis. FEBS Lett (2012) 586(19):3299–308. doi: 10.1016/j.febslet.2012.07.004
4. Coburn K, Melville Z, Aligholizadeh E, Roth BM, Varney KM, Carrier F, et al. Crystal structure of the human heterogeneous ribonucleoprotein A18 RNA-recognition motif. Acta Crystallogr F Struct Biol Commun (2017) 73(Pt 4):209–14. doi: 10.1107/S2053230X17003454
5. Logan SM, Storey KB. Cold-inducible RNA-binding protein cirp, but not Rbm3, may regulate transcript processing and protection in tissues of the hibernating ground squirrel. Cell Stress Chaperones (2020) 25(6):857–68. doi: 10.1007/s12192-020-01110-3
6. Masuda T, Itoh K, Higashitsuji H, Higashitsuji H, Nakazawa N, Sakurai T, et al. Cold-inducible RNA-binding protein (Cirp) interacts with Dyrk1b/Mirk and promotes proliferation of immature male germ cells in mice. Proc Natl Acad Sci United States America (2012) 109(27):10885–90. doi: 10.1073/pnas.1121524109
7. Roilo M, Kullmann MK, Hengst L. Cold-inducible RNA-binding protein (CIRP) induces translation of the cell-cycle inhibitor p27Kip1. Nucleic Acids Res (2018) 46(6):3198–210. doi: 10.1093/nar/gkx1317
8. Sakurai T, Itoh K, Higashitsuji H, Nonoguchi K, Liu Y, Watanabe H, et al. Cirp protects against tumor necrosis factor-alpha-induced apoptosis via activation of extracellular signal-regulated kinase. Biochim Biophys Acta (2006) 1763(3):290–5. doi: 10.1016/j.bbamcr.2006.02.007
9. Zhang Y, Wu Y, Mao P, Li F, Han X, Zhang Y, et al. Cold-inducible RNA-binding protein CIRP/hnRNP A18 regulates telomerase activity in a temperature-dependent manner. Nucleic Acids Res (2016) 44(2):761–75. doi: 10.1093/nar/gkv1465
10. Morf J, Rey G, Schneider K, Stratmann M, Fujita J, Naef F, et al. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science (2012) 338(6105):379–83. doi: 10.1126/science.1217726
11. Zhong P, Peng J, Bian Z, Huang H. The role of cold inducible RNA-binding protein in cardiac physiology and diseases. Front Pharmacol (2021) 12:610792. doi: 10.3389/fphar.2021.610792
12. Zhu X, Bührer C, Wellmann S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell Mol Life Sci CMLS (2016) 73(20):3839–59. doi: 10.1007/s00018-016-2253-7
13. Lujan DA, Ochoa JL, Hartley RS. Cold-inducible RNA binding protein in cancer and inflammation. Wiley Interdiscip Rev RNA (2018) 9(2):1–18. doi: 10.1002/wrna.1462
14. Yang R, Zhan M, Nalabothula NR, Yang Q, Indig FE, Carrier F. Functional significance for a heterogenous ribonucleoprotein A18 signature RNA motif in the 3'-untranslated region of ataxia telangiectasia mutated and Rad3-related (ATR) transcript. J Biol Chem (2010) 285(12):8887–93. doi: 10.1074/jbc.M109.013128
15. Yang R, Weber DJ, Carrier F. Post-transcriptional regulation of thioredoxin by the stress inducible heterogenous ribonucleoprotein A18. Nucleic Acids Res (2006) 34(4):1224–36. doi: 10.1093/nar/gkj519
16. Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med (2013) 19(11):1489–95. doi: 10.1038/nm.3368
17. Zhou Y, Dong H, Zhong Y, Huang J, Lv J, Li J. The cold-inducible RNA-binding protein (CIRP) level in peripheral blood predicts sepsis outcome. PloS One (2015) 10(9):e0137721. doi: 10.1371/journal.pone.0137721
18. Rajayer SR, Jacob A, Yang WL, Zhou M, Chaung W, Wang P. Cold-inducible RNA-binding protein is an important mediator of alcohol-induced brain inflammation. PloS One (2013) 8(11):e79430. doi: 10.1371/journal.pone.0079430
19. Zhou M, Yang WL, Ji Y, Qiang X, Wang P. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim Biophys Acta (2014) 1840(7):2253–61. doi: 10.1016/j.bbagen.2014.02.027
20. Godwin A, Yang WL, Sharma A, Khader A, Wang Z, Zhang F, et al. Blocking cold-inducible RNA-binding protein protects liver from ischemia-reperfusion injury. Shock (Augusta Ga) (2015) 43(1):24–30. doi: 10.1097/SHK.0000000000000251
21. Cen C, Yang WL, Yen HT, Nicastro JM, Coppa GF, Wang P. Deficiency of cold-inducible ribonucleic acid-binding protein reduces renal injury after ischemia-reperfusion. Surgery (2016) 160(2):473–83. doi: 10.1016/j.surg.2016.04.014
22. McGinn J, Zhang F, Aziz M, Yang WL, Nicastro J, Coppa GF, et al. The protective effect of a short peptide derived from cold-inducible RNA-binding protein in renal ischemia-reperfusion injury. Shock (Augusta Ga) (2018) 49(3):269–76. doi: 10.1097/SHK.0000000000000988
23. McGinn JT, Aziz M, Zhang F, Yang WL, Nicastro JM, Coppa GF, et al. Cold-inducible RNA-binding protein-derived peptide C23 attenuates inflammation and tissue injury in a murine model of intestinal ischemia-reperfusion. Surgery (2018) 164(6):1191–7. doi: 10.1016/j.surg.2018.06.048
24. Cen C, McGinn J, Aziz M, Yang W-L, Cagliani J, Nicastro JM, et al. Deficiency in cold-inducible RNA-binding protein attenuates acute respiratory distress syndrome induced by intestinal ischemia-reperfusion. Surgery (2017) 162(4):917–27. doi: 10.1016/j.surg.2017.06.004
25. Yang W-L, Sharma A, Wang Z, Li Z, Fan J, Wang P. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci Rep (2016) 6:26571. doi: 10.1038/srep26571
26. Ode Y, Aziz M, Wang P. CIRP increases ICAM-1(+) phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J leukocyte Biol (2018) 103(4):693–707. doi: 10.1002/JLB.3A0817-327RR
27. Jin H, Aziz M, Ode Y, Wang P. CIRP induces neutrophil reverse transendothelial migration in sepsis. Shock (Augusta Ga) (2019) 51(5):548–56. doi: 10.1097/SHK.0000000000001257
28. Bolognese AC, Sharma A, Yang WL, Nicastro J, Coppa GF, Wang P. Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner. Cell Mol Immunol (2018) 15(1):38–47. doi: 10.1038/cmi.2016.43
29. Zhang F, Brenner M, Yang WL, Wang P. A cold-inducible RNA-binding protein (CIRP)-derived peptide attenuates inflammation and organ injury in septic mice. Sci Rep (2018) 8(1):3052. doi: 10.1038/s41598-017-13139-z
30. Gong J-D, Qi X-F, Zhang Y, Li H-L. Increased admission serum cold-inducible RNA-binding protein concentration is associated with prognosis of severe acute pancreatitis. Clin Chim Acta (2017) 471:135–42. doi: 10.1016/j.cca.2017.06.002
31. Sharma A, Brenner M, Wang P. Potential role of extracellular CIRP in alcohol-induced alzheimer's disease. Mol Neurobiol (2020) 57(12):5000–10. doi: 10.1007/s12035-020-02075-1
32. Murao A, Aziz M, Wang H, Brenner M, Wang P. Release mechanisms of major DAMPs. Apoptosis (2021) 26(3-4):152–62. doi: 10.1007/s10495-021-01663-3
33. Denning N-L, Aziz M, Gurien SD, Wang P. DAMPs and NETs in sepsis. Front Immunol (2019) 10:2536. doi: 10.3389/fimmu.2019.02536
34. Li Z, Fan EK, Liu J, Scott MJ, Li Y, Li S, et al. Cold-inducible RNA-binding protein through TLR4 signaling induces mitochondrial DNA fragmentation and regulates macrophage cell death after trauma. Cell Death Dis (2017) 8(5):e2775. doi: 10.1038/cddis.2017.187
35. Aziz M, Brenner M, Wang P. Extracellular CIRP (eCIRP) and inflammation. J leukocyte Biol (2019) 106(1):133–46. doi: 10.1002/JLB.3MIR1118-443R
36. De Leeuw F, Zhang T, Wauquier C, Huez G, Kruys V, Gueydan C. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Exp Cell Res (2007) 313(20):4130–44. doi: 10.1016/j.yexcr.2007.09.017
37. Waris S, Wilce MC, Wilce JA. RNA Recognition and stress granule formation by TIA proteins. Int J Mol Sci (2014) 15(12):23377–88. doi: 10.3390/ijms151223377
38. Lu B, Wang C, Wang M, Li W, Chen F, Tracey KJ, et al. Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Rev Clin Immunol (2014) 10(6):713–27. doi: 10.1586/1744666X.2014.909730
39. Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, et al. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol (2006) 174(7):997–1007. doi: 10.1083/jcb.200605004
40. Murray RZ, Stow JL. Cytokine secretion in macrophages: SNAREs, rabs, and membrane trafficking. Front Immunol (2014) 5:538. doi: 10.3389/fimmu.2014.00538
41. Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology (2004) 29(8):1426–31. doi: 10.1038/sj.npp.1300439
42. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (2020) 367(6478):1–40. doi: 10.1126/science.aau6977
43. Murao A, Brenner M, Aziz M, Wang P. Exosomes in sepsis. Front Immunol (2020) 11:2140. doi: 10.3389/fimmu.2020.02140
44. Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinf (2015) 13(1):17–24. doi: 10.1016/j.gpb.2015.02.001
45. de la Torre Gomez C, Goreham RV, Bech Serra JJ, Nann T, Kussmann M. "Exosomics"-a review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front Genet (2018) 9:92. doi: 10.3389/fgene.2018.00092
46. Banerjee N, Talcott S, Safe S, Mertens-Talcott SU. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res Treat (2012) 136(1):21–34. doi: 10.1007/s10549-012-2224-0
47. Sheedy FJ. Turning 21: Induction of miR-21 as a key switch in the inflammatory response. Front Immunol (2015) 6:19. doi: 10.3389/fimmu.2015.00019
48. Murao A, Tan C, Jha A, Wang P, Aziz M. Exosome-mediated eCIRP release from macrophages to induce inflammation in sepsis. Front Pharmacol (2021) 12:791648. doi: 10.3389/fphar.2021.791648
49. Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, et al. Regulation of exosome production and cargo sorting. Int J Biol Sci (2021) 17(1):163–77. doi: 10.7150/ijbs.53671
50. Catalano M, O'Driscoll L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J extracellular vesicles (2020) 9(1):1703244. doi: 10.1080/20013078.2019.1703244
51. Jadli AS, Ballasy N, Edalat P, Patel VB. Inside(sight) of tiny communicator: Exosome biogenesis, secretion, and uptake. Mol Cell Biochem (2020) 467(1-2):77–94. doi: 10.1007/s11010-020-03703-z
52. Khan MM, Yang W-L, Brenner M, Bolognese AC, Wang P. Cold-inducible RNA-binding protein (CIRP) causes sepsis-associated acute lung injury via induction of endoplasmic reticulum stress. Sci Rep (2017) 7:41363. doi: 10.1038/srep41363
53. Liu W, Fan Y, Ding H, Han D, Yan Y, Wu R, et al. Normothermic machine perfusion attenuates hepatic ischaemia-reperfusion injury by inhibiting CIRP-mediated oxidative stress and mitochondrial fission. J Cell Mol Med (2021) 25(24):11310–21. doi: 10.1111/jcmm.17062
54. Bolourani S, Sari E, Brenner M, Wang P. Extracellular CIRP induces an inflammatory phenotype in pulmonary fibroblasts via TLR4. Front Immunol (2021) 12:721970. doi: 10.3389/fimmu.2021.721970
55. Chen K, Cagliani J, Aziz M, Tan C, Brenner M, Wang P. Extracellular CIRP activates STING to exacerbate hemorrhagic shock. JCI Insight (2021) 6(14):1–14. doi: 10.1172/jci.insight.143715
56. Denning NL, Aziz M, Murao A, Gurien SD, Ochani M, Prince JM, et al. Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis. JCI Insight (2020) 5(5):1–16. doi: 10.1172/jci.insight.134172
57. Tan C, Gurien SD, Royster W, Aziz M, Wang P. Extracellular CIRP induces inflammation in alveolar type II cells via TREM-1. Front Cell Dev Biol (2020) 8:579157. doi: 10.3389/fcell.2020.579157
58. Zhou M, Aziz M, Denning NL, Yen HT, Ma G, Wang P. Extracellular CIRP induces macrophage endotoxin tolerance through IL-6R-mediated STAT3 activation. JCI Insight (2020) 5(5):1–16. doi: 10.1172/jci.insight.133715
59. Sharma A, Brenner M, Jacob A, Marambaud P, Wang P. Extracellular CIRP activates the IL-6Rα/STAT3/Cdk5 pathway in neurons. Mol Neurobiol (2021) 58(8):3628–40. doi: 10.1007/s12035-021-02368-z
60. Borjas T, Jacob A, Yen H, Patel V, Coppa GF, Aziz M, et al. Inhibition of the interaction of TREM-1 and eCIRP attenuates inflammation and improves survival in hepatic Ischemia/Reperfusion. Shock (Augusta Ga) (2022) 57(2):246–55. doi: 10.1097/SHK.0000000000001894
61. Murao A, Arif A, Brenner M, Denning NL, Jin H, Takizawa S, et al. Extracellular CIRP and TREM-1 axis promotes ICAM-1-Rho-mediated NETosis in sepsis. FASEB J Off Publ Fed Am Societies Exp Biol (2020) 34(7):9771–86. doi: 10.1096/fj.202000482R
62. Denning NL, Aziz M, Ochani M, Prince JM, Wang P. Inhibition of a triggering receptor expressed on myeloid cells-1 (TREM-1) with an extracellular cold-inducible RNA-binding protein (eCIRP)-derived peptide protects mice from intestinal ischemia-reperfusion injury. Surgery (2020) 168(3):478–85. doi: 10.1016/j.surg.2020.04.010
63. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
64. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet (London England) (2020) 395(10219):200–11. doi: 10.1016/S0140-6736(19)32989-7
65. Ode Y, Aziz M, Wang P. CIRP increases ICAM-1 phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J leukocyte Biol (2018) 103(4):693–707. doi: 10.1002/JLB.3A0817-327RR
66. Ode Y, Aziz M, Jin H, Arif A, Nicastro JG, Wang P. Cold-inducible RNA-binding protein induces neutrophil extracellular traps in the lungs during sepsis. Sci Rep (2019) 9(1):6252. doi: 10.1038/s41598-019-42762-1
67. Woodfin A, Voisin M-B, Beyrau M, Colom B, Caille D, Diapouli F-M, et al. The junctional adhesion molecule JAM-c regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol (2011) 12(8):761–9. doi: 10.1038/ni.2062
68. Xu Q, Wang M, Guo H, Liu H, Zhang G, Xu C, et al. Emodin alleviates severe acute pancreatitis-associated acute lung injury by inhibiting the cold-inducible RNA-binding protein (CIRP)-mediated activation of the NLRP3/IL-1/CXCL1 signaling. Front Pharmacol (2021) 12:655372. doi: 10.3389/fphar.2021.655372
69. Zhong P, Peng J, Yuan M, Kong B, Huang H. Cold-inducible RNA-binding protein (CIRP) in inflammatory diseases: Molecular insights of its associated signalling pathways. Scand J Immunol (2021) 93(1):e12949. doi: 10.1111/sji.12949
70. Chen L, Ran D, Xie W, Xu Q, Zhou X. Cold-inducible RNA-binding protein mediates cold air inducible airway mucin production through TLR4/NF-κB signaling pathway. Int Immunopharmacol (2016) 39:48–56. doi: 10.1016/j.intimp.2016.07.007
71. Juan Y, Haiqiao W, Xie W, Huaping H, Zhong H, Xiangdong Z, et al. Cold-inducible RNA-binding protein mediates airway inflammation and mucus hypersecretion through a post-transcriptional regulatory mechanism under cold stress. Int J Biochem Cell Biol (2016) 78:335–48. doi: 10.1016/j.biocel.2016.07.029
72. Hozumi H, Kataoka K, Kondoh Y, Isayama T, Okada J, Sugiura K, et al. Clinical significance of cold-inducible RNA-binding protein in idiopathic pulmonary fibrosis. Chest (2021) 160(6):2149–57. doi: 10.1016/j.chest.2021.06.067
73. Ran D, Chen L, Xie W, Xu Q, Han Z, Huang H, et al. Cold-inducible RNA binding protein regulates mucin expression induced by cold temperatures in human airway epithelial cells. Arch Biochem Biophys (2016) 603:81–90. doi: 10.1016/j.abb.2016.05.009
74. Zhang W, Wang Y, Li C, Xu Y, Wang X, Wu D, et al. Extracellular CIRP-impaired Rab26 restrains EPOR-mediated macrophage polarization in acute lung injury. Front Immunol (2021) 12:768435. doi: 10.3389/fimmu.2021.768435
75. Zhou K, Cui S, Duan W, Zhang J, Huang J, Wang L, et al. Cold-inducible RNA-binding protein contributes to intracerebral hemorrhage-induced brain injury via TLR4 signaling. Brain Behav (2020) 10(6):e01618. doi: 10.1002/brb3.1618
76. Kumar K, Singh N, Jaggi AS, Maslov L. Clinical applicability of conditioning techniques in ischemia-reperfusion injury: A review of the literature. Curr Cardiol Rev (2021) 17(3):306–18. doi: 10.2174/1573403X16999200817170619
77. Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med (2011) 17(11):1391–401. doi: 10.1038/nm.2507
78. Zhang F, Yang W-L, Brenner M, Wang P. Attenuation of hemorrhage-associated lung injury by adjuvant treatment with C23, an oligopeptide derived from cold-inducible RNA-binding protein. J Trauma Acute Care Surg (2017) 83(4):690–7. doi: 10.1097/TA.0000000000001566
79. Denning N-L, Yang W-L, Hansen L, Prince J, Wang P. C23, an oligopeptide derived from cold-inducible RNA-binding protein, suppresses inflammation and reduces lung injury in neonatal sepsis. J Pediatr Surg (2019) 54(10):2053–60. doi: 10.1016/j.jpedsurg.2018.12.020
80. Denning NL, Aziz M, Diao L, Prince JM, Wang P. Targeting the eCIRP/TREM-1 interaction with a small molecule inhibitor improves cardiac dysfunction in neonatal sepsis. Mol Med (Cambridge Mass) (2020) 26(1):121. doi: 10.1186/s10020-020-00243-6
81. Siskind S, Royster W, Brenner M, Wang P. A novel eCIRP/TREM-1 pathway inhibitor attenuates acute kidney injury. Surgery (2022) 172(2):639–47. doi: 10.1016/j.surg.2022.02.003
82. Gibot S, Kolopp-Sarda M-N, Béné M-C, Bollaert P-E, Lozniewski A, Mory F, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med (2004) 200(11):1419–26. doi: 10.1084/jem.20040708
83. Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res (2011) 39(16):7223–33. doi: 10.1093/nar/gkr254
84. Gurien SD, Aziz M, Jin H, Wang H, He M, Al-Abed Y, et al. Extracellular microRNA 130b-3p inhibits eCIRP-induced inflammation. EMBO Rep (2020) 21(1):e48075. doi: 10.15252/embr.201948075
85. Liu B, Piao X, Niu W, Zhang Q, Ma C, Wu T, et al. Kuijieyuan decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-dependent PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut microbiota. Front Pharmacol (2020) 11:1036. doi: 10.3389/fphar.2020.01036
86. Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J ethnopharmacology (2018) 225:342–58. doi: 10.1016/j.jep.2018.05.019
87. Hong X, Zhao X, Wang G, Zhang Z, Pei H, Liu Z. Luteolin treatment protects against renal ischemia-reperfusion injury in rats. Mediators Inflammation (2017) 2017:9783893. doi: 10.1155/2017/9783893
88. Zhang Y, Zhang J, Ren Y, Li T, Bi J, Du Z, et al. Luteolin suppresses sepsis-induced cold-inducible RNA-binding protein production and lung injury in neonatal mice. Shock (Augusta Ga) (2021) 55(2):268–73. doi: 10.1097/SHK.0000000000001624
89. Fang B, Chen X, Wu M, Kong H, Chu G, Zhou Z, et al. Luteolin inhibits angiogenesis of the M2−like TAMs via the downregulation of hypoxia inducible factor−1α and the STAT3 signalling pathway under hypoxia. Mol Med Rep (2018) 18(3):2914–22. doi: 10.3892/mmr.2018.9250
90. Yu Q, Zhang M, Qian L, Wen D, Wu G. Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway. Life Sci (2019) 225:1–7. doi: 10.1016/j.lfs.2019.03.073
91. Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics (2016) 6(9):1306–23. doi: 10.7150/thno.14858
92. Toledano Furman NE, Lupu-Haber Y, Bronshtein T, Kaneti L, Letko N, Weinstein E, et al. Reconstructed stem cell nanoghosts: A natural tumor targeting platform. Nano Lett (2013) 13(7):3248–55. doi: 10.1021/nl401376w
93. Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS nano (2016) 10(8):7738–48. doi: 10.1021/acsnano.6b03148
94. Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett (2014) 14(4):2181–8. doi: 10.1021/nl500618u
95. Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol (2014) 29:116–25. doi: 10.1016/j.ceb.2014.05.004
96. Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun (2019) 10(1):3838. doi: 10.1038/s41467-019-11718-4
97. Luo R, Liu M, Tan T, Yang Q, Wang Y, Men L, et al. Emerging significance and therapeutic potential of extracellular vesicles. Int J Biol Sci (2021) 17(10):2476–86. doi: 10.7150/ijbs.59296
98. Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics (2021) 13(1):1–20. doi: 10.3390/pharmaceutics13010122
99. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics (2021) 11(7):3183–95. doi: 10.7150/thno.52570
100. Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol Cancer (2020) 19(1):160. doi: 10.1186/s12943-020-01278-3
101. Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther J Am Soc Gene Ther (2010) 18(9):1606–14. doi: 10.1038/mt.2010.105
102. Aqil F, Jeyabalan J, Agrawal AK, Kyakulaga AH, Munagala R, Parker L, et al. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct (2017) 8(11):4100–7. doi: 10.1039/C7FO00882A
103. Zhang J, Zhang HD, Yao YF, Zhong SL, Zhao JH, Tang JH. β-elemene reverses chemoresistance of breast cancer cells by reducing resistance transmission via exosomes. Cell Physiol Biochem Int J Exp Cell physiology biochemistry Pharmacol (2015) 36(6):2274–86. doi: 10.1159/000430191
104. Yang J, Gao F, Zhang Y, Liu Y, Zhang D. Buyang huanwu decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. J Mol Neurosci MN (2015) 56(4):898–906. doi: 10.1007/s12031-015-0539-0
105. Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics (2019) 9(6):1714–27. doi: 10.7150/thno.30716
106. Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, et al. Exosome-mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol Ther J Am Soc Gene Ther (2016) 24(10):1836–47. doi: 10.1038/mt.2016.126
107. Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res (2012) 40(17):e130. doi: 10.1093/nar/gks463
108. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for parkinson's disease therapy. J Controlled release Off J Controlled Release Soc (2015) 207:18–30. doi: 10.1016/j.jconrel.2015.03.033
109. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell (2019) 177(2):428–445.e418. doi: 10.1016/j.cell.2019.02.029
110. Mir B, Goettsch C. Extracellular vesicles as delivery vehicles of specific cellular cargo. Cells (2020) 9(7):1–19. doi: 10.3390/cells9071601
111. Joshi BS, de Beer MA, Giepmans BNG, Zuhorn IS. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano (2020) 14(4):4444–55. doi: 10.1021/acsnano.9b10033
112. Zhang YF, Shi JB, Li C. Small extracellular vesicle loading systems in cancer therapy: Current status and the way forward. Cytotherapy (2019) 21(11):1122–36. doi: 10.1016/j.jcyt.2019.10.002
113. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine nanotechnology biology Med (2016) 12(3):655–64. doi: 10.1016/j.nano.2015.10.012
114. Chen C, Wang J, Sun M, Li J, Wang HD. Toward the next-generation phyto-nanomedicines: Cell-derived nanovesicles (CDNs) for natural product delivery. Biomed pharmacotherapy = Biomed pharmacotherapie (2022) 145:112416. doi: 10.1016/j.biopha.2021.112416
115. Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics (2018) 10(4):1–40. doi: 10.3390/pharmaceutics10040218
Glossary
Keywords: cold-inducible RNA-binding protein, exosome, immune response, inflammation, targeted therapy
Citation: Han J, Zhang Y, Ge P, Dakal TC, Wen H, Tang S, Luo Y, Yang Q, Hua B, Zhang G, Chen H and Xu C (2023) Exosome-derived CIRP: An amplifier of inflammatory diseases. Front. Immunol. 14:1066721. doi: 10.3389/fimmu.2023.1066721
Received: 11 October 2022; Accepted: 26 January 2023;
Published: 14 February 2023.
Edited by:
Haichao Wang, Feinstein Institute for Medical Research, United StatesReviewed by:
Thierry Mp Gauthier, National Institutes of Health (NIH), United StatesMax Brenner, Feinstein Institute for Medical Research, United States
Copyright © 2023 Han, Zhang, Ge, Dakal, Wen, Tang, Luo, Yang, Hua, Zhang, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caiming Xu, eHVjYWltaW5nQGRtdS5lZHUuY24=; Hailong Chen, Y2hlbmhhaWxvbmdAZG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Jingrun Han
Jingrun Han Yibo Zhang1,2,3†
Yibo Zhang1,2,3† Peng Ge
Peng Ge Haiyun Wen
Haiyun Wen Shuangfeng Tang
Shuangfeng Tang Yalan Luo
Yalan Luo Qi Yang
Qi Yang Hailong Chen
Hailong Chen Caiming Xu
Caiming Xu