
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 March 2023
Sec. Autoimmune and Autoinflammatory Disorders: Autoinflammatory Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1062919
This article is part of the Research Topic Pathogenesis and Target-Treatments of Systemic Lupus Erythematosus View all 18 articles
Objectives: IGU (IGU), a novel immunomodulatory agent for rheumatoid arthritis, has been shown to be effective and safe as monotherapy in a small population with refractory lupus nephritis (LN). The aim of this prospective study was to evaluate the efficacy and safety of IGU as an add-on therapy in patients with refractory LN in the context of clinical practice.
Methods: This is a single-arm observational study. We have enrolled LN patients since 2019 at Renji Hospital. All participants should have recurrent or refractory LN with at least one immunosuppressant (IS) and have a baseline urine protein/creatinine ratio (UPCR) >1.0. After enrollment, we added IGU (25 mg twice daily) to one of their previous immunosuppressants (IS) without increasing the dose of steroids. The primary outcome was the complete renal response (CRR) in the 6th month. UPCR decrease of over 50% was defined as partial response (PR). Extended follow-up was performed after the initial 6 months.
Results: We enrolled 26 eligible participants. 11/26 patients had chronic kidney disease (CKD) stage 2/3 at the baseline. The IS combined with IGU included mycophenolate mofetil, tacrolimus, and cyclosporin A. No IS change was allowed. 80.7% of patients had baseline steroids less than 0.5mg/kg daily and there was no steroids escalation during the IGU treatment. The CRR rate was 42.3% (11/26) at month 6. With a median follow-up of 52 weeks (range: 23-116 weeks), the CRR rate at the last visit was 50% (13/26) and 73.1% (19/26) of patients had UPCR decrease of over 50%. Six patients withdrew, three for no response and three for renal flare after initial CRR. One patient had an estimated glomerular filtration rate worsening of over 20% and was classified as renal flare. Three mild to moderate adverse events were recorded.
Conclusions: Our investigation merits further investigation in IGU as a potentially tolerable component of combination therapy for refractory LN.
Systemic lupus erythematosus (SLE) is an autoimmune disease that can involve multiple organs or systems (1–3). Lupus nephritis (LN) is associated with high mortality and morbidity rates. Over recent decades, substantial progress has been made in developing immunosuppressant agents and biologic therapies (4). However, a significant proportion of patients either do not respond to first-line immunosuppressive drugs or quickly relapse after initial remission. Approximately 10% of patients with LN will experience continued worsening of renal function and go on to develop end-stage renal disease (5).
To treat refractory LN, the European League Against Rheumatism (EULAR) recommendation suggests a switch either from cyclophosphamide (CYC) to mycophenolate mofetil (MMF) or vice versa. Moreover, combinational therapy is a common strategy in a series of observational studies for refractory LN (6–8). And recently, multi-targeted therapy such as MMF combined with a calcineurin inhibitor (CNI) has been recommended (9–12). The combination therapy of a study agent and a conventional immunosuppressant (IS) is a popular design of present trials for LN: such as trials for belimumab (13), voclosporin (14), obinutuzumab (15), and anifrolumab (16). However, in the context of clinical practice, the efficacy and safety of an agent in combinational treatment need a more cautious interpretation without a control arm.
IGU (IGU), a new immunomodulatory drug, has been approved for treating rheumatoid arthritis (RA) in northeast Asia. According to data from RA clinical trials in Japan and China, IGU is superior to placebo and non-inferior to methotrexate and sulfasalazine (17–20). Mechanically, as a disease-modifying drug for RA, IGU has been discovered to reduce inflammation via the nuclear factor-κB (NF-κB). IGU interferes with TNF-α-induced translocation of NF-κB and suppresses TNF-α-induced production of IL-6, IL-8, and monocyte chemoattractant protein 1 (MCP1) (21, 22). Besides, IGU selectively disturbs Act1-TRAF5 connections and TRAF5-Ikki interactions, interrupting IL-17 signaling (23). IGU inhibits macrophage migration inhibitory factor (MIF) tautomerase activity and prevents MIF-induced proinflammatory effects, therefore sparing steroids (24). COX-2 activity and transcription are both inhibited by IGU (25).
IGU has shown efficacy in LN-like disease of MRL/lpr mice (26). Interestingly, we further found IGU interference human B cell terminal differentiation via PKC/EGR1 axis (27). Recently, we reported 13/14 patients with refractory LN responded to IGU monotherapy at week 24 (28). In this study, we aimed to explore the efficacy and safety of IGU as a component of combination therapy for refractory LN. For this, we applied an add-on design, which we believe is effective for elucidating.
We have screened the medical records from Renji Hospital since 2019. All participants should have recurrent or refractory LN with at least one IS and have a baseline urine protein/creatinine ratio (UPCR) >1.0. Failure was defined as no remission (not achieving complete renal response (CRR) or having UPCR decrease over 50%, see below in outcomes) on one agent for at least 6 months. Once a patient had been enrolled, he or she was prescribed oral IGU at a dose of 25mg twice daily in addition to one of the IS that the patient previously used with an insufficient response (failure or flare). Meanwhile, the patients continued other medications, such as steroids, anti-malaria drugs, or angiotensin converting enzyme/receptor inhibitor (ACEI/ARB), without dose adjustment. All patients gave written informed consent. The study was approved by the Ethics Committee of Renji Hospital, Shanghai, China.
The complete renal response (CRR) (14) at 6 months was used as the primary outcome, i.e., UPCR ≤0.5 with estimated glomerular filtration rate (eGFR) ≥60 mL/min/1·73 m2 or no confirmed decrease from baseline in eGFR of >20% (14). UPCR decrease of over 50% was assessed as partial response (PR) at each visit as a key supplementary treatment target (9), especially for refractory LN. After 6 months, the renal response would be continuously assessed.
Other outcomes evaluated included renal flares, extra-renal flares, and safety. A renal flare was defined according to Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and pediatric lupus nephritis (29). An extra-renal flare was defined as the presence of manifestations that could be attributed to SLE that required high-dose steroids. Any need for treatment escalation over one week, including daily prednisone ≥ 1mg/kg or add/switch to another IS/targeted therapy would be counted as treatment failure and lead to termination of the follow-up.
Grading of the severity of adverse events was carried out using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 (grade scale 0–5).
Baseline clinical characteristics of the study participants were summarized using medians with ranges for continuous variables and proportions for categorical outcomes. Mann-Whitney test was used for group comparison of continuous variables. Fisher exact chi-squared test or likelihood-ratio test was used for group comparison of categorical outcomes. The alternative hypothesis was accepted at a statistical significance level of P<0.05 on all applied statistical tests. Analyses were conducted using IBM SPSS Statistics 25.0. Transition plots for renal outcomes were performed using R language software (Version R 4.2.1).
From 2019 to 2022, we screened 32 patients for this study. Five of these patients failed to meet the inclusion criteria and one patient withdrew her consent. A total of 26 participants were eligible and enrolled. 24/26 participants were female. The median disease duration of LN was 5 years (range: 0.8-19 years). 80.8% (21/26) of the nephritis had been confirmed by biopsy (WHO class III/IV/V) (30). Major clinical characteristics are shown in Table 1.
The median amount of baseline UPCR was 2.808 (range: 1.13-17.76). None of the patients had detectable evidence of active extra-renal organ involvement, probably because of long-term steroid/immunosuppressive therapy and long disease duration (31). At enrollment, the median eGFR was 97 ml/minute/1.73 m2 (range: 52-132), ten patients had chronic kidney disease (CKD) stage 2, and one was in stage 3. 25/26 patients had renal pathology results, most of which were performed at the diagnosis of LN. One patient had too few glomeruli to calculate a reliable acute or chronic index (AI/CI). Among the 24 left patients, the median AI of renal pathology was 8.00 (IQR: 6.25-11.00), and the median CI was 3.00 (IQR 3.00-5.75). The IS combined with IGU included mycophenolate mofetil, tacrolimus, and cyclosporin A. 80.7% (21/26) of patients had steroids less than 0.5mg/kg daily and the steroids dose of the other five patients was in the range of 0.5mg/kg - 1.0mg/kg daily at baseline. (Table 1)
The baseline median anti-dsDNA antibody level was 37.47 IU/mL (range: 6.4-100) by Farr method. The baseline median serum C3 was 0.73g/L (range: 0.36-1.20) by quantitative turbidimetric assay.
The CRR rate was 42.3% (11/26) at month 6. With a median follow-up of 52 weeks (range: 23-116 weeks), the CRR rate at the last visit was 50% (13/26) and 76.9% (20/26) of patients had a UPCR decrease of over 50%. (Table 1) The renal outcome transition from the baseline to the 12th month is illustrated in Figures 1A, B. Five patients (Patient 2, 4, 6, 14, and 21) did not achieve response up to 12 months yet had decreased UPCR >50% of baseline, so they were still with IGU treatment. (Figure 1C) Three patients developed renal relapse and exited the follow-up. Of these three, one patient (Patient 8) had an estimated glomerular filtration rate (eGFR) worsening of over 20%, despite of remission in proteinuria. Most patients kept eGFR stable. (Figure 1D). No steroids escalation was recorded during the IGU treatment. 18 patients experienced at least one reduction of steroids during follow-up. At the last visit, 24/26 patients had steroids ≤ 10mg/d (calculated as prednisone).
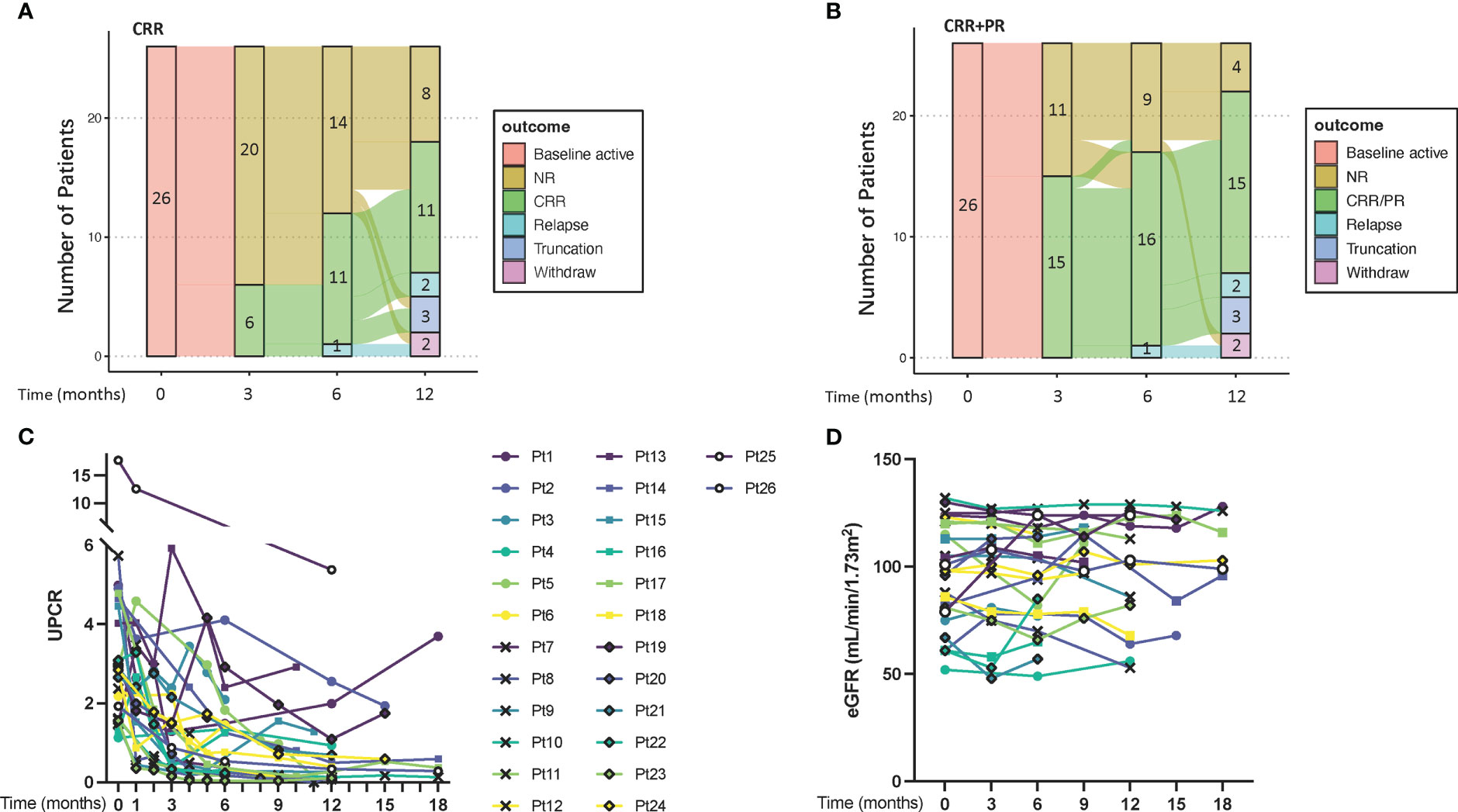
Figure 1 The transition and renal outcomes of the patients. (A, B), transition plots for renal outcomes defined by CRR and CRR+PR, respectively. (C) UPCR of each patient. (D) eGFR of each patient. CRR, complete renal response; PR, partial response; eGFR, estimated glomerular filtration rate; NR, no response; UPCR, urine protein/creatinine ratio.
Two mild to moderate adverse events were recorded. One was leukocytopenia and another was an elevation of alanine aminotransferase. Both events were transient and recovered after symptomatic treatment. Among the five patients receiving the combination of IGU and CNIs, who had more concern for renal function (32), four patients in CKD 1 had eGFR stable and one patient with baseline CKD 2 (Patient 23) experienced a transient eGFR worsening and recovered automatically in the 12th month. Another patient receiving MMF+IGU (Patient 8), had slowly worsened eGFR with a complete remission in proteinuria. Given the decreasing manner and the increase in anti-dsDNA antibody from 6.4IU/L to 46.4IU/L, we classified this patient as a renal flare. No side effects lead to treatment stopping or withdrawal.
In this study, we showed for the first time the feasibility of IGU as a component of combinational therapy for LN management. Previously we showed IGU monotherapy for refractory LN with a 92.3% (12/13) response rate at week 24 (28). The 6-month response rate was 46.2% (12/26) in this study, as the response criteria changed from traditional partial/complete response (11, 28, 33) to CRR and as a higher proportion of CKD in eligible patients (42.3% vs 8.3%).
We had 23 patients eligible for a 12-month analysis, 47.8% (11/23) of whom had CRR and 65.2% (15/23) had CRR+PR. In a recent randomized controlled trial of refractory LN, a combination of three agents, cyclophosphamide, rituximab, and belimumab, achieved 38% CRR and 52% CRR+PR at week 48 (34). In another small observational cohort of refractory lupus, 67% of twelve LN patients had CRR with combinational therapy of rituximab and belimumab (35). Despite different renal remission definitions, the response rate of our study is comparable to those previously reported in observational studies on several agents of refractory LN, including calcineurin inhibitors (9, 10), rituximab (6–8), and stem cell transplantation (25).
The common conventional IS for LN treatment (9), including MMF, CNIs, and azathioprine, were involved in this study. There was no observable discrepancy among these combinations, implying IGU is a versatile component. Of note, all the IS in this study were required to have insufficient response prior to IGU treatment. Moreover, most patients (84.6%) had steroids less than 0.5mg/kg and no patients escalated steroids during IGU treatment. Therefore, the efficacy of IGU could be effectively assessed under these circumstances, which also made the results of the study compelling and reliable despite the absence of a control arm.
Given the missing data, we did not perform further analysis on serum C3 or anti-dsDNA. Other subgroup comparisons between CRR and non-CRR patients showed some trends but not significantly, probably due to the limited number of patients, including sex, LN duration, renal pathology type, previous IS number, as well as baseline UPCR, and the stage of CKD (Supplementary Figure).
Recently, several new treatments, such as belimumab, voclosporin, obinutuzumab, and anifrolumab, have achieved positive primary endpoints or key secondary endpoints in phase II or phase III trials for general LN. These agents represent the current target of LN treatment, including B cell (obinutuzumab and belimumab), T cell (voclosporin), and innate immunity (anifrolumab). However, the CRR in these clinical trials is still far from satisfactory. The 1-year CRR is 35%-41% (13–16), with a conventional IS background treatment in each trial. With a composite effect on all the targets above, IGU inhibits B cell termination differentiation (27), NF-κB and IL-17 signaling in T cells (23, 36), and macrophage activator MIF (24). These features make IGU a competitive candidate for general LN treatment.
Our study had several limitations. 1) The major limitation of the current study is the small sample size with only 26 cases, partially because of the difficulty in collecting refractory LN patients, which is even true for a multicentered clinical trial (34). 2) This study did not contain a control arm. To minimize this shortness, we carefully controlled every confounding factor that might interfere with interpreting the results. The patients needed not only to have active baseline renal manifestations but to meet the criteria for refractory. No steroids or IS escalation over one week was allowed during follow-up. With these, we believe that the results show some value of IGU in this add-on design for LN treatment. 3) Although with a common difficulty for LN management in repeated renal biopsy (37), more differential diagnoses could be done, such as a repeated renal biopsy at the baseline of IGU use to rule out podocytopathy. 4) This study only enrolled patients from East Asia, an ethnic origin in which lupus is less severe as compared to other groups (38), therefore the results cannot be generalized to other patient such as Caucasians or Afro-Americans. 5) The median follow-up time was 52 weeks, which might miss the long-term outcome. To overcome these limitations, we are performing a randomized controlled clinical trial to compare the efficacy of IGU in the induction therapy of active LN with a longer follow-up period (NCT02936375).
Our findings imply the potential feasibility to explore IGU as a component of combination therapy for LN.
The data analyzed in this study is subject to the following licenses/restrictions: The raw data are internal data of Renji Hospital of Shanghai Jiaotong University School of Medicine and are not available to the public. Requests to access these datasets should be directed to QY (eWFucWluZ3JhbkByZW5qaS5jb20=).
The study was approved by the Ethics Committee of Renji Hospital, Shanghai, China. The patients/participants provided their written informed consent to participate in this study.
QY, MZ, and FD carried out the study with support from YK, PY, QL, and BL. CB and MD supervised the project. QY wrote the manuscript. All authors discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
This work is supported by Shanghai Municipal Commission of Health and Family Planning (20204Y0088).
We thank Ms. Jing Zhu and Mr. Shuwen Bao from Jiangsu Simcere Pharmaceutical Company, Ltd. for their support of this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1062919/full#supplementary-material
Supplementary Figure | Subgroup analysis between CRR and non-CRR patients on age, sex, LN duration, renal pathology type, previous IS number, as well as baseline UPCR, and the stage of CKD. None of these statistics are significant. CRR, complete renal response; UPCR, urine protein/creatinine ratio; CKD, chronic kidney disease; LN, lupus nephritis; IS, immunosuppressants.
1. Schwartzman-Morris J, Putterman C. Gender differences in the pathogenesis and outcome of lupus and of lupus nephritis. Clin Dev Immunol (2012) 2012:1–9. doi: 10.1155/2012/604892
2. Mina R, Brunner HI. Pediatric lupus–are there differences in presentation, genetics, response to therapy, and damage accrual compared with adult lupus? Rheumatic Dis Clinics North America (2010) 36(1):53–80. doi: 10.1016/j.rdc.2009.12.012
3. Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol (2017) 12(5):825–35. doi: 10.2215/CJN.05780616
4. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis (2019) 78(6):736–45. doi: 10.1136/annrheumdis-2019-215089
5. Alarcón GS. Multiethnic lupus cohorts: What have they taught us? Reumatología Clínica (2011) 7(1):3–6. doi: 10.1016/j.reuma.2010.11.001
6. Gunnarsson I, Sundelin B, Jonsdottir T, Jacobson SH, Henriksson EW, van Vollenhoven RF. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheumatol (2007) 56(4):1263–72. doi: 10.1002/art.22505
7. Lateef A, Lahiri M, Teng GG, Vasoo S. Use of rituximab in the treatment of refractory systemic lupus erythematosus: Singapore experience. Lupus (2010) 19(6):765–70. doi: 10.1177/0961203309358599
8. Catapano F, Chaudhry AN, Jones RB, Smith KG, Jayne DW. Long-term efficacy and safety of rituximab in refractory and relapsing systemic lupus erythematosus. Nephrol Dial Transplant (2010) 25(11):3586–92. doi: 10.1093/ndt/gfq256
9. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 Update of the joint European league against rheumatism and European renal association-European dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis (2020) 79(6):713–23. doi: 10.1136/annrheumdis-2020-216924
10. Choi CB, Won S, Bae SC. Outcomes of multitarget therapy using mycophenolate mofetil and tacrolimus for refractory or relapsing lupus nephritis. Lupus (2018) 27(6):1007–11. doi: 10.1177/0961203318758505
11. Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med (2015) 162(1):18–26. doi: 10.7326/M14-1030
12. Jesus D, Rodrigues M, da Silva JAP, Ines L. Multitarget therapy of mycophenolate mofetil and cyclosporine a for induction treatment of refractory lupus nephritis. Lupus (2018) 27(8):1358–62. doi: 10.1177/0961203318758508
13. Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med (2020) 383(12):1117–28. doi: 10.1056/NEJMoa2001180
14. Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (2021) 397(10289):2070–80. doi: 10.1016/S0140-6736(21)00578-X
15. Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis (2022) 81(1):100–7. doi: 10.1136/annrheumdis-2021-220920
16. Jayne D, Rovin B, Mysler EF, Furie RA, Houssiau FA, Trasieva T, et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann Rheum Dis (2022) 81(4):496–506. doi: 10.1136/annrheumdis-2021-221478
17. Tanaka K. Iguratimod (T-614): A novel disease modifying anti-rheumatic drug. Rheumatol Rep (2009) 1(1):e4. doi: 10.4081/rr.2009.e4
18. Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, et al. Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Modern rheumatology/the Japan Rheumatism Assoc (2007) 17(1):1–9. doi: 10.3109/s10165-006-0542-y
19. Lu LJ, Teng JL, Bao CD, Han XH, Sun LY, Xu JH, et al. Safety and efficacy of T-614 in the treatment of patients with active rheumatoid arthritis: a double blind, randomized, placebo-controlled and multicenter trial. Chin Med J (2008) 121(7):615–9. doi: 10.1097/00029330-200804010-00008
20. Lu LJ, Bao CD, Dai M, Teng JL, Fan W, Du F, et al. Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheumatol (2009) 61(7):979–87. doi: 10.1002/art.24643
21. Aikawa Y, Yamamoto M, Yamamoto T, Morimoto K, Tanaka K. An anti-rheumatic agent T-614 inhibits NF-kappaB activation in LPS- and TNF-alpha-stimulated THP-1 cells without interfering with IkappaBalpha degradation. Inflammation Res (2002) 51(4):188–94. doi: 10.1007/PL00000291
22. Kohno M, Aikawa Y, Tsubouchi Y, Hashiramoto A, Yamada R, Kawahito Y, et al. Inhibitory effect of T-614 on tumor necrosis factor-alpha induced cytokine production and nuclear factor-kappaB activation in cultured human synovial cells. J Rheumatol (2001) 28(12):2591–6.
23. Luo Q, Sun Y, Liu W, Qian C, Jin B, Tao F, et al. A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J Immunol (2013) 191(10):4969–78. doi: 10.4049/jimmunol.1300832
24. Bloom J, Metz C, Nalawade S, Casabar J, Cheng KF, He M, et al. Identification of iguratimod as an inhibitor of macrophage migration inhibitory factor (MIF) with steroid-sparing potential. J Biol Chem (2016) 291(51):26502–14. doi: 10.1074/jbc.M116.743328
25. Tanaka K, Kawasaki H, Kurata K, Aikawa Y, Tsukamoto Y, Inaba T. T-614, a novel antirheumatic drug, inhibits both the activity and induction of cyclooxygenase-2 (COX-2) in cultured fibroblasts. Jpn J Pharmacol (1995) 67(4):305–14. doi: 10.1254/jjp.67.305
26. Yan Q, Du F, Huang X, Fu Q, Chen S, Dai D, et al. Prevention of immune nephritis by the small molecular weight immunomodulator iguratimod in MRL/lpr mice. PloS One (2014) 9(10):e108273. doi: 10.1371/journal.pone.0108273
27. Ye Y, Liu M, Tang L, Du F, Liu Y, Hao P, et al. Iguratimod represses b cell terminal differentiation linked with the inhibition of PKC/EGR1 axis. Arthritis Res Ther (2019) 21(1):92. doi: 10.1186/s13075-019-1874-2
28. Kang Y, Yan Q, Fu Q, Wang R, Dai M, Du F, et al. Iguratimod as an alternative induction therapy for refractory lupus nephritis: a preliminary investigational study. Arthritis Res Ther (2020) 22(1):65. doi: 10.1186/s13075-020-02154-7
29. Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JHM, et al. Joint European league against rheumatism and European renal association–European dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheumatic Dis (2012) 71(11):1771–82. doi: 10.1136/annrheumdis-2012-201940
30. Aikawa Y, Tanuma N, Shin T, Makino S, Tanaka K, Matsumoto Y. A new anti-rheumatic drug, T-614, effectively suppresses the development of autoimmune encephalomyelitis. J Neuroimmunol (1998) 89(1-2):35–42. doi: 10.1016/S0165-5728(98)00056-3
31. Yan Q, Liu B, Yang M, Li Q, Wang J, Li T, et al. Duration biased distribution of clinical and immunological phenotypes in active SLE. Front Immunol (2022) 13:1044184. doi: 10.3389/fimmu.2022.1044184
32. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. (2009) 4(2):481–508. doi: 10.2215/CJN.04800908
33. Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. (2009) 20(5):1103–12. doi: 10.1681/ASN.2008101028
34. Atisha-Fregoso Y, Malkiel S, Harris KM, Byron M, Ding L, Kanaparthi S, et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol (2021) 73(1):121–31. doi: 10.1002/art.41466
35. Kraaij T, Arends EJ, van Dam LS, Kamerling SWA, van Daele PLA, Bredewold OW, et al. Long-term effects of combined b-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol Dial Transplant (2021) 36(8):1474–83. doi: 10.1093/ndt/gfaa117
36. Li G, Yamasaki R, Fang M, Masaki K, Ochi H, Matsushita T, et al. Novel disease-modifying anti-rheumatic drug iguratimod suppresses chronic experimental autoimmune encephalomyelitis by down-regulating activation of macrophages/microglia through an NF-κB pathway. Sci Rep (2018) 8(1):1933. doi: 10.1038/s41598-018-20390-5
37. Arriens C, Chen S, Karp DR, Saxena R, Sambandam K, Chakravarty E, et al. Prognostic significance of repeat biopsy in lupus nephritis: Histopathologic worsening and a short time between biopsies is associated with significantly increased risk for end stage renal disease and death. Clin Immunol (2017) 185:3–9. doi: 10.1016/j.clim.2016.11.019
Keywords: IGU, refractory lupus nephritis, combinational therapy, add-on, prospective study
Citation: Yan Q, Zhang M, Du F, Kang Y, Ye P, Li Q, Liu B, Dai M and Bao C (2023) Efficacy and safety of Iguratimod as an add-on therapy for refractory lupus nephritis: A preliminary investigational study. Front. Immunol. 14:1062919. doi: 10.3389/fimmu.2023.1062919
Received: 06 October 2022; Accepted: 27 February 2023;
Published: 08 March 2023.
Edited by:
Maria Giovanna Danieli, Università Politecnica delle Marche, ItalyReviewed by:
Beatriz Tejera Segura, Insular University Hospital of Gran Canaria, SpainCopyright © 2023 Yan, Zhang, Du, Kang, Ye, Li, Liu, Dai and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunde Bao, YmFvY2h1bmRlXzE2NzhAMTI2LmNvbQ==; Min Dai, ZGFpbWluQHJlbmppLmNvbQ==; Qingran Yan, eWFucWluZ3JhbkByZW5qaS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.