
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 16 March 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1052657
This article is part of the Research Topic Methods in Cancer Immunity and Immunotherapy: 2022 View all 17 articles
 Rui Han1,2,3,4,5*†
Rui Han1,2,3,4,5*† Jiayin Li6
Jiayin Li6 Jing Hony1,2
Jing Hony1,2 Zhiwei Xiao6
Zhiwei Xiao6 Jinghui wang7
Jinghui wang7 Man Yao1,2
Man Yao1,2 Shufang Liang1,2
Shufang Liang1,2 Lingeng Lu3,4,5*†
Lingeng Lu3,4,5*†Hepatocellular carcinoma (HCC) is a lethal malignancy with a lack of effective treatments particularly for the disease at an advanced stage. Even though immune checkpoint inhibitors (ICIs) have made great progress in the treatment of HCC, durable and ideal clinical benefits still cannot be achieved in plenty of patients with HCC. Therefore, novel and refined ICI-based combination therapies are still needed to enhance the therapeutic effect. The latest study has reported that the carbonic anhydrase XII inhibitor (CAXIIi), a novel type of anticancer drug, can modify the tumor immunosuppression microenvironment by affecting hypoxic/acidic metabolism and alter the functions of monocytes and macrophages by regulating the expression of C-C motif chemokine ligand 8 (CCL8). These observations shine a light on improving programmed cell death protein 1 (PD-1)/programmed cell death ligand-1 (PD-L1) immunotherapy in combination with CAXIIis. This mini-review aims to ignite enthusiasm to explore the potential application of CAXIIis in combination with immunotherapy for HCC.
Hepatocellular carcinoma (HCC) is the most common histological type in primary liver cancer, which occurs predominantly in individuals with chronic liver disease or cirrhosis. HCC is the fourth leading cause of cancer-related death worldwide, and its incidence has been rising globally over the last 20 years (1, 2). It has been expected to keep increasing until 2030 in some countries including the United States, and it has become the fourth of the most common malignancies in China (3, 4). Patients with early-stage HCC may be curable by receiving radical treatment, such as local ablation, surgical resection, or liver transplantation. However, a high recurrence rate still exists (5-year survival rate after surgery is only approximately 35%) (2). In addition, over one-half of HCC patients have already been at an advanced stage when diagnosed due to the lack of sensitive and specific diagnostic tools in the clinic. More importantly, the options of available therapeutic strategies for those patients are also limited (5). For instance, a multikinase inhibitor (MKI), a newly developed anticancer agent compared to traditional chemotherapy, has already made significant progress in HCC treatment in recent years. MKIs such as lenvatinib, cabozantinib, and regorafenib have been approved for treating advanced or metastatic HCC (6–8). However, a considerable number of HCC patients are still unable to gain durable and ideal clinical benefits (7–10).
Since most HCCs are derived from chronic inflammatory liver damage (e.g., hepatitis B virus-related) (11, 12), such a disease is considered an inflammation-related cancer. Therefore, HCC patients are theoretically considered to benefit from immunotherapy (5). In addition, notable advances in the comprehension of HCC immunogenicity have been achieved over the last few years, leading to the evaluation of immune checkpoint inhibitors (ICIs) as a frontline treatment in this setting. However, based on the results of clinical trials, ICI monotherapy has been found to provide limited efficacy against HCC, with the treatment being beneficial in a limited cohort of patients (13, 14). Additionally, the role of ICIs in combination with other anticancer agents (including MKIs) in unresectable treatment-naive HCC has also been assessed in some phase I–III clinical trials (Table 1) (6, 14, 18–21). For instance, in the IMbrave150 trial (phase III study), which randomized HCC patients to atezolizumab plus bevacizumab or to sorafenib monotherapy, the overall survival (OS) and independent review facility-assessed progression-free survival (PFS) both were superior in patients receiving the immunotherapy-based combination compared to those of the monotherapy group (22). Furthermore, as reported in the phase III HIMALAYA trial, the risk of death for HCC patients in the durvalumab plus tremelimumab group was significantly lower than that of the sorafenib monotherapy group (Table 1) (16). Such evidence not only suggests a novel standard of care in HCC patients but also proves the superiority of ICI-combined therapy. However, several unanswered questions remain in such settings, including the lack of biomarkers predictive of response to immunotherapy and the presence of a non-negligible proportion of patients who do not gain benefit from ICIs (21, 23, 24). Therefore, how to explore a novel and effective ICI-based combination therapeutic strategy to obtain better clinical benefits has become a hot and difficult issue in the current international frontier of HCC treatment (25–29).
Carbonic anhydrase XII (CAXII) is a transmembrane zinc metalloenzyme involved in the regulation of the tumor microenvironment, contributing to tumor cell proliferation, invasion, migration, and pluripotency (30). It has been reported that CAXII is overexpressed in HCC, and its level is significantly negatively correlated with the prognosis of HCC patients, which may act as an independent prognostic factor (31–33).
A highly hypoxic tumor microenvironment is a hallmark for human cancer including HCC, which results from the Warburg effect and the surrounding fibrotic tissues caused by persistent chronic inflammation. The overproduction of pyruvate, lactate, and carbonic acid in response to hypoxia aggravates the hypoxic/acidic microenvironment, leading to the enhancement of tumor invasion, tumor immune surveillance escape, and local inflammation. As a regulator of hypoxic stress and acidity, CAXII can affect the tumor microenvironment by regulating proteins such as 14V-ATPase and 15V-ATPase, thereby promoting HCC progression (32, 33). Therefore, a CAXII inhibitor (CAXIIi) is thought to be a novel anti-HCC agent, controlling HCC progression and reducing immunosuppressive stress via the regulation of hypoxic/acidic metabolism. However, the investigation of the antitumor effects and mechanisms of CAXIIis is still in fragmentation. The development and exploration of antitumor CAXIIis have become a new global research hot spot (30, 32, 33).
The initiation of CAXIIis on cancer treatment has started recently. A phase I clinical trial of the first highly selective small-molecule inhibitor of both CAIX and CAXII (SLC-0111) recently has been completed with promising results (34). Briefly, 17 patients with 10 different cancer types including one HCC patient were recruited to be on the inhibitor. The results showed that the inhibitor was safe in patients with previously treated advanced solid tumors (Figure 1) (34). Moreover, a multicenter, open-label, phase Ib study of SLC-0111 in combination with gemcitabine for metastatic pancreatic ductal cancer in subjects positive for CAIX has been conducted since 2018 (Figure 1). Acetazolamide, as a multiple carbonic anhydrase inhibitor (including CAXII), and its combination with radiochemotherapy have been tested in lung cancer (NCT03467360), while the combination of acetazolamide and temozolomide has also been trialed for brain cancer (NCT03011671) (Figure 1) (35, 36). To our knowledge, no other attempt has been made to investigate the therapeutic effect of CAXIIis in HCC treatment so far, which is still a desertlike field.
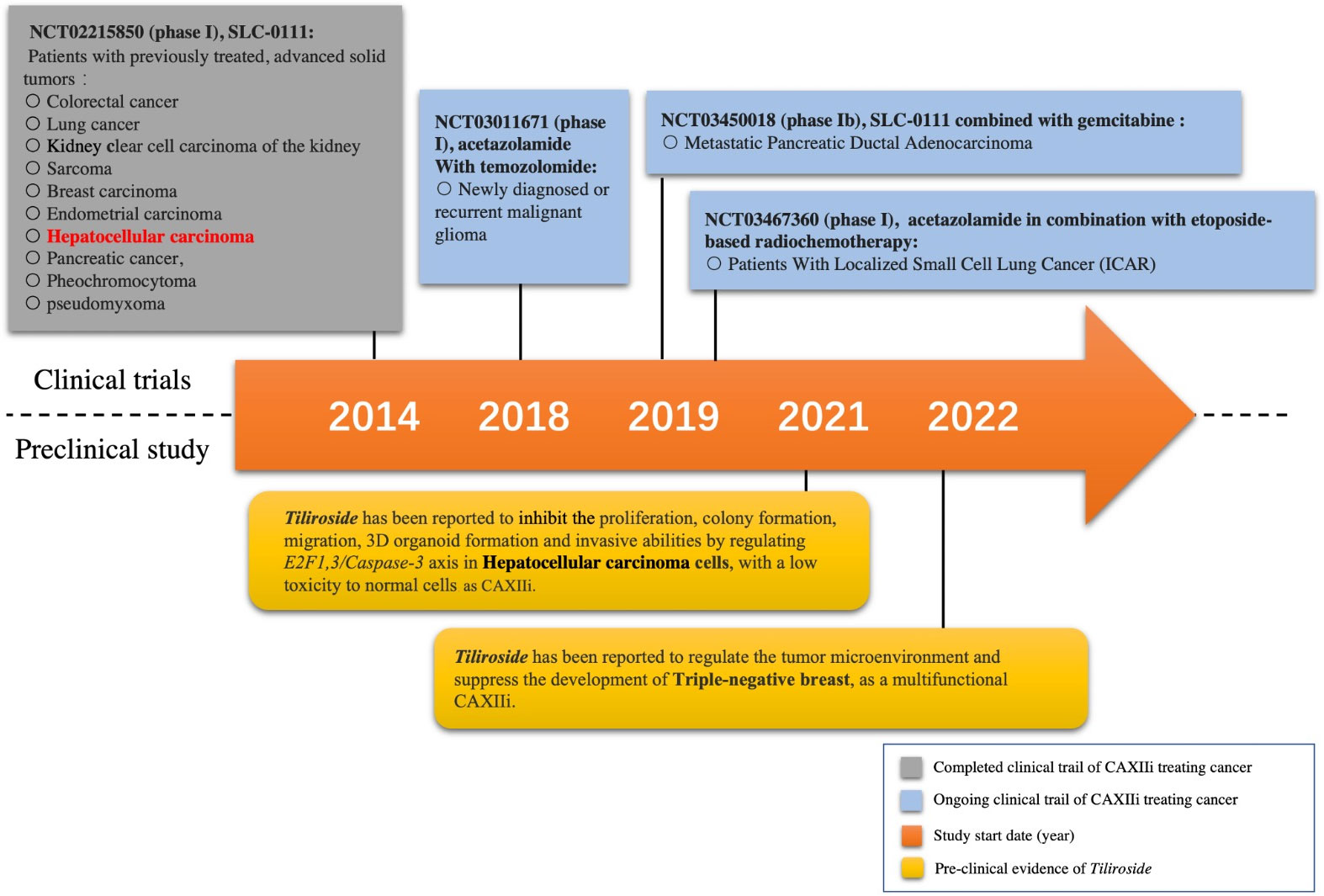
Figure 1 Milestone of selected CAXII inhibitor (CAXIIi) research and development. Currently, only one CAXIIi has been tested in a clinical trial for hepatocellular carcinoma treatment. Tiliroside monotherapy has been found to inhibit the development of hepatocellular carcinoma cells by a preclinical study.
Macrophages, some of the most abundant immune cells in tissues, are highly heterogeneous and can switch between different functions in the context of their niches where they are located. Their functions are determined by their polarized types (M1 or M2). Generally, the M1 subtype secretes inflammatory cytokines and reactive oxygen intermediates and presents antigen to tumor-suppressive T cells, stimulating the immune response (37, 38). In contrast, the M2 subtype is a tumor-promoting macrophage, inducing T-cell anergy (or exhaustion) and angiogenesis, producing extracellular matrix components, and repairing damaged tissues (37, 38). Although the origins of macrophages in many cancers remain uncertain, most of the macrophages recruited to the tumor microenvironment, known as the tumor-associated macrophages (TAMs), are the tumor-supportive M2 subtype (39). Abundant M2 macrophages were positively associated with poor survival in patients with breast cancer (40, 41). Recent studies have found that effectively interfering with macrophages is a potential strategy to treat cancer (42–44).
Evidence has shown that the metabolic reprogramming of macrophages can eventually inhibit tumor growth by regulating T cells (45). With tumors developing, the response protein of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) is produced by the interaction between macrophages and cancer cells, which participates in the remodeling of several key metabolic pathways of macrophages. Blocking the expression of PERK can inhibit the downstream metabolic signaling in tumor-infiltrating macrophages, resulting in more effector T cells to fight the cancer cells and consequently enhancing the efficacy of PD-1 inhibitors (45). Therefore, targeting or editing the metabolism of macrophages has been thought to be a novel therapeutic treatment in combination with PD-1 inhibitors. Furthermore, it has been reported recently that CAXIIis can interfere with the metabolism of macrophages by regulating CCL8 and PERK expression (46) and promote the therapeutic effect of PD-1 inhibitors in HCC treatment (47).
Evidence has shown that CAXII was the most significantly upregulated gene among all αCA family genes in tumor-infiltrating monocytes when comparing to the ones in the paired non-tumor liver tissues (45). Moreover, the expression level of CAXII mRNA increased in tumor-infiltrating monocytes but not in other CD14+ cell components in both tumor tissue and non-tumor liver tissue, indicating that CAXII might contribute to HCC progression (45). It was also shown that a positive correlation existed between the expression level of CAXII and glucose transporter GLUT1 in tumor-purified CD14+ cells, which may affect the glycolytic switch in tumor-infiltrating monocytes and macrophages (45).
In addition, the glycolysis inhibitor 2-deoxyglucose (2-DG) or 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3, a key glycolytic enzyme) can effectively reduce the expression of CAXII mRNA and protein levels in HepG2 tumor culture supernatant (TSN)-treated monocytes (peripheral blood purified CD14+ cells from healthy subjects) (45, 46). Meanwhile, the tumor-triggered glycolytic switch in monocytes has been found to induce the activation of hypoxia-inducible factor (HIF)1α and the production of tumor necrosis factor (TNF)-α, interleukin (IL)-10, and IL-1β, which in turn synergistically upregulates the CAXII expression in monocytes (45, 46). Therefore, it has been considered that aerobic glycolysis can induce the CAXII upregulation through HIF1α and the autocrine cytokine-dependent pathways in monocytes and macrophages, and CAXII was also found to mediate the survival of macrophages and monocytes in an acidic microenvironment in HepG2 cells (45).
The C-C motif chemokine ligand 8 (CCL8), a member of the CC chemotactic protein family, can recruit monocytes, T cells, eosinophils, basophils, natural killer (NK) cells, and dendritic cells by binding to the receptors of CCR1, CCR2, CCR3, and CCR5. It acts as an important immune regulator in inflammatory response, antitumor immunity, and acute graft-versus-host disease (aGVHD) (48). Evidence has shown that the levels of matrix metalloproteinase (MMP)9, vascular endothelial growth factor A (VEGFA), and CCL8 are all increased in tumor monocytes, with CCL8 showing the most pronounced upregulation compared to non-tumor monocytes. It has been demonstrated that the glycolysis-induced upregulation of CAXII expression was related to the CCL8 production in tumor-associated monocytes and macrophages (47, 48). Moreover, CCL8, as a CC chemokine that utilizes multiple cellular receptors to attract and activate human leukocytes, was reported to significantly promote the migration of HepG2 cells and increase the expression levels of vimentin (VIM) and SNAI1, two epithelial–mesenchymal transition (EMT)-related markers (48). The mRNA level of CCL8 in tumor-infiltrating monocytes was also found to be positively correlated with VIM, negatively with cadherin 1 (CDH1), and positively with the metastatic potential of HCC. Therefore, the CAXII/CCL8 axis has been thought to be involved in the progression and metastasis of tumor and a potential therapeutic target (47, 48).
Further evidence displayed that CAXIIis significantly increased the therapeutic effects (including suppressing tumor growth, attenuating tumor metastasis, and enhancing OS of mice) of anti–PD-1 antibodies on HCC compared to either single CAXII inhibitor group or single anti–PD-1 antibody group alone (P < 0.05) in vivo, respectively (47). In addition, CAXIIis have also been found to increase the apoptosis of macrophages, reduce the ratio of macrophages in total CD45+ cells, and increase the ratio of CD8+ T cells in total tumor lymphocytes (47). Such a result is partly consistent with our previous study in which targeting CAXII can effectively inhibit the development of liver cancer and triple-negative breast cancer cells (32, 49). What is noteworthy is that even though CAXII was mainly concentrated on tumor-infiltrating macrophages in the majority of tumor samples, it has also been found that CAXII might be important for the survival and function of M2-subtype macrophages in the tissues of HCC (47). Overexpression of CAXII may mediate the accumulation of M2 cells in tumor tissues via regulating PERK and CCL8 (47, 50, 51).
Since the immunoregulation ability of CAXIIis has been revealed, it has become a crucial issue to choose an appropriate CAXIIi that can properly synergize with the therapeutic effect of ICIs. In addition, some CAXIIis have already displayed excellent anticancer effects on different types of cancer cells (32, 34). The CAXII inhibitor SLC-0111 has been found to enhance the cisplatin antitumor activity and suppress the growth and invasion of head and neck squamous cell carcinoma (52). Meanwhile, it has also been found to sensitize patients with either melanoma or breast cancer to PD-1/PD-L1 inhibitors by enhancing the Th-1 response (53). In addition, the therapeutic strategy of acetazolamide combined with radiotherapy has been tested in lung cancer (NCT03467360), and the combination of acetazolamide plus temozolomide has also been trialed for brain cancer (NCT03011671) (Figure 1) (35, 36). However, few studies have been conducted to show the combination of CAXIIis with PD-1/PD-L1 ICIs in HCC thus far. It would be more intriguing if the tumor-suppressive effect of CAXIIis could be further enhanced by combining with PD-1 inhibitors.
The plant Tribulus terrestris L. (TT) can be found in many regions of Asia and Africa and has been used in traditional Chinese medicine and Ayurvedic medicine as an herb to treat liver diseases for thousands of years. Tiliroside (TS), one of the main extractions from this herb (54, 55), has been found to possess anti-inflammatory, anticholinesterase, and antioxidant activities (56). Moreover, our recent study has further revealed its multiple anticancer effects on HCC cells and, more importantly, its property of CAXIIi (32). Evidence has displayed that TS can inhibit the proliferation, colony formation, migration, three-dimensional (3D) organoid formation, and invasive abilities by regulating apoptosis and stemness in HCC cells while having low toxicity to normal cells (32). The study also found that TS can regulate the tumor microenvironment by modulating the levels of pHi, pHe, and lactate, therefore inhibiting the development of triple-negative breast cells, and act as a multifunctional CAXIIi (Figure 1) (49).
Therefore, TS is a promising candidate in combination with PD-1 inhibitors to improve the immunotherapy efficacy (Figure 2).
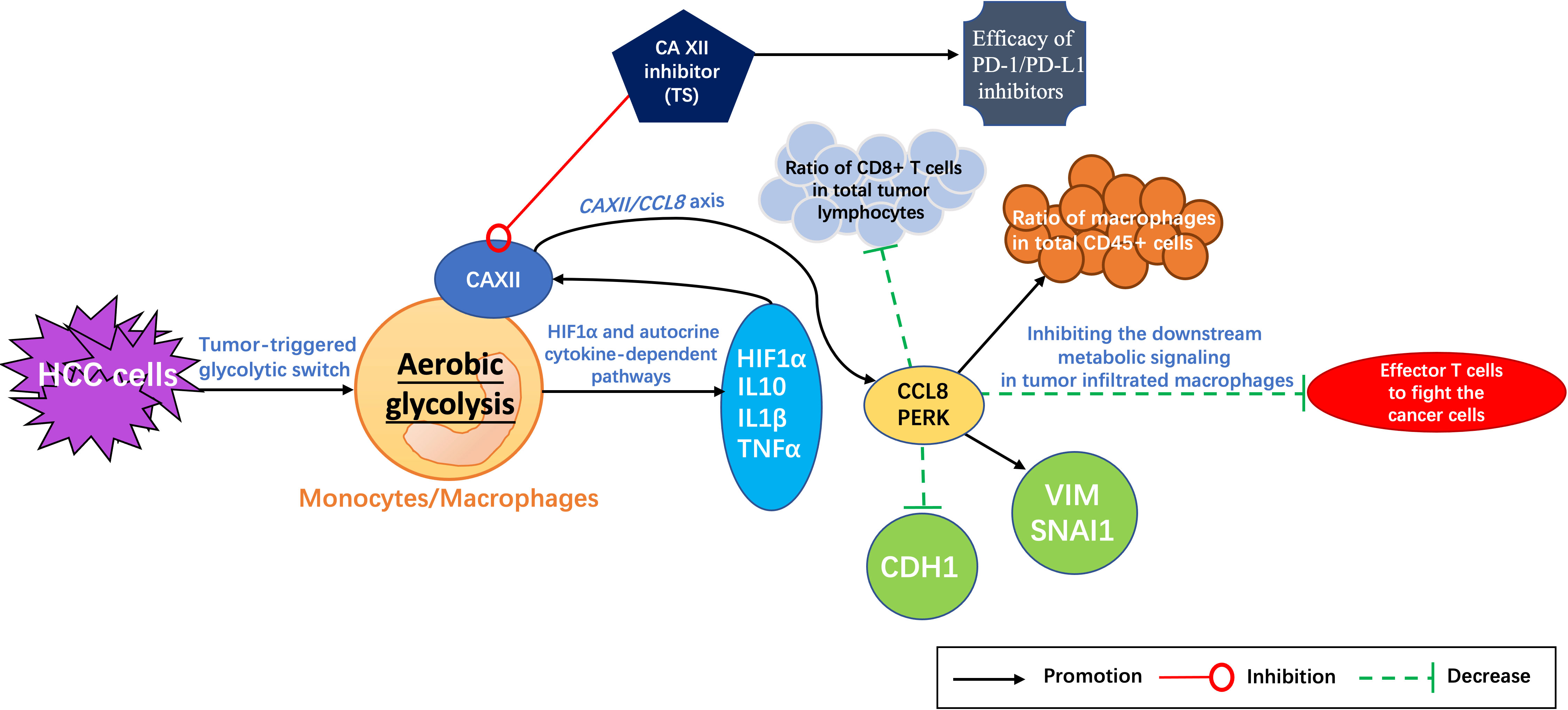
Figure 2 Potential mechanism of tiliroside (TS) enhancing the therapeutic effect of PD-1/PD-L1 inhibitors. HCC cells trigger the metabolic switch from oxidative phosphorylation to aerobic glycolysis in tumor-infiltrating monocytes and macrophages. The activation of HIF1α is induced and consequently the cytokines of TNF-α, IL-10, and IL-1β are produced, which in turn synergistically upregulate the CAXII expression in monocytes and macrophages, and then increase the expression of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). CAXII inhibitors (such as TS) can regulate the infiltration of lymphocytes (reducing the ratio of macrophages in total CD45+ cells and increasing the ratio of CD8+ T cells in total tumor lymphocytes) and suppress the expression of vimentin (VIM) and SNAI1 but increase CDH1 by regulating the expression of C-C motif chemokine ligand 8 (CCL8) and PERK, consequently increasing the therapeutic effect of PD-1/PD-L1 inhibitors. HCC, hepatocellular carcinoma; HIF1α, hypoxia-inducible factor (HIF)1α; TNF-α, Tumour necrosis factor α; IL-10, Interleukin 10; IL-1*β, Interleukin-1β; CAXII, carbonic anhydrase XII; SNAI1, Snail Family Transcriptional Repressor 1; CDH1, cadherin 1; PERK, Protein kinase RNA-like endoplasmic reticulum kinase.
At present, there are a few studies on the safety of CAXIIis. For instance, a novel CAXIIi named 6A10-Fab-fragment is currently being trialed in a phase I study to evaluate its maximum tolerated dose and patient-specific dosimetry in a combined therapeutic strategy for patients with glioblastoma (NCT05533242) (57). Moreover, for treating advanced solid tumors, the safety and tolerability of a highly selective small-molecule inhibitor of CAIX/CAXII (SLC-0111) have already been evaluated by a phase I study (NCT02215850) with very promising results (34). For details, as reported by this study, SLC-0111 was generally well tolerated at doses of 1,000 mg daily or below, but frequent early discontinuation was observed at doses of 2,000 mg. However, as displayed by pharmacokinetic (PK) assessments, both the 1,000- and 2,000-mg doses gained similar levels of drug exposure following single doses of SLC-0111. Moreover, majority of the reported drug-related adverse events (AEs) were grade 1 or 2 in severity, and the most frequent AEs were fatigue, nausea, anorexia, diarrhea, and vomiting, all of which were reversible (34). Only one patient has been reported to undergo a grade 3 hepatobiliary disorder, which was considered to be a serious AE related to SLC-0111 but not a dose-limiting toxicity (DLT) (34). Furthermore, the toxicities of SLC-0111 will be continually evaluated by other ongoing clinical trials, such as NCT03450018. Apparently, the safety evaluation of CAXIIis still requires more research investment.
Cancer is not a simple disease but a complex product of changes in the genome and the body’s internal environmental response. Therefore, the current cancer immunotherapy targeting a single target, such as PD-1/PD-L1 inhibitor, Cytotoxic T-lymphocyte Antigen-4 (CTLA-4) inhibitor, and Chimeric antigen receptor (CAR) T-cell therapy (CAR-T), has very limited therapeutic effect on most cancers (58, 59). Thus, new combination therapies have been sought to improve clinical benefits. As mentioned above, CAXIIis, novel and strong anticancer agents, can enhance the therapeutic effect of PD-1 inhibitors by regulating the antitumor immunity in HCC (47), providing a new thought for cancer treatment. Therefore, the roles of CAXIIis in the integrated metabolic space of tumor and immune cells should be further explored in a future study for exerting their anticancer and immunomodulatory effects in a more efficient way. On the other hand, based on a more comprehensive and clear regulatory network of CAXIIis, more chances could be gained by expanding their application in different combination therapies with multiple targets for treating cancer, as shown in a preclinical study that combined CAIX and CAXII dual inhibition with immune checkpoint blockade resulting in improved efficacy of immune therapy in melanoma and breast cancer (53). Meanwhile, the effects of CAXIIis combined with different or multiple immune checkpoint blockades should also be evaluated for seeking novel ICI-based treatment (53). Moreover, adding a safe and targeted drug delivery system, which is a pattern of specifically designed carriers, to the application of CAXIIis in cancer treatment is also a recommended potential research direction (60, 61). Those nanoparticles were created to encapsulate and deliver agents to specific lesion sites with enhanced solubility and efficacy of drugs and reduced interaction with untargeted tissues (60, 61). For instance, our previous study has reported the capability of promoting the therapeutic effect, possessed by a novel lipid-based nanoparticle, LNP-DP1, in treating HCC (60). Furthermore, currently, to the best of our knowledge, none of the biomarkers has been reported to be applied in predicting the effectiveness, safety, or toxicity of CAXIIi treatment. Such unsolved issue is like an invisible barrier that might impede the potential application of CAXIIis in cancer treatment, including cancer immunotherapy (62). Therefore, the assessment of biomarkers for CAXIIis is encouraged in a future study. The same future investigation is also required for evaluating the safety and tolerability of CAXIIis.
Accumulating evidence has demonstrated the anticancer effect of CAXIIis on different types of cancers, including HCC. Therefore, such agents are considered promising novel anti-HCC drugs that can suppress HCC progression (32). Moreover, CAXIIis have been found to reduce the immunosuppressive stress mediated by hypoxic/acidic metabolism, regulate the expression of CCL8, and affect the functions of monocytes and macrophages, thereby improving the antitumor immunity and enhancing the therapeutic effect of PD-1 inhibitors in HCC (47). In summary, CAXIIis are not only effective single anticancer agents but also potential sensitizers of PD-1 inhibitors. Thus, a candidate such as TL, a novel CAXIIi with high efficiency against tumors and low toxicity to normal cells, has been considered to hold untapped potential in ICI-based combination therapy for HCC treatment. Furthermore, more promising candidates of CAXIIis are warranted to be included in future studies to explore more effective therapeutic strategies and novel therapeutic targets for HCC. Additionally, the combination setting of PD-1 inhibitors plus CAXIIis and antiangiogenic agents may offer more effective therapeutic options to patients if the function of CAXIIis as a PD-1 inhibitor sensitizer has been fully evaluated.
RH generated the original concept of this manuscript with the help of LL. JL, JH, ZX, JW, helped to write the paper, MY and SL helped to generate the figure. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China (Youth foundation) (No.82204864) project (to RH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Garcia-Monaco RD, Chung JW, Vilgrain V, Bouattour M, Covey AM. Summary of key guidelines for locoregional treatment of HCC in Asia, Europe, south and north America. Br J Radiol (2022) p:20220179. doi: 10.1259/bjr.20220179
2. Amini M, Looha MA, Zarean E, Pourhoseingholi MA. Global pattern of trends in incidence, mortality, and mortality-to-incidence ratio rates related to liver cancer, 1990-2019: a longitudinal analysis based on the global burden of disease study. BMC Public Health (2022) 22(1):604. doi: 10.1186/s12889-022-12867-w
3. Chen YG, Yang CW, Chung CH, Ho CL, Chen WL, Chien WC. Correction to: The association between metabolic risk factors, nonalcoholic fatty liver disease, and the incidence of liver cancer: a nationwide population-based cohort study. Hepatol Int (2022) 16(2):488. doi: 10.1007/s12072-022-10308-9
4. Rutherford MJ, Arnold M, Bardot A, Ferlay J, De P, Tervonen H, et al. Comparison of liver cancer incidence and survival by subtypes across seven high-income countries. Int J Cancer (2021) 149(12):2020–31. doi: 10.1002/ijc.33767
5. Yuan G, Xie F, Song Y, Li Q, Li R, Hu X, et al. Hepatic tumor stiffness measured by shear wave elastography is prognostic for HCC progression following treatment with anti-PD-1 antibodies plus lenvatinib: A retrospective analysis of two independent cohorts. Front Immunol (2022) 13:868809. doi: 10.3389/fimmu.2022.868809
6. Feng MY, Chan LL, Chan SL. Drug treatment for advanced hepatocellular carcinoma: First-line and beyond. Curr Oncol (2022) 29(8):5489–507. doi: 10.3390/curroncol29080434
7. Deng S, Solinas A, Calvisi DF. Cabozantinib for HCC treatment, from clinical back to experimental models. Front Oncol (2021) 11:756672. doi: 10.3389/fonc.2021.756672
8. Bornschein J, Schlosser S. [Regorafenib - a revolution in the systemic treatment options of HCC?]. Z Gastroenterol (2017) 55(7):685–6. doi: 10.1055/s-0043-106861
9. Roviello G, Sohbani N, Petrioli R, Rodriquenz MG. Ramucirumab as a second line therapy for advanced HCC: a significant achievement or a wasted opportunity for personalised therapy? Invest New Drugs (2019) 37(6):1274–88. doi: 10.1007/s10637-019-00760-0
10. Cinnamon E, Pikarsky E. Are we ready for targeted therapy combinations in HCC? Gut (2020) 69(4):613–4. doi: 10.1136/gutjnl-2019-319780
11. Campbell C, Wang T, McNaughton AL, Barnes E, Matthews PC. Risk factors for the development of hepatocellular carcinoma (HCC) in chronic hepatitis b virus (HBV) infection: a systematic review and meta-analysis. J Viral Hepat (2021) 28(3):493–507. doi: 10.1111/jvh.13452
12. Shen Y, Risch H, Lu L, Ma X, Irwin ML, Lim JK, et al. Risk factors for hepatocellular carcinoma (HCC) in the northeast of the united states: results of a case-control study. Cancer Causes Control (2020) 31(4):321–32. doi: 10.1007/s10552-020-01277-1
13. Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen YL, et al. FDA Approval summary: Atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res (2021) 27(7):1836–41. doi: 10.1158/1078-0432.CCR-20-3407
14. Ouyang T, Kan X, Zheng C. Immune checkpoint inhibitors for advanced hepatocellular carcinoma: Monotherapies and combined therapies. Front Oncol (2022) 12:898964. doi: 10.3389/fonc.2022.898964
15. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
16. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol (2022) 40(4_suppl):379–9. doi: 10.1200/JCO.2022.40.4_suppl.379
17. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6
18. Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J Clin Oncol (2021) 39(27):2991–3001. doi: 10.1200/JCO.20.03555
19. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol (2020) 21(6):808–20. doi: 10.1016/S1470-2045(20)30156-X
20. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
21. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76(4):862–73. doi: 10.1016/j.jhep.2021.11.030
22. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
23. Han R, Chen X, Li Y, Zhang S, Li R, Lu L. MicroRNA-34a suppresses aggressiveness of hepatocellular carcinoma by modulating E2F1, E2F3, and caspase-3. Cancer Manag Res (2019) 11:2963–76. doi: 10.2147/CMAR.S202664
24. Rizzo A, Ricci AD, Di Federico A, Frega G, Palloni A, Tavolari S, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: Where do we stand? Front Oncol (2021) 11:803133. doi: 10.3389/fonc.2021.803133
25. Hercun J, Vincent C, Bilodeau M, Lapierre P. Immune-mediated hepatitis during immune checkpoint inhibitor cancer immunotherapy: Lessons from autoimmune hepatitis and liver immunology. Front Immunol (2022) 13:907591. doi: 10.3389/fimmu.2022.907591
26. Zhang Y, Zhang X, Kuang M, Yu J. Emerging insights on immunotherapy in liver cancer. Antioxid Redox Signal (2022) 37:1325–38. doi: 10.1089/ars.2022.0047
27. Di Federico A, Rizzo A, Carloni R, De Giglio A, Bruno R, Ricci D, et al. Atezolizumab-bevacizumab plus y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs (2022) 31(4):361–9. doi: 10.1080/13543784.2022.2009455
28. Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol (2021) 15(11):1245–51. doi: 10.1080/17474124.2021.1973431
29. Rizzo A, Cusmai A, Gadaleta-Caldarola G, Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol (2022) 16(4):333–9. doi: 10.1080/17474124.2022.2064273
30. Huang T, Tang L, Wang H, Lin L, Fu J. Carbonic anhydrase 12 gene silencing reverses the sensitivity of paclitaxel in drug-resistant breast cancer cells. Bioengineered (2021) 12(2):9806–18. doi: 10.1080/21655979.2021.1995575
31. Hwang S, Shin DM, Hong JH. Drug repurposing as an antitumor agent: Disulfiram-mediated carbonic anhydrase 12 and anion exchanger 2 modulation to inhibit cancer cell migration. Molecules (2019) 24(18):1–14. doi: 10.3390/molecules24183409
32. Han R, Yang H, Lu L, Lin L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates E2Fs/Caspase-3 axis. Sci Rep (2021) 11(1):8626. doi: 10.1038/s41598-021-88133-7
33. Gu XF, Shi CB, Zhao W. Prognostic value of carbonic anhydrase XII (CA XII) overexpression in hepatocellular carcinoma. Int J Clin Exp Pathol (2019) 12(6):2173–83.
34. McDonald PC, Chia S, Bedard PL, Chu Q, Lyle M, Tang L, et al. A phase 1 study of SLC-0111, a novel inhibitor of carbonic anhydrase IX, in patients with advanced solid tumors. Am J Clin Oncol (2020) 43(7):484–90. doi: 10.1097/COC.0000000000000691
35. Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs (2018) 27(12):963–70. doi: 10.1080/13543784.2018.1548608
36. Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, et al. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front Cell Dev Biol (2021) 9:641469. doi: 10.3389/fcell.2021.641469
37. Yin C, Han Q, Xu D, Zheng B, Zhao X, Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology (2019) 8(7):1601479. doi: 10.1080/2162402X.2019.1601479
38. Cui Z, Li W, Wang Y, Zhao M, Liu K, Yang Y, et al. M2 macrophage-derived exosomal ferritin heavy chain promotes colon cancer cell proliferation. Biol Trace Elem Res (2022). doi: 10.1007/s12011-022-03488-w
39. Liu J, Geng X, Hou J, Wu G. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int (2021) 21(1):389. doi: 10.1186/s12935-021-02089-2
40. Turova AD, Trutneva EA. [Effect of cardiac glycosides associated with barbamil and valerian on the heart in frogs]. Farmakol Toksikol (1957) 20(6):54–5.
41. Dai Q, Wu W, Amei A, Yan X, Lu L, Wang Z. Regulation and characterization of tumor-infiltrating immune cells in breast cancer. Int Immunopharmacol (2021) 90:107167. doi: 10.1016/j.intimp.2020.107167
42. Morales V, Soto-Ortiz L. Modeling macrophage polarization and its effect on cancer treatment success. Open J Immunol (2018) 8(2):36–80. doi: 10.4236/oji.2018.82004
43. Huang D, Qiu H, Miao L, Guo L. Cdc42 promotes thyroid cancer cell proliferation and migration and tumor-associated macrophage polarization through the PTEN/AKT pathway. J Biochem Mol Toxicol (2022) p:e23115. doi: 10.1002/jbt.23115
44. Ando T, Komatsu T, Naiki Y, Takahashi K, Yokochi T, Watanabe D, et al. GSK2656157, a PERK inhibitor, reduced LPS-induced IL-1beta production through inhibiting caspase 1 activation in macrophage-like J774.1 cells. Immunopharmacol Immunotoxicol (2016) 38(4):298–302. doi: 10.1080/08923973.2016.1192191
45. Raines LN, Zhao H, Wang Y, Chen HY, Gallart-Ayala H, Hsueh PC, et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat Immunol (2022) 23(3):431–45. doi: 10.1038/s41590-022-01145-x
46. Buddingh EP, Kuijjer ML, Duim RA, Burger H, Agelopoulos K, Myklebost O, et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res (2011) 17(8):2110–9. doi: 10.1158/1078-0432.CCR-10-2047
47. Ning WR, Jiang D, Liu XC, Huang YF, Peng ZP, Jiang ZZ, et al. Carbonic anhydrase XII mediates the survival and prometastatic functions of macrophages in human hepatocellular carcinoma. J Clin Invest (2022) 132(7). doi: 10.1172/JCI153110
48. Farmaki E, Chatzistamou L, Kaza V, Kiaris H. A CCL8 gradient drives breast cancer cell dissemination. Oncogene (2016) 35(49):6309–18. doi: 10.1038/onc.2016.161
49. Han R, Yang H, Ling C, Lu L. Tiliroside suppresses triple-negative breast cancer as a multifunctional CAXII inhibitor. Cancer Cell Int (2022) 22(1):368. doi: 10.1186/s12935-022-02786-6
50. Pratap UP, Vadlamudi RK. PERK promotes immunosuppressive M2 macrophage phenotype by metabolic reprogramming and epigenetic modifications through the PERK-ATF4-PSAT1 axis. Immunometabol (Cobham) (2022) 4(3):e00007. doi: 10.1097/IN9.0000000000000007
51. Farmaki E, Kaza V, Chatzistamou I, Kiaris H. CCL8 promotes postpartum breast cancer by recruiting M2 macrophages. iScience (2020) 23(6):101217. doi: 10.1016/j.isci.2020.101217
52. Sarnella A, Ferrara Y, Auletta L, Albanese S, Cerchia L, Alterio V, et al. Inhibition of carbonic anhydrases IX/XII by SLC-0111 boosts cisplatin effects in hampering head and neck squamous carcinoma cell growth and invasion. J Exp Clin Cancer Res (2022) 41(1):122. doi: 10.1186/s13046-022-02345-x
53. Chafe SC, McDonald PC, Saberi S, Nemirovsky O, Venkateswaran G, Burugu S, et al. Targeting hypoxia-induced carbonic anhydrase IX enhances immune-checkpoint blockade locally and systemically. Cancer Immunol Res (2019) 7(7):1064–78. doi: 10.1158/2326-6066.CIR-18-0657
54. Fabova Z, Adam T, Abdel HH, Saleh A, Jan K, Alexander V. Tribulus terrestris can suppress the adverse effect of toluene on bovine and equine ovarian granulosa cells. Reprod Domest Anim (2022) 57:1307–18. doi: 10.1111/rda.14204
55. Abbas MW, Hussain M, Akhtar S, Ismail T, Qamar M, Shafiq Z, et al. Bioactive compounds, antioxidant, anti-inflammatory, anti-cancer, and toxicity assessment of tribulus terrestris-in vitro and in vivo studies. Antioxidants (Basel) (2022) 11(6):372–79. doi: 10.3390/antiox11061160
56. Wang X, Cao J, Wu Y, Wang Q, Xiao J. Flavonoids, antioxidant potential, and acetylcholinesterase inhibition activity of the extracts from the gametophyte and archegoniophore of marchantia polymorpha l. Molecules (2016) 21(3):360. doi: 10.3390/molecules21030360
57. Fiedler L, Kellner M, Oos R, Boning G, Ziegler S, Bartenstein P, et al. Fully automated production and characterization of (64) Cu and proof-of-Principle small-animal PET imaging using (64) Cu-labelled CA XII targeting 6A10 fab. ChemMedChem (2018) 13(12):1230–7. doi: 10.1002/cmdc.201800130
58. Lu Y, Huntoon K, Lee D, Wang Y, Ha J, Qie Y, et al. Immunological conversion of solid tumours using a bispecific nanobioconjugate for cancer immunotherapy. Nat Nanotechnol (2022) 17(12):1332–41. doi: 10.1038/s41565-022-01245-7
59. Franzen AS, Raftery MJ, Pecher G. Implications for immunotherapy of breast cancer by understanding the microenvironment of a solid tumor. Cancers (Basel) (2022) 14(13). doi: 10.3390/cancers14133178
60. Han R, Nusbaum O, Chen X, Zhu Y. Valeric acid suppresses liver cancer development by acting as a novel HDAC inhibitor. Mol Ther Oncolytics (2020) 19:8–18. doi: 10.1016/j.omto.2020.08.017
61. Rajasekaran D, Srivastava J, Ebeid K, Gredler R, Akiel M, Jariwala N, et al. Combination of nanoparticle-delivered siRNA for astrocyte elevated gene-1 (AEG-1) and all-trans retinoic acid (ATRA): An effective therapeutic strategy for hepatocellular carcinoma (HCC). Bioconjug Chem (2015) 26(8):1651–61. doi: 10.1021/acs.bioconjchem.5b00254
Keywords: CAXII inhibition, sensitizer, antitumor immunity, ICIs based therapy, hepatocellular carcinoma, Tiliroside, synergistic effect
Citation: Han R, Li J, Hony J, Xiao Z, wang J, Yao M, Liang S and Lu L (2023) CAXII inhibitors: Potential sensitizers for immune checkpoint inhibitors in HCC treatment. Front. Immunol. 14:1052657. doi: 10.3389/fimmu.2023.1052657
Received: 24 September 2022; Accepted: 01 March 2023;
Published: 16 March 2023.
Edited by:
Manel Juan, Hospital Clinic of Barcelona, SpainReviewed by:
Jiaxiang Ye, Affiliated Tumor Hospital of Guangxi Medical University, ChinaCopyright © 2023 Han, Li, Hony, Xiao, wang, Yao, Liang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Han, ZGlhbnhpcWlhb0Bmb3htYWlsLmNvbQ==; Lingeng Lu, bGluZ2VuZy5sdUB5YWxlLmVkdQ==
†ORCID: Rui Han, orcid.org/0000-0001-8856-3681
Lingeng Lu, orcid.org/0000-0001-9871-0809
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.