- 1Non-Communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 2Research Unit of General Practice, Department of Public Health, University of Southern Denmark, Odense, Denmark
- 3Endocrinology and Metabolism Population Sciences Institute, Non-Communicable Diseases Research Center, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Pediatric, Iran University of Medical Sciences, Tehran, Iran
Background: Individuals with normal weight could suffer from obesity based on their body fat percentage (also known as normal weight obesity (NWO)), thus being at risk of significant morbidity and mortality compared to the general population. It seems that inflammatory pathways and chronic inflammation are significant contributors to the pathogenicity of NWO. This study aimed to assess and pool the association of proinflammatory and anti-inflammatory cytokines with NWO.
Methods: In this systematic review and meta-analysis, online international databases (PubMed, Scopus, EMBASE, Web of Science, and Google Scholar) were searched until August 2022. All observational studies with an English full text comparing the mean levels of proinflammatory and anti-inflammatory cytokines (e.g., C-reactive protein (CRP), various types of interleukins (IL) s, tumor necrosis factor-alpha (TNF)) and white blood cell (WBC) count, in subjects with NWO and “normal weight non-obese (NWNO)” were included. Two researchers independently screened, reviewed and assessed the quality of included studies. The remaining articles’ data were extracted post-screening. The heterogeneity between studies was assessed using the I2 and Cochran’s Q tests. A random effect model meta-analysis was used to pool the standardized mean difference (SMD) as an effect size.
Results: From the initial 559 studies, 21 and 19 were included in the qualitative and quantitative synthesis, respectively. In the systematic review, 8 studies reported a significant association between various proinflammatory cytokines (CRP, IL6, IL1β, and TNFα) and NWO. According to random-effect meta-analysis, the association between NWO with CRP (SMD: 0.60, 95% CI: 0.30, 0.91) and IL6 (SMD: 0.90, 95%CI: 0.14, 1.66) was statistically significant. Moreover, the mean level of TNFα in subjects with NWO and NWNO did not differ significantly (SMD: 0.67, 95% CI: -0.36, 1.70).
Conclusion: The findings of this study show that NWO was associated with high levels of CRP and IL6. Therefore, inflammatory pathways may play a role in the pathogenicity of NWO.
Background
Obesity has been extensively studied as one of the most prominent causes of morbidity and mortality (1, 2). Despite such evaluations in different target populations, new findings still emerge in this topic (3). These findings are particularly important in preventing and treating obesity as its prevalence, morbidity, and mortality are increasing globally (4). It should be noted that lately, morbidity and mortalities attributed to obesity are being seen in individuals who, based on previous definitions of obesity, a body mass index (BMI) above 30 Kg/m2, are not considered obese (5, 6). Hence new definitions and types of obesity have been defined (6).
One of these relatively new definitions regards those with normal BMI values and yet high body fat percentage (6–9). These individuals are regarded as Normal Weight Obese (NWO) (9). Studies indicate that NWOs are at an increased risk of cardiometabolic conditions similar to obese individuals and may suffer from the same morbidity and mortality-related conditions (10–13). Some studies suggest that one contributing factor to this increased risk of cardiometabolic conditions in obesity and NWO could be chronic inflammation, as inflammation has been observed in increased adiposity (14, 15). Despite inflammation being an essential process in the body, chronic inflammation can have adverse cardiometabolic effects, since pro-inflammatory cytokines in a chronic inflammatory status can contribute to the development of atherosclerosis, insulin resistance, type 2 diabetes, hypertension and hypercholesterolemia (14). Since obesity is a chronic condition, the resulting inflammation persists, resulting in chronic inflammation and subsequently the aforementioned conditions (15, 16). Similar to obesity (based on BMI) the association of inflammation with NWO has been highlighted in a review (9). It seems that in NWO the secretions of the adipose tissue itself contributes to inflammation; these studies argue that the underlying cause of this inflammation is the increased fat mass and lipid accumulation resulting in increased oxidative stress, and NF-κB pathway (a major pathway in the innate inflammatory response) activation (9, 17). Although the number of studies addressing the association of NWO and inflammation has been increasing throughout the years (9), no systematic review on the inflammatory aspect of NWO has been published so far. This systematic review and meta-analysis aimed to summarize and pool the association of NWO phenotype with inflammatory markers in published studies.
Methods
This study was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) checklist (18).
Search strategy
A comprehensive systematic search was conducted on all available online databases (Scopus, EMBASE, Web of Science, PubMed, and Google Scholar) until August 2022. One of the investigators conducted the search, and another reviewed the findings. Terms such as “normal weight obesity”, “NWO”, “high fat percentage” and their MeSH term equivalents alongside proinflammatory and anti-inflammatory cytokines such as “CRP” “C-reactive protein” and “interleukin” and white blood cell (WBC) count were searched. The entire search (terms and strategy) can be seen as Supplementary Table 1. Moreover, reference lists of included studies or reviews were hand-searched to identify more potentially eligible studies.
Study selection criteria and eligibility
All observational studies with an English full text that assessed the association of proinflammatory and anti-inflammatory cytokines such as C-reactive protein (CRP), various types of interleukins (IL) s, tumor necrosis factor (TNF) alpha with NWO were included in this study. Regardless of their various definitions of NWO (based on fat percentage, waist circumference, etc.), all studies were included. All studies included studies had represented the targeted population and compared them with normal-weight, non-obese (NWNO) individuals. Studies that failed to meet the inclusion criteria were excluded. Furthermore, duplicates, non-peer-reviewed publications, and studies without sufficient information to determine eligibility were excluded.
Two investigators independently carried out the screening process of included studies, including titles, abstracts and full texts. Upon removing the irrelevant entries, the full texts of the remaining articles were assessed. Moreover, to find the missed relevant studies (if any), the reference lists of the included studies were hand-searched as well. Discrepancies were referred to a third investigator for resolution.
Data extraction strategy
Two investigators separately extracted the data using a pre-designed data extraction sheet. The extracted data were composed of the name of the first author and publication year, the number of participants, age and sex, the definition of normal weight obesity, the studied cytokines, and the outcome as standardized mean difference (SMD) alongside their 95% confidence interval (CI) of the outcomes were extracted as the effect size of dichotomous and continuous respectively. Moreover, discrepancies were referred to a third investigator for resolution.
Quality assessment
We used the Newcastle-Ottawa Scale for quality assessment. This scale consists of seven items, scoring based on selection, comparability, exposure (case-control studies), and outcome (cohort studies). The total score ranges from 0 to 9 for cohort studies or 0 to 10 for case-control studies and is calculated by summing the scores of each item of this assessment tool (19, 20). We categorized the scores as 0 to 4, 5-6, 7 and above, indicating the studies’ quality (low, middle, and high-quality studies, respectively). Two investigators independently assessed the quality of the studies, and discrepancies were referred to the third investigator.
Statistical analysis
The I2 and Cochran’s Q tests were used to assess the heterogeneity between the studies. A random-effect model was adapted for analyses if the heterogeneity was statistically significant (P-value<0.1). Otherwise, a fixed model was used. The SMDs of the included studies were calculated and pooled as an effect size for NWO association with the mean levels of proinflammatory and anti-inflammatory cytokines. Meta-Analysis was performed for outcomes with at least 3 reports within the studies. If applicable, sub-group analysis was performed for proinflammatory and anti-inflammatory cytokines (stratified by sex, quality and adjustment for confounding variables and type of CRP (high-sensitivity (hs-CRP) and CRP excluding hs-CRP) as well. Egger’s test was adapted to assess publication bias for each inflammatory factor, and trim fill analysis was performed if publication bias was present. STATA (Stata Corporation, College Station, Texas, USA) version 17 was used to analyze the data.
Results
Search results
Figure 1 shows the flowchart of the selection of studies for inclusion in the meta-analysis. From the 559 studies found in the initial search, 263 duplicate studies were removed. Out of 296 remaining articles, 227 irrelevant studies were excluded after titles and abstracts screening. The full texts of the 69 articles were assessed, and 45 studies were excluded due to failing the eligibility criteria. Finally, 21 articles remained in the current systematic review (21–41). However,19 were eligible for inclusion in the meta-analysis (one study reported OR as the effect size (36), and one study exclusively evaluated complement C3 (23), which did not reach the minimum number of three studies needed to enter the meta-analysis)
Study characteristics
The included studies were conducted worldwide (Canada, Iran, Italy, Japan, Poland, South Korea, Spain, Sweden, USA) with a total number of 19,857 participants aged ≥13 years. Twenty studies were conducted on the adult population (age ≥ 18 years), one study was conducted on adolescents aged 13 to 18 (34), and one was conducted exclusively on the elderly aged above 60 (41). Most studies were conducted in Italy (5 studies) and South Korea (4 studies). Canada, Poland, Spain, Sweden and Switzerland had the least number of studies (1 study). The greatest sample size was from a study conducted in the USA (4116 individuals), and the smallest was from Brazil (52 individuals). In most of the included studies, NWO was defined as normal BMI values with a body fat percentage above 30% in women (8 out of 16 reports) and above 25% in men (4 out of 8 reports). These general characteristics are shown in summery in Table 1. Three studies (24, 38, 40) had adjusted their findings for possible confounders; the other included studies were not adjusted for any confounding factors.
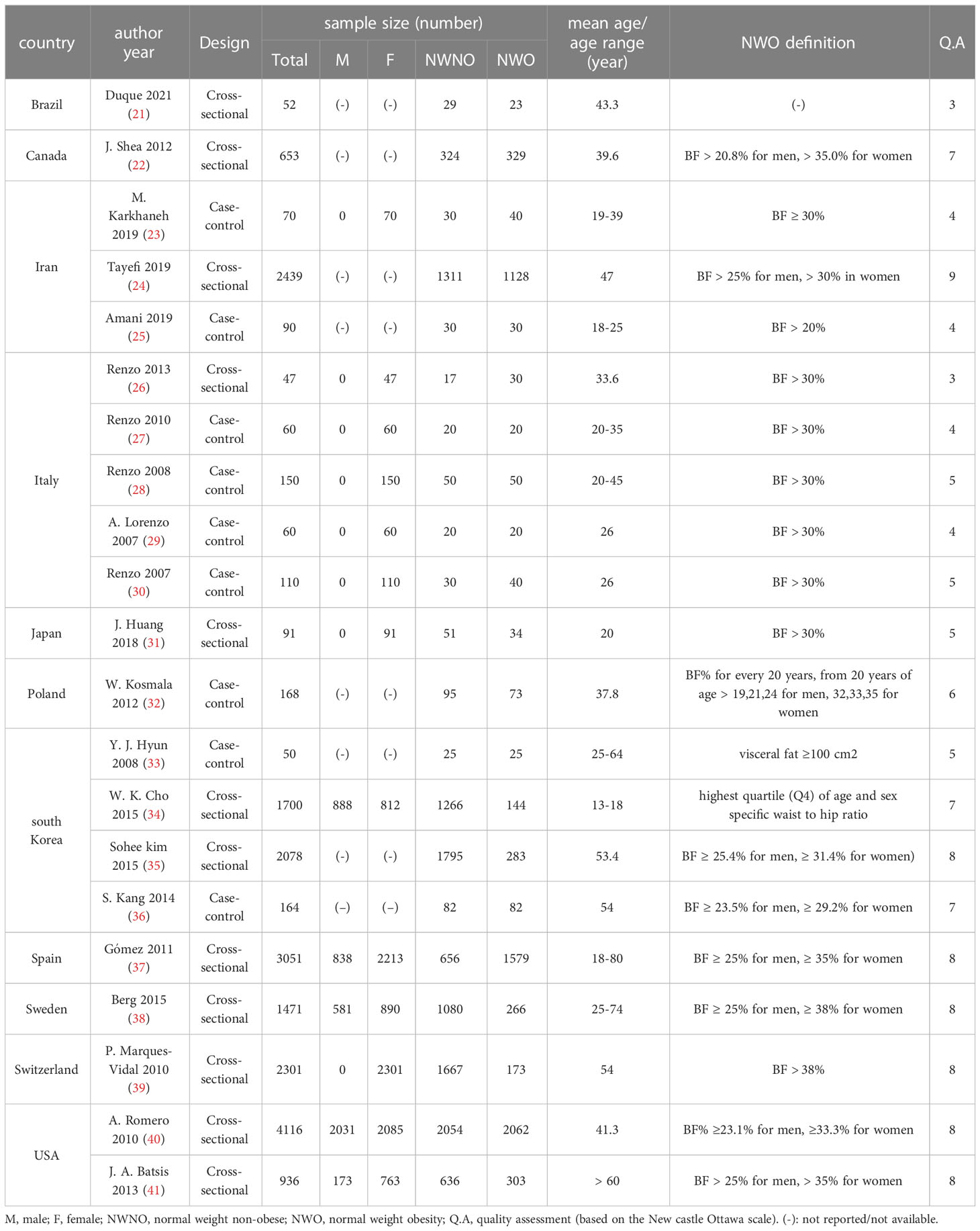
Table 1 General Characteristics of included studies for association of NWO and inflammatory factors.
Qualitative synthesis
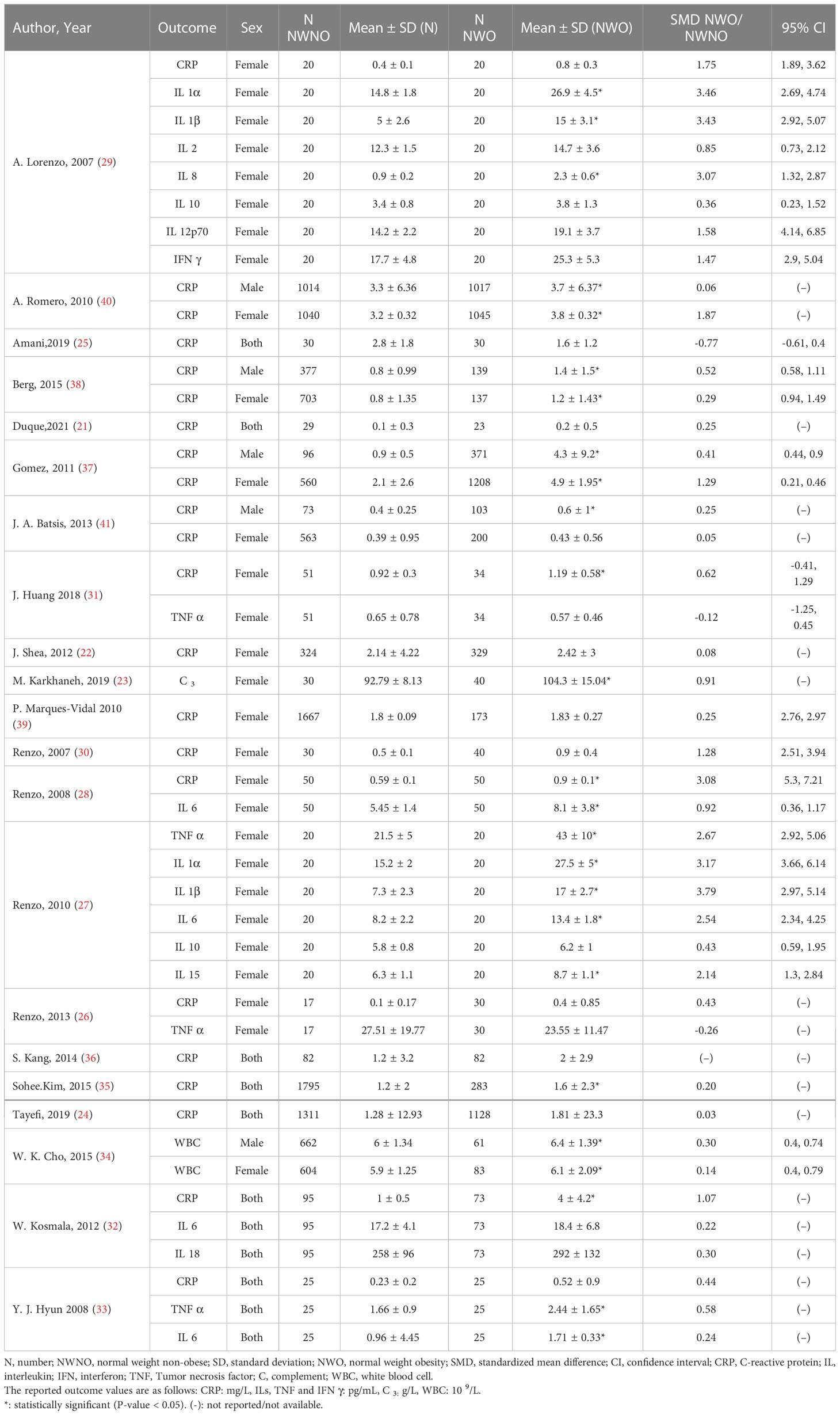
The included studies evaluated the associations of NWO with the mean levels of proinflammatory and anti-inflammatory cytokines (CRP, IFNγ, TNFα, IL1α, IL1β, IL2, IL6, IL8, IL10, IL12p70, IL15, IL18) as well as complement C3, and white blood cell count (WBC), compared with normal-weight non-obese (NWNO) individuals; and their effect sizes are shown in Table 2. As can be seen, in 11 out of 22 reports of CRP, 2 out of 3 reports of IL6, 1 out of 3 reports of TNFα, 2 out of 2 reports of WBC, IL1α and IL1β, and 1 out of 1 reports of IL15 and complement C3, significant differences between NWO and NWNO individuals were observed; with the greatest effect size regarding IL1β, SMD: 3.79, 95% CI (2.75-4.83). However, in the two reports evaluating IL10 and singular reports evaluating IL2, IL8, IL12p70, IL18 and IFNγ, no significant differences between NWO and NWNO individuals were seen. Moreover, one study (36) reported that NWOs, in comparison with NWNOs, have significantly increased odds of vascular inflammation (OR:3.07 95%CI (1.29-7.29)).
Quantitative synthesis
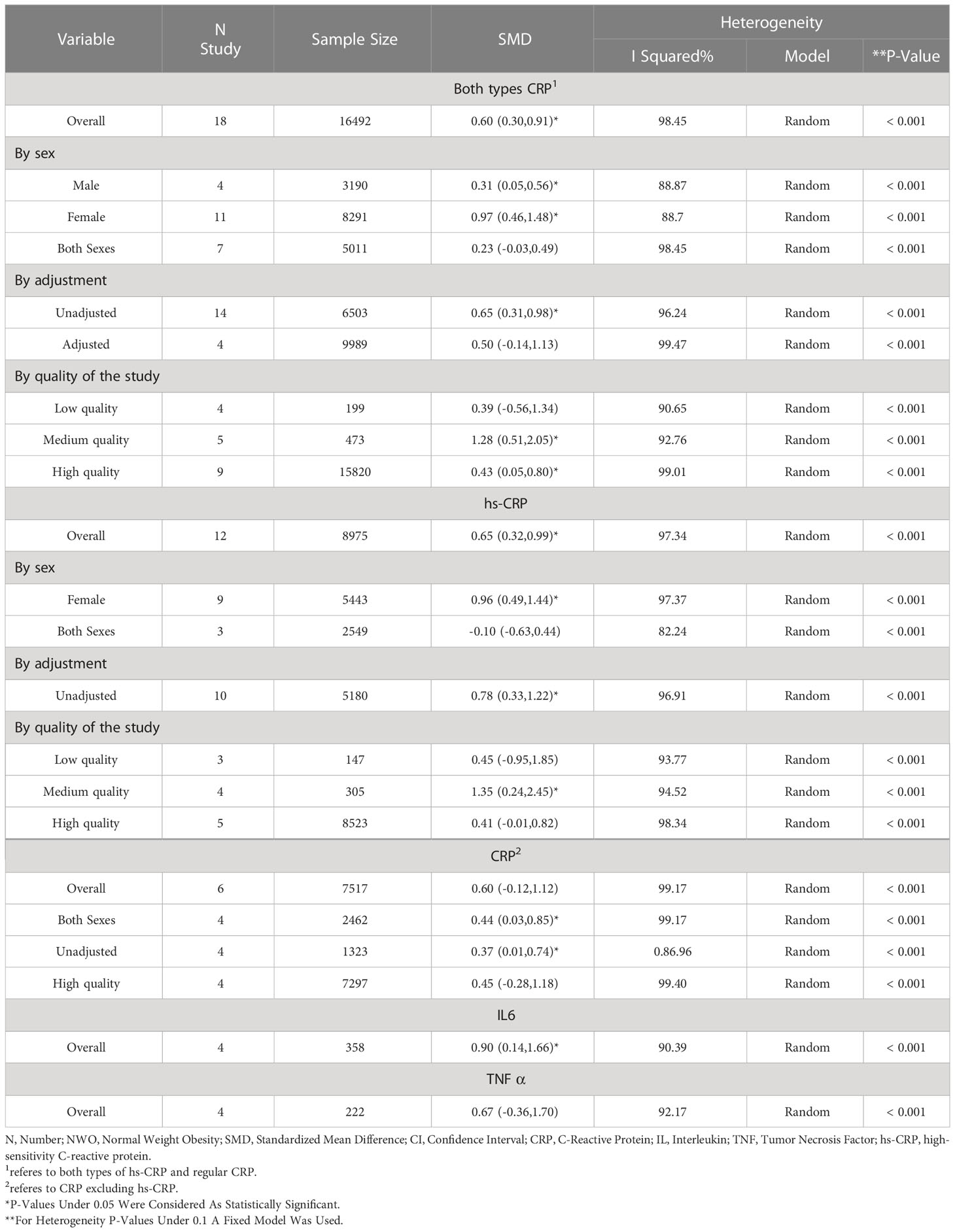
Significant heterogeneity among the studies assessing the association between NWO and CRP, IL6 and TNFα was seen (for all associations I2: ≥ 88%, P-value < 0.001). The overall association between NWO, and CRP, IL6 and TNFα are shown in Table 3. Based on the random effect models meta-analysis, the pooled association of CRP (SMD: 0.60, 95% CI: 0.30, 0.91) and IL6 (SMD: 0.90, 95%CI: 0.14, 1.66) was significantly higher among NWO individuals compared with NWNOs. TNF-α (SMD: 0.67, 95% CI: -0.36, 1.70) was also higher among NWO individuals; however, this association was not statistically significant. Figures 2–4, illustrate the included studies and their overall relationships between NWO and CRP, IL6 and TNFα, respectively.
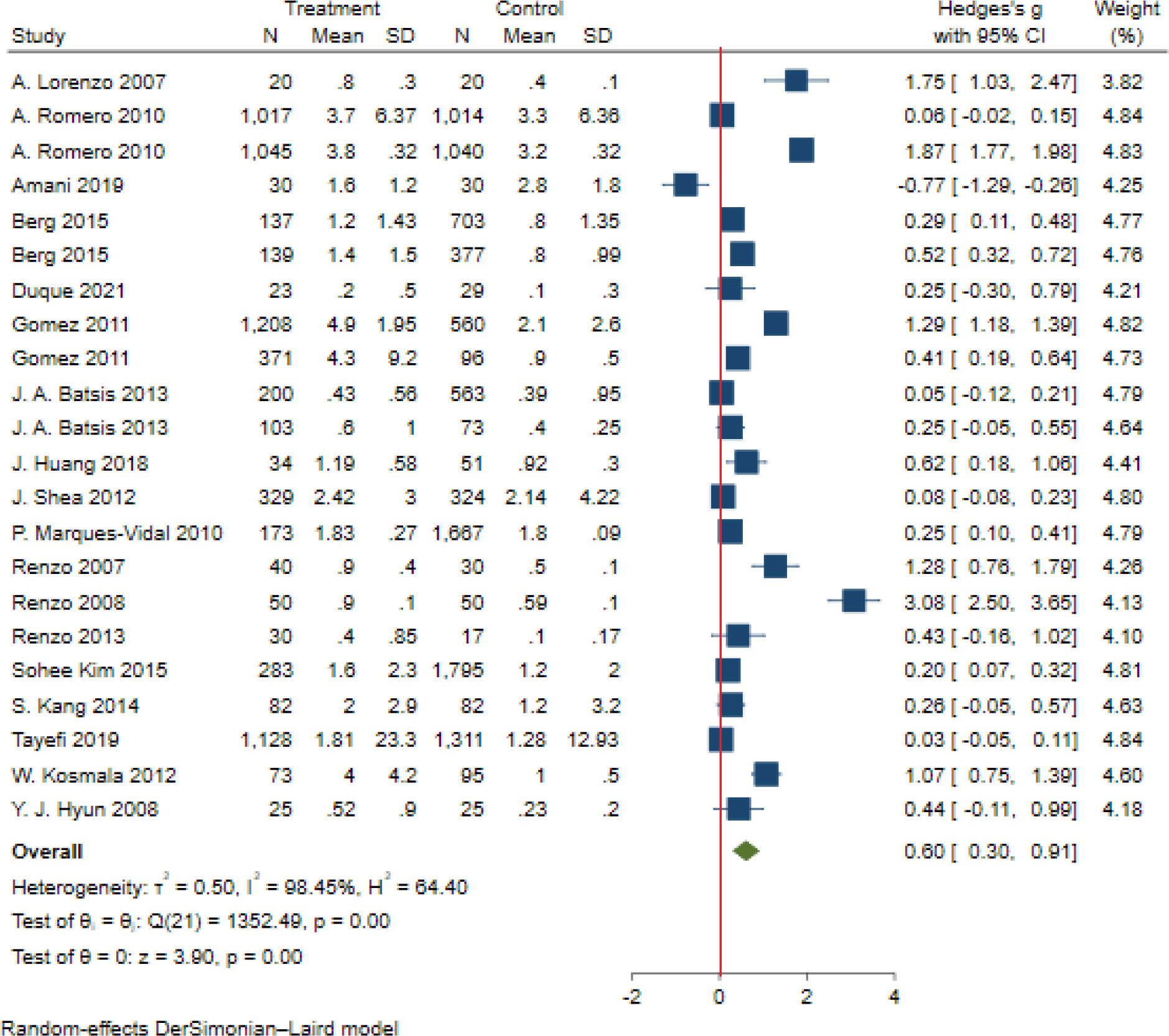
Figure 2 The extracted and overall association between NWO and CRP. (The red line represents null effect).
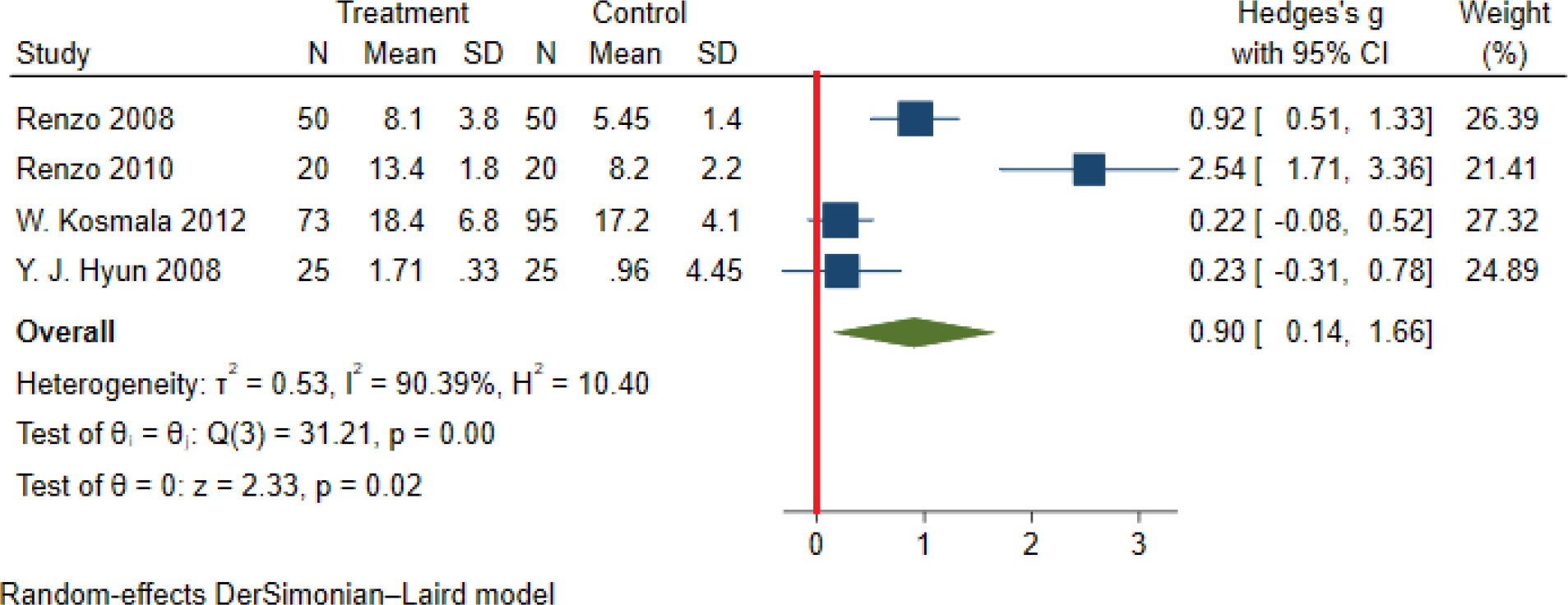
Figure 3 The extracted and overall association between NWO and IL 6. (The red line represents null effect).
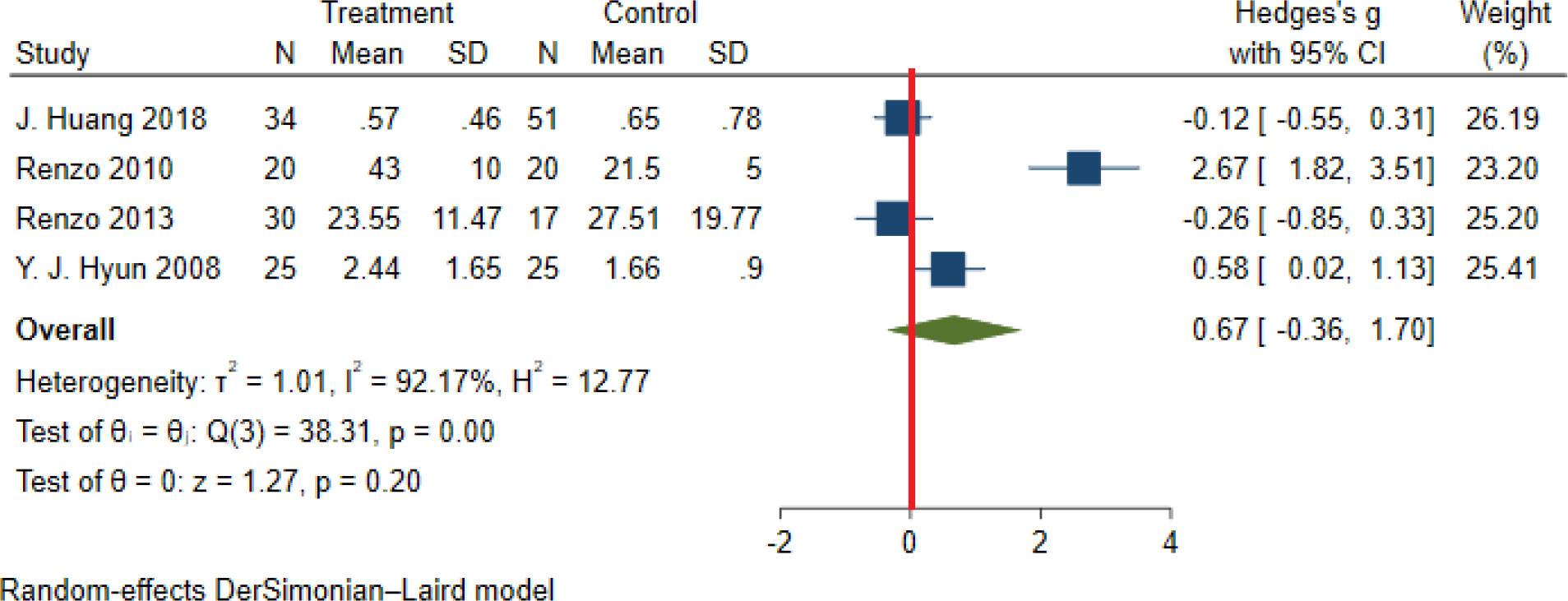
Figure 4 The extracted and overall association between NWO and TNF-α. (The red line represents null effect).
Stratified meta-analysis
Based on the random effect model, the means of CRP sub-grouped by sex in males and females were (SMD: 0.31, 95% CI 0.05, 0.56) and (SMD: 0.97, 95% CI 0.31, 0.98), respectively.
The pooled association between NWO and CRP sub-grouped by quality assessment was (SMD: 0.39, 95% CI -0.57, 1.35), (SMD: 1.28, 95% CI 0.51, 2.06), and (SMD: 0.43, 95% CI 0.05, 0.81) for low quality (articles which had a high risk of bias), satisfactory (articles with moderate risk of bias) and high-quality studies (articles with a low risk of bias) respectively. The pooled association between NWO and CRP sub-grouped by study adjustment (whether studies adjusted their findings for confounding variables) was (SMD:0.50 95%CI -0.14,1.13) and (SMD:0.65 95%CI 0.31,0.98) for adjusted and unadjusted studies respectively. The pooled stratified association between NWO and CRP sub-types according to sex, quality assessment and study adjustment are reported in Table 3. The association of NWO with hs-CRP was statistically significant (SMD:0.65 95%CI 0.32,0.99), and this association was not statistically significant for CRP excluding hs-CRP (SMD:0.60 95% CI -0.12,1.12).
Meta-regression
Meta-regression was performed with sex, quality of the studies and study adjustment (whether studies adjusted their findings for confounding variables) as covariates across CRP, IL6 and TNFα; no significant results were seen across them except for IL6; in which sex and quality of the studies were recognized as possible causes of heterogeneity. (Coefficient: -0.69, P-value: 0.005 and coefficient: -1.61, P-value: 0.001 respectively.)
Quality assessment
Based on the New-Castle Ottawa scale, six of the included studies were of low quality studies (indicating a high possibility of bias), Five studies had middle quality (with acceptable risk of bias) and ten studies had high quality (with low risk of bias). The overall quality score of the studies can be seen in Table 1.
Publication bias
No publication bias was seen in articles studying the association between NWO and CRP (coefficient: 2.22, P-value: 0.16); however, publication bias was seen in articles assessing NWO and TNF α and IL6 (coefficient: 13.42, P-value: 0.004 and coefficient: 7.62, P-value: 0.017 respectively)
Trim fill analysis
Sensitivity analysis was performed on studies assessing NWO and TNFα (SMD: 0.67, 95%CI (-0.36, 1.70) and IL6 (SMD: 0.90, 95%CI (0.14, 1.66), indicating that publication bias did not have a substantial effect on the results.
Discussion
To the best of our knowledge, the current study is the only systematic review and meta-analysis that compared the mean levels of proinflammatory and anti-inflammatory cytokines among NWO and NWNO individuals across the population. Nineteen studies were included in our meta-analysis to address the research questions. And the findings of this study showed that the mean levels of CRP and IL6 were significantly higher in NWO individuals compared to the NWNO individuals.
Although inflammation is one of the body’s essential processes, chronic inflammation is not desirable (42). As chronic inflammation could cause damage to the inflamed site, resulting in metabolic dysregulation, homeostatic mechanisms alteration and even result in some diseases (anemia, various tissue damages, malnutrition, and autoimmune diseases) (42–44). It should be kept in mind that chronic inflammation itself can cause progressive atherosclerosis and cardiovascular diseases through various mechanisms (43, 45). In this regard, although IL6 is an essential modulator of the immune system and has a wide range of biological activities such as modulating immune responses (46), inflammation and hematopoiesis (46, 47); its increase has been associated with renal injury (48), autoimmune conditions (e.g. rheumatoid arthritis and Crohn’s disease), increased risk of cardiovascular disease and increased mortality due to cardiovascular diseases (49, 50). Similarly, it seems that CRP is an important regulator of inflammation and not just a marker (51). Elevated levels of CRP have been seen in autoimmune conditions (e.g. rheumatoid arthritis) and infections and inflammation (51). Furthermore, elevated CRP has been associated with cardiovascular conditions (52), atherothrombosis (53) and atherosclerosis (54). As studies suggest, elevated levels of both IL6 and CRP have been associated with cardiovascular diseases (55). Hence it seems that alongside the increased adiposity which is a significant risk factor for cardiovascular diseases (56), the increased IL6 and CRP associated with NWO could have a significant effect on atherosclerosis and cardiovascular diseases as well. Thus by reducing inflammation with various methods (proper nutrition, exercise, etc.), the cardiovascular risk imposed by NWO may be reduced to some degree (8, 16). Moreover, to reduce the comorbidities of NWO, the public should be educated on various types of obesity and the risks that they impose; they should know that the BMI system has its flaws, and a normal BMI does not indicate the absence of obesity.
It should be kept in mind that CRP could be affected by many environmental factors; thus, IL levels could be more suitable for association assessment and research purposes (57).
Limitations and strength
Despite this study being the aggregated data of all studies on inflammation and NWO with a more precise estimation, the majority of the included studies were not adjusted for potential confounders and had a relatively small sample size; hence studies with a greater population and proper adjustments (e.g. age, sex, underlying diseases, inflammatory and infectious conditions prior to testing, etc) are needed to properly evaluate the association of IL6, CRP and TNFα with NWO; and since many of the included studies were unadjusted the findings of these studies, should be interpreted with caution. Moreover, only IL6, CRP and TNFα had enough studies making meta-analysis rational. More studies on other aspects of inflammation are needed to evaluate their association with NWO as well.
Conclusion
The present study highlighted the significant association of NWO with CRP and IL6 and showed that these cytokines were significantly higher in NWO individuals compared to the NWNO individuals, pertaining to the presence of some degrees of inflammation among NWOs. Regarding the aligned effect of inflammation and adiposity in the progression of cardiovascular diseases and, most importantly, the flaws in the current BMI system, using other measures alongside BMI, and implementing preventive measures to reduce adiposity and inflammation is needed. Moreover, more studies on inflammatory markers in NWO individuals are needed to understand their association better.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
NM and MQ, FB, designed the study. NM and SM searched the databases. NM and SA screened and extracted the data. NM and FB analyzed the data and prepared the results. NM and MQ wrote the paper. FB, OT-M and AE edited and revised the manuscript. All other authors contributed to the article and approved the submitted version.
Funding
Alborz University of Medical Sciences funded this study. The funder had no role in study design, data collection, analysis and preparation of the manuscript and decision to publish
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1044178/full#supplementary-material
References
1. Finer N. Medical consequences of obesity. Medicine (2015) 43(2):88–93. doi: 10.1016/j.mpmed.2014.11.003
2. Ortega Francisco B, Lavie Carl J, Blair Steven N. Obesity and cardiovascular disease. Circ Res (2016) 118(11):1752–70. doi: 10.1161/CIRCRESAHA.115.306883
3. He C, Zhang M, Li J, Wang Y, Chen L, Qi B, et al. Novel insights into the consequences of obesity: A phenotype-wide mendelian randomization study. Eur J Hum Genet (2022) 30:540–6. doi: 10.1038/s41431-021-00978-8
4. Lim HJ, Xue H, Wang Y. Global trends in obesity. In: Meiselman H. Eds. Handbook of Eating and Drinking (Cham: Springer (2020). doi: 10.1007/978-3-030-14504-0_157
5. García-Hermoso A, Agostinis-Sobrinho C, Camargo-Villalba GE, González-Jiménez NM, Izquierdo M, Correa-Bautista JE, et al. Normal-weight obesity is associated with poorer cardiometabolic profile and lower physical fitness levels in children and adolescents. Nutrients (2020) 12(4):1171. doi: 10.3390/nu12041171
6. Khonsari NM, Khashayar P, Shahrestanaki E, Kelishadi R, Nami SM, Heidari-Beni M, et al. Normal weight obesity and cardiometabolic risk factors: A systematic review and meta-analysis. Front Endocrinol (2022) 13:857930. doi: 10.3389/fendo.2022.857930
7. Anoop S, Kapoor N. Normal-weight obesity: A hidden pandemic. In: Faintuch J., Faintuch S. Eds. Obesity and Diabetes Cham: Springer (2020). doi: 10.1007/978-3-030-53370-0_26
8. Franco LP, Morais CC, Cominetti C. Normal-weight obesity syndrome: diagnosis, prevalence, and clinical implications. Nutr Rev (2016) 74(9):558–70. doi: 10.1093/nutrit/nuw019
9. Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis (2014) 56(4):426–33. doi: 10.1016/j.pcad.2013.10.003
10. Xu S, Ming J, Jia A, Yu X, Cai J, Jing C, et al. Normal weight obesity and the risk of diabetes in Chinese people: A 9-year population-based cohort study. Sci Rep (2021) 11(1):1–8. doi: 10.1038/s41598-021-85573-z
11. Shirasawa T, Ochiai H, Yoshimoto T, Nagahama S, Kobayashi M, Ohtsu I, et al. Associations between normal weight central obesity and cardiovascular disease risk factors in Japanese middle-aged adults: A cross-sectional study. J Health Population Nutr (2019) 38(1):1–7. doi: 10.1186/s41043-019-0201-5
12. Perini W, Kunst AE, Snijder MB, Peters RJG, van Valkengoed IGM. Nutrition , metabolism & cardiovascular diseases ethnic differences in metabolic cardiovascular risk among normal weight individuals : Implications for cardiovascular risk screening. HELIUS study. Nutr Metab Cardiovasc Dis (2019) 29(1):15–22. doi: 10.1016/j.numecd.2018.09.004
13. Kim S, Joo HJ, Shim W-J, Lee J. Normal weight obesity and metabolic syndrome risk in Korean adults: 5-year longitudinal health checkup study. Circulation (2018) 138(Suppl_1):A13448–A.
14. Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Investig (2018) 48(9):e12997. doi: 10.1111/eci.12997
15. Karczewski J, Śledzińska E, Baturo A, Jończyk I, Maleszko A, Samborski P, et al. Obesity and inflammation. Eur Cytokine Netw (2018) 29(3):83–94. doi: 10.1684/ecn.2018.0415
16. Ferrante A. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J Internal Med (2007) 262(4):408–14. doi: 10.1111/j.1365-2796.2007.01852.x
17. Elks CM, Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr Hypertens Rep (2010) 12(2):99–104. doi: 10.1007/s11906-010-0096-4
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
19. Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res methodol (2014) 14(1):1–5. doi: 10.1186/1471-2288-14-45
20. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford (2000).
21. Duque AP, Barbosa IM, Lins AS, de Jesus FG, Araújo CF, Id N, et al. Abstract P028: Increased cardiometabolic risk and autonomic function alterations in normal weight obesity. Circulation (2021) 143(Suppl_1):AP028–AP. doi: 10.1161/circ.143.suppl_1.P028
22. Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis (2012) 22(9):741–7. doi: 10.1016/j.numecd.2010.11.009
23. Karkhaneh M, Qorbani M, Ataie-Jafari A, Mohajeri-Tehrani MR, Asayesh H, Hosseini S. Association of thyroid hormones with resting energy expenditure and complement C3 in normal weight high body fat women. Thyroid Res (2019) 12:9. doi: 10.1186/s13044-019-0070-4
24. Tayefi M, Tayefi B, Darroudi S, Mohammadi-Bajgiran M, Mouhebati M, Heidari-Bakavoli A, et al. There is an association between body fat percentage and metabolic abnormality in normal weight subjects: Iranian large population. Trans Metab Syndrome Res (2019) 2(1):11–6. doi: 10.1016/j.tmsr.2019.08.001
25. Amani R, Parohan M, Jomehzadeh N, Haghighizadeh MH. Dietary and biochemical characteristics associated with normal-weight obesity. Int J Vitamin Nutr Res (2019) 89(5-6):331–6. doi: 10.1024/0300-9831/a000477
26. Di Renzo L, Sarlo F, Petramala L, Iacopino L, Monteleone G, Colica C, et al. Association between -308 G/A TNF-alpha polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis Mark. (2013) 35(6):615–23. doi: 10.1155/2013/983424
27. Di Renzo L, Galvano F, Orlandi C, Bianchi A, Di Giacomo C, La Fauci L, et al. Oxidative stress in normal-weight obese syndrome. Obesity (2010) 18(11):2125–30. doi: 10.1038/oby.2010.50
28. Di Renzo L, Bertoli A, Bigioni M, Gobbo VD, Premrov MG, Calabrese V, et al. Body composition and -174G/C interleukin-6 promoter gene polymorphism: association with progression of insulin resistance in normal weight obese syndrome. Curr Pharm Des (2008) 14(26):2699–706. doi: 10.2174/138161208786264061
29. De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr (2007) 85(1):40–5. doi: 10.1093/ajcn/85.1.40
30. Di Renzo L, Bigioni M, Del Gobbo V, Premrov MG, Barbini U, Di Lorenzo N, et al. Interleukin-1 (IL-1) receptor antagonist gene polymorphism in normal weight obese syndrome: relationship to body composition and IL-1 alpha and beta plasma levels. Pharmacol Res (2007) 55(2):131–8. doi: 10.1016/j.phrs.2006.11.002
31. Huang J, Fukuo K, Yoshino G, Kazumi T, Basetty C, Huang Y, et al. (2018). Body composition and biochemical characteristics of normal weight obesity in Japanese young women with different physical activities, in: 2018 IEEE international conference on bioinformatics and biomedicine (BIBM), Madrid, Spain, pp. 1480–3. doi: 10.1109/BIBM.2018.8621153
32. Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: Contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circulation: Cardiovasc Imaging. (2012) 5(3):349–56. doi: 10.1161/CIRCIMAGING.111.969956
33. Hyun YJ, Koh SJ, Chae JS, Kim JY, Kim OY, Lim HH, et al. Atherogenecity of LDL and unfavorable adipokine profile in metabolically obese, normal-weight woman. Obesity (2008) 16(4):784–9. doi: 10.1038/oby.2007.127
34. Cho WK, Kim H, Lee HY, Han KD, Jeon YJ, Jung IA, et al. Insulin resistance of normal weight central obese adolescents in Korea stratified by waist to height ratio: results from the Korea national health and nutrition examination surveys 2008–2010. Int J Endocrinol (2015) 2015:158758. doi: 10.1155/2015/158758
35. Kim S, Kyung C, Park JS, Lee S-P, Kim HK, Ahn CW, et al. Normal-weight obesity is associated with increased risk of subclinical atherosclerosis. Cardiovasc diabetol (2015) 14(1):1–9. doi: 10.1186/s12933-015-0220-5
36. Kang S, Kyung C, Park JS, Kim S, Lee S-P, Kim MK, et al. Subclinical vascular inflammation in subjects with normal weight obesity and its association with body fat: an 18 f-FDG-PET/CT study. Cardiovasc Diabetol (2014) 13(1):1–12. doi: 10.1186/1475-2840-13-70
37. Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Millán D. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (2011) 36(2):286–94. doi: 10.1038/ijo.2011.100
38. Berg C, Strandhagen E, Mehlig K, Subramoney S, Lissner L, Björck L. Normal weight adiposity in a Swedish population: how well is cardiovascular risk associated with excess body fat captured by BMI? Obes Sci Pract (2015) 1(1):50–58. doi: 10.1002/osp4.4
39. Marques-Vidal P, Pécoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, et al. Normal weight obesity: Relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutrition Metab Cardiovasc Dis (2010) 20(9):669–75. doi: 10.1016/j.numecd.2009.06.001
40. Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J (2010) 31(6):737–46. doi: 10.1093/eurheartj/ehp487
41. Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in united states subjects >/=60 years of age (from the third national health and nutrition examination survey). Am J Cardiol (2013) 112(10):1592–8. doi: 10.1016/j.amjcard.2013.07.014
42. Pahwa R, Goyal A, Jialal I. Chronic inflammation. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2021). Available from: https://www.ncbi.nlm.nih.gov/books/NBK493173/.
43. Hotamisligil GS. Inflammation and metabolic disorders. Nature (2006) 444(7121):860–7. doi: 10.1038/nature05485
44. Straub R. The origin of chronic inflammatory systemic diseases and their sequelae. Elsevier Inc (2015). doi: 10.1016/C2014-0-04588-1
45. Lopez-Candales A, Burgos PMH, Hernandez-Suarez DF, Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci (2017) 3(4):e341.
46. Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol (2010) 40:1830–5. doi: 10.1002/eji.201040391
47. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). (2012) 122(4):143–59. doi: 10.1042/CS20110340
48. Su H, Lei CT, Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front Immunol (2017) 8:405. doi: 10.3389/fimmu.2017.00405
49. Patterson CC, Smith AE, Yarnell JW, Rumley A, Ben-Shlomo Y, Lowe GD. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: the Caerphilly study. Atherosclerosis (2010) 209(2):551–7. doi: 10.1016/j.atherosclerosis.2009.09.030
50. Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation (2001) 103(7):947–53. doi: 10.1161/01.cir.103.7.947
51. Sproston NR, Ashworth JJ. Role of c-reactive protein at sites of inflammation and infection. Front Immunol (2018) 9:754. doi: 10.3389/fimmu.2018.00754
52. Wilson AM, Ryan MC, Boyle AJ. The novel role of c-reactive protein in cardiovascular disease: risk marker or pathogen. Int J Cardiol (2006) 106(3):291–7. doi: 10.1016/j.ijcard.2005.01.068
53. Devaraj S, Singh U, Jialal I. The evolving role of c-reactive protein in atherothrombosis. Clin Chem (2009) 55(2):229–38. doi: 10.1373/clinchem.2008.108886
54. Libby P, Ridker PM. Inflammation and atherosclerosis: role of c-reactive protein in risk assessment. Am J Med (2004) 116 Suppl 6A:9S–16S. doi: 10.1016/j.amjmed.2004.02.006
55. Nadrowski P, Chudek J, Skrzypek M, Puzianowska-Kuźnicka M, Mossakowska M, Więcek A, et al. Associations between cardiovascular disease risk factors and IL-6 and hsCRP levels in the elderly. Exp Gerontol. (2016) 85:112–7. doi: 10.1016/j.exger.2016.10.001
56. Poirier P. Adiposity and cardiovascular disease: are we using the right definition of obesity? Eur Heart J (2007) 28(17):2047–8. doi: 10.1093/eurheartj/ehm321
Keywords: NWO, normal weight obesity, inflammation, inflammatory markers, CRP, interleukin
Citation: Mohammadian Khonsari N, Baygi F, Tabatabaei-Malazy O, Mohammadpoor Nami S, Ehsani A, Asadi S and Qorbani M (2023) Association of normal weight obesity phenotype with inflammatory markers: A systematic review and meta-analysis. Front. Immunol. 14:1044178. doi: 10.3389/fimmu.2023.1044178
Received: 14 September 2022; Accepted: 13 February 2023;
Published: 27 February 2023.
Edited by:
Tuo Deng, Central South University, ChinaReviewed by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroYing Meng, University of Rochester, United States
Copyright © 2023 Mohammadian Khonsari, Baygi, Tabatabaei-Malazy, Mohammadpoor Nami, Ehsani, Asadi and Qorbani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mostafa Qorbani, TXFvcmJhbmkxMzc5QHlhaG9vLmNvbQ==; Sahar Mohammadpoor Nami, c21vaGFtbWFkcG9vcm5hbWlAZ21haWwuY29t
 Nami Mohammadian Khonsari
Nami Mohammadian Khonsari Fereshteh Baygi
Fereshteh Baygi Ozra Tabatabaei-Malazy
Ozra Tabatabaei-Malazy Sahar Mohammadpoor Nami
Sahar Mohammadpoor Nami Amir Ehsani
Amir Ehsani Sasan Asadi1
Sasan Asadi1 Mostafa Qorbani
Mostafa Qorbani