
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 15 September 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.998516
This article is part of the Research Topic Assessment and Management of Adverse Drug Reactions in Oncology View all 12 articles
Immune-checkpoint inhibitors (ICI) targeting programmed cell death 1 (PD-1) and its ligand 1 (PD-L1) have quickly changed the treatment landscape in advanced non-small cell lung cancer. However, any patient treated with an immune checkpoint inhibitor is at risk for immune-related adverse events (irAEs). Checkpoint inhibitor pneumonitis (CIP) is a rare but potentially severe pulmonary toxicity of immunotherapy. Since the imaging features and symptoms are not specific, the diagnosis of CIP is challenging. In addition, CIP may mimic other lung diseases. Due to these characteristics, proper patient management may be delayed. So, a comprehensive understanding of imaging features is essential for a prompt detection and correct management of these drug-induced lung diseases. We presented a patient with lung squamous cell cancer who has clinical symptoms preceding imaging evidence of pneumonitis after immunotherapy and radiotherapy. We also discussed the safety of immunotherapy, the complexity and management of immune pneumonitis.
Immune-checkpoint inhibitors (ICI) have transformed the treatment of multiple cancer, which significantly improved survival (1–3). One of the remarkable characteristics of ICIs is their relatively mild toxicity profile. Although uncommon, immune-related adverse events (irAEs) may occur in patients receiving immunotherapy, especially in those receiving combined ICI. The mechanisms leading to Checkpoint inhibitor pneumonitis (CIP) are still being clarified. Some potential mechanisms include increasing T-cell activity in response to cross-antigens, increasing levels of autoantibodies and inflammatory cytokines and enhancing complement mediated inflammation (4). Most of irAEs are low grade and can involve almost any organ system. They have uncertain features, but in most cases can be improved by drug immunosuppression and discontinuation of treatment. CIP is uncommon but potentially fatal toxicity of immunotherapy.
The clinical manifestations of CIP are variable, it might show asymptomatic disease, or it may present with respiratory symptoms such as cough, shortness of breath and respiratory failure, in some cases can lead to death. The incidence of CIP in combined therapy (6.5%–10%) was higher than that in monotherapy (3%–4%) (4–6). However, due to the lack of typical imaging findings, the radiological diagnosis of CIP is challenging. In most cases, the main radiologic patterns of CIP include several abnormalities with cryptogenic organizing pneumonia (COP), nonspecific interstitial pneumonitis (NSIP), hypersensitive pneumonitis (HP), ground-glass opacities, and acute interstitial pneumonitis for more severe cases. In some cases, CIP can display a special imaging feature that cannot be classified in any of the above-mentioned specific modes (7). Moreover, CIP is a dynamic process that evolves over time. Most studies reflect the incidence of CIP, but do not clarify the course of it. Here, we report a patient with CIP whose imaging features lag behind clinical symptoms after immunotherapy and radiotherapy.
A 66-year-old man with stage IIIIC lung squamous cell carcinoma (SCC) (cT4N3M0) presented with cough and chest pain for four months (Figure 1). He had a history of smoking for more than 40 years (40 cigarettes a day, quit > 3 months). His body weight was 71 kg, with no significant change from the previous weight. No sensitive gene mutations were detected and the percentage of tumor cells with membranous PD-L1 staining (tumor proportion score) was 30%. Four cycles of tislelizumab plus nab-paclitaxel and carboplatin chemotherapy were performed. The fourth cycle of treatment were synchronized with thoracic radiotherapy (TRT) (60 Gy/30 fractions), the mean lung dose (MLD) was 8.07 Gy, 22.5% of the lung received 20 Gy (V20), and 36% of the lung received 5 Gy (V5), which ended in Jan 2022 (Figure 2C). The patient’s symptoms of cough and chest pain improved.
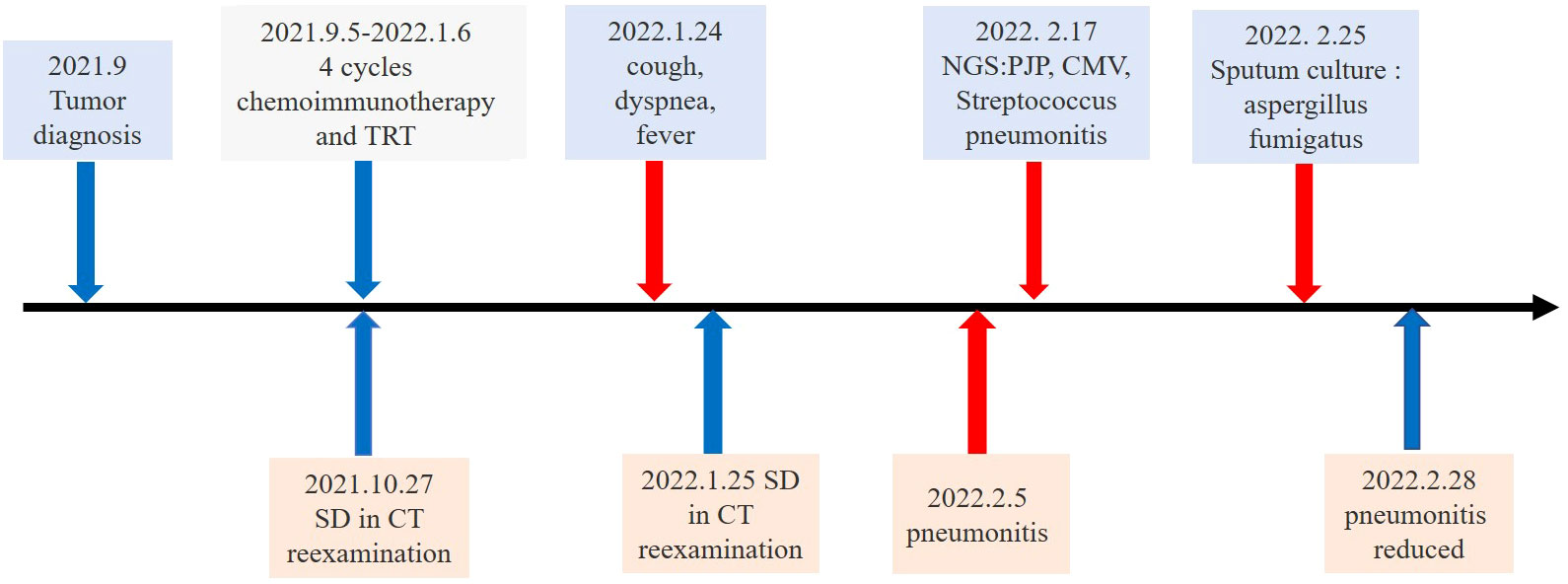
Figure 1 Timeline of the major treatment process and CT evaluation of the patient since diagnosis. SD means “stable disease”.
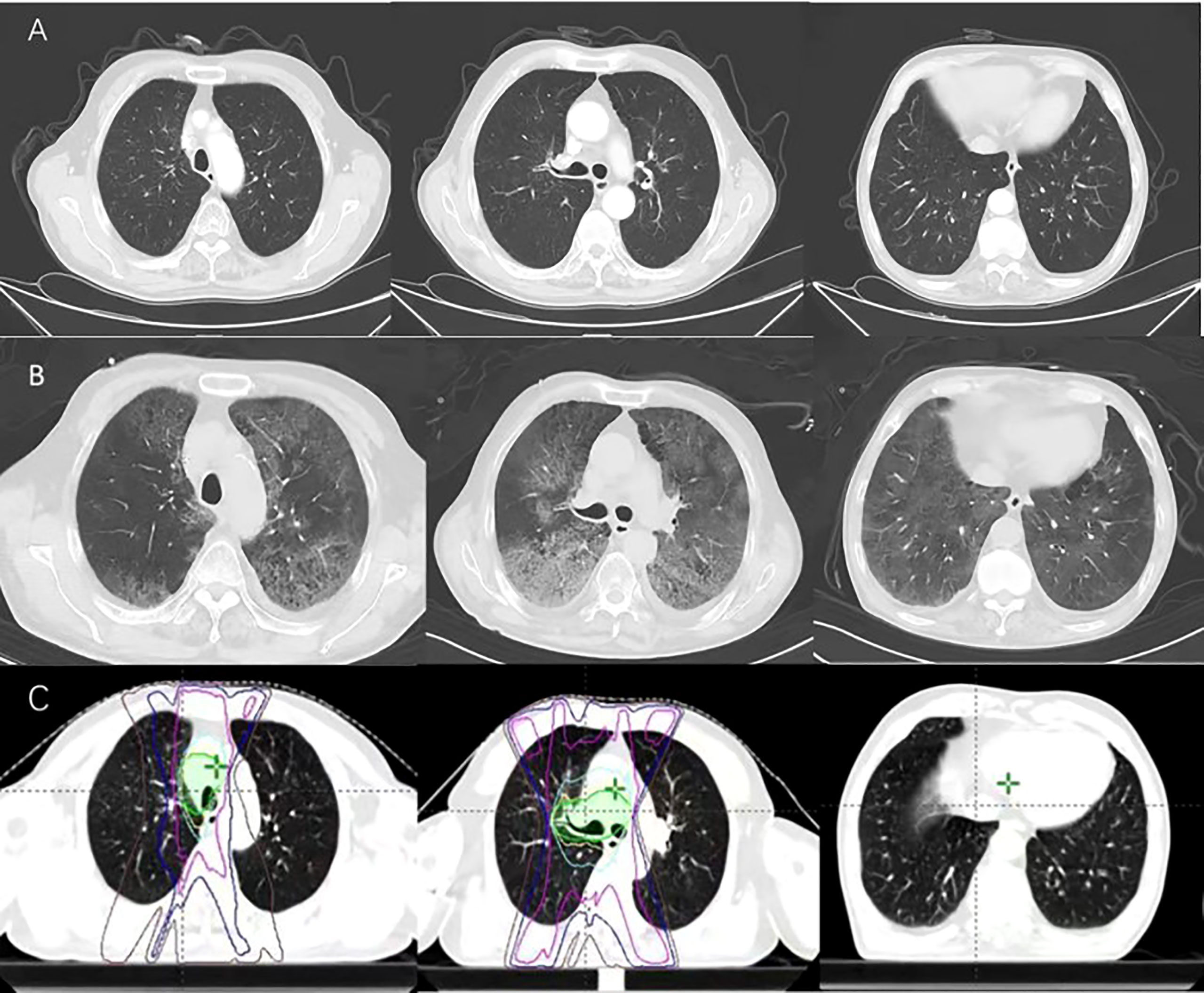
Figure 2 (A) 20 days after treatment ends, dyspnea, cough and fever occurred. (B) 27 days after treatment ends. (C) Radiation field.
After 20 weeks of the initial tislelizumab administration (20 days after radiotherapy ended), dyspnea and cough developed. The patient presented to our hospital, the laboratory tests including hemogram and inflammatory markers were normal except for the white blood cell of 14.79×109/L. Computerized tomography (CT) of the chest showed the tumor was reduced in size and no pneumonitis was observed (Figure 2A). We administered an inhaled acetylcysteine solution for the symptomatic treatment of dyspnea. However, 1 week later, the patient had a fever with a worsening cough and expectation. Due to the Spring Festival, he did not seek medical attention in time. Then his temperature continued to rise. Only 3 days later, in the emergency department, CT was notable for extensive exudation of both lungs, diagnosis of interstitial pneumonia (Figure 2B). The patient’s condition continued to deteriorate and he was transferred to the intensive care unit (ICU). His temperature was 42°C, the pulse was 123 beats per minute, mask oxygen was given to the patient. Then his partial pressure of oxygen (PaO2) was only 60 mmHg and the fraction of inspiration O2 (FiO2) was 57%. Serum (1–3)-ß-d-glucan level was 327.12pg/mL, and the galactomannan was 0.25μg/L. According to the multidisciplinary team (MDT), the patient had a history of previous immunotherapy. CT showed large blurred shadow with unclear margins, and his serum (1–3)-ß-d-glucan level are high. It was thought to be pulmonary complications related to immune checkpoint inhibitor therapy and possible coinfection. Then methylprednisolone (40 mg per 12 hours) was administered intravenously. And antibiotics such as Compound Sulfamethoxazole Tablets (Trimethoprim 0.16g and Sulfamethoxazole 0.8g per 6 hours), Sulperazon (3g per 8 hours) and voriconazole (0.2g per 12 hours) were empirically administered. After a week, the patient’s condition did not improve, and voriconazole was replaced by caspofungin (70mg once→50mg/day). Bronchoscopy with bronchoalveolar lavage (BAL) was performed. On the 12th hospital day, next-generation sequencing (NGS) analysis of BALF specimens identified Pneumocystis jirovecii(PJP), streptococcus pneumonia and cytomegalovirus (CMV). Ganciclovir (375mg/day for seven days → 0.25g per 12 hours) was added to his treatment and increased the dose of Compound Sulfamethoxazole Tablets (Trimethoprim 0.24g and Sulfamethoxazole 1.2g per 6 hours). At the same time, the patient with sustained grade III/IV thrombocytopenia, immunoglobulin (25 g/day for five days) was injected intravenously. On the 20th hospital day, the fungal and bacterial sputum culture obtained 7 days earlier grew Aspergillus fumigatus. The patient improved after 23 days in ICU and CT showed reduced inflammation.
We described a case of CIP after radiotherapy combined with immunotherapy whose symptoms predate imaging findings, complicated by Pneumocystis, Aspergillus and other infections during the treatment of CIP. Previous studies have shown that during PD-1 inhibitor monotherapy, the incidence of all-grade pneumonia in NSCLC was 4.1%, and the incidence of grade 3 and above pneumonia was 1.8% (6). ICIs may overstimulate the immune system, change the homeostasis of the host, and lead to excessive inflammatory response. During radiotherapy, ionizing radiation induces free radicals and DNA damage, promoting oxidative stress, vascular damage, and inflammation on normal tissues (8). A pool analysis showed, about 11% of patients who received stereotactic body radiotherapy (SBRT) developed grade ≧̸2 radioactive pneumonia within a few months (9). The combination of radiation therapy(RT) and ICI works not only by directly tumor-cell killing, but also by stimulating the immune system response through radiation, so as to enhance the ICI effect (10). Many preclinical studies have shown that there is synergistic activity between ICIS and RT. The combination of thoracic radiotherapy and ICI may have synergistic pulmonary toxicity. The occurrence of pneumonitis is an important point worth exploring.
Some studies have demonstrated the safety of radiotherapy combined with immune checkpoint inhibition. In the KEYNOTE-001 trial, the secondary analysis found that prior thoracic radiotherapy was associated with an increased risk of treatment-related pulmonary toxicities (13% vs. 1%, P = 0.046), while there was no significant difference in high-grade pulmonary toxicity (11). The phase 3 PACIFIC trial showed the incidence of any grade pneumonitis/radiation pneumonitis was 33.9% in the immunotherapy group and 24.8% in the placebo group (P value not reported), and the incidence of grade 3 or higher pneumonitis/radiation pneumonitis occurred in 3.4% and 2.6%, respectively (P value not reported) (12). They mainly demonstrated the pulmonary toxicities of sequential combination of ICI with a previous history of radiotherapy. A recent retrospective study in patients who underwent TRT after ICI evaluated the incidence and severity of treatment-related pneumonitis. It showed that the incidence and severity of treatment-related pneumonitis was significantly higher in lung cancer patients who received TRT after ICI (13). As is known, acute “radiation pneumonitis” usually occurs within 4 to 12 weeks after thoracic irradiation. Recently, some studies revealed that pneumonitis in radio-immunotherapy patients usually occurs in previously irradiated areas, regardless of how much time has elapsed after RT, even after a few years. In some patients receiving ICIS, the time interval between radiation therapy and pneumonitis raises the hypothesis of “radiation recall pneumonitis”. A recent pooled analysis found that the patients received radiotherapy within 90 days (RT ≤ 90) after ICI had slightly numerically higher rates of pneumonitis than those more than 90 days (RT >90) before the start of ICI therapy. These differences were due to low grade (grade 1-2) AEs (14). In addition, a phase 1 trial of Pembrolizumab combination with chemoradiotherapy for locally advanced NSCLC found pneumonitis of ≥grade 2 occurred in 33% patients, and risk of pneumonitis was higher in patients who started pembrolizumab on day 1 of chemotherapy (15). In summary, the combination of ICI and radiotherapy have a slightly higher rate of pneumonitis than monotherapy, but the difference may be owing to low grade (grade 1-2) AEs pneumonitis that is mostly tolerated. In addition, timing of combination therapy is critical. Longer intervals seem to portend better security. If the interval exceeds 90 days, there is no significant increase in the probability of pneumonitis.
The most common symptoms of pneumonitis are dyspnea and cough, fever and chest pain were less common, some patients were asymptomatic at the onset of pneumonitis (16). Several cases of CIP have been reported, indicating that the clinical course of pneumonitis also varies among patients, with some requiring ICU admission, intubation and even death, while others are successfully treated with oral corticosteroid. In addition, a few patients experience recurrent pneumonitis after restarting their immunotherapy (5, 7, 17). Retrospective case studies have inconsistently identified risk factors for underlying lung disease, such as ILD, history of radiation therapy, history of COPD and V20. However, the factors involved are highly variable for individual patients (18).
As far as we know, we present the first case of patient’s pneumonitis symptoms prior to radiologic manifestation. In this case, when the patient returned to the hospital with cough, dyspnea, and fever, a CT scan on him found no obvious signs of pneumonitis. However, 1 week later, his symptoms gradually worsened and CT also showed severe pneumonitis. The patient was treated with methylprednisolone (80mg per 12 hours) and empirically administered with antibiotics. His condition had not significantly improved but found coinfection. Regarding infectious complications, it has emerged that the blockade of the PD-1/PD-L1 axis does not appear to inherently increase the risk of infection because it promotes T-cell effector functions. Nevertheless, they can induce irAEs. The treatment of these irAEs require additional immunosuppressive with corticosteroids which can lead to opportunistic infection (19). However, unlike the majority of reported cases, the new reports showed that some infections can be triggered by ICIs without immunosuppressive treatment. It seems that hyperinflammatory dysregulated immunity associated with ICIs drives pathogenesis. These infections are characterized by a dysregulated inflammatory immune response (20).
The patient's NGS analysis also found CMV, there is evidence that CMV may be a potential trigger for severe irAE. A retrospective cohort study comparing CMV infection in patients with different degrees of CIP showed that the CMV positive rate was much higher in patients with severe CIP than in patients with no or mild ICI pneumonitis (91.7 vs. 20%). There are reports that immunotherapy exacerbates progressive fungal infections, such as aspergillosis, in the absence of immunosuppression. Clinically, this infection can mimic the progression of cancer. Therefore, immune checkpoint inhibition may exaggerate the immune response to fungal colonization, which may promote fungal growth (21).
With rare exceptions, CIP may also be accompanied by other irAE, such as this patient developed thrombocytopenia with normal hemoglobin and normal white cell counts. Bone marrow biopsy showed no obvious morphological abnormalities, no hemophagocytic cells, and no malignant invasion in the patient. Laboratory tests such as antinuclear antibodies were negative, but antiphospholipid and antiplatelet antibodies were abnormal. Thrombocytopenia caused by chemotherapy, infection, or other drugs, was excluded, and the final diagnosis was immune-induced thrombocytopenia. While steroid therapy, he received five platelet transfusions and intravenous immunoglobulin (25 g/day for five days), but the platelets did not recover. In the meantime, the lowest platelet level was 7 × 109/L. Fortunately, his platelets returned to normal after discharge. This also reminds us that in the face of patients after immunotherapy, the adverse reactions may be complex, and we should comprehensively evaluate and designate a comprehensive and individualized treatment plan.
CIP is essentially interstitial pneumonia and opportunistic infections are prone to occur in CIP patients. However, early diagnosis is difficult due to the acute course of infection and the heterogeneity of clinical manifestations. Bronchoscopy and bronchoalveolar lavage (BAL) are key to confirming infection, and NGS should be performed if necessary. We should pay attention to distinguishing between bacterial infection, viral infection, fungal infection and heart failure. Occasionally, patients may present with symptoms without imaging findings, which deserves our vigilance.
The diagnosis of CIP is exclusionary, which is usually a combination of clinical assessment, imaging findings, and laboratory analysis. BAL analysis is also crucial as it can differentiate inflammation and tumor. The BAL cytology shows a predominantly lymphocytic or a mixed pattern (7). Inflammatory markers such as C-reactive protein were also elevated. In some patients who can’t have a BAL, imaging findings are crucial to the diagnosis of disease. In imaging, the mainly differential diagnoses of CIP are infection and tumor progression, which can share similar clinical symptoms. The relationship between the radiation field and pneumonia deserves our attention.
To date, the treatment of irAEs has mostly been empirical. Several published guidelines recommended similar treatments for different grades of pneumonitis. Grade 1 pneumonitis is treated by discontinuation of ICI therapy without steroids. Grade 2 pneumonitis can be managed by withholding the ICI therapy and initiating steroids therapy. Grades 3 and 4 pneumonitis should permanent discontinuation of ICI therapy and intravenous steroids along with empirical antibiotic therapy. Therefore, the most common initial treatment for CIP is steroids. But a small proportion of patients are refractory or become resistant to steroids, which is rare but is associated with higher morbidity and mortality (5, 22). For these patients, treatment guidelines suggest second-line immunosuppressive therapy including infliximab, mycophenolate, intravenous immunoglobulin and cyclophosphamide (23–25). Since the clinical course and response pattern to steroids are often individualized. The timing of initiation of additional immunomodulatory agents varies among patients. At present, it is considered that a more effective treatment strategy is to give additional immunomodulatory agents as soon as possible once the patient has the initial signal of refractory response (26). Other promising strategies, such as targeting the microbiome, are emerging and have yet to be included in these guidelines. Multiple case reports of opportunistic infections after ICI treatment by a variety of different pathogens proved the necessity of having a low threshold of investigation for opportunistic infection (20).
As for patients who develop CIP, it has been demonstrated that the use of high-dose glucocorticoids increases the risk of pneumocystis. Thus, PJP prophylaxis has to be considered (27). Increased vigilance and timely identification of pneumonia are prerequisites for early initiation of treatment and prevention of further morbidity and mortality in these patients (28). Because CIP has no clear onset time, patients may have no imaging findings, and even a few patients have symptoms prior to imaging findings. It is recommended that continues to strengthen the monitoring of patient symptoms and signs and laboratory results during immunotherapy (29).
The case has several limitations. The main limitation is due to the serious condition of the patient, methylprednisolone was given before there have a BAL analysis or other microbiology result, which may interfere the BAL cytology. Antibiotics are also used empirically, which prevents us from accurately assessing the initial infection status. In addition, we lack the serial blood gas analysis of the patients, which prevent us to understand the treatment response through PaO2/FiO2. Given these limitations, more CIP patients deserve our attention.
In patients with CIP, a new feature that the clinical symptoms predate imaging findings deserves our attention. CIP is gradually increasing following the use of immune checkpoint inhibitors. When thoracic radiotherapy is combined with ICI, timing of combine is critical, longer intervals seem to portend better security. Clinicians should strengthen the surveillance and management of pneumonitis. Opportunistic infections are prone to occur in CIP patients and steroid therapy should be used earlier when pulmonary toxicity occurs. It has been demonstrated that the use of high-dose glucocorticoids increases the risk of PJP. Thus, PJP prophylaxis has to be considered. There are critical needs to clarify the mechanisms of CIP and to formulate individualized treatment strategy to improve the safety of tumor immunotherapy. Looking into the future, radiologists must strengthen collaboration with multidisciplinary teams to provide optimal treatment and management for patients with ICI-associated pneumonia, which is so complicated in diagnosis and treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
XM proposed, edited, and approved the final manuscript. MY collected clinical information. YW analyzed the data and drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Academic Promotion Program of Shandong First Medical University (2019ZL002), Research Unit of Radiation Oncology, Chinese Academy of Medical Sciences (2019RU071), the foundation of National Natural Science Foundation of China (81627901, 81972863 and 82030082), the foundation of Natural, Science Foundation of Shandong (ZR201911040452). Natural Science Foundation of Shandong (ZR2019MH010), CSCO-Haosen Foundation (Y-HS202102-0089), and CSCO-Xinda Foundation (Y-XD202001-0008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-Small-Cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol (2021) 7(5):709–17. doi: 10.1001/jamaoncol.2021.0366
2. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
3. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
4. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
5. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/Programmed death ligand 1 therapy. J Clin Oncol (2017) 35(7):709–17. doi: 10.1200/JCO.2016.68.2005
6. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol (2016) 2(12):1607–16. doi: 10.1001/jamaoncol.2016.2453
7. Pozzessere C, Lazor R, Jumeau R, Peters S, Prior JO, Beigelman-Aubry C. Imaging features of pulmonary immune-related adverse events. J Thorac Oncol (2021) 16(9):1449–60. doi: 10.1016/j.jtho.2021.05.017
8. Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: Assessment and management. Chest (2019) 156(1):150–62. doi: 10.1016/j.chest.2019.03.033
9. Zhao J, Yorke ED, Li L, Kavanagh BD, Li XA, Das S, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: A pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys (2016) 95(5):1357–66. doi: 10.1016/j.ijrobp.2016.03.024
10. Vanpouille-Box C, Formenti SC, Demaria S. Toward precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res (2018) 24(2):259–65. doi: 10.1158/1078-0432.CCR-16-0037
11. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7
12. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-Small-Cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
13. Chen Y, Liu X, Huang Z, Zhao K, Wang Y, Ren F, et al. Safety of thoracic radiotherapy after PD-(L)1 inhibitor treatment in patients with lung cancer. Cancer Med (2021) 10(23):8518–29. doi: 10.1002/cam4.4363
14. Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: A pooled analysis of trials in the US food and drug administration database. JAMA Oncol (2022) 8(2):232–40. doi: 10.1001/jamaoncol.2021.6439
15. Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: A nonrandomized controlled trial. JAMA Oncol (2020) 6(6):848–55. doi: 10.1001/jamaoncol.2019.6731
16. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
17. Hsu PC, Li SH, Yang CT. Recurrent pneumonitis induced by atezolizumab (Anti-programmed death ligand 1) in NSCLC patients who previously experienced anti-programmed death 1 immunotherapy-related pneumonitis. J Thorac Oncol (2018) 13(11):e227–e30. doi: 10.1016/j.jtho.2018.06.022
18. Perol M. Multidisciplinary approach of immune checkpoint inhibitor-related pneumonitis: A key to address knowledge and management gaps. J Thorac Oncol (2020) 15(8):1261–4. doi: 10.1016/j.jtho.2020.05.007
19. Redelman-Sidi G, Michielin O, Cervera C, Ribi C, Aguado JM, Fernandez-Ruiz M, et al. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors). Clin Microbiol Infect (2018) 24(Suppl 2):S95–107. doi: 10.1016/j.cmi.2018.01.030
20. Morelli T, Fujita K, Redelman-Sidi G, Elkington PT. Infections due to dysregulated immunity: an emerging complication of cancer immunotherapy. Thorax (2022) 77(3):304–11. doi: 10.1136/thoraxjnl-2021-217260
21. Lin X, Lu T, Li S, Xie X, Chen X, Jiang J, et al. Cytomegalovirus infection as an underestimated trigger for checkpoint inhibitor-related pneumonitis in lung cancer: a retrospective study. Clin Transl Oncol (2021) 23(2):389–96. doi: 10.1007/s12094-020-02432-5
22. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: Incidence and risk factors. J Thorac Oncol (2018) 13(12):1930–9. doi: 10.1016/j.jtho.2018.08.2035
23. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
24. Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J For Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
25. Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol (2017) 28(suppl_4):iv119–iv42. doi: 10.1093/annonc/mdx225
26. Beattie J, Rizvi H, Fuentes P, Luo J, Schoenfeld A, Lin IH, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer (2021) 9(2):e001884. doi: 10.1136/jitc-2020-001884
27. Reynolds KL, Sullivan RJ, Fintelmann FJ, Mansour MK, England J. Case 9-2020: A 64-Year-Old man with shortness of breath, cough, and hypoxemia. N Engl J Med (2020) 382(12):1150–9. doi: 10.1056/NEJMcpc1909621
28. Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune checkpoint inhibitor therapy-related pneumonitis: Patterns and management. Radiographics (2019) 39(7):1923–37. doi: 10.1148/rg.2019190036
Keywords: immune checkpoint inhibitors (ICI), immune-related adverse event(irAE), pneumonitis, programmed cell death 1 inhibitor, cancer immunotherapy
Citation: Wang Y, Wang Y, Yu J and Meng X (2022) Case report: Pneumonia with clinical symptoms precedes imaging evidence after immune checkpoint inhibitors combined with radiotherapy in lung squamous cell cancer. Front. Immunol. 13:998516. doi: 10.3389/fimmu.2022.998516
Received: 20 July 2022; Accepted: 01 September 2022;
Published: 15 September 2022.
Edited by:
Kevin Sheng-Kai Ma, University of Pennsylvania, United StatesReviewed by:
Da-Chun Hsu, Taipei Veterans General Hospital, TaiwanCopyright © 2022 Wang, Wang, Yu and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangjiao Meng, bWVuZ3hpYW5namlhb0AxMjYuY29t; Jinming Yu, c2R5dWppbm1pbmdAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.