- Department of Aquatic Animal Medicine, College of Fisheries, Huazhong Agricultural University, Wuhan, China
Cottonseed protein concentrate (CPC) has been proven to partially replace fishmeal without adverse effects on fish growth performance, while little information is known about the effects on liver health during bacterial infection. In the present study, 15% CPC was included into the diet of juvenile largemouth bass (32.12 ± 0.09g) to replace fishmeal for 8 weeks, with fish growth potential and hepatic inflammatory responses during Nocardia seriolae (N. seriolae) infection systemically evaluated. After adaptation to dietary CPC inclusion, largemouth bass even exhibited better growth potential with higher SGR and WGR during the last three weeks of whole feeding trial, which was accompanied with higher phosphorylation level of TOR signaling and higher mRNA expression level of myogenin (myog). At the end of 8-weeks feeding trial, the histological structure of largemouth bass liver was not significantly affected by dietary CPC inclusion, accompanied with the similar expression level of genes involved in innate and adaptive immunity and comparable abundance of T cells in bass liver. N.seriolae infection induced the pathological changes of bass liver, while such hepatic changes were more serious in CPC group than that in FM group. Additionally, RT-qPCR results also suggested that largemouth bass fed with CPC experienced much higher inflammatory potential both in liver and gill during N. seriolae infection, which was accompanied with higher expression level of genes involved in pyroptosis. Therefore, this study demonstrated that the application of CPC in largemouth bass diet should be careful, which may induce higher inflammatory potential during N. seriolae infection.
Introduction
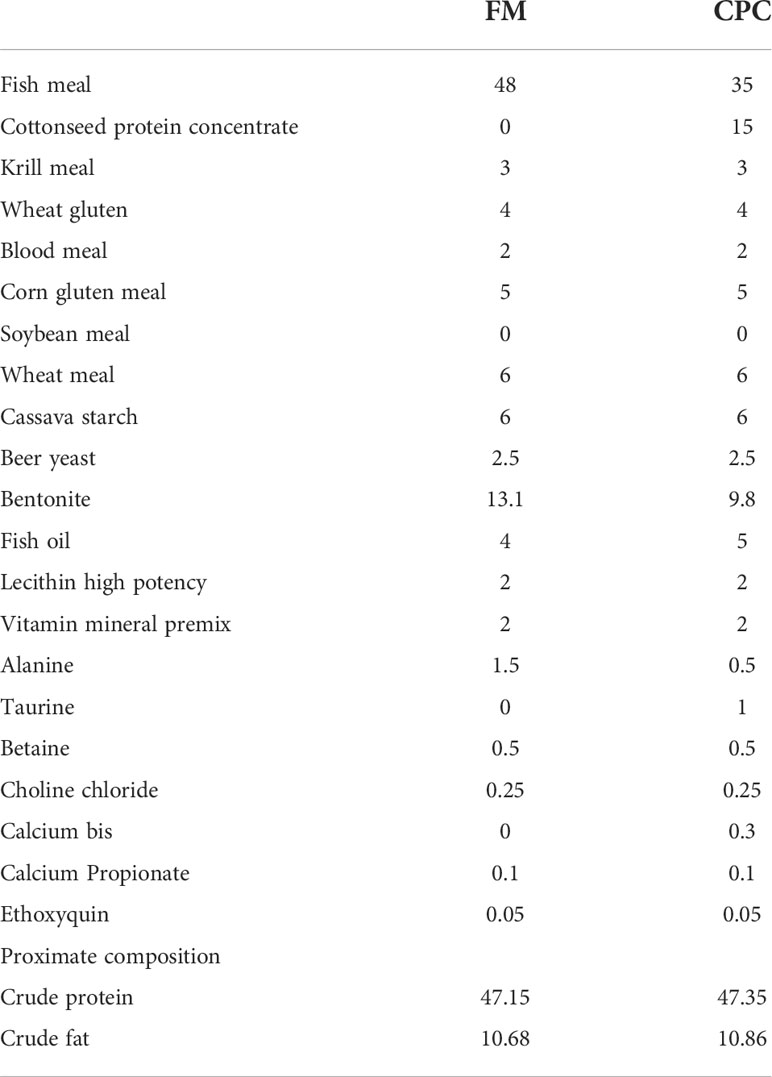
Aquaculture supplies plenty of high protein food for human beings and has become the fastest animal-protein producing industry among all animal husbandry in the past 30 years (1). Feed expenses have always been the main cost for fish farmers, and fish meal has been traditionally regarded as the highest quality protein raw material for aquafeed due to its high-quality amino acid ratio, good palatability and perfect digestibility (2). However, global fishmeal production cannot reach a stable increase like the aquaculture production and the fishmeal price has significantly increased year by year (3). It is important to search for alternative protein sources to reduce fishmeal utilization in fish diet, and soybean meal has been widely used in advanced aquafeed because of its high protein content and balanced amino acid composition (4). In China and other main aquaculture countries, the soybean self-production capacity is relatively weak and the global soybean application has been pledged to be restricted which are in the expense of deforestation. Thus, it has become an industry consensus to develop new raw materials and reduce the use of fish meal and soybean meal in aquafeed. Many protein sources have also been tested in fish diet, including rapeseed meal, peanut meal and many others (5). However, the main constraint for these alternative sources remains in their much lower production than soybean meal. Cotton is one of the most important crops in the world, with large output but low production cost, which makes the price of cotton products relatively low (6). During the process of cotton production, a large number of by-products such as cottonseed can be produced, and developing them as feed protein raw materials not only has important economic value, but also has important ecological significance for reducing carbon emissions. With cottonseed as the basic raw material, cottonseed protein concentrate (CPC) is produced through low-temperature oil extraction, removal of gossypol and other process stages, based on the “liquid-liquid-solid” extraction technology production processes. The crude protein level of CPC could reach up to 60%, which is rich in arginine, leucine, phenylalanine and other essential fish amino acids. Additionally, some water-soluble non-starch polysaccharides, oligosaccharides, and anti-nutritional factors such as phenol and tannin has been removed to significantly improve its nutritional value and palatability (7). CPC has been proved to effectively replace partial FM without adverse effects on growth of silver sillago (Sillago sihama), golden pompano (Trachinotus ovatus), hybrid grouper (Mycteroperca tigris × Epinephelus lanceolatus) (8), red drum (Sciaenops ocellatus) (9), and rainbow trout (Oncorhynchus mykiss) (10).
With the urbanization, the demand for high-nutritional value and bone-less fish species is significantly increased in many countries including China (11). Largemouth bass (Micropterus salmoides), a carnivorous fish species originating from Canada and America, has been widely cultured all over the world. Ever since it was introduced to China in 1980s, the aquaculture production of largemouth bass has significantly increased year by year and has reached 619, 519 tons in 2020 (12). During aquaculture, the liver health of largemouth bass has received much attention. Liver structure was reported to be significantly affected by dietary glucose level (13). Additionally, N. seriolae infection, which is rather common in largemouth bass aquaculture, also caused serious hepatic damage (14). In fact, liver receives dual blood supply from the portal vein and hepatic artery, and both acts as the metabolic center and performs immune-regulatory roles (15). The liver is constantly bombarded by a stream of dietary and commensal bacterial products with inflammatory potential even in healthy individuals, which results in persistent, regulated inflammation (16). Such inflammation creates the potential for excessive immune activation during challenge by pathogens or tissue damage, which could be resolved during appropriate immune activation to maintain liver homeostasis. Failure to clear ‘dangerous’ stimuli or regulate appropriately activated immune mechanisms leads to chronic pathological inflammation and disrupted tissue homeostasis characterized by the progressive development of fibrosis, cirrhosis and eventual liver failure (17). Especially, during fishmeal replacement by plant proteins, the existing anti-nutritional factors in plant sources would be transferred to liver, which may cause continual inflammatory stimulus to liver and even immune fatigue in liver. When fish was exposed to pathogenic infection, the chronic pathological inflammation may occur which results in the liver failure. In largemouth bass, CPC has been reported to replace fishmeal at different ratios in different studies without adverse effects on fish growth performance (18–20). However, the immune status and tissue health have been rarely evaluated, especially the immune status during bacterial challenge.
As mentioned above, N. seriolae infection seriously affected the health of largemouth bass and resulted in the damage of internal organs including liver and eventual high mortality (14). N. seriolae infection has been reported to induce high inflammatory responses in bass liver (21) while little information is known about the metabolic control of such inflammatory responses in liver. Considering the high production of CPC and its application potential in diet for largemouth bass, it is important to study the influences of CPC inclusion on liver structure and function, especially the inflammatory responses during bacterial infection. Thus, the influences of dietary cottonseed protein concentrate replacing fishmeal on growth potential, liver health status and challenge resistance of largemouth bass (Micropterus salmoides) were systemically evaluated in the present study. To our acknowledge, this is the first study to evaluate the effects of CPC on fish hepatic inflammatory responses during nocardiosis.
Materials and methods
Ethics statement
All the fish care and experimental procedures were approved by the Animal Experiment Committee of Huazhong Agricultural University (permit number HZAUFI-2016-007).
Feed formulation, fish husbandry and sampling
Two isoproteic (50%) and isolipidic (12.5%) diets were formulated based on fish meal (FM) or cottonseed protein concentrate (CPC), respectively (Table 1). In order to balance the amino acid composition, krill meal, blood cells, wheat gluten and corn gluten meal were also included in each diet. Attractant (betaine) and taurine were also added to improve the food palatability.
Juvenile largemouth bass were acclimated to our laboratory conditions and experimental feeds for two weeks before being randomly distributed into fibre-glass tanks of 400-L capacity. Experiment was done in wet lab of College of Fisheries, Huazhong Agricultural University. Fish with initial weight of 32.12 ± 0.09 g were distributed into 6 experimental tanks (400-L) which were connected to a circulating water system. The water parameters in circulating water system were kept constant with water flow at 0.5 L/min, water temperature at 26 ± 2 °C, and oxygen content of outlet water at over 85% saturation. Day length and dark ranged over the course of the trial following natural changes. Each diet was randomly allocated to triplicate groups of fish for 8 weeks. Feed was offered by hand to apparent satiety twice per day, with feed consumption was recorded. After 2 weeks, 5 weeks and 8 weeks, all fish were counted and group-weighed under moderate anaesthesia (3-aminobenzoic acid ethyl ester, MS 222; 100 μg/mL) for growth parameters calculation.
At the end of the feeding trial, total fish number and body weight in each tank were counted and measured after being anesthetized by MS222, respectively. Blood samples were collected from the caudal vessels of 9 fish from each group with heparinized syringe. Then fish were perfused with PBS to discard the inference of blood on liver and gill immune responses. Liver and gill tissues were separated, with partial segments stored in paraformaldehyde. Other tissue samples (serum, gill, liver and others) were immediately frozen in liquid nitrogen and then stored at -80°C for further assay.
Bacterial challenge
N. seriolae strain HG2101, was isolated from an infected largemouth bass and preserved in our laboratory. The whole genome sequence has been sequenced by our laboratory to confirm the accurate (unpublished). Before the formal experiment, a preliminary study was conducted to determine the LD50 of this bacterial strain in largemouth bass. Thus the infection concentration in the formal experiment was finally determined as 1.0 × 107 CFU/mL. Before bacterial challenge experiment, the N. seriolae was taken out from −80°C refrigerator and recovered on plate medium at 28°C. Then the single colony was cultured in a tryptic soy broth soybean-casein digest medium (pancreatic digest of casein 17.0 g L−1, papaic digest of soybean 3.0 g L−1, dextrose 2.5 g L−1, sodium chloride 5.0 g L−1, dipotassium phosphate 2.5 g L−1) in a shaker incubator at 180 rpm at 28°C. The colony forming units per milliliter (CFU/mL) in the final culture were measured by plate counting under a microscope. Bacterial suspensions at 1.0 × 107 CFU/mL were prepared in normal saline.
For the challenge experiment, largemouth bass in all groups were firstly anaesthetized with MS 222 and then artificially injected with the N. seriolae (0.10 mL) by intraperitoneal injection. After challenge experiment, all fish was transferred back to each tank with the same water conditions. Fish samples were obtained from FM and CPC group at 0 day, 1 day, and 3 days post infection (dpi). Fish sampling methods were similar to previous in 2.3.
Histological assay
The liver and gill tissues of largemouth bass in two groups at different time points post infection were fixed with polyformaldehyde. After fixing, the tissues were transfered into gradient alcohol for dehydration, xylene for transparent, and then paraffin for section slicing. The obtained 5-μm sections of liver tissues were then used for H.&E. staining, following the producers’ introduction. The stained slides were examined under a light microscope (Olympus, DP72) equipped with a camera (Nikon E600) and CellSens Standard Software (Olympus) for image acquisition.
TUNEL assay
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was conducted to precisely detect cells experiencing programmed cell death (PCD) with the one-step TUNEL Assay kit (Beyotime, China) following the manufacturer’s instructions. Briefly, tissue sections were twice dewaxed in xylene for 5-10 minutes. Then tissues were immersed into anhydrous ethanol for 5 minutes, 90% ethanol for 2 minutes, 70% ethanol for 2 minutes, and distilled water for 2 minutes. Tissues were treated with DNase-free proteinase K (Beyotime, 20μg/mL) at room temperature for 15-30 minutes and then washed with PBS for three times to remove excess proteinase K. Afterwards, the labelling solution containing terminal deoxynucleotidyl transferase, buffer, and fluorescein dUTP was added to tissues for labelling reaction at 37 °C for 60 minutes in a humidity chamber. Following incubation, excess labelling solution was washed off smears with PBS, and then cell smears were mounted with fluorescent microscopy mounting solution. Images were captured and analyzed using a CCD camera (Olympus, Japan).
RNA extraction and RT-qPCR analysis
Total RNA samples were extracted from liver and gill tissues using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. The purity and concentration of RNA were detected with Nanodrop 2000 spectrophotometer (Thermo scientific, USA), accompanied with the integrity of RNA checked by 1.0% agarose gel electrophoresis. One microgram of the resulting total RNA was reverse transcribed into cDNA using the SuperScript III RNaseH-Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA) and oligo dT primers (Promega, Charbonnie `res, France) according to the manufacturers’ instructions.
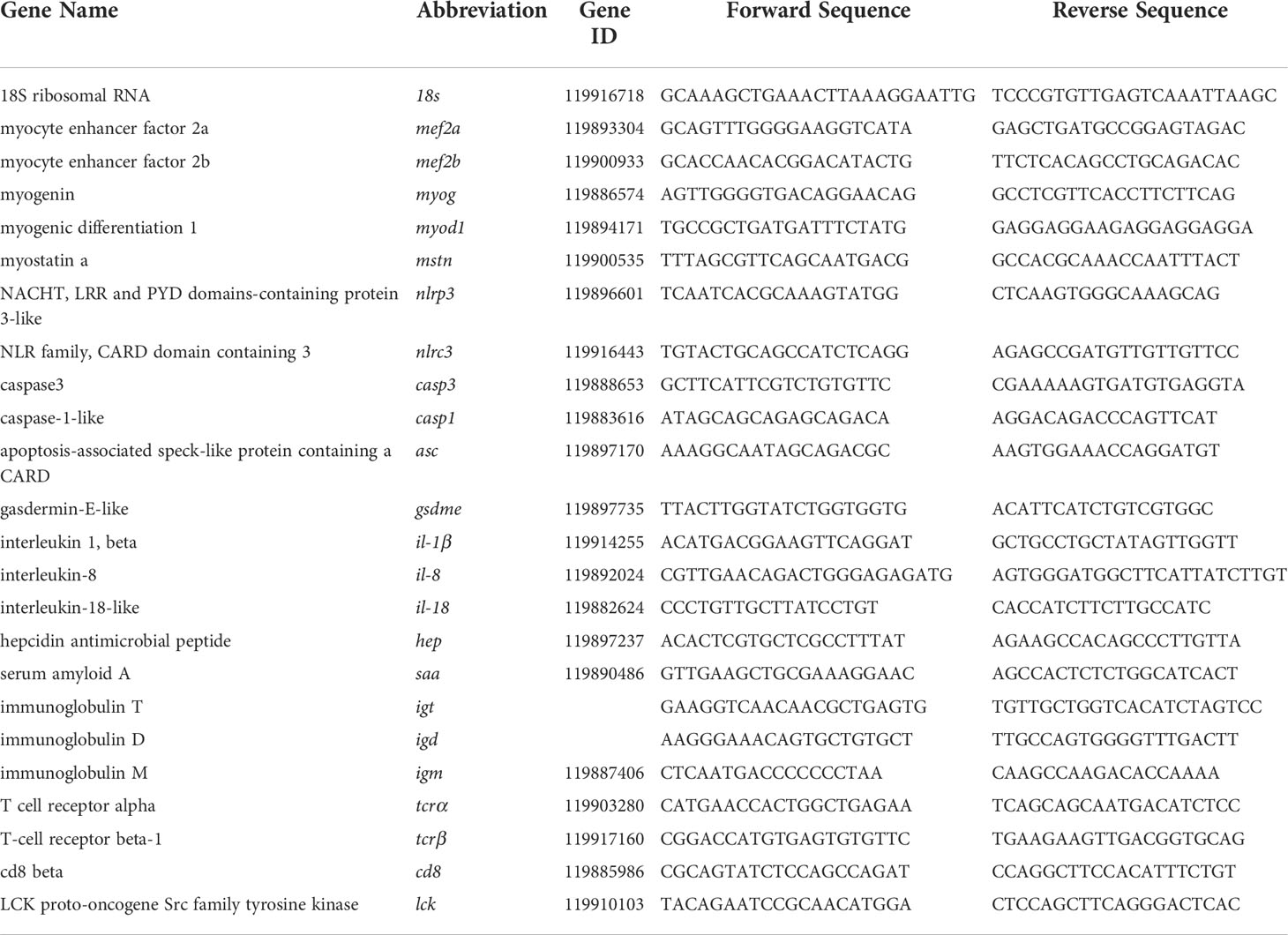
Quantitative RT-PCR was carried out on an iCycler iQTM real-time PCR detection system (BIO-RAD, Hercules, CA, USA) using the Eva Green 2 × qPCR Master mix (ABM, Canada). The program was as follows: 95 °C for 5min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. In addition, a melt curve analysis was performed after amplification to verify the accuracy of each amplicon. 18S was employed as a non-regulated reference gene and no changes in 18S gene expression were observed in our investigations (data not shown). The relative quantification of the target genes was determined via the comparative CT method (2-ΔΔCt method). Primer used in the present study was shown in Table 2.
Western blotting analysis
Proteins from liver homogenates were separated on SDS-PAGE. For each assay, all samples were analyzed at the same time on four-wide gels to eliminate inter-assay variation. Proteins were electrophoretically transferred to polyvinylidene difluoride transfer membranes (Pall Corporation), which were incubated with appropriate primary antibodies, washed and exposed to an appropriate secondary antibody. Blots were developed using an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology) and visualized. Primary antibodies for Phospho-S6 (Ser235/236) (cat # 4856) and S6 (cat # 2217) used in the present study were purchased from Cell Signaling Technology (U.S.A.) and primary antibody for β-actin (AC004) was purchased from ABlonal Biotechnology (China).
Immunofluorescent assay
In order to detect T cells, liver paraffin sections were stained with Alexa Fluor 647 anti-Lck (pAb, 1: 900) overnight at 4°C. After washing four times, all sections were stained with DAPI (4′,6-diamidino-2-phenylindole, 1 μg/mL, Invitrogen) before mounting. Images were captured and analyzed using a CCD camera (Olympus, Japan).
Calculations and statistical analysis
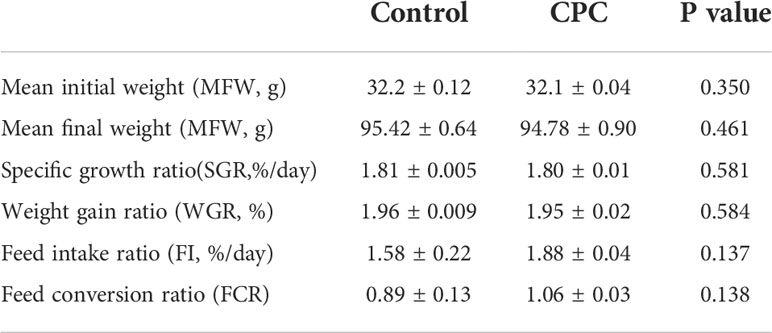
Mean final weight (MFW, g) = total final weight/fish number; Weight gain ratio (WGR, %) = 100*(final body weight – initial body weight)/initial body weight; Specific growth rate (SGR, %/day) = 100* (ln final body weight − ln initial body weight)/days; Feed intake ratio (FI, %/day) = 100 *dry feed intake/[(initial body weight + final body weight)/2]/days; Feed conversion ratio (FCR) = dry feed intake/(final body weight – initial body weight).
All statistical analyses were performed using SPSS 17.0. Data about fish growth performance, feed utilization and western blotting were analyzed using student’s t-test. All gene-expression results were tested for normality using the Shapiro Wilk’s-W’s test, and normally distributed data were analyzed by Factorial (two-way) analysis of variance (ANOVA) to determine the main effects of period and group, and their interactions on gene expression. When significant interaction of period and group were observed, data were analyzed by one-way ANOVA followed by Tukey’s multiple range tests to inspect differences among all the treatments. When only a significant interaction and significant main effects of period or group were observed, data were analyzed by one-way ANOVA followed by Tukey’s multiple range tests to inspect differences among periods within each group and vice versa. When the significance is only with the main effects of period or group, the data were analyzed by the two-way ANOVA followed by Tukey’s multiple range tests to assess the main effects of period or group only. Differences were considered significant when P < 0.05. All data were expressed as mean ± standard deviation of the mean (SD), except the specific statement.
Results
Largemouth bass growth performance and feed utilization were not affected after CPC replacing dietary fishmeal for 8 weeks
The growth performance of juvenile largemouth bass after 8-weeks feeding trial in the present study is shown in Table 3. Mean final weight (MFW) of juvenile largemouth bass in two groups could reach 95.42 ± 0.64 and 94.78 ± 0.90, respectively, which showed no significant difference between two groups (P>0.05). Accordingly, the weight gain ratio (WGR) and specific growth ratio (SGR) of bass were also around 1.96 and 1.80 in two groups, respectively. Moreover, the feed intake ratio (FI) also reached 0.23 ± 0.03 and 0.28 ± 0.06, which difference was also not significant (P>0.05). Feed conversion ratio was 0.89 ± 0.13 and 1.06 ± 0.03 for FM and CPC, which were not significantly different (P>0.05). Thus, the growth performance and feed utilization of largemouth bass was not significantly affected after CPC replacing dietary fishmeal for an 8-weeks feeding period.
Largemouth bass exhibited better growth potential after adaptation to dietary CPC
In order to have a more systematic overlook of bass growth performance with two diets, the growth parameters were separately evaluated from 0-2 weeks, 3-5 weeks and 6-8 weeks (Figure 1A). In the first two weeks, juvenile largemouth bass in FM group exhibited better MFW, WGR and SGR than bass in CPC group. However, there were no significant differences in WGR and SGR between two groups (P>0.05) during the period from 3rd weeks to 5th weeks. Furthermore, both WGR and SGR of bass in CPC group was even significantly higher than that in FM group (P<0.05) in the last 3 weeks, and MFW in two groups showed no significant difference (P>0.05). Thus, largemouth bass exhibited adaptation to dietary CPC and then showed better growth potential in the prolonged rearing period.
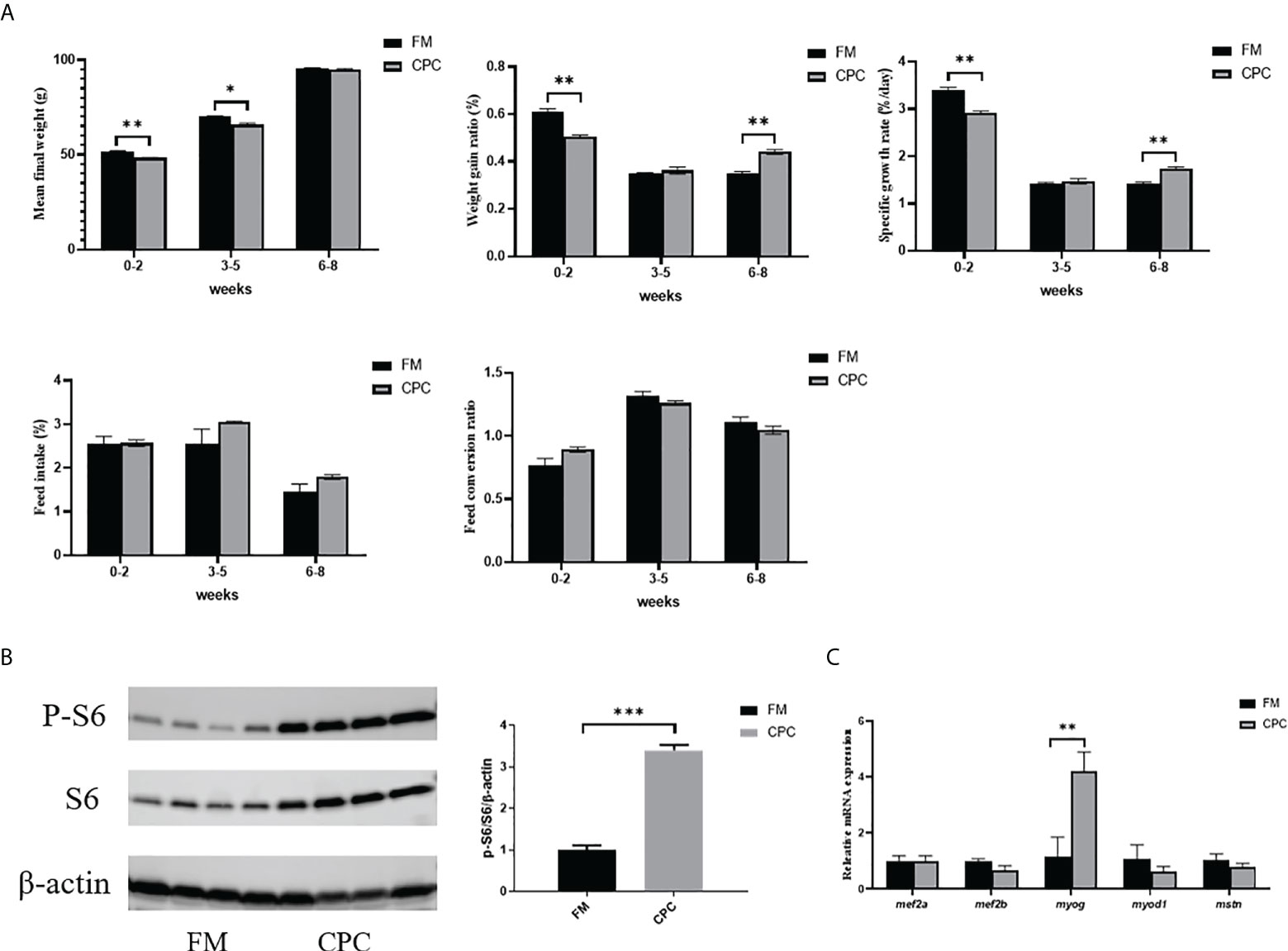
Figure 1 Largemouth bass exhibited better growth potential after adaptation to dietary CPC. (A) The growth parameters including mean final weight (MFW, g), weight gain ratio (WGR, %), specific growth ratio (SGR, %/day), feed intake ratio (FI, %/day) and feed conversion ratio (FCR) of juvenile largemouth bass fed with FM and CPC in 0-2 weeks, 3-5weeks and 6-8 weeks. (B) The relative protein level of S6 and P-S6 in liver of juvenile largemouth bass fed with FM and CPC. (C) The relative mRNA expression levels of genes involved in muscle formation (mef2a, mef2b, myog, myod1, mstn) in the liver of juvenile largemouth bass in both FM group and CPC group. All data was analyzed with student’s t-test and data are means ± SD (n = 6). *P< 0.05; **P< 0.01; ***P< 0.001.
In order to confirm the growth potential at 8 weeks after adaptation to dietary CPC, the activation status of TOR signaling pathway was evaluated via western blotting analysis. As shown in Figure 1B, the phosphorylation level of S6 was significantly higher in bass liver of CPC group than that of FM group (P<0.05). Additionally, the relative mRNA expression level of genes involved in myogenesis were also examined by RT-qPCR. As shown in Figure 1C, the expression of myocyte enhancer factor 2a (mef2a), myocyte enhancer factor 2b (mef2b), myostatin a (mstn), and myogenic differentiation 1 (myod1) showed no significant difference between two groups (P>0.05), while the expression of myogenin (myog) in bass liver of CPC group was also significantly higher than that of FM group (P<0.05).
Largemouth bass liver structure along with the programmed cell death was not significantly affected after dietary CPC replacing fishmeal
The liver structure of bass in two groups was evaluated via H.&E. staining (Figure 2A). As shown, the bass liver exhibited cuboidal hepatocytes with rounded nuclei which are arranged in cords and supported by reticulated fibers and connective tissue. The sinusoidal gaps between the cords are also known as hepatic blood sinuses (S), which are the sites of blood filtration into the liver. Moreover, veins (V) and bile ducts (B) are also detected to be accompanied with each other while arterial (A) features were rarely observed. No significant differences were detected in liver interior (left), hepatic blood sinuses (middle) and edge (right) of juvenile largemouth bass in FM and CPC group.
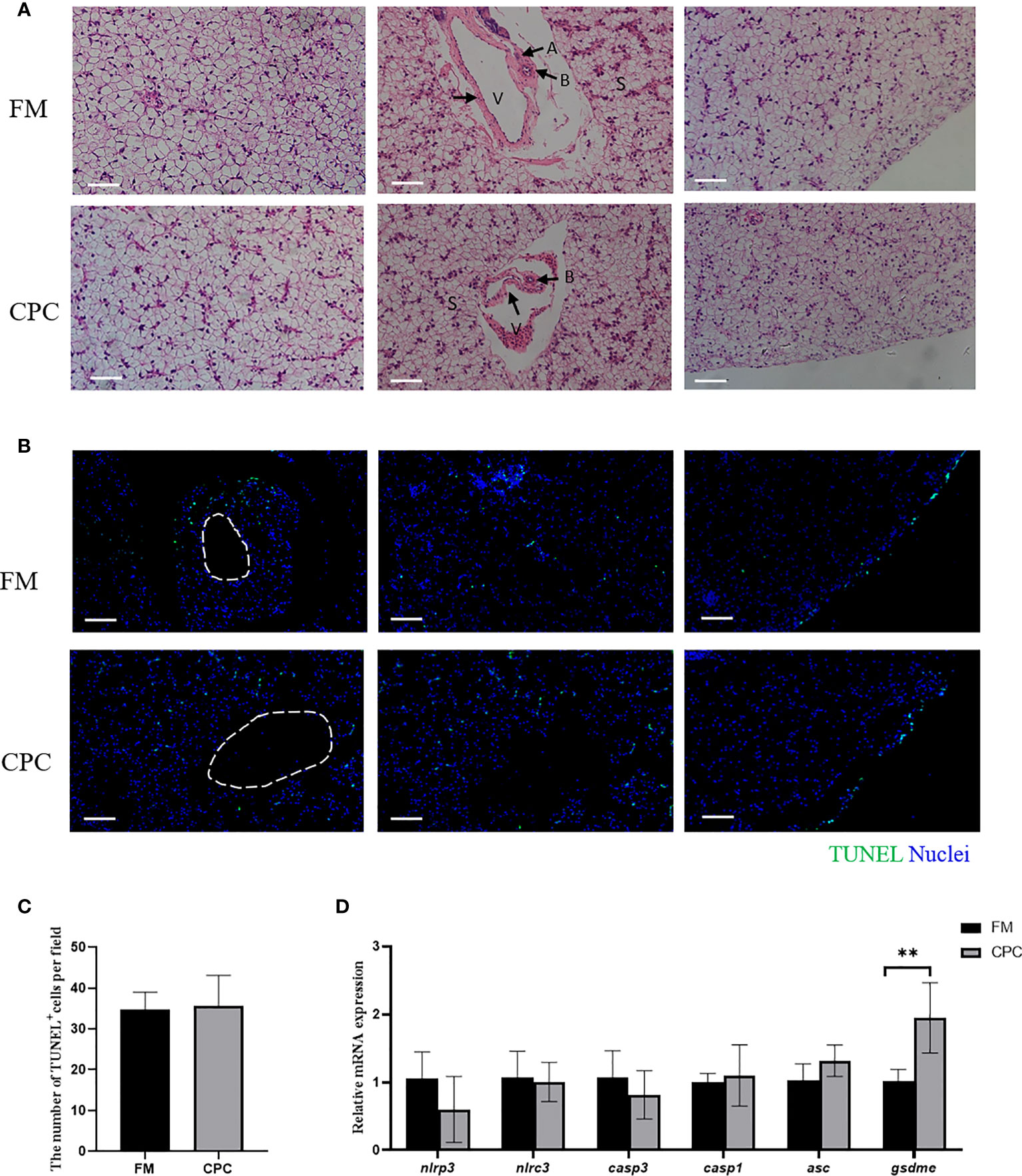
Figure 2 Largemouth bass liver structure along with the programmed cell death was not significantly affected after dietary CPC replacing fishmeal. (A) The H.E. staining results of juvenile largemouth bass liver fed with FM and CPC for 8 weeks. V: veins; B: bile ducts; A: arterial; S: sinusoid. Scale bars, 50μm. (B) TUNEL assay with immunofluorescence microscope on liver tissue to detect the cells experiencing programmed cell death. Scale bars, 100μm. (C) The calculated number of TUNEL+ cells in liver of two groups. (D) The relative mRNA expression levels of genes involved in apoptosis and pyroptosis (nlrp3, nlrc3, casp1, casp3, asc and gsdme) in the liver of juvenile largemouth bass in both FM group and CPC group. All data was analyzed with student’s t-test and data are means ± SD (n = 6). *P< 0.05; **P< 0.01; ***P< 0.001.
Moreover, the programmed cell death (PCD) including apoptosis and pyroptosis in bass liver was also detected by TUNEL. As shown in Figure 2B, TUNEL+ cells could be mainly detected at the edge of the liver tissue in juvenile largemouth bass of two groups. Additionally, few cells experiencing PCD could be detected in the interior of liver tissue. Statistical analysis showed that the number of TUNEL+ cells in two groups were not significantly different (P>0.05) (Figure 2C). Additionally, the mRNA expression levels of genes involved in apoptosis and pyroptosis were also evaluated in bass liver of two groups. As shown in Figure 2D, the expression of NACHT, LRR and PYD domains-containing protein 3-like (nlrp3), NLR family CARD domain containing 3 (nlrc3), caspase-1-like (casp1), caspase-3-like (casp3) and apoptosis-associated speck-like protein containing a CARD (asc) showed no significant difference in two groups (P>0.05). However, the expression of gasdermin-E-like (gsdme) in CPC group was significantly higher than that in FM group (P<0.05).
Largemouth bass hepatic immunity was not significantly affected after CPC replacing fishmeal
The expression of genes involved in the innate immunity of largemouth bass liver in two groups were evaluated by RT-qPCR. As shown in Figure 3A, the expression of interleukins including interleukin-1beta (il-1β), interleukin-18-like (il-18), and interleukin-8 (il-8) showed no significant differencein bass liver of two groups (P>0.05). Similarly, the expression of hepcidin antimicrobial peptide (hep) was also not significantly affected by dietary CPC inclusion (P>0.05). However, dietary CPC inclusion significantly increased the expression of serum amyloid A (saa) (P<0.05).
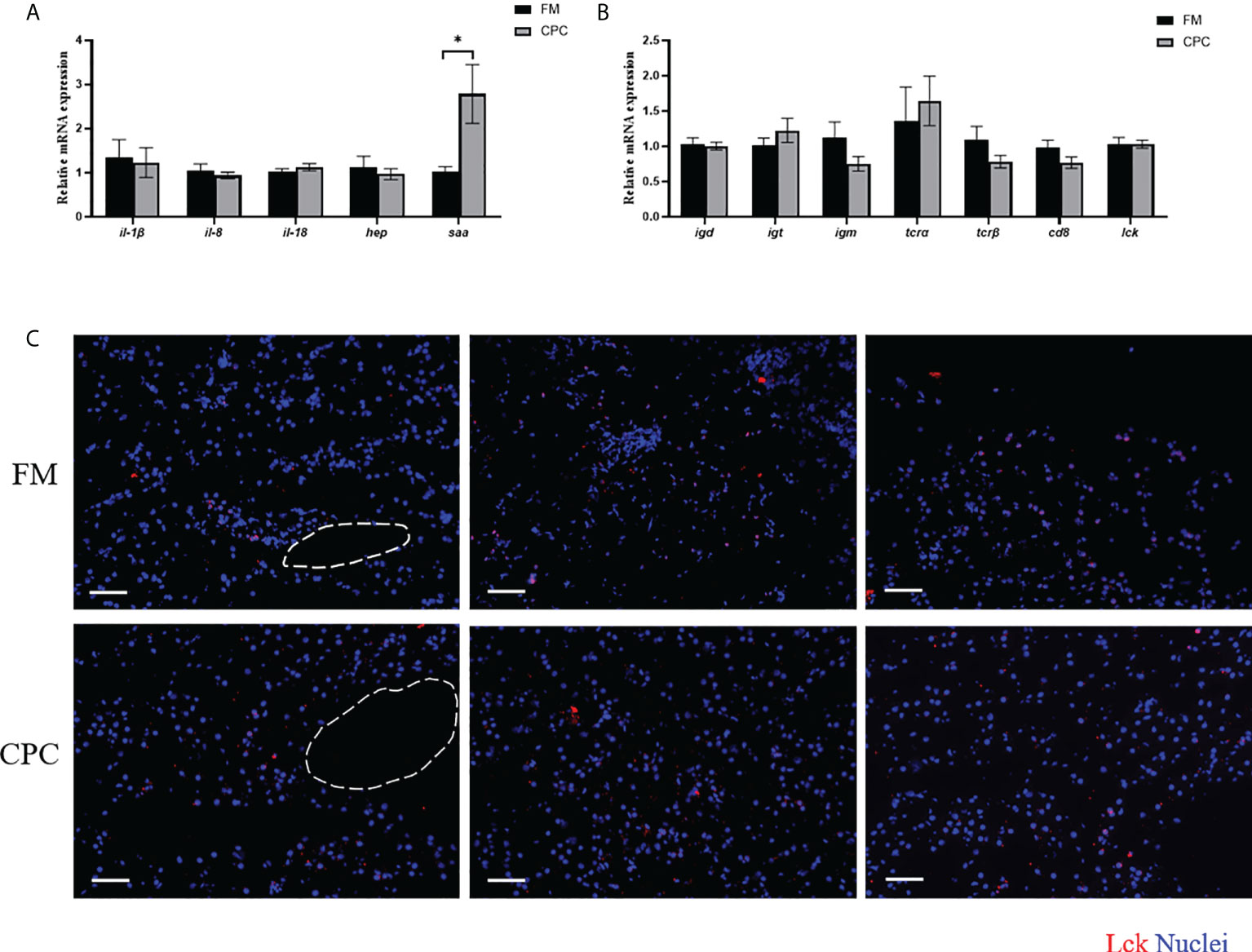
Figure 3 Largemouth bass hepatic immunity was not significantly affected after CPC replacing fishmeal. (A) The relative mRNA expression levels of genes involved in innate immunity (il-1β, il-18, il-8, hep and saa) in the liver of juvenile largemouth bass in both FM group and CPC group. (B) The relative mRNA expression levels of genes involved in adaptive immunity (igm, igd, igt, tcrα, tcrβ, lck, and cd8) in the liver of juvenile largemouth bass in both FM group and CPC group. (C) Immunofluorence results of T cells in liver of juvenile largemouth bass in both FM group and CPC group with antibody against Lck. Scale bars, 100μm. All data was analyzed with student’s t-test and data are means ± SD (n = 6). *P< 0.05; **P< 0.01; ***P< 0.001.
Meanwhile, the adaptive immunity in bass liver was also evaluated via both detecting the expression of related genes and the relative abundance of T cells. As shown in Figure 3B, the expression of immunoglobulins including immunoglobulin M (igm), igd, and igt showed no significant difference in largemouth bass liver of two groups (P>0.05). Similarly, the expression levels of T cell receptor alpha (tcrα), T-cell receptor beta-1 (tcrβ), LCK proto-oncogene Src family tyrosine kinase (lck), and cd8 beta (cd8) were also not significantly (P>0.05) affected by dietary CPC inclusion. Moreover, the relative abundance of T cells in bass liver were also determined using immunofluorescent assay with antibody against Lck, a cytoplasmic protein bound to CD4 and CD8 in T lymphocytes. As shown in Figure 3C, Lck+ cells could be detected in bass liver tissue of two groups, which are diffusely distributed in the hepatic sinusoids. The number of Lck+ B cells in bass liver showed no significant difference between two groups (P>0.05).
Higher inflammatory responses were induced in both liver and gill of largemouth bass fed with CPC after N. seriolae infection
N. seriolae infection induced serious pathological changes in liver of largemouth bass, which were detected via H.&E. staining. As shown in Figure 4A, lipogranulomas (black dotted circles) composed of a mixture of lipid droplets (blue arrows), macrophages (black arrows), and lymphocytes (yellow arrows) could be detected in liver of FM and CPC group at 1 dpi. Meanwhile, the number of inflammatory cells (black triangles) in liver of CPC group were more than that in FM group, accompanied with the irregularly arranged cells and ruptured membranes (white dotted circles) in some area. At 3 dpi, the pathological changes were more serious, with chaotic arrangement of hepatocytes and aggravated inflammatory response. Accordingly, a large number of eosinophils infiltrating in liver tissue could be detected in CPC group, which is more serious than that in FM group. Severe cellular vacuolar degeneration (black circles) could also be detected in the CPC group. Moreover, the expression of genes involved in inflammatory responses and pyroptosis in liver were also evaluated via RT-qPCR. As shown in Figure 4B, N. seriolae infection induced the upregulated expression of il-1β in FM group at 3 dpi, while CPC group induced earlier upregulation of il-1β expression at 1 dpi (P<0.05). Moreover, N. seriolae infection induced the upregulated expression of il-18 in liver of CPC group at 1 dpi and 3 dpi, whose expression was significantly higher than that of FM group (P<0.05). Accordingly, the expression of casp1 and casp3 at 1 dpi was also significantly higher in liver of CPC group than that of FM group. Similarly, the expression of gsdme at both 0 dpi and 1 dpi was also higher in liver of CPC group than that of FM group (P<0.05). Thus, dietary CPC inclusion may trigger high inflammatory potential in bass liver during N. seriolae infection, which is in accordance with the histological structure.
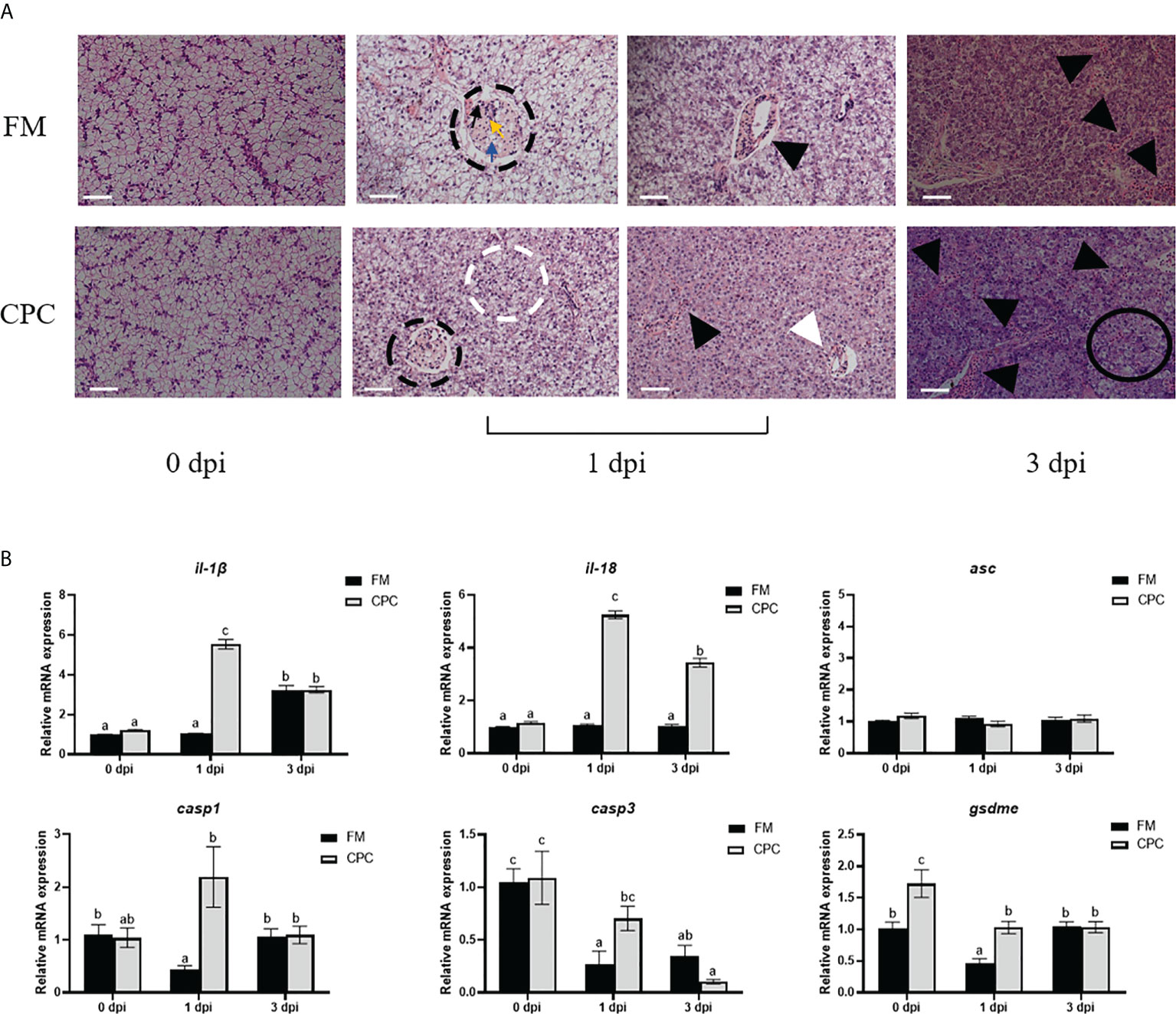
Figure 4 Higher inflammatory responses were induced in liver of largemouth bass fed with CPC after N. seriolae infection. (A) The H.E. staining results of juvenile largemouth bass liver fed with FM and CPC at different days post N. seriolae infection (dpi). Blue arrows: lipid droplets; black arrows: macrophages; yellow arrows: lymphocytes; black dotted circles: lipogranulomas; white dotted circles: ruptured cell membranes; white triangles: lymphocytic infiltration; black triangles: inflammatory cells; black circles:cell vacuolar degeneration. Scale bars, 50μm. (B) The relative mRNA expression levels of genes involved in inflammation and pyroptosis (il-1β, il-18, asc, casp1, casp3 and gsdme) in the liver of largemouth bass fed with FM and CPC at different days post N. seriolae infection (dpi). All data was analyzed with two-way ANOVA and data are means ± SD (n = 6). Mean values with different letters indicated significant difference among groups, P < 0.05.
In order to further confirm the pro-inflammatory effects of CPC during bacterial infection, the histological structures and expression of genes involved in inflammatory responses of gill were also evaluated (Figure 5). As shown in Figure 5A, N. seriolae infection induced serious histological structural changes in gill, as the primary gill lamella (PL) thickened, which could be detected in two groups. Statistical analysis indicated that the width of PL was significantly higher in CPC group than that in FM group at 1dpi and 3 dpi (P<0.05). Meanwhile, the secondary gill lamella (SL) turned to be short and bending after infection in both groups. The ratio of length to width of SL in CPC group was significantly higher than that in FM group before infection, but turned to be much lower in CPC group at both 1 dpi and 3 dpi (P<0.05). Moreover, N. seriolae infection also induced the upregulated expression of il-1β and casp1 in the gill of largemouth bass in FM group (Figure 5B). The expression of il-18 and il-1β in the gill of CPC group was even significantly higher than that of FM group at 3 dpi, while CPC group also exhibited higher expression of il-18 at 0 dpi (P<0.05). The expression of casp1 and asc in the gill were also significantly higher in CPC group than FM group at both 1 dpi and 3 dpi. Accordingly, the expression of casp3 and gsdme in the gill were also significantly higher in CPC group than FM group at 3 dpi (P<0.05).
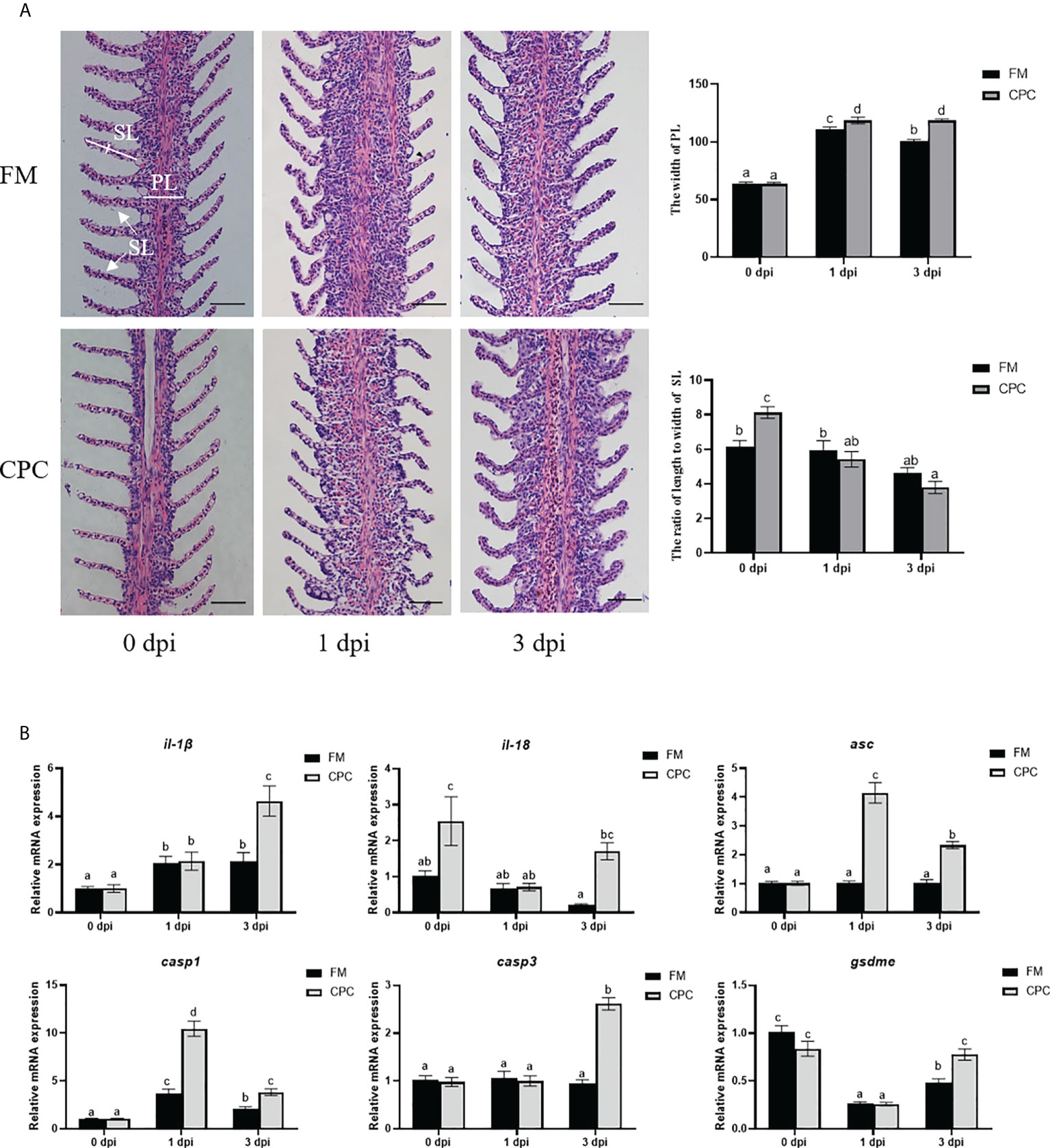
Figure 5 Higher inflammatory responses were induced in gill of largemouth bass fed with CPC after N. seriolae infection. (A) The H.E. staining results of juvenile largemouth bass gill fed with FM and CPC at different days post N. seriolae infection (dpi). PL: primary gill lamella; SL: secondary gill lamella. Scale bars, 50μm. (B) The relative mRNA expression levels of genes involved in inflammation and pyroptosis (il-1β, il-18, asc, casp1, casp3 and gsdme) in the gill of largemouth bass fed with FM and CPC at different days post N. seriolae infection (dpi). All data was analyzed with two-way ANOVA and data are means ± SD (n = 6). Mean values with different letters indicated significant difference among groups, P < 0.05.
Discussion
Cottonseed is a sustainable source of plant protein, producing ~10 million metric tons of protein globally (22). In aquaculture practice, cottonseed meal has been widely tested in many fish species, while the existing gossypol seriously restricted its application (23). Reducing the gossypol content to permissible limits (450 ppm, World Health Organization) via physical, chemical and biological methods makes cottonseed a good candidate for use in food and feed formulations, with a balanced amino acid composition and functional properties comparable to those of soy protein (24). Recently, the new product, CPC, has been proved to effectively replace partial FM without adverse effects on growth of several aquatic animal species (10, 25). Our present study illustrated the dietary inclusion of CPC (15%) in largemouth bass diet without affecting fish growth performance when fishmeal decreased from 48% to 35% (Table 3). Early studies have also tested the effects of CPC replacing fishmeal in largemouth diet, while the replacing level varied in different studies (18–20). Considering the basic fishmeal level and other protein sources varied in different studies, it will be more convincible to use the real inclusion level of CPC in fish diet. For example, He et al. (18) reported that CPC inclusion of up to 13% showed no adverse effects on weight gain, specific growth rate and protein efficiency ratio of largemouth bass, while Xie et al. (19) also reported that CPC inclusion (23.5%) even promoted largemouth bass growth performance. Another study reported that CPC inclusion level (no more than 36.23%) could replace fishmeal in bass diet (initial body weight of 95.3g) without compromising the growth, flesh composition, texture, flavor characteristics and antioxidant capacity (20). Such phenomenon is rather common in many studies of fishmeal replacement by other protein sources. For example, in hybrid grouper (Mycteroperca tigris × Epinephelus lanceolatus), dietary CPC could replace fishmeal of up to 60% without significantly altering the growth performance but adversely influenced fish intestinal immune responses and microbial profiles (26), while the same research group showed that dietary CPC inclusion at low levels (15.76% and 18.18%) even promote hybrid grouper growth performance (27). One important question remains why results varied in the different reports. It has been speculated that differential results may originate from the varied fish growth period, the different basic diet formula, and different rearing environment. Here, in order to explain the question, the growth parameters in different feeding period were systemically evaluated. As shown in Figure 1A, bass in CPC group showed lower WGR and SGR than that in FM group in the first two weeks, while no difference was detected between two groups in the next 3weeks. However, both WGR and SGR of bass in CPC group was even significantly higher than that in FM group in the last 3 weeks (Figure 1A), which suggested that largemouth bass may have adapted to CPC diet and exhibited better growth potential at the end of 8 weeks feeding trial. A recent study reported that largemouth bass even showed adaptation of dietary glucose after 8 weeks feeding trial (28). Former studies in rainbow trout have also showed that rainbow trout can be adapted and even trained to plant-sourced proteins and exhibited good performance after several generations of selection (29), which all supported our hypothesis. Additionally, our previous studies have proved that TOR functions as the central regulator of fish growth and nutrient metabolism, and the activation status could be selected as the marker of fish growth potential (30). For example, the inhibition of TOR by rapamycin significantly resulted in the decreased fish body weight, while the adverse growth performance during fishmeal replacement by plant proteins could also be attributed to the inhibition of TOR signaling. In order to further confirm the previous assumption that largemouth bass showed better growth potential after 8-weeks adaptation to dietary CPC, the activation status of TOR signaling pathway was evaluated. In accordance with the higher SGR and WGR from 6-8 weeks, the phosphorylation level of S6 was significantly higher in bass liver of CPC group than that of FM group (Figure 1B). Moreover, the RNA expression level of myog, which plays a key role in muscle differentiation by controlling myoblast fusion and myofiber formation, was also significantly higher in bass liver of CPC group than that of FM group (Figure 1C). All these results may suggest that largemouth bass has adapted to dietary CPC and then exhibited even better growth potential with prolonged feeding period. Such results may also explain that fish fed with plant proteins in some studies may exhibit better growth performance than that fed with fishmeal (27), which have adapted to such plant protein sources.
Besides the growth performance, fish health has received attention in recent years, especially during the fishmeal replacement by plant proteins. In the aquaculture practice of largemouth bass, liver health has been given special attention from fish farmers and scientists, which needs a comprehensive overlook of liver structures and functions. As known in mammals, liver is an essential organ controlling nutrient metabolism and immune responses, which is closely related to its unique pattern of vascular distribution, namely, the venous-biliary-artery tract (VBAT) structure (31). Such mammalian-like VBAT structures also exist in some teleost species, such as Atlantic salmon (Salmo salar), rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta) (32). Here in the present study, the typical VBAT structure was also detected in bass liver via H.&E. staining. As shown in Figure 2A bass liver, cuboidal hepatocytes with rounded nuclei were arranged in cords and supported by reticulated fibers and connective tissue. Moreover, veins (V) and bile ducts (B) are also detected to be accompanied with each other while arterial (A) features were rarely observed. Moreover, no significant difference was found in liver structure of juvenile largemouth bass between two groups. The tissue homeostasis could be due to the controlled proliferation and programmed cell death (PCD) of inner cells (33). Here, TUNEL assay was conducted to evaluate the programmed cell death (Figure 2B). Results also showed that TUNEL+ cells were mainly distributed at the edge of liver with few apoptotic cells in the interior of bass liver and no significant difference was detected between FM and CPC group (Figure 2C). Moreover, the expression levels of several PCD-related genes were also evaluated. As shown in Figure 2C, excepting gsdme, the expression of genes including nlrp3, nlrc3, casp1, casp3 and asc showed no significant difference between two groups. Thus, H.&E. staining, TUNEL assay and RT-qPCR results all suggested that bass liver structures were not significantly affected after dietary CPC inclusion at 15% for 8 weeks.
As mentioned above, fish liver is also involved in the immune responses, as several types of immune cells have been identified in the hepatic blood sinuses (34). Dietary and commensal bacterial products from the gastrointestinal tract enters liver from portal vein, which exert liver to continuous inflammatory potential (35). In fact, fish is the first bony vertebrate to develop both innate and adaptive immunity among all vertebrates (36), thus both innate and adaptive immune responses in liver were also systemically evaluated. Inflammatory responses are soon induced after pathogenic infection or other damage signals exposure. The expression levels of genes involved in the inflammatory responses including il-1β, il-8 and il-18 showed no significant difference between two groups. Moreover, the expression levels of several antibacterial peptide including hep and saa were also evaluated. The expression level of saa, which plays a key role in bacterial clearance and inflammatory regulation, in CPC group was significantly higher than that in FM group, which suggested that bass liver in CPC group was activated to control the inflammatory responses. On the other hand, the adaptive immunity in fish is executed by B cells and its secreted immunoglobulins which are responsible for humoral immunity, and T cells which are responsible for cellular immunity (37). As reported, three types of immunoglobulins (Igs) have been identified in teleosts (36). In the present study, the expression of Igs including igm, igt and igd, also showed no significant difference between two groups. Moreover, T cells are characterized by the presence of T-cell receptors (TCRs), which consists of an α (TCRα) chain and a β (TCRβ) chain in the conventional T cells. Such T lymphocoytes could be distinguished by the expression of mutually exclusive CD4+ or CD8+ co-receptors (38). Additionally, Lck is a cytoplasmic protein bound to CD4 and CD8 in T cells, which participates in antigen-induced T cell activation (39). Here, in the present study, the expression of tcra, tcrβ, lck, and cd8 also showed no significant difference between two groups, which indicated that dietary CPC inclusion did not significantly affect the expression of T cell markers. Additionally, immunofluorescence study with antibody against Lck also showed that T cells also exist in the liver interior with no Lck+ cells detected in the blood vessel, which indicated the successful perfusion procedure. Importantly, the number of T cells showed no significant difference between two groups. Thus, the innate and adaptive immunity in bass liver under normal status were not significantly affected during dietary CPC inclusion.
Largemouth bass is rather sensitive to N. seriolae infection, which caused serious hepatic damage and even mortality (14). In order to further illustrate the immune responses of bass liver during pathogenic infection, all bass was injected with N.a seriolae. As shown in Figure 4, the liver pathological structures showed rather serious damages. Lipogranulomas could be detected in liver of FM and CPC group at 1 dpi, while severe cellular vacuolar degeneration (black circles) could also be detected in the CPC group at 3 dpi. Moreover, the number of inflammatory cells (black triangles) in liver increased with the prolonged infection period, and such inflammatory cells in liver of CPC group were more than that in FM group, accompanied with the irregularly arranged cells and ruptured membranes. As mentioned above, fish liver is continuously exposed to dietary and commensal bacterial products, which results in persistent, regulated inflammation (16). When fishmeal was replaced by plant proteins in fish diet, the plant sources-originated anti-nutritional factors would cause further continual inflammatory stimulus to liver. Failure to clear such ‘dangerous’ stimuli may lead to chronic pathological inflammation and even disrupted tissue homeostasis such as fibrosis, cirrhosis and eventual liver failure (17). Recently, one new type of PCD, pyroptosis which is executed by gasdermin family proteins, has been discovered to closely correlate with the inflammation (40). Under the stimulation of pathogen- and/or damage-associated molecular patterns, pattern recognition receptors (PRRs) such as Nod like receptors could recruit apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspases to form inflammasomes and then activate caspases, which then cleave gasdermin E in fish to form oligomeric pores, and also promote the maturation and release of inflammatory cytokines such as il-1β and il-18 (40). In the present study, N. seriolae infection induced the upregulated expression of il-1β at 3 dpi in the FM group, while il-1β expression in CPC group was significantly induced much earlier at 1 dpi. Moreover, N. seriolae infection induced the upregulated expression of il-18 in the liver of CPC group at both 1 dpi and 3 dpi, whose expression was significantly higher than that of FM group. Besides the inflammatory cytokines, the expression of casp1 and casp3 was also significantly higher in bass liver of CPC group than that of FM group at 1 dpi. Similar results were detected in the expression of gsdme in liver at 0 dpi and 1 dpi. Thus, dietary CPC inclusion may trigger high inflammatory potential after N. seriolae infection, which is in accordance with the histological structure.
In order to further confirm the pro-inflammatory effects of CPC during bacterial infection, the histological structures and expression of genes in gill involved in inflammatory responses were also evaluated. As shown in Figure 5A, N. seriolae infection induced serious histological structural changes in gill, as the primary gill lamella (PL) thickened and the secondary gill lamella (SL) shortened, which could be detected in two groups. Earlier studies in hybrid snakehead (Channa maculata ♀ × Channa argus ♂) also detected that the gill filament was become short, twisted and shrunk seriously after N. seriolae infection (41), with a number of pale yellow nodules could be detected on the serosal surface, mesentery and internal organs (gill, heart, spleen, swim bladder, kidney and liver). Especially, more serious gill damages could be detected in CPC group, with much higher the width of PL but lower ratio of length to width of SL than that in FM group. Moreover, N. seriolae infection also induced the upregulated expression of il-1β, and casp1 in the gill of largemouth bass (Figure 5B). Moreover, the expression of il-18 and il-1β in the gill of CPC group was even significantly higher than that of FM group at 3dpi, while CPC group also exhibited higher expression of il-18 at 0 dpi. The expression of casp1 and asc in the gill were also significantly higher in CPC group than FM group at both 1 dpi and 3 dpi. Accordingly, the expression of casp3 and gsdme in the gill were also significantly higher in CPC group than FM group at 3 dpi. All these results in gill further confirmed that dietary CPC inclusion in largemouth bass significantly increased inflammatory potential and pyroptosis during bacterial infection, which may further influence fish health and survival during infection.
In summary, our present study indicated the potential of dietary CPC as replacement sources for fish meal, and that largemouth bass can adapt to it and even showed better growth potential. However, special concern should be given during the application of CPC in largemouth bass diet as it may induce higher inflammatory risk during N. seriolae infection.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Animal Experiment Committee of Huazhong Agricultural University (permit number HZAUFI-2016-007).
Author contributions
QW designed and wrote the main context. MW conducted most experimental protocol. ZC and YW joined the experimental analysis. JZ, SL, and XG conducted the experimental analysis. JG supplied the relevant materials. All authors contributed to the article and approved the submitted version.
Funding
This article was funded by National Natural Science Foundation of China (Grant No. 32172996; 31802317) and China Scholarship Council (Grant No. CSC[2021]109).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. FAO. The state of world fisheries and aquaculture. Rome: Food and Agricultural Organization (2020). Available at: http://www.fao.org/3/ca9229en/ca9229en.pdf.
2. Zhou Q, Mai K, Liu Y, Tan B. Research progress of animal and plant protein substitutes for fish meal. J Fisheries (2005) 3:404–10. doi: 10.3321/j.issn:1000-0615.2005.03.021
3. Tacon AGJ, Metian M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture (2008) 285:146–58. doi: 10.1016/j.aquaculture.2008.08.015
4. Bruce TJ, Oliver LP, Ma J, Small BC, Hardy RW, Brown ML, et al. An initial evaluation of fishmeal replacement with soy protein sources on growth and immune responses of burbot (Lota lota maculosa). Aquaculture (2021) 545:737157. doi: 10.1016/j.aquaculture.2021.737157
5. Sallam EA, Matter AF, Mohammed LS, Azam AE, Shehab A, Mohamed SM. Replacing fish meal with rapeseed meal: potential impact on the growth performance, profitability measures, serum biomarkers, antioxidant status, intestinal morphometric analysis, and water quality of Oreochromis niloticus and Sarotherodon galilaeus fingerlings. Vet Res Commun (2021) 45:223–41. doi: 10.1007/s11259-021-09803-5
6. Feng L, Chi B, Dong H. Cotton cultivation technology with Chinese characteristics has driven the 70-year development of cotton production in China. J Integr Agr (2022) 21:597–609. doi: 10.1016/S2095-3119(20)63457-8
7. Xue M. Processing technology of cottonseed protein concentrate and its nutritional value in aquatic feed. Feed industry (2021) 42:1–5. doi: 10.13302/j.cnki.fi.2021.12.001
8. Chen G, Yin B, Liu H, Tan B, Dong X, Yang Q, et al. Effects of fishmeal replacement with cottonseed protein concentrate on growth, digestive proteinase, intestinal morphology and microflora in pearl gentian grouper (♀ epinephelus fuscoguttatus×♂ epinephelus lanceolatu). Aquac Res (2020) 51:2870–84. doi: 10.1111/are.14626
9. Wang Q, Ji W, Xu Z. Current use and development of fish vaccines in China. Fish Shellfish Immunol (2020) 96:223–34. doi: 10.1016/j.fsi.2019.12.010
10. Zhao W, Liu Z, Niu J. Growth performance, intestinal histomorphology, body composition, hematological and antioxidant parameters of Oncorhynchus mykiss were not detrimentally affected by replacement of fish meal with concentrated dephenolization cottonseed protein. Aquac Rep (2021) 19:100557. doi: 10.1016/j.aqrep.2020.100557
11. Wang W, Gong Y, Greenfield BK, Nunes LM, Yang Q, Lei P, et al. Relative contribution of rice and fish consumption to bioaccessibility-corrected health risks for urban residents in eastern China. Environ Int (2021) 155:106682. doi: 10.1016/j.envint.2021.106682
12. Ministry of Agriculture and Rural Affairs of the People’s Republic of China. China Fishery statistical yearbook. (Beijing; China Agriculture Press). (2021)
13. Guo J, Kuang W, Zhong Y, Zhou Y, Chen Y, Lin S. Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass(Micropterus salmoides)fed high-starch diet. Fish Shellfish Immunol (2020) 97:602–7. doi: 10.1016/j.fsi.2019.12.087
14. Huang X, Liu S, Chen X, Zhang H, Yao J, Geng Y, et al. Comparative pathological description of nocardiosis in largemouth bass (Micropterus salmoides) and other perciformes. Aquaculture (2020) 534:736193. doi: 10.1016/j.aquaculture.2020.736193
15. Wu G. Amino acids: biochemistry and nutrition, (2nd ed.). Boca Raton: CRC Press (2021). doi: 10.1201/9781003092742
16. Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol (2009) 27:147–63. doi: 10.1146/annurev.immunol.021908.132629
17. Robinson MW, Harmon C, Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol (2016) 13:267–76. doi: 10.1038/cmi.2016.3
18. He G, Zhang T, Zhou X, Liu X, Sun H, Chen Y, et al. Effects of cottonseed protein concentrate on growth performance, hepatic function and intestinal health in juvenile largemouth bass. Micropterus salmoides Aquac Rep (2022) 23:101052. doi: 10.1016/j.aqrep.2022.101052
19. Xie S, Zhou Q, Zhang X, Zhu T, Guo C, Yang Z, et al. Effect of dietary replacement of fish meal with low-gossypol cottonseed protein concentrate on growth performance and expressions of genes related to protein metabolism for swimming crab (Portunus trituberculatus). Aquaculture (2022) 549:737820. doi: 10.1016/j.aquaculture.2021.737820
20. Xu X, Yang H, Zhang C, Bian Y, Yao W, Xu Z, et al. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture (2022) 548:737551. doi: 10.1016/j.aquaculture.2021.737551
21. Feng J, Han T, Zhang Y, Zhang B, Huang D, Wang T, et al. Molecular characterization and biological function of CXCR1 in nocardia seriolae-infected largemouth bass (Micropterus salmoides). Tissue Cell (2021) 72:101551. doi: 10.1016/j.tice.2021.101551
22. Wedegaertner T, Rathore K. Elimination of gossypol in cottonseed will improve its utilization. Proc Environ Sci (2015) 29:124–5. doi: 10.1016/j.proenv.2015.07.212
23. Rinchard J, Lee KJ, Dabrowski K, Ciereszko A, Blom JH, Ottobre JS. Influence of gossypol from dietary cottonseed meal on haematology, reproductive steroids and tissue gossypol enantiomer concentrations in male rainbow trout (Oncorhynchus mykiss). Aquac Nutr (2003) 9:275–82. doi: 10.1046/j.1365-2095.2003.00253.x
24. Kumar M, Tomar M, Punia S, Grasso S, Arrutia F, Choudhary J, et al. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci Technol (2021) 111:100–13. doi: 10.1016/j.tifs.2021.02.058
25. Wang H, Hu X, Zheng Y, Chen J, Tan B, Shi L, et al. Effects of replacing fish meal with cottonseed protein concentrate on the growth, immune responses, digestive ability and intestinal microbial flora in. Litopenaeus vannamei Fish Shellfish Immunol (2022) 128:91–100. doi: 10.1016/j.fsi.2022.07.067
26. Ye G, Dong X, Yang Q, Chi S, Liu H, Zhang H, et al. Low-gossypol cottonseed protein concentrate used as a replacement of fish T meal for juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂): Effects on growth performance, immune responses and intestinal microbiota. Aquaculture (2020) 524:735309. doi: 10.1016/j.aquaculture.2020.735309
27. He Y, Guo X, Tan B, Dong X, Yang Q, Liu H, et al. Replacing fishmeal with cottonseed protein concentrate in feed for pearl gentian groupers (Epinephelus fuscoguttatus♀ × e. lanceolatus♂): Effects on growth and expressions of key genes involved in appetite and hepatic glucose and lipid metabolism. Aquac Rep (2021) 20:100710. doi: 10.1016/j.aqrep.2021.100710
28. Chen P, Zhu Y, Wu X, Gu X, Xue M, Liang X. Metabolic adaptation to high starch diet in largemouth bass (Micropterus salmoides) was associated with the restoration of metabolic functions via inflammation, bile acid synthesis and energy metabolism. Brit J Nutr (2022) 27: 1–38. doi: 10.1017/S0007114522001180
29. Abernathy J, Overturf K. Expression of antisense long noncoding RNAs as potential regulators in rainbow trout with different tolerance to plant-based diets. Anim Biotechnol (2019) 30:87–94. doi: 10.1080/10495398.2017.1401546
30. Wang Q, He G, Mai K, Xu W, Zhou H. Fishmeal replacement by mixed plant proteins and maggot meal on growth performance, target of rapamycin signalling and metabolism in juvenile turbot (Scophthalmus maximus l.). Aquac Nutr (2016) 22:752–8. doi: 10.1111/anu.12296
31. Giari L, Manera M, Simoni E, Dezfuli BS. Cellular alterations in different organs of European sea bass dicentrarchus labrax (L.) exposed to cadmium. Chemosphere (2007) 67:1171–81. doi: 10.1016/j.chemosphere.2006.10.061
32. Rocha E, Monteiro RA, Pereira C. Presence of rodlet cells in the intrahepatic biliary ducts of the brown trout, salmo trutta fario Linnaeus, 1758. Can J Zool (2011) 72:1683–7. doi: 10.1139/z94-225
33. Wang J. Programmed cell death in aging. Ageing Res Rev (2015) 23:90–100. doi: 10.1016/j.arr.2015.04.002
34. Cheng D, Morsch M, Shami GJ, Chung RS, Braet F. Observation and characterisation of macrophages in zebrafish liver. Micron (2020) 132:102851. doi: 10.1016/j.micron.2020.102851
35. Möller AM, Korytář T, Köllner B, Schmidt-Posthaus H, Segner H. The teleostean liver as an immunological organ: Intrahepatic immune cells (IHICs) in healthy and benzo pyrene challenged rainbow trout (Oncorhynchus mykiss). Dev Comp Immunol (2014) 46:518–29. doi: 10.1016/j.dci.2014.03.020
36. Wang J, Clark G, Ju M, Castillo S, Gatlin DM. Effects of replacing menhaden fishmeal with cottonseed flour on growth performance, feed utilization and body composition of juvenile red drum sciaenops ocellatus. Aquaculture (2020) 523:735217. doi: 10.1016/j.aquaculture.2020.735217
37. Zhu L, Nie L, Zhu G, Xiang L, Shao J. Advances in research of fish immune-relevant genes: a comparative overview of innate and adaptive immunity in teleosts. Dev Comp Immunol (2012) 39:39–62. doi: 10.1016/j.dci.2012.04.001
38. Jung JW, Chun JH, Lee JS, Kim SW, Lee AR, Kim J, et al. Characterization of CD4-positive lymphocytes in the antiviral response of olive flounder (Paralichthys oliveceus) to nervous necrosis virus. Int J Mol Sci (2020) 21:4180. doi: 10.3390/ijms21114180
39. Dai P, Liu X, Li Q. Function of the lck and fyn in T cell development. Hereditas (2012) 34:289–95. doi: 10.3724/sp.j.1005.2012.00289
40. Song Z, Zou J, Wang M, Chen Z, Wang Q. A comparative review of pyroptosis in mammals and fish. J Immunol Res (2022) 15:2323–31. doi: 10.2147/JIR.S361266
Keywords: cottonseed protein concentrate, largemouth bass, liver health, Nocardia seriolae, inflammation
Citation: Wang M, Chen Z, Wang Y, Zou J, Li S, Guo X, Gao J and Wang Q (2022) Largemouth bass (Micropterus salmoides) exhibited better growth potential after adaptation to dietary cottonseed protein concentrate inclusion but experienced higher inflammatory risk during bacterial infection. Front. Immunol. 13:997985. doi: 10.3389/fimmu.2022.997985
Received: 12 August 2022; Accepted: 29 August 2022;
Published: 15 September 2022.
Edited by:
Mingchun Ren, Chinese Academy of Fishery Sciences, ChinaReviewed by:
Hualiang Liang, Chinese Academy of Fishery Sciences, ChinaYabing Wang, Chinese Academy of Fishery Sciences, China
Copyright © 2022 Wang, Chen, Wang, Zou, Li, Guo, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingchao Wang, cWN3YW5nQG1haWwuaHphdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Mengya Wang†
Mengya Wang† Jian Gao
Jian Gao Qingchao Wang
Qingchao Wang