- Hepatobiliary Pancreatic Surgery Department, Affiliated Huadu Hospital, Southern Medical University, Guangzhou, China
Background: Red blood cell distribution width (RDW) is a common biomarker of bacterial infections, and it can be easily obtained from a routine blood test. We investigate the diagnostic value of RDW for the prediction of mortality in adult sepsis patients through a review and meta-analysis. We registered this review in PROSPERO (Registration Number: CRD42022357712), and the details of the registration are included in Appendix 1.
Methods: We searched PubMed, Cochrane Library, Springer, and Embase between Jan. 1, 2000, and May 30, 2022, for primary studies about this research. We collected articles that investigated RDW for varying degrees of sepsis patients—those who suffered from sepsis, severe sepsis, or sepsis shock. Studies of healthy people and sepsis of children and neonates were excluded from our research. The definition of study characteristics and data extraction were finished by two independent researchers and discrepancies resolved by consensus. The combined sensitivities and specificities were calculated by meta-analysis using STATA14.0. The sensitivity of the included studies was analyzed by excluding studies that had potential heterogeneity. A summary operating characteristic curve was made to evaluate the diagnostic value for the prediction of mortality in adult sepsis patients. The Fagan test was used to explore likelihood ratios and posttest probabilities. Finally, we investigated the source of heterogeneity using meta-regression.
Results: Twenty-four studies, including 40,763 cases altogether, were included in this analysis. Bivariate analysis indicated a combined sensitivity of 0.81 (95% CI 0.73–0.86) and specificity of 0.65 (95% CI 0.54–0.75). The area under the summary receiver operating characteristic curve was 0.81 (95% CI 0.77–0.84). Substantial heterogeneity resided in the studies (I2 =96.68, 95% CI 95.95–97.4). Meta-regression showed that the reference description, prospective design, and blinded interpretation of the included studies could be responsible for the heterogeneity.
Conclusions: RWD is an available and valuable biomarker for prediction of mortality in adult sepsis patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022357712.
Introduction
Sepsis is one of the most serious diseases, and it threatens human health. It represents an abnormal immune reaction to infection, which leads to organ dysfunction (1–3). Up to now, the pathogenesis of sepsis has not been understood sufficiently. The currently accepted theory of its pathogenesis is that once endo- or exotoxins are released by the pathogen, immune cell receptors may activate a series of signaling pathways, which release pro- and anti-inflammatory mediators, such as acute-phase proteins, cytokines, chemokines. Under normal physiological conditions, exotoxins plays an important role in the body’s defense against pathogenic microorganisms; however, the excessive release of cytokines and the consequent chain reaction could cause endothelial damage and intestinal permeability. If prompt and effective treatment is not carried out, it may lead to systemic inflammatory response syndrome, organ dysfunction, or even sequential multiple organ failure, which increase patient mortality during hospitalization significantly (4, 5). Early identification and timely therapy are critical for the treatment of sepsis. Therefore, approaches to identifying sepsis and estimating its prognosis are developing (4, 6, 7). Red blood cell distribution width (RDW) is a common erythrocyte index, which we can easily obtain from a routine blood test. Its inexpensive characteristic and low technical requirements mean that it can be easily accessible in most medical institutions. Research indicates that RDW is an powerful biomarker for predicting some clinical illnesses, such as cardiovascular diseases (8), respiratory disease (9), hepatitis B (10), or acute appendicitis (11). For the study of the relationship between RDW and sepsis morbidity and mortality, several studies show that RDW not only has a diagnostic value, but also has noteworthy prediction of mortality for sepsis (12–14).
Recently, a meta-analysis about the prognostic role of RDW in sepsis indicated that patients with increased RDW are more likely to have higher mortality (7). However, the limitations of this research were territorial and potential publication bias. Therefore, more scientific, rigorous, and multicenter studies about the prognostic role of RDW in sepsis need to performed, and our understanding of RDW needs to be developing continually.
On the base of previous studies, we did a meta-analysis about the diagnostic value of RDW for the prediction of mortality in adult sepsis patients and did further investigation about the clinical diagnostic value of RDW.
Methods
Search strategy and selection criteria
We searched PubMed, Cochrane Library, Springer, and Embase for studies that involved RDW for the prediction of mortality in adult sepsis patients. The following MeSH terms and their combinations were searched: “(red blood cell distribution width OR RDW) and (sepsis OR “severe sepsis” OR “sepsis shock”) and (prognosis OR mortality).” The time period of our search was from Jan. 1, 2000, to May 30, 2022.
To ensure the rigorousness of the literature search, the reference standard for sepsis was defined based on the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (15) or SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference (Sepsis-2) (16). For comparison of the research, a positive result was defined as the sepsis patient who had an ending of death or no survival during the observation period, and the comparators for the analyzed studies were a negative result, defined as sepsis patient who had a survival ending. Furthermore, a 2×2 contingency table should be provided from the studies, and if not available for 2×2 contingency, the receiver operating characteristic (ROC) curve about sensitivity and specificity prediction of sepsis mortality, the numbers on survival and nonsurvival, must be indicated so that we can convert the data mentioned above into true positive, false positive, true negative, and false negative statistics. Only adult patients who suffered from sepsis were included in our research; healthy people and sepsis about children and neonates were excluded.
Procedures
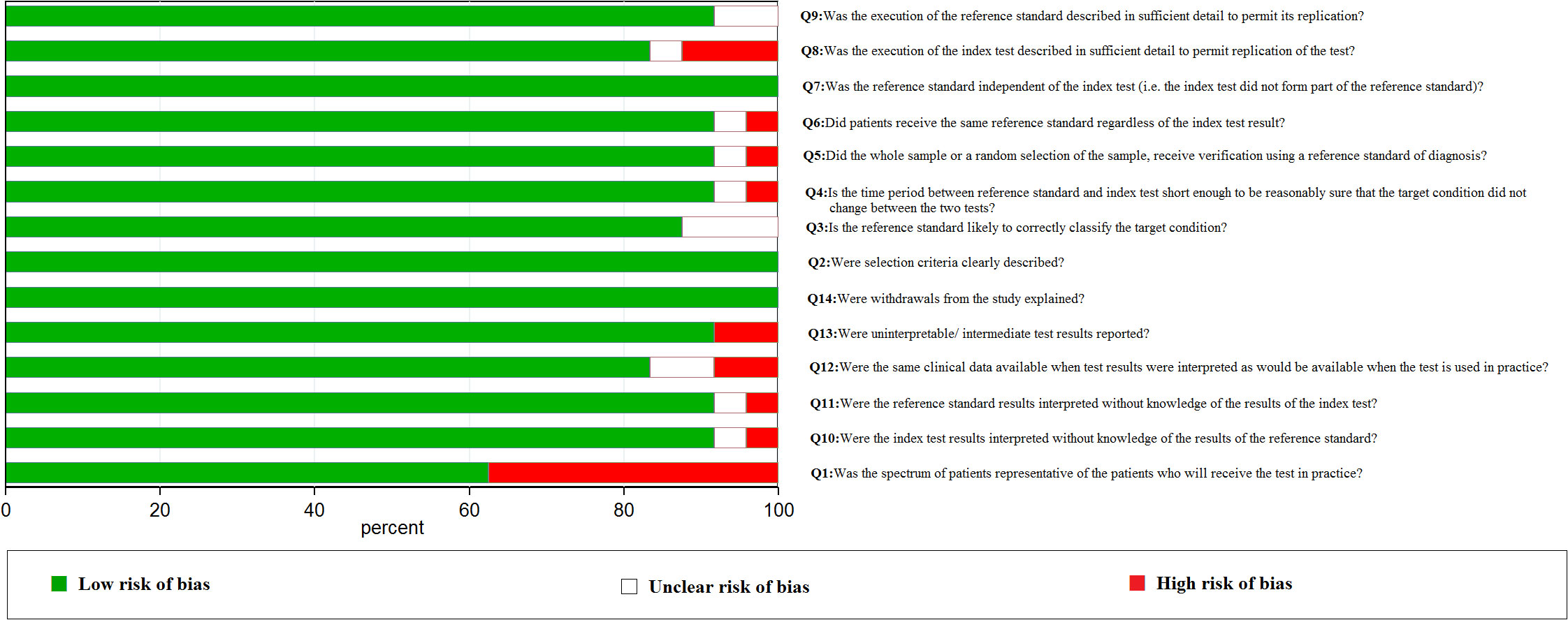
Two researchers (Hongsheng Wu and Biling Liao) extracted the data from the included studies independently. When a discrepancy occurred, a consensus meeting was held to resolve the problem. The evaluation scale of QUADAS was used for assessing the methodological quality of the included studies (17). The 14 quality evaluation criteria included the following: Q1. Was the spectrum of patients representative of the patients who will receive the test in practice? Q2. Were selection criteria clearly described? Q3. Is the reference standard likely to correctly classify the target condition? Q4. Is the time period between the reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? Q5. Did the whole sample or a random selection of the sample receive verification using a reference standard of diagnosis? Q6. Did patients receive the same reference standard regardless of the index test result? Q7. Was the reference standard independent of the index test (i.e., the index test did not form part of the reference standard)? Q8. Was the execution of the index test described in sufficient detail to permit replication of the test? Q9. Was the execution of the reference standard described in sufficient detail to permit its replication? Q10. Were the index test results interpreted without knowledge of the results of the reference standard? Q11. Were the reference standard results interpreted without knowledge of the results of the index test? Q12. Were the same clinical data available when test results were interpreted as would be available when the test was used in practice? Q13. Were uninterpretable/intermediate test results reported? Q14. Were withdrawals from the study explained? We used “yes,” “no,” or “unclear” as the risk of quality assessment.
Statistical analysis
According to the sepsis patient death or not, we calculated true positives, false positives, true negatives, and false negatives of sepsis from the included studies and figured out the sensitivity and specificity and their 95% CI.
The “MIDAS” module was used for synthesizing the data to explore the combined sensitivity and specificity and their 95% CI. The “metaninf” order was used for evaluating the sensitivity of the data synthesis. The summary ROC (SROC) was used for calculating the area under the curve (AUC) of the diagnostic value. A Fagan plot showed the relationship among prior probability, likelihood ratio, and posterior probability. Finally, a funnel plot was used for investigating the publication bias, and meta-regression was used to explore the source of heterogeneity from the included studies. All of the statistical and graphical methods mentioned above were finished by STATA version 14.0.
Results
Literature selection and quality assessment
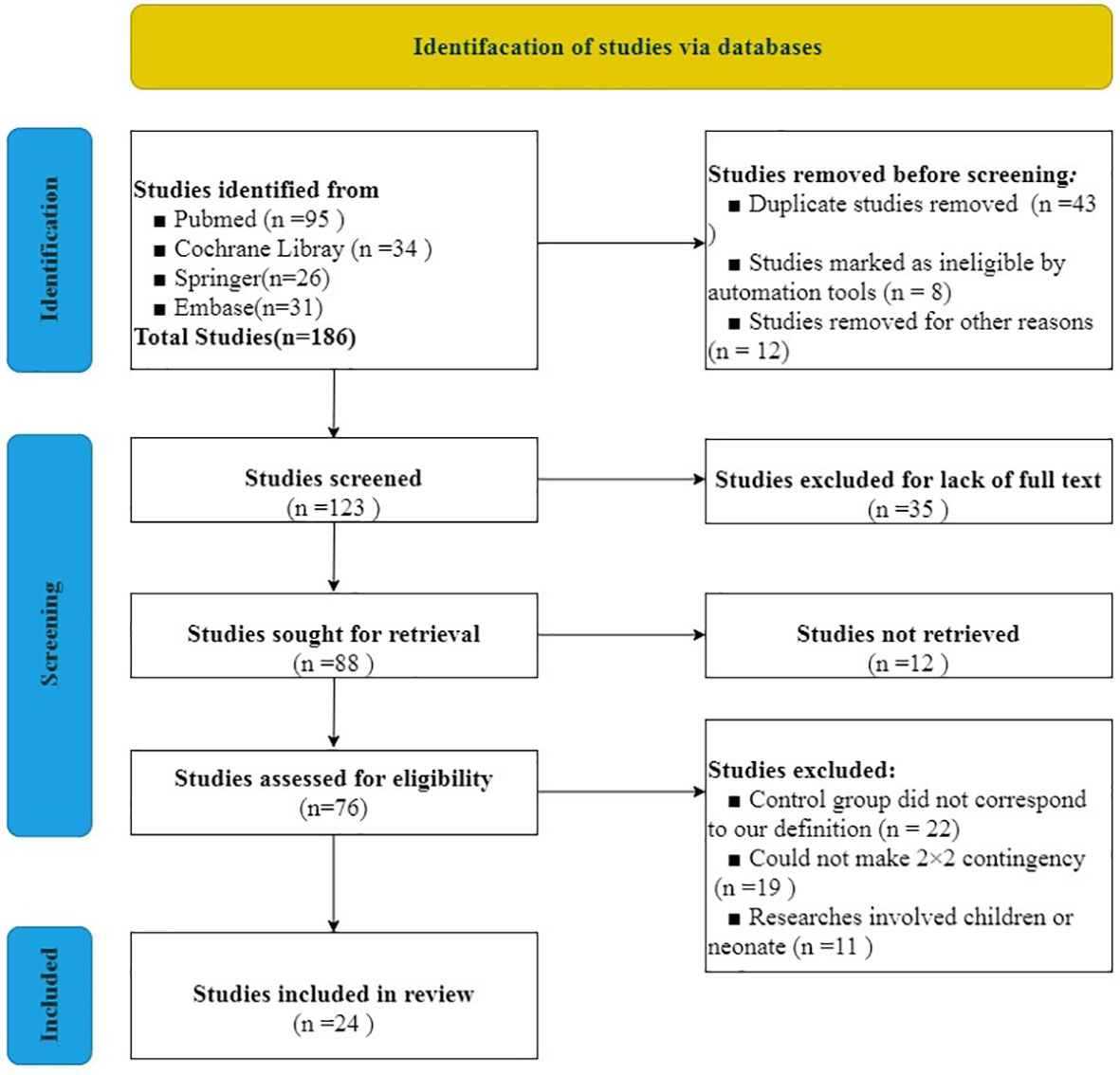
We used the PRISMA 2020 (18) statement to finish the selection of included studies. After evaluating and screening carefully from all of the studies from the databases, 24 studies including 40,763 cases were included in our analysis. The literature screening process is indicated in Figure 1. Literature quality assessment was estimated by QUADAS (17). We made great efforts to score each item according to this scale and how the items were assessed. The results of the included studies’ quality assessment is shown in Figure 2.
Characteristics of the included studies
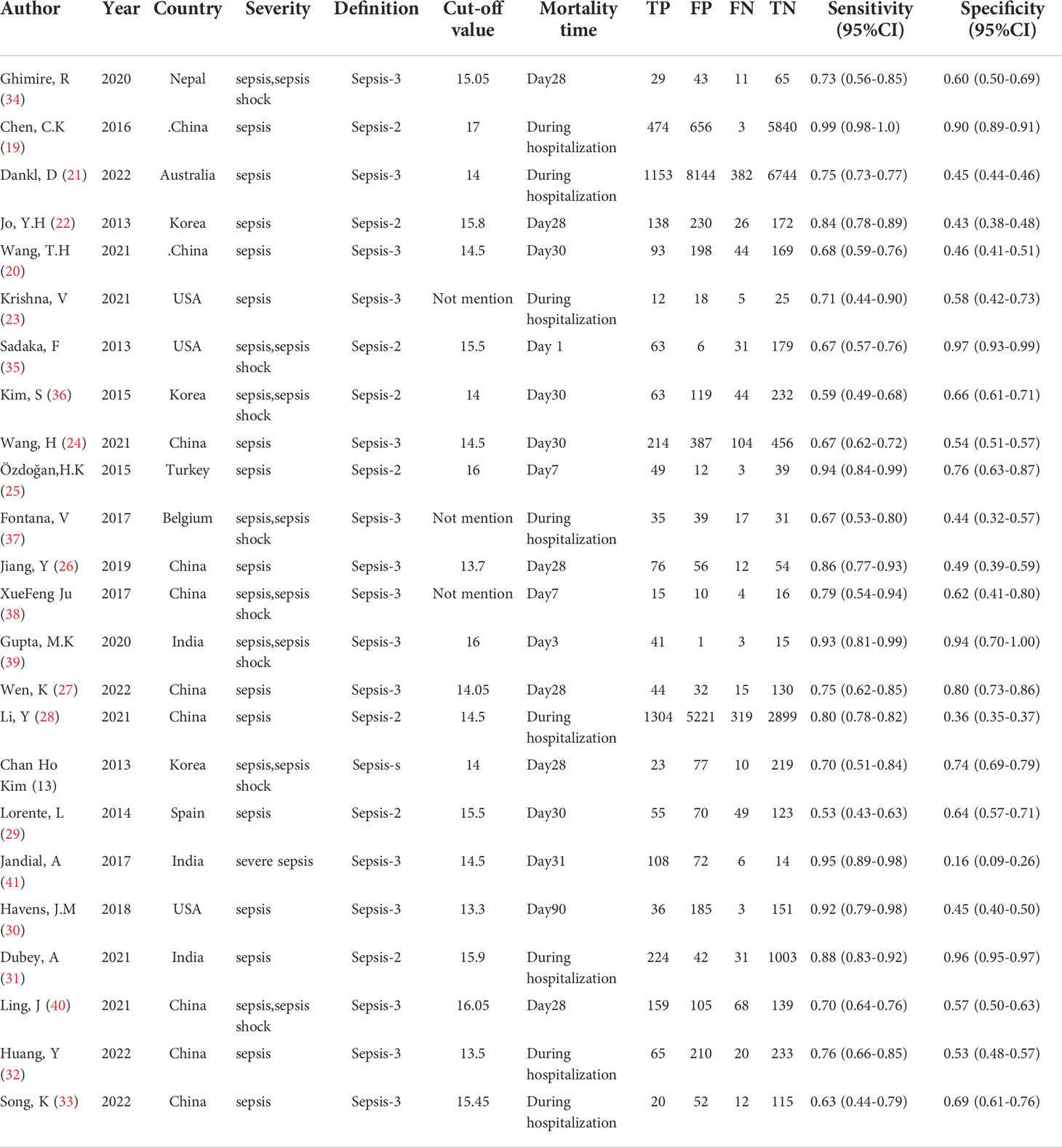
From all of the included studies, the severity of 15 studies (19–33) was defined as “sepsis,” eight studies (13, 34–40) were defined as “sepsis or sepsis shock,” and one study (41) was defined as “severe sepsis.” Twenty-one studies (13, 19–22, 24–36, 39–41) explicitly mentioned the cutoff value of RDW, and the other three studies (23, 37, 38) had no mention about the cutoff value of RDW. The cutoff value of RDW ranged from 13.3 to 17.0 in the included studies that mentioned it. For the mortality time, the longest observation time published was 90 days in just one study (30), yet the shortest observation time published was 1 day (35). Eight studies (19, 21, 23, 28, 31–33, 37) did not provide a definite mortality time and just defined it as “during ICU hospitalization” or “during hospitalization.” The characteristics of the included studies are shown in Table 1.
Results of combined sensitivity, combined specificity
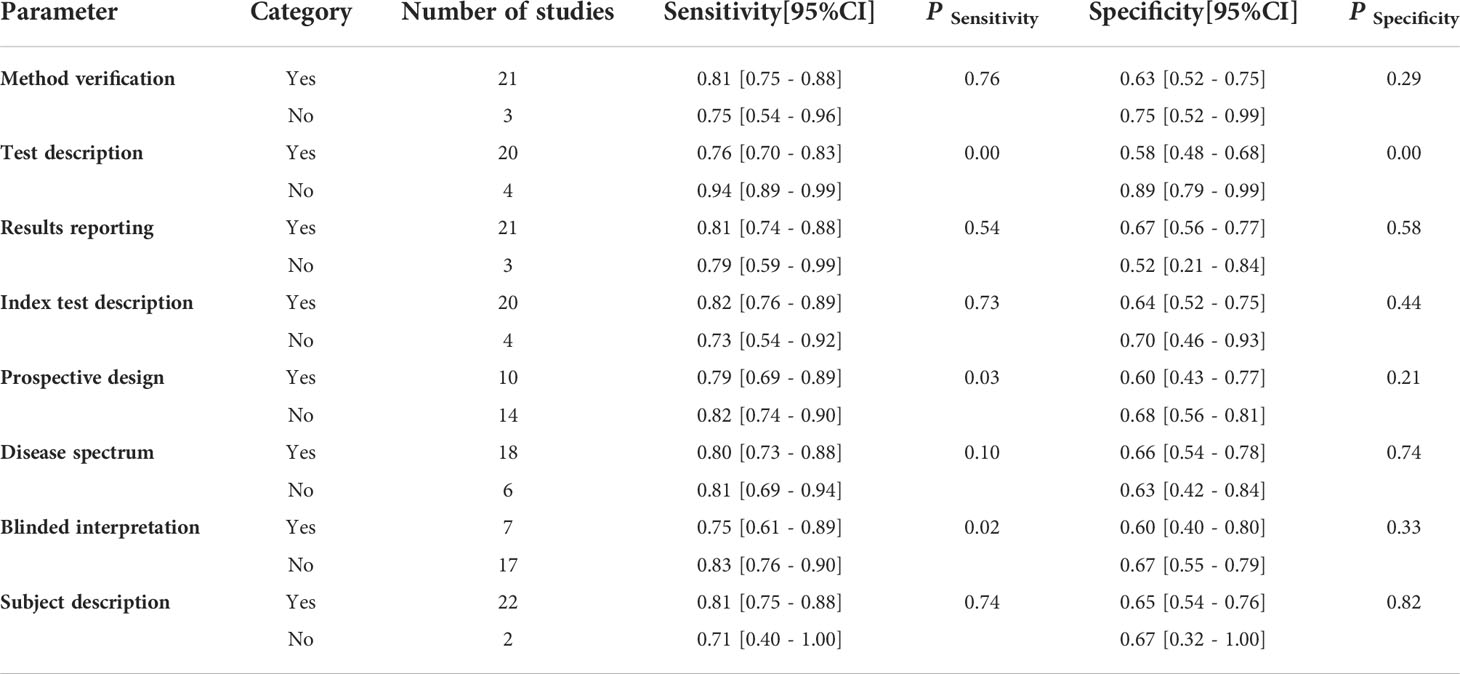
Through pooling all of the included studies together through meta-analysis, the combined sensitivity was 0.81 (95% CI 0.73–0.86), and the combined specificity was 0.65 (95% CI 0.54–0.75). The result of the pooled studies is shown in Figure 3. To explore the diagnostic value, the SROC was made, and the results indicate that AUC and its corresponding 95% CI was 0.81 (95% CI 0.77–0.84; Figure 4).
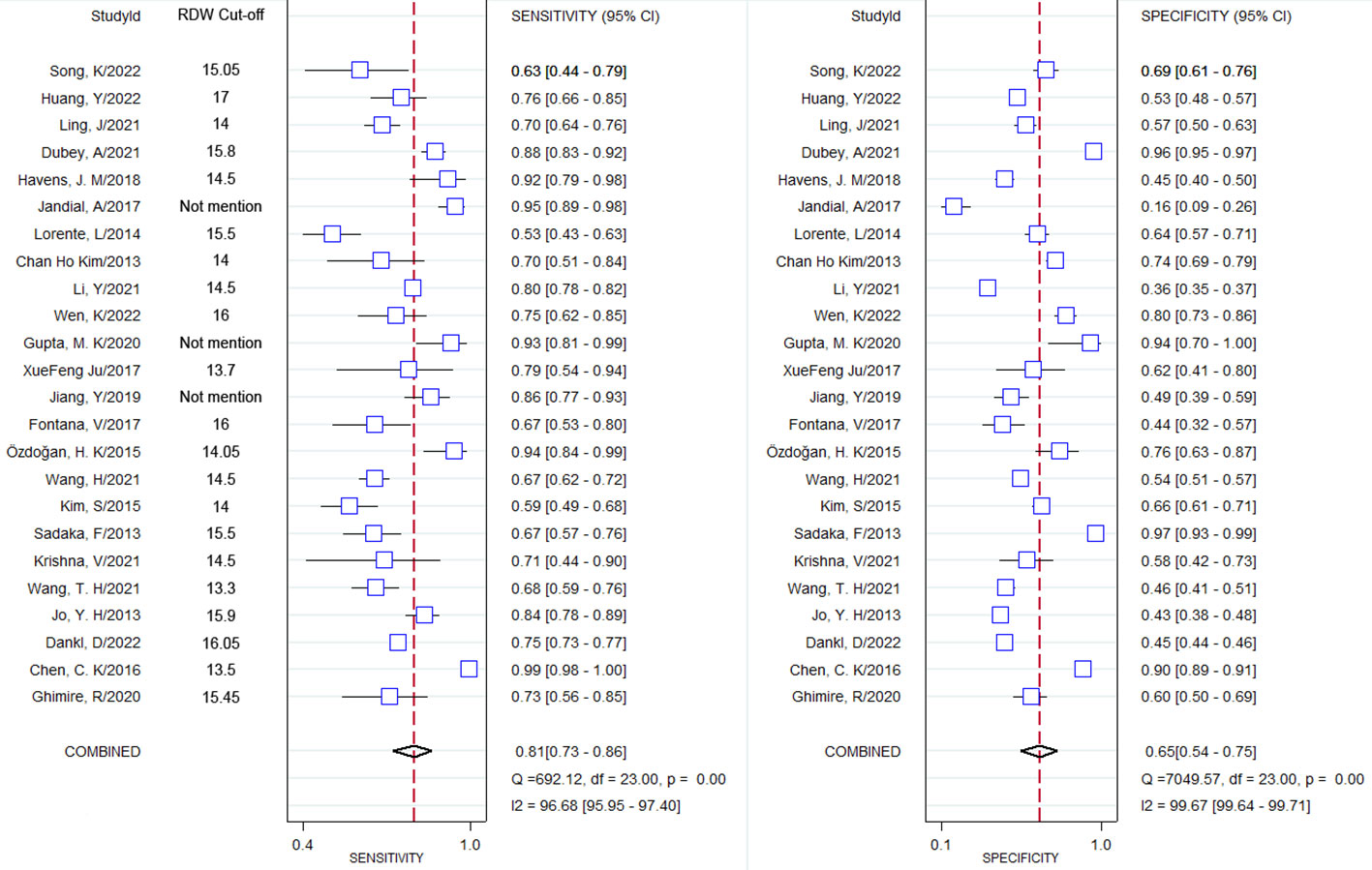
Figure 3 Forest plot of combined sensitivity and combined specificity and their corresponding 95% Cl.
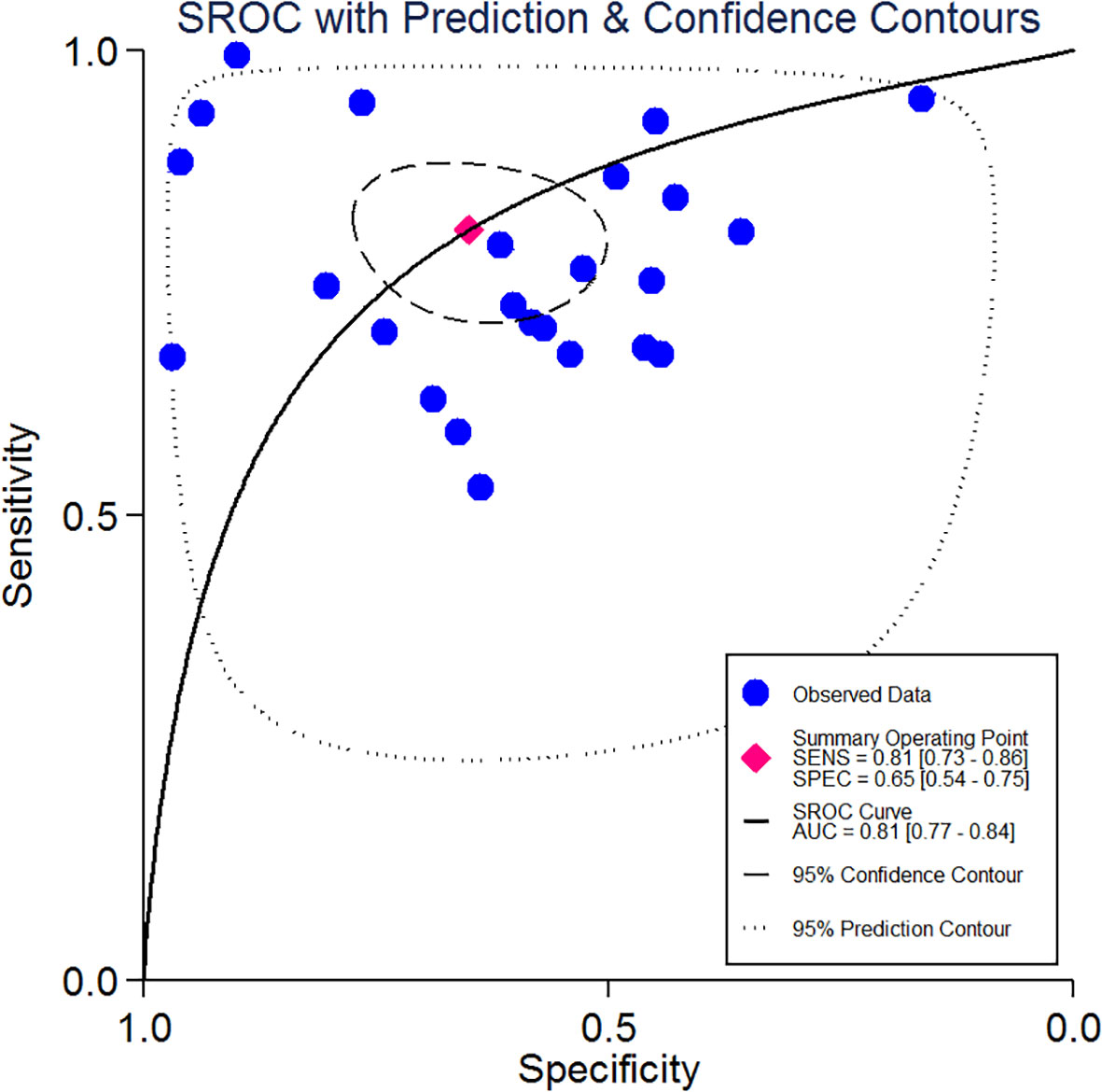
Figure 4 Summary receiver operating characteristics curve and the 95% confidence contour and 95% prediction contour.
Sensitivity analysis of the pooled studies
Based on the revision of Sepsis-2 (publication in 2001), a new definition and new assessing clinical criteria for sepsis (Sepsis-3) was proposed and reached a consensus in 2016 (15, 42). According to which of the included studies are using the Sepsis-2 definition and which the Sepsis-3 definition, we also made a sensitivity analysis of this research, the aim of which was to investigate the stability of the results. We did sensitivity analysis using “METANINF.” Nine studies (13, 19, 22, 25, 28, 29, 31, 35, 36) that were defined as Sepsis-s and had potential influence on the results were excluded after removing the above four studies that had significantly impacted on sensitivity. The rest of the included studies were distributed between upper and lower 95% confidence intervals with the estimation line as the center. The sensitivity analysis is shown in Figure 5.
Figure 5 Sensitivity analysis of the result base on the new definition and clinical criteria for sepsis (Sepsis-s and Sepsis-3). Also showed the lower and upper 95% Cl.
Fagan test and Deek’s test
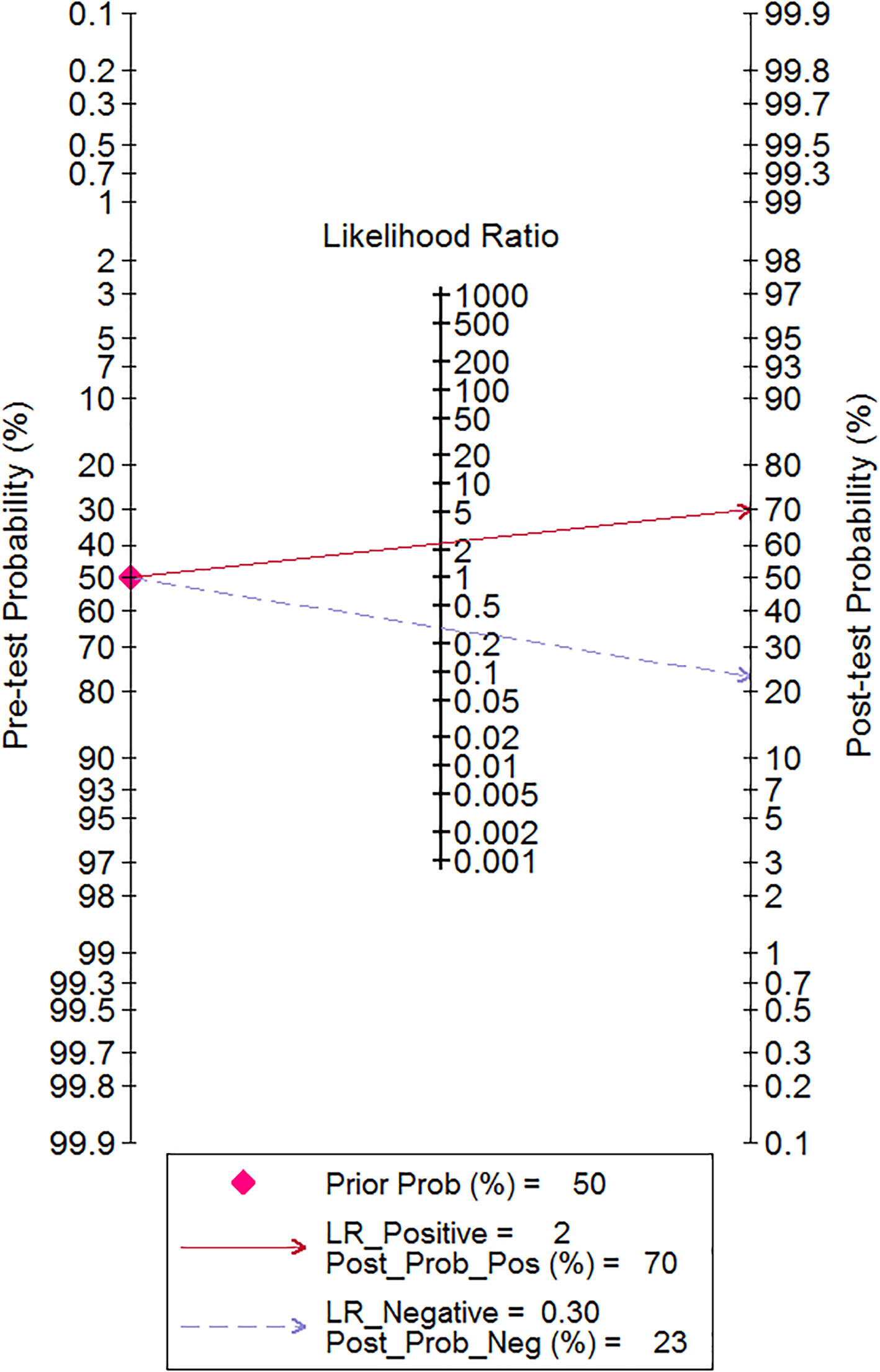
We also used the Fagan test to evaluate likelihood ratios and posttest probabilities. The results of our research show that both likelihood ratio and posttest probability were moderate, which means that, if a sepsis patient had a high-level RDW, the prediction of mortality was 70%. Likewise, if a sepsis patient had a lower level RDW, the possibility of mortality was just 23% (Figure 6). For the publication bias of this meta-analysis, the funnel plot in Figure 7 indicates that the publication bias was low (p value=.52).
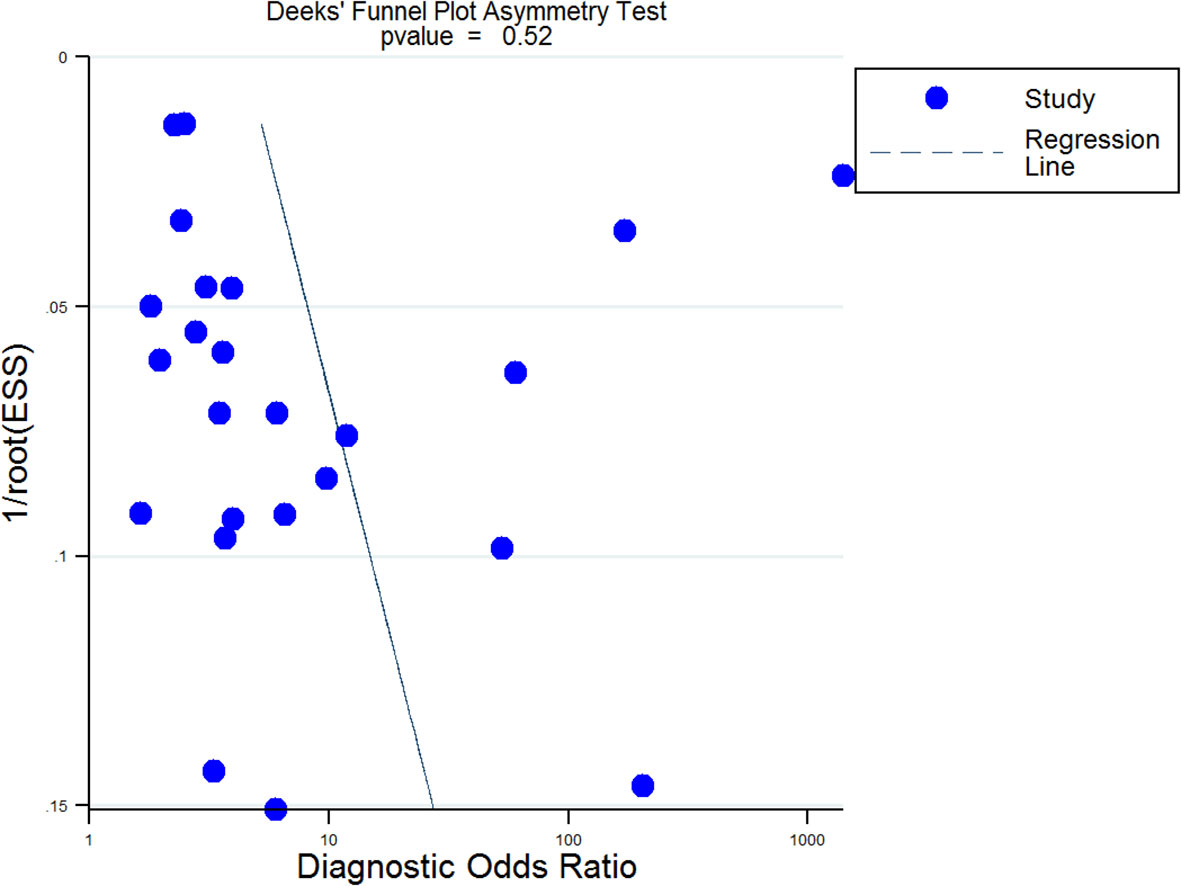
Figure 7 Deek’s funnel plot for publication bias analysis. A p value=0.52 suggested that the publication bias of this Meta-analysis was low. EES, Effective Sample Size.
Heterogeneity exploration
To explore the source of heterogeneity, meta-regression was employed. From the subgroup of the sensitivity analysis, the results indicate that reference description (sensitivity: 0.76, 95% CI: 0.70–0.83, P=.00), prospective design (sensitivity: 0.79, 95% CI: 0.69–0.89, P=.03), and the blinded interpretation of the study (sensitivity: 0.75, 95% CI: 0.61–0.89, P=.02) could be responsible for the heterogeneity. Nevertheless, from the subgroup of the specificity analysis, only the reference description (specificity: 0.58, 95% CI: 0.48–0.68, P=.00) was a significant factor affecting heterogeneity (Table 2). The forest plot of univariable meta-regression of subgroup analysis is shown in Figure 8.
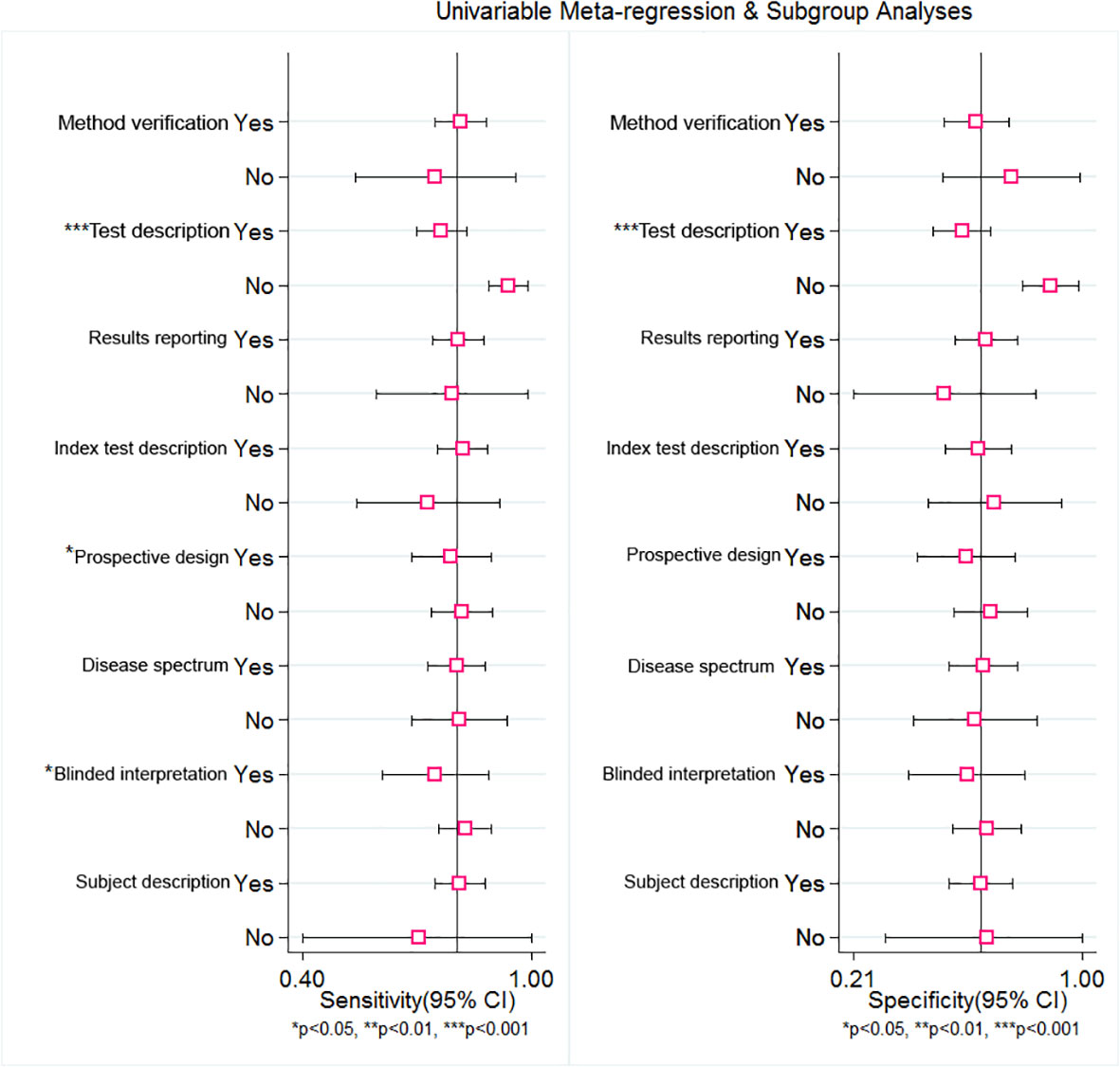
Figure 8 Univariable Meta-regression and subgroup analysis of RDW for predicting of mortality in adult sepsis patients. The forest plot indicated that Test description, Prospective design and Blinded may be responded for the heterogeneity.
Discussion
Sepsis is a serious clinical syndrome caused by abnormal host immune response to infections, which may lead to organ failure or mortality (3, 4). In an estimate based on research data, the incidence of sepsis and severe sepsis were 31 and 19 million, respectively, each year and lead to about 5 million deaths annually (43). One recent review published in 2021 indicates that, from the epidemiologic analysis of the global incidence of sepsis, hospital-treated adult sepsis was 189 cases per 100,000 persons from high-income countries (44). Nevertheless, if applying the Global Burden of Diseases, Injuries, and Risk Factors Study as the reference to compare with sepsis incidence, the incidence of sepsis can be as high as 677.5 cases per 100,000 persons worldwide (45). Previous research shows that RDW is a useful prediction of mortality in adult sepsis patients (46, 47). In a meta-analysis from 2020, the result of synthesis of 11 included studies indicated that high-level RDW was associated with mortality (HR = 1.14, 95% CI 1.09–1.20, Z=5.78, P<.001). However, the potential heterogeneity (I2 =80%, Pheterogeneity <.001) was obvious (7), and the meta-analysis lacked the mortality predicting ability, such as the sensitivity and specificity.
In our meta-analysis, the study search was performed through strict screening between Jan. 1, 2000, and May 30, 2022, and all of the studies were published in English. The pooled sensitivity was 0.81 (95% CI 0.73–0.86), and the pooled specificity was 0.65 (95% CI 0.54–0.75), respectively, and had the equivalent predicted value. To evaluate the RDW diagnostic value for predicting mortality in sepsis, SROC and the Fagan test were performed. The SROC curve indicated that the AUC was 0.81 (95% CI 0.77–0.84) with moderate predicted value. Several previous studies indicate the RDW for the prediction of mortality in adult sepsis patients. Wang, A.Y. et al. (48) show that the AUC of RDW in predicting mortality was 0.63 (95% CI 0.52–0.74); however, the included cases were elderly people whose age was more than 65, and the number of included cases was small. Another study (49) showed that RDW was a significant independent prognosis factor of 30-day mortality for sepsis patients, but the AUC of ROC was just 0.66 (0.59–0.73), which was inferior to our meta-analysis. Furthermore, we explored the likelihood ratios and posttest probabilities of RDW for the diagnostic value in predicting mortality, and the result of the Fagan test indicated that, given a pretest probability of 50%, the posttest probability for a positive test result was 70%. Likewise, there was a posttest probability of 23% for a negative test result.
Although this meta-analysis had a useful predicted value of mortality in adult sepsis patients, some limitations were inevitable. First, the research had certain heterogeneity, and the heterogeneity of combined sensitivity and specificity were 96.68 (95% CI 95.95–97.40) and 99.67 (95% CI 99.64–99.71), respectively. We used meta-regression to explore the origin of the heterogeneity, and the results showed that reference description, prospective design, and the blinded interpretation were responsible for the heterogeneity. The main reasons may be as follows (1): Discrepancy references of Sepsis-2 and Sepsis-3 could be responsible for the heterogeneity of reference description, which was consistent with our sensitivity analysis (2). Blindness methods lacked in most studies, and this may be the main reason for heterogeneity of blinded interpretation. (3) For reasons of heterogeneity of prospective design, different research designs from all the included studies (prospective or retrospective design) may be the cause of heterogeneity. Second, the cutoff values of RDW were not consistent, and the cutoff value of RDW in three included studies were not mentioned. This situation may have a negative influence on the diagnostic combined sensitivity and specificity.
In conclusion, RDW is a helpful marker for prediction of mortality in adult sepsis patients. In view of sepsis being an extremely complex syndrome with complex pathophysiological mechanisms, RDW should not be considered as the single definitive test for predicting mortality of sepsis patients, and other useful factors, such as medical history, physical examination, and pathogenic microorganism tests, should be taken into consideration during the clinical process.
Data availability statement
The datasets presented in this article are not readily available. Because the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available. Requests to access the datasets should be directed to Y3Jhenl3dTIwMDdAMTI2LmNvbQ==.
Author contributions
HW had the idea and designed this meta-analysis, and undertook literature search, collection, analysis and writing of this manuscript. BL undertook the data statistical work and the preparation of statistical chart. TJ, JH were responsible for searching relevant literatures. TC and KM had full access to all the data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Funding
The funds for research design, data collection and data analysis in this study from the internal medicine research fund of Affiliated Huadu Hospital, Southern Medical University (People’s Hospital of Huadu District). Fund Number: 2020A01. Construction of Major Subject of Affiliated Huadu Hospital, Southern Medical University (YNZDXK202201, 2022-2025).
Acknowledgments
We acknowledge the support from the Internal Medicine Research Fund of Affiliated Huadu Hospital, Southern Medical University (People’s Hospital of Huadu District).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Esposito S, De Simone G, Boccia G, De Caro F, Pagliano P. Sepsis and septic shock: New definitions, new diagnostic and therapeutic approaches. J Global antimicrob. resist. (2017) 10:204–12. doi: 10.1016/j.jgar.2017.06.013
2. Bracht H, Hafner S, Weiß M. Sepsis update: Definition and epidemiology. Anasthesiol. Intensivmedizin Notfallmedizin Schmerztherapie AINS. (2019) 54(1):10–20. doi: 10.1055/a-0625-5492
3. Salomão R, Ferreira BL, Salomão MC, Santos SS, Azevedo LCP, Brunialti MKC. Sepsis: evolving concepts and challenges. Braz J Med Biol Res = Rev Bras pesquisas medicas e biologicas. (2019) 52(4):e8595. doi: 10.1590/1414-431X20198595
4. Thompson K, Venkatesh B, Finfer S. Sepsis and septic shock: Current approaches to management. Internal Med J (2019) 49(2):160–70. doi: 10.1111/imj.14199
5. Purcarea A, Sovaila S. Sepsis, a 2020 review for the internist. Romanian J Internal Med = Rev roumaine med interne. (2020) 58(3):129–37. doi: 10.2478/rjim-2020-0012
6. Opal SM, Wittebole X. Biomarkers of infection and sepsis. Crit Care clinics. (2020) 36(1):11–22. doi: 10.1016/j.ccc.2019.08.002
7. Zhang L, Yu CH, Guo KP, Huang CZ, Mo LY. Prognostic role of red blood cell distribution width in patients with sepsis: a systematic review and meta-analysis. BMC Immunol (2020) 21(1):40. doi: 10.1186/s12865-020-00369-6
8. Li N, Zhou H, Tang Q. Red blood cell distribution width: A novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis markers. (2017) 2017:7089493. doi: 10.1155/2017/7089493
9. Yčas JW. Toward a blood-borne biomarker of chronic hypoxemia: Red cell distribution width and respiratory disease. Adv Clin Chem (2017) 82:105–97. doi: 10.1016/bs.acc.2017.06.002
10. Fan X, Deng H, Wang X, Fu S, Liu Z, Sang J, et al. Association of red blood cell distribution width with severity of hepatitis b virus-related liver diseases. Clinica Chimica Acta; Int J Clin Chem (2018) 482:155–60. doi: 10.1016/j.cca.2018.04.002
11. Anand S, Krishnan N, Jukić M, Križanac Z, Llorente Muñoz CM, Pogorelić Z. Utility of red cell distribution width (RDW) as a noninvasive biomarker for the diagnosis of acute appendicitis: A systematic review and meta-analysis of 5222 cases. Diagn (Basel Switzerland) (2022) 12(4):1–11. doi: 10.3390/diagnostics12041011
12. Zhang HB, Chen J, Lan QF, Ma XJ, Zhang SY. Diagnostic values of red cell distribution width, platelet distribution width and neutrophil-lymphocyte count ratio for sepsis. Exp Ther Med (2016) 12(4):2215–9. doi: 10.3892/etm.2016.3583
13. Kim CH, Park JT, Kim EJ, Han JH, Han JS, Choi JY, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care (London England). (2013) 17(6):R282. doi: 10.1186/cc13145
14. Dogan P, Guney Varal I. Red cell distribution width as a predictor of late-onset gram-negative sepsis. Pediatr Int Off J Japan Pediatr Society. (2020) 62(3):341–6. doi: 10.1111/ped.14123
15. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
16. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med (2003) 29(4):530–8. doi: 10.1007/s00134-003-1662-x
17. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res methodol. (2003) 3:25. doi: 10.1186/1471-2288-3-25
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1186/s13643-021-01626-4
19. Chen CK, Lin SC, Wu CC, Chen LM, Tzeng IS, Chen KF. STARD-compliant article: The utility of red cell distribution width to predict mortality for septic patients visiting the emergency department. Medicine (2016) 95(24):e3692. doi: 10.1097/MD.0000000000003692
20. Wang TH, Hsu YC. Red cell distribution width as a prognostic factor and its comparison with lactate in patients with sepsis. Diagn (Basel Switzerland) (2021) 11(8):1–9. doi: 10.3390/diagnostics11081474
21. Dankl D, Rezar R, Mamandipoor B, Zhou Z, Wernly S, Wernly B, et al. Red cell distribution width is independently associated with mortality in sepsis. Med Princ Pract (2022) 31(2):187–94. doi: 10.1159/000522261
22. Jo YH, Kim K, Lee JH, Kang C, Kim T, Park HM, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emergency Med (2013) 31(3):545–8. doi: 10.1016/j.ajem.2012.10.017
23. Krishna V, Pillai G, Velickakathu Sukumaran S. Red cell distribution width as a predictor of mortality in patients with sepsis. Cureus (2021) 13(1):e12912. doi: 10.7759/cureus.12912
24. Wang H, Huang J, Liao W, Xu J, He Z, Liu Y, et al. Prognostic value of the red cell distribution width in patients with sepsis-induced acute respiratory distress syndrome: A retrospective cohort study. Dis Markers. (2021) 2021:5543822. doi: 10.1155/2021/5543822
25. Özdoğan HK, Karateke F, Özyazıcı S, Özdoğan M, Özaltun P, Kuvvetli A, et al. The predictive value of red cell distribution width levels on mortality in intensive care patients with community-acquired intra-abdominal sepsis. Ulusal travma ve acil cerrahi dergisi = Turkish J Trauma Emerg Surg TJTES. (2015) 21(5):352–7. doi: 10.5505/tjtes.2015.26737
26. Jiang Y, Jiang FQ, Kong F, An MM, Jin BB, Cao D, et al. Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: a preliminary observational study. Ann Intensive Care (2019) 9(1):67. doi: 10.1186/s13613-019-0542-7
27. Wen K, Du H, Tang B, Xiong B, Zhang A, Wang P. Complete blood count and myocardial markers combination with sequential organ failure assessment score can effectively predict the mortality in sepsis: A derivation and validation study. Int J Gen Med (2022) 15:3265–80. doi: 10.2147/IJGM.S349751
28. Li Y, She Y, Fu L, Zhou R, Xiang W, Luo L. Association between red cell distribution width and hospital mortality in patients with sepsis. J Int Med Res (2021) 49(4):3000605211004221. doi: 10.1177/03000605211004221
29. Lorente L, Martín MM, Abreu-González P, Solé-Violán J, Ferreres J, Labarta L, et al. Red blood cell distribution width during the first week is associated with severity and mortality in septic patients. PloS One (2014) 9(8):e105436. doi: 10.1371/journal.pone.0105436
30. Havens JM, Seshadri AJ, Salim A, Christopher KB. Red cell distribution width predicts out of hospital outcomes in critically ill emergency general surgery patients. Trauma Surg acute Care Open (2018) 3(1):e000147. doi: 10.1136/tsaco-2017-000147
31. Dubey A, Kumar S, Acharya S, Wanjari AK, Bawankule S, Agrawal S, et al. Impact of red cell and platelet distribution width in patients of medical intensive care unit. J Lab Physicians (2021) 13(4):309–16. doi: 10.1055/s-0041-1730883
32. Huang Y, Jiang S, Li W, Fan Y, Leng Y, Gao C. Establishment and effectiveness evaluation of a scoring system-RAAS (RDW, AGE, APACHE II, SOFA) for sepsis by a retrospective analysis. J Inflamm Res (2022) 15:465–74. doi: 10.2147/JIR.S348490
33. Song K, Guo C, Zeng Z, Li C, Ding N. Factors associated with in-hospital mortality in adult sepsis with escherichia coli infection. BMC Infect Dis. (2022) 22(1):197. doi: 10.1186/s12879-022-07201-z
34. Ghimire R, Shakya YM, Shrestha TM, Neupane RP. The utility of red cell distribution width to predict mortality of septic patients in a tertiary hospital of Nepal. BMC Emergency Med (2020) 20(1):43. doi: 10.1186/s12873-020-00337-8
35. Sadaka F, O'Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med (2013) 28(5):307–13. doi: 10.1177/0885066612452838
36. Kim S, Lee K, Kim I, Jung S, Kim MJ. Red cell distribution width and early mortality in elderly patients with severe sepsis and septic shock. Clin Exp Emergency Med (2015) 2(3):155–61. doi: 10.15441/ceem.15.037
37. Fontana V, Spadaro S, Bond O, Cavicchi FZ, Annoni F, Donadello K, et al. No relationship between red blood cell distribution width and microcirculatory alterations in septic patients. Clin Hemorheol Microcirculation (2017) 66(2):131–41. doi: 10.3233/CH-160154
38. Ju X, Wang F, Wang Li. Dynamic change of red cell distribution width levels in prediction of hospital mortality in Chinese elderly patients with septic shock. Chin Med J (2017) 130(10):1189–95. doi: 10.4103/0366-6999.205858
39. Gupta MK, Yadav G, Singh Y, Bhalekar A. Correlation of the changing trends of red cell distribution width and serum lactate as a prognostic factor in sepsis and septic shock. J Anaesthesiol Clin Pharmacol (2020) 36(4):531–4. doi: 10.4103/joacp.JOACP_105_19
40. Ling J, Liao T, Wu Y, Wang Z, Jin H, Lu F, et al. Predictive value of red blood cell distribution width in septic shock patients with thrombocytopenia: A retrospective study using machine learning. J Clin Lab Analysis (2021) 35(12):e24053. doi: 10.1002/jcla.24053
41. Jandial A, Kumar S, Bhalla A, Sharma N, Varma N, Varma S. Elevated red cell distribution width as a prognostic marker in severe sepsis: A prospective observational study. Indian J Crit Care Med peer-reviewed Off Publ Indian Soc Crit Care Med (2017) 21(9):552–62. doi: 10.4103/ijccm.IJCCM_208_17
42. Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama (2016) 315(8):775–87. doi: 10.1001/jama.2016.0289
43. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Curr Estimates Limitations. Am J Respir Crit Care Med (2016) 193(3):259–72. doi: 10.1164/rccm.201504-0781OC
44. Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol (2021) 34(2):71–6. doi: 10.1097/ACO.0000000000000958
45. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet (London England) (2020) 395(10219):200–11. doi: 10.1016/S0140-6736(19)32989-7
46. Zhang L, Huang T, Xu F, Li S, Zheng S, Lyu J, et al. Prediction of prognosis in elderly patients with sepsis based on machine learning (random survival forest). BMC Emergency Med (2022) 22(1):26. doi: 10.1186/s12873-022-00582-z
47. Jain K, Sharma D, Patidar M, Nandedkar S, Pathak A, Purohit M. Red cell distribution width as a predictor of mortality in patients with clinical sepsis: Experience from a single rural center in central India. Clin Pathol (Thousand Oaks Ventura County Calif) (2022) 15:2632010x221075592. doi: 10.1177/2632010X221075592
48. Wang AY, Ma HP, Kao WF, Tsai SH, Chang CK. Red blood cell distribution width is associated with mortality in elderly patients with sepsis. Am J Emergency Med (2018) 36(6):949–53. doi: 10.1016/j.ajem.2017.10.056
Keywords: red blood cell distribution width, sepsis, diagnostic value, mortality, prognosis, meta-analysis
Citation: Wu H, Liao B, Cao T, Ji T, Huang J and Ma K (2022) Diagnostic value of RDW for the prediction of mortality in adult sepsis patients: A systematic review and meta-analysis. Front. Immunol. 13:997853. doi: 10.3389/fimmu.2022.997853
Received: 19 July 2022; Accepted: 23 September 2022;
Published: 17 October 2022.
Edited by:
Yuan-Qiang Lu, Zhejiang University, ChinaReviewed by:
Evangelos Giamarellos-Bourboulis, National and Kapodistrian University of Athens, GreeceI-Shiang Tzeng, National Taipei University, Taiwan
Copyright © 2022 Wu, Liao, Cao, Ji, Huang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongsheng Wu, Y3Jhenl3dTIwMDdAMTI2LmNvbQ==; Keqiang Ma, bWtxOTkyOEBob3RtYWlsLmNvbQ==
 Hongsheng Wu
Hongsheng Wu Biling Liao
Biling Liao Tengfei Ji
Tengfei Ji Keqiang Ma
Keqiang Ma