- 1Department of Neurology, Shandong Provincial Qianfoshan Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 3The Second School of Medicine, Wenzhou Medical University, Wenzhou, China
- 4The First School of Medicine, School of Information and Engineering, Wenzhou Medical University, Wenzhou, China
- 5Department of Emergency, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Background and Purpose: White blood cell count to mean platelet volume ratio (WMR) is increasingly recognized as a promising biomarker. However, its predictive capability for acute ischemic stroke (AIS) patients is relatively less researched. The primary aim of this study is to explore its prognostic value in AIS patients after reperfusion regarding 3-month poor functional outcome.
Methods: A total of 549 AIS patients who had undergone vascular reperfusion procedure with complete 3-month follow-up were retrospectively recruited in this study. White blood cell count, mean platelet volume at 24 h of admission were recorded. Stroke severity had been estimated using the National Institutes of Health Stroke Scale (NIHSS) and poor outcome was defined as modified Rankin Scale (mRS) 3–6 at 3 months.
Results: AIS patients with poor functional outcome at 3 months displayed higher WMR. A positive correlation between WMR and NIHSS score was found (r = 0.334, p < 0.001). After adjusting potential confounders, WMR was still an independent risk factor for poor prognosis at 3 months (OR = 2.257, 95% CI [1.117-4.564], p = 0.023) in multivariate logistic regression model. Subgroup analyses further suggested a significant association between WMR and poor outcome in high baseline NIHSS (per 0.1-point increase: OR = 1.153, 95% CI [1.014-1.312], p = 0.030) group. Receiver operating characteristic (ROC) curves analysis was utilized to assess the predictive ability of WMR, indicating a cut-off value of 0.86. A nomogram that includes age, sex, NIHSS on admission, high WMR for predicting 1-year all-cause survival was also developed (C-index = 0.628).
Conclusions: WMR is significantly correlated with stroke severity on admission and is proved to be an important prognostic indicator for AIS outcomes, especially in high NIHSS on admission group. Additionally, the developed nomogram that includes high WMR for predicting 1-year survival provides us with an effective visualization tool.
Introduction
Acute ischemic stroke (AIS) is a major type of stroke that can cause high mortality and morbidity (1). As a currently accepted and recognized management by rapid vascular reperfusion within 4.5 h of stroke onset, intravenous thrombolysis has been reported to yield a powerful effect on clinical outcomes and reduce disability in AIS patients (2). However, due to the following pathophysiological process after therapy, a proportion of patients are still inclined to develop poor functional outcomes (3). Therefore, seeking an accurate and reliable prognostic indicator to distinguish AIS patients after vascular reperfusion that may have a higher risk of poor functional outcomes is of great clinical value (4).
Systemic inflammation response, in which white blood cell (WBC) plays a vital role, has been proved to be involved in the pathogenesis in ischemic stroke (5). Previous studies among AIS patients have found an association between early elevation of leukocyte and the volume of infarcted area, which has been proven to be positively correlated to poor functional outcomes (6, 7). Also, several studies have suggested that WBC on admission is correlated with greater degree of neurological impairment and unfavorable long-term outcome (8, 9).
Mean platelet volume (MPV), which reflects platelet size, can provide information of platelet function and activation (10). Hyperactive platelets play a pivotal role in thrombus formation and propagation (4), which is one of the pathological processes of AIS. In inflammatory conditions, MPV is also associated with increased percentage of large platelets (11). The predictive value of MPV in ischemic stroke has been explored and confirmed in other studies (4, 12).
As a combination of the two above-mentioned biomarkers, white blood cell count to mean platelet volume ratio (WMR) indicates to be more stable and comprehensive regarding the pathology of AIS (13). It has been recognized in other literature as a relatively new marker for prognosis in atherosclerotic diseases and myocardial infarction (14). Moreover, recent evidence suggested an association between platelet-to-white blood cell ratio (PWR), a similar index to WMR, and the National Institutes of Health Stroke Scale (NIHSS) on admission and on 7 days after admission in AIS patients (15). This previous study has also provided important information on the clinical value of the two combined biomarkers. However, the predicting value of WMR in the prognosis of AIS patients still remains less researched. Therefore, in this retrospective observatory cohort study, we sought to analyze whether WMR would be able to indicate patients at high risk for poor outcomes at 3 months after vascular reperfusion.
Materials and methods
Study population
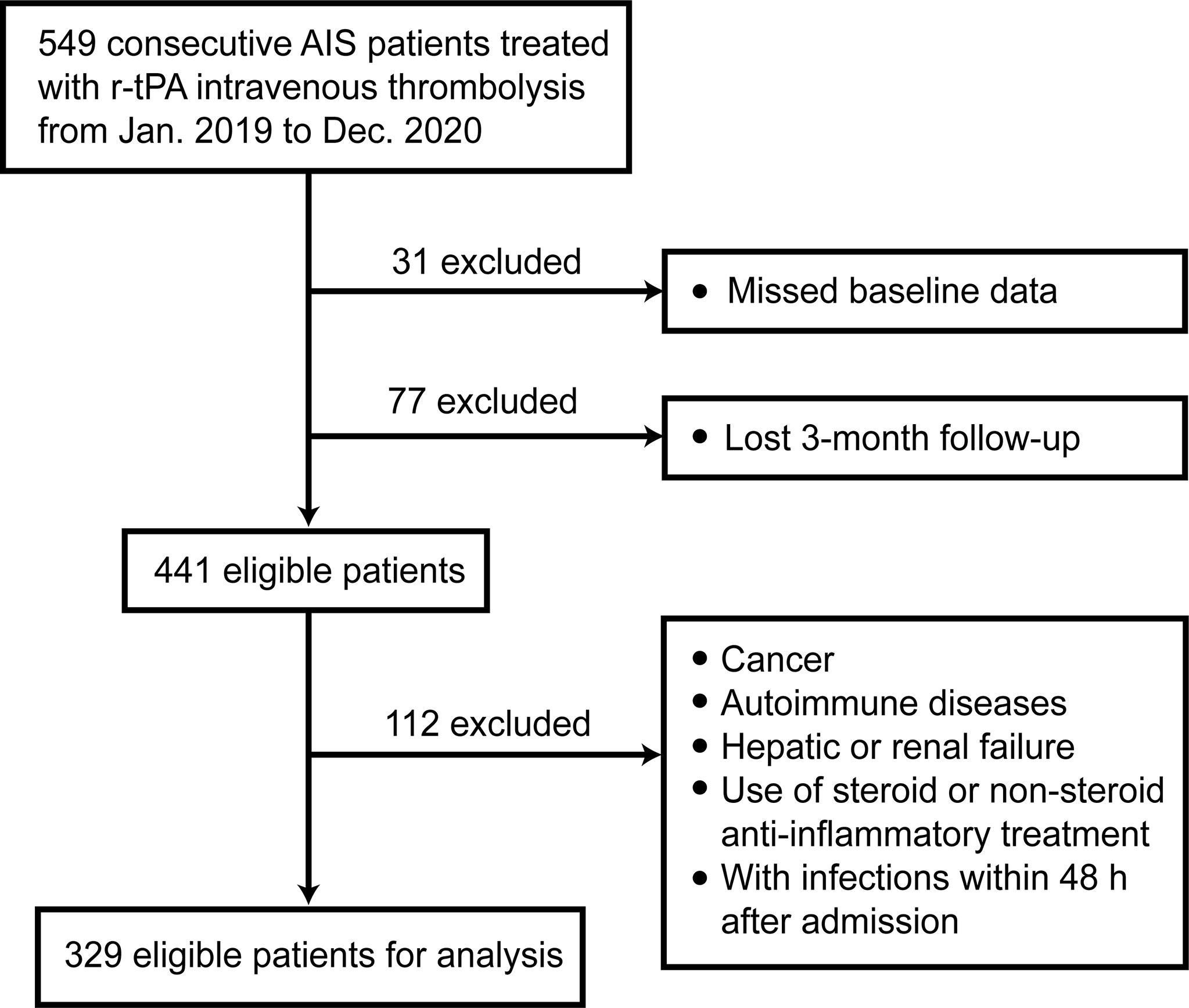
In this retrospective study, a total of 549 AIS patients receiving intravenous thrombolysis therapy were consecutively recruited from the First Affiliated Hospital of Wenzhou Medical University from January 2019 to December 2020. The exclusion criteria were set as follows (1): with cancer; (2) with autoimmune diseases; (3) with severe hepatic failure or renal failure; (4) use of steroid or non-steroid anti-inflammatory treatment (14, 16); (5) with infections within 48h after admission.
We followed up these patients for 3 months and 1 year after AIS onset. After excluding 108 patients with missing data, there remained 441 patients. Finally, a total of 329 patients were eligible for analysis with complete 3-month follow-up. Figure 1 shows the exclusion and inclusion procedure in the form of flow chart.
The study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and was performed in accordance with the Declaration of Helsinki. All patients signed a written informed consent form.
Data collection
Demographic data (age, sex) and medical history (smoking, alcohol, hypertension, diabetes, atrial fibrillation, coronary artery disease and prior stroke) of patients were obtained from medical records. Serum biomarkers included WBC count, MPV collected at 24 h of admission. WMR was calculated with WBC counts divided by MPV. Stroke severity was measured by NIHSS on admission and 24 h after admission. At 3 months and 1 year after the onset of AIS, the prognoses of patients were assessed using modified Rankin Scale (mRS) through telephone follow-up by two experienced clinicians.
Clinical assessment
The etiology of AIS was classified based on the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria: large artery atherosclerosis (LAA), small artery occlusion (SAO), cardio-embolism (CE), and others (17). Primary outcome included death or major disability at 3 months, defined as mRS score at 3-6. Secondary outcomes included poor functional outcome at 3 months defined by mRS score 2-6, early neurological improvement (ENI) defined as NIHSS score at 0-1 within 24 h after thrombolysis (18), early neurological deterioration decrease (END) defined as a decrease ≥ 4 points in the NIHSS score (19), death or major disability (mRS 3-6) at 1 year and all-cause mortality at 1 year.
Statistical analysis
We used SPSS Statistics 26.0.0.0, MedCalc Statistical Software version 18.2.1, GraphPad Prism version 9.0.0 and R version 4.1.3 for plots and statistical analyses. Data were first analyzed for normality of distribution using the Kolmogorov-Smirnov test of normality. Continuous variables with normal distribution were expressed as mean with standard deviation (mean ± SD) while continuous variables with non-normal distribution were expressed as medians and interquartile range (median, IQR). Patients were attributed into high/low WMR groups according to the cut-off value and inter-group differences were compared using Mann-Whitney U test. Participants were then divided into four groups according to WMR quartile (Q1 < 0.581, Q2 0.581-0.728, Q3 0.728-0.919, Q4 > 0.919). Differences of baseline characteristics across WMR quartile were compared using Kruskal-Wallis test. Categorical variables were presented as frequency and percentages (n, %) and differences among groups were compared through chi-squared test or Fisher’s exact test.
Case distribution across quartile-based categories of WMR and mRS score at 3 months was presented using density plots. To test the correlation between WMR and NIHSS, we calculated Spearman’s correlation coefficient. To account for differences in low WMR distributions across stroke subtype, and differences in mRS distribution in high and low WMR groups classified using WMR cutoff value, chi-squared test was performed.
Univariate and multivariate logistic analyses were utilized to demonstrate the association between WMR and primary outcome. The crude model, model 1, was carried out by univariate analysis. In model 2, we adjusted for covariates with a p-value < 0.1 in model 1. To further explore the potential predictive ability disparity in different subgroups, stratified analysis was undertaken. With WMR median as a node undergoing binary logistic regression, restricted cubic splines with 3 knots (at 0.6, 0.9 1.2 respectively) were also plotted to demonstrate the correlation between WMR and AIS outcomes.
Receiver operating characteristic (ROC) analysis was employed to determine the optimal cutoff value and the ability of WMR to distinguish poor functional outcomes. Multivariable time-to-event analysis was performed using Cox proportional hazards regression models to develop a nomogram using weighted estimators corresponding to each covariate that was found statistically significant in predicting 1-year mortality using multivariate regression model. A p-value of less than 0.05 was considered statistically significant.
Results
Characteristics of study patients
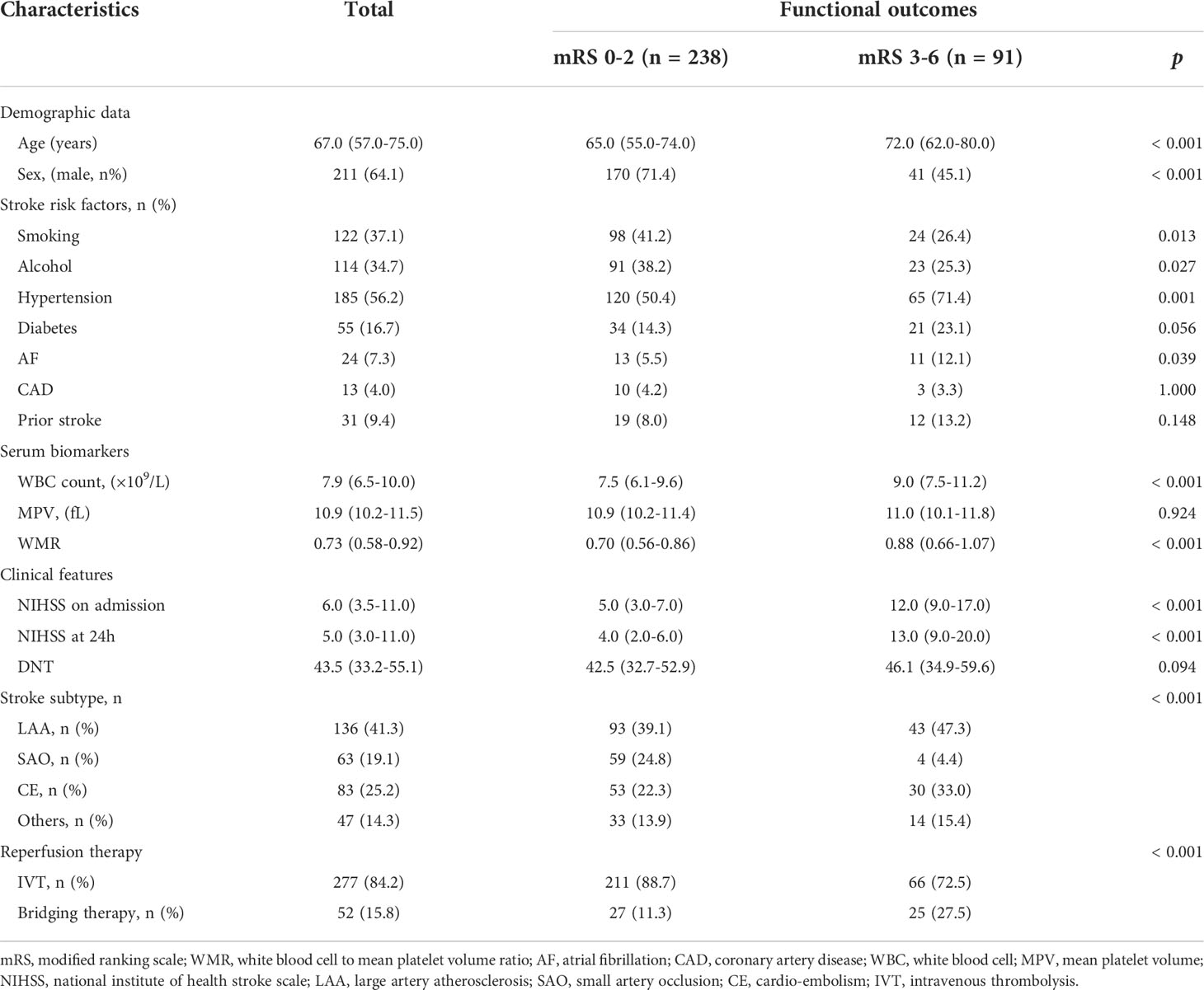
A total of 329 AIS patients after intravenous thrombolysis were retrospectively included for analysis in this study, with a median age of 67 years and men accounting for 64.1% (211). The median NIHSS score on admission for all study patients was 6, and median WMR was 0.73. A total of 91 (27.7%) patients had poor functional outcomes at 3 months, 31 patients (9.4%) died during the 1-year follow-up. The clinical characteristics of AIS patients were displayed in Table 1. Compared with patents with a good functional outcome, those in the poor outcome group had significantly older age (median age: 72.0 vs. 65.0, p < 0.001), higher proportion of hypertension (71.4% vs. 50.4%, p = 0.001), higher proportion of atrial fibrillation (AF) (12.1% vs. 5.5%, p = 0.039), higher WBC count (median: 9.0 vs. 7.5, p < 0.001), higher WMR (median: 0.88 vs. 0.70, p < 0.001), higher NIHSS on admission (median: 12.0 vs. 5.0, p < 0.001), and at 24 h (median: 13.0 vs. 4.0, p < 0.001). Those with a good functional outcome were more likely to be male (71.4% vs. 45.1%, p < 0.001) and the small artery occlusion (SAO) subtype (24.8% vs. 4.4%, p < 0.001). Furthermore, smoking history (41.2% vs. 26.4%, p = 0.013), alcohol drinking history (38.2% vs. 25.3%, p = 0.027) were more prevalent in patients with a good outcome in comparison with those with a poor outcome.
As illustrated in Table 2, it was found that smoking, WBC count, MPV, NIHSS on admission and at 24 h, SAO subtype distribution, mRS score 3-6, 2-6, mRS 3-6 at 1 year mortality rate at 1 year differed by WMR quartile.
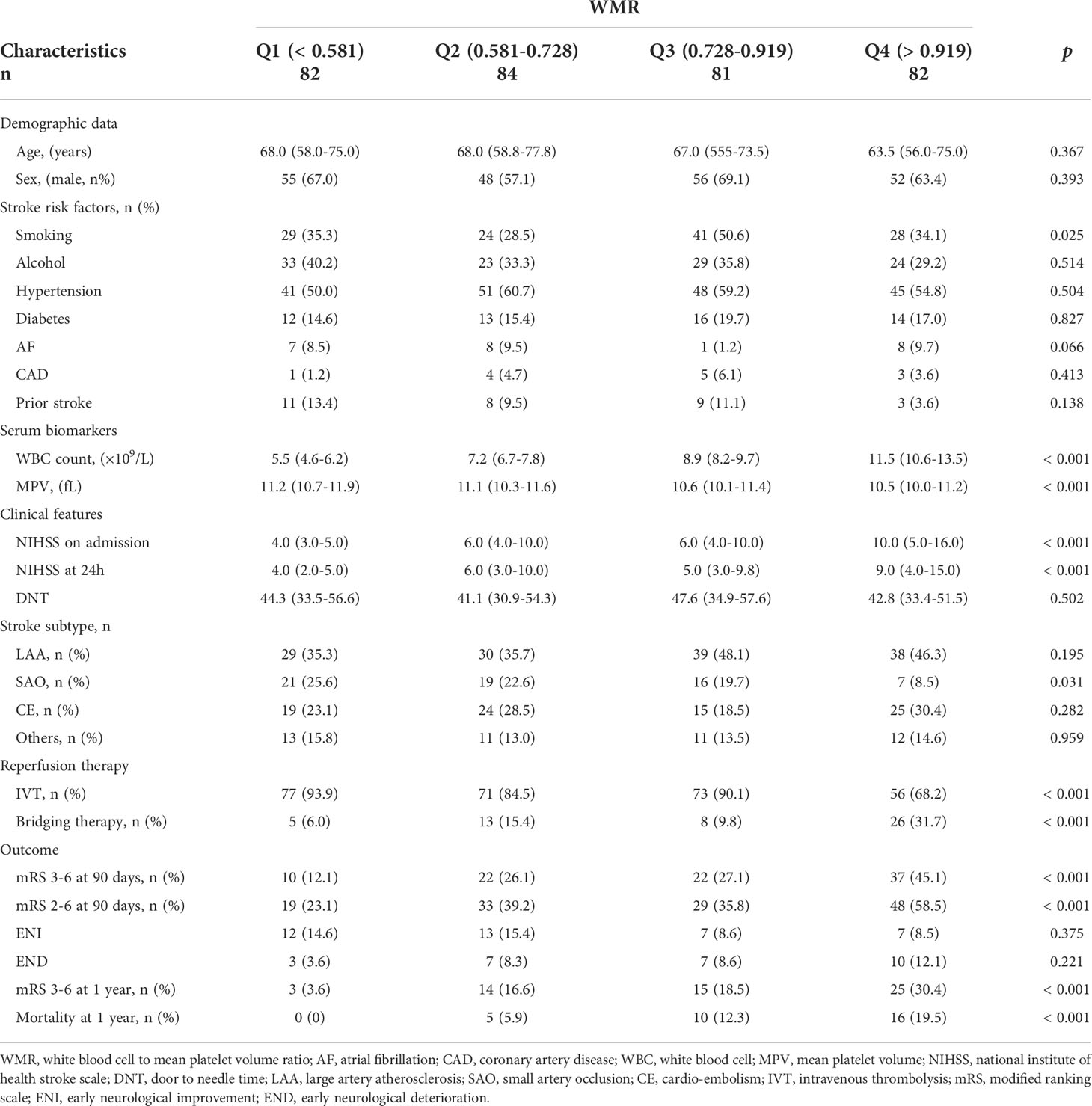
Table 2 Comparisons of baseline characteristics and outcomes between groups divided according to WMR quartile.
The correlation between WMR and clinical status
We plotted a density plot to display patient distribution cross WMR quartile. The intensity of color represented the frequency of cases. It can be observed in Figure 2A, that in general, 200 out of the total 329 cases had the outcome mRS 0-1 (60.8%). In Q1, cases with mRS score at 1 took up the highest proportion (46.3%), while in Q4, 15 cases developed a mRS score at 6 (18.3%). The case distribution across WMR quartile was statistically significant (p < 0.001). Correlation analysis demonstrated a significant linear trend between WMR and baseline NIHSS (r = 0.334, p < 0.001). The size of circles represented the relative weighting of mRS score at 3 months. It can be seen in Figure 2B, that patients with higher WMR showed a higher NIHSS score on admission and a higher mRS score at 3 months. We further investigated the difference in distribution of mRS score between patients in high WMR group (the outer layer of the circle) and low WMR group (the inner layer). High WMR was found to be positively related to death or major disability (mRS 3-6) (r = 0.251, p < 0.001), and proportion of mRS 3-6 in high WMR group was more prominent compared with that in low WMR group (Figure 2C). In addition, we plotted Figure 2D, to visually display the ratio of low WMR group in different stroke subtypes. Chi-squared test verified a significant difference in low WMR distributions across stroke subtype (p = 0.007), and proportion of low WMR was reported to be the largest in SAO subtype (85.7%).
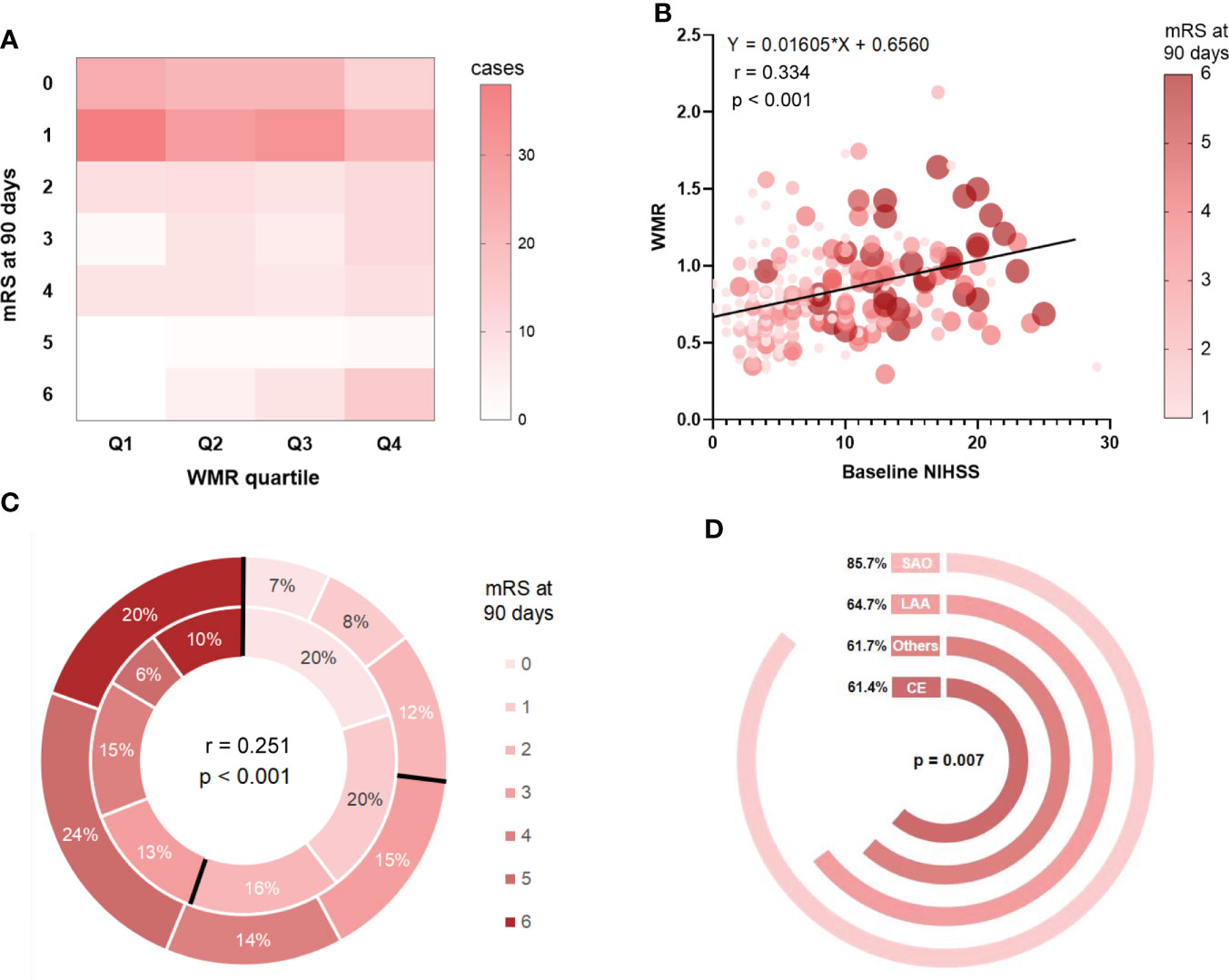
Figure 2 Correlation analyses between WMR and patient features and outcome. (A): Case distribution across quartile-based categories of WMR and mRS score at 90 days. (B): The relationships between WMR and baseline NIHSS as well as mRS at 3 months. (C) mRS distribution at 3 months for high WMR group v.s. low WMR group. The outer layer represented distribution of mRS score in high WMR group while the inner layer represented that in low WMR group. (D): Ratio of low WMR group in different stroke subtypes.
Predictive value of WMR for primary outcome
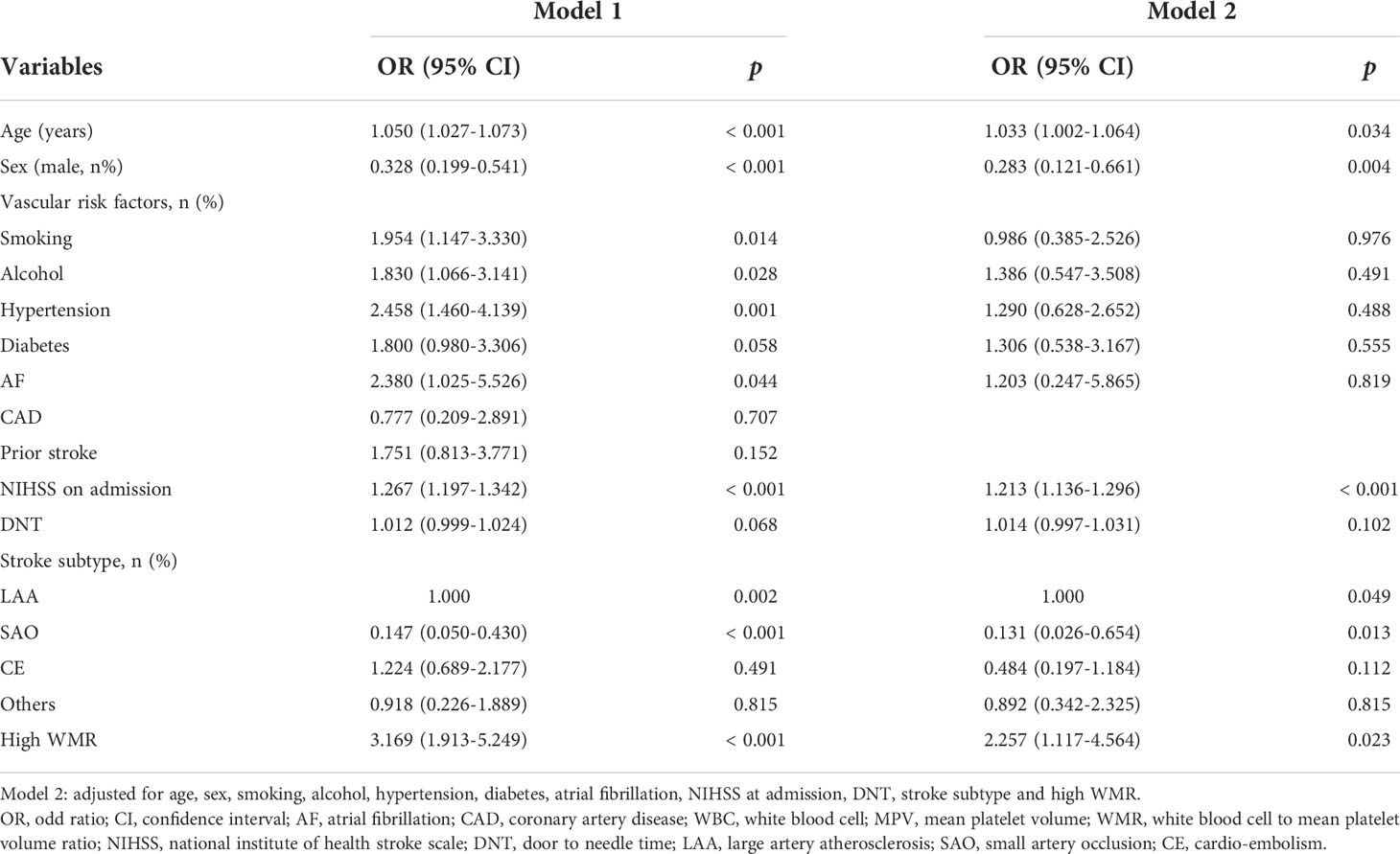
As shown in model 1 conducted under the univariate regression analyses, age, smoking, alcohol, hypertension, AF, NIHSS on admission, stroke subtypes and high WMR (OR = 3.169, 95% CI [1.913-5.249], p < 0.001) were found significantly associated with primary outcome (mRS 3-6) at 3 months. To determine whether high WMR possessed the ability to independently indicate the prognosis for poor outcome at 3 months, multivariate logistic regression was then performed. In model 2, after adjusting for potential confounders with a p-value < 0.1 in model 1, high WMR remained to have an independent association with poor 3-month function outcome (OR = 2.257, 95% CI [1.117-4.564], p = 0.023) (Table 3). Univariate logistic regression model with restricted cubic splines revealed a positive association between WMR level and the odds of 3-month poor outcomes, including mRS 3-6 and mRS 2-6 (both p for linearity < 0.001, Figure 3).
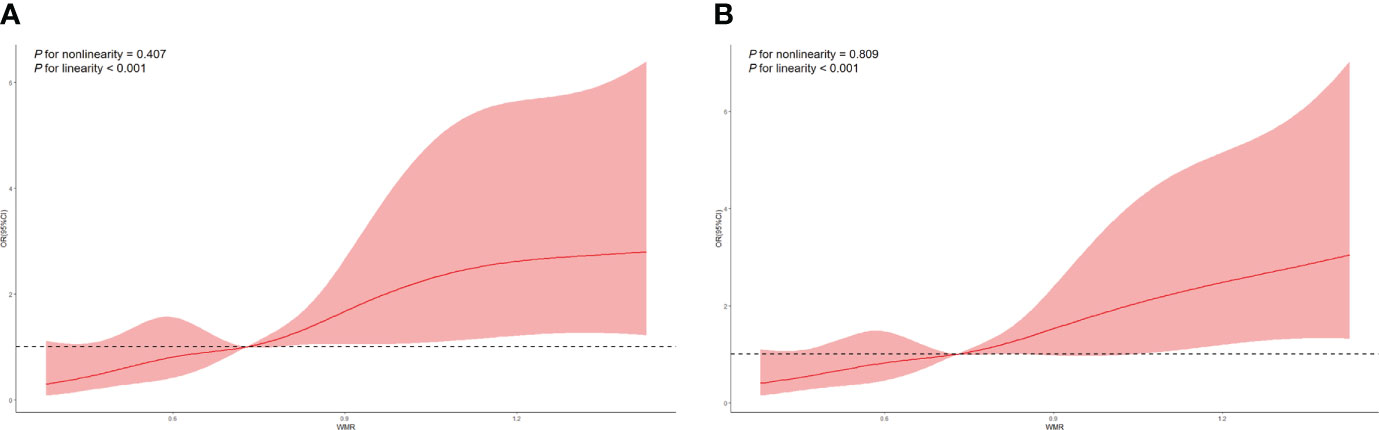
Figure 3 Restricted cubic spline regression analyses of WMR and poor outcomes. (A) mRS 3-6 at 3 months. (B) mRS 2-6 at 3 months.
In terms of the ROC analysis for the prediction of poor 3-month outcome using WMR, the cut-off value with the optimal distinguishing capacity of this indicator was 0.86 (a sensitivity of 51.6 and a specificity of 75.2). Additionally, The area under curve (AUC) was 0.662 (95% CI [0.608-0.713], p < 0.001) (Figure S1).
Subgroup analyses
In subgroup analyses stratified by age, sex, smoking, alcohol, hypertension, diabetes, NIHSS on admission and stroke subtype (LAA), an attenuated association between WMR and poor functional outcomes in patients with lower NIHSS score on admission was identified (Table 4). WMR per 0.1-point increase was independently associated with poor functional outcomes in age ≥ 67 (OR = 1.207, 95% CI [1.015–1.436], p = 0.034) and NIHSS on admission ≥ 6 (OR = 1.153, 95% CI [1.014–1.312], p = 0.030) groups (Table 4).
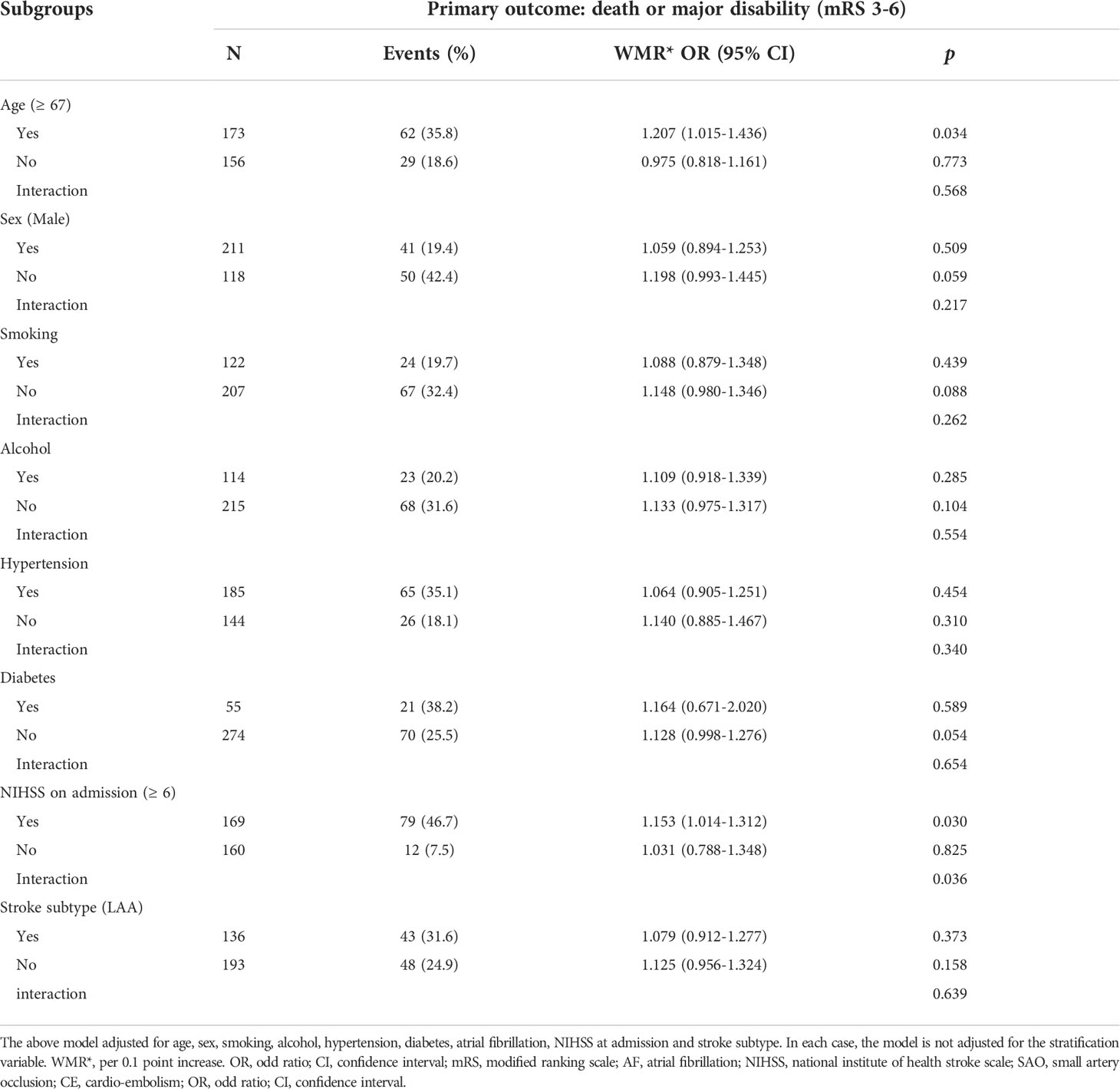
Table 4 Subgroup analyses of the association between WMR and primary outcome (death or major disability).
Predictive value of WMR for all-cause mortality
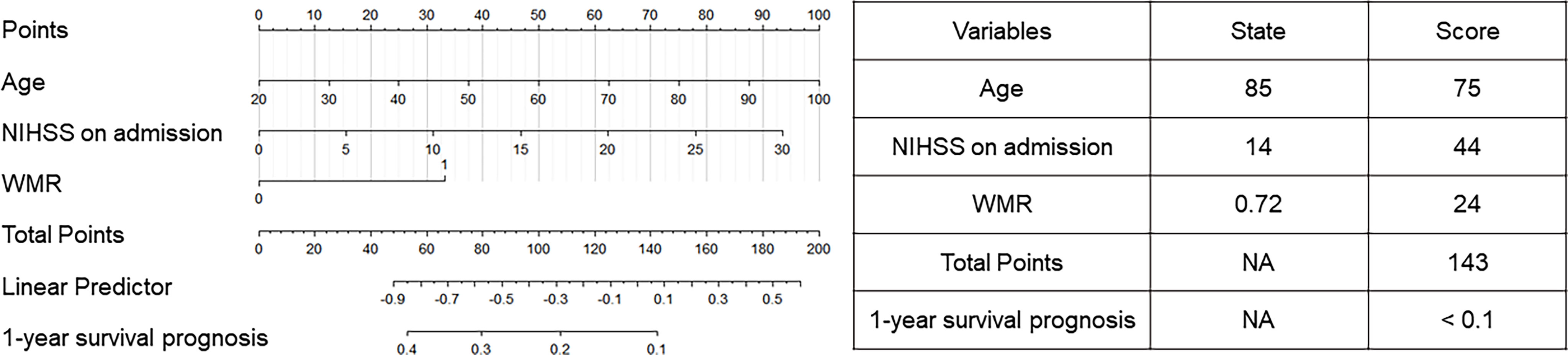
Univariate and multivariate analyses (Table S2) were performed to spot prognostic factors to build the nomogram. In the univariate analysis, age (OR = 1.092, 95% CI [1.050–1.135], p < 0.001), sex (OR = 0.383, 95% CI [0.176–0.836], p = 0.016), hypertension (OR = 3.843, 95% CI [1.502–9.833], p = 0.005), AF (OR = 8.064, 95% CI [2.290–28.400], p = 0.001), NIHSS on admission (OR = 1.260, 95% CI [1.163–1.364], p < 0.001) and high WMR (OR = 4.007, 95% CI [1.795–8.943], p = 0.001) were found to be independent risk factors for 1-year mortality. After including these factors with p < 0.05 into the multivariate logistic regression model, age (OR = 1.076, 95% CI [1.028–1.126], p = 0.002), NIHSS on admission (OR = 1.190, 95% CI [1.085–1.305], p < 0.001), and high WMR (OR = 3.251, 95% CI [1.145–9.227], p = 0.027) still remained significant. By including those, a nomogram was constructed (Figure 4) and was evaluated to possess a relatively high accuracy (C-index = 0.628). Table on the right showed the use of the nomogram with one death case as an example. For an 85-year-old patient with NIHSS score on admission at 14 and WMR at 0.72, the total score (143) was calculated with corresponding scores on the nomogram and presented the 1-year survival prognosis score (< 0.1).
Discussion
This study set out to explore the association between the relatively new biomarker WMR and the functional outcomes in patients with AIS after intravenous thrombolysis therapy. Overall, our current study mainly established that (1) WMR was significantly higher in patients with poor functional outcomes; (2) There was a linear relationship between WMR and NIHSS on admission, mRS 3-6, and mRS 2-6; (3) After adjusting for potential confounders, high WMR was still significantly correlated with primary outcome; (4) WMR was independently associated with poor functional outcome in older age and high NIHSS score on admission group, and the association was attenuated in patients with lower NIHSS score on admission. (5) WMR could be utilized to predict 1-year survival with a nomogram.
Vascular reperfusion as a therapy to restore the blood supply and limit the brain damage plays an important role in the management of AIS. Currently, available types of therapy to realize the purpose of vascular reperfusion in AIS patients mainly include intravenous thrombolysis and endovascular treatment (20). However, due to the complex stroke pathobiology, these strategies may have limited effectiveness and induce serious side effects (21). In the past few decades, blood-based biomarkers for prognosis of poor functional outcomes following reperfusion therapy in AIS patients has been an object of research (22). Therefore, it is of great importance to identify reliable and accurate biomarkers that could facilitate disease management and clinical decision making.
The impact of inflammatory processes in the progression of AIS has been widely recognized (5). Previous studies have validated that an elevated WBC count is independently associated with stroke severity on admission, poor functional outcomes at discharge, and mortality rates in AIS patients (6). Our data suggested a significantly elevated WBC count at 24 h of admission in the group with poor functional outcome, which also supported the prognostic capability of WBC count.
Platelets play a role in the formation of atherosclerosis and atherothrombosis, which is a critical process during the pathophysiology of stroke (23). They not only participate in clot formation, but are also involved in inflammatory processes (24). During the inflammatory course, the proportion of larger platelets was found to increase, probably because of a synthesis of factors that promote coagulation and inflammation, and a release of platelet stored in the spleen (11). At the same time, these platelets are fast recruited to the inflammation site where they might be activated and worn down, which can possibly account for the decrease of MPV in patients in an inflammation condition (25). Besides, ischemia-reperfusion injury (IRI) is also a prominent pathological process after reperfusion therapy in AIS patients, which may result in a consumption and sequestration of platelets, and thus be a part of the explanation (24).
WMR as a composite marker comprised of WBC count and MPV, indicates a promising value for the prognosis of cardiovascular outcomes (14). The interaction between the two single biomarkers is suggested to be related to the inflammatory response and stroke infarction development, where their counts are mutually affected (15). The elevation of WMR has been demonstrated by other study to reliably identify lower extremity artery disease (LEAD) patients at high risk for chronic limb-threatening ischemia (CLTI), and an association with the endpoint of stroke, which has not been elaborated (13). Additionally, WMR has also been researched in the prediction for patients with non-ST elevation myocardial infarction (14). Though previous research has identified an association between PWR with the severity of AIS and also divided patients according to stroke etiology, it did not examine the long-term prognostic value of the index and did not consider reperfusion therapy (15). In the current study, we explored the predictive value of WMR for different outcomes in a group of patients that all had undergone reperfusion therapy and conducted a further subgroup analysis. However, the prognostic value of WMR in AIS remains less researched. Therefore, more trials concentrating on AIS patients regarding the efficacy of WMR are supposed to be carried out in the future to validate our findings.
Our findings of the elevation in WMR might be explained by an increase in the WBC count and an decrease in the MPV in the inflammation process, which can provide indications for the disease development for AIS patients (13). Sensitivity and specificity analysis demonstrated that compared to WBC as an independent predictor, the value of sensitivity decreased but specificity for prognosis improved after adding MPV to form the WMR parameter, which reduces the probability of false positive rate (Table S1). At the same time, it can be easily calculated using the two cheap and available biomarkers from a complete blood cell count test. Thus, it can be of help for clinicians to make relatively accurate prognosis for AIS patients at an early stage.
However, it should be noted that several limitations still exist in our study. First, considering the retrospective and observational nature of this study, it was unable to determine the causality between WMR and poor functional outcomes. Second, the sample size was relatively small. Among the 549 patients who were initially enrolled under the inclusion criteria, 31 (5.6%) patients were excluded for missing baseline data and 77 (14.0) were excluded for the loss of 3 months follow-up. Partly due to the sample size, the ROC curve for the prediction of a 3-month poor outcome using WMR did not reach a high prediction capability (AUC 0.662, sensitivity 51.6, specificity 75.2), which needs to be further researched with a larger data base. Additionally, this study was monocentric so selection bias might still exist and an external validation of the nomogram could not be conducted.
Conclusion
Our study demonstrated that there is a linear correlation between WMR and NIHSS on admission. High WMR is positively related to a poor functional outcome, and possesses a prognostic potential for AIS outcomes. The predictive capability is especially prominent in older and neurologically more severe groups. Furthermore, as an easily obtainable and cost-effective parameter, WMR can also be utilized to predict 1-year mortality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XL and XZ conceptualized this work. XL and XZ supervised the study. YW, YG, MZ, TZ, JH, HX, JH, YC, XH, JX, JZ, SL, TK acquisition of data, YW, YG, MZ performed the statistical analysis and interpreted data. YW, YG, MZ prepared the manuscript. XL, XZ, YW, YG, MZ, TZ, JH, HX, JH, YC, XH, JX, JZ, SL, TK revised the manuscript. All authors approved the protocol.
Acknowledgments
We sincerely thank the participating hospitals, patients, their families and colleagues who have provided valuable suggestions for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.995911/full#supplementary-material
References
1. Campbell BCV, Khatri P. Stroke. Lancet (2020) 396(10244):129–42. doi: 10.1016/S0140-6736(20)31179-X
2. Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers (2019) 5(1):70. doi: 10.1038/s41572-019-0118-8
3. Orellana-Urzua S, Rojas I, Libano L, Rodrigo R. Pathophysiology of ischemic stroke: Role of oxidative stress. Curr Pharm Design (2020) 26(34):4246–60. doi: 10.2174/1381612826666200708133912
4. Staszewski J, Pogoda A, Data K, Walczak K, Nowocien M, Frankowska E, et al. The mean platelet volume on admission predicts unfavorable stroke outcomes in patients treated with IV thrombolysis. Clin Interventions Aging (2019) 14:493–503. doi: 10.2147/CIA.S195451
5. Stoll G, Nieswandt B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat Rev Neurol (2019) 15(8):473–81. doi: 10.1038/s41582-019-0221-1
6. Furlan JC, Vergouwen MDI, Fang J, Silver FL. White blood cell count is an independent predictor of outcomes after acute ischaemic stroke. Eur J Neurol (2014) 21(2):215–22. doi: 10.1111/ene.12233
7. Zaidi SF, Aghaebrahim A, Urra X, Jumaa MA, Jankowitz B, Hammer M, et al. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke (2012) 43(12):3238–44. doi: 10.1161/STROKEAHA.112.671594
8. Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and white men and women - atherosclerosis risk in communities study. Am J Epidemiol (2001) 154(8):758–64. doi: 10.1093/aje/154.8.758
9. Zheng X, Zeng N, Wang A, Zhu Z, Zhong C, Xu T, et al. Prognostic value of white blood cell in acute ischemic stroke patients. Curr Neurovascular Res (2018) 15(2):151–7. doi: 10.2174/1567202615666180626154857
10. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: A link between thrombosis and inflammation? Curr Pharm Design (2011) 17(1):47–58. doi: 10.2174/138161211795049804
11. Schwertz H, Koster S, Kahr WHA, Michetti N, Kraemer BF, Weitz DA, et al. Anucleate platelets generate progeny. Blood (2010) 115(18):3801–9. doi: 10.1182/blood-2009-08-239558
12. Xie D, Xiang W, Weng Y, Li J, Xu L, Zhang X, et al. Platelet volume indices for the prognosis of acute ischemic stroke patients with intravenous thrombolysis. Int J Neurosci (2019) 129(4):344–9. doi: 10.1080/00207454.2018.1536054
13. Guetl K, Raggam RB, Muster V, Gressenberger P, Vujic J, Avian A, et al. The white blood cell count to mean platelet volume ratio for the prediction of chronic limb-threatening ischemia in lower extremity artery disease. J Clin Med (2019) 8(10):1593. doi: 10.3390/jcm8101593
14. Sivri S, Sokmen E, Celik M, Ozbek SC, Yildirim A, Boduroglu Y. Usefulness of white blood cell count to mean platelet volume ratio in the prediction of SYNTAX score in patients with non-ST elevation myocardial infarction. Pakistan J Med Sci (2019) 35(3):824–9. doi: 10.12669/pjms.35.3.1017
15. Amalia L, Dalimonthe NZ. Clinical significance of platelet-to-White blood cell ratio (PWR) and national institute of health stroke scale (NIHSS) in acute ischemic stroke. Heliyon (2020) 6(10):e05033. doi: 10.1016/j.heliyon.2020.e05033
16. Cicek G, Acikgoz SK, Yayla C, Kundi H, Ileri M. White blood cell count to mean platelet volume ratio: A novel and promising prognostic marker for ST-segment elevation myocardial infarction. Cardiol J (2016) 23(3):225–35. doi: 10.5603/CJ.a2016.0001
17. Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. trial of org 10172 in acute stroke treatment. Stroke (1993) 24(1):35–41. doi: 10.1161/01.str.24.1.35
18. Wang M, Farouki Y, Hulscher F, Mine B, Bonnet T, Elens S, et al. Early neurological improvement predicts clinical outcome after thrombectomy for distal medium vessel occlusions. Front Neurol (2022) 13. doi: 10.3389/fneur.2022.809066
19. Saleem Y, Nogueira RG, Rodrigues GM, Kim S, Sharashidze V, Frankel M, et al. Acute neurological deterioration in Large vessel occlusions and mild symptoms managed medically. Stroke. (2020) 51(5):1428–34. doi: 10.1161/STROKEAHA.119.027011
20. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome - a meta-analysis. Stroke. (2007) 38(3):967–73. doi: 10.1161/01.STR.0000258112.14918.24
21. Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, et al. Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovascular Diseases. (2008) 25(3):268–78. doi: 10.1159/000118039
22. Montellano FA, Ungethüm K, Ramiro L, Nacu A, Hellwig S, Fluri F, et al. Role of blood-based biomarkers in ischemic stroke prognosis. Stroke (2021) 52(2):543–51. doi: 10.1161/STROKEAHA.120.029232
23. Alexandru N, Andrei E, Dragan E, Georgescu A. Interaction of platelets with endothelial progenitor cells in the experimental atherosclerosis: Role of transplanted endothelial progenitor cells and platelet microparticles. Biol Cell (2015) 107(6):189–204. doi: 10.1111/boc.201400071
24. Burkard P, Voegtle T, Nieswandt B. Platelets in thrombo-inflammation: Concepts, mechanisms, and therapeutic strategies for ischemic stroke. Hamostaseologie (2020) 40(2):153–64. doi: 10.1055/a-1151-9519
Keywords: WMR, ischemic stroke, inflammation, intravenous thrombolysis, prognosis
Citation: Weng Y, Gao Y, Zhao M, Zeng T, Huang J, Xie H, Huang J, Chen Y, Hu X, Xu J, Zhu J, Lin S, Ke T, Li X and Zhang X (2022) The white blood cell count to mean platelet volume ratio for ischemic stroke patients after intravenous thrombolysis. Front. Immunol. 13:995911. doi: 10.3389/fimmu.2022.995911
Received: 16 July 2022; Accepted: 01 September 2022;
Published: 03 October 2022.
Edited by:
Paolo Immovilli, Guglielmo da Saliceto Hospital, ItalyReviewed by:
Alina Gonzalez-Quevedo, Instituto de Neurología y Neurocirugía, La Habana, CubaYicheng Xu, Aerospace Center Hospital, China
Chiara Terracciano, Gugliemo da Saliceto Hospital, Italy
Copyright © 2022 Weng, Gao, Zhao, Zeng, Huang, Xie, Huang, Chen, Hu, Xu, Zhu, Lin, Ke, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Zhang, ZHJ6aGFuZ3h1QDEyNi5jb20=; Xiang Li, d3ptdWxpeGlhbmdAMTI2LmNvbQ==
†These authors share first authorship
 Yiyun Weng1,2†
Yiyun Weng1,2† Mingyue Zhao
Mingyue Zhao Xiang Li
Xiang Li Xu Zhang
Xu Zhang