- Center of Reproductive Medicine, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, China
Recurrent spontaneous abortion (RSA) is defined as two or more pregnancy loss, affecting the happiness index of fertility couples. The mechanisms involved in the occurrence of RSA are not clear to date. The primary problem for the maternal immune system is how to establish and maintain the immune tolerance to the semi-allogeneic fetuses. During the pregnancy, decidual macrophages mainly play an important role in the immunologic dialogue. The purpose of this study is to explore decidual macrophages, and to understand whether there is a connection between these cells and RSA by analyzing their phenotypes and functions. Pubmed, Web of Science and Embase were searched. The eligibility criterion for this review was evaluating the literature about the pregnancy and macrophages. Any disagreement between the authors was resolved upon discussion and if required by the judgment of the corresponding author. We summarized the latest views on the phenotype, function and dysfunction of decidual macrophages to illuminate its relationship with RSA.
Introduction
Recurrent spontaneous abortion (RSA) is an early pregnancy complication, which is defined as two or more spontaneous pregnancy loss with the same couple (1). The European Society of Human Reproduction and Embryology (ESHRE) considers it often occur continuously prior to 24 gestational weeks (2). In recent years, RSA becomes one of the formidable challenges for the doctors and infertile patients, as it may govern the fate of the whole family. However, miscarriage is a relatively common problem, occurring in 12 to 15 percent of clinically recognized pregnancies. Its risk increases with maternal age (3) and each previous prenancy losses stepwise. Patients suffered from recurrent abortion account for 1-3% (4). Large amount of data shows that RSA patients are a high-risk population for obstetrical and perinatal complications (5, 6). As the number of miscarriages has increased, the more damage to the maternal endometrium and the emergence of pelvic inflammatory disease, lead to secondary infertility. Equally, the risk of fetal growth restriction, placental abruption, premature delivery and stillbirth in future pregnancies are also raised. What can not be ignored are the following issues, such as venous thromboembolism, mental health and economic costs. Thus, closer surveillance of the RSA patients in late pregnancy must be introduced in clinical practice.
The causes of RSA are connected with anatomic defects, chromosomal abnormalities, immune dysregulation, thrombophilia, endocrine disease, infection, environmental and psychological factors (7, 8). Until now, approximately 50% of RSA cases remain elusive, leaving us away from an accurate examination and treatment. Actually, the follow-up studies of the exact etiology and pathogenesis are frequently difficult. Because of practical feasibility and ethical limitations, mouse models with higher conception rate and shorter gestation are always used in the studies of RSA (9). However, the relationship between unexplained RSA and immune system has increasingly drawn more attention in clinical practice. The latest research about a single-cell RNA sequencing showed macrophages have been observed in human yolk sac both morphologically and transcriptionally, which is essential for fetal development in early pregnancy (10). And numerous immunomodulatory therapies for RSA have been suggested.
Given the relationship with RSA, researches mostly support that the immunological factor is a prerequisite for a successful pregnancy (11). Compared to the more explicit role of NK cells in pregnancy (12), the roles of decidual macrophages in pregnancy have not been fully investigated. Macrophages are the second largest group of immune cells and account for 20 percent of the total leukocytes at the maternal-fetal interface (13). They participate in all physiological events in the female reproductive system, such as menstruation, implantation and deliver (14). On account of the polarization and plasticity of macrophages, they differentiate into specific phenotypes as a response to the microenvironmental stimuli (15, 16). The number and function of macrophages in the non-pregnant uterus are regulated by the estrogen and progesterone during the menstrual cycle (17). When the endometrium falls off at menstruation, macrophages with numbers peaking promote “wound healing” through phagocytosis and tissue remodel (18). Before implanting, macrophages are recruited to exhibit M1 phenotype to reply the inflammatory response resulting from seminal fluid. As extravillous trophoblasts (EVTs) begin to invade the decidua, decidual macrophages convert to a mixed profile of M1/M2 macrophages (19). Then, for the establishment of fetal immune tolerance, macrophages transform into an overwhelming M2 phenotype (20). By releasing proangiogenic growth factors such as interleukin 8 (IL-8), vascular endothelial growth factor (VEGF)-A and VEGF-C, M2 macrophages act as ‘bridge cells’. They jointly facilitate unique vascularization and immunosuppression in the placental microenvironment (21, 22). In the process of tissue remodeling, decidual macrophages protect embryos from phagocytosis and infection (23, 24). Therefore, decidual macrophages are indispensable in pregnancy and its dysfunction will lead to pregnancy loss.
Phenotypes of decidual macrophages
Plasticity and polarization are landmarks of macrophages (16, 25). At the maternal-fetal interface, notable changes have occurred in the decidual macrophages. Next, we will brief the classification and possible mechanisms of macrophage in terms of its phenotype.
M1/M2
Based on their cytokines secretion, chemokines expression and functional characteristics, decidual macrophages can be classified into two subsets: classically activated macrophages (M1 macrophages), and alternatively activated macrophages (M2 macrophages) (26–28).
Bacterial lipopolysaccharide (LPS) recognition or induction of Th1 cytokines, such as tumor necrosis factor α (TNF-α), interferon-γ (INF-γ), can drive M1 polarization of macrophages. These macrophages secrete pro-inflammatory cytokines and chemokines IL-1α, IL-1β, IL-6, IL-12, TNF-α, CXCL9, CXCL10 and express surface markers CD80, CD86, TLR-2, TLR-4, and major histocompatibility complex (MHC) class II. With the capacity of presenting antigen, M1 macrophages produce T helper type 1 (Th1) responses. It is characterized by maximizing the ability of immune cells to make cytotoxic or inflammatory reaction to viral infections, tumors or grafts. In early pregnancy, M1 macrophages promote embryo implantation and protect the fetus from infection (29–33).
Moreover, IL-4 and IL-13 directly induce M2 macrophage activation, IL-10 and transforming growth factor-β (TGF-β) make macrophages polarized toward the M2 phenotype. As the part of a polarized Th2 response, M2 macrophages are involved in apoptotic cells clearance and tissue remodeling. The release of a distinct set of chemokines, such as CCL17, CCL22 and CCL24 and their corresponding chemokine receptors CCR4 and CCR3, can also cause the recruitment of Th2 cells and amplification of polarized Th2 responses. They have immunosuppressive properties with higher levels of CD206, CD209 and CD163 expression. M2 macrophages provide an immune-tolerant environment for the fetus throughout pregnancy (29, 34–36).
Macrophages are typical plastic cells which can switch phenotypes and be subject to environmental disturbances (37, 38). Therefore, it is necessary to ensure the balance of M1 and M2 macrophages, so that the embryo can implant and develop smoothly at the maternal-fetal interface (39–42).
CD11chigh/CD11clow
Distinct from the traditional M1 and M2 macrophages, Houser classified decidual macrophages into CD11c high and CD11c low subpopulations on the basis of CD11c expression (43, 44). Moreover, Jiang et al. subdivided macrophages into three decidual subsets, CCR2-CD11cLO, CCR2-CD11cHI, and CCR2+CD11cHI by flow cytometry analysis (45, 46). CD11c low decidual macrophages and CCR2-CD11cLO subset expressed highly phagocytic receptors, such as CD209 and CD206 (43, 47). CCR2-CD11cLO macrophages also specifically exhibits heme oxygenase-1 (HMOX1) (48, 49), which may be in favor of protecting the fetus from being affected by possible infections during the early pregnancy (42). While CCR2+CD11cHI and CCR2-CD11cHI subsets posses pro-inflammatory and anti-inflammatory characteristics respectively (46, 50). They maintain an immnue balance to facilitate the clearance of pathogen infection and keep the homeostasis of the maternal-fetal interface (Table 1).
Functions of decidual macrophages in normal pregnancy
Decidual macrophages have drawn remarkable attention for their functional characteristics of plasticity and polarization (16). Throughout the maternal adaptations to pregnancy, decidual macrophages also play critical roles (56). Decidual macrophages coordinate tissue remodeling and angiogenesis, induce apoptosis of damaged cells, facilitate trophoblasts invasion and suppress inflammation (57–62). Consequently, they are indispensable in contributing to the maternal-fetal immune tolerance.
As we all know, endometrial macrophages act as determinants of uterine receptivity. Owing to the characteristics of immune tolerance, they became the research focus in the medical community (63). Gorczynski pointed out that CD200 and MD-1 have immune regulatory activity toward macrophages, which is beneficial to successful pregnancy (64). If the expression of CD200R in macrophages increases, it can stimulate the activity of indoleamine2,3-dioxygenase (IDO) (65). Thus, establishing an immunosuppressive environment is necessary for successful implantation (66). Furthermore, there are many kinds of Toll receptors on decidual macrophages, such as TLR2, TLR3 and TLR4. In response to TLRs activation, decidual macrophages facilitated the secretion of pro-inflammatory cytokines IL-1β, TNF-α, IL-6, IL-8 and the production of the anti-inflammatory cytokines IL-1RA and IL-10. Among these cytokines, IL-10 was the most easily induced. Along with the higher secretion of IL-10 increased by TLRs activation, it might help sustain immune tolerance by curbing the action of pro-inflammatory cytokines (67). In addition, the inhibitory receptors expressed on invading extravillous trophoblasts, such as immunoglobulin-like transcript 2 (ILT2) and ILT4 for human leukocyte antigen (HLA)-G (68, 69), can be combined with the decidual macrophages. As a negative signal that be delivered to the decidual macrophages, they are in favor of the production of anti-inflammatory cytokines and tolerance to the trophoblast (70).
Human decidual MMP-9+ macrophages can degrade the extracellular matrix (ECM) and promote endovascular trophoblast invasion, and they are enriched in the vicinity of the trophoblast invasion during early pregnancy (71). IL-33, as a cytokine of the IL-1 family, is found to be associated with Th2 and M2 polarization (72, 73). It can accelerate the development of primary trophoblasts, villous cytotrophoblast (74). Granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophages colony-stimulating factor (M-CSF) are secreted from first trimester decidual cells. These activated cells can promote macrophages activation, and induce extravillous trophoblasts (EVTs) apoptosis through the caspase 3/7 dependent pathway (75). Conversely, trophoblasts-derived IL-6 (76), CXCL16 (77), and hyaluronan (HA) (78) could induce M2 macrophages polarization. In addition, macrophages could also secrete exosomes or extracellular vesicles and deliver miRNAs to affect the invasion and migration capabilities of trophoblasts, thereby participating in the occurrence of RSA (51, 79).
Placental macrophages are a special M2-like polarized phenotype, which don’t possess all properties of M2 cells. However, the expression intensity of CD163, CD80, CD11c, CCR5, CXCR4 on M2-like macrophages are lower than M2 macrophages. They were shown to regulate gap junction communication and promote decidualization (80).
Decidual macrophages may be the major APCs in the decidua (81). It is thought to be the sentinels of the immune system that initiate and regulate the immune response (82). M2 macrophages can wipe out infection by switching gene expression toward anti-inflammatory cytokines including IL-10, TGF-β and IL-1Ra (83). It also express high levels of scavenger receptors CD163, Stabilin-1 and c-type lectins receptors CD206 and CD209 (84). Phagocytosis of damaged and apoptotic cells are fundamental M2 macrophage functions, which also apply to decidual macrophages at the maternal-fetal interface.
The relationship between decidual macrophages and RSA
It has been fully elaborated that RSA have an immune background. What’s the relationship between decidual macrophages and the aetiology of RSA? How do dysfunction of decidual macrophages lead to RSA (Figure 1)?
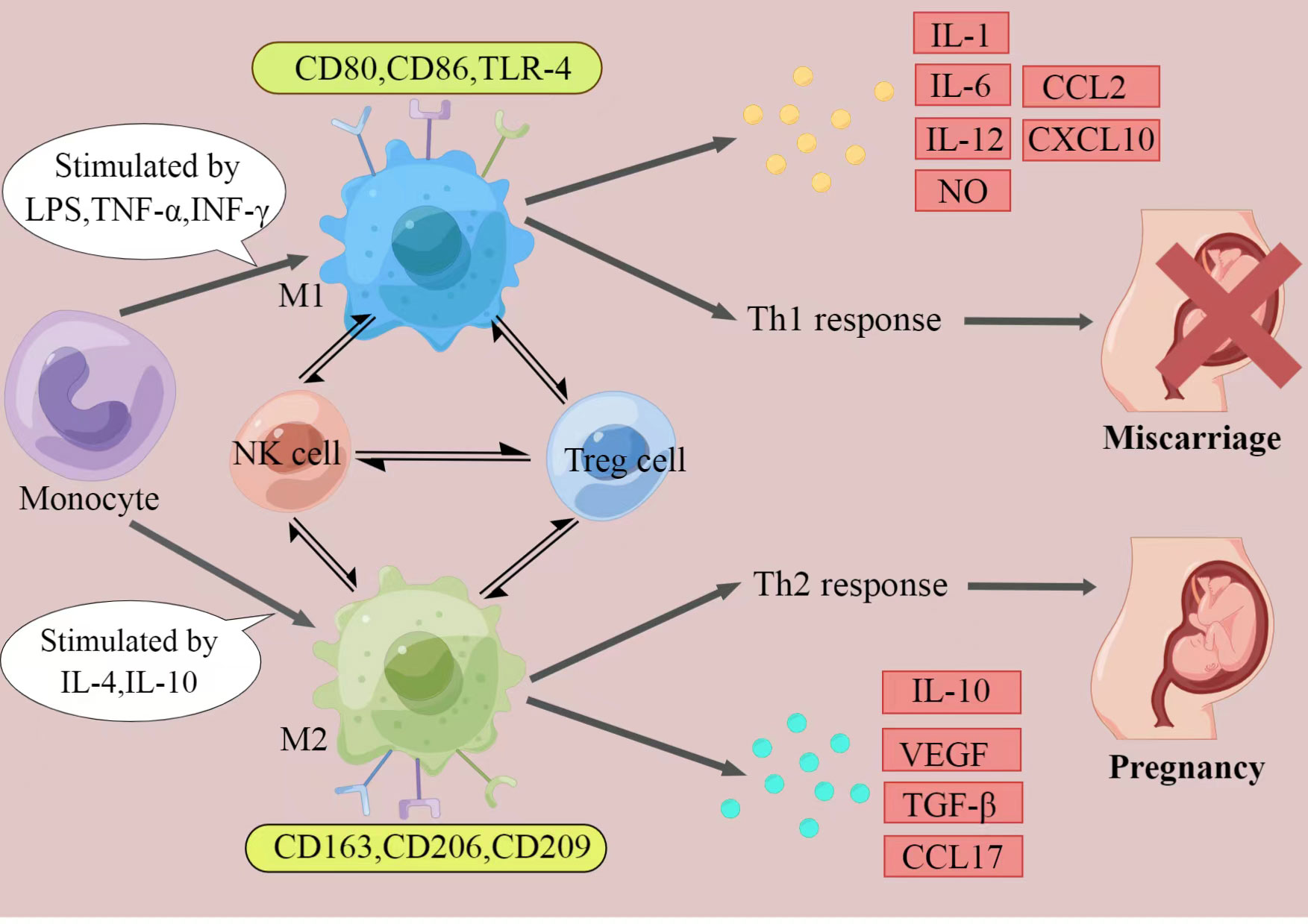
Figure 1 The polarization of macrophages and their characteristics. The figure displays a general principle of polarized M1 and M2 macrophage. M1 and M2 phenotypes represent two extremes of macrophage polarization and display distinct functions, thereby result in different pregnancy outcomes. In response to different stimuli, decidual macrophages undergo M1-like, or M2-like activation. M1 macrophages are stimulated by LPS, TGF-α, or IFN-γ. They express CD80, CD86, and TLR-4, secrete IL-1, IL-6, IL-12, NO, CCL2 and CXCL10, and produce Th1 responses, exert pro-inflammatory effects. In contrast, M2 macrophages are activated by IL-4 or IL-10. They express CD163, CD206, and CD209, secrete IL-10, VEGF, TGF-β and CCL17, and promote Th2 responses, provide an immune-tolerant environment for the fetus. Thus, if M2 macrophages play the major role at the maternal-fetal interface, pregnancy would continue. When M1 macrophages are absolutely dominant, it will ultimately lead to miscarriage. (Created by Figdraw).
M1/M2 macrophages balance
As it was mentioned above, the disturbed M1/M2 macrophages balance came to light at the maternal-fetal interface of RSA (40). It seems that the M1 subtype predominates over the M2 subtype in those cases, accompanied by pregnancy complications (85, 86). M1 macrophages can suppress epithelial-mesenchymal transition (EMT), migration, and invasion of trophoblasts by transporting miR-146b-5p to directly inhibit TRAF6 expression, thereby participating in the pathogenesis of RSA (87). ChunYan Wei have tested that JAK2 inhibitor adjusted the proportion of M1/M2 macrophages, further affecting the pregnancy outcome through the CCL2/CCR2/JAK2 pathway (88). Decreased programmed death-1 (PD-1) protein expression on decidual macrophages, accompanied with reduced programmed cell death ligand-1 (PD-L1) expression on placental villi, was observed in RSA. Meanwhile, the disturbed PD-1/PD-L1 axis induced M1 differentiation (89). Knockdown of CYP26A1 in mice uterine can decrease the number of embryo implantation. It can be also discovered that the protein levels of M1 markers TNF-α, IL-6 and CD86 were significantly decreased, thus leading to the insufficient M1 polarization (90). Additionally, in the immune atlas of RSA without chromosomal aberrations, pro-inflammatory subsets of CD11chigh macrophages increased remarkably (91). The present research illustrated that the abnormally increased MNSFβ expression can promote the secretion of TNF-α, inducing the polarization of decidual macrophages toward a pro-inflammatory phenotype (92). More intuitive studies on mouse experiments confirmed that Cathepsin E-deficient mice displayed compromised immune reactions with higher susceptibility to bacterial infection (93).
Cytokines
Several studies have demonstrated that the expression of CD80, CD86 and HLA-DR, but not CD163 on decidual macrophages were higher in RSA patients compared to normal pregnancies, accompanied with higher production of TNF-α and lower secretion of IL-10 and IL-33 (40, 73, 94). In LPS-induced mice abortion model, the expression IL-1, IL-6, TNF-α, IFN-γ, IL-17a was significantly raised (95). Macrophage depletion was also proved to prevent CpG-induced embryonic resorption in an abortion mice model and in IL-10-/- mice (96, 97). The experiment as early as the 18th century has confirmed that the depletion of macrophags results in the loss of pregnancy and recurrent abortion (57). CSF-1-deficient mice displayed few decidual macrophages, with lower implantation at day 7 and 8, and always had aberrant fertility with smaller size (98). On day 0.5 or day 3.5 post-coitum, injection of diphtheria toxin (DT) to Cd11b-Dtr mouse model caused implantation failure and infertility. But implantation failure can be alleviated by administration of bone marrow-derived CD11b+F4/80+ monocytes/macrophages (99). And injection on day 14 and 16 led to fetal mortality without cervix ripening (100). However, it could be alleviated by administration of RANK+ macrophages (101). RANKL derived from trophoblasts could make macrophage polarization to M2 by activating AKT/STAT6-Jmjd3/IRF4 signaling pathway. The knockout model of RANK−/− mice can lead to the decreased expression of TGF-β and the increased pregnancy loss (102). Mice with uterine deficiency of high-mobility group box-1 (HMGB1) protein, showed impaired implantation and severe subfertility (103). But highly expressed HMGB1 was actively secreted by macrophages and then activated pyroptosis, leading to the occurrence and development of RSA (104).Therefore, restricting macrophages accumulation is also needed.
T-cell immunoglobulin and mucin domain containing protein 3 (Tim-3) blockade down-regulated the phagocytosis of decidual macrophages, leading to accumulation of inflammatory granulocytes and macrophages at the maternal-fetal interface (105). Therefore, high level of pro-inflammatory cytokines establishes a pro-inflammatory microenvironment and impairs normal pregnancy. It also has been indicated that dysregulation of decidual macrophages activation by regulatory T cells (Treg cells) may lead to RSA. When Treg cells regulate aberrant cell-cell contact, there will be a problem with decidual macrophages. The abnormity of decidual macrophages was indicated to be regulated by Treg cells through aberrant cell-cell contact and TGF-β secretion (106). Moreover, Jiayu Wang’s studies showed that abnormally expressed USP2a may be found in the placental villous samples of RSA patients. Further studies have confirmed that TGF-β could collaborate with USP2a to promote trophoblasts migration and invasion via its interaction with TGFBR1 (107). Thrombospondin1 (TSP1) needs to interact with CD36, CD47 and heparin sulphate proteoglycanto enhance the ability of macrophages (108). They are engaged in regulating IL-10 secretion and boost the tolerance of the immune system at the maternal-fetal interface (109). Thus, low expression of TSP1 along with decreased IL-10 could appear in RSA.
Therapies
Owing to the uncertainty of the pathogenesis, the likelihood of recurrence, recent studies suggest that various treatment of RSA may work (110, 111). Clinicians often use progesterone to support or supplement the pregnancy, by oral, vaginal, intramuscular, or other ways. It is considered to be essential for successful embryo implantation. But now, it is increasingly becoming the psychological support to patients (112). Besides, all treatments for RSA are almost based on immunomodulation for their effects (2, 113). A meta-analysis of the treament of APS-related RSA showed that aspirin plus low-molecular-weight heparin (LMWH) can significantly reduce the rate of repeated pregnancy loss (114). Another study proved the combination of anticoagulant and anti-inflammatory could contribute to a better pregnancy outcome (115). Prednisone, hydroxychloroquine (116) and cyclosporine A (117) are also part of the clinical therapy regimen of RSA.
When a semi-allogeneic fetus appears at maternal-fetal interface, maternal tolerance is required to avoid the miscarriage. Patients with RSA may lack this capacity. Therefore, alloimmunization was born in response to the condition. It has been suggested that the effect of lymphocyte immunotherapy (LIT) was probably positive. And a higher success rate was likely observed in those immunized with paternal lymphocytes (118). Some experts recommend immunotherapy before and during pregnancy with low dose of lymphocytes. It can break the balance between Th1 and Th2 cytokines, reducing the level of Th1 cytokines (IL-2, INF-γ, TNF-α, and IL-6), while increasing the level of Th2 cytokines (IL-4, IL-10) (119). As opposed to “active immunization” with allogenic lymphocytes which was introduced previously, intravenous immunoglobulin (IVIg) was termed as “passive immunization”. The effect of IVIg on Treg/Th17 cells ratio enhances Treg cells function, and thereby improve the live birth rate in pregnancy to some extent (120). The RSA mice models with intraperitoneally administration of G-CSF certified that the absence of G-CSF weakened the inhibitory effects on macrophages, leading to more M1-type differentiation and overexpressing NLRP3 inflammasomes at the maternal-fetal interface. It implies that G-CSF may improve pregnancy success rate by modulating the inflammatory state (121).
It is worth noting that immunotherapy is not a panacea for treating all patients with RSA. The choice of therapeutic plans should have certain indications (122). For unexplained RSA, we should make efforts to seek the pathogenesis. Testing for inherited thrombophilia and hyperhomocysteinemia should be performed. If necessary, screening for immunological factors such as Human Leukocyte Antigen (HLA), cytokines, antinuclear antibodies (ANA), Natural Killer (NK) cells, anti-HLA antibodies and antisperm antibodies, Lupus anticoagulant (LAC), Anticardiolipin antibodies (ACL) and anti-β2 glycoprotein-I antibodies (β2-GPI). So, further research in personalised treatment options is warranted.
Conclusion
Take together, polarized macrophages can influence the reception of maternal to embryo through the secretion of various cytokines and chemokines. The specific etiology and pathogenesis among them is very complicated, which is an emerging field that needs to be explored urgently. How to maintain M1/M2 macrophages balance? Even we know the correlation of decidual macrophages with RSA, what can we do to help them? Collectively, there are a large number of challenges to be overcome, and further efforts are needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
Q-YZ and Q-HL wrote and discussed the manuscript. Y-YF discussed the manuscript. Q-YZ and A-FJ designed and created tables. C-ER and Y-HM reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81501275), Shandong Province College Science and Technology Plan Project (J18KA252), National Natural Science Foundation of Shangdong Province (ZR2019BH037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril (2020) 113:533–5. doi: 10.1016/j.fertnstert.2019.11.025
2. Bender AR, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open (2018) 2018:y4. doi: 10.1093/hropen/hoy004
3. de Ziegler D, Frydman RF. Recurrent pregnancy losses, a lasting cause of infertility. FERTIL STERIL (2021) 115:531–2. doi: 10.1016/j.fertnstert.2020.12.004
4. Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet (2021) 397:1658–67. doi: 10.1016/S0140-6736(21)00682-6
5. Ticconi C, Pietropolli A, Specchia M, Nicastri E, Chiaramonte C, Piccione E, et al. Pregnancy-related complications in women with recurrent pregnancy loss: A prospective cohort study. J Clin Med (2020) 9:2833. doi: 10.3390/jcm9092833
6. Rasmark RE, Christiansen OB, Kallen K, Hansson SR. Women with a history of recurrent pregnancy loss are a high-risk population for adverse obstetrical outcome: A retrospective cohort study. J Clin Med (2021) 10:179. doi: 10.3390/jcm10020179
7. Hong LY, Marren A. Recurrent pregnancy loss: A summary of international evidence-based guidelines and practice. Aust J Gen Pract (2018) 47:432–6. doi: 10.31128/AJGP-01-18-4459
8. van Dijk MM, Kolte AM, Limpens J, Kirk E, Quenby S, van Wely M, et al. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? a systematic review of the literature and meta-analysis. Hum Reprod Update (2020) 26:356-67. doi: 10.1093/humupd/dmz048
9. Yi X, Zhang J, Liu H, Yi T, Ou Y, Liu M, et al. Suppressed immune-related profile rescues abortion-prone fetuses: A novel insight into the CBA/J x DBA/2J mouse model. Reprod Sci (2019) 26:1485–92. doi: 10.1177/1933719119828042
10. Jardine L, Haniffa M. Reconstructing human DC, monocyte and macrophage development in utero using single cell technologies. Mol Immunol (2020) 123:1–6. doi: 10.1016/j.molimm.2020.04.023
11. Vomstein K, Feil K, Strobel L, Aulitzky A, Hofer-Tollinger S, Kuon RJ, et al. Immunological risk factors in recurrent pregnancy loss: Guidelines versus current state of the art. J Clin Med (2021) 10:869. doi: 10.3390/jcm10040869
12. Fu YY, Ren CE, Qiao PY, Meng YH. Uterine natural killer cells and recurrent spontaneous abortion. Am J Reprod Immunol (2021) 86:e13433. doi: 10.1111/aji.13433
13. Stephanie EA, Michael SD, Carolyn BC. Immune responses at the maternal-fetal interface. Sci Immunol (2019) 4:eaat6114. doi: 10.1126/sciimmunol.aat6114
14. Chambers M, Rees A, Cronin JG, Nair M, Jones N, Thornton CA. Macrophage plasticity in reproduction and environmental influences on their function. Front Immunol (2020) 11:607328. doi: 10.3389/fimmu.2020.607328
15. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest (2012) 122:787–95. doi: 10.1172/JCI59643
16. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol (2018) 233:6425–40. doi: 10.1002/jcp.26429
17. Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol (2015) 15:217–30. doi: 10.1038/nri3819
18. Cousins FL, Kirkwood PM, Saunders PT, Gibson DA. Evidence for a dynamic role for mononuclear phagocytes during endometrial repairand remodelling. Sci Rep (2016) 6:36748. doi: 10.1038/srep36748
19. Jaiswal MK, Mallers TM, Larsen B, Kwak-Kim J, Chaouat G, Gilman-Sachs A, et al. V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction (2012) 143:713–25. doi: 10.1530/REP-12-0036
20. Ning F, Liu H, Lash GE. The role of decidual macrophages during normal and pathological pregnancy. Am J Reprod Immunol (2016) 75:298–309. doi: 10.1111/aji.12477
21. Schmidt T, Carmeliet P. Blood-vessel formation: Bridges that guide and unite. Nature (2010) 465:697–9. doi: 10.1038/465697a
22. Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood (2010) 116:829–40. doi: 10.1182/blood-2009-12-257832
23. Mege JL, Mehraj V, Capo C. Macrophage polarization and bacterial infections. Curr Opin Infect Dis (2011) 24:230–4. doi: 10.1097/QCO.0b013e328344b73e
24. Patel U, Rajasingh S, Samanta S, Cao T, Dawn B, Rajasingh J. Macrophage polarization in response to epigenetic modifiers during infection andinflammation. Drug Discovery Today (2017) 22:186–93. doi: 10.1016/j.drudis.2016.08.006
25. Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res (2012) 53:11–24. doi: 10.1007/s12026-012-8291-9
26. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature (2013) 496:445–55. doi: 10.1038/nature12034
27. Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev (2014) 262:153–66. doi: 10.1111/imr.12218
28. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front Immunol (2014) 5:514. doi: 10.3389/fimmu.2014.00514
29. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
30. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol (2006) 177:7303–11. doi: 10.4049/jimmunol.177.10.7303
31. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep (2014) 6:13. doi: 10.12703/P6-13
32. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol (2014) 5:614. doi: 10.3389/fimmu.2014.00614
33. Chiara P, Elena R, Alessandro I, Antonio S. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol (2015) 27:237-48. doi: 10.1016/j.smim.2015.10.003
34. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol (2009) 27:451–83. doi: 10.1146/annurev.immunol.021908.132532
35. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med (1992) 176:287–92. doi: 10.1084/jem.176.1.287
36. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity (2010) 32:593–604. doi: 10.1016/j.immuni.2010.05.007
37. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol (2008) 8:958–69. doi: 10.1038/nri2448
38. Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity (2005) 23:344–6. doi: 10.1016/j.immuni.2005.10.001
39. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol (2000) 164:6166–73. doi: 10.4049/jimmunol.164.12.6166
40. Tsao FY, Wu MY, Chang YL, Wu CT, Ho HN. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J Formos Med Assoc (2018) 117:204–11. doi: 10.1016/j.jfma.2017.03.011
41. Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol (2009) 29:1419–23. doi: 10.1161/ATVBAHA.108.180497
42. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol (2010) 11:889–96. doi: 10.1038/ni.1937
43. Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. Two unique human decidual macrophage populations. J Immunol (2011) 186:2633–42. doi: 10.4049/jimmunol.1003153
44. Houser BL. Decidual macrophages and their roles at the maternal-fetal interface. Yale J Biol Med (2012) 85:105–18.
45. Jiang X, Wang H. Macrophage subsets at the maternal-fetal interface. Cell Mol Immunol (2020) 17:889–91. doi: 10.1038/s41423-020-0435-6
46. Jiang X, Du MR, Li M, Wang H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell Mol Immunol (2018) 15:1027–37. doi: 10.1038/s41423-018-0008-0
47. Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, et al. Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol (2017) 57:66–76. doi: 10.1165/rcmb.2016-0361OC
48. Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol (2010) 50:323–54. doi: 10.1146/annurev.pharmtox.010909.105600
49. Ozen M, Zhao H, Lewis DB, Wong RJ, Stevenson DK. Heme oxygenase and the immune system in normal and pathological pregnancies. Front Pharmacol (2015) 6:84. doi: 10.3389/fphar.2015.00084
50. Callewaert L, Michiels CW. Lysozymes in the animal kingdom. J Biosci (2010) 35:127–60. doi: 10.1007/s12038-010-0015-5
51. Zhang YH, He M, Wang Y, Liao AH. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol (2017) 8:120. doi: 10.3389/fimmu.2017.00120
52. Ali S, Majid S, Ali MN, Taing S, Rehman MU, Arafah A. Cytokine imbalance at materno-embryonic interface as a potential immune mechanism for recurrent pregnancy loss. Int Immunopharmacol (2021) 90:107118. doi: 10.1016/j.intimp.2020.107118
53. Wheeler KC, Jena MK, Pradhan BS, Nayak N, Das S, Hsu CD, et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PloS One (2018) 13:e191040. doi: 10.1371/journal.pone.0191040
54. Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol (2003) 162:887–96. doi: 10.1016/S0002-9440(10)63884-9
55. Mills CD. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit Rev Immunol (2001) 21:399–425. doi: 10.1615/CritRevImmunol.v21.i5.10
56. Yang Z, Kong B, Mosser DM, Zhang X. TLRs, macrophages, and NK cells: our understandings of their functions in uterusand ovary. Int Immunopharmacol (2011) 11:1442–50. doi: 10.1016/j.intimp.2011.04.024
57. Jena MK, Nayak N, Chen K, Nayak NR. Role of macrophages in pregnancy and related complications. Arch Immunol Ther Exp (Warsz) (2019) 67:295–309. doi: 10.1007/s00005-019-00552-7
58. Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol (2009) 174:1959–71. doi: 10.2353/ajpath.2009.080995
59. Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest (2001) 81:1143–52. doi: 10.1038/labinvest.3780326
60. Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol (2003) 1:119. doi: 10.1186/1477-7827-1-119
61. Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancydecidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol (2003) 50:444–52. doi: 10.1046/j.8755-8920.2003.00112.x
62. Redline RW, Lu CY. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol (1988) 140:3947–55.
63. Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PloS One (2008) 3:e2078. doi: 10.1371/journal.pone.0002078
64. Gorczynski RM, Hadidi S, Yu G, Clark DA. The same immunoregulatory molecules contribute to successful pregnancy and transplantation. Am J Reprod Immunol (2002) 48:18–26. doi: 10.1034/j.1600-0897.2002.01094.x
65. Huang HL, Yang HL, Lai ZZ, Yang SL, Li MQ, Li DJ. Decidual IDO(+) macrophage promotes the proliferation and restricts the apoptosis of trophoblasts. J Reprod Immunol (2021) 148:103364. doi: 10.1016/j.jri.2021.103364
66. Suzuki Y, Suda T, Asada K, Miwa S, Suzuki M, Fujie M, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol (2012) 19:436–42. doi: 10.1128/CVI.05402-11
67. Duriez M, Quillay H, Madec Y, El CH, Cannou C, Marlin R, et al. Human decidual macrophages and NK cells differentially express toll-like receptors and display distinct cytokine profiles upon TLR stimulation. Front Microbiol (2014) 5:316. doi: 10.3389/fmicb.2014.00316
68. Petroff MG, Sedlmayr P, Azzola D, Hunt JS. Decidual macrophages are potentially susceptible to inhibition by class ia and class ib HLA molecules. J Reprod Immunol (2002) 56:3–17. doi: 10.1016/S0165-0378(02)00024-4
69. Rouas-Freiss N, Moreau P, LeMaoult J, Papp B, Tronik-Le RD, Carosella ED. Role of the HLA-G immune checkpoint molecule in pregnancy. Hum Immunol (2021) 82:353–61. doi: 10.1016/j.humimm.2021.01.003
70. Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim J. The role of decidual immune cells on human pregnancy. J Reprod Immunol (2017) 124:44–53. doi: 10.1016/j.jri.2017.10.045
71. Helige C, Ahammer H, Moser G, Hammer A, Dohr G, Huppertz B, et al. Distribution of decidual natural killer cells and macrophages in the neighbourhood of the trophoblast invasion front: a quantitative evaluation. Hum Reprod (2014) 29:8–17. doi: 10.1093/humrep/det353
72. Hazlett LD, McClellan SA, Barrett RP, Huang X, Zhang Y, Wu M, et al. IL-33 shifts macrophage polarization, promoting resistance against pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci (2010) 51:1524–32. doi: 10.1167/iovs.09-3983
73. Sheng YR, Hu WT, Wei CY, Tang LL, Liu YK, Liu YY, et al. IL-33/ST2 axis affects the polarization and efferocytosis of decidual macrophages in early pregnancy. Am J Reprod Immunol (2018) 79:e12836. doi: 10.1111/aji.12836
74. Fock V, Mairhofer M, Otti GR, Hiden U, Spittler A, Zeisler H, et al. Macrophage-derived IL-33 is a critical factor for placental growth. J Immunol (2013) 191:3734–43. doi: 10.4049/jimmunol.1300490
75. Wu ZM, Yang H, Li M, Yeh CC, Schatz F, Lockwood CJ, et al. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta (2012) 33:188–94. doi: 10.1016/j.placenta.2011.12.007
76. Ding J, Yang C, Cheng Y, Wang J, Zhang S, Yan S, et al. Trophoblast-derived IL-6 serves as an important factor for normal pregnancy by activating Stat3-mediated M2 macrophages polarization. Int Immunopharmacol (2021) 90:106788. doi: 10.1016/j.intimp.2020.106788
77. Wang XQ, Zhou WJ, Hou XX, Fu Q, Li DJ. Correction: Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell Mol Immunol (2019) 16:313. doi: 10.1038/s41423-018-0194-9
78. Wang S, Sun F, Han M, Liu Y, Zou Q, Wang F, et al. Trophoblast-derived hyaluronan promotes the regulatory phenotype of decidual macrophages. Reproduction (2019) 157:189–98. doi: 10.1530/REP-18-0450
79. Ding J, Zhang Y, Cai X, Diao L, Yang C, Yang J. Crosstalk between trophoblast and macrophage at the maternal-fetal interface: Current status and future perspectives. Front Immunol (2021) 12:758281. doi: 10.3389/fimmu.2021.758281
80. Rajakumar A, Kane MA, Yu J, Jones JW, Qu H, Badell M, et al. Alternatively activated macrophages are the primary retinoic acid-producing cells in human decidua. Reprod Sci (2020) 27:334–41. doi: 10.1007/s43032-019-00030-7
81. Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod (2003) 69:1438–46. doi: 10.1095/biolreprod.103.017574
82. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflammation Allergy (2005) 4:281–6. doi: 10.2174/1568010054022024
83. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol (2013) 229:176–85. doi: 10.1002/path.4133
84. Mendoza-Coronel E, Ortega E. Macrophage polarization modulates FcgammaR- and CD13-mediated phagocytosis and reactive oxygen species production, independently of receptor membrane expression. Front Immunol (2017) 8:303. doi: 10.3389/fimmu.2017.00303
85. Brown MB, von Chamier M, Allam AB, Reyes L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol (2014) 5:606. doi: 10.3389/fimmu.2014.00606
86. Liu X, Jiang M, Ren L, Zhang A, Zhao M, Zhang H, et al. Decidual macrophage M1 polarization contributes to adverse pregnancy induced by toxoplasma gondii PRU strain infection. Microb Pathog (2018) 124:183–90. doi: 10.1016/j.micpath.2018.08.043
87. Ding J, Zhang Y, Cai X, Zhang Y, Yan S, Wang J, et al. Extracellular vesicles derived from M1 macrophages deliver miR-146a-5p and miR-146b-5p to suppress trophoblast migration and invasion by targeting TRAF6 inrecurrent spontaneous abortion. Theranostics (2021) 11:5813–30. doi: 10.7150/thno.58731
88. Wei CY, Li MQ, Zhu XY, Li DJ. Immune status of decidual macrophages is dependent on the CCL2/CCR2/JAK2 pathwayduring early pregnancy. Am J Reprod Immunol (2021) 86:e13480. doi: 10.1111/aji.13480
89. Zhang Y, Ma L, Hu X, Ji J, Mor G, Liao A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum Reprod (2019) 34:25–36. doi: 10.1093/humrep/dey347
90. Ji WH, Li DD, Wei DP, Gu AQ, Yang Y, Peng JP. Cytochrome P450 26A1 modulates the polarization of uterine macrophages during the peri-implantation period. Front Immunol (2021) 12:763067. doi: 10.3389/fimmu.2021.763067
91. Wu Z, Wang M, Liang G, Jin P, Wang P, Xu Y, et al. Pro-inflammatory signature in decidua of recurrent pregnancy loss regardless of embryonic chromosomal abnormalities. Front Immunol (2021) 12:772729. doi: 10.3389/fimmu.2021.772729
92. Zhen XX, Yang L, Gu Y, Yang Q, Gu WW, He YP, et al. MNSFbeta regulates TNFalpha production by interacting with RC3H1 in human macrophages, and dysfunction of MNSFbeta in decidual macrophages is associated with recurrent pregnancy loss. Front Immunol (2021) 12:691908. doi: 10.3389/fimmu.2021.691908
93. Goto S, Ozaki Y, Suzumori N, Yasukochi A, Kawakubo T, Furuno T, et al. Role of cathepsin e in decidual macrophage of patients with recurrent miscarriage. Mol Hum Reprod (2014) 20:454–62. doi: 10.1093/molehr/gau008
94. Shimada S, Ebina Y, Iijima N, Deguchi M, Yamada H. Decidual CD68(+) HLA-DR(+) CD163(-) M1 macrophages increase in miscarriages withnormal fetal chromosome. Am J Reprod Immunol (2018) 79:e12791. doi: 10.1111/aji.12791
95. Cui L, Xu F, Wang S, Li X, Lin H, Ding Y, et al. Pharmacological activation of rev-erbalpha suppresses LPS-induced macrophage M1 polarization and prevents pregnancy loss. BMC Immunol (2021) 22:57. doi: 10.1186/s12865-021-00438-4
96. Kang X, Zhang X, Zhao A. Macrophage depletion and TNF-alpha inhibition prevent resorption in CBA/J x DBA/2 model of CpG-induced abortion. Biochem Bioph Res Co (2016) 469:704–10. doi: 10.1016/j.bbrc.2015.12.024
97. Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol (2009) 183:1144–54. doi: 10.4049/jimmunol.0900788
98. Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, Stanley ER. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol (1991) 148:273–83. doi: 10.1016/0012-1606(91)90336-2
99. Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest (2013) 123:3472–87. doi: 10.1172/JCI60561
100. Yellon SM, Greaves E, Heuerman AC, Dobyns AE, Norman JE. Effects of macrophage depletion on characteristics of cervix remodeling and pregnancy in CD11b-dtr mice. Biol Reprod (2019) 100:1386–94. doi: 10.1093/biolre/ioz002
101. Meng YH, Zhou WJ, Jin LP, Liu LB, Chang KK, Mei J, et al. RANKL-mediated harmonious dialogue between fetus and mother guarantees smooth gestation by inducing decidual M2 macrophage polarization. Cell Death Dis (2017) 8:e3105. doi: 10.1038/cddis.2017.505
102. Chang RQ, Shao J, Meng YH, Wang J, Li DJ, Li MQ. Decidual RANKL/RANK interaction promotes the residence and polarization of TGF-beta1-producing regulatory gammadelta T cells. Cell Death Dis (2019) 10:113. doi: 10.1038/s41419-019-1380-0
103. Aikawa S, Deng W, Liang X, Yuan J, Bartos A, Sun X, et al. Uterine deficiency of high-mobility group box-1 (HMGB1) protein causes implantation defects and adverse pregnancy outcomes. Cell Death Differ (2020) 27:1489–504. doi: 10.1038/s41418-019-0429-z
104. Zhu D, Zou H, Liu J, Wang J, Ma C, Yin J, et al. Inhibition of HMGB1 ameliorates the maternal-fetal interface destruction in unexplained recurrent spontaneous abortion by suppressing pyroptosis activation. Front Immunol (2021) 12:782792. doi: 10.3389/fimmu.2021.782792
105. Chabtini L, Mfarrej B, Mounayar M, Zhu B, Batal I, Dakle PJ, et al. TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J Immunol (2013) 190:88–96. doi: 10.4049/jimmunol.1202176
106. Wang WJ, Hao CF, Lin QD. Dysregulation of macrophage activation by decidual regulatory T cells in unexplained recurrent miscarriage patients. J Reprod Immunol (2011) 92:97–102. doi: 10.1016/j.jri.2011.08.004
107. Wang J, Ding J, Zhang S, Chen X, Yan S, Zhang Y, et al. Decreased USP2a expression inhibits trophoblast invasion and associates with recurrent miscarriage. Front Immunol (2021) 12:717370. doi: 10.3389/fimmu.2021.717370
108. Yamauchi Y, Kuroki M, Imakiire T, Abe H, Uchida H, Beppu R, et al. Thrombospondin-1 differentially regulates release of IL-6 and IL-10 by human monocytic cell line U937. Biochem Biophys Res Commun (2002) 290:1551–7. doi: 10.1006/bbrc.2002.6386
109. Jin Y, Wang X, Xiao Y, Lv C, Ding C, Lin Q. The role of TSP-1 on decidual macrophages involved in the susceptibility to unexplained recurrent spontaneous abortion. Am J Reprod Immunol (2009) 61:253–60. doi: 10.1111/j.1600-0897.2009.00689.x
110. Coomarasamy A, Dhillon-Smith RK, Papadopoulou A, Al-Memar M, Brewin J, Abrahams VM, et al. Recurrent miscarriage: evidence to accelerate action. Lancet (2021) 397:1675–82. doi: 10.1016/S0140-6736(21)00681-4
111. Deng T, Liao X, Zhu S. Recent advances in treatment of recurrent spontaneous abortion. Obstet Gynecol Surv (2022) 77:355–66. doi: 10.1097/OGX.0000000000001033
112. Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst Rev (2019) 11:CD003511. doi: 10.1002/14651858.CD003511.pub5
113. Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev (2014) 10:CD000112. doi: 10.1002/14651858.CD000112.pub3
114. Lu C, Liu Y, Jiang HL. Aspirin or heparin or both in the treatment of recurrent spontaneous abortion inwomen with antiphospholipid antibody syndrome: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med (2019) 32:1299–311. doi: 10.1080/14767058.2017.1404979
115. Ye SL, Gu XK, Tao LY, Cong JM, Wang YQ. Efficacy of different treatment regimens for antiphospholipid syndrome-related recurrent spontaneous abortion. Chin Med J (Engl) (2017) 130:1395–9. doi: 10.4103/0366-6999.207471
116. Ghasemnejad-Berenji H, Ghaffari NM, Hajshafiha M, Nazarian H, Hashemi SM, Ilkhanizadeh B, et al. Immunomodulatory effects of hydroxychloroquine on Th1/Th2 balance in women with repeated implantation failure. BioMed Pharmacother (2018) 107:1277–85. doi: 10.1016/j.biopha.2018.08.027
117. Azizi R, Ahmadi M, Danaii S, Abdollahi-Fard S, Mosapour P, Eghbal-Fard S, et al. Cyclosporine a improves pregnancy outcomes in women with recurrent pregnancy loss and elevated Th1/Th2 ratio. J Cell Physiol (2019) 234:19039–47. doi: 10.1002/jcp.28543
118. Liu Z, Xu H, Kang X, Wang T, He L, Zhao A. Allogenic lymphocyte immunotherapy for unexplained recurrent spontaneous abortion: A meta-analysis. Am J Reprod Immunol (2016) 76:443–53. doi: 10.1111/aji.12511
119. Yokoo T, Takakuwa K, Ooki I, Kikuchi A, Tamura M, Tanaka K. Alteration of TH1 and TH2 cells by intracellular cytokine detection in patients with unexplained recurrent abortion before and after immunotherapy with the husband's mononuclear cells. Fertil Steril (2006) 85:1452–8. doi: 10.1016/j.fertnstert.2005.10.058
120. Muyayalo KP, Li ZH, Mor G, Liao AH. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol (2018) 80:e13018. doi: 10.1111/aji.13018
121. Gao P, Zha Y, Wei L, Zhou X, Zhu S, Zhang H, et al. G-CSF: A vehicle for communication between trophoblasts and macrophages which may cause problems in recurrent spontaneous abortion. Placenta (2022) 121:164–72. doi: 10.1016/j.placenta.2022.03.125
Keywords: decidual macrophages, recurrent spontaneous abortion (RSA), M1/M2 balance, CD11clow/CD11chigh, the maternal-fetal interface
Citation: Zhao Q-Y, Li Q-H, Fu Y-Y, Ren C-E, Jiang A-F and Meng Y-H (2022) Decidual macrophages in recurrent spontaneous abortion. Front. Immunol. 13:994888. doi: 10.3389/fimmu.2022.994888
Received: 15 July 2022; Accepted: 22 November 2022;
Published: 08 December 2022.
Edited by:
Raj Raghupathy, Kuwait University, KuwaitReviewed by:
Shanmuga Priyaa Madhukaran, University of Texas Southwestern Medical Center, United StatesFernando Gómez-Chávez, (IPN), Mexico
Copyright © 2022 Zhao, Li, Fu, Ren, Jiang and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Han Meng, eWhtZW5nNkAxNjMuY29t
 Qiu-Yan Zhao
Qiu-Yan Zhao Yu-Han Meng
Yu-Han Meng