- 1Peking University, China-Japan Friendship School of clinical medicine, Beijing, China
- 2Department of Rheumatology, China-Japan Friendship Hospital, Beijing, China
- 3Data and Project Management Unit, Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, China
- 4Department of Rheumatology, Beijing Key Lab for Immune-Mediated Inflammatory Diseases, China-Japan Friendship Hospital, Beijing, China
Objective: To explore the clinical features and prognoses of dermatomyositis (DM) associated with a double-positive anti-MDA5 and anti-aminoacyl-tRNA synthetase (anti-ARS) antibody presentation.
Methods: We retrospectively analyzed 1280 consecutive patients with idiopathic inflammatory myopathy (IIM). Individuals with anti-MDA5 and anti-ARS antibodies (anti-MDA5+/ARS+) were compared to anti-MDA5-/ARS+ and anti-MDA5+/ARS- control individuals based on clinical, pulmonary radiological characteristics, treatment, and follow-up information.
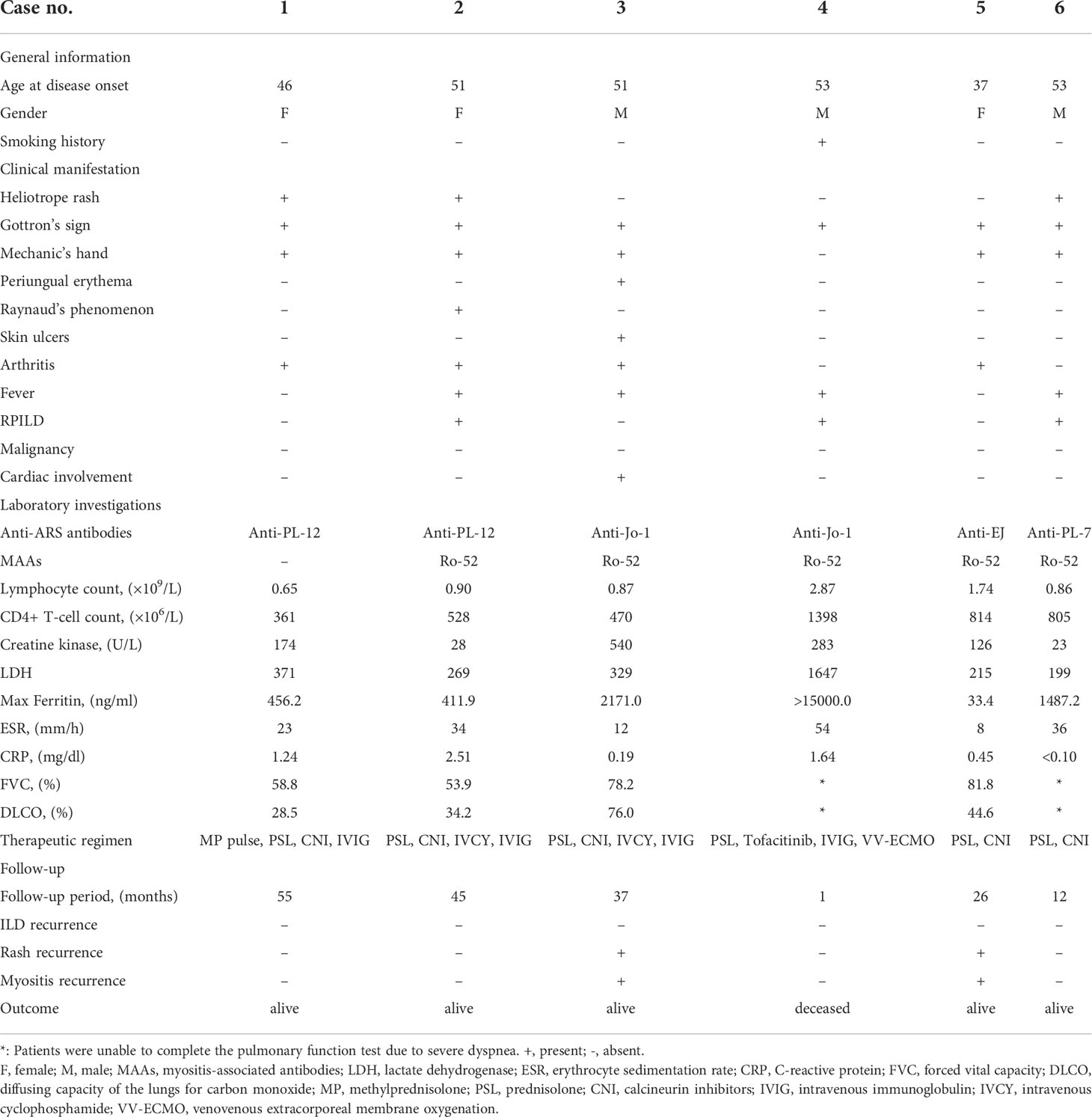
Results: Six individuals (0.47%) presented with anti-MDA5+/ARS+; of these, 2 (33.3%) were anti-PL-12+, 2 (33.3%) were anti-Jo-1+, 1 (16.7%) was anti-EJ+, and 1 (16.7%) was anti-PL-7+. Hallmark cutaneous manifestations, including Gottron’s sign (100%), heliotrope rash (50%), mechanic’s hand (66.7%), and skin ulcers (16.7%) were common. Anti-MDA5+/ARS+ patients tended to have higher ferritin levels (p = 0.038) than anti-MDA5-/ARS+ group, and higher CD4+ T-cell counts (p = 0.032) compared to the anti-MDA5+/ARS- group. Radiologically, NSIP with OP overlap was predominant (60%). Consolidation (60%), ground-glass attenuation (GGA) (80%), traction bronchiectasis (80%), and intralobular reticulation (100%) were common in anti-MDA5+/ARS+ individuals. All were diagnosed with ILD and 50% were categorized as RPILD. All patients received glucocorticoids combined with one or more immunosuppressants. Most (83.3%) had a good prognosis following treatment, but there was no difference in the survival rate between the three subgroups.
Conclusion: Presentation with anti-MDA5+/ARS+ DM was rare. The clinical and radiological characteristics of anti-MDA5+/ARS+ DM combined the features of anti-MDA5+ and anti-ARS+ individuals. Individuals with anti-MDA5+/ARS+ antibodies may respond well to glucocorticoid therapy; glucocorticoids combined with one or more immunosuppressants may be considered a basic treatment approach.
Introduction
Idiopathic inflammatory myopathies (IIM) are a heterogeneous group of autoimmune disorders usually characterized by chronic muscle inflammation with varying clinical manifestations, treatment responses, and prognoses. IIM can be classified into several subgroups: dermatomyositis (DM), anti-synthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), inclusion body myositis (IBM), polymyositis (PM), and overlap myositis (1). A major advance in the field of myositis was the discovery of auto-antibodies, called myositis-specific antibodies (MSA). As previous studies have reported (1, 2), MSAs are strongly associated with distinct clinical phenotypes and are therefore predictive of organ manifestations and potentially of prognosis.
ASS is characterized by the presence of unique anti-aminoacyl-tRNA synthetase (ARS) antibodies, which can be further sub-classified into: anti-histidyl (anti-Jo-1), anti-threonyl (anti-PL-7), anti-alanyl (anti-PL-12), anti-glycyl (anti-EJ), anti-isoleucyl (anti-OJ), etc (2). Anti-melanoma differentiation-associated gene 5 (MDA5) DM is a distinct subtype of DM. Patients with anti-MDA5 typically exhibit characteristic cutaneous manifestations, including palmar papules and deep ulcerations over joints, and have clinical amyopathic DM (CADM) with few muscular symptoms (3, 4). Anti-MDA5 DM is strongly associated with interstitial lung disease (ILD) in most regions and ethnicities, especially rapidly progressive ILD (RPILD) which has a poor clinical prognosis (5). ASS is a relatively homogeneous multisystem disease (6), characterized by fever, myositis, arthritis, mechanic’s hands, Raynaud’s phenomenon, and chronic relapsing ILD, and responds well to glucocorticoid and immunosuppressive agents. The coexistence of anti-MDA5 and anti-ARS antibodies is very rare; in fact, they are believed to be mutually exclusive (1). Very few such cases have been reported (7–11). In this study, we attempted to identify cases that are positive for anti-MDA5 and anti-ARS antibodies and explore the clinical features and prognosis of dermatomyositis in these individuals. This work will assist physicians in better understanding this disease and guide clinical decision-making.
Methods
Study design
We retrospectively analyzed the clinical data of 1280 consecutive patients with IIM hospitalized in the Department of Rheumatology at the China-Japan Friendship Hospital from January 2016 to September 2021. A diagnosis of IIM was based on the Bohan and Peter criteria (12) or 2004 European Neuromuscular Centre (ENMC) criteria (13). Patients with anti-MDA5 and anti-ARS antibodies (anti-MDA5+/ARS+) were enrolled. In addition, we selected controls by using a randomly generated number table; these included 24 (1:4) ARS antibody-positive patients without anti-MDA5 antibodies (anti-MDA5-/ARS+) and 24 (1:4) MDA5 antibody-positive patients without anti-ARS antibodies (anti-MDA5+/ARS-). The presence of ILD was evaluated via chest radiography or high-resolution computed tomography (HRCT). RPILD was defined as previously published (14). Patient demographic data, laboratory tests, therapy regimens, and follow-up information were captured and recorded in detail. In addition, we also conducted a literature review of the condition. The study protocol was approved by the Ethics Committee of the China-Japan Friendship Hospital (reference number: 2019-25-K19) and written informed consent was obtained from each participant. The study was conducted per the declaration of Helsinki, 2000.
Clinical findings, laboratory parameters, and treatment
Patient background, clinical findings, and the treatment regimen were evaluated. Laboratory tests included lymphocyte count, creatine kinase (CK), lactic acid dehydrogenase (LDH), serum ferritin (FET), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Pulmonary function test (PFT) results [forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO)] were also evaluated. In addition, we evaluated the overall HRCT score based on the classification by Ichikado (15). Patient treatment parameters including dosages of glucocorticoids (GC), steroid pulse therapy, immunosuppressive agents, intravenous immunoglobulin (IVIG), and biological agents were also recorded.
Autoantibody detection
Anti-MDA5 and anti-ARS antibodies (antigens including Jo-1, PL-7, PL-12, EJ, and OJ) were quantified via immunoblotting according to the manufacturer’s instruction (EUROIMMUN, Lübeck, Germany). Myositis-associated antibodies (MAAs) (antigens including Ku, Ro-52, PM-Scl 100, and PM-Scl 75) were quantified in the same way. Sera that were positive (2+ or 3+) for anti-MDA5 and anti-ARS antibodies via immunoblotting were considered as positive. In order to avoid false positives, anti-synthetase ELISA Kit (MBL, Nagoya, Japan) was used to detect the presence of anti-ARS antibodies (including anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ and anti-KS antibodies) and anti-ARS antibodies’ level greater than 8.1 U/ml was considered as positive. Anti-MDA5 antibody was also detected using an ELISA Kit (MBL, Nagoya, USA) with a cut-off value of 32 U/ml (16).
Prognosis and relapse
The follow-up period was calculated from the date of initial treatment to either the date of death or of the last investigation/evaluation. We evaluated patient prognoses and relapses during the follow-up period. Relapses were classified as ILD relapse, rash relapse, or myositis relapse. ILD relapse was recorded when all the following were present: aggravated respiratory status, deterioration in ILD based on radiological findings, and the need to institute treatment with GC or immunosuppressants. Rash and myositis relapses were recorded when a recurrence of rash or myositis necessitated the commencement of intensive treatment.
Statistical analyses
All analyses were performed using IBM SPSS software (version 23.0, Armonk, NY, USA). Continuous data are presented as mean ± SD. Categorical variables are presented as frequencies and percentages. The continuous variables were compared via ANOVA with a Bonferroni post-hoc test when normally and homogeneous distributed, Kruskal Wallis H test with subsequent pairwise comparisons when not normally or inhomogeneous distributed. We used Fisher’s exact test to compare categorical variables and Chi-square with Bonferroni adjustment for post hoc tests. Survival among three groups was evaluated by applying the Kaplan-Meier (log-rank) test. All analyses were 2-tailed and p values <0.05 were considered to indicate statistical significance.
Results
Clinical characteristics of anti-MDA5+/ARS+ DM
We identified 6 individuals (0.47%) presenting as double positive for anti-MDA5 and anti-ARS antibodies. Of these, 2 (33.3%) were anti-PL-12+, 2 (33.3%) were anti-Jo-1+, 1 (16.7%) was anti-EJ+, and 1 (16.7%) was anti-PL-7+. A summary of the baseline clinical characteristics of the 6 double-positive patients is provided in Table 1. Comparisons between the clinical characteristics of the three subgroups (anti-MDA5+/ARS+, anti-MDA5-/ARS+, and anti-MDA5+/ARS-) are shown in Table 2.
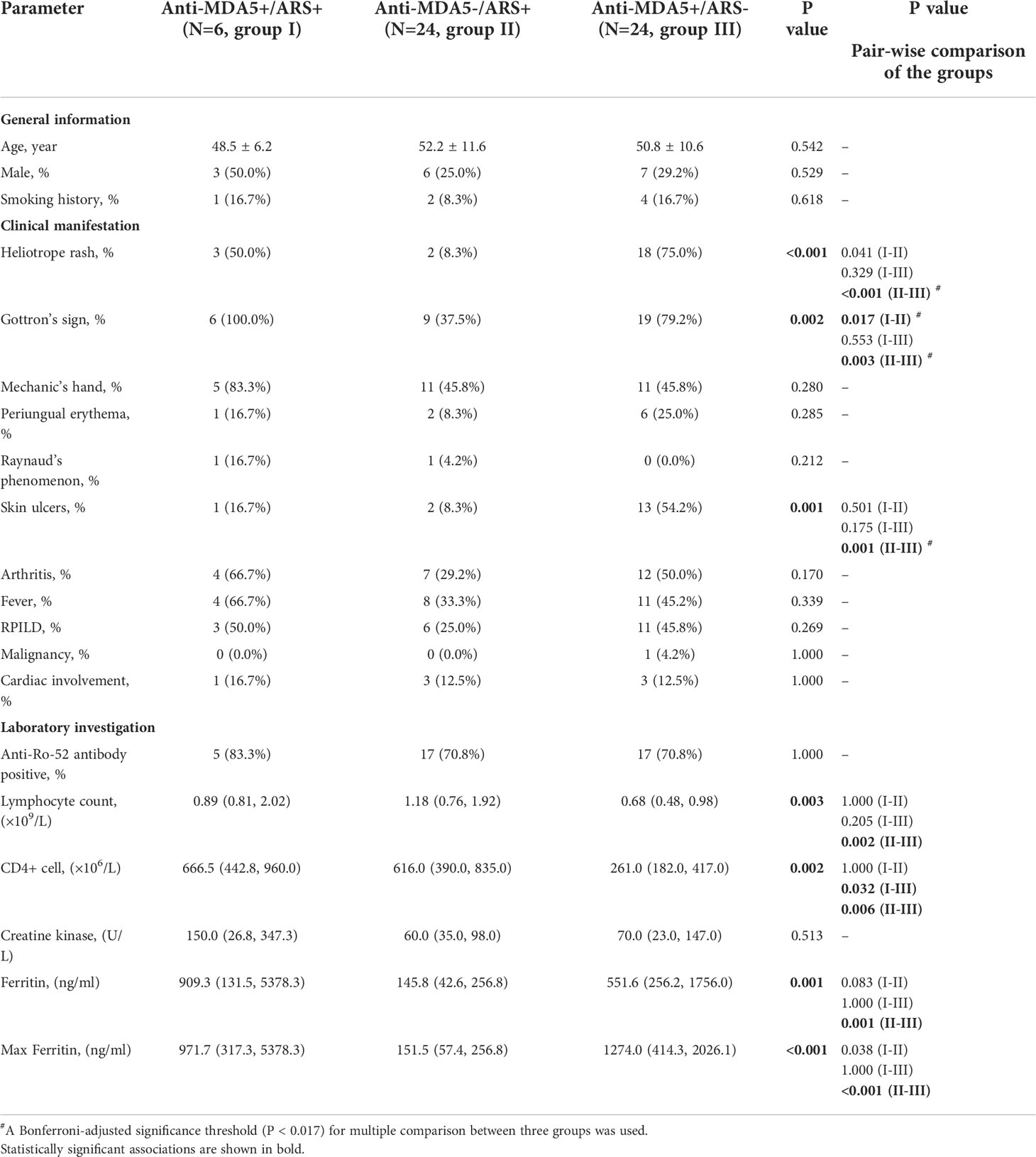
Table 2 Comparison of clinical manifestations in different groups (anti-MDA5+/ARS+, anti-MDA5-/ARS+ and anti-MDA5+/ARS-) of IIM patients .
The anti-MDA5+/ARS+ group included 3 males and 3 females with a median age of 51 years (range 37–53). All showed the hallmark cutaneous manifestations of DM and presented with Gottron’s sign, 3 (50%) showed a Heliotrope rash, 1 (16.7%) showed periungual erythema and skin ulcers, 1 (16.7%) showed typical Raynaud’s phenomenon, 4 (66.7%) had arthritis, and 5 (83.3%) had mechanic’s hand. Compared to anti-MDA5-/ARS+, the anti-MDA5+/ARS+ group had a greater frequency of Heliotrope rash (p = 0.041) and Gottron’s sign (p = 0.017), but the between-group difference in Heliotrope rash was not significant after post hoc correction. All anti-MDA5+/ARS+ individuals were diagnosed with ILD and 3 (50%) were categorized as RPILD. Furthermore, none of them complicated with malignancy, while one patient (16.7%) presented with tachycardia without other manifestations of cardiac involvement.
In terms of laboratory investigations, five anti-MDA5+/ARS+ individuals (83.3%) were Ro-52 positive. At the initial visit, roughly half showed an elevated level of LDH, FET, ESR, CRP, and a decreased lymphocyte count. CD4+ T-cell counts in anti-MDA5+/ARS+ were higher than in anti-MDA5+/ARS- individuals (p = 0.032) but there was no difference when compared to anti-MDA5-/ARS+ (p = 1.000). Serum FET levels were elevated in anti-MDA5+/ARS+ compared to anti-MDA5-/ARS+ individuals (p = 0.038). PFT was performed in 4 anti-MDA5+/ARS+ individuals but could not be completed in the remaining 2 due to severe dyspnea. The former 4 showed a restrictive pattern and a decreased diffusing capacity.
Radiological findings during anti-MDA5+/ARS+ DM
One individual (Case 4) was unable to complete HRCT detection due to severe dyspnea and instead received bedside chest radiography. Others had HRCT on admission and during follow-up. Supplementary Table S1 compares HRCT findings among the three subgroups (anti-MDA5+/ARS+, anti-MDA5-/ARS+, and anti-MDA5+/ARS-). Although there was no significant difference in HRCT findings between anti-MDA5+/ARS+ and the two control groups, we found that NSIP with OP overlap dominated (3/5, 60%), and most presented with consolidation in the subpleural area of the lung (Figure 1). The regions of consolidation were symmetrically distributed, mainly in both lower lobes. In addition, we observed ground-glass attenuation (GGA) in 4 (80%) individuals, reticulation in 5 (100%), and traction bronchiectasis in 4 (80%). Ichikado has suggested that an HRCT score > 230 is independently associated with a risk of death (15). In the present study, no patients with anti-MDA5+/ARS+ antibodies had an HRCT score >230. During the follow-up period, 3 individuals (Cases 1, 2, and 6) showed a remarkable improvement in ILD, while 2 showed no marked change (Cases 3 and 5).
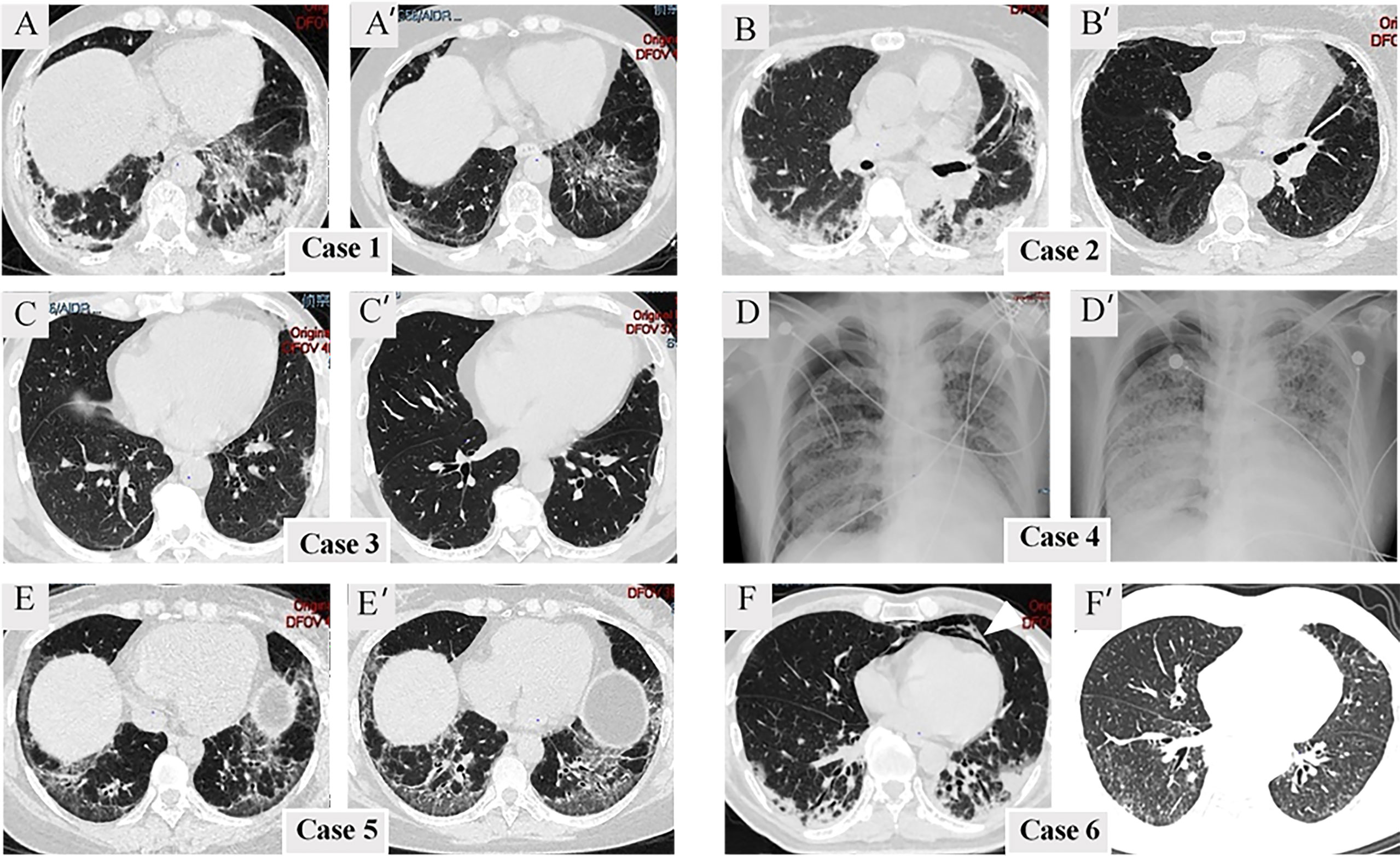
Figure 1 Radiological finding. (A–F) Radiological imaging at admission. (A’–F’) Radiological imaging following treatment. Mediastinal emphysema is indicated by the arrowheads.
Treatment and follow-up
All anti-MDA5+/ARS+ DM individuals received combination therapy (Table 1). At the initial visit, 1 received methylprednisolone pulse therapy (500mg×3d), while the others were treated with medium or high doses of prednisolone (PSL). Immunosuppressants were simultaneously administered; these included calcineurin inhibitors (CNI) (tacrolimus or cyclosporin A), intravenous cyclophosphamide (IVCY), or tofacitinib. When comparing the treatment regimens among the three subgroups (Supplementary Table S2), we found that compared to anti-MDA5-/ARS+, anti-MDA5+/ARS+ individuals showed a higher tendency to require triple therapy (PSL, CNI, and IVCY; p = 0.034) and IVIG (p = 0.007), although a significant difference was not observed on triple therapy after post hoc correction. Following treatment, 5/6 (83.3%) experienced relief of respiratory symptoms and showed a good prognosis except for 1 failure of intensive treatment resulting in death (Case 4). During the follow-up period, 2 (33.3%) experienced rash and myositis recurrence, while none had a recurrence of ILD. Follow-up FET, CK, lymphocyte counts, and HRCT scores improved over time (Supplementary Figure S1). There was no difference in the overall survival rate between the three subgroups (p > 0.05) (Figure 2).
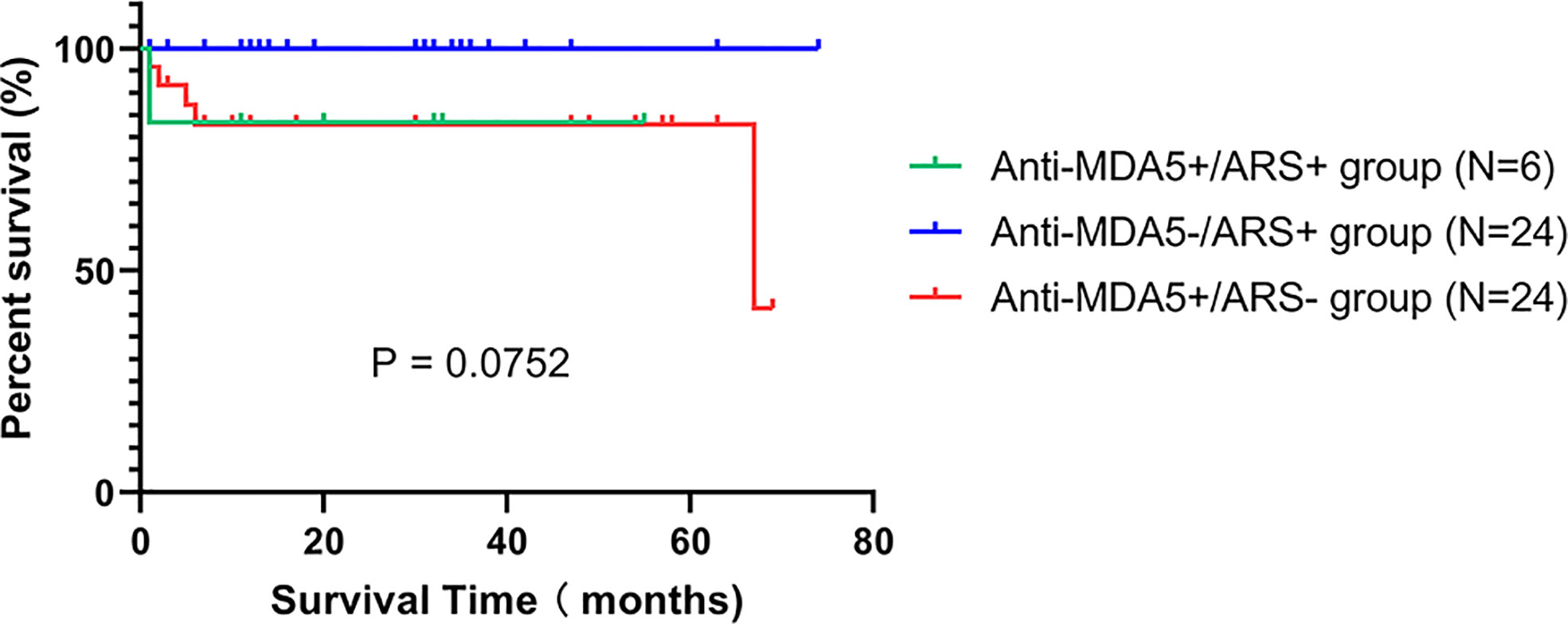
Figure 2 Kaplan-Meier curves for the anti-MDA5+/ARS+, anti-MDA5-/ARS+, and anti-MDA5+/ARS- subgroups.
Complications during treatment
Compared with anti-MDA5-/ARS+, infection was more common in anti-MDA5+/ARS+ individuals (Supplementary Table S3). The clinical course was complicated in 66.7% of the anti-MDA5+/ARS+ individuals by the presence of at least one pathogen. We identified bacterial infection in 2 (33.3%), fungal infection in 2 (33.3%), and cytomegalovirus (CMV) in 1 (16.7%) of the cases. The infections were well controlled with intensive treatment in most of these cases. Mediastinal emphysema is a frequent complication of anti-MDA5+ DM (17) and was observed in 1/6 (16.7%) cases in this study.
Literature review
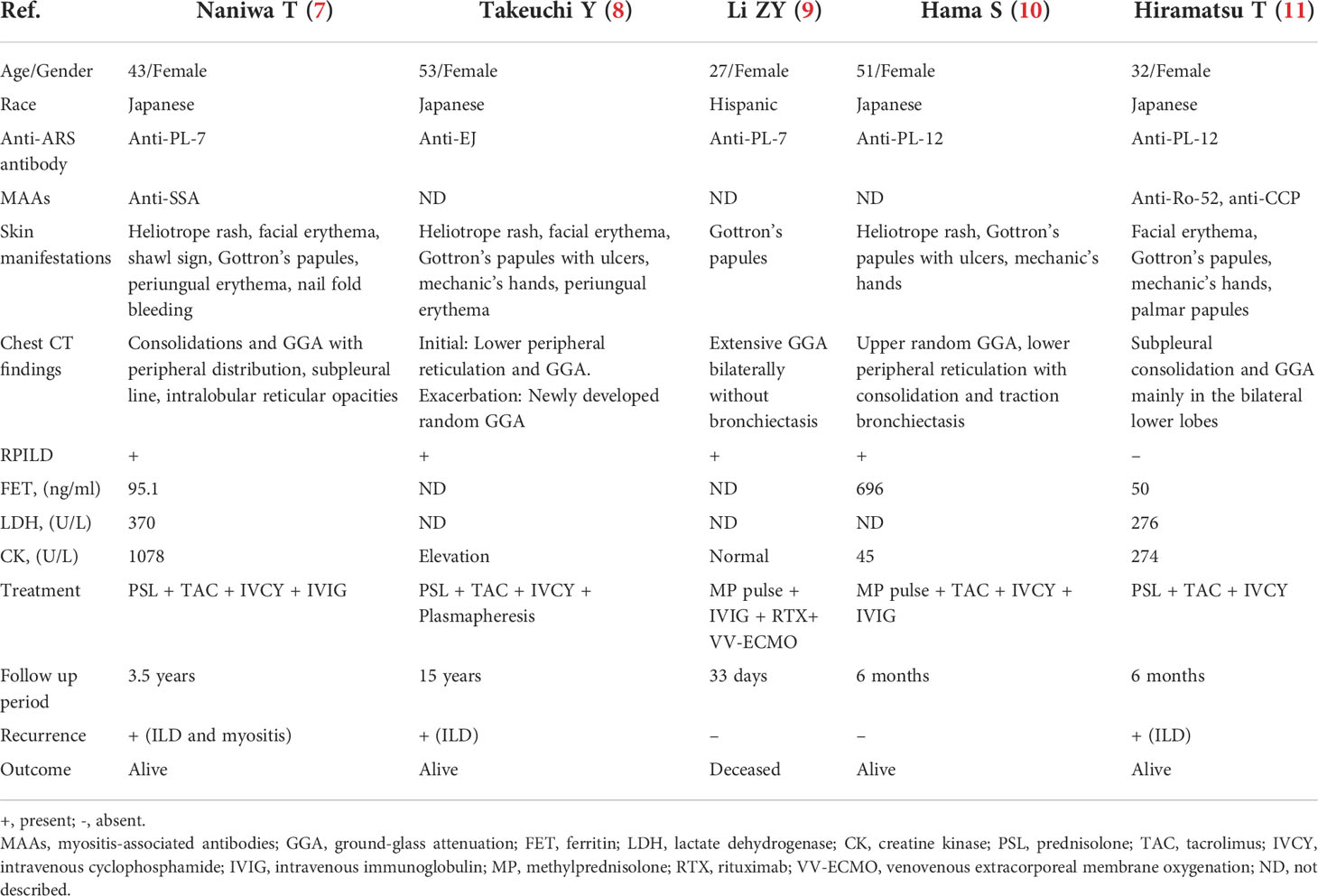
We analyzed five published case reports (7–11). Their characteristics are summarized in Table 3. Together with our cohort, 11 anti-MDA5+/ARS+ were identified, including 3 adult males and 8 adult females with a median age of 51 years (range 27–53). 10 (90.9%) were Asian. The main anti-ARS antibodies were anti-PL-12 (4/11, 36.3%), anti-PL-7 (3/11, 27.3%), anti-Jo-1 (2/11, 18.2%), and anti-EJ (2/11, 18.2%). Cutaneous lesions (100%) and ILD (100%) were the most common reported symptoms. 7/11 (63.6%) presented with RPILD and anti-Ro-52 antibodies were detected in 75% (6/8) of cases. Radiologically, consolidations (6/10, 70%) and GGA (9/10, 90%) with a peripheral distribution were prevalent. Traction bronchiectasis (5/10, 50%) and intralobular reticulation (8/10, 80%) were also frequently seen. All cases were treated with glucocorticoids and immunosuppressants. Some had additional treatments, such as IVIG, rituximab, plasmapheresis, and VV-ECMO. Following treatment, 9 (81.8%) recovered and 2 (18.2%) failed intensive treatment and died of respiratory failure. During follow-up, 3/9 (33.3%) experienced a recurrence of ILD, 2 (22.2%) of rash, and 3 (33.3%) of myositis.
Discussion
In the present study, we identified and reviewed six anti-MDA5+/ARS+ DM cases. Our analyses raised three important clinical issues. First, anti-MDA5+/ARS+ DM shows clinical characteristics that combine the features of anti-MDA5+ DM and anti-ARS+ DM. Second, NSIP with OP overlap is predominant in anti-MDA5+/ARS+ associated ILD. Third, anti-MDA5+/ARS+ patients appear to respond to glucocorticoid therapy and glucocorticoid combined with one or more immunosuppressants; as such, this approach should be considered in cases of anti-MDA5+/ARS+ DM.
A clinical presentation with combined anti-MDA5 and anti-ARS antibodies in DM is very rare. As such we know little about the characteristics of this disease subgroup. To our knowledge, the prevalence of anti-MDA5+/ARS+ in IIM patients is approximately 0.47%. In the present study, we found that cutaneous lesions and ILD were the most common symptoms in anti-MDA5+/ARS+ DM. Moreover, we found that periungual erythema, skin ulceration (characteristic of anti-MDA5+ DM), and mechanic’s hands (characteristic of anti-ARS+ DM) were common in anti-MDA5+/ARS+ DM (18). Previous studies reported that the prevalence of ILD in anti-MDA5+ DM ranges from 50 to 100% (19, 20) and that anti-MDA5-associated RPILD is more common than anti-ARS-associated RPILD (21). In our cohort, all anti-MDA5+/ARS+ patients were diagnosed with ILD and 50% were categorized as RPILD. Together with the published cases, 63.6% of the double-positive individuals presented with RPILD, which is more closely aligned with anti-MDA5+ DM (22). However, Yuhui Li et al. (21, 23) reported mortality rates of 30–40% in anti-MDA5 antibody-associated RPILD, which is higher than the mortaility rate of anti-MDA5+/ARS+ associated RPILD in our cohort (28.6%). Therefore, the clinical course of anti-MDA5+/ARS+ may be better and more closely resembled that of anti-ARS+ DM.
To date, numerous biomarkers have been reported to be associated with DM-ILD (15, 24). Ferritin is a well-characterized serum biomarker for anti-MDA5+ DM-ILD and correlates with the severity and prognosis of ILD; reported cutoff values vary from 500 to 1500 ng/ml (25). We showed that the maximum ferritin levels of anti-MDA5+/ARS+ DM were significantly higher than in anti-MDA5-/ARS+ DM but not different from anti-MDA5+/ARS- DM. Notably, one patient in our cohort died of respiratory failure with extremely high levels of ferritin (> 15,000.0 ng/mL). Systemic macrophage activation, which is correlated with hyperferritinemia, is potentially related to the pathogenesis of anti-MDA5 antibody-associated RPILD (26, 27). Whether macrophage activation is also involved in the pathophysiology of anti-MDA5+/ARS+ DM and directly modulates ferritin levels remains to be tested. Apart from hyperferritinemia, lymphocytopenia is also reported as a risk factor in anti-MDA5+ DM. There are several possible explanations for the decrease of lymphocytes in DM. Shu et al. found that DM is associated with suppressed CD3+ T-cell autophagy, leading to increased T-lymphocyte apoptosis and lymphocytopenia (28). Kamphuis E et al. demonstrated that increased type 1 interferons can downregulate central memory and naïve T lymphocytes, activate B lymphocytes, and eventually result in lymphocytopenia (29). We found that CD4+ T-cell counts were higher in anti-MDA5+/ARS+ DM than anti-MDA5+/ARS- DM patients, implying a favorable prognostic immune state in the former group. Based on these characteristics of anti-MDA5+/ARS+ DM, we concluded that anti-MDA5+/ARS+ DM patients show a combined presentation of anti-MDA5+ DM and anti-ARS+ DM; we investigated this hypothesis further through radiological analyses.
Using HRCT, most anti-MDA5+/ARS+ patients (60%) were diagnosed as having NSIP with OP overlap and two (40%) had NSIP. Consolidation, GGA, traction bronchiectasis, and intralobular reticulation were frequently observed in these patients. As described in previous studies (25), lower consolidation/GGA patterns and random GGA patterns are mainly found in anti-MDA5+ DM, whereas a lower reticulation pattern is the main finding among anti-MDA5- DM. The latter HRCT pattern corresponds with NSIP and likely with anti-ARS+ DM patients. Therefore, we thought that the radiological characteristics of anti-MDA5+/ARS+ DM also combined the features of anti-MDA5+ DM and anti-ARS+ DM. Although some studies suggested that NSIP with OP overlap may correlate with the acute or subacute form of onset of ILD and become resistant to therapy and progress to fibrosis (30), most anti-MDA5+/ARS+ patients in our cohort showed a remarkable improvement or remained stable following treatment.
In terms of treatment, there is no generally accepted therapy guideline for MSA antibody-positive or double-antibody positive cases. Based on the conventional treatment of anti-MDA5+ DM, glucocorticoid monotherapy may be inadequate, and more intensive therapy might improve prognosis (31). Together with published case reports, all identified anti-MDA5+/ARS+ DM cases received glucocorticoids and additional treatments, including CNI, IVCY, IVIG, tofacitinib, rituximab, plasmapheresis, and VV-ECMO. Most responded to these treatments and showed good prognosis, except for two who died of respiratory failure despite intensive immunosuppressive therapy. Hiramatsu T (11) reported on a female case with anti-MDA5+/ARS+ DM, who was initially refractory to glucocorticoids combined with tacrolimus; her condition was subsequently stabilized by IVCY. Therefore, glucocorticoids combined with one or more immunosuppressants may offer a basic treatment approach in cases of anti-MDA5+/ARS+ DM. In our cohort, IVIG was more commonly used in anti-MDA5+/ARS+ DM compared to anti-MDA5-/ARS+ DM. Its value arises from the inhibition of cytokine production and enhancement of the clearance of pathogenic autoantibodies (32). Thus, IVIG can also be considered in these patients. Furthermore, plasma exchange, VV-ECMO, and lung transplantation might be considered in life-threatening situations although their efficacy remain controversial (33, 34).
In our cohort, two individuals (33.3%) experienced a recurrence of rash and myositis but none had a recurrence of ILD during the follow-up. In our literature review, we identified three of five cases that presented with an ILD recurrence; one of these also had a recurrence of myositis. How disease recurrence factors into the clinical course of anti-MDA5+/ARS+ DM is still unknown. In our survival analyses, there was no significant difference between the three subgroups, which partly stems from the unavoidably small sample size. Thus, the prognosis of anti-MDA5+/ARS+ DM remains uncertain and warrants further investigation. Additionally, we observed infection and mediastinal emphysema in anti-MDA5+/ARS+ DM. Many studies have shown that infection and mediastinal emphysema can increase the risk of death in IIM (17, 35, 36). Therefore, clinicians also need to be highly vigilant of these complications in anti-MDA5+/ARS+ DM.
This study presents critically important data but we recognize some limitations. First, this was a small, retrospective study conducted at a single institution. Second, the small sample size limited statistical power. Although anti-MDA5+/ARS+ DM is very rare, we believe that as this and other studies are published, we can develop an increasingly precise understanding of this condition and its most effective therapies.
In summary, anti-MDA5+/ARS+ DM is very rare. The clinical and radiological characteristics of anti-MDA5+/ARS+ DM show combined features of anti-MDA5+ DM and anti-ARS+ DM. Anti-MDA5+/ARS+ patients might respond well to glucocorticoid therapy and glucocorticoid combined with one or more immunosuppressants. Further studies and meta-analyses will gradually help us to improve the precision of these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YG conceived of the review and supervised the project. XC wrote the manuscript. LZ, XL and QJ contributed to literature review and editing. JL and QP contributed to literature review and compiled figures. GW contributed to content and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National High Level Hospital Clinical Research Funding [2022-NHLHCRF-YS-02]; and National Natural Science Foundation of China [81971521, 82171788].
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.987841/full#supplementary-material
References
1. Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers (2021) 7(1):86. doi: 10.1038/s41572-021-00321-x
2. Gono T, Kuwana M. Current understanding and recent advances in myositis-specific and -associated autoantibodies detected in patients with dermatomyositis. Expert Rev Clin Immunol (2020) 16(1):79–89. doi: 10.1080/1744666X.2019.1699059
3. Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, et al. RNA Helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheumatol (2009) 60(7):2193–200. doi: 10.1002/art.24621
4. Sontheimer RD. Dermatomyositis: An overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin (2002) 20(3):387–408. doi: 10.1016/S0733-8635(02)00021-9
5. Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatol (Oxford) (2012) 51(7):1278–84. doi: 10.1093/rheumatology/ker518
6. Mahler M, Miller FW, Fritzler MJ. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: A comprehensive review. Autoimmun Rev (2014) 13(4-5):367–71. doi: 10.1016/j.autrev.2014.01.022
7. Naniwa T, Tamechika S, Okazaki Y, Maeda S, Kuwana M. Coexistence of anti-melanoma differentiation-associated gene 5 and anti-aminoacyl-transfer RNA synthetase antibodies in a patient with dermatomyositis and rapidly progressive and relapsing interstitial lung disease. Modern Rheumatol Case Rep (2017) 1(1):3–8. doi: 10.1080/24725625.2016.1253650
8. Takeuchi Y, Hashimoto M, Nakashima R, Tanaka M, Kuramoto N, Murakami K, et al. Anti-EJ, anti-MDA5 double-positive chronic clinically amyopathic dermatomyositis: A case report. Rheumatol Adv Pract (2018) 2(2):rky022. doi: 10.1093/rap/rky022
9. Li ZY, Gill E, Mo F, Reyes C. Double anti-PL-7 and anti-MDA-5 positive amyopathic dermatomyositis with rapidly progressive interstitial lung disease in a Hispanic patient. BMC Pulm Med (2020) 20(1):220. doi: 10.1186/s12890-020-01256-x
10. Hama S, Higashida-Konishi M, Akiyama M, Shimada T, Takei H, Izumi K, et al. Dermatomyositis which was double positive for anti-MDA5 and anti-ARS antibodies that was successfully treated by intensive immunosuppressive therapy. Intern Med (2022) 61(7):1085–91. doi: 10.2169/internalmedicine.8579-21
11. Hiramatsu T, Murano M, Nakai S, Murakami Y, Nishimoto K, Matsushima S, et al. Clinically amyopathic dermatomyositis with interstitial lung disease double-positive for anti-MDA5 and anti-PL12 antibodies. Respir Med Case Rep (2022) 36:101606. doi: 10.1016/j.rmcr.2022.101606
12. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med (1975) 292(7):344–7. doi: 10.1056/NEJM197502132920706
13. Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: Trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, naarden, the Netherlands. Neuromuscul Disord (2004) 14(5):337–45. doi: 10.1016/j.nmd.2004.02.006
14. Won Huh J, Soon Kim D, Keun Lee C, Yoo B, Bum Seo J, Kitaichi M, et al. Two distinct clinical types of interstitial lung disease associated with polymyositis-dermatomyositis. Respir Med (2007) 101(8):1761–9. doi: 10.1016/j.rmed.2007.02.017
15. Ichikado K, Suga M, Muranaka H, Gushima Y, Miyakawa H, Tsubamoto M, et al. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: validation in 44 cases. Radiology (2006) 238(1):321–9. doi: 10.1148/radiol.2373041515
16. Sato S, Murakami A, Kuwajima A, Takehara K, Mimori T, Kawakami A, et al. Clinical utility of an enzyme-linked immunosorbent assay for detecting anti-melanoma differentiation-associated gene 5 autoantibodies. PloS One (2016) 11(4):e0154285. doi: 10.1371/journal.pone.0154285
17. Zhou M, Ye Y, Yan N, Lian X, Bao C, Guo Q. Noninvasive positive pressure ventilator deteriorates the outcome of pneumomediastinum in anti-MDA5 antibody-positive clinically amyopathic dermatomyositis. Clin Rheumatol (2020) 39(6):1919–27. doi: 10.1007/s10067-019-04918-2
18. Concha JSS, Merola JF, Fiorentino D, Werth VP. Re-examining mechanic's hands as a characteristic skin finding in dermatomyositis. J Am Acad Dermatol (2018) 78(4):769–75.e2. doi: 10.1016/j.jaad.2017.10.034
19. Motegi SI, Sekiguchi A, Toki S, Kishi C, Endo Y, Yasuda M, et al. Clinical features and poor prognostic factors of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol (2019) 29(5):511–7. doi: 10.1684/ejd.2019.3634
20. Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) (2016) 68(5):689–94. doi: 10.1002/acr.22728
21. Li Y, Gao X, Li Y, Jia X, Zhang X, Xu Y, et al. Predictors and mortality of rapidly progressive interstitial lung disease in patients with idiopathic inflammatory myopathy: A series of 474 patients. Front Med (Lausanne) (2020) 7:363. doi: 10.3389/fmed.2020.00363
22. Gui X, Shenyun S, Ding H, Wang R, Tong J, Yu M, et al. Anti-Ro52 antibodies are associated with the prognosis of adult idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatol (Oxford) (2022). doi: 10.1093/rheumatology/keac090
23. Li Y, Li Y, Wu J, Miao M, Gao X, Cai W, et al. Predictors of poor outcome of anti-MDA5-Associated rapidly progressive interstitial lung disease in a Chinese cohort with dermatomyositis. J Immunol Res (2020) 2020:2024869. doi: 10.1155/2020/2024869
24. Lian X, Zou J, Guo Q, Chen S, Lu L, Wang R, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: The FLAIR model. Chest (2020) 158(4):1535–45. doi: 10.1016/j.chest.2020.04.057
25. Mimori T, Nakashima R, Hosono Y. Interstitial lung disease in myositis: clinical subsets, biomarkers, and treatment. Curr Rheumatol Rep (2012) 14(3):264–74. doi: 10.1007/s11926-012-0246-6
26. Stewart JA, Price T, Moser S, Mullikin D, Bryan A. Progressive, refractory macrophage activation syndrome as the initial presentation of anti-MDA5 antibody positive juvenile dermatomyositis: A case report and literature review. Pediatr Rheumatol Online J (2022) 20(1):16. doi: 10.1186/s12969-022-00675-w
27. Zuo Y, Ye L, Liu M, Li S, Liu W, Chen F, et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatol (Oxford) (2020) 59(10):2829–37. doi: 10.1093/rheumatology/keaa034
28. Shu X, Chen F, Peng Q, Lu X, Tian X, Wang Y, et al. Potential role of autophagy in t−cell survival in polymyositis and dermatomyositis. Mol Med Rep (2017) 16(2):1180–8. doi: 10.3892/mmr.2017.6693
29. Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood (2006) 108(10):3253–61. doi: 10.1182/blood-2006-06-027599
30. Debray MP, Borie R, Revel MP, Naccache JM, Khalil A, Toper C, et al. Interstitial lung disease in anti-synthetase syndrome: Initial and follow-up CT findings. Eur J Radiol (2015) 84(3):516–23. doi: 10.1016/j.ejrad.2014.11.026
31. Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, Ruiz-Rodríguez JC, Castellvi I, Rodriguez-Nieto MJ, et al. Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheumatol (2020) 50(4):776–90. doi: 10.1016/j.semarthrit.2020.03.007
32. Wang LM, Yang QH, Zhang L, Liu SY, Zhang PP, Zhang X, et al. Intravenous immunoglobulin for interstitial lung diseases of anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Rheumatol (Oxford) (2021). doi: 10.1093/rheumatology/keab928
33. Shirakashi M, Nakashima R, Tsuji H, Tanizawa K, Handa T, Hosono Y, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatol (Oxford) (2020) 59(11):3284–92. doi: 10.1093/rheumatology/keaa123
34. Gu Q, Diao M, Hu W, Huang M, Zhu Y. Case report: Extracorporeal membrane oxgenation for rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis in a post-partum woman. Front Med (Lausanne) (2021) 8:742823. doi: 10.3389/fmed.2021.742823
35. Ge YP, Shu XM, He LR, Wang GC, Lu X. Infection is not rare in patients with idiopathic inflammatory myopathies. Clin Exp Rheumatol (2022) 40(2):254–9. doi: 10.55563/clinexprheumatol/yps7ai
Keywords: dermatomyositis, anti-MDA5 antibody, anti-aminoacyl-tRNA synthetase, myositis-specific auto-antibody, interstitial lung disease
Citation: Chen X, Zhang L, Jin Q, Lu X, Lei J, Peng Q, Wang G and Ge Y (2022) The clinical features and prognoses of anti-MDA5 and anti-aminoacyl-tRNA synthetase antibody double-positive dermatomyositis patients. Front. Immunol. 13:987841. doi: 10.3389/fimmu.2022.987841
Received: 08 July 2022; Accepted: 15 August 2022;
Published: 30 August 2022.
Edited by:
Mitsuhiro Takeno, Nippon Medical School Musashi Kosugi Hospital, JapanReviewed by:
Hideaki Tsuji, Kyoto University Hospital, JapanShuang Ye, Shanghai Jiao Tong University, China
Copyright © 2022 Chen, Zhang, Jin, Lu, Lei, Peng, Wang and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongpeng Ge, Z3lwMjAxNkAxNjMuY29t
†These authors have contributed equally to this work and share senior authorship
‡These authors have contributed equally to this work and share last authorship
 Xixia Chen
Xixia Chen Lu Zhang
Lu Zhang Qiwen Jin
Qiwen Jin Xin Lu
Xin Lu Jieping Lei
Jieping Lei Qinglin Peng
Qinglin Peng Guochun Wang
Guochun Wang Yongpeng Ge
Yongpeng Ge