- Department of Urology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Radical cystectomy and removal of pelvic lymph nodes (RC-PLND) is a recommended treatment for high-risk non-muscle-invasive and muscle-invasive non-metastatic bladder cancer (BC). However, 50% of patients relapse after RC-PLND. This study aimed to evaluate the effect of programmed cell death ligand-1 (PD-L1) on the prognosis of bladder urothelial carcinoma (BUC) after RC-PLND.
Methods: We present this meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-Analyses Guidelines. The main outcomes were overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS) of 3 and 5 years after RC-PLND.
Results: Overall, 11 studies and 1393 BUC cases were included in our meta-analysis. In tumor cells (TCs), the PD-L1 negative group had statistically significant advantage in 5-year OS (risk ratio [RR]: 0.85, 95% confidence interval [CI]: 0.74–0.97, P = 0.02), RFS (RR: 0.76, 95% CI: 0.58–0.99, P = 0.04), and CSS (RR: 0.73, 95% CI: 0.58–0.92, P = 0.009) compared with the PD-L1 positive group. But, no statistically significant difference in 5-year OS and RFS was observed between the PD-L1 negative and positive groups in tumor-infiltrating immune cells.
Conclusions: Our study found that patients with BUC who tested positive for PD-L1 in TCs had a poor prognosis after RC-PLND. PD-1 or PD-L1 inhibitors could be used as a adjuvant medication for patients with BUC after RC-PLND who exhibit PD-L1 overexpression in TCs.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022301424.
Introduction
Bladder cancer (BC) is one of the most common malignant tumors in the world and accounts for more than 500,000 new cases and 200,000 deaths every year (1). Urothelial carcinoma is the most common type of BC and accounts for more than 90% of BC cases (2). According to the latest data of the American Cancer Society in 2022, the incidence and mortality of BC rank second among the tumors of the urinary system (3). Radical cystectomy and removal of pelvic lymph nodes (RC-PLND) is a recommended treatment for high-risk non-muscle-invasive and muscle-invasive non-metastatic BC (4). However, 50% of patients relapse after RC-PLND (5).
Programmed death ligand-1 (PD-L1) expression measured by immunohistochemistry (IHC) is a predictor of the treatment outcome of tumors and has been used in clinical practice. PD-L1 is also the most studied biomarker in tumors (6). Given that BC is an immunogenic tumor (7), blocking the interaction between programmed death-1 (PD-1) and PD-L1 has positively affected BC by restoring T cell-mediated immune response (8). However, PD-L1 expression in tumor cells (TCs) or tumor-infiltrating immune cells (ICs) have different effects on BC prognosis (9–11). Therefore, we need to additional more research and data to obtain a unified result. This biomarker has a high clinical value for the prognosis evaluation and recurrence prevention of patients after radical cystectomy.
This study aimed to evaluate the effect of PD-L1 on the prognosis of bladder urothelial carcinoma (BUC) after RC-PLND. We present this meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-Analyses Guidelines (12).
Materials and methods
This study was registered in the International Prospective Register of Systematic Reviews (number: CRD42022301424).
Search strategy
All studies written in English language and published from PubMed, Embase, and Cochrane Library up to February 15, 2022 were searched independently by two reviewers using the same search formula: (“urothelial carcinoma” [Title/Abstract] OR “bladder carcinoma” OR “bladder cancer” [Title/Abstract] OR “bladder tumor” [Title/Abstract]) AND (“PD-1” [Title/Abstract] OR “programmed death 1” [Title/Abstract] OR “programmed death ligand 1” [Title/Abstract] OR “PD-L1” [Title/Abstract]). The reference lists from published studies were also searched.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) patients with BUC had completed RC-PLND, (2) PD-L1 expression was measured by IHC after RC-PLND, and (3) written in English language. Exclusion criteria were as follows: (1) case report and case series, (2) conference abstract, and (3) incomplete data or duplicated data.
Data extraction and quality assessment
All records from the three electronic databases were selected using the Endnote software. The data of the included studies were extracted independently by two reviewers using Engauge Digitizer 12.1 software. The quality of included studies was also evaluated. Any disagreements were resolved through discussion with a third reviewer. Newcastle–Ottawa Scale (NOS) (13) was employed to evaluate the quality of all included retrospective studies (Supplementary Table 1). The NOS comprises eight items with a maximum of nine scores, with high scores indicating good quality.
Outcomes of interest
The main outcomes were overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS) of 3 and 5 years after RC-PLND.
Statistical analysis
All data analyses were performed using the Review Manager version 5.3 and Stata SE 14.0 tools. The Risk ratio (RR) was used to describe the results of dichotomous variables. p < 0.05 was regarded as statistically significant. Heterogeneities among studies were assessed by heterogeneity (I2) and chi-squared (χ2) tests. When I2 < 50%, a fixed-effect model was used. For I2 ≥ 50%, a random-effects model was employed.
Results
Selected studies
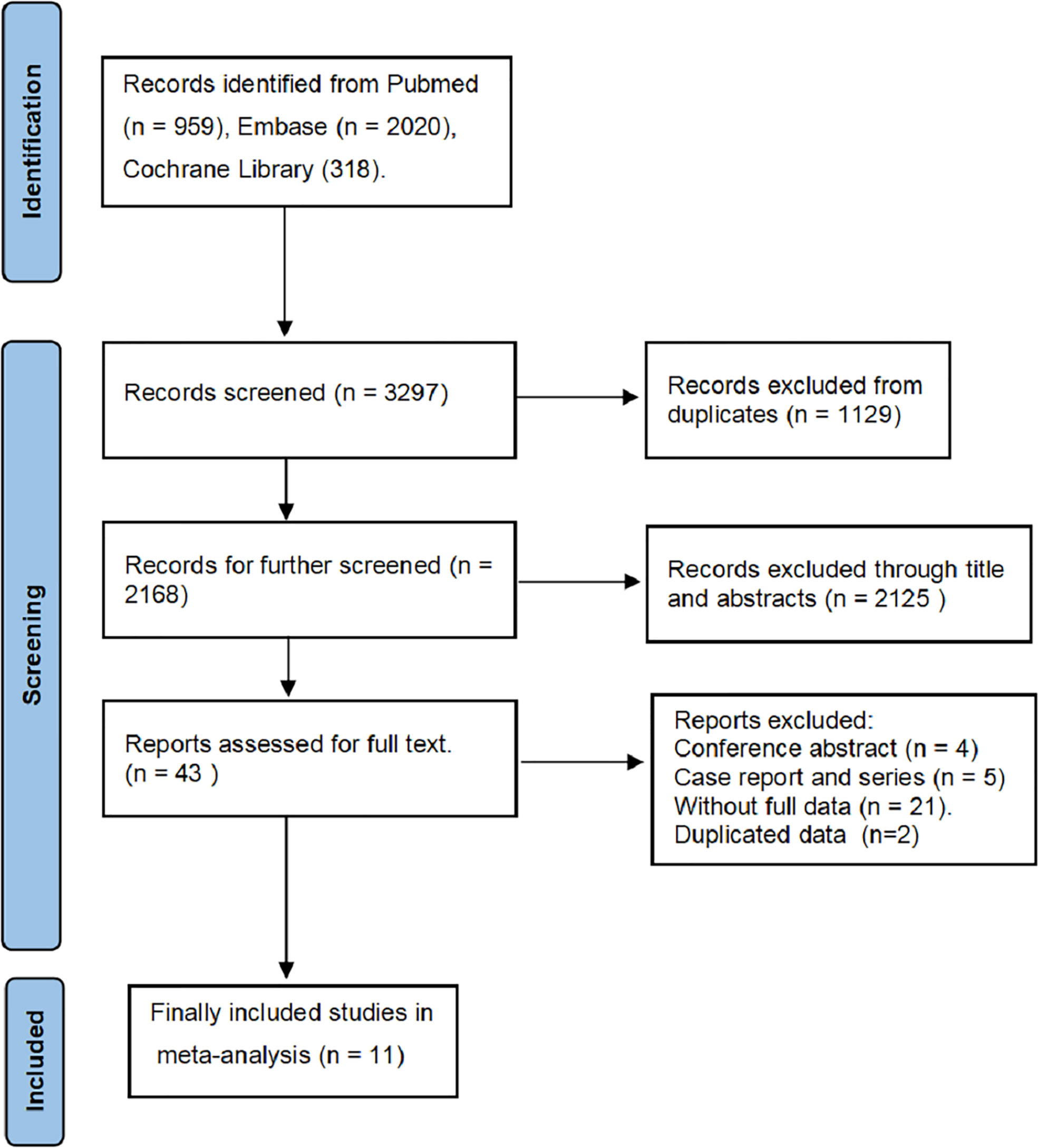
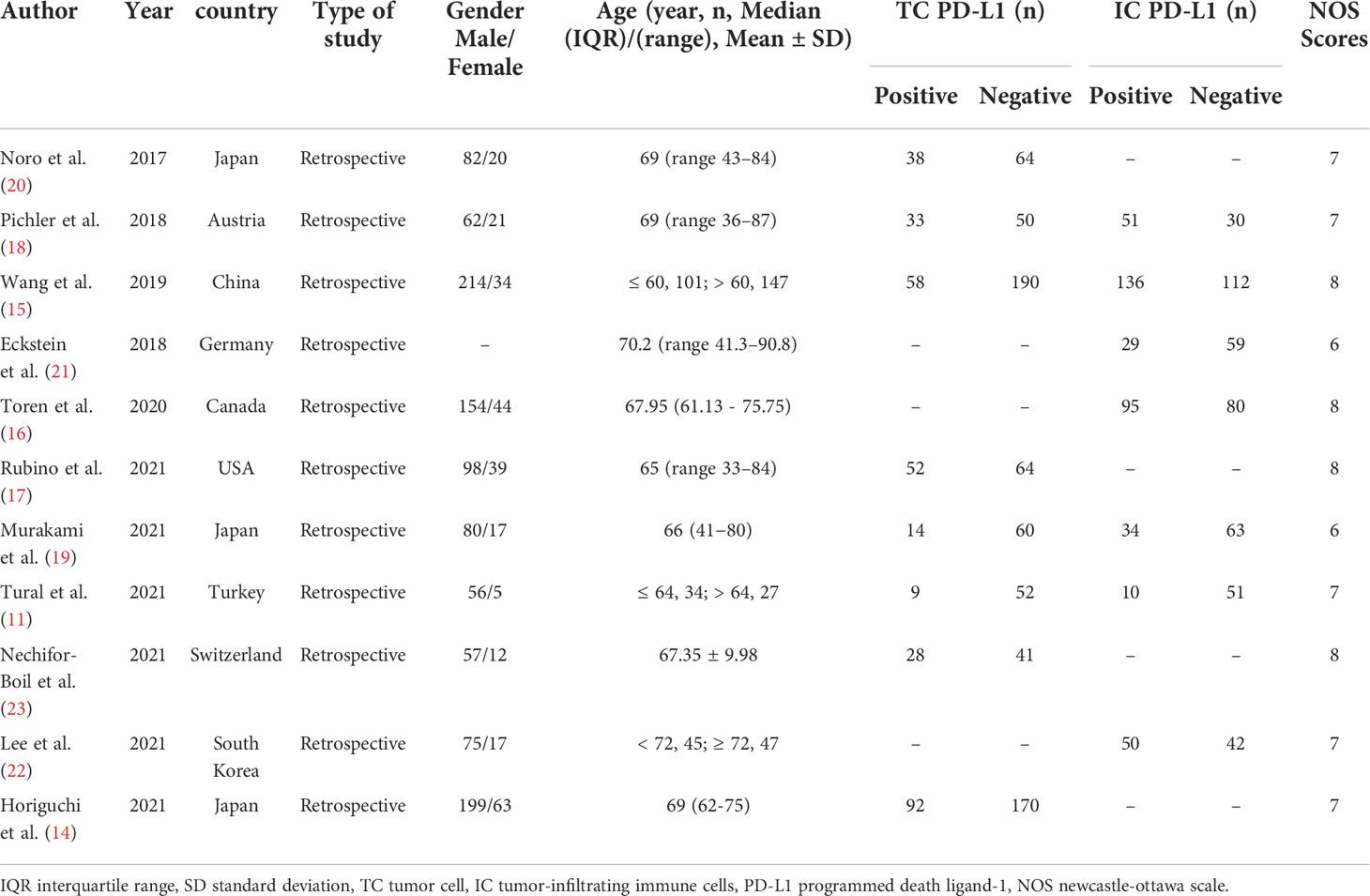
We searched 3297 records from the three electronic databases (PubMed, Embase, and Cochrane Library). Finally, 11 studies (11, 14–23) and 1393 BUC cases were included in our meta-analysis (Figure 1). This study is the first meta-analysis about the prognostic value of PD-L1 expression in patients with BUC after RC-PLND. Table 1 summarizes the characteristics and quality of all included studies that were published within the past 5 years. The patients were divided into PD-L1 positive group (PD-L1+) and PD-L1 negative group (PD-L1—) for TCs or ICs.
OS
For TCs, the PD-L1 negative group had a statistically significant better OS of 3 years (RR: 0.84, 95% CI: 0.75–0.95, P = 0.004) (Figure 2A) and 5 years (RR: 0.85, 95% CI: 0.74–0.97, P = 0.02) (Figure 2B) compared with the PD-L1 positive group. For ICs, no statistically significant difference in 3-year (RR: 0.94, 95% CI: 0.70–1.28, P = 0.72) and 5-year (RR: 0.88, 95% CI: 0.59–1.32, P = 0.54) OS was found between the PD-L1 negative and positive groups (Figures 2C, D).
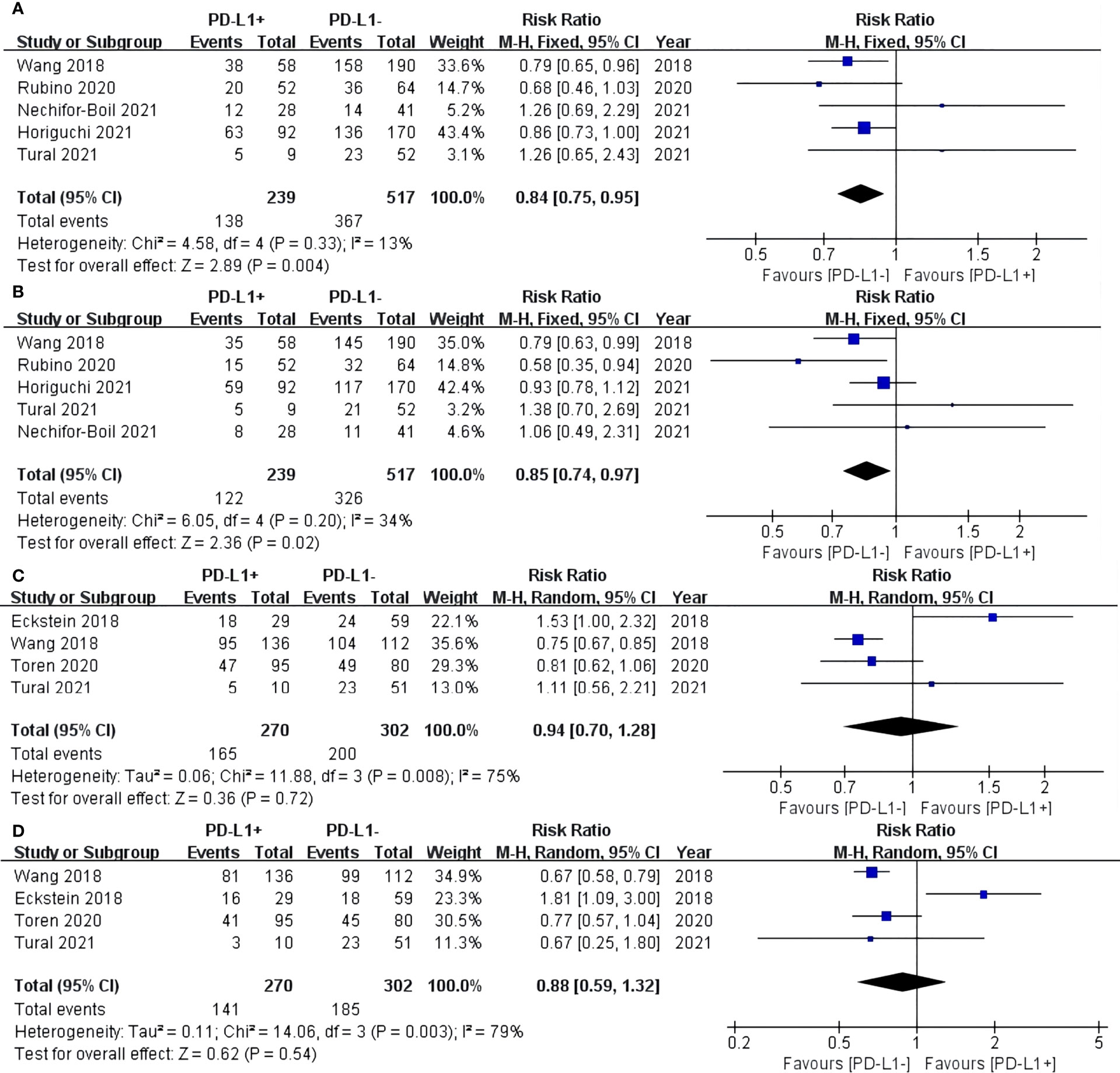
Figure 2 Forest plot of overall survival (OS) between the PD-L1 negative and positive groups in tumor cells (TCs): OS after surgery 3 years (A) and 5 years (B). Forest plot of OS between two group in tumor-infiltrating immune cells (ICs): OS after surgery 3 years (C) and 5 years (D).
RFS
For TCs, the PD-L1 negative group had a statistically significant advantage in 3-year (RR: 0.70, 95% CI: 0.61–0.81, P < 0.00001) (Figure 3A) and 5-year (RR: 0.76, 95% CI: 0.58–0.99, P = 0.04) RFS after RC-PLND (Figure 3B). For ICs, no statistically significant difference in 3-year (RR: 0.75, 95% CI: 0.48–1.17, P = 0.20) and 5-year (RR: 0.71, 95% CI: 0.43–1.17, P = 0.18) RFS was found between the two groups (Figures 3C, D).
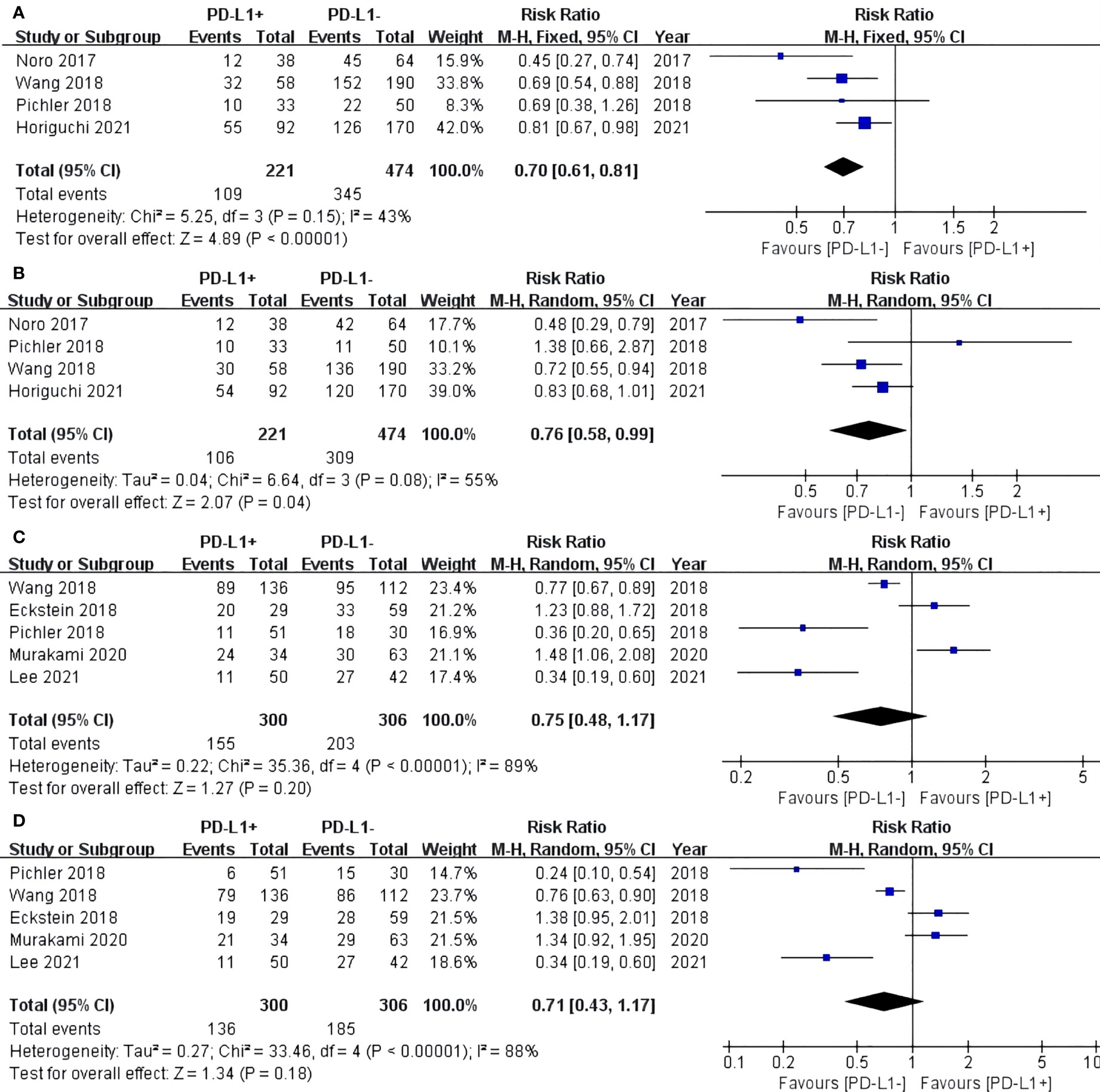
Figure 3 Forest plot of recurrence-free survival (RFS) between two group in TCs: RFS after surgery 3 years (A) and 5 years (B). Forest plot of RFS between two group in ICs: RFS after surgery 3 years (C) and 5 years (D).
CSS
Three included studies reported the CSS of patients with BUC after RC-PLND with PD-L1 detected on TCs. For TCs, the PD-L1 negative group had a statistically significant advantage in 5-year (RR: 0.73, 95% CI: 0.58–0.92, P = 0.009) CSS (Figure 4).
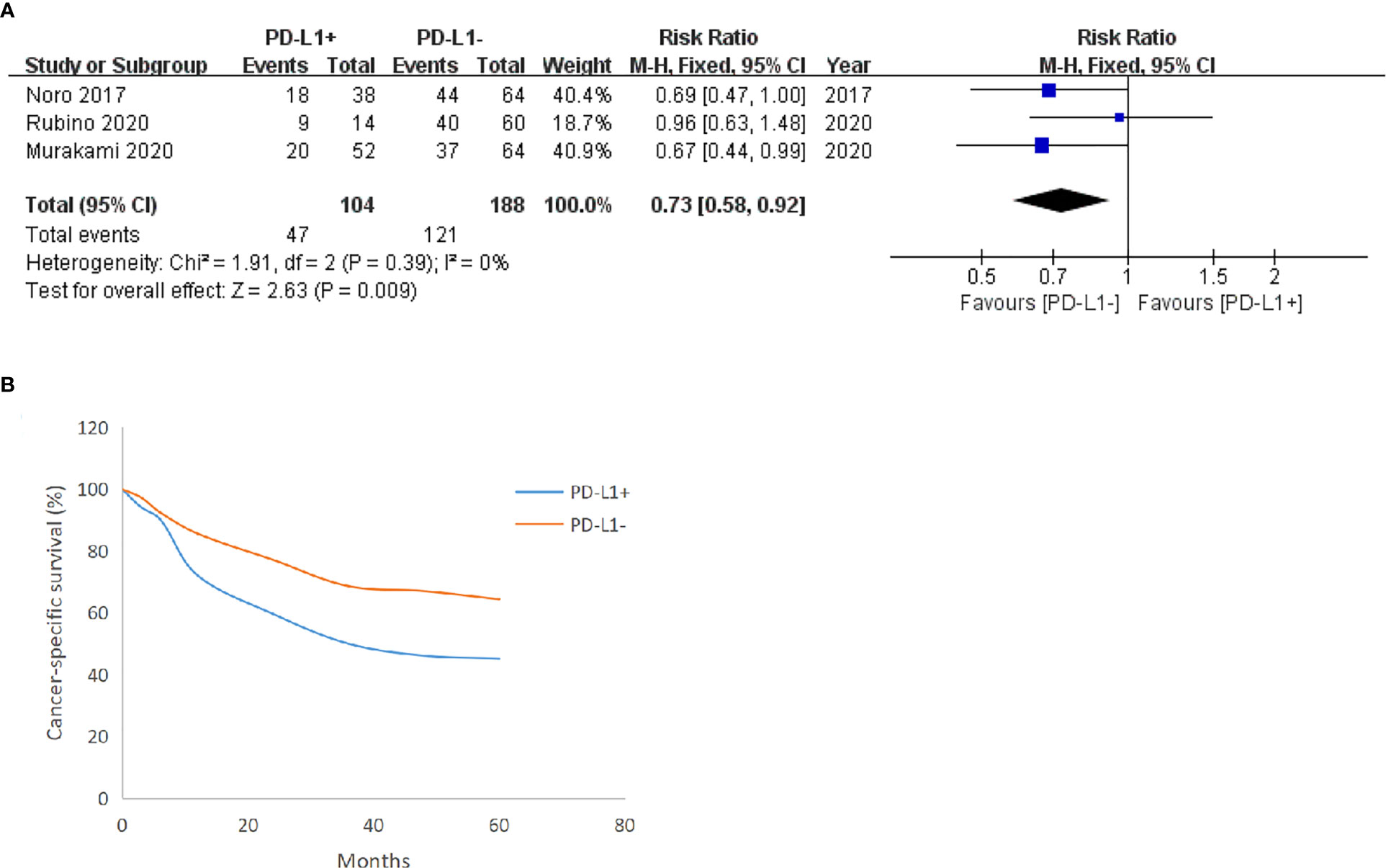
Figure 4 Forest plot of cancer-specific survival (CSS) between two group in TCs after surgery 5 years (A), CSS trend chart between the group in TCs (B).
Sensitivity analysis
We conducted the sensitivity analysis of OS (Figure 5A), RFS (Figure 5B), and CSS (Figure 5C) for PD-L1 expression on TCs at 5 years after surgery by separately removing each study and then merging the effect quantity. The results showed no remarkable changes in the overall results after the exclusion of each study from the main analysis.
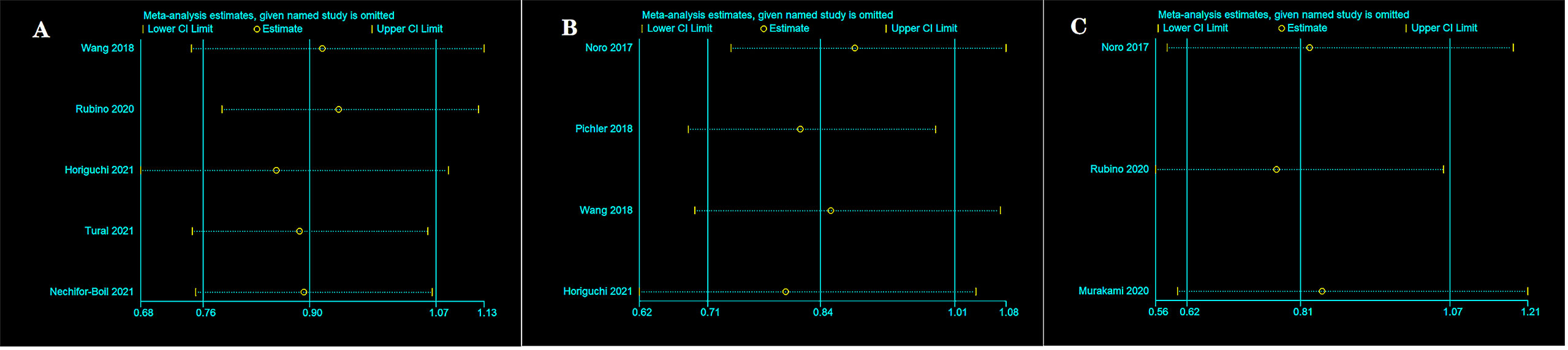
Figure 5 Sensitivity analysis between two group in TCs after surgery 5 years. OS (A), RFS (B), and CSS (C).
Publication bias
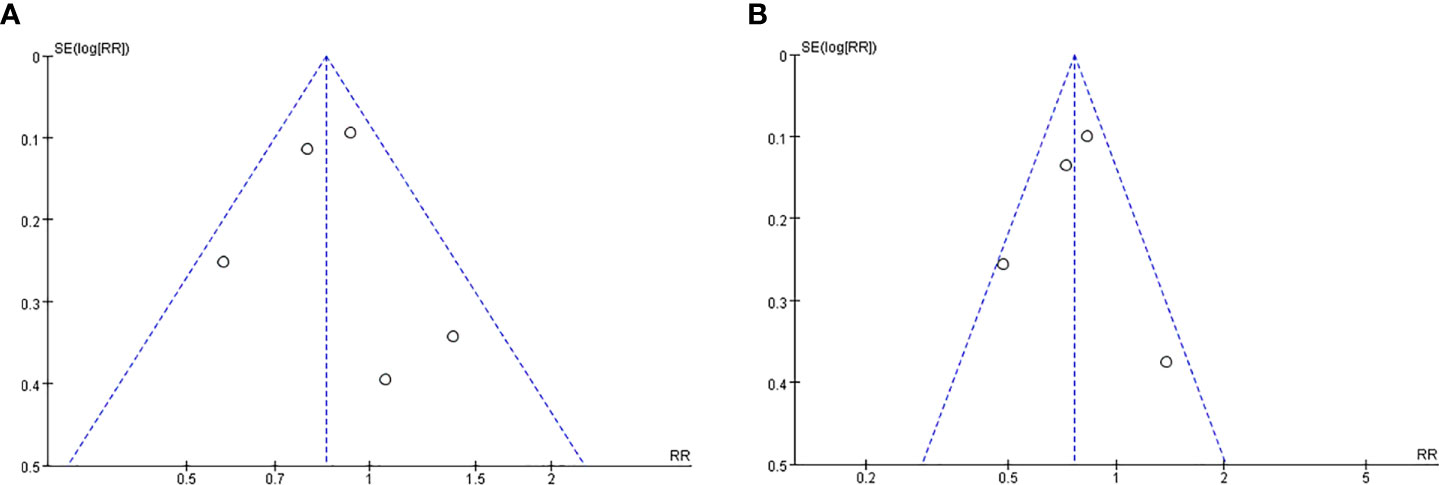
We constructed the funnel plot of OS (Figure 6A) and RFS (Figure 6B) for PD-L1 expression in TCs at 5 years after surgery. The funnel plot was basically symmetrical, indicating the low probability of publication bias.
Discussion
The high recurrence rate after radical cystectomy for BC has become a problem for doctors and patients. This study is the first meta-analysis of retrospective cohort studies for patients with BUC after radical cystectomy with long clinical follow-up time. The results showed the prognostic value of PD-L1 staining in bladder tumors.
In TCs, the PD-L1 negative group had statistically significant advantage in 5-year OS (63.1% vs. 51.0%), RFS (65.2% vs. 48.0%), and CSS (64.4% vs. 45.2%) compared with the PD-L1 positive group. This finding indicated that the prognosis of PD-L1 positive patients is worse after radical surgery due to the ability of PD-L1 to promote tumor immune escape (24). Overexpressed PD-L1 on TCs binds to PD-1 on ICs, and the TCR-signaling cascade is counteracted by SHP-2 phosphorylation. Finally, the activation of T cells is impaired (25).
However, no statistically significant difference in 5-year OS and RFS was observed between the PD-L1 negative and positive groups in ICs. This finding confirmed that the PD-L1 expression is more correlated with patient prognosis in TCs than in ICs (26).
Different results for PD-L1 expression have been reported in TCs or ICs removed by radical cystectomy (14, 17–19) due to the insufficient population included in single-center studies or the use of different PD-L1 positive determination methods that may interfere with the results from no correlation to poor prognosis. Our meta-analysis included many patients and studies conducted in recent years. The current results showed that patients with BUC who tested positive for PD-L1 in TCs had a poor prognosis after RC-PLND. Meanwhile, the PD-L1 expression in ICs had no statistical significance in the prognosis of patients with BUC after RC-PLND. Therefore, PD-1 or PD-L1 inhibitors can be used as a postoperative medication for patients with BUC after RC-PLND who exhibit PD-L1 overexpression in their TCs.
Shen et al. reported that compared with traditional drugs, PD-1 or PD-L1 inhibitors prolong the OS time of patients as second or later lines of treatment in advanced solid tumors with PD-L1 positive or negative expression (27). Funt et al. reported that neoadjuvant atezolizumab with gemcitabine and cisplatin chemotherapy effectively reduced the pathological stage of patients with muscle-invasive BC prior to RC-PLND and improved their OS and RFS rates (28). PD-1 or PD-L1 inhibitors have been used in advanced BC or neoadjuvant BC treatment and have achieved good results (27, 28). We expect that this regime will be utilized for patients who underwent radical cystectomy as a preventive rather than a salvage treatment. Molecular pathology and metabolomics have driven efforts to classify BC into subtypes, which could be potentially valuable in predictions of clinical outcomes and benefit to early diagnosis and treatment (29).
Our study had several limitations. First, no RCTs were included. Second, further subgroup analysis according to clinical or pathological stages was not conducted due to lack of data. Third, when a patient relapses after surgery, the use of different treatment methods may affect the patient’s OS and CSS. However, our results are still credible and have passed the heterogeneity test, publication bias test, and sensitivity analysis.
Conclusion
Our meta-analysis found that patients with BUC who tested positive for PD-L1 in TCs had a poor prognosis after RC-PLND. Meanwhile, PD-L1 expression in ICs had no statistical significance in the prognosis of patients after RC-PLND. PD-1 or PD-L1 inhibitors could be used as a adjuvant medication for patients with BUC after RC-PLND who exhibit PD-L1 overexpression in TCs; however, randomized controlled trials are needed for further verification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JZ and LS contributed to the conception and design this study. JZ, HZ and QL were responsible for the development of the methodology and data interpretation. JZ analyzed and interpreted the data. JZ wrote the paper. DW revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0732), and Chongqing Science and Technology Commission and Technology Commission (cstc2017shms-zdyf0319).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.986911/full#supplementary-material
References
1. Tran L, Xiao JF, Agarwal N, Duex JE, Theodorescu D. Advances in bladder cancer biology and therapy. Nat Rev Cancer. (2021) 21(2):104–21. doi: 10.1038/s41568-020-00313-1
2. Heath E, Rosenberg JE. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol. (2021) 18(2):93–103. doi: 10.1038/s41585-020-00394-5
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
4. Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur Urol. (2021) 79(1):82–104. doi: 10.1016/j.eururo.2020.03.055
5. Yoo SH, Kim H, Kwak C, Kim HH, Jung JH, Ku JH. Late recurrence of bladder cancer following radical cystectomy: Characteristics and outcomes. Urol Int (2019) 103(3):291–6. doi: 10.1159/000502656
6. Savic Prince S, Bubendorf L. Predictive potential and need for standardization of PD-L1 immunohistochemistry. Virchows Arch (2019) 474(4):475–84. doi: 10.1007/s00428-018-2445-7
7. van Wilpe S, Gerretsen ECF, van der Heijden AG, de Vries IJM, Gerritsen WR, Mehra N. Prognostic and predictive value of tumor-infiltrating immune cells in urothelial cancer of the bladder. Cancers (Basel). (2020) 12(9):2692. doi: 10.3390/cancers12092692
8. Thomas Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cristina C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. (2014) 515(7528):558–62. doi: 10.1038/nature13904
9. Hahn AW, Sirohi D, Agarwal N. The role of PD-L1 testing in advanced genitourinary malignancies. Eur Urol Focus. (2020) 6(1):11–3. doi: 10.1016/j.euf.2019.03.003
10. Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol (2015) 26(4):812–7. doi: 10.1093/annonc/mdv009
11. Tural D, Akar E, Baytekin HF, Canoglu D, Yilmaz M, Tugcu V, et al. Relationship between survival outcomes and microsatellite instability, tumor infiltrating lymphocytes and programmed cell death ligand-1 expression in patients with bladder cancer and radical cystectomy. J BUON. (2021) 26(5):2117–25.
12. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-p) 2015 statement. Syst Rev (2015) 4:1. doi: 10.1186/2046-4053-4-1
13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
14. Horiguchi H, Hatakeyama S, Yoneyama T, Yoneyama MS, Tanaka T, Fujita N, et al. Prognostic significance of the Ki67 index and programmed death-ligand 1 expression after radical cystectomy in patients with muscle-invasive bladder cancer. Urol Oncol (2021) 39(4):238. e9–238. e17. doi: 10.1016/j.urolonc.2020.11.029
15. Wang B, Pan W, Yang M, Yang W, He W, Chen X, et al. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci (2019) 110(2):489–98. doi: 10.1111/cas.13887
16. Toren p, Brisson H, Simonyan D, Hovington H, Lacombe L, Bergeron A, et al. Androgen receptor and immune cell PD-L1 expression in bladder tumors predicts disease recurrence and survival. World J Urol. (2021) 39(5):1549–58. doi: 10.1007/s00345-020-03358-x
17. Rubino S, Kim Y, Zhou J, Dhilon J, Li R, Spiess P, et al. Positive ki-67 and PD-L1 expression in post-neoadjuvant chemotherapy muscle-invasive bladder cancer is associated with shorter overall survival: A retrospective study. World J Urol. (2021) 39(5):1539–47. doi: 10.1007/s00345-020-03342-5
18. Pichler R, Fritz J, Lackner F, Sprung S, Brunner A, Horninger W, et al. Prognostic value of testing PD-L1 expression after radical cystectomy in high-risk patients. Clin Genitourin Cancer (2018) 16(5):e1015–24. doi: 10.1016/j.clgc.2018.05.015
19. Murakami Y, Matsumoto K, Shimizu Y, Ikeda M, Amano N, Shimura S, et al. PD-L1 expression in tumor-infiltrating lymphocytes (TILs) as an independent predictor of prognosis in patients with pN0 bladder cancer undergoing radical cystectomy. Urol Oncol (2021) 39(3):195. doi: 10.1016/j.urolonc.2020.09.034
20. Noro D, Hatakeyama S, Yoneyama T, Hashimoto Y, Koie T, Kawaguchi T, et al. Post-chemotherapy PD-L1 expression correlates with clinical outcomes in Japanese bladder cancer patients treated with total cystectomy. Med Oncol (2017) 34(6):117. doi: 10.1007/s12032-017-0977-3
21. Eckstein M, Wirtz RM, Pfannstil C, Wach S, Stoehr R, Breyer J, et al. A multicenter round robin test of PD-L1 expression assessment in urothelial bladder cancer by immunohistochemistry and RT-qPCR with emphasis on prognosis prediction after radical cystectomy. Oncotarget. (2018) 9(19):15001–14. doi: 10.18632/oncotarget.24531
22. Lee CU, Lee DH, Song W. Prognostic role of programmed death ligand-1 on tumor-infiltrating immune cells in “High-risk” patients following radical cystectomy: A retrospective cohort study. Front Oncol (2021) 11:706503. doi: 10.3389/fonc.2021.706503
23. Nechifor-Boilă IA, Loghin A, Nechifor-Boilă A, Decaussin-Petrucci M, Voidăzan S, Chibelean BC, et al. PD-L1 expression in muscle invasive urothelial carcinomas as assessed via immunohistochemistry: Correlations with specific clinical and pathological features, with emphasis on prognosis after radical cystectomy. Life (Basel). (2021) 11(5):404. doi: 10.3390/life11050404
24. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. (2019) 18(1):10. doi: 10.1186/s12943-018-0928-4
25. Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol (2021) 14(1):10. doi: 10.1186/s13045-020-01027-5
26. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res (2014) 20(19):5064–74. doi: 10.1158/1078-0432.CCR-13-3271
27. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: Meta-analysis. BMJ (2018) 362:k3529. doi: 10.1136/bmj.k3529
28. Funt SA, Lattanzi M, Whiting K, Al-Ahmadie H, Quinlan C, Teo MY. Neoadjuvant atezolizumab with gemcitabine and cisplatin in patients with muscle-invasive bladder cancer: A multicenter, single-arm, phase II trial. J Clin Oncol (2022), 40(12):1313–22. doi: 10.1200/JCO.21.01485
Keywords: meta-analysis, bladder urothelial carcinoma, programmed cell death ligand-1, prognostic value, radical cystectomy
Citation: Zhang J, Song L, Zhu H, Liu Q and Wang D (2022) Prognostic value of programmed cell death ligand-1 expression in patients with bladder urothelial carcinoma undergoing radical cystectomy: A meta-analysis. Front. Immunol. 13:986911. doi: 10.3389/fimmu.2022.986911
Received: 05 July 2022; Accepted: 14 September 2022;
Published: 28 September 2022.
Edited by:
Yuchen Liu, Shenzhen University, ChinaReviewed by:
Albino Eccher, Integrated University Hospital Verona, ItalyFelice Crocetto, Federico II University Hospital, Italy
Liangkuan Bi, Second Affiliated Hospital of Anhui Medical University, China
Copyright © 2022 Zhang, Song, Zhu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delin Wang, d2FuZGVsaW5AaG9zcGl0YWwuY3FtdS5lZHUuY24=
†ORCID ID: Delin Wang, orcid.org/0000-0003-0689-3199
 Jindong Zhang
Jindong Zhang Liangdong Song
Liangdong Song Delin Wang
Delin Wang