- 1Clinical Pharmacology Modeling & Simulation, GlaxoSmithKline (GSK), Stevenage, Hertfordshire, United Kingdom
- 2Department of Microbiology and Immunology, Emory University, Atlanta, GA, United States
- 3Department of Biology, Emory University, Atlanta, GA, United States
Currently, vaccines for SARS-CoV-2 and influenza viruses are updated if the new vaccine induces higher antibody-titers to circulating variants than current vaccines. This approach does not account for complex dynamics of how prior immunity skews recall responses to the updated vaccine. We: (i) use computational models to mechanistically dissect how prior immunity influences recall responses; (ii) explore how this affects the rules for evaluating and deploying updated vaccines; and (iii) apply this to SARS-CoV-2. Our analysis of existing data suggests that there is a strong benefit to updating the current SARS-CoV-2 vaccines to match the currently circulating variants. We propose a general two-dose strategy for determining if vaccines need updating as well as for vaccinating high-risk individuals. Finally, we directly validate our model by reanalysis of earlier human H5N1 influenza vaccine studies.
Introduction
SARS-CoV-2 (‘CoV-2’ hereafter) has caused the most severe pandemic since influenza in 1918 (approximately half a billion confirmed cases and 6 million deaths as of 28th April 2022 - WHO). In contrast with the 1918 influenza pandemic, where no vaccines or therapeutics were available and immunity was only gained following recovery from infection, vaccination has played a key role in mitigating the morbidity and mortality of CoV-2 (1, 2). However, as is the case with other circulating human coronaviruses, immunity does not provide lifelong protection from reinfection (3–5) and we are witnessing waves of infection with new virus variants. These variants arise and spread due to a combination of factors such as waning immunity (6–9) and virus evolution (10–12). The latter results in both more transmissible viruses (13–16), and viruses able to escape immunity to earlier variants and vaccines (16–18). For example, the Omicron (OM) variant of CoV-2, that arose in late 2021, is much more transmissible than the ancestral Wuhan (WU) (13, 15), and in addition, OM has a panoply of mutations in the spike protein (12, 16) that allow it to partially escape antibody responses to earlier variants as well as Wuhan (WU) based vaccines (2).
Prima facie, we might expect that it is best to keep the vaccine updated with the current strain. For example, we might expect the updated vaccine to generate higher antibody-titers to the currently circulating virus in unvaccinated individuals. Indeed, the experimental data from the animal model studies support this (19). However, over time, most of the population will have either been immunized or naturally infected. Studies on influenza have shown that prior immunity can skew responses to subsequent infection and immunization and the phenomenon has been termed original antigenic sin (OAS) (20–25). Understanding of the implications of OAS for CoV-2 vaccination requires integrating experimental and clinical studies with mathematical models. A number of elegant experimental and observational studies show that prior immunity has unexpected effects on the outcome following boosting with different vaccines (26–28), and in particular suggest that updating the vaccine to match the circulating variant does not enhance the antibody titer to the circulating variant any more than the original vaccine. We focus on the OM-vaccine study by Gagne et al. (28) as the pattern of boosting observed was very similar to the study using the vaccine based on the Beta variant (27).
The Gagne study (28) used a macaque primate model system to compare the boosting of immunity with a WU- versus an updated OM-vaccine. Primates were first given two vaccine doses of the currently used mRNA-1273 vaccine (WU-vaccine), which encodes a spike protein derived from the ancestral Wuhan virus variant, to mimic prior immunity of vaccinated humans. These two vaccinations (#1 and #2) resulted in a high titer of antibodies against the WU virus, and significantly lower titers against the OM variant (see Figure 3A). Over time the antibody titers to both WU and OM viruses waned significantly, and at week 41 the animals were boosted with a third vaccination, either with the original WU-vaccine (vaccination regime WU-WU-WU) or an updated OM-vaccine that incorporated spike protein antigen from the OM virus (regime WU-WU-OM). This allowed them to determine whether updating the vaccine would produce higher titers to the OM-virus. Surprisingly, their results showed that both WU-WU-WU and WU-WU-OM resulted in similar antibody titers to the OM-virus. Also surprising was the finding that both these vaccination regimes resulted in similar antibody titers against the WU-virus (albeit at higher levels than to the OM-virus, as shown in Figure 3A). Furthermore, as neutralization titers followed a very similar pattern to the total antibody titers, these observations implied that it might not be necessary to reformulate the vaccine to match the OM virus variant.
In this paper, we use computational models to better understand the rules of boosting of responses to new virus variants. As the available data is restricted to measurements of only a few immune biomarkers (cells and antibodies) at limited time points, we use simple computational models for the dynamics of immunity following vaccination. In these circumstances, the results from simpler models are typically more robust than that from more complex models (29). We show that our models generate robust qualitative results consistent with data from a number of CoV-2 studies (8, 19, 30, 31). Analyzing the dynamics of antigen, B cells, and antibodies in our simulations allows us to understand the reason for the initially surprising observation from Gagne et al. (28) that vaccination #3 with either the WU- or the OM-vaccine results in similar antibody titers to the OM virus. We then use this model to rapidly explore different scenarios for subsequent vaccinations. We find that while the level of immunity to the WU and OM viruses appears equal following the initial booster with either the WU- or OM-vaccines, using the OM-vaccine may have significant advantages with subsequent vaccinations or infections. Based on model predictions, we suggest critical experiments that will allow us to determine whether the vaccine strain should be updated to that of the circulating virus variants. Finally, we directly validate our predictions for boosting by reanalysis of earlier human H5N1 influenza vaccine studies (32, 33).
Results
The immunodynamics model
We consider an immunodynamics model for the interaction between the vaccine and the humoral immune response. The model extends earlier multi-epitope models for the dynamics of antibody levels following vaccination (25, 34) in the following ways. First, we incorporate two different vaccines, the WU- and OM-vaccines. Second, we incorporate differences in the boosting of naïve and memory cells to antigenically altered epitopes that underlie the phenomenon of original antigenic sin (23). We then used the model to explore how the boosting of immune responses to the new virus variants is affected by the interplay between prior immunity to the old variant and the antigens expressed by the updated vaccines.
The model is shown schematically in Figure 1. The WU- and OM-vaccines have unique as well as shared or conserved epitopes. We keep track of three types of epitopes: C, W and O denote conserved epitopes and epitopes unique to WU- and OM-vaccines, respectively. We also keep track of B cells and antibodies specific to these epitopes. B cells specific to an epitope are stimulated by cognate antigen, undergo clonal expansion, and produce antibodies specific to that epitope. The response wanes once the antigen is cleared. Further details, equations and parameters are described in the Materials and Methods. We do not include more complex features of the selection and differentiation of B cell clones and interactions with other immune cells such as follicular dendritic cells and T cells in germinal centers (35, 36). This is because, at this stage, the experimental data does not include precise measurements of these quantities after CoV-2 vaccination. Under these circumstances, the results of simpler models can typically be more robust than those of complex models (29), and we focus on qualitative patterns observed in the data rather than specific values.
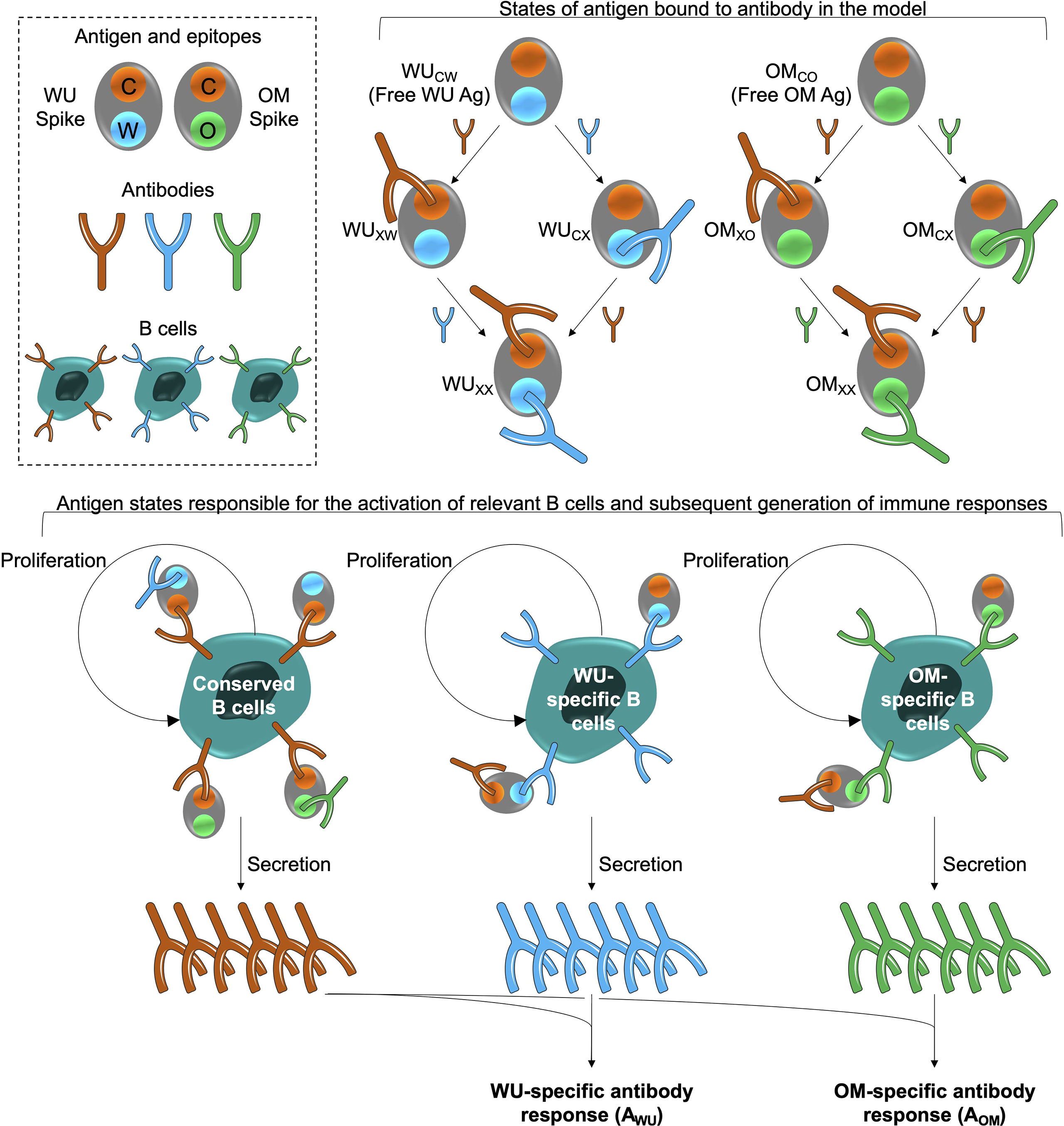
Figure 1 Model schematic. The box at the top left shows the epitopes of the WU- and OM-vaccine antigens. Epitope C (shown in orange) is common to both vaccines. Epitopes W (blue) and O (green) are unique to the WU and OM respectively. Antibodies specific to these epitopes can bind to these epitopes and prevent them from stimulating B cells for the same epitope. A bound epitope is denoted by ‘X’ in the schematic (for example, WUCX indicates that the ‘W’ epitope of the WU antigen is bound to its cognate antibody). The different antigen states generated and the B cells they stimulate are shown in the top right and bottom panels respectively. The bottom panel illustrates that binding of antigen to B cells stimulates their clonal expansion and the production of antibodies.
Model recapitulates a number of studies on CoV-2 responses following vaccination and boosting
Our model recapitulates the broad patterns of immunity generated both by natural infections and vaccination with CoV-2. A wealth of data show that both natural infection with circulating CoV-2 as well as vaccination induce antigen-specific humoral immune responses. We next describe how the model can qualitatively describe the pattern of the humoral immune response observed in a number of studies.
As mentioned in the Introduction, prima facie we would expect that boosting of naïve individuals with a vaccine based on the circulating variant will elicit higher antibody titers to this strain rather than a vaccine based on an earlier variant. This simple observation was demonstrated by Ying et al. (19) as seen in the right panel of Figure 2A. In their experiment, groups of mice were immunized with two doses of either the WU-vaccine (WU-WU) or the OM-vaccine (OM-OM), and the generated WU and OM antibody titers were compared between the groups. The WU-WU group elicited orders of magnitude higher WU titers than OM titers, while the OM-OM group exhibited exactly the opposite response, much higher OM titers than WU titers. Our model recapitulates this observation (left panel of Figure 2A).
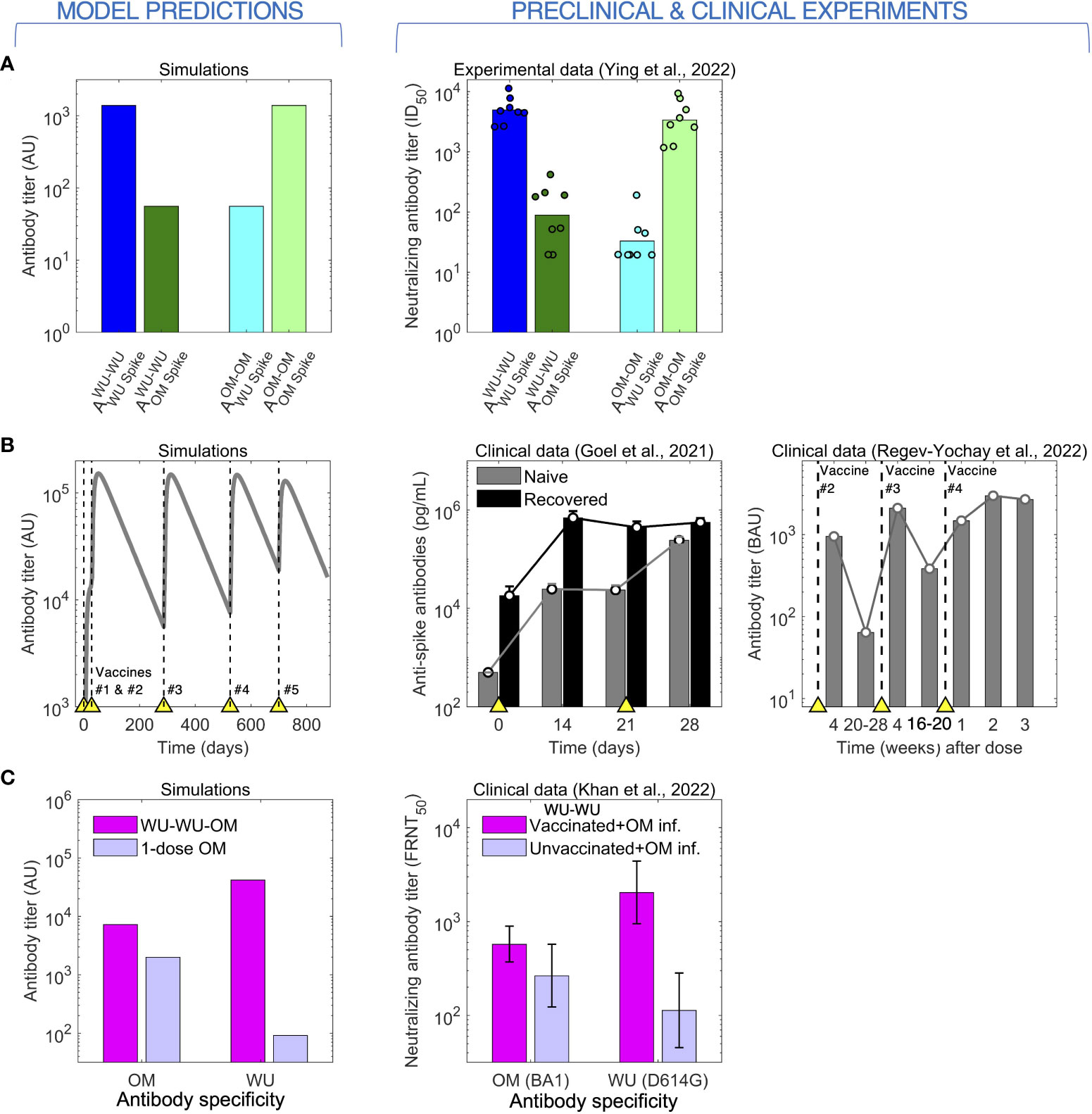
Figure 2 The model predicts (left) antibody responses to CoV-2 following vaccination and infection (right). (A) Immunization of mice with 2 doses of either the WU- or the OM-vaccine. High antibody titers to a given antigen (WU or OM) requires 2 vaccinations with that antigen. (B) Antibody titers saturate following repeated immunization or infection. Left panel: Simulations of repeated immunizations with the WU-vaccine at times indicated by the yellow triangles. The antibody response to the WU-virus increases substantially after the first two vaccinations. Further boosts with the same vaccine results in little further increases in the titer of antibody. Center panel: data for the virus titer in naive (grey) and CoV-2 infected and recovered (black) individuals following two doses of the WU-vaccine. The titer of antibodies in recovered individuals saturates after a single vaccination, while that in naive individuals is boosted by the second vaccination. Right panel: antibody titers following four doses of the WU-vaccine shows plateauing after vaccination #3. (C) Two stimulations with the WU-antigen enhance the subsequent responses to OM (following OM stimulation).
A characteristic of humoral immunity is that while antibody responses can be boosted by repeated vaccination, the antibody titer saturates when immunity is high and subsequent vaccinations lead to only very modest increases in antibody titers, as is shown in both in the clinical data for CoV-2 and model simulations (Figure 2B) (8, 31). We note that in our model, the saturation in the magnitude of the responses occurs due to antibody binding to an epitope sterically preventing B cells specific for that epitope from binding to and being stimulated by that antigen (24, 25). This saturation in antibody titers has also been widely observed for other pathogens such as influenza (24, 32, 33).
Immune responses get more complex when individuals are exposed to different virus variants or vaccines. These complexities have been discussed in the context of OAS following infections with different strains of influenza. OAS also plays a role for CoV-2 infections, and this is seen in the clinical dataset described by Khan et al. (30) (right panel of Figure 2C). Khan et al. show measured antibody titers to both the WU and OM variants in two human cohorts who were infected by the OM (BA.1 variant) virus. The first cohort comprised naïve individuals, and the second comprised individuals previously immunized with two doses of WU-vaccines. Vaccinees showed boosting of both WU and OM antibody titers compared with naïve individuals. Interestingly, the WU-vaccine also imprinted responses to the WU-variant, and following OM-infection, these responses reached higher titers compared with antibodies to the OM variant. This is a signature of OAS, and our model reproduces a similar pattern as shown in the left panel of Figure 2C.
Model explains the experimental vaccine study of Gagne et al.
The most comprehensive and elegant study of boosting by vaccines with new variants are studies which followed vaccination of previously immunized individuals with the original-vaccine versus the updated vaccine (26–28). We focus on the OM-vaccine study by Gagne et al. (28) as the pattern of boosting observed was very similar to the studies based on the Beta variant (26, 27).
We used the model to simulate the experiments of Gagne et al., focusing on the responses to the WU and OM viruses (responses to other variants such as Beta and Delta fall in between the responses to WU and OM, as might be expected). Primates were first immunized with two doses of the WU-vaccine and antibody titers were allowed to wane for just under a year. The authors then compared how vaccination #3 with the WU- versus the OM-vaccine boosted responses to both WU and OM virus variants. As mentioned earlier and shown in Figure 3A, Gagne et al. show that the initial two vaccinations (WU-WU) induce higher titers to WU than OM, and that the subsequent vaccination #3 with either WU- or OM-vaccines induce very similar fold-increases in the antibody titers to both WU and OM viruses.
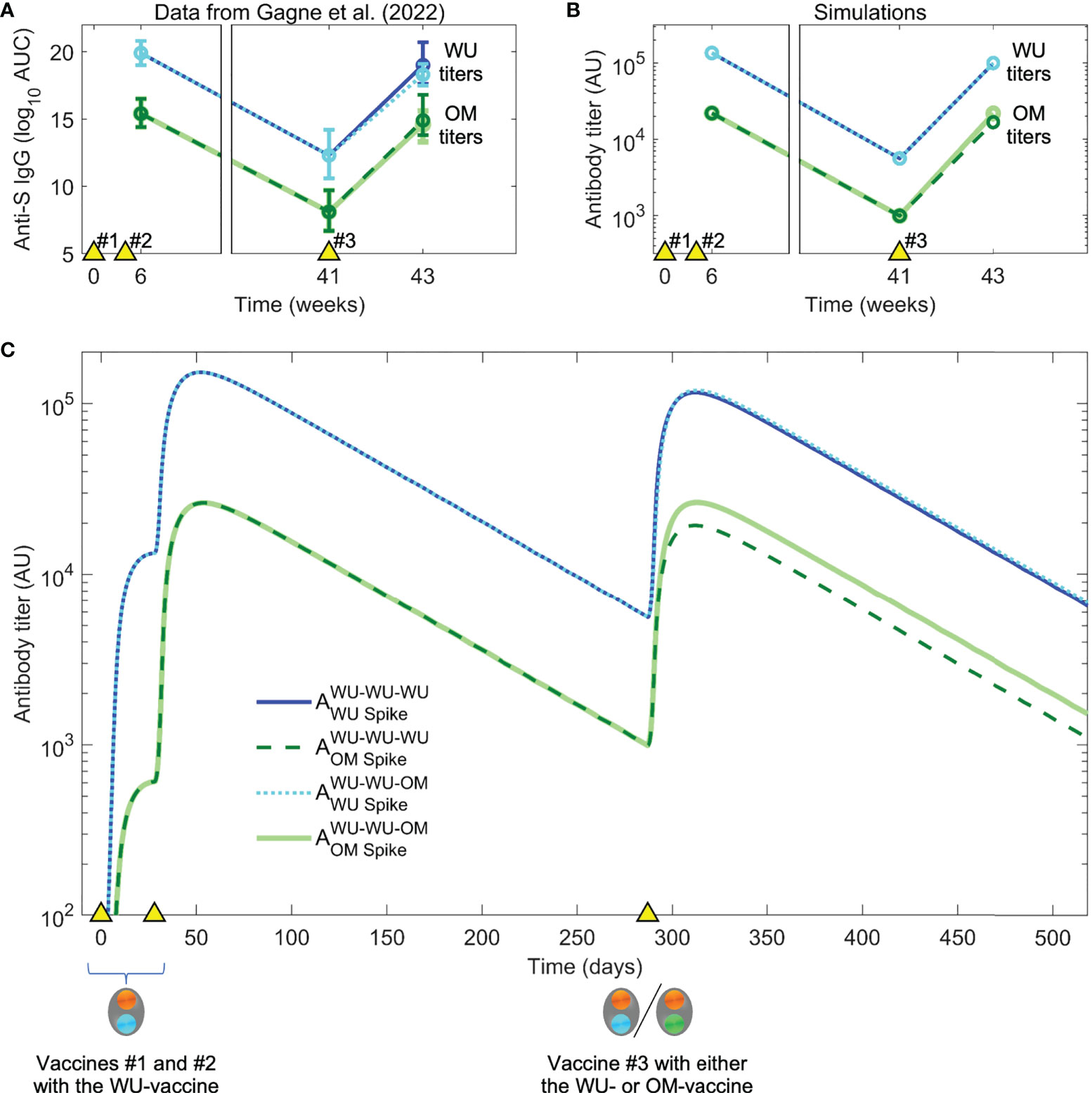
Figure 3 Data and simulation of vaccination with the WU-vaccine followed by boosting with WU- vs OM-vaccines. (A) In Gagne et al. (28), individuals were vaccinated at times indicated by the yellow triangles), initially with the WU-vaccine on days 0 and 28. The authors found that a second boost (vaccination #3) at day 287 (week 41) with either the WU- or OM-vaccine resulted in comparable titers to the OM virus two weeks later. (Color coding same as in the legend for panel C). (B) Simulations with the same dosing regimen as Gagne et al. (28) reproduce the experimental trends seen in panel (A). (C) Simulated dynamics of antibody titers to WU- vs OM-antigens (corresponding to panel B).
Our model simulations generated the pattern observed experimentally (Figure 3A), and simulations are shown in Figures 3B, C. We then used the model to explore what gives rise to these results. At first glance, there are two surprising observations. First, vaccination #3 with the OM-vaccine does not elicit higher antibody titers to OM than vaccination #3 with the WU-vaccine. Second, vaccination #3 with the OM-vaccine boosts the titer of antibodies to the WU-virus to the same extent as vaccination #3 with the WU-vaccine. From the simulations, we notice that the first observation arises as a consequence of the relationship between the final titer, precursor frequency (i.e., the frequency of specific B cells prior to immunization), and fold boost of B cells caused by the immunization. Since the antibody titer is proportional to the B cell response, the final titer equals the product of the initial titer and the fold boost. Vaccination #3 with OM (which is the first exposure to OM) results in a significant clonal expansion of B cells unique to OM. However, since the precursor frequency of these cells prior to this immunization is low, the final titer of the response to unique epitopes on OM is relatively modest. In contrast, the precursor frequency of the response to conserved epitopes is high, and even though the fold boost is smaller than that to the epitopes unique to OM (due to epitope masking), these responses to conserved epitopes form most of the total OM-specific response (see Figures 3B, C).
The model also recapitulated the second observation, namely that vaccination #3 with the OM-vaccine induced similar increases in antibody titers to WU as WU-vaccination #3. This is due to low affinity WU-specific antibodies on memory B cells (B cell receptors) being able to capture OM-antigen via multivalent binding even though they may not bind strongly to it in solution and memory B cells being easier to activate and recruit into immune responses compared with naïve B cells. This is along the lines of observations of original antigenic sin observed for influenza as has been proposed earlier (23).
The model thus shows that though the titer of antibodies to the OM epitope is similar following immunization #3 with either the WU- or OM-vaccines, there are important differences. Vaccination #3 with the OM-vaccine results in a modest increase in OM-specific B cells and antibodies. While these form a small fraction of the total response to OM, we show next that they may have a profound effect following subsequent vaccinations or infections with OM.
Model predicts scenarios that reveal the benefit of updating the vaccine
We now use our model simulations to examine what would occur if we were to give additional vaccinations (#4 and #5) with OM versus WU. The results are shown in Figure 4A. We see that while the size of the OM response following vaccination #3 is similar whether the OM- or the WU-vaccine is used (the first two bars of the bar plot on the right in Figure 4A), the same does not hold following subsequent vaccinations. Additional OM vaccinations (#4 and #5) result in progressively higher antibody titers to OM compared with a scenario where all vaccinations are with the WU-vaccine. The simulations shown in Figure 4B show that the higher OM-specific response following vaccination #4 & #5 with the OM-vaccine arise due to the generation of antibodies to epitopes unique to OM. These predictions can be experimentally tested if the experimental design of Gagne et al. and similar studies on the Beta variant had included at least one further vaccination (#4). We would expect similar results if exposures #4 and #5 were infections rather than vaccinations.
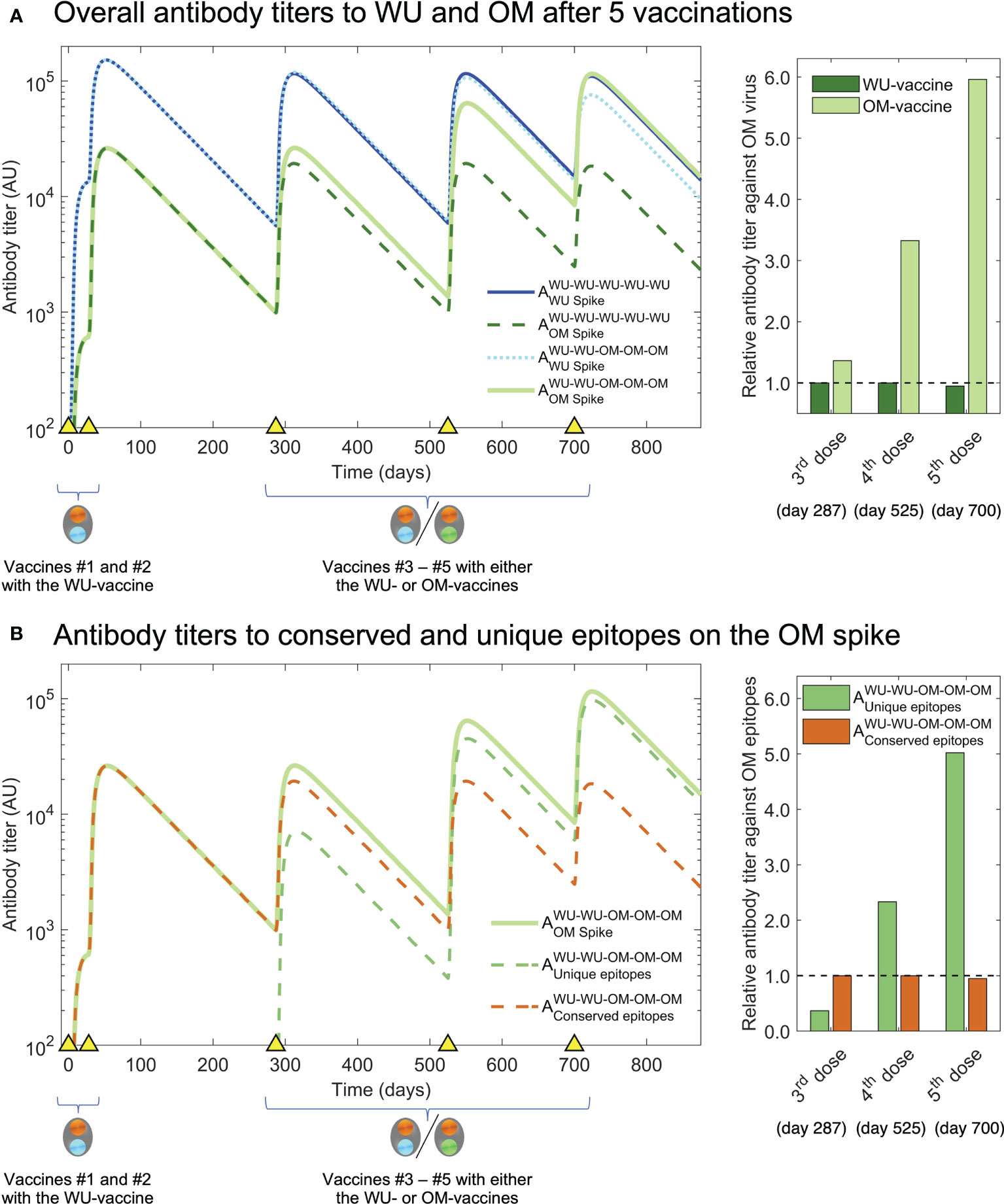
Figure 4 Simulation of third and fourth boosts with the OM-vaccine show generation of higher titers to OM antigen than boosting with the WT-vaccine. (A) We plot antibody titers to WU and OM (subscripts) after WU-WU-WU-WU-WU and WU-WU-OM-OM-OM vaccinations. Titers to OM are similar after vaccination #3 with the WU-vaccine and OM-vaccine (solid light green and dashed dark green lines). However, a further vaccination #4 results in substantially higher antibody titers to OM when the OM-vaccine is used rather than the WU-vaccine (compare solid light green and dashed dark green lines). The bar graph at the right shows that vaccinations #3-#5 with the OM-vaccine (light green bars) result in much higher antibody titers to OM compared to when the WU-vaccine is used (dark green bars). (B) We plot antibody titers to the OM-specific versus conserved epitopes following WU-WU-OM-OM-OM vaccination. The overall increase in titers to OM (light green line) following vaccinations #4 & #5 arises from increases in the OM-specific antibody titer (dashed green line) and responses to the conserved epitopes do not increase (dashed brown line). This is shown in the bar-plot to the right where we see the antibody titer to the OM-specific epitopes (green bars) and shared epitopes (brown bars).
The model can be used to consider alternate vaccination and immunization scenarios. Two potentially relevant scenarios are shown in Figure S1: (i) WU-WU-OM-WU, corresponding to individuals who received WU-vaccinations #1 and #2, got infected by the OM-virus (#3), and then received a WU-vaccine booster (#4), and, (ii) WU-WU-WU-OM, corresponding to individuals who received WU-vaccinations #1 and #2, followed by a WU-booster (#3), and were subsequently infected or vaccinated with OM (#4).
In summary, our model predicts that if vaccination #3 is followed by subsequent vaccinations or infections with the OM variant, it will result in a much higher titer of OM-specific antibodies compared with a scenario where these vaccinations are with the WU-vaccine.
Predictions are validated by analysis of data for vaccination with H5N1 influenza
The strongest independent support for the prediction that two vaccinations with a new virus strain is needed to reveal the boosting of antibodies to new epitopes comes from influenza H5N1 studies. In Figure 5, we show clinical data from an earlier study (32) for immunization with an influenza H5N1 vaccine. Volunteers were immunized with two doses of the hemagglutinin (HA) envelope protein from the H5N1 strain of influenza (which had not circulated in the human population). The HA protein of H5N1 has head and stem domains. The head of H5N1-HA is novel and very different from that of currently circulating influenza strains, while the stem shares conserved epitopes with influenza H1N1, which is circulating in the human population and to which individuals have prior immunity. We see that the first dose of the H5 vaccine results in an increase in the antibody to the shared stem region of HA, and little discernible increase in antibody to the new head region of HA (left panel of Figure 5). This occurs because the antibody titer to elicited to novel epitopes on the head of HA is small compared to the “background” level of antibody. However, the situation is reversed following the second immunization with H5. A booster with the H5 vaccine results in substantial increase in the titer to the head of H5, but little further increase in titers to the stem of HA (left panel of Figure 5). This is consistent with the results of our model (see right panel of Figure 5) and supports the hypothesis that generating high antibody titers to novel antigenic sites on a virus protein that exhibits antigenic changes requires two immunizations. Very similar results are obtained from the analysis of another H5N1 vaccine study, which compared adjuvanted vs unadjuvanted H5N1 vaccines in humans (33).
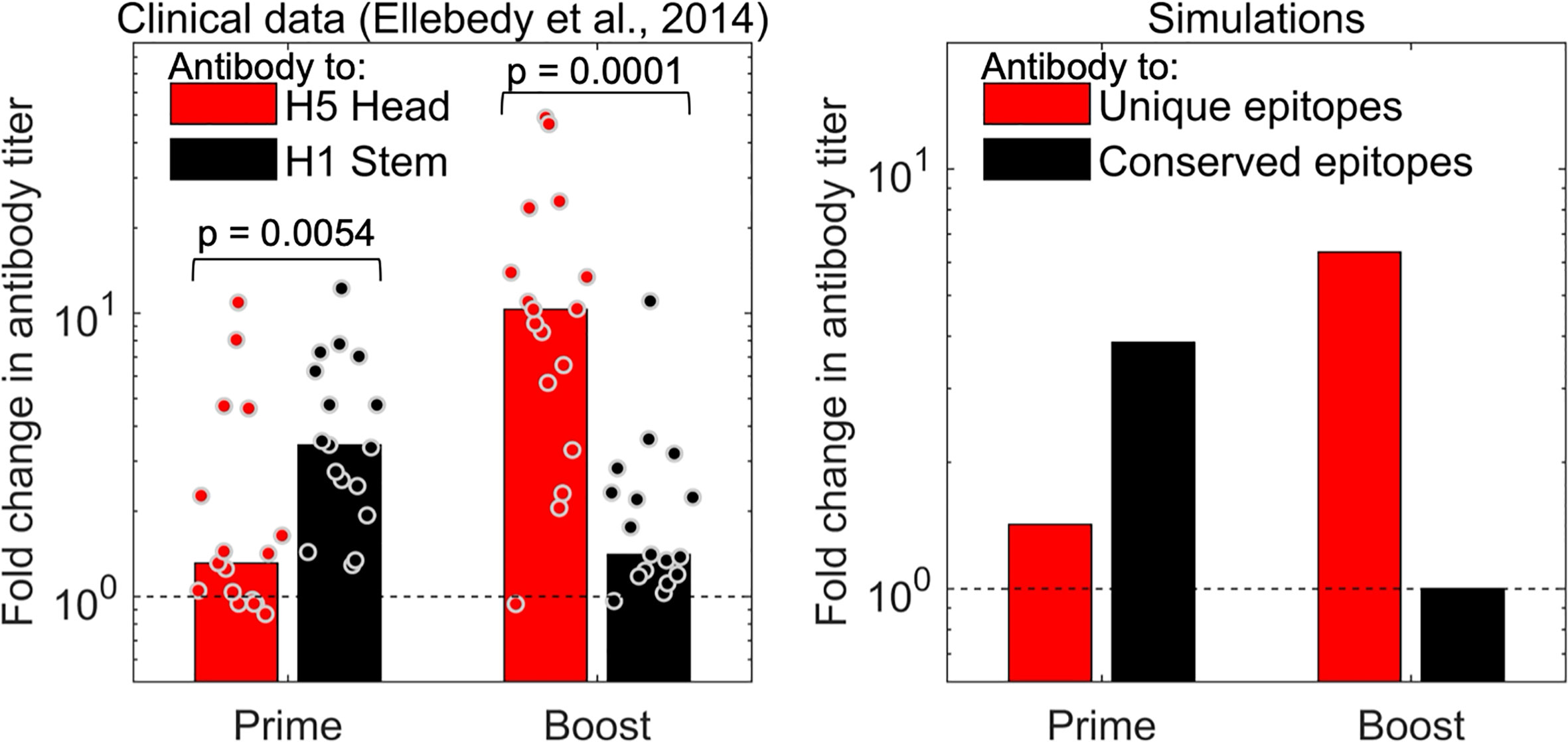
Figure 5 The model captures the observations for influenza vaccination. The left plot shows data for the fold change in antibody titer to epitopes on the head (red) and stem (black) of influenza hemagglutinin (HA) antigen following prime and boost with a H5 vaccine. The first immunization with H5 results in a larger fold increase in antibodies to the conserved stem (shared with H1 viruses), and a significant fold-increase in antibody titers to the unique head epitopes is only seen following the second H5 immunization (boost) (32). Right panel: model simulations capture trends from experiments.
Discussion
Vaccination has played a critical role in the control of the CoV-2 pandemic worldwide (1, 2). However, a combination of waning immunity and virus evolution has resulted in large waves caused by new virus variants, in particular the Delta and Omicron variants, that partly evade immunity elicited by the vaccine (2, 16, 17). The question then is, when do we need to modify the vaccine to match the circulating virus variant?
Understanding the dynamics of immunity to CoV-2 and influenza are particularly challenging because pre-existing immunity from earlier vaccinations and infections impacts the outcome of subsequent exposures to new virus variants (20–25). The utility of computational models such as the one we use is their ability to explain complex outcomes that arise from the interactions between multiple factors. The integration of computational modeling to recapitulate patterns observed in multiple datasets can thus play an important role, and ideally should be done in an iterative manner where the models are used to understand the existing data and propose experimental tests that can allow rejection or refinement of the models.
The most important findings of our paper arise from computational modeling of the patterns observed in the elegant experimental study of Gagne et al. (28), which compared how the original WU-vaccine versus an updated OM-vaccine boosts immunity to the currently circulating OM virus. Surprisingly, their results showed that WU-WU-WU vaccination was as effective as WU-WU-OM as measured by antibody titers to OM, suggesting that it was not necessary to update the vaccine at the current time. We use mathematical models to address the following: what accounts for this observation, what are the consequences for subsequent immunizations or infections, and how can the model be empirically tested?
Our model suggested that WU-WU-WU and WU-WU-OM result in similar antibody titers to OM because this response is dominated by relatively large secondary (or recall) responses to shared epitopes common to OM and WU. The magnitude of this secondary response obscures the much smaller primary response to new epitopes on OM that occur for the first-time following vaccination #3 with the OM-vaccine (but not with the WU-vaccine).
We then used our models to forecast what would happen if vaccination #3 was followed by further vaccinations or infections. We found that repeated boosts (#3, #4, #5) with OM resulted in much higher titers of antibodies for epitopes unique to OM, and this resulted in a much higher overall titer to OM. This is because, while the first OM-vaccination (dose #3) primed a pool of OM-specific B cells, further boosts are required to expand this population to contribute significantly to the overall immune response. Our models thus predict that repeated vaccinations with the updated vaccine are needed to enhance the responses to the new epitopes present in the antigens of new variants. Furthermore, our model suggests a key experiment to allow validation or rejection of the model. The key experiment involves giving one additional vaccine dose (#4) with OM to the primates used by Gagne et al. The model predicts that the group getting WU-WU-OM-OM vaccinations will have much higher antibody titers to OM than the group getting WU-WU-WU-WU. We would expect a similar result (much higher antibody titers to OM) following natural infection with the OM virus after WU-WU-OM vaccination.
We then showed that our conclusions are directly supported by in vivo studies, and in particular, two human vaccination trials for the novel H5N1 influenza vaccines (32, 33). The H5 HA protein in the vaccine has head and stem domains. The stem of HA shares epitopes with those on the H1N1 influenza virus that circulates in the population and to which we have antibodies, while the head of H5 HA is almost entirely novel, and we do not have pre-existing immune responses to this domain. Our model predicts the results seen in the clinical data: (i) primary immunization results in a large increase in antibody titers to the stem of HA but little discernable increase in the titers to the head; and (ii) a second immunization results in a more substantial increase in antibodies to the epitopes on the head of H5 HA but little further boosting of antibodies to the stem of HA due to saturation in the magnitude of responses.
Based on the combination of the analysis of the CoV-2 and influenza vaccination data, we suggest the general prediction that most of the response generated following the first dose of a CoV-2 vaccine updated to match a new virus variant consists of antibodies specific to the shared antigens, and that high titer responses specific for epitopes unique to the new variant are revealed only following a second immunization with the same vaccine. There may be additional advantages to updating the vaccine to match new virus variants. In particular, it allows the antibody response of individuals to better match future variants that arise from the current OM variant. These variants may correspond to the newly arising lineages of OM (e.g., BA2, BA4, BA5), and antibody responses generated by two doses of the OM-vaccine would be expected to have higher titers to these new variants than if the WU-vaccine were used. Finally, we note that it may be worth considering giving two doses of updated vaccines to vulnerable individuals, not only for CoV-2 but potentially also for influenza.
We now briefly mention several caveats pertaining to our study. At the current stage, we have intentionally used a relatively simple model that focuses on the magnitude of the antibody response following WU- and OM-vaccination. This is because at present, data on the dynamics following immunization and boosting is largely limited to titers of antibodies (6, 8, 37–40), serum biomarkers (8, 37, 38), and the virus inoculum (41, 42). We have much more limited data on the dynamics of different populations of cells responsible for the generation of humoral immune responses in the lymph nodes (39, 43). These would include different populations of dendritic cells, follicular CD4 T cells, as well as different populations of B cells and plasma cells (35, 36, 44–50). Further complexities specific to CoV-2 include the spatial aspect of infections of the respiratory tract (51–54) as well as the dynamics of production and distribution of antigen by mRNA based vaccines (55) as well as infections. As more data becomes available, it will be possible to construct more nuanced and refined models of the dynamics of humoral immunity to different neutralizing and non-neutralizing epitopes as well as affinity maturation (56–62). Other directions that could be taken include modeling how protection is lost as the antibody titers elicited by the different immunizations wane. Gagne et al. showed that shortly after vaccination #3, both vaccines elicited similar levels of protection following virus challenge, and it will be important to know if and how this protection declines over time as antibody titers wane (7, 63, 64). In particular, we would like to know if the protection against OM infections generated by WU-WU-OM-OM would decline slower than protection following WU-WU-WU-WU. Furthermore we would like to evaluate this for different components of protection, namely, protection from infection versus protection from severe disease (65). Another direction is to explore the roles of CD8 T cells (66–68), particularly those specific to the CoV-2 nucleocapsid protein (69) and other viral proteins which may be conserved across CoV-2 strains and might thus play a valuable role in inducing potent cross-reactive immunity. Finally, the model can be extended to compare immune responses and protection across different vaccine modalities such as adjuvanted protein vaccines, mRNA vaccines, inactivated virus vaccines, as well as live attenuated virus vaccines and natural infection.
In summary, the current study uses models to explore some of the complexities associated with choosing when to update the CoV-2 vaccine to match antigenic changes in the virus. Model simulations explain the outcomes of multiple studies of boosting of immunity to CoV-2 and generate qualitatively robust predictions that have implications for determining when to update the CoV-2 vaccine. Based on our results, we suggest that it is not sufficient to monitor the level of immunity to the new variant after a single boost, but that further vaccinations with the updated vaccine should be administered in studies that evaluate the benefit of updating vaccines. This general conclusion may also be relevant for the boosting of immunity to other respiratory viruses such as influenza. An important function of models is that they not only guide the design of vaccination regimes, but also that they are falsifiable, and we have suggested experimental tests that can either confirm or reject the model. Applied to the current debate on updating the CoV-2 vaccine, we propose that a second boost with the OM vaccine be incorporated in studies would result in substantially higher OM-specific antibody titers than if the WU vaccine strain were used.
Materials and methods
As mentioned in the text, we extend a multi-epitope model developed earlier (25, 34) to consider the dynamics of boosting responses to new strains of influenza. As mentioned in the Results, the model has the following extensions. First, we incorporate two different vaccines, the WU- and the OM-vaccines. Second, we incorporate differences in the boosting of naïve and memory cells to antigenically altered epitopes that underlie the phenomenon of original antigenic sin (23). We now describe the model in detail.
Because the available longitudinal data focuses on antibody titers, we use a minimal model that considers 2 vaccine antigens for the WU-vaccine and the OM-vaccine. The antigens WU and OM each have two types of epitopes (Figure 1): the ‘C’ epitopes are conserved across both WU and OM, and the ‘W’ and ‘O’ epitopes are unique to the WU and OM respectively. We let the ratio of conserved to unique antigen epitopes equal m:n (m = 1, n = 5; results are qualitatively similar for other values of m and n). Binding of antibody to the different epitopes on the antigen gives us antigen states as shown in Figure 1. We consider different states for antigen with antibody bound to antigen, for example OMco and OMxo represents OM antigen with no antibody bound (both C and O epitopes free) and OM antigen with antibody bound to the C epitope, respectively. The model also keeps track of B cells Bc, Bw and Bo which make antibodies Ac, Aw and Ao specific for C, W and O epitope sites, respectively. We use the usual mass action terms for binding of antigen to antibody. B cells are stimulated by cognate antigen (antigen with the relevant epitope free). We further allow previously stimulated (but not naïve) B cells to be stimulated by the altered epitope at a low rate. This arises as a consequence of: i) low affinity antibodies on the cell surface (B cell receptors) being able to capture antigen via multivalent binding even though they may not bind to it in solution and ii) memory B cells being easier to activate and differentiate to plasma cells compared to naive B cells. This mechanism for original antigenic sin has been described previously (23) and is also validated by the ability of the model to recapitulate CoV-2 boosting data by Khan et al. (2022) shown in Figure 2C. We use standard mass action terms for binding of antibody to antigen and a saturating dose response function for the stimulation of B cells (25, 34). This general model structure may be adapted to other vaccines and virus infections, albeit with changes in the relevant parameters for different vaccination regimes and infections. The relevant equations for the response to the WU antigen are below (similar equations for the response to the OM antigen are not shown).
Model parameters used in simulations are shown in Table 1.
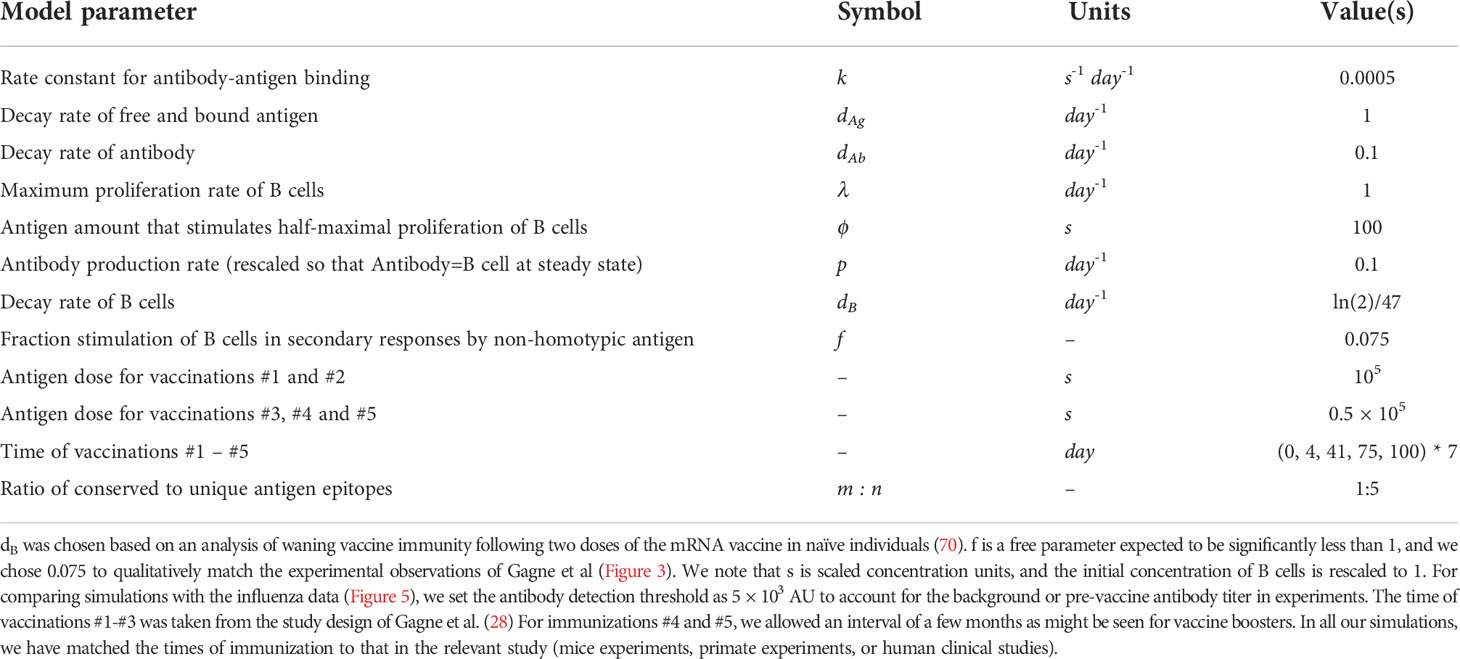
Table 1 Parameter values employed in the model. Parameter values are similar to our previous model (25).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. All model simulation and plotting codes (tested with MATLAB R2022a) along with all the raw data from published experiments are included as Supplementary Material, thus making all our results and figures entirely reproducible. Further inquiries can be directed to the corresponding authors.
Author contributions
RD: conceptualization, methodology, software, formal analysis, data curation, writing – original draft, writing – review and editing, and visualization. SL: methodology, formal analysis, and writing – review and editing. CD: methodology, formal analysis, and writing – review and editing. VZ: formal analysis and writing – review and editing. HA: formal analysis, writing – review and editing, and visualization. RA: conceptualization, methodology, formal analysis, writing – original draft, writing – review and editing, visualization, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge funding from the National Institutes of Health (NIH) grants U01 AI150747, U01 HL139483, and U01 AI144616.
Conflict of interest
Author RD is employed by GSK.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.985478/full#supplementary-material
Supplementary Figure 1 | Simulation of additional vaccination scenarios. Top panel: we simulate the WU- and OM-titers following WU-WU-OM-WU. This pertains to individuals who received WU-vaccinations #1 and #2, got infected by the OM-virus (#3), and then received a WU-vaccine booster (#4). Bottom panel: we simulate the WU- and OM-titers following WU-WU-WU-OM. This pertains to individuals who received WU-vaccinations #1 and #2, followed by a WU-booster (#3), and were subsequently infected or vaccinated with OM (#4). To facilitate comparison with , we include simulations of WU-WU-WU-WU and WU-WU-OM-OM in both panels.
Abbreviations
WU, Wuhan; OM, Omicron; WU-vaccine, original vaccines encoding the ancestral Wuhan strain (WU virus) spike; OM-vaccine, updated vaccine encoding the Omicron strain (OM virus) spike; OAS, original antigenic sin.
References
1. Koelle K, Martin MA, Antia R, Lopman B, Dean NE. The changing epidemiology of SARS-CoV-2. Science (2022) 375:1116–21. doi: 10.1126/science.abm4915
2. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol (2021) 21:626–36. doi: 10.1038/s41577-021-00592-1
3. Callow KA, Parry HF, Sergeant M, Tyrrell D a. J. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infection (1990) 105:435–46. doi: 10.1017/S0950268800048019
4. Edridge AWD, Kaczorowska J, Hoste ACR, Bakker M, Klein M, Loens K, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med (2020) 26:1691–3. doi: 10.1038/s41591-020-1083-1
5. Ren X, Zhou J, Guo J, Hao C, Zheng M, Zhang R, et al. Reinfection in patients with COVID-19: a systematic review. Global Health Res Policy (2022) 7:12. doi: 10.1186/s41256-022-00245-3
6. Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory b and T cells. CR Med (2021) 2:100354. doi: 10.1016/j.xcrm.2021.100354
7. Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol (2021) 21:395–404. doi: 10.1038/s41577-021-00550-x
8. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science (2021) 374:abm0829. doi: 10.1126/science.abm0829
9. Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. New Engl J Med (2022) 386:1207–20. doi: 10.1056/NEJMoa2118691
10. Bushman M, Kahn R, Taylor BP, Lipsitch M, Hanage WP. Population impact of SARS-CoV-2 variants with enhanced transmissibility and/or partial immune escape. Cell (2021) 184:6229–6242.e18. doi: 10.1016/j.cell.2021.11.026
11. Grubaugh ND, Cobey S. Of variants and vaccines. Cell (2021) 184:6222–3. doi: 10.1016/j.cell.2021.11.013
12. Simon-Loriere E, Schwartz O. Towards SARS-CoV-2 serotypes? Nat Rev Microbiol (2022) 20:187–8. doi: 10.1038/s41579-022-00708-x
13. Burki TK. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med (2022) 10:e17. doi: 10.1016/S2213-2600(21)00559-2
14. Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell (2020) 182:812–827.e19. doi: 10.1016/j.cell.2020.06.043
15. Liu Y, Rocklöv J. The effective reproductive number of the omicron variant of SARS-CoV-2 is several times relative to delta. J Travel Med (2022) 29:taac037. doi: 10.1093/jtm/taac037
16. Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature (2022) 603:679–86. doi: 10.1038/s41586-022-04411-y
17. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature (2021) 596:276–80. doi: 10.1038/s41586-021-03777-9
18. Wang R, Zhang Q, Ge J, Ren W, Zhang R, Lan J, et al. Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity (2021) 54:1611–1621.e5. doi: 10.1016/j.immuni.2021.06.003
19. Ying B, Scheaffer SM, Whitener B, Liang C-Y, Dmytrenko O, Mackin S, et al. Boosting with variant-matched or historical mRNA vaccines protects against omicron infection in mice. Cell (2022) 185:1572–1587.e11. doi: 10.1016/j.cell.2022.03.037
20. Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, et al. Antibody landscapes after influenza virus infection or vaccination. Science (2014) 346:996–1000. doi: 10.1126/science.1256427
21. Henry C, Palm A-KE, Krammer F, Wilson PC. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol (2018) 39:70–9. doi: 10.1016/j.it.2017.08.003
22. Kucharski AJ, Lessler J, Read JM, Zhu H, Jiang CQ, Guan Y, et al. Estimating the life course of influenza A(H3N2) antibody responses from cross-sectional data. PloS Biol (2015) 13:e1002082. doi: 10.1371/journal.pbio.1002082
23. Linderman SL, Hensley SE. Antibodies with ‘Original antigenic sin’ properties are valuable components of secondary immune responses to influenza viruses. PloS Pathog (2016) 12:e1005806. doi: 10.1371/journal.ppat.1005806
24. Linderman SL, Ellebedy AH, Davis C, Eberhardt CS, Antia R, Ahmed R, et al. Influenza immunization in the context of preexisting immunity. Cold Spring Harb Perspect Med (2021) 11:a040964. doi: 10.1101/cshperspect.a040964
25. Zarnitsyna VI, Lavine J, Ellebedy A, Ahmed R, Antia R. Multi-epitope models explain how pre-existing antibodies affect the generation of broadly protective responses to influenza. PloS Pathog (2016) 12:e1005692. doi: 10.1371/journal.ppat.1005692
26. Choi A, Koch M, Wu K, Chu L, Ma L, Hill A, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med (2021) 27:2025–31. doi: 10.1038/s41591-021-01527-y
27. Corbett KS, Gagne M, Wagner DA, O’ Connell S, Narpala SR, Flebbe DR, et al. Protection against SARS-CoV-2 beta variant in mRNA-1273 vaccine–boosted nonhuman primates. Science (2021) 374:1343–53. doi: 10.1126/science.abl8912
28. Gagne M, Moliva JI, Foulds KE, Andrew SF, Flynn BJ, Werner AP, et al. mRNA-1273 or mRNA-omicron boost in vaccinated macaques elicits similar b cell expansion, neutralizing responses, and protection from omicron. Cell (2022) 185:1556–71.e18. doi: 10.1016/j.cell.2022.03.038
29. May RM. Uses and abuses of mathematics in biology. Science (2004) 303:790–3. doi: 10.1126/science.1094442
30. Khan K, Karim F, Cele S, Reedoy K, San JE, Lustig G, et al. Omicron infection enhances delta antibody immunity in vaccinated persons. Nature (2022) 607:356–9. doi: 10.1038/s41586-022-04830-x
31. Regev-Yochay G, Gonen T, Gilboa M, Mandelboim M, Indenbaum V, Amit S, et al. Efficacy of a fourth dose of covid-19 mRNA vaccine against omicron. N Engl J Med (2022) 386:1377–80. doi: 10.1056/NEJMc2202542
32. Ellebedy AH, Krammer F, Li G-M, Miller MS, Chiu C, Wrammert J, et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci (2014) 111:13133–8. doi: 10.1073/pnas.1414070111
33. Ellebedy AH, Nachbagauer R, Jackson KJL, Dai Y-N, Han J, Alsoussi WB, et al. Adjuvanted H5N1 influenza vaccine enhances both cross-reactive memory b cell and strain-specific naive b cell responses in humans. Proc Natl Acad Sci (2020) 117:17957–64. doi: 10.1073/pnas.1906613117
34. Zarnitsyna VI, Ellebedy AH, Davis C, Jacob J, Ahmed R, Antia R. Masking of antigenic epitopes by antibodies shapes the humoral immune response to influenza. Philos Trans R Soc B: Biol Sci (2015) 370:20140248. doi: 10.1098/rstb.2014.0248
35. Abbott RK, Crotty S. Factors in b cell competition and immunodominance. Immunol Rev (2020) 296:120–31. doi: 10.1111/imr.12861
36. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol (2022) 40:413–42. doi: 10.1146/annurev-immunol-120419-022408
37. Mateus J, Dan JM, Zhang Z, Rydyznski Moderbacher C, Lammers M, Goodwin B, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science (2021) 374:eabj9853. doi: 10.1126/science.abj9853
38. Ogata AF, Cheng C-A, Desjardins M, Senussi Y, Sherman AC, Powell M, et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis (2022) 74:715–8. doi: 10.1093/cid/ciab465
39. Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature (2021) 596:109–13. doi: 10.1038/s41586-021-03738-2
40. Yang Y, Yang M, Peng Y, Liang Y, Wei J, Xing L, et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol (2022) 7:423–33. doi: 10.1038/s41564-021-01051-2
41. Ke R, Zitzmann C, Ho DD, Ribeiro RM, Perelson AS. In vivo kinetics of SARS-CoV-2 infection and its relationship with a person’s infectiousness. Proc Natl Acad Sci (2021) 118:e2111477118. doi: 10.1073/pnas.2111477118
42. Néant N, Lingas G, Le Hingrat Q, Ghosn J, Engelmann I, Lepiller Q, et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci (2021) 118:e2017962118. doi: 10.1073/pnas.2017962118
43. Kim W, Zhou JQ, Horvath SC, Schmitz AJ, Sturtz AJ, Lei T, et al. Germinal centre-driven maturation of b cell response to mRNA vaccination. Nature (2022) 604:141–5. doi: 10.1038/s41586-022-04527-1
44. Crotty S. T Follicular helper cell biology: A decade of discovery and diseases. Immunity (2019) 50:1132–48. doi: 10.1016/j.immuni.2019.04.011
45. De Silva NS, Klein U. Dynamics of b cells in germinal centres. Nat Rev Immunol (2015) 15:137–48. doi: 10.1038/nri3804
46. Elsner RA, Shlomchik MJ. Germinal center and extrafollicular b cell responses in vaccination, immunity, and autoimmunity. Immunity (2020) 53:1136–50. doi: 10.1016/j.immuni.2020.11.006
47. Laidlaw BJ, Ellebedy AH. The germinal centre b cell response to SARS-CoV-2. Nat Rev Immunol (2022) 22:7–18. doi: 10.1038/s41577-021-00657-1
48. Meyer-Hermann M, FIgGe MT, Toellner KM. Germinal centres seen through the mathematical eye: B-cell models on the catwalk. Trends Immunol (2009) 30:157–64. doi: 10.1016/j.it.2009.01.005
49. Pae J, Jacobsen JT, Victora GD. Imaging the different timescales of germinal center selection*. Immunol Rev (2022) 306:234–43. doi: 10.1111/imr.13039
50. Shlomchik MJ, Luo W, Weisel F. Linking signaling and selection in the germinal center. Immunol Rev (2019) 288:49–63. doi: 10.1111/imr.12744
51. Michael Lavigne G, Russell H, Sherry B, Ke R. Autocrine and paracrine interferon signalling as ‘ring vaccination’ and ‘contact tracing’ strategies to suppress virus infection in a host. Proc R Soc B: Biol Sci (2021) 288:20203002. doi: 10.1098/rspb.2020.3002
52. Quirouette C, Younis NP, Reddy MB, Beauchemin CAA. A mathematical model describing the localization and spread of influenza a virus infection within the human respiratory tract. PloS Comput Biol (2020) 16:e1007705. doi: 10.1371/journal.pcbi.1007705
53. Smith AM. Host-pathogen kinetics during influenza infection and coinfection: insights from predictive modeling. Immunol Rev (2018) 285:97–112. doi: 10.1111/imr.12692
54. Tan H-X, Juno JA, Esterbauer R, Kelly HG, Wragg KM, Konstandopoulos P, et al. Lung-resident memory b cells established after pulmonary influenza infection display distinct transcriptional and phenotypic profiles. Sci Immunol (2022) 7:eabf5314. doi: 10.1126/sciimmunol.abf5314
55. Giorgi M, Desikan R, Graaf PH, Kierzek AM. Application of quantitative systems pharmacology to guide the optimal dosing of COVID-19 vaccines. CPT Pharmacometrics Syst Pharmacol (2021) 10:1130–3. doi: 10.1002/psp4.12700
56. De Boer RJ, Perelson AS. How germinal centers evolve broadly neutralizing antibodies: The breadth of the follicular helper T cell response. J Virol (2017) 91(22):e00983-17. doi: 10.1128/JVI.00983-17
57. Garg AK, Desikan R, Dixit NM. Preferential presentation of high-affinity immune complexes in germinal centers can explain how passive immunization improves the humoral response. Cell Rep (2019) 29:3946–57. doi: 10.1016/j.celrep.2019.11.030
58. Garg AK, Mittal S, Padmanabhan P, Desikan R, Dixit NM. Increased b cell selection stringency in germinal centers can explain improved COVID-19 vaccine efficacies with low dose prime or delayed boost. Front Immunol (2021) 12:776933. doi: 10.3389/fimmu.2021.776933
59. Luo S, Perelson AS. Competitive exclusion by autologous antibodies can prevent broad HIV-1 antibodies from arising. Proc Natl Acad Sci USA (2015) 112:11654–9. doi: 10.1073/pnas.1505207112
60. Meyer-Hermann M. Injection of antibodies against immunodominant epitopes tunes germinal centers to generate broadly neutralizing antibodies. Cell Rep (2019) 29:1066–1073.e5. doi: 10.1016/j.celrep.2019.09.058
61. Meyer-Hermann M, Mohr E, Pelletier N, Zhang Y, Victora GD, Toellner KM. A theory of germinal center b cell selection, division, and exit. Cell Rep (2012) 2:162–74. doi: 10.1016/j.celrep.2012.05.010
62. Wang S, Mata-Fink J, Kriegsman B, Hanson M, Irvine DJ, Eisen HN, et al. Manipulating the selection forces during affinity maturation to generate cross-reactive HIV antibodies. Cell (2015) 160:785–97. doi: 10.1016/j.cell.2015.01.027
63. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med (2021) 27:1205–11. doi: 10.1038/s41591-021-01377-8
64. Padmanabhan P, Desikan R, Dixit NM. Modeling how antibody responses may determine the efficacy of COVID-19 vaccines. Nat Comput Sci (2022) 2:123–31. doi: 10.1038/s43588-022-00198-0
65. Antia R, Halloran ME. Transition to endemicity: Understanding COVID-19. Immunity (2021) 54:2172–6. doi: 10.1016/j.immuni.2021.09.019
66. Heitmann JS, Bilich T, Tandler C, Nelde A, Maringer Y, Marconato M, et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature (2022) 601:617–22. doi: 10.1038/s41586-021-04232-5
67. Jarjour NN, Masopust D. Jameson SC. T Cell memory: Understanding COVID-19. Immun (2021) 54:14–8. doi: 10.1016/j.immuni.2020.12.009
68. Tarke A, Sidney J, Kidd CK, Dan JM, Ramirez SI, Yu ED, et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. CR Med (2021) 2:100204. doi: 10.1016/j.xcrm.2021.100204
69. Matchett WE, Joag V, Stolley JM, Shepherd FK, Quarnstrom CF, Mickelson CK, et al. Cutting edge: Nucleocapsid vaccine elicits spike-independent SARS-CoV-2 protective immunity. J Immunol (2021) 207:376–9. doi: 10.4049/jimmunol.2100421
Keywords: Vaccine, variants, SARS-CoV-2, COVID-19, omicron, modeling, simulations
Citation: Desikan R, Linderman SL, Davis C, Zarnitsyna VI, Ahmed H and Antia R (2022) Vaccine models predict rules for updating vaccines against evolving pathogens such as SARS-CoV-2 and influenza in the context of pre-existing immunity. Front. Immunol. 13:985478. doi: 10.3389/fimmu.2022.985478
Received: 03 July 2022; Accepted: 16 September 2022;
Published: 03 October 2022.
Edited by:
Rong Hai, University of California, Riverside, United StatesReviewed by:
Yimin Huang, Biogen Idec, United StatesJuan Manuel Carreño Qurioz, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2022 Desikan, Linderman, Davis, Zarnitsyna, Ahmed and Antia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajat Desikan, cmFqYXRkZXNpa2FuQGdtYWlsLmNvbQ==; Rustom Antia, cmFudGlhQGVtb3J5LmVkdQ==
†These authors have contributed equally to this work
 Rajat Desikan
Rajat Desikan Susanne L. Linderman2
Susanne L. Linderman2 Veronika I. Zarnitsyna
Veronika I. Zarnitsyna Rustom Antia
Rustom Antia