
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 24 August 2022
Sec. Immunological Tolerance and Regulation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.984984
Background: Since the discovery of the immunomodulatory functions of mesenchymal stem cells (MSCs), their application in immunomodulation has attracted considerable attention, and an increasing number of studies have been conducted worldwide. Our research aimed to investigate the global status and trends in this field.
Methods: Publications on the immunomodulatory functions of MSCs from 1 January 2000 to 7 March 2022 were retrieved from the Web of Science Core Collection. The data were studied and indexed using the bibliometric methodology. Visualization analysis, co-authorship, co-occurrence analysis, and publication trends in MSC immunomodulation were conducted using the VOSviewer software.
Results: In total, 4,227 papers were included in the study. The number of publications and research interests has significantly increased globally. China published the highest number of related articles, while the US published articles with the highest number of citations. Stem Cell Research & Therapy had the highest number of publications. Sun Yat-sen University, Shanghai Jiao Tong University, Harvard University, and Seoul National University were the most contributive institutions. Furthermore, the studies were divided into four research hotspots for MSC immunomodulation: MSC immunomodulation in regenerative medicine, the effects and mechanisms of MSC immunomodulation, MSC therapy for immune diseases, and the cell source of MSCs.
Conclusion: This study indicates that the number of publications on MSC immunomodulation will increase in the future, and MSC immunomodulation mechanisms and clinical applications of MSC immunotherapy should be the next hotspots in this research field.
Mesenchymal stem cells (MSCs) were first identified and isolated from the bone marrow as an adherent fibroblast-like population (1). Although bone marrow MSCs (BMMSCs) are recognized as classic MSCs, non-marrow-derived MSCs are identified in other tissues, including the placenta, umbilical cord blood, adipose tissue, fetal liver, muscle, lung, dermis, amniotic fluid, orofacial tissue and so on (2). MSCs are rapidly adherent to tissue culture vessels, with multiple differentiation potentials, positive expression of CD73, CD90, CD105, CD44 and Sca1, and negative expression of hematopoietic markers, CD45, CD34, CD14, CD11b, CD79a, and human leukocyte antigen DR isotype (HLA-DR) (3, 4). Based on their key properties of self-renewal and differentiation into mesenchymal and non-mesenchymal lineages, MSCs have been widely used therapeutically as a promising cell source for regenerative medicine.
Emerging evidence indicates a unique immunoregulatory effect of MSCs on several subsets of immune cells, including T lymphocytes, B lymphocytes, natural killer cells, and dendritic cells (5, 6). In vitro, MSCs induce cell apoptosis of T lymphocytes and significantly inhibit immune cell proliferation and pro-inflammatory cytokine production (7–9). Moreover, MSCs reduce antibody production of B lymphocytes and suppress the generation and function of antigen-presenting cells (10–12). From 2000 to the present, there have been numerous in vivo studies and clinical trials on the therapeutic effects of MSCs immunomodulation (13). These studies indicated that systemic transplantation of MSCs has been successfully used to treat various autoimmune diseases, including graft-versus-host disease (GVHD), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and inflammatory bowel disease (IBD) (14–17). MSCs regulate the local immune environment and provide suitable “soil” for tissue regeneration (18, 19). Multiple therapeutic mechanisms contribute to MSC-based cell therapies, including paracrine secretion and interactions between MSCs and immune cells (20). The immunomodulatory effect of MSCs renders them significant for translational applications in tissue regeneration and autoimmune conditions.
Bibliometric analysis is a mathematical and statistical method for assessing the quantitative fluctuations, distributions, and change rules of published literature (21). It enables the quantitative measurement of profile distribution and the relationship and clustering of studies. It provides objective scientific indicators for evaluating research trends, developing guidelines, and treating diseases (22). Bibliometrics has been used in various fields of medicine, including stem cell fields, such as stem cells in cardiovascular diseases (23) and osteoarthritis (24). MSCs have received significant attention in immunotherapy (25). However, to the best of our knowledge, quantitative and qualitative analyses of this research topic have not been reported. Therefore, this study analyzed publications on MSC immunomodulation in the 21st century, highlighting the current status of global research, evidence of trends, and topics requiring further exploration.
Bibliometric analysis was ultimately performed online using the Web of Science (WoS) database, given it is a commonly widely used database with rich bibliometric indicators for analysis. This analysis was performed on a single day to avoid daily updating bias since the database remains open.
The search strategy was shown in Figure 1. Publications in research of the immunomodulation of MSCs were searched for in Web of Science Core Collection (WoSCC) on 7 March 2022. The search formula was set as follows: TS= (“Mesenchymal Stem Cell*” OR “Bone Marrow Stromal Cell*” OR “Mesenchymal Stromal Cell*” OR “Mesenchymal Progenitor Cell*” OR “Wharton* Jelly Cell*” OR “Bone Marrow Stromal Stem Cell*”) AND TS= (“Immunotherap*” OR “immune therap*” OR “immunomodulation*” OR “immunoregulator*” OR “immunomodulator*” OR “immune suppression*” OR “Immunosuppression*”). The time period of article publication was from 1 January 2000 to 7 March 2022. For manuscript types, only peer-reviewed articles in English were included. The full records of each publication, including title, year of publication, authors’ names, nationalities, affiliations, name of the journal, keywords, and abstract, were downloaded from the WoS database and imported into Microsoft Excel 2016.

Figure 1 Search strategy for retrieving publications on MSC immunomodulation. The search formula was set as follows: TS = “Mesenchymal Stem Cell*” OR “Bone Marrow Stromal Cell*” OR “Mesenchymal Stromal Cell*” OR “Mesenchymal Progenitor Cell*” OR “Wharton* Jelly Cell*” OR “Bone Marrow Stromal Stem Cell*” AND TS = (“Immunotherap*” OR “immune therap*” OR “immunomodulation*” OR “immunoregulator*” OR “immunomodulator*” OR “immune suppression*” OR “Immunosuppression*”). The publication date was from 1 January 2000, to 7 March 2022. The 6,655 studies were identified from the Web of Science Core Collection (WoSCC), and 54 non-English studies were excluded. The search strategy identified 4,227 studies and excluded 2347 non-articles.
VOSviewer is the classic bibliometric analysis software, which was used to perform visualized analysis in this study (26). VOSviewer (version 1.6.18) was used to analyze the co-authorship, co-occurrence, established co-authorship network visualization map, keyword network visualization map, and overlay visualization map. Additionally, a descriptive analysis was also conducted which included publication years, journals, highly-cited papers, countries, institutions and authors.
A total of 4,227 articles from 2000 to 2022 met the search criteria (excluding 54 non-English and 2,347 non-article publications). As shown in Figure 2, there was a significant upward trend in global publications on MSC immunomodulation per year (publications in 2022 were excluded because the statistical data were not completed as of 7 March 2022). Global publications were divided into three stages, according to the development of this research field. The “cultivation period” was from 2000 to 2009, with a small number of publications per year, ranging from several to dozens. From 2010 to 2016 was the “unk development period”, with the number of publications exceeding 100 in 2010 and 300 in 2015 (3.04 times that in 2010). From 2017 to 2021 was the “unk boom period”, with 2,254 articles, which accounted for 53.32% of all publications. The number of publications increased annually to a peak in 2020 (503 publications, 11.90%).
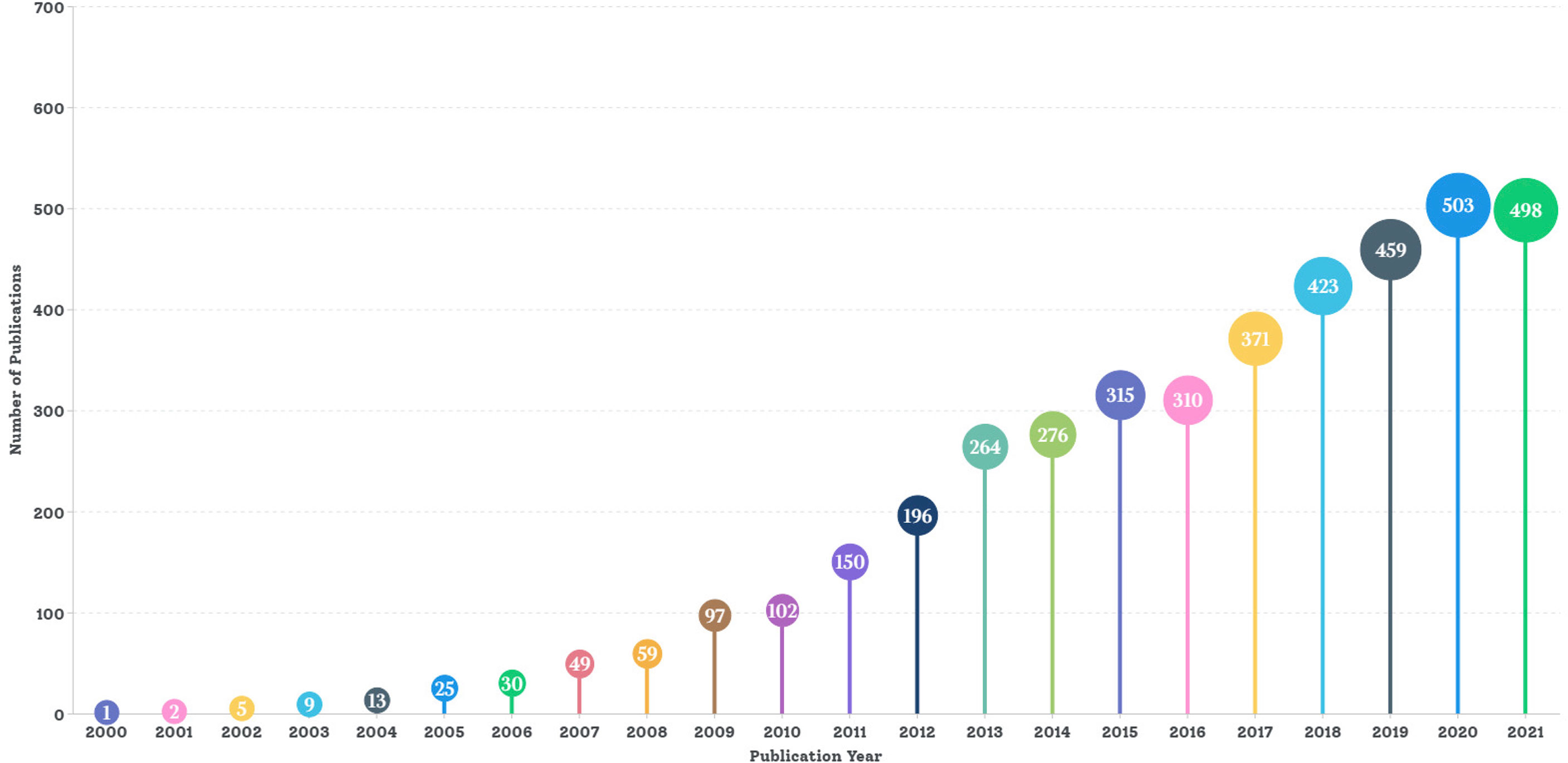
Figure 2 Trends in number of global publications on MSC immunomodulation 2000−2022. A total of 4,227 articles from 2000 to 2022 met the search criteria. Starting from one article in 2000, the number of articles published in 2010 exceeded 100, with more than 300 articles in 2015 and more than 400 articles in 2018 until 2021.
A total of 82 countries and regions contributed to the field of MSC immunomodulation, as shown by the geographic distribution of global publications in Figure 3. The countries of published articles were mainly distributed in Asia, led by China, and North America, led by the US. The top ten contributive countries with publications in this research field are shown in Table 1. China published the largest number of articles (1,054, 24.93%), followed by the US (1,000, 23.66%) and Italy (342, 8.09%). Publications from the US had the highest citation frequency (53,856 citations), followed by China (30,671 citations), Italy (17,148 citations), and Germany (10,345 citations). Regarding average citations, articles published from France had the highest average citation frequencies (64 citations), indicating that publications from France were of high quality. Additionally, Iran (the average publication year was 2018.14) had the latest publication year among the top ten contributive countries.
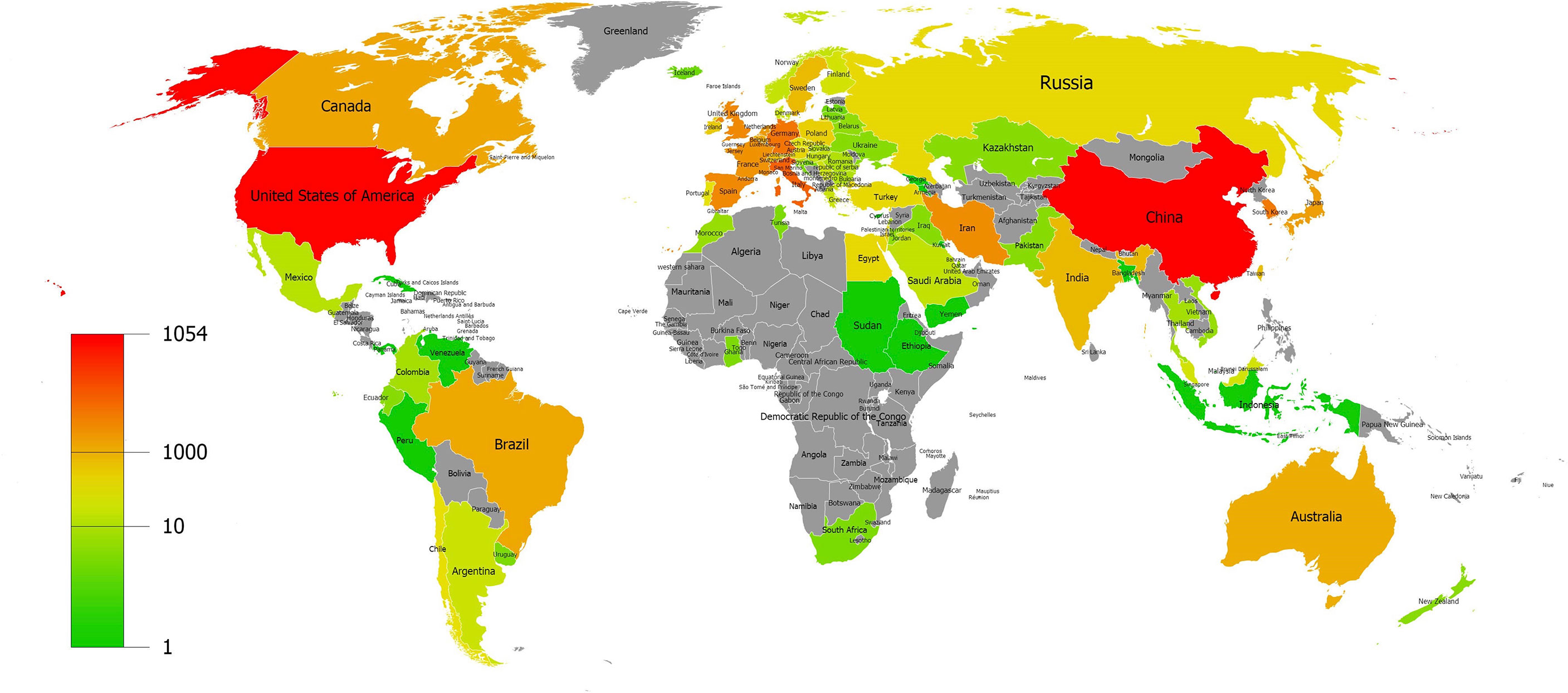
Figure 3 Geographic distribution of global publications on MSC immunomodulation. World map showing the distribution of global publications on MSCs immunomodulation.
4227 articles on the immunomodulation of MSCs were published by 884 journals in total. There were 1,168 articles (27.63%) published in the top 10 journals (Table 2). Stem Cell Research & Therapy (IF=6.832, 2020) published the most relative articles (240 publications). Stem Cells and Development (IF=3.272, 2020) ranked second with 123 publications, followed by PLOS ONE (IF=3.240, 2020) with 119 publications. The Publications on the immunomodulation of MSCs in Stem Cells (IF=6.277, 2020) had the highest total citations (12808 times) and average citations (110 times). About the latest average publication year in the top 10 journals, in Stem Cell Research & Therapy was 2018.32 and in Frontiers in Immunology was 2018.30, which indicating these two journals published lots of articles in recent years.
The top ten articles with the highest citations on MSC immunomodulation are shown in Table 3. Among them, five articles, including the article with the highest citation frequency (3,591 citations), were published in Blood, Cell Stem Cell, Transplantation, Experimental Hematology and Stem Cells respectively. The article with the highest citation frequency, “Human mesenchymal stem cells modulate allogeneic immune cell responses”, was published by Aggarwal et al. in 2005. The latest publication with a high number of citations (1,037 citations) was “The MSC: An Injury Drugstore”, published in Cell Stem Cell in 2011.
A total of 4,186 institutions contributed to the research field of MSC immunomodulation. As shown in Table 4, the most contributive institution was Sun Yat-sen University (113 publications), followed by Shanghai Jiao Tong University (99 publications), Harvard University (86 publications), Seoul National University (71 publications), and Chinese Academy of Sciences (69 publications). Moreover, among the top ten institutions, Harvard University had the article with the highest total and average citation frequency (8,872 and 103 citations, respectively).
The minimum number of institutional publications was set as 10, and 224 contributive institutions were selected for institutional co-authorship analysis using the VOSviewer software (Figure 4). Among them, 223 institutions formed the largest institutional co-authorship network and were divided into 11 clusters. The largest cluster (red cluster), consisting of 42 institutions, was centered at the University of São Paulo and the Karolinska Institute. The green cluster, consisting of 49 institutions mainly located in China, ranked second and was centered at Sun Yat-sen University, Shanghai Jiao Tong University, and the Chinese Academy of Sciences. The top three institutions with the largest total link strength were Harvard University (total link strength = 51 times), Chinese Academy of Sciences (total link strength = 43 times), and Sun Yat-sen University (total link strength = 34 times), indicating that they were the most contributive institutions.
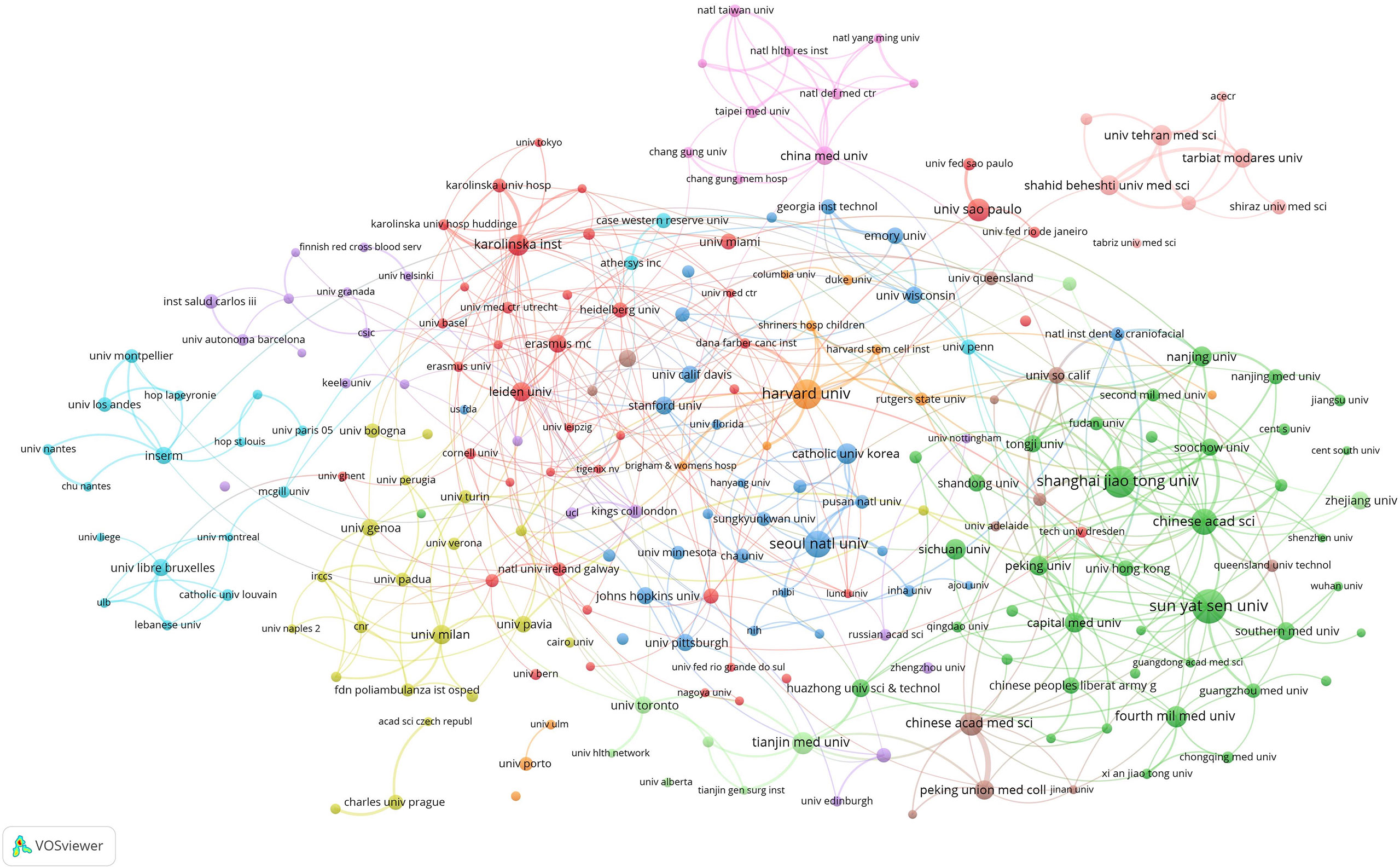
Figure 4 Mapping institutional co-authorship on MSC immunomodulation publications. The size of the points represents the institutional co-authorship frequency. The line between the two points indicates established collaboration between the two institutions. The thickening of the line indicates the collaboration degree between the two institutions.
A total of 24,049 authors have published articles on MSC immunomodulation from 2000 to 2022. The top ten most productive authors are listed in Table 5. The two authors with the most publications were Lagneaux Laurence (37 publications) and Najar Mehdi (37 publications). Shi Yufang had the highest citation frequency (4,474 citations). In addition, the average publication year of Hashemi Seyed Mahmoud was 2017.89, which was the latest average publication year among the top ten contributive authors.
The minimum number of author publications was set as five, and 648 contributive authors were selected for author co-authorship analysis using the VOSviewer software. In addition, 458 authors formed the largest author co-authorship network, which was divided into 19 clusters (Figure 5). The red cluster (58 authors) was the largest co-authorship cluster. The dark blue cluster (14 authors), centered on Lagneaux Laurence, Najar Mehdi, and Bron Dominique, was a close-knit research team. These three authors collaborated several times; for example, Lagneaux Laurence and Najar Mehdi collaborated 35 times, and Lagneaux Laurence and Bron Dominique collaborated 23 times. The top three authors with the largest total link strength were Shi Yufang (total link strength = 39 times), Shi Songtao (total link strength = 35 times), and Hoogduijn Martin (total link strength = 31 times).
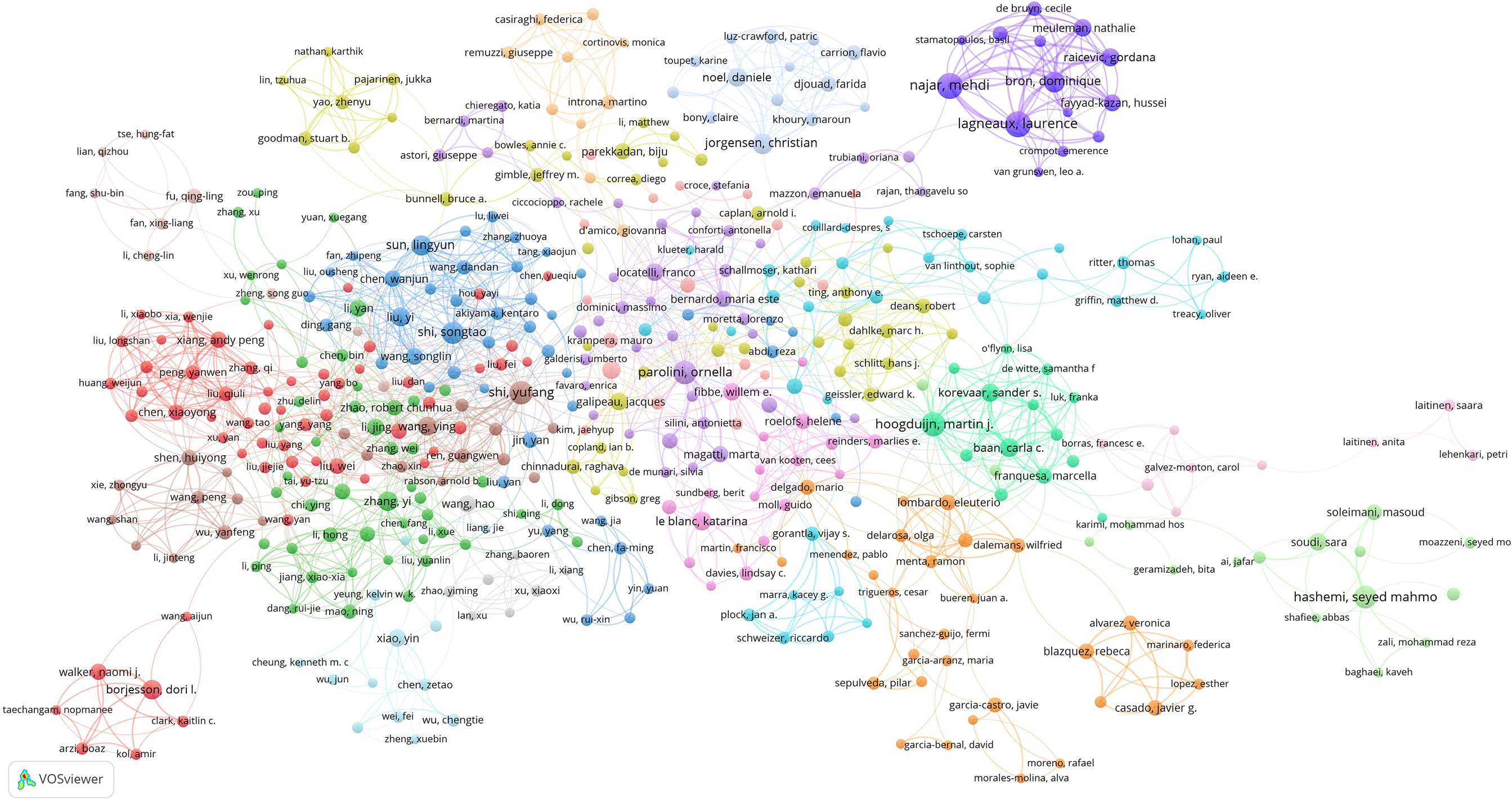
Figure 5 Mapping author co-authorship on MSC immunomodulation publications. The size of the points represents the co-authorship frequency of authors. The line between the two points represents established collaboration between the two authors. The thickening of the line represents the collaboration degree between the two authors.
There were 10,599 keywords in the 4,227 publications on MSC immunomodulation. The top ten keywords with the highest frequencies were MSCs (2,567 times), stem cell (1,995 times), differentiation (904 times), immunomodulation (861 times), inflammation (440 times), macrophage (266 times), cytokine (263 times), mechanisms (239 times) and tissue regeneration (202 times). A total of 111 keywords (Supplementary Table 1), defined as high-frequency keywords used more than 40 times, were selected for co-occurrence analysis and classified into four clusters using the VOSviewer software. As shown in Figure 6A, the largest cluster (red cluster), including 43 keywords, focused on “MSCs immunomodulation in regenerative medicine”. The keywords in the other three clusters concentrated on “the effects and mechanisms of MSCs immunomodulation” (green cluster), “MSC therapy for immune diseases” (blue cluster), and “the cell source of MSCs” (yellow cluster).
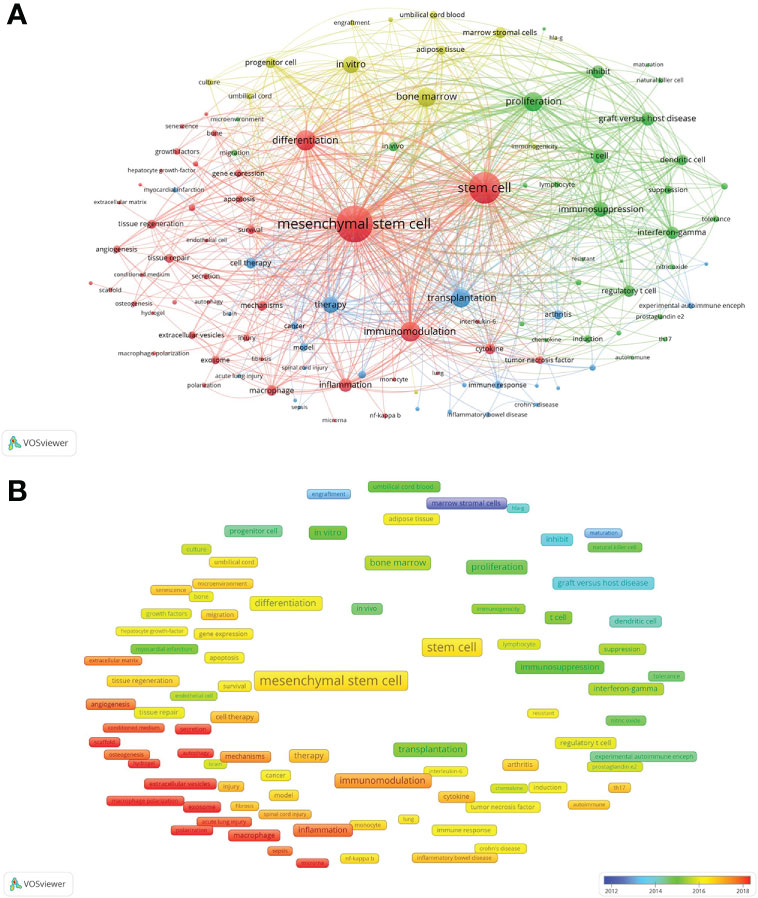
Figure 6 Mapping co-occurrence of high-frequency keywords on MSC immunomodulation publications. (A) Mapping of keywords appeared in the publications. (B) Distribution of keywords according to the average publication year.
A total of 111 high-frequency keywords were color-coded based on the average year of appearance in the publications (Figure 6B). The blue color-coded keywords indicated early publications, and the red color-coded keywords indicated the latest publications. The top five latest keywords were exosome (2019.29), extracellular vesicles (2019.15), macrophage polarization (2019.15), polarization (2018.56), and scaffold (2018.45).
Bibliometrics combined with visualization analysis produces an overview of certain research fields of interest, guiding further research. This study was performed to evaluate the contribution of countries, institutions, authors, core literature, and research hotspots and identify the developmental trend of the research field of MSC immunomodulation. The main outcomes of the analysis are discussed in subsequent subsections.
Although MSCs were first isolated and characterized in 1974 (27), the immunosuppressive effects of BMMSCs were not reported until 1995 (28). This study demonstrates that there has been a sustained increase in the number of publications on the immunomodulatory properties of MSCs per year. More than half of the related studies were published from 2018 to 2022, indicating increased research interest and a continuous upward trend. We predict that more studies with in-depth knowledge and insightful understanding of MSC immunomodulation will be published subsequently. Moreover, of the total 82 countries that contributed to global publications, China published the most relevant articles (1,054 articles), followed by the US (1,000 articles). Analysis of the citations representing the academic impact and quality of publications indicated that the total citation frequency of the US (53,856 citations) was the highest, much higher than that of China (30,671 citations), indicating that the US could be regarded as the leading and principal contributive country in this research field. These data indicate an assignable imbalance between the number and quality of studies in China.
Considering the quantity and quality of journals, the core publishing journals on MSCs immunomodulation were listed. Authors interested in this field should focus on these journals and consider them when contributing to a relevant paper. Moreover, Stem Cells ranked first in total and average citation frequencies, indicating that their publications are of great significance and reference value; Stem Cell Research & Therapy ranked first in the average publication years and the number of publications, indicating that it updates publications actively and grasps the frontiers of dynamic research.
The top five productive institutions from China, the US, and Korea were the leading institutions in the field of MSC immunomodulation. Among them, Harvard University in the US produced many high-quality articles corresponding with the top country in citation frequency. This demonstrates that advanced research institutions may be a determining factor at the academic level of a country. Furthermore, a co-authorship analysis was performed to evaluate the collaboration between institutions and authors; a higher total link strength indicated that the research institutions/authors preferred collaboration. The most predominant institutions were Harvard University, Chinese Academy of Sciences, and Sun Yat-sen University. The top three contributive authors were Shi Yufang, Shi Songtao, and Hoogduijn Martin. It was also revealed that the collaborative institutions/authors as the cluster centeres usually belonged to the top contributive section, suggesting that collaboration with other institutions/authors would promote the development of certain scientific fields, and the interest and enthusiasm for studies may also be important subjective factors.
Co-occurrence analysis was applied to determine interests and popular topics in the research field, which will guide researchers in in-depth studies. From the co-occurrence mapping of keywords in the field of MSC immunomodulation, it was revealed that keywords including stem cells, MSC, immunomodulation, and differentiation were conspicuously shown with higher weight in size and relation strength than other keywords. The research hotspots extracted from all included studies were divided into four clusters for different research directions.
MSCs participate in the whole tissue regeneration process as the primary stem cell source. The immunoregulatory effects of MSCs are primarily involved in this research direction, supporting their application in regenerative medicine. This cluster suggested that prominent keywords, such as “MSC differentiation”, “tissue inflammation” and “immunomodulation”, were the main research directions. MSCs significantly promote tissue regeneration by regulating the subtype distribution and phenotypes of immune cells, including macrophage polarization (29). Moreover, MSCs immunosuppress inflammatory response in the early phase of tissue injury, resulting in less scarring and tissue regeneration (30). The immunomodulatory properties of MSCs also rely on the paracrine effects, including cytokines and extracellular vesicles (31, 32). Emerging evidence has demonstrated that MSC-derived exosomes have immunomodulatory functions similar to those of donor MSCs (33–35). The immunomodulation of MSC-derived exosomes is hypothesized to be a future research hotspot.
Since Hamburger et al. first reported that BMMSCs produce immuno-cytokines to contribute to the suppression of hematopoiesis (36), the immunomodulatory effects of MSCs and their underlying mechanisms have been of interest to investigators. In 2005, the first most-cited article innovatively described the phenomenon of MSCs regulating allogeneic immune cells, suggesting that prostaglandin E2 (PGE2) may be an important MSC immunomodulator (5). There were several similar keywords, including “immunosuppression”, “inhibit” and “suppression”, among the more prevalent ones in the literature. These keywords were identified as the principal effects of MSC immunomodulation. Cluster mapping also demonstrated the mechanism of MSC-developed immunosuppressive effects, such as involved “immune cells”, “proliferation” and “inflammatory cytokines”. The investigators reported that MSCs directly induced the apoptosis of T lymphocytes, inhibited the proliferation of immune cells, and reduced cytokine secretion via soluble factors and cell-to-cell contact (37, 38), involving the participation of immune cells, including T lymphocytes, regulatory T cells, B cells, and dendritic cells (39–42). TNFα/TNFR2 signaling pathway, as an immune checkpoint axis, plays a critical role in the immunomodulatory effects of MSCs through direct suppression of T cells and indirect induction of active Foxp3+ Tregs (43, 44).
MSCs have quickly attracted considerable attention in the clinical field considering their promising features including regenerative properties and immunomodulatory ability. To 2016, 493 MSC-based clinical trials were registered in the database of the US National Institutes of Health, and the number of new trials with MSC therapy is still growing exponentially (45). Until 2022, 416 published MSC-based clinical trials have evaluated MSCs’ effectiveness in treating various diseases (46). Some of the diseases that MSCs have been utilized to treat in clinical trials are summarized as follows (47). Cardiovascular diseases: cardiomyopathy, chronic heart failure, myocardial infarction and atherosclerotic plaque. Neurological diseases: hypoxic-ischemic brain lesions, Parkinson’s disease, stroke and Alzeheimer’s disease. Orthopedic diseases: osteochondral defects and osteoarthritis. Rheumatologic diseases: RA, ankylosing spondylitis, systemic sclerosis, lupus erythematosus, polymyositis and dermatomyositis and Sjögren’s syndrome. Endocrine diseases: Type 1 diabetes mellitus. Increasing investigations, regardless of animal studies or clinical trials, offer considerable evidence that MSCs transplantation is promising immunotherapy for autoimmune or systemic diseases.
In addition, the immune microenvironment has a particular impact on the occurrence, development, and metastasis of tumors, which are potential targets of MSCs in oncology (48). MSCs can be polarized by TLR signaling into two homogenously acting phenotypes, including pro-inflammatory MSC1 and anti-inflammatory MSC2 (49). Furthermore, MSC2 supported cancer growth and spread while MSC1 had anti-tumor effects (50). MSC1/MSC2 polarization is a convenient way to define a heterogeneous population of cells that may help in the further studies, as well as provides important guidance in the improvement of MSC-based therapies (49). Hashemi Seyed Mahmoud, considered a contributive scientist in this field, reported that MSCs isolated from breast cancer and normal breast adipose tissues shared similar morphology and immunophenotype but exhibited a diverse profile of immunoregulatory capacities and inflammatory cytokines (51). Cluster analysis also suggested future research directions on the functional elements of MSCs and how they could be converted to clinical and therapeutic advantages.
Although BMMSCs are recognized as the classic and principal source of MSCs in most scientific investigations and preclinical studies, non-marrow tissue-derived MSCs are identified in almost all adult tissues (52). Analysis of this cluster revealed that most MSC immunomodulation studies still focused on BMMSCs while exploring MSCs isolated from other tissues, including adipose tissue, umbilical cord, umbilical cord blood, placenta, and dental tissue. Numerous comparative studies have demonstrated heterogeneity of cytokine profiles, immunomodulatory effects, cell proliferation, and differentiation among different tissue-derived MSCs (53–58). Standardized studies on in vitro culture systems and stem cell sources will guide more precise clinical applications of MSCs in the future.
Based on the overlap analysis of keyword prevalence over time, research directions were monitored, and emerging hotspots were determined. The color of the node from blue to red represents the average publication year of keywords from 2012 to 2022. According to the results, exosomes, extracellular vesicles, secretion, extracellular matrix, scaffold, polarization, hypoxia, and inflammation were colored red. These keywords are the most popular topics in the field of MSC immunomodulation. Studies on MSC-based immunotherapy have shifted from cell therapy to cell-free therapy, which is a promising application in future medicine. Furthermore, studies exploring the mechanisms and effects of MSC immunomodulation remain the focus of this field.
As a bibliometric analysis, this study had several limitations. First, one database could not identify all studies. This study analyzed publications included in the WoSCC, which might represent the main research in the discipline. Second, only English literature was retrieved and included in this study; therefore, some important studies in other languages could have been omitted. Third, some recently published high-quality papers might have been omitted due to low citation frequencies shortly after publication. Therefore, the latest primary studies and non-English literature should be explored in future research.
This study reveals global trends in MSC immunomodulation, which indicates that the immunomodulatory properties of MSCs have important research value and clinical application prospects. Since the 21st century, the total number of published studies has steadily increased, and China and the US are the two most productive regions in the world. Stem Cell Research & Therapy has the most publications in this field. Furthermore, the focus of studies has gradually shifted from “immunoregulatory effects” to “underlying mechanisms and immunotherapy”, indicating that further research might be focused on exploring the therapeutic potential of MSC immunomodulation. In particular, “exosomes”, “extracellular vesicles”, “secretion”, “polarization”, and “autophagy” are the recent promising hotspots.
WZ: Data collection, data analysis and interpretation, final approval of manuscript. SY: Data collection, data analysis and interpretation. TX: Data analysis and interpretation, manuscript writing. SR: Data analysis and interpretation, manuscript writing. XY: Data collection, data analysis. ZY: Data collection, data analysis. LY: Conception and design, financial support, data analysis and interpretation, final approval of manuscript. All authors read and approved the final manuscript.
This study was supported by the Key Research and Development Program of Liaoning Province (2021JH2/10300038 to LY).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.984984/full#supplementary-material
Mesenchymal stem cells, MSCs; bone marrow mesenchymal stem cells, BMMSCs; human leukocyte antigen DR isotype, HLA-DR; graft-versus-host disease, GVHD; rheumatoid arthritis, RA; systemic lupus erythematosus, SLE; inflammatory bowel disease, IBD; Web of Science Core Collection, WoSCC.
2. Gonzalez-Vilchis RA, Piedra-Ramirez A, Patiño-Morales CC, Sanchez-Gomez C, Beltran-Vargas NE. Sources, characteristics, and therapeutic applications of mesenchymal cells in tissue engineering. Tissue Eng Regener Me (2022) 19:325–61. doi: 10.1007/s13770-021-00417-1
3. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy (2006) 8:315–7. doi: 10.1080/14653240600855905
4. Tropel P, Noël D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res (2004) 295:395–406. doi: 10.1016/j.yexcr.2003.12.030
5. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood (2005) 105:1815–22. doi: 10.1182/blood-2004-04-1559
6. Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, et al. Bone marrow derived mesenchymal stem cells induce both polyclonal expansion and differentiation of b cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells (2008) 26:562–9. doi: 10.1634/stemcells.2007-0528
7. Legg K. MSCs can inhibit T-cell proliferation in vitro but not in vivo. Nat Rev Rheumatol (2010) 6:244. doi: 10.1038/nrrheum.2010.55
8. Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells (2017) 35:766–76. doi: 10.1002/stem.2509
9. Ghannam S, Pène J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol (2010) 185:302–12. doi: 10.4049/jimmunol.0902007
10. Franquesa M, Mensah FK, Huizinga R, Strini T, Boon L, Lombardo E, et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory b cells independently of T helper cells. Stem Cells (2015) 33:880–91. doi: 10.1002/stem.1881
11. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood (2005) 105:2214–9. doi: 10.1182/blood-2004-07-2921
12. Gur-Wahnon D, Borovsky Z, Beyth S, Liebergall M, Rachmilewitz J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp Hematol (2007) 35:426–33. doi: 10.1016/j.exphem.2006.11.001
13. Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther (2021) 12:192. doi: 10.1186/s13287-021-02265-1
14. Kelly K, Rasko JEJ. Mesenchymal stromal cells for the treatment of graft-versus-host disease. Front Immunol (2021) 12:761616. doi: 10.3389/fimmu.2021.761616
15. Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R, et al. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab (2015) 22:606–18. doi: 10.1016/j.cmet.2015.08.018
16. Liu Y, Chen C, Liu S, Liu D, Xu X, Chen X, et al. Acetylsalicylic acid treatment improves differentiation and immunomodulation of SHED. J Dent Res (2015) 94:209–18. doi: 10.1177/0022034514557672
17. Hwang JJ, Rim YA, Nam Y, Ju JH. Recent developments in clinical applications of mesenchymal stem cells in the treatment of rheumatoid arthritis and osteoarthritis. Front Immunol (2021) 12:631291. doi: 10.3389/fimmu.2021.631291
18. Wei ST, Huang YC, Chiang JY, Lin CC, Lin YJ, Shyu WC, et al. Gain of CXCR7 function with mesenchymal stem cell therapy ameliorates experimental arthritis via enhancing tissue regeneration and immunomodulation. Stem Cell Res Ther (2021) 12:314. doi: 10.1186/s13287-021-02402-w
19. Lian M, Sun B, Han Y, Yu B, Xin W, Xu R, et al. A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration. Biomaterials (2021) 274:120841. doi: 10.1016/j.biomaterials.2021.120841
20. Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci (2020) 41:653–64. doi: 10.1016/j.tips.2020.06.009
21. Thompson DF, Walker CK. And historical review of bibliometrics with applications to medical sciences. Pharmacotherapy (2015) 35:551–9. doi: 10.1002/phar.1586
22. Abd-Alrazaq A, Schneider J, Mifsud B, Alam T, Househ M, Hamdi M, et al. A comprehensive overview of the COVID-19 literature: machine learning-based bibliometric analysis. J Med Internet Res (2021) 23:e23703. doi: 10.2196/23703
23. Chen C, Lou Y, Li XY, Lv ZT, Zhang LQ, Mao W. Mapping current research and identifying hotspots on mesenchymal stem cells in cardiovascular disease. Stem Cell Res Ther (2020) 11:498. doi: 10.1186/s13287-020-02009-7
24. Xing D, Zhao Y, Dong S, Lin J. Global research trends in stem cells for osteoarthritis: a bibliometric and visualized study. Int J Rheum Dis (2018) 21:1372–84. doi: 10.1111/1756-185X.13327
25. Fayyad-Kazan M, Fayyad-Kazan H, Lagneaux L, Najar M. The potential of mesenchymal stromal cells in immunotherapy. Immunotherapy (2016) 8:839–42. doi: 10.2217/imt-2016-0037
26. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
27. Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol (1974) 2:83–92.
28. Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant (1995) 16:557–64.
29. Liu J, Feng B, Xu Y, Zhu J, Feng X, Chen W, et al. Immunomodulatory effect of mesenchymal stem cells in chemical-induced liver injury: a high-dimensional analysis. Stem Cell Res Ther (2019) 10:262. doi: 10.1186/s13287-019-1379-6
30. Zhang Y, Zhang X, Zhang H, Song P, Pan W, Xu P, et al. Mesenchymal stem cells derived extracellular vesicles alleviate traumatic hemorrhagic shock induced hepatic injury via IL-10/PTPN22-mediated M2 kupffer cell polarization. Front Immunol (2022) 12:811164. doi: 10.3389/fimmu.2021.811164
31. Kudinov VA, Artyushev RI, Zurina IM, Zorina ES, Lapshin RD, Snopova LB, et al. Inhaled placental mesenchymal stromal cell secretome from two- and three-dimensional cell cultures promotes survival and regeneration in acute lung injury model in mice. Int J Mol Sci (2022) 23:3417. doi: 10.3390/ijms23073417
32. Jin QH, Kim HK, Na JY, Jin C, Seon JK. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci Rep (2022) 12:4754. doi: 10.1038/s41598-022-08398-4
33. Clark K, Zhang S, Barthe S, Kumar P, Pivetti C, Kreutzberg N, et al. Placental mesenchymal stem cell-derived extracellular vesicles promote myelin regeneration in an animal model of multiple sclerosis. Cells (2019) 8:1497. doi: 10.3390/cells8121497
34. Tian J, Zhu Q, Zhang Y, Bian Q, Hong Y, Shen Z, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and treg cell responses. Front Immunol (2020) 11:598322. doi: 10.3389/fimmu.2020.598322
35. Shahir M, Mahmoud Hashemi S, Asadirad A, Varahram M, Kazempour-Dizaji M, Folkerts G, et al. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J Cell Physiol (2020) 235:7043–55. doi: 10.1002/jcp.29601
36. Hamburger AW. Impaired cytokine production by bone marrow stromal cells of immunodeficient mice. Br J Haematol (1995) 90:750–6. doi: 10.1111/j.1365-2141.1995.tb05192.x
37. Bulati M, Miceli V, Gallo A, Amico G, Carcione C, Pampalone M, et al. The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-γ produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front Immunol (2020) 11:54. doi: 10.3389/fimmu.2020.00054
38. Suzdaltseva Y, Goryunov K, Silina E, Manturova N, Stupin V, Kiselev SL. Equilibrium among inflammatory factors determines human MSC-mediated immunosuppressive effect. Cells (2022) 11:1210. doi: 10.3390/cells11071210
39. Kadle RL, Abdou SA, Villarreal-Ponce AP, Soares MA, Sultan DL, David JA, et al. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PloS One (2018) 13:e0193178. doi: 10.1371/journal.pone.0193178
40. Lim JY, Park MJ, Im KI, Kim N, Jeon EJ, Kim EJ, et al. Combination cell therapy using mesenchymal stem cells and regulatory T-cells provides a synergistic immunomodulatory effect associated with reciprocal regulation of TH1/TH2 and th17/treg cells in a murine acute graft-versus-host disease model. Cell Transplant (2014) 23:703–14. doi: 10.3727/096368913X664577
41. Luk F, Carreras-Planella L, Korevaar SS, de Witte SFH, Borràs FE, Betjes MGH, et al. Inflammatory conditions dictate the effect of mesenchymal stem or stromal cells on b cell function. Front Immunol (2017) 8:1042. doi: 10.3389/fimmu.2017.01042
42. Schülke S. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front Immunol (2018) 9:455. doi: 10.3389/fimmu.2018.00455
43. Beldi G, Khosravi M, Abdelgawad ME, Salomon BL, Uzan G, Haouas H, et al. TNFα/TNFR2 signaling pathway: an active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res Ther (2020) 11:281. doi: 10.1186/s13287-020-01740-5
44. Razazian M, Khosravi M, Bahiraii S, Uzan G, Shamdani S, Naserian S. Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells. World J Stem Cells (2021) 13:971–84. doi: 10.4252/wjsc.v13.i8.971
45. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant (2016) 25:829–48. doi: 10.3727/096368915X689622
46. Galderisi U, Peluso G, Di Bernardo G. Clinical trials based on mesenchymal stromal cells are exponentially increasing: where are we in recent years? Stem Cell Rev Rep (2022) 18:23–36. doi: 10.1007/s12015-021-10231-w
47. Baykal B. Mesenchymal stem cells for the treatment of various diseases. J Stem Cell Res Med (2016) 1:64–5. doi: 10.15761/JSCRM.1000110
48. Mohme M, Maire CL, Geumann U, Schliffke S, Dührsen L, Fita K, et al. Local intracerebral immunomodulation using interleukin-expressing mesenchymal stem cells in glioblastoma. Clin Cancer Res (2020) 26:2626–39. doi: 10.1158/1078-0432.CCR-19-0803
49. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PloS One (2010) 5:e10088. doi: 10.1371/journal.pone.0010088
50. Waterman RS, Henkle SL, Betancourt AM. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PloS One (2012) 7:e45590. doi: 10.1371/journal.pone.0045590
51. Sineh Sepehr K, Razavi A, Hassan ZM, Fazel A, Abdollahpour-Alitappeh M, Mossahebi-Mohammadi M, et al. Comparative immunomodulatory properties of mesenchymal stem cells derived from human breast tumor and normal breast adipose tissue. Cancer Immunol Immunother (2020) 69:1841–54. doi: 10.1007/s00262-020-02567-y
52. Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L. Adult mesenchymal stem cells and their exosomes: sources, characteristics, and application in regenerative medicine. Life Sci (2020) 256:118002. doi: 10.1016/j.lfs.2020.118002
53. Yamada Y, Nakamura-Yamada S, Umemura-Kubota E, Baba S. Diagnostic cytokines and comparative analysis secreted from exfoliated deciduous teeth, dental pulp, and bone marrow derived mesenchymal stem cells for functional cell-based therapy. Int J Mol Sci (2019) 20:5900. doi: 10.3390/ijms20235900
54. Sarıkaya A, Aydın G, Özyüncü Ö, Şahin E, Uçkan-Çetinkaya D, Aerts-Kaya F. Comparison of immune modulatory properties of human multipotent mesenchymal stromal cells derived from bone marrow and placenta. Biotech Histochem (2022) 97:79–89. doi: 10.1080/10520295.2021.1885739
55. Bajek A, Olkowska J, Walentowicz-Sadłecka M, Sadłecki P, Grabiec M, Porowińska D, et al. Human adipose-derived and amniotic fluid-derived stem cells: a preliminary in vitro study comparing myogenic differentiation capability. Med Sci Monit (2018) 24:1733–41. doi: 10.12659/msm.905826
56. Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med (2014) 34:695–704. doi: 10.3892/ijmm.2014.1821
57. Yi X, Chen F, Liu F, Peng Q, Li Y, Li S, et al. Comparative separation methods and biological characteristics of human placental and umbilical cord mesenchymal stem cells in serum-free culture conditions. Stem Cell Res Ther (2020) 11:183. doi: 10.1186/s13287-020-01690-y
Keywords: mesenchymal stem cells, immunomodulation, immunosuppression, immunotherapy, bibliometric analysis, immunoregulation
Citation: Wang ZQ, Sun YQ, Shen R, Tang X, Xu YX, Zhang Y and Liu Y (2022) Global scientific trends on the immunomodulation of mesenchymal stem cells in the 21st century: A bibliometric and visualized analysis. Front. Immunol. 13:984984. doi: 10.3389/fimmu.2022.984984
Received: 03 July 2022; Accepted: 05 August 2022;
Published: 24 August 2022.
Edited by:
Leonardo M.R. Ferreira, Medical University of South Carolina, United StatesReviewed by:
Philippe Lewalle, Jules Bordet Institute, BelgiumCopyright © 2022 Wang, Sun, Shen, Tang, Xu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Liu, bGl1eWFvQGNtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.