- 1Division of Rheumatology and Clinical Immunology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 2Division of Haematology, Medical Oncology and Haemopoietic Stem Cell Transplantation, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Division of Clinical Immunology, Department of Pathology, Queen Mary Hospital, Hong Kong, Hong Kong SAR, China
Background: Adult antibody deficiency remains under-recognised and under-studied – especially among Asian populations. Patterns of immunoglobulin use and the feasibility of subcutaneous immunoglobulin (SCIg) replacement among Chinese patients remains unclear.
Objective: To investigate the trends of immunoglobulin use, burden of adult antibody deficiency and the outcomes of patients on SCIg compared to intravenous immunoglobulin (IVIg) replacement in Hong Kong through a retrospective observational study.
Methods: Population-wide data of immunoglobulin recipients in Hong Kong between 2012 and 2021, and longitudinal clinical data of adult immunodeficiency patients at Queen Mary Hospital were collected and analysed.
Results: Total immunoglobulin consumption and recurrent immunoglobulin recipients increased continuously from 175,512g to 298,514g (ρ=0.99, p<0.001) and 886 to 1,508 (ρ=0.89, p=0.001) between 2012-21 in Hong Kong. Among 469 immunoglobulin recipients at Queen Mary Hospital in 2021, 344 (73.3%) were indicated for replacement. Compared to those on IVIg (n=14), patients on SCIg replacement (n=8) had fewer immunodeficiency-related hospitalisations (IRR=0.11) and shorter duration of hospitalisation stay (IRR=0.10) per year, as well as better quality of life (SF-36v2 Health Survey and Life Quality Index). Estimated annual healthcare cost of SCIg replacement per patient was lower than that of IVIg (HKD196,850 [USD25,096] vs HKD222,136 [USD28,319]).
Conclusion: There was a significantly increasing burden of adult antibody deficiency and immunoglobulin consumption in Hong Kong. SCIg was feasible and more cost-effective when compared to IVIg, with SCIg patients experiencing better clinical outcomes and quality of life. Future prospective studies to confirm the long-term efficacy and superiority of SCIg are required.
1 Introduction
Immunodeficiency among adults remains an important but severely under-recognised entity, with antibody deficiency being the most common subtype (1, 2). Antibody deficiencies can be classified as either primary or secondary – with secondary being much more common than their primary counterparts among adult patients (3, 4). Moreover, the prevalence of antibody deficiency continues to increase due to the popularising use of novel immunosuppressants and B-cell depleting therapies (e.g. anti-CD20 monoclonal antibodies such as rituximab) (3, 4). Although primary antibody deficiencies among paediatric patients have been extensively studied, the burden of adult antibody deficiency has not been well-characterised – especially among Asians and Chinese populations (1, 2, 5–10).
Normal human immunoglobulin, a pooled human blood product consisting of mainly IgG, has been used as a form of replacement in patients with antibody deficiency or as immunomodulation for various immune-mediated diseases (such as chronic inflammatory demyelinating polyradiculoneuropathy, multifocal motor neuropathy etc.) (11, 12). For replacement, immunoglobulin is usually administered with a relatively lower dose at regular intervals; while in immunomodulation, a relatively high dose is usually administered as single or short-term course (13–15). As a plasma-derived product from blood donors, immunoglobulin remains a precious and valuable resource especially during the Coronavirus disease (COVID-19) era with substantial declines in blood donations (16–18). Various resource-saving measures, such as establishment of immunoglobulin governance committees, have been implemented in many countries to optimise immunoglobulin use and stewardship (19–21). However, such measures do not currently exist in Hong Kong and the trends of immunoglobulin use remains unknown.
Traditionally, immunoglobulin has been administered intravenously (IVIg) or, uncommonly, intramuscularly. Subcutaneous immunoglobulin (SCIg) is a newer route of immunoglobulin replacement which was only introduced to Hong Kong since recently. In contrast to the IVIg which requires recurrent venous access and is administered during monthly hospital/day centre admissions, SCIg can be self-administrated by patients (or their carers) once every 1 to 2 weeks in the comfort of their own homes (22). SCIg has been shown to achieve at least comparable clinical outcomes compared with IVIg replacement, but with fewer systemic side effects and better health-related quality of life (HRQoL) (23–30). However, these findings have mostly been reported from Western cohorts, and the effectiveness and feasibility of SCIg replacement in Asia and among Chinese are unknown.
In view of these shortcomings, we took advantage of the availability of population-wide data and conducted this study to investigate the ten-year trends of immunoglobulin use and burden of adult antibody deficiency in Hong Kong. We also studied the feasibility of SCIg replacement by comparing the clinical outcomes and HRQoL of patients on SCIg and IVIg replacement in the real-world setting among Hong Kong Chinese.
2 Materials and methods
2.1 Study participants
In this retrospective observational cohort study, anonymised data were systematically retrieved from the Hong Kong Hospital Authority Clinical Data Analysis and Reporting System to identify patients of Hong Kong who received normal immunoglobulin between 2012 and 2021. The Hospital Authority is the sole publicly funded health care system in Hong Kong that serves a population of more than 7 million patients through 43 hospitals, 49 specialist outpatient clinics, and 73 general outpatient clinics. These facilities are organised into 7 clusters (Hong Kong East, Hong Kong West, Kowloon Central, Kowloon East, Kowloon West, New Territories East, and New Territories West) on the basis of geographical locations and provide approximately 90% of inpatient care in Hong Kong (31).
For subgroup analysis, longitudinal clinical data from all adult patients who received normal immunoglobulin and immunodeficiency patients who have attended the Immunology Clinic of Queen Mary Hospital (belonging to the Hong Kong West Cluster) were retrieved. Queen Mary Hospital remains the only centre with a Specialist in Immunology & Allergy and is responsible for the clinical care of immunodeficiency patients in Hong Kong. Therefore, this cohort likely represents the entire burden of adult immunodeficiency patients of the territory during the study period. Paediatric immunodeficiency patients and patients receiving immunoglobulin for non-immunodeficiency disorders (such as dermatological, haematological, neurological, rheumatological, etc.) would be seen throughout other geographical clusters of Hong Kong. As a tertiary referral centre and the only centre with a Specialist in Immunology & Allergy, Queen Mary Hospital also manages patients from other geographical clusters throughout the territory. However, not all patients receive immunoglobulin replacement in Queen Mary Hospital and may be referred to their nearest regional hospital (according to geographical location) for IVIg to facilitate patient convenience.
The study protocol was approved by the institutional review board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster.
2.2 Whole-population trends of immunoglobulin use in Hong Kong
The total number of patients who received normal human immunoglobulin, as well as the number of courses per patient, amount and cost of normal immunoglobulin used in each year were recorded. Recurrent recipients were defined as patients who received at least two courses of normal immunoglobulin within one year (i.e. patients recurrently prescribed immunoglobulin as replacement therapy), while single-time recipients were defined as patients who received only one course of normal immunoglobulin within one year (i.e. patients prescribed ‘on-demand’ immunoglobulin as immunomodulatory therapy).
2.3 Burden of adult antibody deficiency in Hong Kong
Patients’ diagnoses and medication history were recorded. For patients who had received normal human immunoglobulin, their treatment indications were reviewed and categorised as either ‘replacement’ or ‘immunomodulation’) by a Specialist in Immunology & Allergy (11, 12).
2.4 Comparison between SCIg and IVIg
There were no local guidelines or recommendations regarding the choice between IVIg vs. SCIg during the study period. All adult immunodeficiency patients requiring immunoglobulin replacement during the study period were freely offered either route of administration. The final choice on route of administration was a jointly agreed decision between patients and Specialist in Immunology & Allergy. For the comparison between SCIg and IVIg, demographics and clinical data including immunodeficiency diagnosis, age of onset of immunodeficiency, age of diagnosis of immunodeficiency, clinical manifestations, serum immunoglobulin levels and treatment details were obtained. Serum immunoglobulin levels measured at the time of diagnosis were taken as baseline. Trough serum immunoglobulin levels were measured right before the latest immunoglobulin infusion. Clinical outcomes within one year before the study end date, including number of infection episodes, number of immunodeficiency-related and infection hospitalisation episodes, total duration of immunodeficiency-related and infection hospitalisations, as well as adverse events were recorded. Immunodeficiency-related hospitalisation was defined as hospitalisation due to infection or immunoglobulin replacement. HRQoL was assessed using the SF-36v2 Health Survey (SF-36v2) and Life Quality Index (LQI) during patients’ latest clinic visit (32–34). Validated English and Traditional Chinese (Hong Kong) SF-36v2 questionnaires were used (35–38). For LQI, the validated English questionnaire was translated into Traditional Chinese using forward-backward translation (Table S1).
An exploratory comparison of the annual healthcare cost of regular SCIg and IVIg replacement per patient was performed. The healthcare cost was the combined annual costs of normal immunoglobulin and immunodeficiency-related hospitalisation, using the immunoglobulin cost per gram, mean dose per body weight per month, mean body weight, median annual duration of immunodeficiency-related hospitalisation and mean daily cost of hospitalisation. All costs were expressed in Hong Kong Dollars (HKD) and United States Dollars (USD). Cost of SCIg per gram at Queen Mary Hospital (Hong Kong) was HKD530 (USD68), while that of IVIg per gram was HKD316 (USD40) during the study period. Mean daily cost of hospitalisation in Hong Kong was HKD6,020 (USD767) (39). In Hong Kong, all SCIg infusion equipment and consumables are provided by the suppliers without additional charge, and thus considered to be included as part of the SCIg drug cost.
2.5 Statistical analysis
All data were analysed using IBM SPSS Statistics 27.0. For continuous variables, normality of data was checked using the Shapiro–Wilk test. Data were presented as number, number (percentage), mean ± standard deviation or median (25th percentile to 75th percentile), as appropriate. Spearman’s correlation was used in the analysis of whole-population trends of immunoglobulin use. In the comparison of SCIg and IVIg, the distribution of demographics was examined using the chi-square test, Fisher exact test (if >5% of the cells have expected count <5), student’s T-test or Mann-Whitney U test, as appropriate. Multiple imputation with 20 imputed data sets was performed for the missing data of baseline laboratory values using the bar procedure (40). For these variables, multiple imputation was used for the main analyses and complete-case analysis was used for the sensitivity analyses. In all statistical tests, two-sided p-values <0.05 were considered significant.
3 Results
3.1 Continuous increase in immunoglobulin consumption over the past decade in Hong Kong, with majority being recurrent recipients
Total amount of immunoglobulin consumption in Hong Kong from 2012 to 2021, sorted by Hospital Authority clusters, are shown in Figure 1. The total amount of immunoglobulin consumption significantly increased from 175,512g to 298,514g (Spearman’s correlation coefficient [ρ]=0.99, p<0.001). The average immunoglobulin consumption increased from 128g to 159g per patient (ρ=0.86, p=0.003). The total number of immunoglobulin recipients and expenditure also increased continuously from 1,367 to 1,873 (ρ=0.71, p=0.022) (Figure S1) and from HKD55,541,992 (USD7,080,829) to HKD97,523,880 (USD12,432,939) from 2012 to 2021 (ρ=0.99, p<0.001) (Figure S2), respectively.
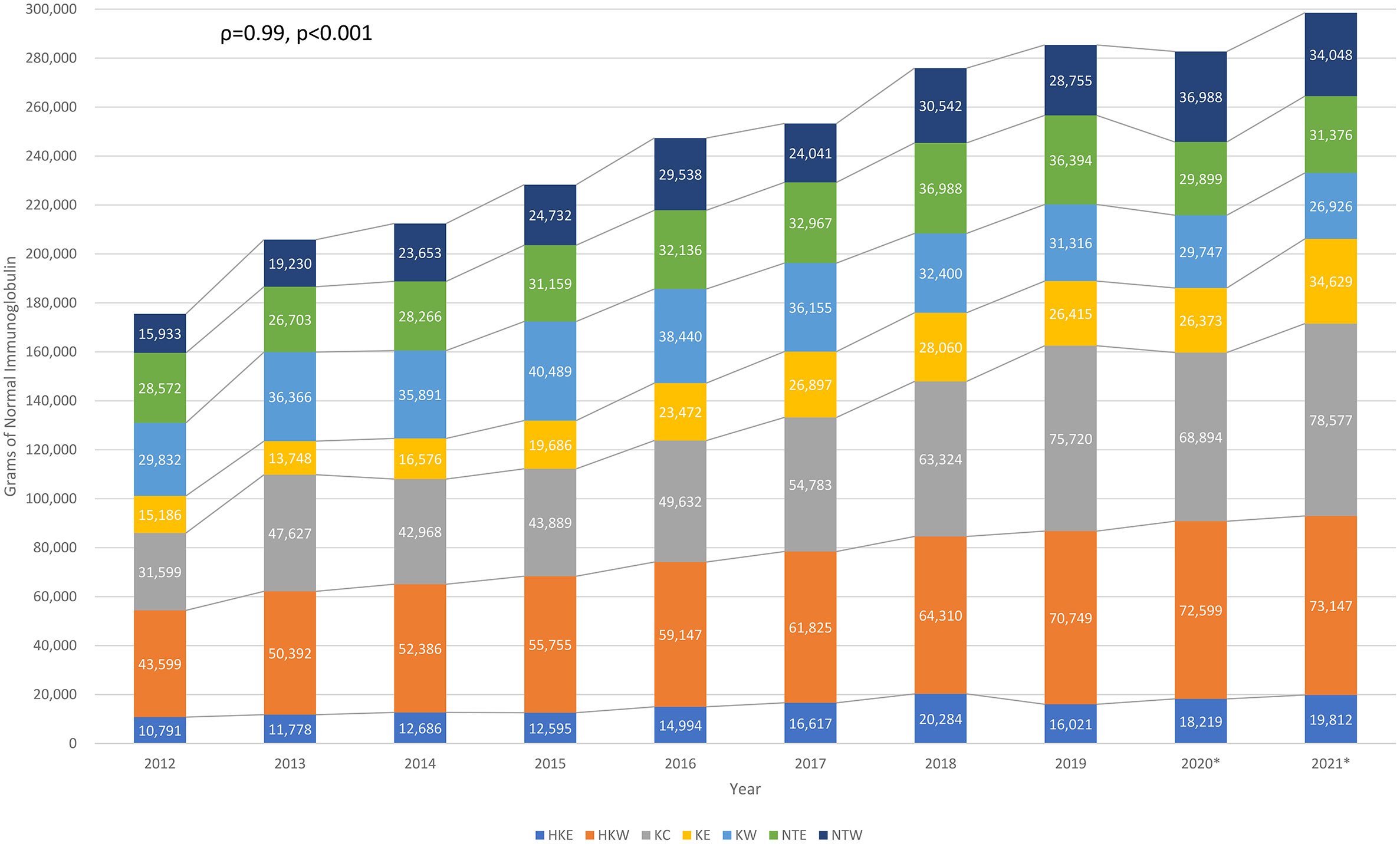
Figure 1 Longitudinal normal immunoglobulin consumption over the past decade in Hong Kong. HKE, Hong Kong East Cluster; HKW, Hong Kong West Cluster; KC, Kowloon Central Cluster; KE, Kowloon East Cluster; KW, Kowloon West Cluster; NTE, New Territories East Cluster; NTW, New Territories West Cluster. *Outbreak of COVID-19 in Hong Kong since January 2020, some of the healthcare services had been temporarily suspended upon waves of outbreak.
The number of recurrent and single-time immunoglobulin recipients per year from 2012 to 2021 are shown in Figure 2. The number of recurrent recipients increased from 886 to 1,508 (ρ=0.89, p=0.001), while the number of single-time immunoglobulin recipients remained relatively stable (ρ=−0.03, p=0.946). The proportion of recurrent immunoglobulin recipients also increased from 65% in 2012 to 77% in 2021 (ρ=0.94, p<0.001).
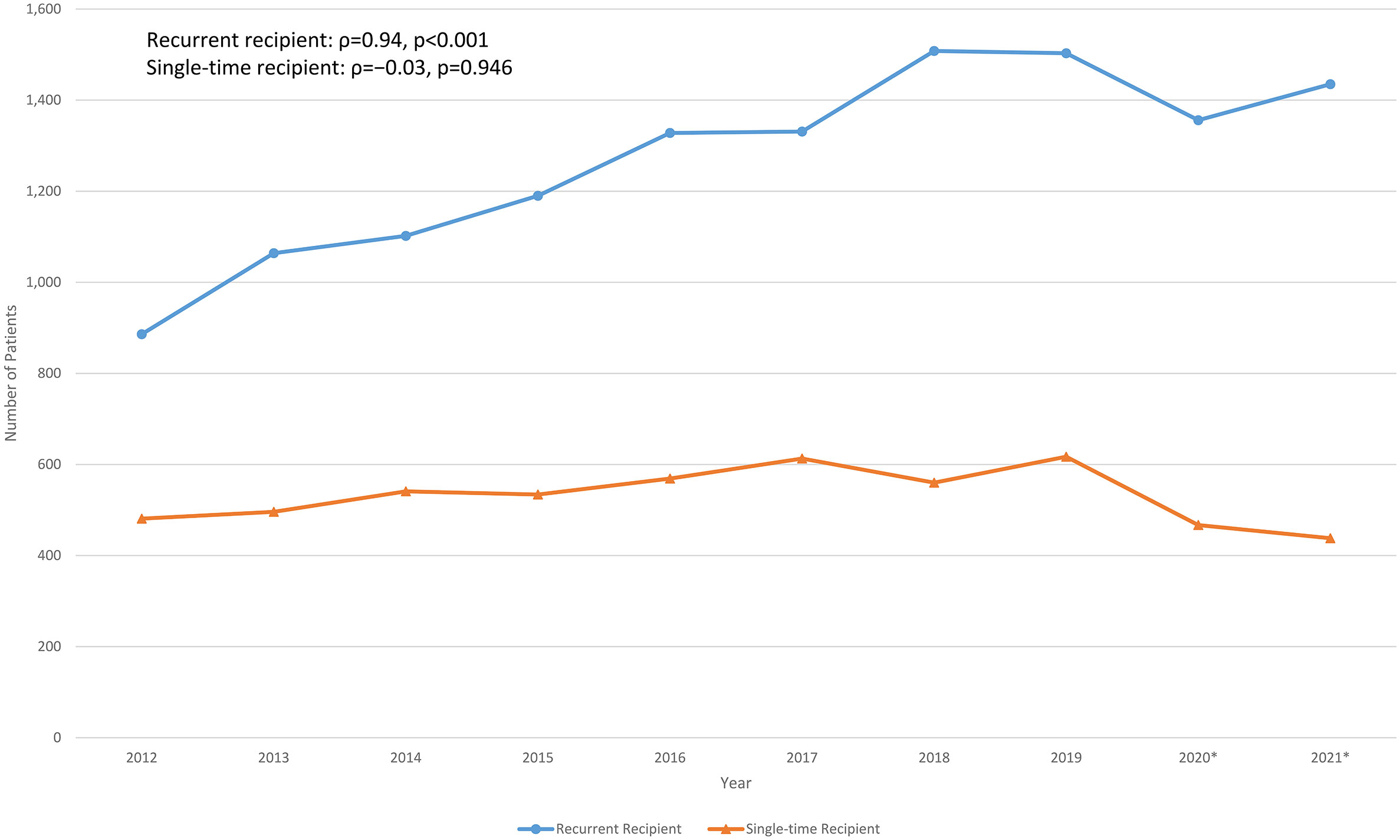
Figure 2 Longitudinal recurrent and single-time normal immunoglobulin recipients over the past decade in Hong Kong. *Outbreak of COVID-19 in Hong Kong since January 2020, some of the healthcare services had been temporarily suspended upon waves of outbreak.
3.2 Majority of immunoglobulin recipients were indicated for replacement, and most commonly for haematology patients
In subgroup analysis, 469 patients received immunoglobulin at Queen Mary Hospital (Hong Kong) in 2021; 344 (73.3%) received immunoglobulin for replacement, while the remaining 125 (26.7%) for immunomodulation. Among those receiving immunoglobulin replacement therapy, the most common were haematology patients (285, 82.8%), followed by solid organ transplant recipients (21, 6.1%) and primary immunodeficiencies (15, 4.4%). Among haematology patients, patients with history of lymphoma and post-haematopoietic stem-cell transplantation accounted for the majority with 36.1% and 33.7%, respectively (Figure S3).
3.3 Significant burden of antibody deficiency among adult immunodeficiency patients
A total of 108 patients with confirmed immunodeficiencies attended the Immunology Clinic at Queen Mary Hospital (Hong Kong). Breakdown of their diagnoses is shown in Figure 3. Forty-four (40.7%) of the patients had antibody deficiency; among them, 29 (65.9%) had primary antibody deficiency and 15 (34.1%) had secondary antibody deficiency. Common variable immunodeficiency was the most common diagnosis among primary antibody deficiency (6, 20.7%).
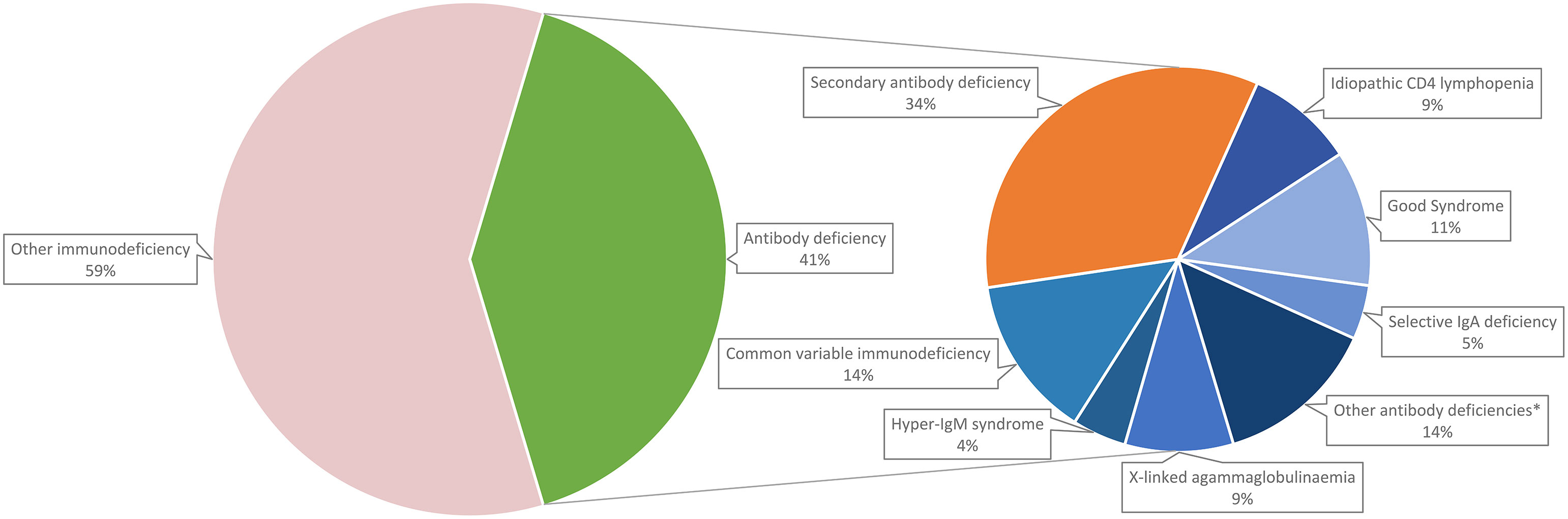
Figure 3 Breakdown of adult immunodeficiency patients in Hong Kong. *Other antibody deficiencies include specific antibody deficiency (2.3%), late-onset combined immunodeficiency (2.3%), ADA deficiency (2.3%), Wiskott-Aldrich syndrome (2.3%), chromosome 22q11.2 deletion syndrome (2.3%) and X-linked immunodeficiency with magnesium defect, Epstein-Barr virus (EBV) infection, and neoplasia (2.3%).
3.4 Better clinical outcomes and HRQoL among SCIg compared to IVIg patients
A total of 22 antibody deficiency patients treated continuously with immunoglobulin replacement were included in the comparison of SCIg and IVIg replacement; 8 (36.4%) were on SCIg and 14 (63.6%) on IVIg. Their diagnoses are shown in Table S2. In this cohort, 16 (72.7%) and 6 (27.3%) of the patients had primary and secondary antibody deficiencies, respectively. Table 1 summarises the clinical characteristics and treatment details of patients on SCIg and IVIg replacement. Demographics, aetiologies, manifestations, laboratory values, duration of immunoglobulin replacement and use of antimicrobial prophylaxis were similar between the two groups. Patients on SCIg required a significantly lower dose of immunoglobulin per body weight per month compared with patients on IVIg (0.52 ± 0.07 vs 0.66 ± 0.23 g/kg/month, p=0.046). Sensitivity analyses yielded similar results (Table S3).
Comparisons of the infection and hospitalisation outcomes are shown in Table 2. Patients on SCIg had less frequent immunodeficiency-related hospitalisations compared to patients on IVIg (0.50 [0.00-1.75] vs 13.0 [12.0-14.3] episodes, incidence rate ratio [IRR]: 0.11). Patients on SCIg also had shorter annual duration of stay of immunodeficiency-related hospitalisation (0.50 [0.00-4.25] vs 13.0 [12.0-27.8] days, IRR: 0.10). The rate of reported adverse events were similar between the two groups (Table S4).
Comparison of the patients’ HRQoL is shown in Table 3. Patients on SCIg had better HRQoL in the Physical Component Summary of SF-36v2 compared with patients on IVIg replacement (54.5 ± 5.66 vs 45.8 ± 8.35). Patients on SCIg also had better immunoglobulin replacement-specific quality of life in the ‘Treatment Interference’ (88.9 ± 9.03 vs 64.3 ± 20.1) and ‘Therapy Setting’ (94.4 [90.3-98.6] vs 63.9 [54.2-94.4]) domains of LQI.
3.5 Lower healthcare cost per patient for SCIg replacement
Using the mean weight of all patients on immunoglobulin replacement (62.2kg), the estimated annual healthcare costs of regular SCIg and IVIg replacement per patient were HKD196,850 (USD25,096) and HKD222,136 (USD28,319) respectively. Projecting from the costs saved from immunodeficiency-related hospitalisation costs alone, HKD25,286 (USD3,221) could be potentially saved per patient per year on SCIg compared to IVIg.
4 Discussion
To our knowledge, this is the first study to characterise the burden of adult antibody deficiency as well as comparing the outcomes of SCIg and IVIg replacement in the East and among Chinese patients. As the only dedicated unit responsible for managing adult immunodeficiency patients in Hong Kong, our cohort is highly representative of the entire immunodeficiency burden of the entire territory. In this retrospective observational study, we observed a substantial burden of adult antibody deficiency in Hong Kong Chinese, as well as better clinical outcomes and HRQoL among patients receiving SCIg replacement compared to those on IVIg.
It has been estimated that the global immunoglobulin consumption has been increasing at an average annual rate of 8% since 2010 (41). Studies in other populations reported increasing trends of immunoglobulin use before the era of COVID-19 (42–45). Similarly, our findings (including data during and following various COVID-19 outbreaks in Hong Kong in 2020) demonstrated significant increase in population-wide immunoglobulin consumption and expenditure over the past decade. This continuous rising trend persisted throughout the COVID-19 pandemic and was mainly among recurrent immunoglobulin recipients (for replacement), while the number of single-time immunoglobulin recipients (for immunomodulation) remained relatively stable (14, 15). Together with the overall increasing immunoglobulin usage and demand in Hong Kong, our findings suggest that there is a steadily growing burden of antibody deficiency in our population. Interestingly, trends in increase immunoglobulin consumption were consistent throughout the 7 geographical clusters of Hong Kong, but most marked in Kowloon Central (2.5x increase) and Kowloon East (2.3x increase). Reasons for disparate increases between geographical regions are likely multifactorial, including disproportionate changes in population densities and demographics, service expansion of immunological/haematological-based specialties or socio-economic variations between different clusters over the past decade. Dedicated studies regarding potential inequalities in prescription and their causes would be of great interest.
In subgroup analysis, we identified that around three quarters of adult immunoglobulin recipients were given immunoglobulin as replacement therapy, which was comparable to findings of our population-wide analysis. Among those indicated for immunoglobulin replacement, the overwhelming majority (96%) had secondary antibody deficiency (most patients being haematology patients), while only 4% had primary immunodeficiency. This supports the observation that there is a much higher prevalence of secondary antibody deficiencies compared to primary antibody deficiencies (4). Furthermore, the indications for immunoglobulin replacement varied across different studies and time. More recent studies tend to report that secondary antibody deficiency are more common than primary antibody deficiency (19, 45), whereas older studies reported the opposite (46, 47). We postulate that this reflects the progressive shift of the burden from primary to secondary antibody deficiencies in recent years.
When comparing between different routes of immunoglobulin replacement in this pragmatic study of real-worldpractice, we observed reduced immunodeficiency-related hospitalisations and duration of stay, as well as better HRQoL among adult patients on SCIg replacement than IVIg. The main difference in hospitalisation rates arose from fewer hospital stays for IVIg administration. These findings are similar to the experience reported by previous studies in other populations (23–30). In addition, we also identified that patients on SCIg required lower average dose of immunoglobulin compared to those on IVIg, while achieving similar infection outcomes. This is contrary to earlier recommendations in the United States, which suggested the use of higher doses (IVIg : SCIg = 1:1.37 or 1:1.53) when calculating the dose of SCIg replacement; while European recommendations suggest the use of equivalent doses (IVIg : SCIg = 1:1) (48–51). Further studies will be required to investigate whether his phenomenon is ethnic- or region-specific. Nonetheless, we propose that in Hong Kong Chinese, SCIg can be considered as a more effective route of immunoglobulin replacement and that we could potentially save on our precious plasma-derived resources by optimising more SCIg use.
Furthermore, we also demonstrated that SCIg is more cost-effective than IVIg as a form of immunoglobulin replacement for adult patients in Hong Kong. Our finding is similar to that of other cost-effective analysis studies in Australia, Canada, Iran, Spain, France, Japan and Switzerland (52–58). Projecting from the costs saved from immunodeficiency-related hospitalisation costs alone, we could potentially save HKD25,286 (USD3,221) per patient per year on SCIg compared to IVIg. This figure is likely an under-estimate, as we did not include indirect costs such as time-off work and productivity lost used for admissions for IVIg, need for healthcare staff during IVIg administration and difference in quality of life. Therefore, we believe that SCIg may be a superior alternative to IVIg replacement among adult patients in Hong Kong, in terms of clinical outcomes, HRQoL and overall healthcare costs. Unfortunately, as of the study period, SCIg remains unsubsidised in Hong Kong and inaccessible by most patients due to financial concerns. Furthermore, unlike in some countries, home administration of IVIg is not recommended in Hong Kong. We therefore strongly advocate for wider adoption and subsidisation of SCIg in Hong Kong.
This study has several limitations. First, immunoglobin use only indirectly reflected the burden of antibody deficiency and milder forms of antibody deficiency which did not warrant immunoglobulin replacement may have been missed. Hence, the actual burden of antibody deficiency may be even larger. Furthermore, there were also numerically a higher proportion of IVIg-treated patients with autoimmune/autoinflammatory disorders (which require higher dosing of immunoglobulin) and SCIg patients had longer duration of overall immunoglobulin replacement (which may have led to better tailoring of dosing regimen), although both variables did not reach statistical significance. Second, the small number of patients in the immunoglobulin replacement cohort did not allow for multivariable statistical analysis. Third, in this descriptive, pragmatic study we aimed to compare SCIg and IVIg replacement among adult antibody deficiency patients; in this pragmatic study, and the drug efficacy was not directly studied. It is possible that differences in real-world clinical practice also contributed to our results, rather than purely differences between the two administration routes alone. Fourth, clinical outcomes were assessed over one year and HRQoL was assessed at one time-point only. Long-term outcomes could not be studied, and HRQoL might vary over time. Fifth, the comparison of annual healthcare cost of SCIg and IVIg replacement per patient was only exploratory. Future formal cost-effectiveness analysis of SCIg replacement in Hong Kong is therefore warranted. Sixth, this was a retrospective observational study and firm conclusions on drug efficacy can not drawn. This highlights the urgent need for dedicated prospective studies in the future. Lastly, subgroup analysis and the comparative analysis between SCIg and IVIg were only performed on adult patients and do not pertain to paediatric immunodeficiency patients and their immunoglobulin use.
In conclusion, there has been significantly increasing immunoglobulin demand in Hong Kong over the past decade. The burden of adult antibody deficiency in Hong Kong is substantial but under-recognised, characterised by the dominance of secondary antibody deficiency with haematological diseases as the most common cause. Around 40% of adult immunodeficiency patients have antibody deficiency, but most patients, especially those with secondary antibody deficiency, did not receive immunologist care. In this retrospective observational study, adult antibody deficiency patients on SCIg replacement had better clinical outcomes and HRQoL compared to those on IVIg. Despite higher drug costs, we demonstrate that SCIg was overall more cost-effective than IVIg among adult patients with antibody deficiency. Although future studies will be required to draw conclusive evidence on the long-term efficacy of SCIg, we strongly advocate for wider adoption and subsidisation of SCIg in Hong Kong.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
AK researched the data, performed statistical analyses and wrote the manuscript. GL, VC and EA reviewed the data and edited the manuscript. CL and PL supervised the project and critically reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.984110/full#supplementary-material
References
1. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: A previously underrecognized common condition. J Allergy Clin Immunol Pract (2016) 4(6):1101–7. doi: 10.1016/j.jaip.2016.09.004
2. Mansouri D, Adimi P, Mirsaedi M, Mansouri N, Tabarsi P, Amiri M, et al. Primary immune deficiencies presenting in adults: Seven years of experience from Iran. J Clin Immunol (2005) 25(4):385–91. doi: 10.1007/s10875-005-4124-0
3. Li PH, Lau CS. Secondary antibody deficiency and immunoglobulin replacement. Hong Kong Bull Rheum (2017) 17(1):1–5. doi: 10.1515/hkbrd-2017-0001
4. Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: Causes, diagnosis, and management. Front Immunol (2019) 10:33. doi: 10.3389/fimmu.2019.00033
5. Takada H. Primary immunodeficiency in japan; epidemiology, diagnosis, and pathogenesis. Pediatr Int (2013) 55(6):671–4. doi: 10.1111/ped.12224
6. Lim DL, Thong BY, Ho SY, Shek LP, Lou J, Leong KP, et al. Primary immunodeficiency diseases in Singapore – the last 11 years. Singapore Med J (2003) 44(11):579–86.
7. Lee WI, Huang JL, Jaing TH, Shyur SD, Yang KD, Chien YH, et al. Distribution, clinical features and treatment in Taiwanese patients with symptomatic primary immunodeficiency diseases (Pids) in a nationwide population-based study during 1985-2010. Immunobiology (2011) 216(12):1286–94. doi: 10.1016/j.imbio.2011.06.002
8. Sigstad HM, Stray-Pedersen A, Frøland SS. Coping, quality of life, and hope in adults with primary antibody deficiencies. Health Qual Life Outcomes (2005) 3:31. doi: 10.1186/1477-7525-3-31
9. Lam DS, Lee TL, Chan KW, Ho HK, Lau YL. Primary immunodeficiency in Hong Kong and the use of genetic analysis for diagnosis. Hong Kong Med J (2005) 11(2):90–6.
10. Duraisingham SS, Buckland M, Dempster J, Lorenzo L, Grigoriadou S, Longhurst HJ. Primary vs. secondary antibody deficiency: Clinical features and infection outcomes of immunoglobulin replacement. PloS One (2014) 9(6):e100324. doi: 10.1371/journal.pone.0100324
11. Nagelkerke SQ, Kuijpers TW. Immunomodulation by ivig and the role of fc-gamma receptors: Classic mechanisms of action after all? Front Immunol (2014) 5:674. doi: 10.3389/fimmu.2014.00674
12. Sewell WA, Jolles S. Immunomodulatory action of intravenous immunoglobulin. Immunology (2002) 107(4):387–93. doi: 10.1046/j.1365-2567.2002.01545.x
13. Negi VS, Elluru S, Sibéril S, Graff-Dubois S, Mouthon L, Kazatchkine MD, et al. Intravenous immunoglobulin: An update on the clinical use and mechanisms of action. J Clin Immunol (2007) 27(3):233–45. doi: 10.1007/s10875-007-9088-9
14. Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol (2005) 142(1):1–11. doi: 10.1111/j.1365-2249.2005.02834.x
15. Hartung HP, Mouthon L, Ahmed R, Jordan S, Laupland KB, Jolles S. Clinical applications of intravenous immunoglobulins (Ivig)–beyond immunodeficiencies and neurology. Clin Exp Immunol (2009) 158 Suppl 1(Suppl 1):23–33. doi: 10.1111/j.1365-2249.2009.04024.x
16. Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am (2008) 28(2):413–37. doi: 10.1016/j.iac.2008.01.008
17. Chiem C, Alghamdi K, Nguyen T, Han JH, Huo H, Jackson D. The impact of covid-19 on blood transfusion services: A systematic review and meta-analysis. Transfus Med Hemother (2021) 30(2):1–12. doi: 10.1159/000519245
18. Al-Riyami AZ, Burnouf T, Wood EM, Devine DV, Oreh A, Apelseth TO, et al. International society of blood transfusion survey of experiences of blood banks and transfusion services during the covid-19 pandemic. Vox Sang (2022) 117(6):822–30. doi: 10.1111/vox.13256
19. Rocchio MA, Schurr JW, Hussey AP, Szumita PM. Intravenous immune globulin stewardship program at a tertiary academic medical center. Ann Pharmacother (2017) 51(2):135–9. doi: 10.1177/1060028016673071
20. Tsapepas D, Der-Nigoghossian C, Patel K, Berger K, Vawdrey DK, Salmasian H. Medication stewardship using computerized clinical decision support: A case study on intravenous immunoglobulins. Pharmacol Res Perspect (2019) 7(5):e00508. doi: 10.1002/prp2.508
21. Derman BA, Schlei Z, Parsad S, Mullane K, Knoebel RW. Changes in intravenous immunoglobulin usage for hypogammaglobulinemia after implementation of a stewardship program. JCO Oncol Pract (2021) 17(3):e445–e53. doi: 10.1200/op.20.00312
22. Skoda-Smith S, Torgerson TR, Ochs HD. Subcutaneous immunoglobulin replacement therapy in the treatment of patients with primary immunodeficiency disease. Ther Clin Risk Manag (2010) 6:1–10. doi: 10.1057/rm.2009.17
23. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: An evidence-based review. Drugs (2013) 73(12):1307–19. doi: 10.1007/s40265-013-0094-3
24. Wasserman RL, Irani AM, Tracy J, Tsoukas C, Stark D, Levy R, et al. Pharmacokinetics and safety of subcutaneous immune globulin (Human), 10% Caprylate/Chromatography purified in patients with primary immunodeficiency disease. Clin Exp Immunol (2010) 161(3):518–26. doi: 10.1111/j.1365-2249.2010.04195.x
25. Desai SH, Chouksey A, Poll J, Berger M. A pilot study of equal doses of 10% igiv given intravenously or subcutaneously. J Allergy Clin Immunol (2009) 124(4):854–6. doi: 10.1016/j.jaci.2009.07.051
26. Wasserman RL, Melamed I, Kobrynski L, Strausbaugh SD, Stein MR, Sharkhawy M, et al. Efficacy, safety, and pharmacokinetics of a 10% liquid immune globulin preparation (Gammagard liquid, 10%) administered subcutaneously in subjects with primary immunodeficiency disease. J Clin Immunol (2011) 31(3):323–31. doi: 10.1007/s10875-011-9512-z
27. Nicolay U, Kiessling P, Berger M, Gupta S, Yel L, Roifman CM, et al. Health-related quality of life and treatment satisfaction in north American patients with primary immunedeficiency diseases receiving subcutaneous igg self-infusions at home. J Clin Immunol (2006) 26(1):65–72. doi: 10.1007/s10875-006-8905-x
28. Compagno N, Cinetto F, Semenzato G, Agostini C. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: A single-center experience in 61 patients. Haematologica (2014) 99(6):1101–6. doi: 10.3324/haematol.2013.101261
29. Windegger TM, English J, Weston H, Morwood K, Kynn M, Scuffham P, et al. Longitudinal study of intravenous versus subcutaneous immunoglobulin replacement therapy in hematological malignancy. Asia Pac J Clin Oncol (2021) 17(6):546–54. doi: 10.1111/ajco.13515
30. Windegger TM, Lambooy CA, Hollis L, Morwood K, Weston H, Fung YL. Subcutaneous immunoglobulin therapy for hypogammaglobulinemia secondary to malignancy or related drug therapy. Transfus Med Rev (2017) 31(1):45–50. doi: 10.1016/j.tmrv.2016.06.006
31. Hospital Authority. Hospital authority annual report (2018). Available at: https://www.ha.org.hk/haho/ho/cc/HA_Annual_Report_2020-21_en.pdf.
32. Maruish ME. User’s manual for the sf-36v2 health survey. Lincoln, RI: QualityMetric Incorporated (2011).
33. Daly PB, Evans JH, Kobayashi RH, Kobayashi AL, Ochs HD, Fischer SH, et al. Home-based immunoglobulin infusion therapy: Quality of life and patient health perceptions. Ann Allergy (1991) 67(5):504–10.
34. Nicolay U, Haag S, Eichmann F, Herget S, Spruck D, Gardulf A. Measuring treatment satisfaction in patients with primary immunodeficiency diseases receiving lifelong immunoglobulin replacement therapy. Qual Life Res (2005) 14(7):1683–91. doi: 10.1007/s11136-005-1746-x
35. Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the sf-36 health survey questionnaire: New outcome measure for primary care. BMJ (1992) (6846) 305:160–4. doi: 10.1136/bmj.305.6846.160
36. Jenkinson C, Stewart-Brown S, Petersen S, Paice C. Assessment of the sf-36 version 2 in the united kingdom. J Epidemiol Community Health (1999) 53(1):46–50. doi: 10.1136/jech.53.1.46
37. Lam CL, Gandek B, Ren XS, Chan MS. Tests of scaling assumptions and construct validity of the Chinese (Hk) version of the sf-36 health survey. J Clin Epidemiol (1998) 51(11):1139–47. doi: 10.1016/s0895-4356(98)00105-x
38. Lam ETP, Lam CLK, Lo YYC, Gandek B. Psychometrics and population norm of the Chinese (Hk) sf-36 health Survey_Version 2. HK Prac (2008) 30:185–98.
39. Food and Health Bureau of the Government of the Hong Kong Special Administrative Region. Average cost (General (Acute & convalescent)) per patient day for each major specialty by hospital cluster for 2019-20 (2021). Available at: https://www.fhb.gov.hk/download/legco/replies/210413_sfc/fhb-h-e.pdf.
40. Baranzini D. The “Bar procedure”: Spss single dataframe aggregating spss multiply imputed split files. (2018). doi: 10.13140/RG.2.2.33750.70722.
41. Prevot J, Jolles S. Global immunoglobulin supply: Steaming towards the iceberg? Curr Opin Allergy Clin Immunol (2020) 20(6):557–64. doi: 10.1097/aci.0000000000000696
42. Murphy MSQ, Tinmouth A, Goldman M, Chassé M, Colas JA, Saidenberg E, et al. Trends in ivig use at a tertiary care Canadian center and impact of provincial use mitigation strategies: 10-year retrospective study with interrupted time series analysis. Transfusion (2019) 59(6):1988–96. doi: 10.1111/trf.15271
43. Hsu LI, Chen JW, Lin DT, Hung YS, Hou SM. Clinical use of intravenous immunoglobulin in Taiwan: A 10-year population study. J Formos Med Assoc (2021) 120(10):1921–5. doi: 10.1016/j.jfma.2021.02.017
44. Modell V, Quinn J, Orange J, Notarangelo LD, Modell F. Primary immunodeficiencies worldwide: An updated overview from the Jeffrey modell centers global network. Immunol Res (2016) 64(3):736–53. doi: 10.1007/s12026-016-8784-z
45. Shemer A, Kivity S, Shoenfeld Y. Clinical indications for intravenous immunoglobulin utilization in a tertiary medical center: A 9-year retrospective study. Transfusion (2018) 58(2):430–8. doi: 10.1111/trf.14427
46. Ruiz-Antorán B, Agustí Escasany A, Vallano Ferraz A, Danés Carreras I, Riba N, Mateu Escudero S, et al. Use of non-specific intravenous human immunoglobulins in Spanish hospitals; need for a hospital protocol. Eur J Clin Pharmacol (2010) 66(6):633–41. doi: 10.1007/s00228-010-0800-y
47. Constantine MM, Thomas W, Whitman L, Kahwash E, Dolan S, Smith S, et al. Intravenous immunoglobulin utilization in the Canadian Atlantic provinces: A report of the Atlantic collaborative intravenous immune globulin utilization working group. Transfusion (2007) 47(11):2072–80. doi: 10.1111/j.1537-2995.2007.01400.x
48. Wasserman RL, Melamed I, Nelson RP Jr., Knutsen AP, Fasano MB, Stein MR, et al. Pharmacokinetics of subcutaneous Igpro20 in patients with primary immunodeficiency. Clin Pharmacokinet (2011) 50(6):405–14. doi: 10.2165/11587030-000000000-00000
49. Haddad E, Berger M, Wang EC, Jones CA, Bexon M, Baggish JS. Higher doses of subcutaneous igg reduce resource utilization in patients with primary immunodeficiency. J Clin Immunol (2012) 32(2):281–9. doi: 10.1007/s10875-011-9631-6
50. Krishnarajah G, Lehmann JK, Ellman B, Bhak RH, DerSarkissian M, Leader D Jr., et al. Evaluating dose ratio of subcutaneous to intravenous immunoglobulin therapy among patients with primary immunodeficiency disease switching to 20% subcutaneous immunoglobulin therapy. Am J Manag Care (2016) 22(15 Suppl):s475–s81.
51. Jolles S, Bernatowska E, de Gracia J, Borte M, Cristea V, Peter HH, et al. Efficacy and safety of hizentra(®) in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol (2011) 141(1):90–102. doi: 10.1016/j.clim.2011.06.002
52. Windegger TM, Nghiem S, Nguyen KH, Fung YL, Scuffham PA. Cost-utility analysis comparing hospital-based intravenous immunoglobulin with home-based subcutaneous immunoglobulin in patients with secondary immunodeficiency. Vox Sang (2019) 114(3):237–46. doi: 10.1111/vox.12760
53. Windegger TM, Nghiem S, Nguyen KH, Fung YL, Scuffham PA. Primary immunodeficiency disease: A cost-utility analysis comparing intravenous vs subcutaneous immunoglobulin replacement therapy in Australia. Blood Transfus (2020) 18(2):96–105. doi: 10.2450/2029.0083-19
54. Shabaninejad H, Asgharzadeh A, Rezapour A, Rezaei N. Cost-effectiveness analysis of subcutaneous immunoglobulin replacement therapy in Iranian patients with primary immunodeficiencies. Med J Islam Repub Iran (2017) 31:94. doi: 10.14196/mjiri.31.94
55. Alsina L, Montoro JB, Moral PM, Neth O, Pica MO, Sánchez-Ramón S, et al. Cost-minimization analysis of immunoglobulin treatment of primary immunodeficiency diseases in Spain. Eur J Health Econ (2022) 23(3):551–8. doi: 10.1007/s10198-021-01378-x
56. Beauté J, Levy P, Millet V, Debré M, Dudoit Y, Le Mignot L, et al. Economic evaluation of immunoglobulin replacement in patients with primary antibody deficiencies. Clin Exp Immunol (2010) 160(2):240–5. doi: 10.1111/j.1365-2249.2009.04079.x
57. Igarashi A, Kanegane H, Kobayashi M, Miyawaki T, Tsutani K. Cost-minimization analysis of Igpro20, a subcutaneous immunoglobulin, in Japanese patients with primary immunodeficiency. Clin Ther (2014) 36(11):1616–24. doi: 10.1016/j.clinthera.2014.08.007
Keywords: antibody deficiency, chinese, primary immunodeficiency disease, immunoglobulin therapy, subcutaneous, adult
Citation: Kan AKC, Leung GMK, Chiang V, Au EYL, Lau CS and Li PH (2022) Ten-year population trends of immunoglobulin use, burden of adult antibody deficiency and feasibility of subcutaneous immunoglobulin (SCIg) replacement in Hong Kong Chinese. Front. Immunol. 13:984110. doi: 10.3389/fimmu.2022.984110
Received: 01 July 2022; Accepted: 29 November 2022;
Published: 14 December 2022.
Edited by:
Hans-Hartmut Peter, University of Freiburg Medical Center, GermanyCopyright © 2022 Kan, Leung, Chiang, Au, Lau and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip Hei Li, bGlwaGlsaXBAaGt1Lmhr
 Andy Ka Chun Kan
Andy Ka Chun Kan Garret Man Kit Leung2
Garret Man Kit Leung2 Valerie Chiang
Valerie Chiang Elaine Yuen Ling Au
Elaine Yuen Ling Au Philip Hei Li
Philip Hei Li