
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 31 August 2022
Sec. Viral Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.982346
This article is part of the Research Topic Updates on Immune Responses to Hepatitis B Infection View all 3 articles
Background: Bruton tyrosine kinase inhibitors (BTKis) interrupt B-cell receptor signaling and thereby could potentially reactivate hepatitis B virus (HBV). However, data about the risk for HBV reactivation (HBVr) of BTKis in relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL) patients are sparse.
Methods: A total of 55 R/R DLBCL patients receiving BTKis therapy in the Tongji Hospital of Tongji University were enrolled. Patient clinical characteristics, treatment outcomes and details of HBVr were collected and analyzed, aiming to demonstrate the risk of HBVr in R/R DLBCL patients post BTKis therapy and the efficacy of BTKis in HBV-associated R/R DLBCL patients.
Results: Of 55 R/R DLBCL patients treated with ibrutinib (N=38) and zanubrutinib (N=17), 4 were with chronic HBV infection (HBsAg positive), 26 with resolved HBV infection (HBsAg negative and HBcAb positive) and 25 without HBV infection (HBsAg negative and HBcAb negative). In resolved HBV infection group, 2 patients developed HBVr after the use of ibrutinib and zanubrutinib respectively. Neither of them developed HBV-related hepatitis. Our finding showed that the incidence of HBVr in resolved HBV infection group was 7.69% (95% CI, 0.9-25.1%). In this study, Overall response rate (ORR) was 70.9%. 1-year overall survival (OS) rate was 80.0%. Median progression-free survival (PFS) was 4 months (95% CI, 3-5 months). In addition, HBV infection was not associated with response rates or survival among R/R DLBCL patients post BTKis treatments.
Conclusion: Our study suggested that HBV infection do not affect the efficacy of BTKis’ treatment. However, R/R DLBCL patients with resolved HBV infection are at a moderate risk of developing HBVr throughout BTKis treatment. Patients should be screened for HBVr during BTKis therapy.
Hepatitis B virus (HBV) infection has been confirmed to relate to the development of diffuse large B-cell lymphoma (DLBCL) at genomic and transcriptomic levels (1). DLBCL patients with concomitant HBV infection have a risk of HBV reactivation (HBVr) when receiving immunotherapy, targeted therapy, chimeric antigen receptor T cell therapy or cytotoxic chemotherapy (2–6). Host status, HBV status at baseline and type of therapy together determine the risk of HBVr (7). After reactivation, clinical manifestations could be totally different. Patients might be asymptomatic, experience hepatitis flares or even develop life-threatening liver failure despite discontinued from treatments (8).
Bruton tyrosine kinase inhibitors (BTKis) are proved to be tolerable and effective in relapsed or refractory DLBCL (R/R DLBCL) (9–11). Among a series of BTKis, ibrutinib is the first-in-class, and zanubrutinib is one of second-generation (12). These agents chould form an irreversible covalent bond at Cys481 within the adenosine triphosphate binding pocket of Bruton tyrosine kinase (BTK), and thereby inhibit BTK, a key component of the B-cell receptor (BCR) signaling pathway (13, 14). As interrupting BCR, which is critical in normal B lymphopoiesis, BTKis could potentially reactivate HBV (8). However, published data about the risk of HBVr induced by BTKis are limited.
Herein, we designed a retrospective study to systematically assess the HBVr rate in R/R DLBCL patients post BTKis therapy in a real world, and explore the efficacy of BTKis in HBV-associated R/R DLBCL patients. To the best of our knowledge, this is the first and largest research to evaluate the HBVr incidence induced by BTKis and the efficacy of BTKis in HBV-associated R/R DLBCL patients. Our research could help physicians to better select R/R DLBCL patients suitable for such a targeted treatment.
This retrospective study enrolled 55 R/R DLBCL patients treated with BTKis at Tongji Hospital of Tongji University from January 2018 to April 2021. Patients were diagnosed with DLBCL by pathology according to 2016 World Health Organization classification for tumors of hematopoietic and lymphoid tissues (15). Relapsed DLBCL was defined as any new lesion or increase by 50% of previously involved sites from nadir (16). Refractory DLBCL was defined as progressive disease or stable disease as best response at any point during chemotherapy or relapsed at ≤12 months from autologous stem cell transplantation (17). Prophylactic nucleos(t)ide analog therapy (NAT) was administered to patients with chronic HBV infection, and patients with resolved HBV infection whose HBV DNA level rose above 100 IU/mL or who could not be able to adhere to HBV DNA monitoring regularly though HBV DNA level is normal. Informed consent was obtained from each patient, and the study adhered to the principles of the Helsinki Declaration.
Data were extracted from patients’ medical records. All patients were observed for HBVr during BTKis therapy. The primary end point was the occurrence of HBVr. The secondary end point was either disease progression or death of any cause. Clinical characteristics including age, sex, international prognostic index, BTKi subtype, time on BTKi, previous therapies, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), lactic acid dehydrogenase (LDH), HBV status (HBsAg, HBsAb, HBeAg, HBeAb, HBcAb and HBV DNA level) at baseline and antiviral prophylaxis were collected before commencing BTKi-containing treatment.
Serum HBV DNA level was measured with the lower limit of detection at 100 IU/mL. HBVr was defined as 1 of the following: >1 log increase in HBV DNA, HBV DNA-positive when previously negative, HBV DNA >2000 IU/mL if no baseline level was available, or reverse seroconversion from HBsAg-negative to -positive (18). Hepatitis flare was defined as serum ALT level >3×upper limit of normal (normal upper limit = 50 U/L in our center) or an ALT increase >100 U/L (19). HBV-related hepatitis was defined as hepatitis accompanying or following HBVr without any acute infection with other hepatitis viruses or any systemic disease (20, 21).
Response evaluation was based on the International Working Group response criteria for non-Hodgkin lymphoma (NHL) (16, 22). Overall survival (OS) was calculated from date of receiving BTKi treatment until June 30, 2021 or death of any cause, and progression-free survival (PFS) was until June 30, 2021, disease progression, or death of any cause.
Normality was assessed using the Shapiro-Wilk test. Equality of variances was assessed using Levene’s test. For normally distributed data with homogeneity of variance, differences between three groups were compared using one-way analysis of variance (ANOVA). For abnormally distributed data or data without homogeneity of variance, nonparametric Kruskal-Wallis test was used. We used Fisher’s exact test for categorical data. If overall comparison among three groups was significant, we would adjust P values for multiple comparisons using the Bonferroni method. OS and PFS were analyzed by the Kaplan–Meier method. Differences between survival curves were tested using log-rank test. We used univariate and multivariate Cox proportional hazard regression models to evaluate predictors of HBVr and OS. Factors with a significance of <0.2 by univariate analysis were included in the multivariate analyses, along with age and sex. All statistical tests were two-sided, and the threshold of significance was set at P<0.05. Analyses were performed using SPSS Version 26.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA).
Between January 2018 and April 2021, 55 R/R DLBCL patients received BTKi-containing therapy, 4 of whom had chronic HBV infection (HBsAg positive), 26 with resolved HBV infection (HBsAg negative and anti-HBc positive) and 25 without HBV infection (Figure 1; Table 1). Median age was 58 years, ranging from 22 years to 78 years. 34 (61.8%) patients were male. 49 (89.1%) cases were non-germinal center B-cell subtype. 38 (69.1%) patients received Ibrutinib therapy, while 17 (30.9%) received zanubrutinib. The median time on BTKi was 3 months (range: 1-24 months). All 4 patients with chronic HBV infection received entecavir as prophylaxis before using BTKi. Among patients with resolved HBV infection group, 9 (34.6%) received entecavir as routine prophylaxis, while 17 patients did not use any NAT strategy. The demographic and clinical characteristics between patients in chronic HBV infection group, resolved HBV infection group and without HBV group were similar. Baseline demographics and disease characteristics for the analysis population were shown in Figure 1 and Table 1.
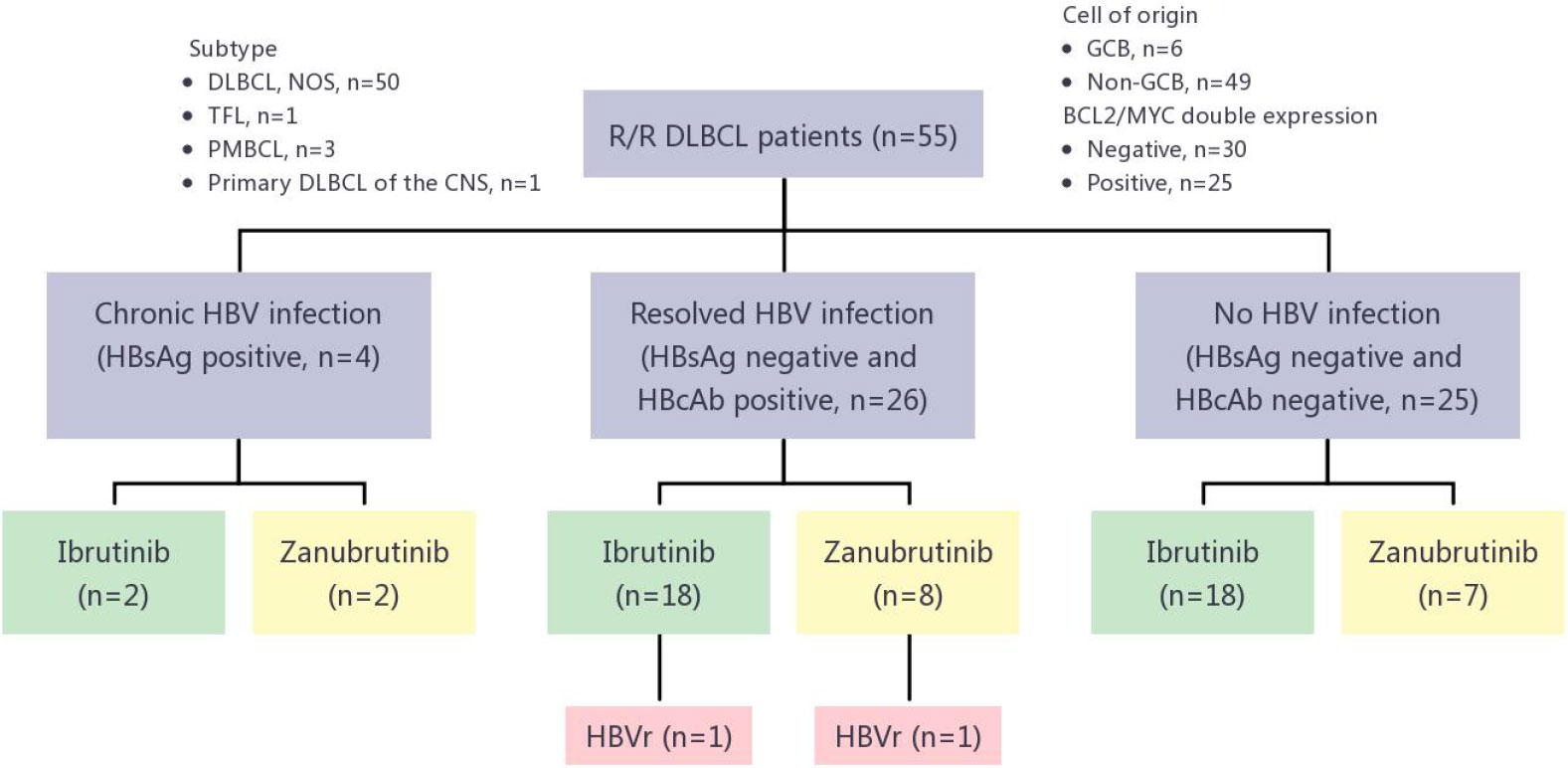
Figure 1 HBVr in R/R DLBCL patients treated with different BTKis. R/R DLBCL, relapsed or refractory diffuse large B-cell lymphoma; DLBCL, NOS, diffuse large B-cell lymphoma, not otherwise specified; TFL, transformed follicular lymphoma; PMBCL, primary mediastinal large B-cell lymphoma; CNS, central nervous system; GCB, germinal center B-cell–like; HBV, hepatitis B virus; HbsAg, hepatitis B surface antigen; HbcAg, hepatitis B core antibody; HBVr, hepatitis B virus reactivation; BTKis, Bruton tyrosine kinase inhibitors.
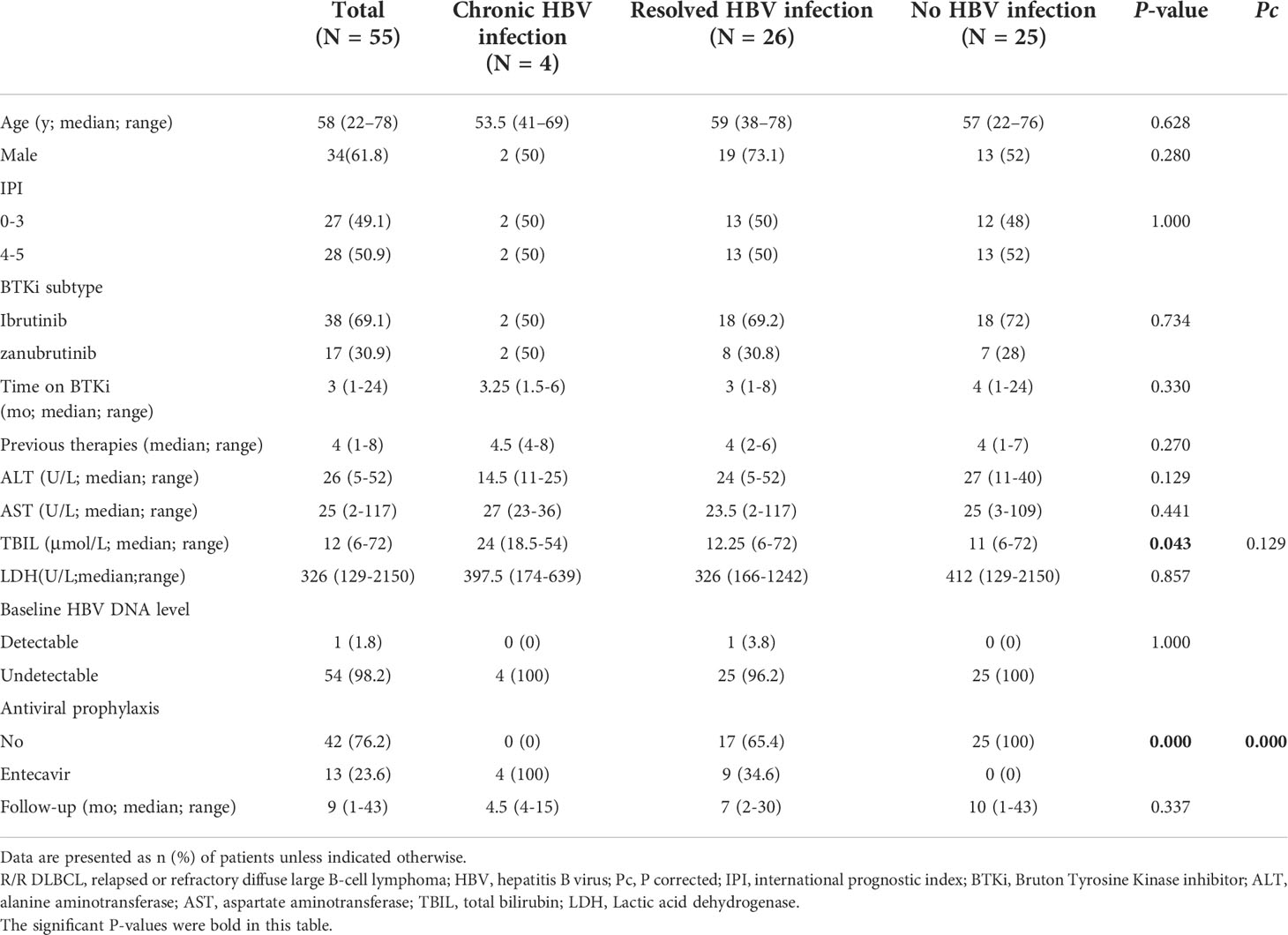
Table 1 Demographics and clinical characteristics of R/R DLBCL patients enrolled in the study (N = 55) .
Of the 26 patients with resolved HBV infection, 2 (7.69%, 95%CI, 0.9-25.1%) had HBVr (Figure 1). Except for these 2 patients, no patient in either chronic HBV infection group (0/4) or without HBV infection group (0/25) developed HBVr. Details for 2 patients with HBVr induced by BTKi were shown in Table 2 and Figure 2.
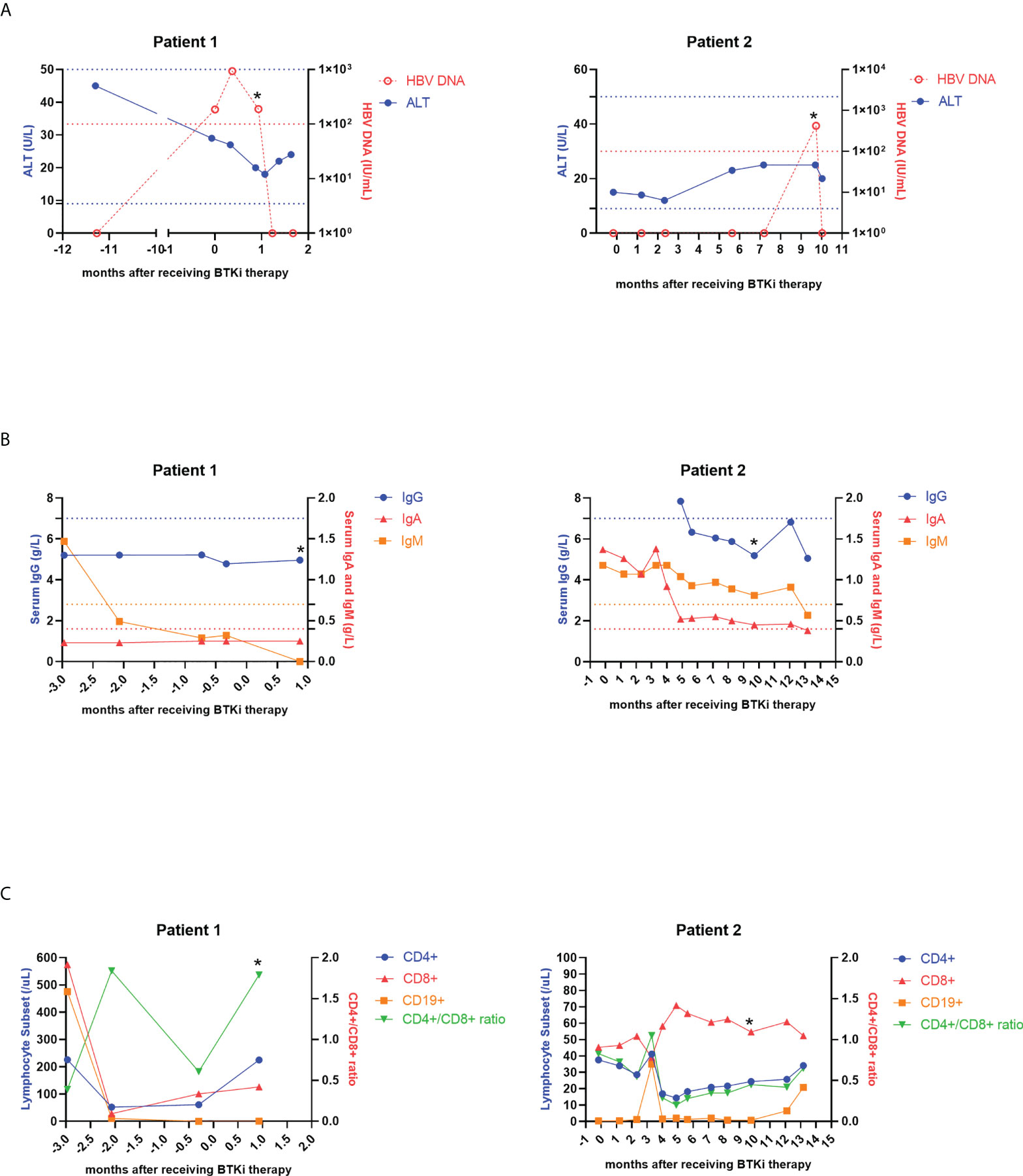
Figure 2 Dynamic changes of ALT, HBV DNA copies (A), serum levels of IgG, IgA, IgM (B) and lymphocyte subsets (C) in two patients with HBVr. An asterisk (*) indicates HBVr. ALT, alanine aminotransferase; HBV, hepatitis B virus; BTKi, Bruton Tyrosine Kinase inhibitor; Ig, immunoglobulin; HBVr, hepatitis B virus reactivation.
Patient 1 was a 58-year-old male, in the advanced stage four, and had received 6 lines of treatments before ibrutinib therapy. Among these 6 regimens, the first two were rituximab-based. The seventh line therapeutic regimen was ibrutinib and rituximab combined with chemotherapy consisting of ifosfamide, cisplatin and etoposide, in which only ibrutinib is the new added agent. At baseline, HbsAg, HbeAg, HbeAb were negative, but HbsAb and HbcAb were positive. HBV-DNA at baseline was 1.86×102IU/mL. He used entecavir as antiviral prophylaxis. After receiving ibrutinib-combined therapy 26 days, the patient’s HBsAg was subsequently reversed from negative to positive. Thus, he was dignosed with HBVr and ibrutinib was stopped. ALT, AST and TBIL were all normal both before using ibrutinib and at diagnosis of HBVr. No HBV-related hepatitis occurred. HBV DNA viral load at HBVr was 1.89×102IU/mL, not elevated compared to baseline (Figure 2A). Besides, immunoglobulins and lymphocyte subsets at HBVr were not declined (Figures 2B, C). Patient 1 achieved partial remission (PR) after seventh line’s treatment, but died 3 months later.
Patient 2 was a 49-year-old male, also in the advanced stage four, and had received 4 lines of treatments previous to receiving zanubrutinib therapy. The first three were rituximab-based, and the fifth regimen was zanubrutinib combined with ESHAP, in which only zanubrutinib and cytarabine are new. At baseline, the patient was HBcAb+/HBsAb−, and HBV DNA cannot be detectable. He also used entecavir as prophylactic anti-virus drug. The patient received zanubrutinib for 2.5 months. Approximately 7.5 months after zanubrutinib therapy ended, HBV DNA of patient 2 elevated to 4.21×102 IU/ml. Then we confirmed that he had developed HBVr. Hepatitis flare was also not observed in patient 2 (Figure 2A). Serum IgG, IgA and IgM concentrations decreased when HBV was reactivated, but lymphocyte concentrations did not change (Figures 2B, C). This patient also achieved PR, however, disease progressed 6 months later.
Univariate Cox regression analysis indicated no association between HBVr risk and sex, age, previous therapies, type of BTKi, HBsAb at baseline and HBeAb at baseline (Table 3). Given no independent factor significantly associated with the risk of HBVr in univariate analysis, we did not conduct multivariate analysis furthermore.
As shown in Table 4, 9 patients achieved complete response and 30 patients achieved PR. Overall response rate was 70.9%. The number of patients that achieved complete response in the chronic HBVr group, resolved HBV infection group and no HBV infection group were 1 (25%), 2 (7.7%) and 6 (24%), respectively. Response rates were similar across the 3 data sets (P=0.281).
Median OS was not reached based on the Kaplan-Meier analysis. 1-year OS rate was 80.0%. Although a higher OS rate among patients in the no HBV infection group was observed, the survival evaluated was similar (P=0.5031) (Figure 3A). PFS was consistently poor in R/R DLBCL patients, with a median PFS of 4 months (95% CI, 3-5 months). PFS rates were also similar across 3 subgroups (P=0.9758) (Figure 3B).
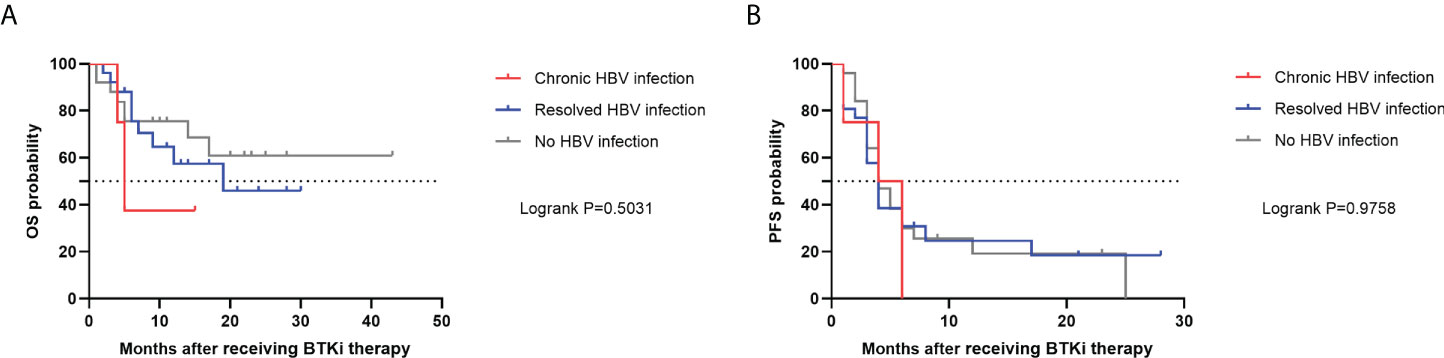
Figure 3 OS (A) and PFS (B) in R/R DLBCL patients after receiving BTKi therapy. OS, overall survival; PFS, progression-free survival; HBV, hepatitis B virus; BTKi, Bruton tyrosine kinase inhibitor; R/R DLBCL, relapsed or refractory diffuse large B-cell lymphoma.
We ran univariate and multivariate Cox regression models to analyze factors influencing the patients’ mortality (Supplementary Table 1). International prognostic index high risk and Age ≤ 60 years were associated with inferior survival (P=0.007 and P=0.033, respectively), whereas sex, HBVr, and HBV infection did not influence OS.
This is the first systematic analysis of HBVr rate and BTKis’ efficacy in R/R DLBCL patients with HBV infection receiving BTKi-containing therapy. The study showed that patients with R/R DLBCL and concomitant past HBV infection do have a moderate risk of HBVr after BTKis therapy as HBVr incidence was 7.69% (95%CI, 0.9-25.1%) in our cohort. Rohit Loomba et al. summarized different classes of biologics associated with HBVr, also considering BTKi is at a moderate-risk (7). Although ibrutinib or zanubrutinib administration has a potential HBVr complication, there was no episode of HBV-hepatitis with a prophylactic NAT, and HBV infection might not affect response rates or survival among R/R DLBCL patients post BTKis treatment. In the era of targeted therapy for hematologic malignancies, it is vital for us to better estimate the risk of HBVr and optimize targeted therapeutic strategies.
In 2015, Patrick de Jésus Ngoma et al. firstly reported a case of HBVr in a 80 years old chronic lymphocytic leukaemia (CLL) patient receiving ibrutinib, revealing that in addition to hemocytopenia and some nonhematologic adverse events such as diarrhea, upper respiratory tract infection, and fatigue, ibrutinib also has a potential risk of reactivating virus (23). With widespread use in a real-world population, a few case reports and case series have reported the HBVr complication of ibrutinib administration in patients with CLL and marginal zone lymphoma (MZL) (24–27). Besides the first-generation BTKi ibrutinib, one of second-generation BTKis zanubrutinib, an highly selective BTKi, also has this infectious diseases adverse event in CLL patients (28). Herein, our study further elucidates that ibrutinib and zanubrutinib could also pose HBVr in R/R DLBCL, revealing that BTKis’ induced HBVr would not only occured in patients with indolent lymphoma, but also in aggressive lymphoma, but the incidence is slightly lower than in CLL. However, given that the size of reported samples were all small, the results must be considered preliminary and should be verified furthermore.
Of 2 patients with HBVr in our study, one case occurred 1 month after receiving BTKi, while HBVr time of another case is almost 7.5 months after BTKi therapy was ended. This phenomenon implies that similar to rituximab and PD-1, the effect of BTKis on viral reactivation would also persist beyond treatment period (29, 30). Thus, longer-term antiviral treatment is also necessary, even after cessation of BTKi treatment. High baseline HBV DNA level is a key risk factor for HBVr, and rituximab may facilitate HBV replication (3). In our study, only one case had high baseline HBV DNA level, who rapidly developed HBVr after only 1 month use of ibrutinib. However, in Cox regression analyses of our cohort, we failed to identify any factor significantly contributed to HBVr, probably because that number of HBVr cases in our study is limited. Of note, the first patient received ibrutinib therapy as the most recent and only new added agent, then HBVr occurred subsequently, supporting that BTKi do have a risk of reactivating HBV. For the second patient, the interval between the last use of rituximab and onset of HBVr was longer than 1 year, suggesting that rituximab is a less likely contributor to the HBVr. In addition, Kusumoto et al’s report about GOYA and GALLIUM demonstrated that the incidence of rituximab- or obinutuzumab-associated HBV reactivation in patients with resolved HBV infection during prophylactic NAT was only 2.1% (3). In view of the B-cell signaling inhibitory activity of BTKis, which might be more potent than rituximab in suppressing B-cells, it should be recognized that BTKis would probably increase the incidence of virus reactivation compared to rituximab.
The mechanisms underlying the increased risk of HBVr reactivation are pivotal but remain unclear. B cell dysfunction and possible T cell dysfunction might be the main cause (31, 32). Loss of BTK function results in a B-cell-dysfunction phenotype with decreased serum immunoglobulin levels and an increased predisposition to infections (33, 34). Of note, first-generation BTKi ibrutinib has off-target effects, inhibiting other tyrosine kinases, such as epidermal growth factor receptor (EGFR), JAK3, TEC, interleukin-2-inducible kinase (ITK), etc., and interacting with other important signaling pathway proteins, which might contribute to an increased risk of adverse events (35, 36). Among these kinases, ITK plays a critical role in T-cell signaling and immune regulation function. Occurrence of HBVr induced by ibrutinib might be associated with the inhibition of ITK, which results in subverting Th2 immunity and potentiating Th1-based immune responses (35, 37). In our research, one patient’s immunoglobulins decreased when HBVr occurred, indicating that humoral immunity might be inhibited by BTKi through interfering with the downstream pathways of BCR signaling. However, we need to knowledge that all patients enrolled in our study are R/R DLBCL who have received multiple lines of treatments. Thus, immunosuppressive effects of upfront and concomitant drugs could not be ignored. Different to immunoglobulins, neither of their lymphocyte concentrations changed. As the number of patients with HBVr in our study was small, the exact associations between HBVr and possible factors could hardly be analyzed. It is possible that with longer follow-up and larger cohort, the reactivation rate will be higher and more outcomes would be observed.
It has been known that PFS and survival between advanced B-cell cancers patients with chronic HBV infection cohort, with resolved HBV infection cohort and without HBV infection cohort post chimeric antigen receptor T cell therapy were similar (38, 39). In our study, response and PFS rates across above 3 data sets post BTKi therapy were also similar. However, though not statistically significant, R/R DLBCL patients with chronic HBV infection seemed to have a poorer prognosis. Additional studies are needed to explain above phenomenon.
This is the first and largest research to evaluate the HBVr incidence induced by BTKis and the efficacy of BTKis in HBV-associated R/R DLBCL patients. We retrospectively collected R/R DLBCL cases receiving BTKis therapy in Tongji hospital of Tongji university, finding that HBVr rate in patients with resolved HBV infection was 7.69% (95% CI, 0.9-25.1%). Regardless of with HBV or not, response rates and survival were similar. Our research could help physicians to better select R/R DLBCL patients suitable for such a targeted treatment, and provide the deeper and comprehensive understanding that BTKis could induce HBVr in different types of B-cell NHL.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Hospital of Tongji University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YN, LG, WQ, AL and PL designed the study and interpreted the data. YN performed the statistical analysis and wrote the manuscript. YN, LG, YL and SY contributed to data collection. All authors recruited patients. All authors contributed to the article and approved the submitted version.
This work was supported by funds from the National Natural Science Foundation of China (Nos. 81830004 and 82070168), Translational Research Grant of NCRCH (2020ZKZC04), the Ministry of Science and Technology of China (2021YFA1100800) and Shanghai Municipal Health Commission (2020CXJQ02).
We thank all people for their valuable help regarding study design and data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.982346/full#supplementary-material
1. Ren W, Ye X, Su H, Li W, Liu D, Pirmoradian M, et al. Genetic landscape of hepatitis b virus-associated diffuse Large b-cell lymphoma. Blood (2018) 131:2670–81. doi: 10.1182/blood-2017-11-817601
2. Gonzalez SA, Perrillo RP. Hepatitis b virus reactivation in the setting of cancer chemotherapy and other immunosuppressive drug therapy. Clin Infect Dis (2016) 62:S306–13. doi: 10.1093/cid/ciw043
3. Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, et al. Risk of HBV reactivation in patients with b-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood (2019) 133:137–46. doi: 10.1182/blood-2018-04-848044
4. Chang C, Tsai C, Yan S. Hepatitis b reactivation in patients receiving targeted therapies. Hematology (2017) 22:592–98. doi: 10.1080/10245332.2017.1321882
5. Yang C, Xie M, Zhang K, Liu H, Liang A, Young KH, et al. Risk of HBV reactivation post CD19-CAR-T cell therapy in DLBCL patients with concomitant chronic HBV infection. Leukemia (2020) 34:3055–59. doi: 10.1038/s41375-020-0913-y
6. Li P, Zhou L, Ye S, Zhang W, Wang J, Tang X, et al. Risk of HBV reactivation in patients with resolved HBV infection receiving anti-CD19 chimeric antigen receptor T cell therapy without antiviral prophylaxis. Front Immunol (2021) 12:638678. doi: 10.3389/fimmu.2021.638678
7. Loomba R, Liang TJ. Hepatitis b reactivation associated with immune suppressive and biological modifier therapies: Current concepts, management strategies, and future directions. Gastroenterology (2017) 152:1297–309. doi: 10.1053/j.gastro.2017.02.009
8. Ogawa E, Wei MT, Nguyen MH. Hepatitis b virus reactivation potentiated by biologics. Infect Dis Clin N Am (2020) 34:341–58. doi: 10.1016/j.idc.2020.02.009
9. Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting b cell receptor signaling with ibrutinib in diffuse Large b cell lymphoma. Nat Med (2015) 21:922–26. doi: 10.1038/nm.3884
10. Sauter CS, Matasar MJ, Schoder H, Devlin SM, Drullinsky P, Gerecitano J, et al. A phase 1 study of ibrutinib in combination with r-ICE in patients with relapsed or primary refractory DLBCL. Blood (2018) 131:1805–08. doi: 10.1182/blood-2017-08-802561
11. Goy A, Ramchandren R, Ghosh N, Munoz J, Morgan DS, Dang NH, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in Relapsed/Refractory non-germinal center b-cell-like DLBCL. Blood (2019) 134:1024–36. doi: 10.1182/blood.2018891598
12. Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton’S tyrosine kinase in cancers: Drug development advances. Leukemia (2021) 35:312–32. doi: 10.1038/s41375-020-01072-6
13. Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma (2013) 54:2385–91. doi: 10.3109/10428194.2013.777837
14. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in b-cell malignancies and safety and efficacy evaluation in CLL. Blood (2019) 134:851–59. doi: 10.1182/blood.2019001160
15. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
16. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol (2007) 25:579–86. doi: 10.1200/JCO.2006.09.2403
17. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse Large b-cell lymphoma: Results from the international SCHOLAR-1 study. Blood (2017) 130:1800–08. doi: 10.1182/blood-2017-03-769620
18. Pauly MP, Tucker L, Szpakowski J, Ready JB, Baer D, Hwang J, et al. Incidence of hepatitis b virus reactivation and hepatotoxicity in patients receiving long-term treatment with tumor necrosis factor antagonists. Clin Gastroenterol H (2018) 16:1964–73. doi: 10.1016/j.cgh.2018.04.033
19. Terrault NA, Lok ASF, McMahon BJ, Chang K, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis b: AASLD 2018 hepatitis b guidance. Hepatology (2018) 67:1560–99. doi: 10.1002/hep.29800
20. Yeo W, Chan TC, Leung NWY, Lam WY, Mo FKF, Chu MT, et al. Hepatitis b virus reactivation in lymphoma patients with prior resolved hepatitis b undergoing anticancer therapy with or without rituximab. J Clin Oncol (2009) 27:605–11. doi: 10.1200/JCO.2008.18.0182
21. Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, et al. Entecavir vs lamivudine for prevention of hepatitis b virus reactivation among patients with untreated diffuse Large b-cell lymphoma receiving r-CHOP chemotherapy. JAMA (2014) 312:2521. doi: 10.1001/jama.2014.15704
22. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The lugano classification. J Clin Oncol (2014) 32:3059–68. doi: 10.1200/JCO.2013.54.8800
23. de Jesus NP, Kabamba B, Dahlqvist G, Sempoux C, Lanthier N, Shindano T, et al. Occult HBV reactivation induced by ibrutinib treatment: A case report. Acta Gastroenterol Belg (2015) 78:424–26.
24. Herishanu Y, Katchman H, Polliack A. Severe hepatitis b virus reactivation related to ibrutinib monotherapy. Ann Hematol (2017) 96:689–90. doi: 10.1007/s00277-016-2917-2
25. Hammond SP, Chen K, Pandit A, Davids MS, Issa NC, Marty FM. Risk of hepatitis b virus reactivation in patients treated with ibrutinib. Blood (2018) 131:1987–89. doi: 10.1182/blood-2018-01-826495
26. Innocenti I, Morelli F, Autore F, Corbingi A, Pasquale R, Sora F, et al. HBV reactivation in CLL patients with occult HBV infection treated with ibrutinib without viral prophylaxis. Leuk Lymphoma (2019) 60:1340–42. doi: 10.1080/10428194.2018.1523401
27. Malek AE, Nieto Y, Szvalb AD, Siddiqui S, Shafi MA, Hwang JP, et al. Hepatitis b virus-associated liver failure in a patient with b-cell non-Hodgkin lymphoma after anti-cancer therapy including ibrutinib. Clin Lymphoma Myeloma Leukemia (2020) 20:e124–27. doi: 10.1016/j.clml.2019.12.006
28. Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of Relapsed/Refractory chronic lymphocytic Leukemia/Small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: Phase 2, single-arm, multicenter study. J Hematol Oncol (2020) 13:48. doi: 10.1186/s13045-020-00884-4
29. Tsai Y, Yang C, Du J, Lin M, Tang S, Wang H, et al. Rituximab increases the risk of hepatitis b virus reactivation in non-Hodgkin lymphoma patients who are hepatitis b surface antigen-positive or have resolved hepatitis b virus infection in a real-world setting: A retrospective study. Peerj (2019) 7:e7481. doi: 10.7717/peerj.7481
30. Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, et al. Hepatitis b virus reactivation in cancer patients with positive hepatitis b surface antigen undergoing PD-1 inhibition. J Immunother Cancer (2019) 7:322. doi: 10.1186/s40425-019-0808-5
31. Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood Rev (2018) 32:387–99. doi: 10.1016/j.blre.2018.03.004
32. Niemann CU, Herman SEM, Maric I, Gomez-Rodriguez J, Biancotto A, Chang BY, et al. Disruption of in vivo chronic lymphocytic leukemia tumor–microenvironment interactions by ibrutinib–findings from an investigator-initiated phase II study. Clin Cancer Res (2016) 22:1572–82. doi: 10.1158/1078-0432.CCR-15-1965
33. Byrd JC, Harrington B O, Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med (2016) 374:323–32. doi: 10.1056/NEJMoa1509981
34. Rawlings DJ, Saffran DC, Jenkins NA, Witte ON, Tsukada S, Largaespada DA, et al. Mutation of unique region of bruton’s tyrosine kinase in immunodeficient XID mice. Science (1993) 261:358–61. doi: 10.1126/science.8332901
35. Berglof A, Hamasy A, Meinke S, Palma M, Krstic A, Mansson R, et al. Targets for ibrutinib beyond b cell malignancies. Scand J Immunol (2015) 82:208–17. doi: 10.1111/sji.12333
36. McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-akt signaling. Blood (2014) 124:3829–30. doi: 10.1182/blood-2014-10-604272
37. Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood (2013) 122:2539–49. doi: 10.1182/blood-2013-06-507947
38. Wang Y, Liu Y, Tan X, Pan B, Ge J, Qi K, et al. Safety and efficacy of chimeric antigen receptor (CAR)-t-cell therapy in persons with advanced b-cell cancers and hepatitis b virus-infection. Leukemia (2020) 34:2704–07. doi: 10.1038/s41375-020-0936-4
Keywords: HBV reactivation, diffuse large B-cell lymphoma, Bruton tyrosine kinase inhibitor, resolved HBV infection, prognosis
Citation: Ni Y, Gao L, Lu Y, Ye S, Zhou L, Qian W, Liang A and Li P (2022) Risk of HBV reactivation in relapsed or refractory diffuse large B-cell lymphoma patients receiving Bruton tyrosine kinase inhibitors therapy. Front. Immunol. 13:982346. doi: 10.3389/fimmu.2022.982346
Received: 30 June 2022; Accepted: 11 August 2022;
Published: 31 August 2022.
Edited by:
Nirupma Trehanpati, The Institute of Liver and Biliary Sciences (ILBS), IndiaReviewed by:
Ting Yang, Fujian Medical University, ChinaCopyright © 2022 Ni, Gao, Lu, Ye, Zhou, Qian, Liang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, bGlseWZvcmV2ZXI3NkAxMjYuY29t; Aibin Liang, bGFiNzE4MkB0b25namkuZWR1LmNu; Wenbin Qian, cWlhbndiQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.