
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 12 September 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.978504
 Xinyu Wu1†
Xinyu Wu1† Dong Liu1†
Dong Liu1† Yanfei Li2
Yanfei Li2 Ya Xie1
Ya Xie1 Liudan Tu1
Liudan Tu1 Yanli Zhang1
Yanli Zhang1 Xi Zhang1
Xi Zhang1 Linkai Fang1
Linkai Fang1 Xiqing Luo1
Xiqing Luo1 Zhiming Lin1
Zhiming Lin1 Zetao Liao1
Zetao Liao1 Limin Rong3
Limin Rong3 Jie Ren4
Jie Ren4 Yuqi Zhou5
Yuqi Zhou5 Niansheng Yang6
Niansheng Yang6 Jian Xu7
Jian Xu7 Hua Zhang8
Hua Zhang8 Baijie Xu9
Baijie Xu9 Zhenbiao Wu10
Zhenbiao Wu10 Feng Zhan11
Feng Zhan11 Zhenbin Li12
Zhenbin Li12 Weiguo Xiao13
Weiguo Xiao13 Shengyun Liu14
Shengyun Liu14 Yi Zhou15
Yi Zhou15 Shanhui Ye16
Shanhui Ye16 Qing Lv17
Qing Lv17 Lijun Zhang18
Lijun Zhang18 Dongbao Zhao19
Dongbao Zhao19 Shanzhi He20
Shanzhi He20 Like Zhao21
Like Zhao21 Lijun Wu22
Lijun Wu22 He Lin23
He Lin23 Yunxiao Zhu24
Yunxiao Zhu24 Donggeng Guo25
Donggeng Guo25 Zehong Yang26
Zehong Yang26 Budian Liu1
Budian Liu1 Kehu Yang2*
Kehu Yang2* Jieruo Gu1*
Jieruo Gu1*Objective: The aim of this review is to provide guidance on the selection of approaches to the screening and assessment of enthesitis in patients with spondyloarthritis (SpA).
Methods: Twenty-four questions regarding the approaches to the screening and assessment of enthesitis and the implementation details were devised, followed by a systemic literature review. The Grading of Recommendations Assessment, Development, and Evaluation methodology was employed in the development of this guideline, with modifications to evaluate non-interventional approaches under comprehensive consideration of costs, accessibility, and evidence strength. A consensus from the voting panel was required for the inclusion of the final recommendations and the strength of each recommendation.
Results: Seventeen recommendations (including five strong recommendations) were included in this guideline. The voting panel expressed unequivocal support for the necessity of screening and assessment of enthesitis in patients with SpA. It was agreed unanimously that symptom evaluation and physical examination should serve as the initial steps to the recognition of enthesitis, whereas Maastricht Ankylosing Spondylitis Enthesitis Score is a reliable tool in both clinical trials and daily medical practice. Ultrasound examination is another reliable tool, with power Doppler ultrasound as an informative addition. Notwithstanding its high resolution, MRI is limited by the costs and relatively low accessibility, whereas radiographs had low sensitivity and therefore should be rendered obsolete in the assessment of enthesitis. PET/CT was strongly opposed in the detection of enthesitis.
Conclusion: This guideline provides clinicians with information regarding the screening and assessment of enthesitis in patients with SpA. However, this guideline does not intend on dictating choices, and the ultimate decisions should be made in light of the actual circumstances of the facilities.
Enthesis refers to the anatomic interface between tendons, ligaments, capsules, fascia, and bones, whereas enthesitis refers to the inflammation at such insertion sites (1, 2). Entheses could be classified as fibrous entheses and fibrocartilaginous entheses (3). Enthesitis is considered the hallmark and characteristic feature of spondyloarthritis (SpA) (4). Persistent enthesitis often leads to regional structural damage such as tendon injuries and bone erosions, and the subsequent repair process could give rise to the formation of enthesophytes and ultimately functional impairment of related anatomic structures (5). Although the importance of enthesitis in the etiology of SpA has been widely acknowledged, it is often overlooked in the management of patients with SpA in clinical practice (6). This guideline is dedicated to the screening and diagnosis of enthesitis in the specific population of patients with SpA, aiming to improve the understanding and awareness of enthesitis detection in rheumatologists.
This guideline was developed using the Grading of Recommendations Assessment, Development, and Evaluation methodology to assess the quality of evidence and the levels of recommendations (7–9). The Core Team, Expert Panel, and Voting Panel generated 17 questions regarding the screening and diagnosis of enthesitis in patients with SpA and the corresponding approaches. The following methods to detect enthesitis were listed as potential approaches: history taking, clinical examination, ultrasound (US), MRI, X-ray, and PET-CT. A patient panel of five patients with SpA reviewed the evidence reports provided with an interpretation from a moderator and gave their personal perspectives. Systemic literature reviews (SLRs) and meta-analyses were conducted to address the questions. Search strategies and study inclusion processes could be found in Supplementary Appendixes 3 and 4.
We devised a framework dedicated to the evaluation of non-interventional approaches to the screening and diagnosis of SpA-related enthesitis. On the basis of the costs and accessibility, all the approaches were categorized as 1) inexpensive and easily accessible, 2) moderately costly and relatively accessible; and 3) expensive and difficult to gain access to. The strengths of each recommendation were classified as strong or conditional. A strong recommendation was given upon the consideration that the approach of screening and examining enthesitis could provide critical information that could educate and modify disease management options with relatively low costs and high accessibility. A conditional recommendation was given when moderate information could be gained with corresponding costs and accessibility. For approaches that are inexpensive and easily accessible, a strong recommendation was also given even in case of low certainty of evidence. For approaches that were moderately costly and relatively accessible, moderate certainty of evidence warranting its necessity was deemed sufficient to support a strong recommendation. For approaches that were expensive and rarely available, high certainty of evidence warrants a strong recommendation. Details of this framework could be seen in Supplementary Appendix 1.
Each question was rewritten into recommendation statement, which were sent to the Voting Panel along with the evidence reports. An online meeting was held, during which the Voting Panel reviewed the evidence reports and the recommendation statements and then voted for or against these recommendations. At least a consensus of 70% of the Voting Panel was required to determine the inclusion of the recommendations in this guideline.
This guideline only applies to patients with SpA, with or without symptomatic enthesitis. Enthesopathy resulting from aging, sports, or mechanical injuries was not addressed in this guideline.
A summary of the recommendations along with the certainty of evidence is presented in Table 1.
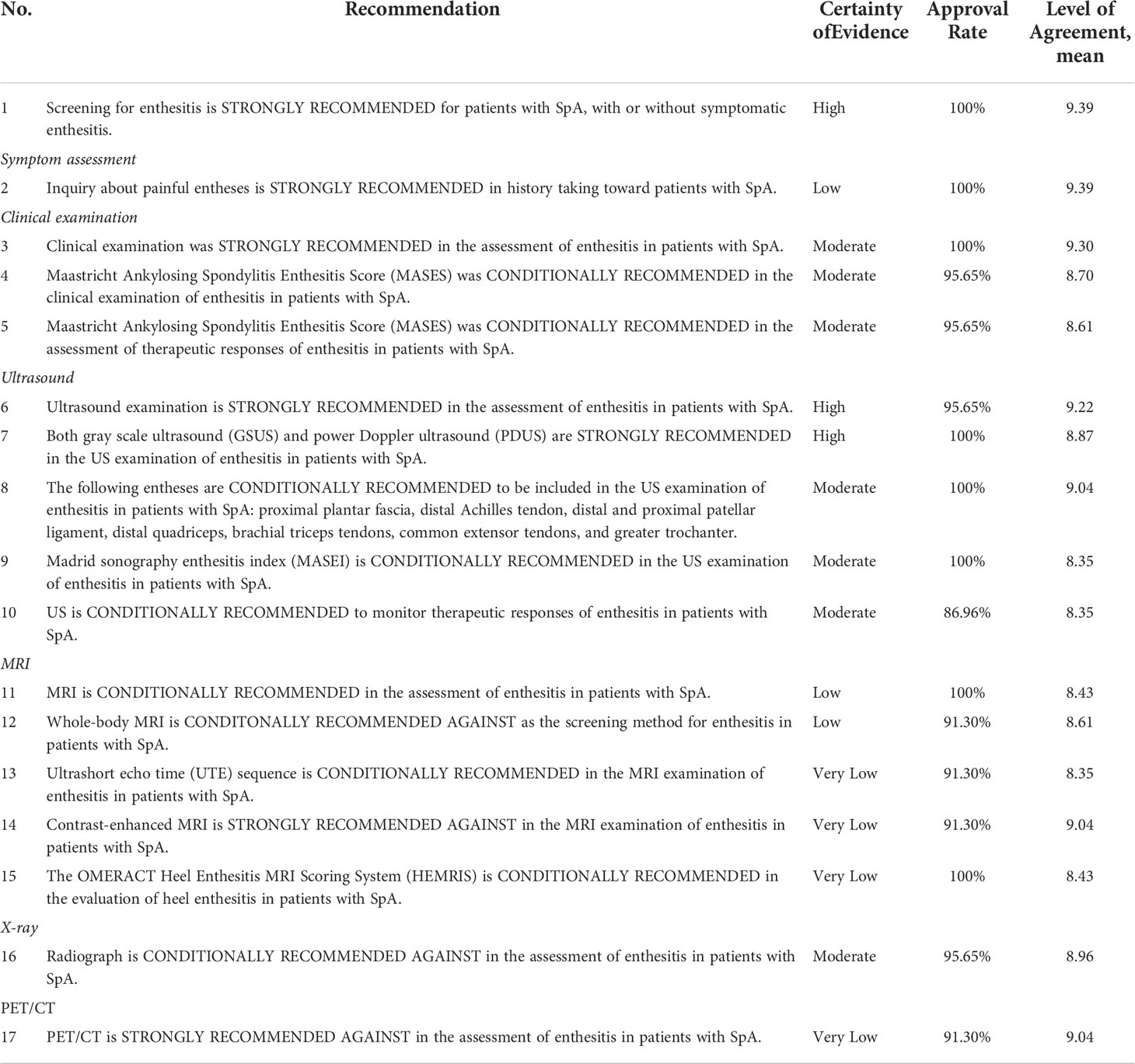
Table 1 Recommendations for approaches pertaining to the screening and evaluation of enthesitis in patients with SpA.
On the basis of questions 1–3 in the evidence report (Supplementary Appendix 6), there was sufficient evidence to support a strong recommendation for the screening for enthesitis in patients with SpA. This recommendation was formulated upon the following three aspects of consideration: 1) Evidence of enthesitis could facilitate the diagnosis of SpA. Enthesitis was listed as a SpA feature in the Assessment in SpondyloArthritis international Society (ASAS) classification criteria for SpA, and its capacity of facilitating an early diagnosis of SpA was confirmed in a prospective study by D’Agostino et al. using power Doppler US (PDUS) to detect enthesitis, even when stratifying patients with or without peripheral symptoms (10). For patients suspected with SpA, findings of enthesitis during the screening process could improve the certainty of diagnosis for clinicians. 2) Clinical enthesitis composed a proportion of the disease burden for patients with SpA. Presence of symptomatic enthesitis is associated with pain, worse quality of life, impaired ability of daily activities, and work capacity (11–14). Screening for enthesitis in this subset of patients could ascertain the presence of enthesitis, differentiating enthesitis from other pathological conditions in adjacent structures (15). 3) Presence of enthesitis is potentially related to severity of disease. Formation of peripheral enthesophytes was associated with the presence and number of axial syndesmophytes, according to the DEvenir des Spondyloarthrites Indifferenciees Recentes (DESIR) cohort and a case-control study by Aydin et al. (16, 17). This finding indicated that peripheral enthesitis could be a marker of disease severity of SpA, even in patients without symptomatic enthesitis.
Symptomatic enthesitis often presents pain at the entheses. Research indicated that there was a delayed diagnosis of approximately 8 years between the first onset of enthesitis symptoms and recognition of enthesitis (18). In clinical practice, asking patients about painful entheses during history taking could serve as the first steps toward recognition of enthesitis. A study showed that, in patients with SpA with long-term pain at the entheses, by using US as the reference standard, the sensitivity of history taking was 72%, whereas specificity was 63%. However, a large number of patients with SpA present asymptomatic enthesitis, whereas fibromyalgia was recognized in 45% of the patients with axial SpA, therefore complicating results of history taking (18). Painful entheses could not serve as confirming evidence of enthesitis, and further examination methods should be chosen on the basis of history taking so as to confirm the presence of enthesitis.
Clinical examination is the most common method in the assessment of enthesitis in patients with SpA, with the advantages of being simple and convenient to conduct and no need of examination equipment (19–21). However, clinical examination has a relatively low sensitivity compared with other imaging examinations, approximately 20% (Supplementary Appendix 6). Enthesitis could be identified in a large number of patients with SpA using imaging methods such as US or MRI, but tenderness could not be elicited during clinical examination (22, 23). On the other hand, tenderness at the entheses does not confirm the presence of enthesitis, because pain could be attributed to synovitis, arthritis, or other pathological conditions in adjacent structures (3). Therefore, clinical examination could only serve as a clue to the discovery of enthesitis, not confirming evidence of enthesitis. Considering that clinical examination is easy to operate in clinical practice, it is advised to apply clinical examination as an initial approach to the identification of enthesitis. After completion of clinical examination, US or MRI is advised as the subsequent examination approach.
Common scoring methods of enthesitis clinical examination include Mander Enthesitis Index (MEI) (21), Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) (19), Leeds Enthesitis Index (LEI) (24), Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index (20), Berlin Index (25), and Gladman Index (24). Among these scoring methods, MASES is considered the most convenient and therefore the most widely applied in clinical practice and clinical trials (26–29). MASES scores the following 13 entheses: first costochondral joint, seventh costochondral joint, posterior superior iliac spine, anterior superior iliac spine, iliac crest, fifth lumbar spinous process, and proximal insertion of Achilles tendon, with each entheses rated as 0 (no tenderness) or 1 (tenderness). It should be noted that, by limiting the number of entheses evaluations, tenderness at certain entheses might be overlooked, because studies showed that 21% of patients with MEI > 0 were rated as 0 with MASES (19). Of all the scoring methods, MASES is considered a relatively convenient and less time-consuming one (6). An overview of the scoring indices of enthesitis with physical examination is presented in Table 2. This guideline endorsed MASES in the screening and assessment of enthesitis in patients with SpA on the account of feasibility and conveniences.
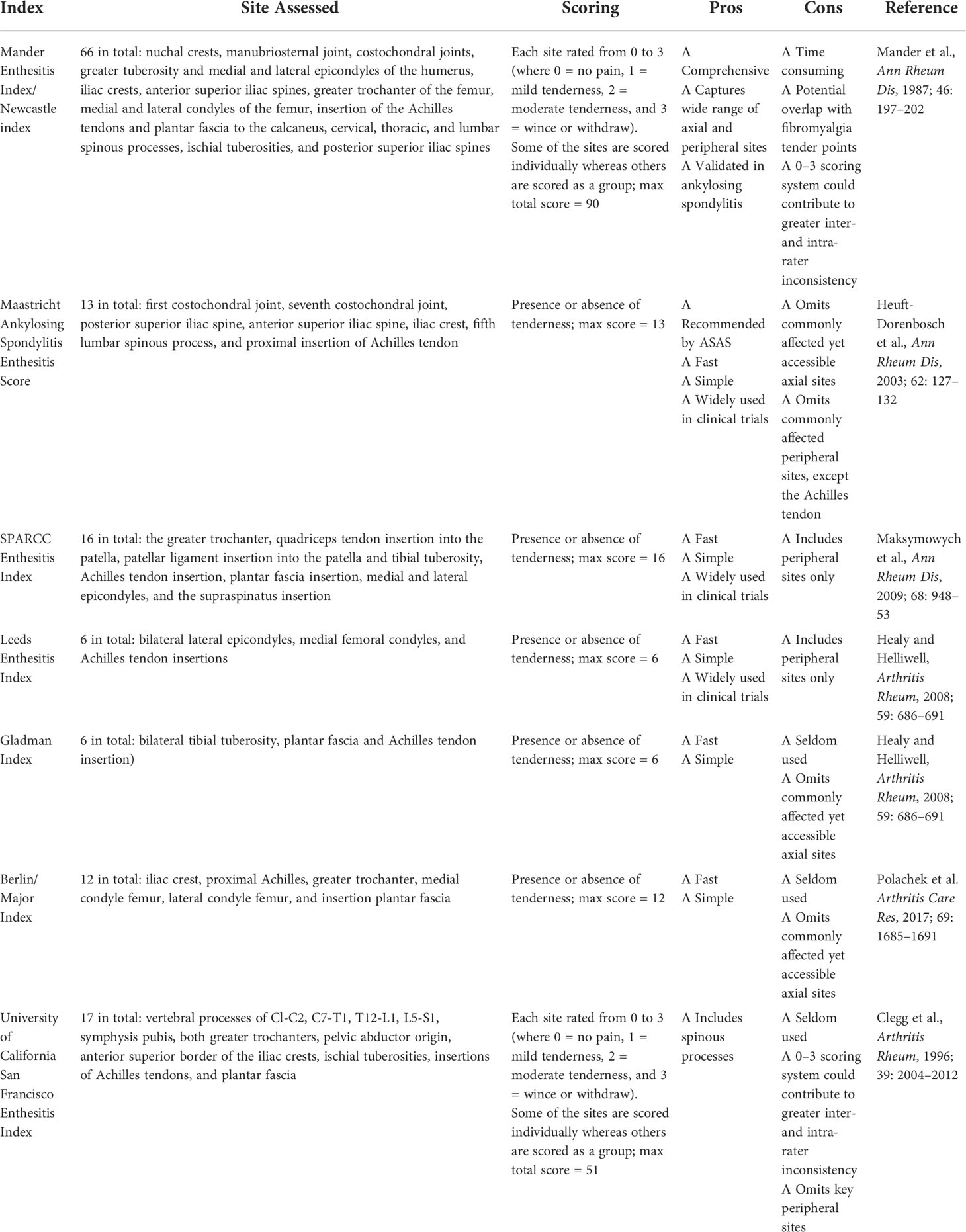
Table 2 Overview of physical examination systems used to assess enthesitis in patients with SpA (Ref. McGonagle et al., Semin Arthritis Rheum., 2021 Jul 9; 51(6): 1147–1161).
Multiple clinical trials employed indices such as MASES and MEI as the study endpoints. Results of the clinical trials showed that, after treatment of csDMARDs or bDMARDs, there was a significant decrease of the MEI score as many as four points, whereas MASES decreased by one to two points (24, 26–32). BE AGILE and BE ACTIVE trials employing MASES as one of the endpoints showed that bimekizumab could lower the MASES index up to three units within 6 months (33, 34). FUTURE 2 and 3 studies also used MASES as one of the endpoints, demonstrating that secukinumab could provide sustained resolution of enthesitis (35). MASES, MEI, SPARCC, and other indices listed above could serve as reliable tools in the assessment of enthesitis therapeutic responses, yet MASES is by far the most widely employed. Upon considerations that MASES is simpler and less time-consuming in the context of clinical practice, this guideline endorsed MASES as the assessment tool of therapeutic responses in patients with SpA. This recommendation does not dictate choices of the assessment tool, and other indices could also be considered.
Because US examination is relatively inexpensive and fairly accessible without exposure of radiation, US has become the first-line approach to assess enthesitis in recent years (1, 2). Compared with clinical examination and X-ray, US possessed a higher sensitivity in the detection of enthesitis, capable of detecting inflammatory lesions and structural lesions at the same time (22, 23, 36–38). Meta-analyses indicated that, compared with clinical examination, a significantly higher number of enthesitis lesions could be found using US (OR = 3.22, 95% CI [2.33, 4.45]) (Supplementary Appendix 6), suggesting that subclinical enthesitis is prevalent in patients with SpA. Systematic literature review showed that the sensitivity of US examination ranged from 50% to 90%, whereas specificity ranged from 60% to 90% (Supplementary Appendix 6). PDUS has a high specificity despite relatively low sensitivity (39, 40). It has been brought to attention that the diagnostic accuracy of US might depend on the individual experiences of the examiners (41, 42), but SLR showed that the inter-observer reliability of US was good with an ICC of 0.7–0.98 (Supplementary Appendix 6). Still, it is still advised to assign training sessions or workshops to the examiners in order to increase the diagnostic accuracy and consistency (41).
According to the OMERACT definitions, elementary lesions of enthesitis in US examinations include hypoechogenicity, increased thickness at enthesis, erosions, calcifications/enthesophytes, and Doppler signal at insertion (Table 3) (43).
Meta-analyses concluded that the occurrence rates of each elementary lesions were 15% for calcifications, 18% for erosions, 12% for enthesophytes, 10% for edema, 16% for thickening, 12% for bursitis, and 8% for PD signal (Supplementary Appendix 6).
Compared with color Doppler US (CDUS), PDUS has a much higher sensitivity because PDUS could detect small blood vessels or vessels with very slow blood flow (44). It should be noted that the vascularization of the entheses is mostly caused by small blood vessels, much to the advantage of PDUS (42). Therefore, PDUS is preferred rather than CDUS in the US examination of enthesitis.
According to an observational study in patients with SpA and healthy volunteers by D’agostino et al., enthesis vascularization was almost exclusive to patients with SpA, whereas PD signal was rarely observed at the entheses of healthy volunteers, suggesting that enthesis vascularization is an important sign in differential diagnosis (39). However, Feydy et al. and Kwiatkowska et al. held the opinion that the occurrence rates of enthesis vascularization were too low to be able to differentiate between healthy individuals and patients with SpA (45, 46). Meta-analyses showed that there was a significantly higher risk of enthesis vascularization in patients with SpA compared with controls (OR = 6.45, 95% CI [1.89, 22.04]) (Supplementary Appendix 6). Therefore, enthesis vascularization should be regarded as a specific sign of enthesitis in patients with SpA.
It has been reported that the occurrence rates of enthesitis at the lower limbs were higher than the upper limbs (10, 30, 39). According to the meta-analyses, occurrence rates of enthesitis at different entheses were as follows:lateral epicondyle of the elbow, 30% (95% CI [0.19, 0.43]); medial epicondyle of the elbow, 7% (95% CI [0.02, 0.21]); greater trochanter, 30% (95% CI [0.16, 0.48]); quadriceps tendons, 38% (95% CI [0.27, 0.50]); patellar ligament, 42% (95% CI [0.25, 0.62]); Achilles tendon, 39% (95% CI [0.24, 0.58]); and plantar fascia, 21% (95% CI [0.08, 0.45]) (Supplementary Appendix 6). Among them, proximal plantar fascia, distal Achilles tendon, distal and proximal patellar ligament, distal quadriceps, brachial triceps tendons, common extensor tendons, and greater trochanter were selected as the common sites of enthesitis, which should be included in the US examination of enthesitis in patients with SpA. It should be noted that it could be difficult to assess a clear Doppler signal at deeper enthesis such as the greater trochanter, with a certain risk of observing enthesopathic alterations.
The following positions should be taken during the US assessment:
Elbow entheses: Elbow flexed at 30°–45°.
Knee entheses: Patient lying in the supine position with the knee flexed at 30°.
Heel entheses: Patient lying prone with the feet hanging over the edge of the bed in a neutral position.
A number of scoring or grading systems have been developed aiming at the assessment of enthesitis. Common scoring or grading systems of enthesitis include Glasgow Ultrasound Enthesitis Scoring System (GUESS) (36), Sonographic Entheseal Index (SEI) (47), Madrid sonography enthesitis index (MASEI) (41), and D’Agostino Scoring System (39) (Table 4). Among them, GUESS evaluates five pairs of entheses at the lower limbs with gray scale ultrasound (GSUS) (36). SEI classified signs of acute injury and chronic lesion, respectively, at five pairs of entheses at the lower limbs with GSUS (47). MASEI scores enthesitis at proximal plantar fascia, distal Achilles tendon, distal and proximal patellar ligament, distal quadriceps, and brachial triceps tendons with GSUS and PDUS (41). D’Agostino Scoring System evaluates enthesitis based on presence or absence of enthesis vascularization, acute injury, and chronic lesions (39).
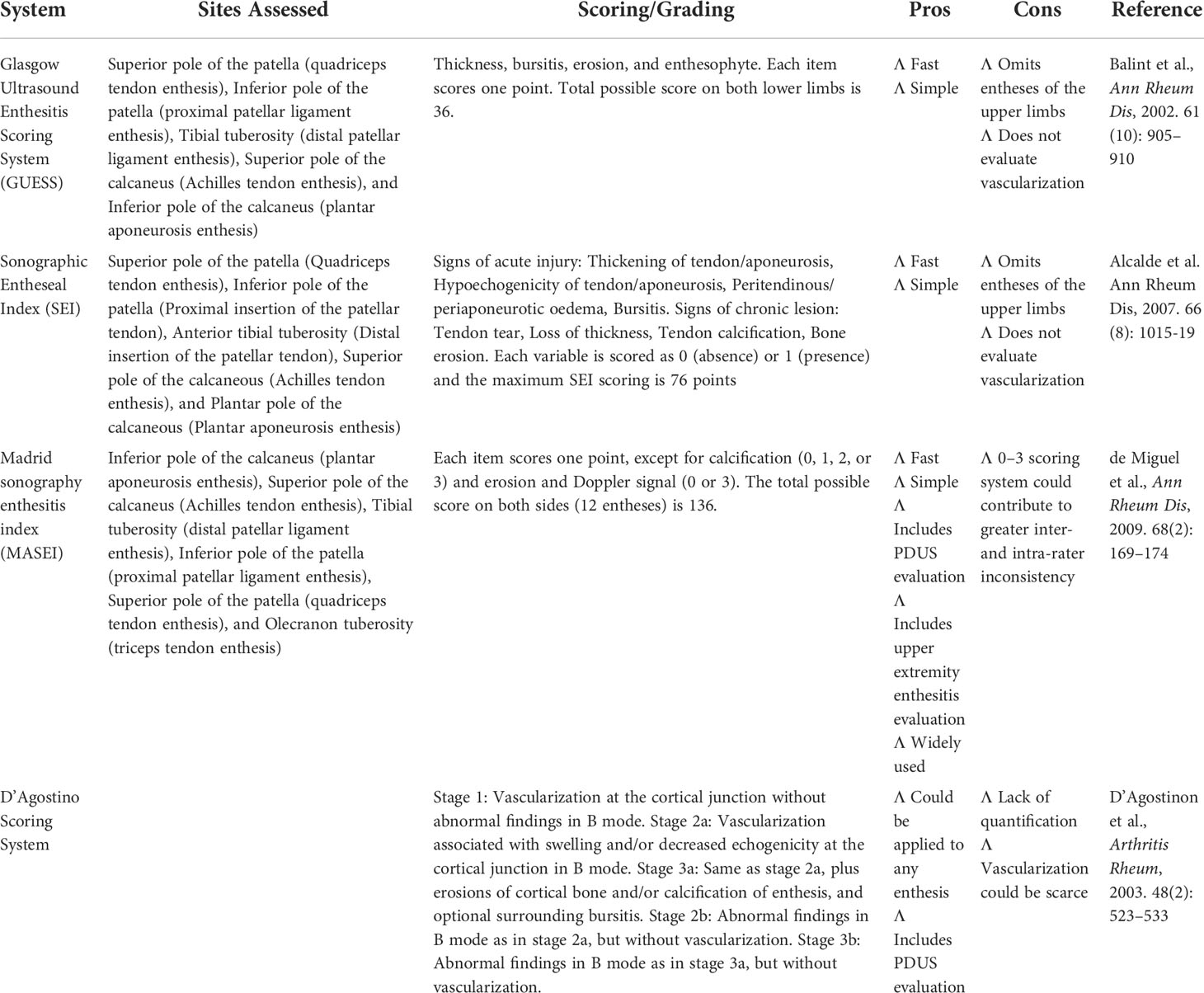
Table 4 Overview of common scoring or grading systems of enthesitis with ultrasound examinations in patients with SpA.
One study comparing different scoring or grading systems of enthesitis exhibited that there was no significant difference in the sensitivity and specificity between different systems (48). However, it has been perceived that MASEI could be superior given its the advantage of PDUS evaluation as well as the inclusion of entheses at the upper limbs (41, 48). This guideline conditionally recommended MASEI in the US assessment of enthesitis in patients with SpA, but other scoring systems are also viable options (Table 5).
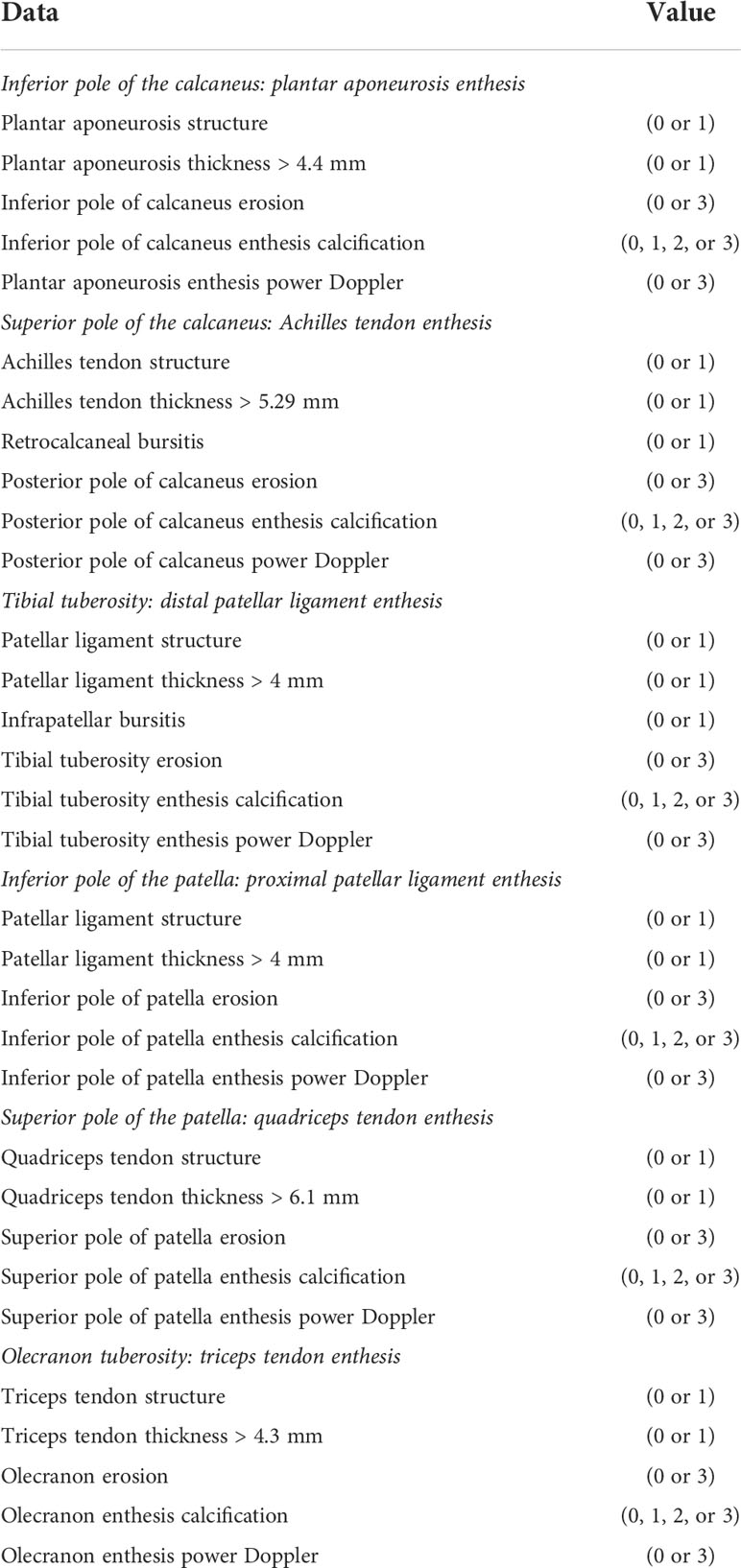
Table 5 Madrid Sonographic Enthesis Index (MASEI) (Ref. de Miguel et al., Ann Rheum Dis, 2009., 68(2): 169–74).
Each item scores one point, except for calcification (0, 1, 2, or 3) and erosion and Doppler signal (0 or 3). The total possible score on both sides (12 entheses) is 136.
Multiple clinical trials employing US as the assessment tool of enthesitis revealed that, after the medication of biologic DMARDs or conventional synthetic DMARDs, US scores decreased significantly (27, 30, 38, 40, 49–51). Aydin et al. and Wang et al. demonstrated that, after medication of tumor necrosis factor-α (TNF-α) inhibitors in patients with ankylosing spondylitis, both GSUS scores and total US scores decreased significantly, whereas there was no significant difference between different TNF-α inhibitors (49, 51). A study by Hartung et al. showed that PDUS scores were significantly lowered after patients with SpA were treated with csDMARDs and bDMARDs (27). Another study by Seven et al. demonstrated that enthesitis was not significantly improved by treatment, which could be attributed to the fact that this study mainly evaluated chronic lesions (30).
Most studies could not identify an association between disease activity indicators and US scores, suggesting that enthesitis might be an indicator independent from systemic inflammation (17, 40, 48, 51, 52).
Overall, US is a reliable tool in the assessment of therapeutic responses of enthesitis in patients with SpA. This guideline endorsed the application of US in the context of both clinical trials and clinical practice.
MRI is a reliable tool in the evaluation of enthesitis, capable of providing high-resolution evidence of tissue abnormalities at the entheses (23, 53, 54). High spatial resolution of MRI could help clinicians differentiate enthesitis from other conditions causing regional pain (15). Apart from the imaging of the inflammatory changes at the soft tissue adjacent to the entheses, MRI is currently the only modality able to present osteitis at the insertion sites (54, 55). Numerous studies have substantiated that sensitivity of MRI in the detection of enthesitis could parallel US examination, and the agreement between MRI and US was satisfactory (23, 56, 57). However, several disadvantages have limited the wide application of MRI in clinical scenarios. Compared with US, MRI is more costly and less available. Conventional MRI sequences could only image entheses at a specific location, incapable of getting the bigger picture, with the exception of whole-body MRI (58, 59). Moreover, the enthesis is mostly composed of tightly packed collagen fibers with little water accumulation, resulting in difficulties in imaging the entheses with conventional fat-saturated water-sensitive MRI sequences (60). With the recent developments of MRI imaging techniques, whole-body MRI and novel sequences such as UTE have shown great promise in the detection of enthesitis (59, 60). Therefore, this guideline conditionally recommended MRI examinations in patients with SpA whose US examinations are inconclusive.
Conventional MRI sequences could only image entheses at a specific location, whereas whole-body MRI could present inflammation at the axial skeleton as well as peripheral entheses (58, 59). By placing multiple coils throughout the body, whole-body MRI could complete scanning from head to toe in one single examination, without the need of repositioning (61, 62). Research showed that the overall readability of whole-body MRI was satisfactory, whereas the agreement between different observers was good (63, 64). OMERACT MRI group standardized the image acquisition and the scanning plane for evaluations in whole-body MRI in 2017 (65). MRI-Whole-Body Score for Inflammation in Peripheral Joints and Entheses in Inflammatory Arthritis (MRI-WIPE) scoring system was devised by Krabbe et al. for the assessment of peripheral joints and entheses in whole-body MRI (64). Credibility of whole-body MRI has been validated and supported by a number of clinical trials (66–69). On the basis of the current evidence of whole-body MRI, common sites of enthesitis included anterior chest wall, pelvis and lower limbs, notably sternoclavicular joint, acromioclavicular joint, ischial tuberosity, and Achilles tendon (70, 71). However, compared with conventional sequences, spatial resolution of whole-body MRI is much lower, especially at the entheses of the distal limbs, resulting in weak confidence of evaluations at these sites (70). Another weakness of whole-body MRI is that the scanning time is much longer, ranging from 40 min to 1 h, whereas reading and scoring of the whole-body MRI images take another hour or so, rendering the whole process time-consuming (63). This guideline conditionally recommended against using whole-body MRI as the routine examination for enthesitis in clinical practice. It is suggested that this modality should only be considered in clinical trials.
The transverse relaxation time (T2) is very short at locations such as tendons and entheses, hence the extremely low signal or no signal on images of conventional MRI sequences, especially T2 fat-saturated images (60). Only advanced enthesitis with very conspicuous edema could be observed on these images, whereas early phase of enthesitis is difficult to visualize and differentiate from normal tissue (72). The echo time of ultrashort echo time (UTE) sequence is much shorter than conventional sequences, enabling it to detect the signal emitted from the short T2 components at the entheses (72). On the basis of this rationale, the UTE spectroscopic imaging sequence, three-dimensional UTE cones sequence are developed, with quantifying measurement of Cones-T2* values and Cones-MTR, which could assist in the differentiation between early enthesitis and normal enthesis (60, 73). According to related studies, UTE sequence enables the clear visualization of entheseal structures, with display of the fibrocartilaginous components and collagenous components of the enthesis (60, 72, 74). However, availability limits the application of this sequence. This guideline conditionally recommended the use of UTE sequence in the MRI examination of enthesitis when UTE is available.
There is limited research on the diagnostic accuracy of contrast-enhanced MRI in the imaging of enthesitis. A few studies demonstrated that contrast-enhanced MRI could identify relatively more sites of enthesitis at lumbar vertebra and pelvis, yet the identification of enthesitis did not provide incremental diagnostic values to bone marrow edema (75, 76). At the peripheral entheses, the administration of contrast agents could help identify only a small number of extra enthesitic lesions, approximately 10% more (62). Conversely, injection of contrast agents exposed patients to threats of impaired renal function and allergies to contrast agents (77). In this case, benefits of contrast-enhanced MRI are outweighed by the potential risks, because contrast-enhanced MRI does not provide much additional information. Therefore, this guideline strongly recommended against contrast-enhanced MRI in the detection of enthesitis.
The Achilles tendon and plantar fascia are one of the most frequent sites of enthesitis in patients with SpA. By using the heel as the prototype, the OMERACT MRI group put forward the Heel Enthesitis MRI Scoring System (HEMRIS), which categorized the pathologies of enthesitis on MRI into inflammatory lesions and structural lesions (Table 6) (78, 79). This scoring system has been validated in patients with enthesitis at the Achilles tendon and plantar fascia and proved to be reliable (80). The inflammatory parameters evaluated intra-tendon hypersignal on T2w/short-tau inversion recovery (STIR) sequences, peri-tendon hypersignal, bone marrow edema, and bursitis, whereas structural lesions were defined as enthesophyte, bone erosion, and tendon thickening. STIR or T2 fat-saturated sequence is recommended in the assessment of inflammatory pathologies. T1-weighted images are recommended when evaluating structural lesions. However, no prospective study has employed this scoring system as the trial endpoints, so its capacity of monitoring disease modification or therapeutic responses was still inconclusive. HEMRIS is currently applied in the evaluation of heel, but its potential in evaluating other entheses awaits further exploring.
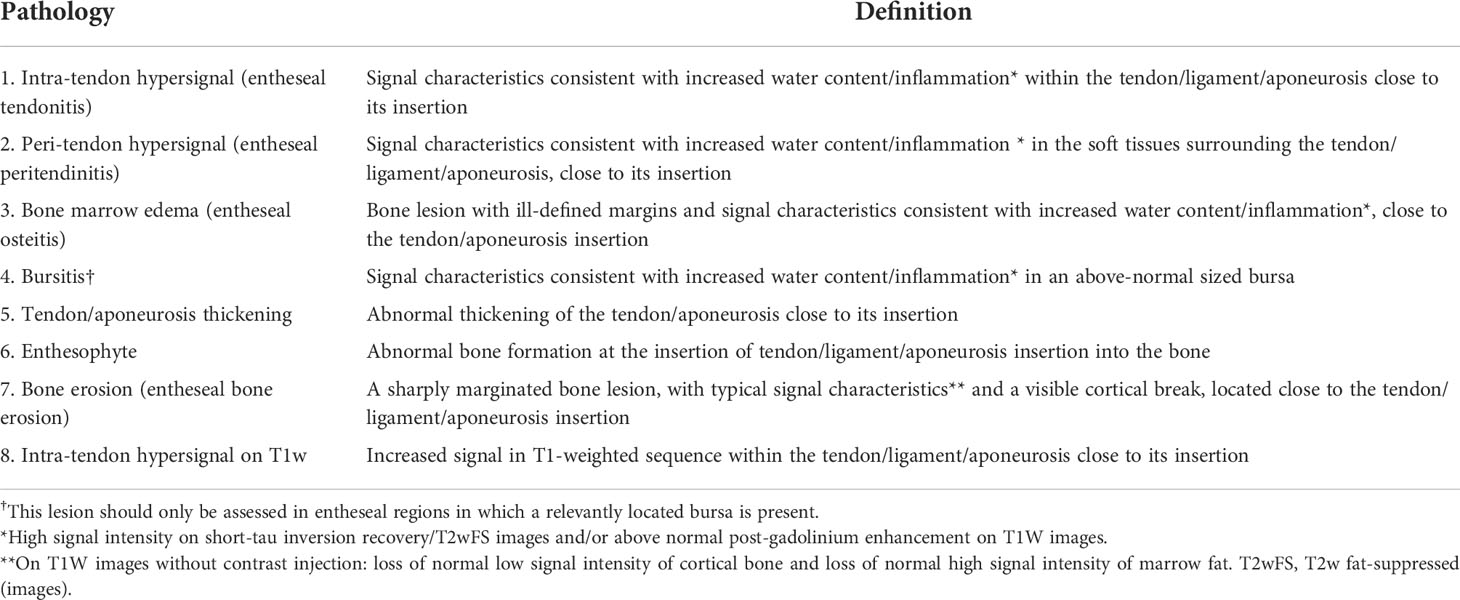
Table 6 Heel Enthesitis Scoring System (HEMRIS) (Ref. Mathew et al., J Rheumatol, 2019., 46(9): 1232–1238).
Radiograph is a viable tool in the detection of enthesitis, but it could only present chronic structural changes such as bone erosions and enthesophytes. Unlike US or MRI, it could not provide information of inflammatory lesions in the acute phase, such as edema and thickening at the enthesis or osteitis. On the other hand, the sensitivity of radiographs in the detection of bone erosions and enthesophytes was relatively low. Radiographs could not compete with US or MRI even in the detection of bone structures and enthesophytes. Only in patients with long-standing enthesitis who present conspicuous cortical bone changes or enthesophytes, radiograph is capable of imaging such changes. Considering that radiograph could provide only limited information about enthesitis, which can rarely instruct therapeutic decision-making but exposes patients to certain amount of radiation, radiograph is conditionally recommended against in this guideline.
It was hypothesized that, because the regional accumulation of 18F-FDG could reflect certain extent of tissue inflammation status, PET/CT could potentially be a promising tool in diagnosing enthesitis (80). However, studies showed that enthesitis as observed on MRI images was not correlated with the accumulation of 18F-FDG (80). Only the structural damage of the Achilles tendon was weakly correlated with the metabolic activity on PET/CT. On the basis of the current evidence, we do not consider PET/CT a reliable tool in the detection of enthesitis in patients with SpA. This is probably due to the relatively low vascularization of the enthesis, which fails to deliver the tracers to the enthesis. Considering that not only PET/CT is expensive and hardly available, but also the tracers of this examination expose patients to unwarranted radiation (81), this guideline strongly recommended against using PET/CT as the routine approach to the diagnosis of enthesitis in clinical practice.
JG, KY, and JR belonged on the core team and were in charge of the overall development of this guideline. XW, DL, YL, YX, LT, Yanli Zhang, XZ, LF, XL, Zhiming Lin, ZetL, and BL conducted the systematic literature review. LR, YuqZ, NY, JX, HZ, BX, ZW, FZ, ZhiL, WX, SL, YiZ, SY, QL, LJZ, DZ, SH, LKZ, LW, HL, DG, YunZ, and ZY served on the voting panel. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Gu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by the grants from the National Natural Science Foundation of China (81871294); the Science and Technology Planning Project of Guangdong Province, China (2019B030316004); Guangdong Clinical Research Center of Immune diseases (2020B1111170008).
We thank Joachim Sieper, David Yu, Buyun Yu, Xiaofeng Zeng, and James Zheng for providing counsel for this guideline. We thank Guangdong Provincial Clinical Studies Center for Immunological Diseases in organizing the online meetings and undertaking the administrative work of this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.978504/full#supplementary-material
1. Gandjbakhch F, Terslev L, Joshua F, Wakefield RJ, Naredo E. And D'Agostino M.A. ultrasound in the evaluation of enthesitis: status and perspectives. Arthritis Res Ther (2011) 13(6):R188. doi: 10.1186/ar3516
2. Terslev L, Naredo E, Iagnocco A, Balint PV, Wakefield RJ, Aegerter P, et al. Defining enthesitis in spondyloarthritis by ultrasound: Results of a Delphi process and of a reliability reading exercise. Arthritis Care Res (Hoboken) (2014) 66(5):741–8. doi: 10.1002/acr.22191
3. D'Agostino MA, Terslev L. Imaging evaluation of the entheses: Ultrasonography, MRI, and scoring of evaluation. Rheum Dis Clin North Am (2016) 42(4):679–93. doi: 10.1016/j.rdc.2016.07.012
4. Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European spondylarthropathy study group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum (1991) 34(10):1218–27.
5. McGonagle D, Marzo-Ortega H, O'Connor P, Gibbon W, Pease C, Reece R, et al. The role of biomechanical factors and HLA-B27 in magnetic resonance imaging-determined bone changes in plantar fascia enthesopathy. Arthritis Rheum (2002) 46(2):489–93. doi: 10.1002/art.10125
6. McGonagle D, Aydin SZ, Marzo-Ortega H, Eder L, Ciurtin C. Hidden in plain sight: Is there a crucial role for enthesitis assessment in the treatment and monitoring of axial spondyloarthritis? Semin Arthritis Rheum (2021) 51(6):1147–61. doi: 10.1016/j.semarthrit.2021.07.011
7. Alper BS, Oettgen P, Kunnamo I, Iorio A, Ansari MT, Murad MH, et al. Defining certainty of net benefit: A GRADE concept paper. BMJ Open (2019) 9(6):e027445. doi: 10.1136/bmjopen-2018-027445
8. Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE working group clarifies the construct of certainty of evidence. J Clin Epidemiol (2017) 87:4–13. doi: 10.1016/j.jclinepi.2017.05.006
9. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Bmj (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
10. D'Agostino MA, Aegerter P, Bechara K, Salliot C, Judet O, Chimenti MS, et al. How to diagnose spondyloarthritis early? accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis (2011) 70(8):1433–40. doi: 10.1136/ard.2010.138701
11. Laatiris A, Amine B, Ibn Yacoub Y, Hajjaj-Hassouni N. Enthesitis and its relationships with disease parameters in Moroccan patients with ankylosing spondylitis. Rheumatol Int (2012) 32(3):723–7. doi: 10.1007/s00296-010-1658-0
12. Mease PJ, Liu M, Rebello S, Hua W, McLean RR, Yi E, et al. Characterization of patients with axial spondyloarthritis by enthesitis presence: Data from the corrona psoriatic Arthritis/Spondyloarthritis registry. ACR Open Rheumatol (2020) 2(7):449–56. doi: 10.1002/acr2.11154
13. Strand V, Deodhar A, Conaghan PG, Gilloteau I, Massey O, Tian H, et al. Assessing the humanistic and economic burden of enthesitis among patients with peripheral and axial spondyloarthritis: Results from a multi-national real world survey database. Arthritis Rheumatol (2019) 71:1082–5. doi: 10.1002/art.41108
14. Sunar I, Ataman S, Nas K, Kilic E, Sargin B, Kasman SA, et al. Enthesitis and its relationship with disease activity, functional status, and quality of life in psoriatic arthritis: A multi-center study. Rheumatol Int (2020) 40(2):283–94. doi: 10.1007/s00296-019-04480-9
15. Aydin SZ, Tan AL, Hodsgon R, Grainger A, Emery P, Wakefield RJ, et al. Comparison of ultrasonography and magnetic resonance imaging for the assessment of clinically defined knee enthesitis in spondyloarthritis. Clin Exp Rheumatol (2013) 31(6):933–6.
16. Aydin SZ, Can M, Alibaz-Oner F, Keser G, Kurum E, Inal V, et al. A relationship between spinal new bone formation in ankylosing spondylitis and the sonographically determined Achilles tendon enthesophytes. Rheumatol Int (2016) 36(3):397–404. doi: 10.1007/s00296-015-3360-8
17. Ruyssen-Witrand A, Jamard B, Cantagrel A, Nigon D, Loeuille D, Degboe Y, et al. Relationships between ultrasound enthesitis, disease activity and axial radiographic structural changes in patients with early spondyloarthritis: Data from DEvenir des Spondyloarthrites Indifferenciees Recentes (DESIR) cohort. RMD Open (2017) 3(2):e000482. doi: 10.1136/rmdopen-2017-000482
18. Klauser AS, Wipfler E, Dejaco C, Moriggl B, Duftner C, Schirmer M. Diagnostic values of history and clinical examination to predict ultrasound signs of chronic and acute enthesitis. Clin Exp Rheumatol (2008) 26(4):548–53.
19. Heuft-Dorenbosch L, Spoorenberg A, Van Tubergen A, Landewé R, van der Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis (2003) 62(2):127–32. doi: 10.1136/ard.62.2.127
20. Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, et al. Development and validation of the spondyloarthritis research consortium of Canada (SPARCC) enthesitis index. Ann Rheum Dis (2009) 68(6):948–53. doi: 10.1136/ard.2007.084244
21. Mander M, Simpson JM, McLellan A. Studies with an enthesis index as a method of clinical assessment in ankylosing spondylitis. Ann Rheum Dis (1987) 46(3):197–202. doi: 10.1136/ard.46.3.197
22. Spadaro A, Iagnocco A, Perrotta FM, Modesti M, Scarno A, Valesini G. Clinical and ultrasonography assessment of peripheral enthesitis in ankylosing spondylitis. Rheumatol (Oxford) (2011) 50(11):2080–6. doi: 10.1093/rheumatology/ker284
23. Wiell C, Szkudlarek M, Hasselquist M, Møller JM, Nørregaard J, Terslev L, et al. Power Doppler ultrasonography of painful Achilles tendons and entheses in patients with and without spondyloarthropathy: A comparison with clinical examination and contrast-enhanced MRI. Clin Rheumatol (2013) 32(3):301–8. doi: 10.1007/s10067-012-2111-4
24. Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: Assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum (2008) 59(5):686–91. doi: 10.1002/art.23568
25. Polachek A, Li S, Chandran V. And gladman D.D. clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: Incidence, prevalence, characteristics, and outcome. Arthritis Care Res (Hoboken) (2017) 69(11):1685–91. doi: 10.1002/acr.23174
26. Gladman DD, Kavanaugh A, Gómez-Reino JJ, Wollenhaupt J, Cutolo M, Schett G, et al. Therapeutic benefit of apremilast on enthesitis and dactylitis in patients with psoriatic arthritis: A pooled analysis of the PALACE 1-3 studies. RMD Open (2018) 4(1):e000669. doi: 10.1136/rmdopen-2018-000669
27. Hartung W, Nigg A, Strunk J, Wolff B. Clinical assessment and ultrasonography in the follow-up of enthesitis in patients with spondyloarthritis: A multicenter ultrasound study in daily clinical practice. Open Access Rheumatol (2018) 10:161–9. doi: 10.2147/oarrr.S179472
28. Lee SH, Park W, Won Lee S, Kim HA, Choe JY, Lee SH, et al. Frequency of peripheral diseases in Korean patients with ankylosing spondylitis and the effectiveness of adalimumab. Int J Rheum Dis (2020) 23(9):1175–83. doi: 10.1111/1756-185x.13917
29. Rudwaleit M, Claudepierre P, Kron M, Kary S, Wong R, Kupper H. Effectiveness of adalimumab in treating patients with ankylosing spondylitis associated with enthesitis and peripheral arthritis. Arthritis Res Ther (2010) 12(2):R43. doi: 10.1186/ar2953
30. Seven S, Pedersen SJ, Østergaard M, Felbo SK, Sørensen IJ, Døhn UM, et al. Peripheral enthesitis detected by ultrasonography in patients with axial spondyloarthritis–anatomical distribution, morphology, and response to tumor necrosis factor-inhibitor therapy. Front Med (2020) 7:341. doi: 10.3389/fmed.2020.00341
31. van der Heijde D, Braun J, Deodhar A, Inman RD, Xu S, Mack ME, et al. Comparison of three enthesitis indices in a multicentre, randomized, placebo-controlled trial of golimumab in ankylosing spondylitis (GO-RAISE). Rheumatol (Oxford) (2013) 52(2):321–5. doi: 10.1093/rheumatology/kes251
32. Zhang J, Huang F, Zhang JL, Zhang H. And zhang Y.M. [The efficacy of etanercept in enthesitis in ankylosing spondylitis and an evaluation method for enthesitis]. Zhonghua nei ke za zhi (2012) 51(5):376–9.
33. Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, et al. Bimekizumab in patients with active psoriatic arthritis: Results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet (2020) 395(10222):427–40. doi: 10.1016/s0140-6736(19)33161-7
34. van der Heijde D, Gensler LS, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: Results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis (2020) 79(5):595–604. doi: 10.1136/annrheumdis-2020-216980
35. Coates LC, Wallman JK, McGonagle D, Schett GA, McInnes IB, Mease PJ, et al. Secukinumab efficacy on resolution of enthesitis in psoriatic arthritis: Pooled analysis of two phase 3 studies. Arthritis Res Ther (2019) 21(1):266. doi: 10.1186/s13075-019-2055-z
36. Balint PV, Kane D, Wilson H, McInnes IB. And sturrock R.D. ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis (2002) 61(10):905–10. doi: 10.1136/ard.61.10.905
37. Hamdi W, Bouaziz Chelli M, Ghannouchi MM, Hawel M, Ladeb MF. And kchir M.M. performance of ultrasounds compared with radiographs to detect chronic enthesitis signs in patients with ankylosing spondylitis. Rheumatol Int (2013) 33(2):497–9. doi: 10.1007/s00296-011-2174-6
38. Zhang H, Liang J, Qiu J, Wang F, Sun L. Ultrasonographic evaluation of enthesitis in patients with ankylosing spondylitis. J Biomed Res (2017) 31(2):162–9. doi: 10.7555/JBR.31.20160088
39. D'Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: A cross-sectional study. Arthritis Rheum (2003) 48(2):523–33. doi: 10.1002/art.10812
40. Naredo E, Batlle-Gualda E, García-Vivar ML, García-Aparicio AM, Fernández-Sueiro JL, Fernández-Prada M, et al. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: Response to therapy of entheseal abnormalities. J Rheumatol (2010) 37(10):2110–7. doi: 10.3899/jrheum.100136
41. de Miguel E, Cobo T, Muñoz-Fernández S, Naredo E, Usón J, Acebes JC, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis (2009) 68(2):169–74. doi: 10.1136/ard.2007.084251
42. Kiris A, Kaya A, Ozgocmen S, Kocakoc E. Assessment of enthesitis in ankylosing spondylitis by power Doppler ultrasonography. Skeletal Radiol (2006) 35(7):522–8. doi: 10.1007/s00256-005-0071-3
43. Balint PV, Terslev L, Aegerter P, Bruyn GAW, Chary-Valckenaere I, Gandjbakhch F, et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann Rheum Dis (2018) 77(12):1730–5. doi: 10.1136/annrheumdis-2018-213609
44. Hu Y, Zhu J, Xue Q, Wang N, Hu B. Scanning of the sacroiliac joint and entheses by color Doppler ultrasonography in patients with ankylosing spondylitis. J Rheumatol (2011) 38(8):1651–5. doi: 10.3899/jrheum.101366
45. Feydy A, Lavie-Brion MC, Gossec L, Lavie F, Guerini H, Nguyen C, et al. Comparative study of MRI and power Doppler ultrasonography of the heel in patients with spondyloarthritis with and without heel pain and in controls. Ann Rheum Dis (2012) 71(4):498–503. doi: 10.1136/annrheumdis-2011-200336
46. Sudoł-Szopińska I, Zaniewicz-Kaniewska K, Kwiatkowska B. Spectrum of ultrasound pathologies of achilles tendon, plantar aponeurosis and flexor digiti brevis tendon heel entheses in patients with clinically suspected enthesitis. Polish J Radiol (2014) 79(1):402–8. doi: 10.12659/PJR.890803
47. Alcalde M, Acebes JC, Cruz M, González-Hombrado L, Herrero-Beaumont G, Sánchez-Pernaute O. A sonographic enthesitic index of lower limbs is a valuable tool in the assessment of ankylosing spondylitis. Ann Rheum Dis (2007) 66(8):1015–9. doi: 10.1136/ard.2006.062174
48. Ozsoy-Unubol T, Yagci I. Is ultrasonographic enthesitis evaluation helpful for diagnosis of non-radiographic axial spondyloarthritis? Rheumatol Int (2018) 38(11):2053–61. doi: 10.1007/s00296-018-4164-4
49. Aydin SZ, Karadag O, Filippucci E, Atagunduz P, Akdogan A, Kalyoncu U, et al. Monitoring Achilles enthesitis in ankylosing spondylitis during TNF-alpha antagonist therapy: An ultrasound study. Rheumatol (Oxford) (2010) 49(3):578–82. doi: 10.1093/rheumatology/kep410
50. Wink F, Bruyn GA, Maas F, Griep EN, van der Veer E, Bootsma H, et al. Ultrasound evaluation of the entheses in daily clinical practice during tumor necrosis factor-α blocking therapy in patients with ankylosing spondylitis. J Rheumatol (2017) 44(5):587–93. doi: 10.3899/jrheum.160584
51. Wang CH, Feng Y, Ren Z, Yang X, Jia JF, Rong MY, et al. Performance of ultrasound to monitor Achilles enthesitis in patients with ankylosing spondylitis during TNF-a antagonist therapy. Clin Rheumatol (2015) 34(6):1073–8. doi: 10.1007/s10067-015-2939-5
52. Lanfranchi MA, Leluc O, Tavano A, Wormser C, Morange S, Chagnaud C, et al. Are ultrasound findings similar in patients with axial spondyloarthritis and in athlete entheses? J Rheumatol (2017) 44(5):609–12. doi: 10.3899/jrheum.160715
53. Eshed I, Bollow M, McGonagle DG, Tan AL, Althoff CE, Asbach P, et al. MRI Of enthesitis of the appendicular skeleton in spondyloarthritis. Ann Rheum Dis (2007) 66(12):1553–9. doi: 10.1136/ard.2007.070243
54. Carron P, De Craemer AS, Van den Bosch F. Peripheral spondyloarthritis: A neglected entity-state of the art. RMD Open (2020) 6(1):e001136. doi: 10.1136/rmdopen-2019-001136
55. Groves C, Chandramohan M, Chew NS, Aslam T, Helliwell PS. Clinical examination, ultrasound and MRI imaging of the painful elbow in psoriatic arthritis and rheumatoid arthritis: Which is better, ultrasound or MR, for imaging enthesitis? Rheumatol Ther (2017) 4(1):71–84. doi: 10.1007/s40744-017-0053-7
56. Aguila Maldonado R, Ruta S, Valuntas ML, García M. Ultrasonography assessment of heel entheses in patients with spondyloarthritis: A comparative study with magnetic resonance imaging and conventional radiography. Clin Rheumatol (2017) 36(8):1811–7. doi: 10.1007/s10067-017-3723-5
57. Kamel M, Eid H, Mansour R. Ultrasound detection of knee patellar enthesitis: A comparison with magnetic resonance imaging. Ann Rheum Dis (2004) 63(2):213–4. doi: 10.1136/ard.2003.010314
58. Krabbe S, Østergaard M, Eshed I, Sørensen IJ, Jensen B, Møller JM, et al. Whole-body magnetic resonance imaging in axial spondyloarthritis: Reduction of sacroiliac, spinal, and entheseal inflammation in a placebo-controlled trial of adalimumab. J Rheumatol (2018) 45(5):621–9. doi: 10.3899/jrheum.170408
59. Poggenborg RP, Eshed I, Østergaard M, Sørensen IJ, Møller JM, Madsen OR, et al. Enthesitis in patients with psoriatic arthritis, axial spondyloarthritis and healthy subjects assessed by 'head-to-toe' whole-body MRI and clinical examination. Ann Rheum Dis (2015) 74(5):823–9. doi: 10.1136/annrheumdis-2013-204239
60. Du J, Chiang AJ, Chung CB, Statum S, Znamirowski R, Takahashi A, et al. Orientational analysis of the Achilles tendon and enthesis using an ultrashort echo time spectroscopic imaging sequence. Magn Reson Imaging (2010) 28(2):178–84. doi: 10.1016/j.mri.2009.06.002
61. Weber U, Pfirrmann CW, Kissling RO, Hodler J, Zanetti M. Whole body MR imaging in ankylosing spondylitis: A descriptive pilot study in patients with suspected early and active confirmed ankylosing spondylitis. BMC Musculoskelet Disord (2007) 8:20. doi: 10.1186/1471-2474-8-20
62. Weckbach S, Schewe S, Michaely HJ, Steffinger D, Reiser MF, Glaser C. Whole-body MR imaging in psoriatic arthritis: Additional value for therapeutic decision making. Eur J Radiol (2011) 77(1):149–55. doi: 10.1016/j.ejrad.2009.06.020
63. Krabbe S, Eshed I, Gandjbakhch F, Pedersen SJ, Bird P, Mathew AJ, et al. Development and validation of an OMERACT MRI whole-body score for inflammation in peripheral joints and entheses in inflammatory arthritis (MRI-Whole-Body Score for Inflammation in Peripheral Joints and Entheses in Inflammatory Arthritis). J Rheumatol (2019) 46(9):1215–21. doi: 10.3899/jrheum.181084
64. Poulsen AEF, Axelsen MB, Poggenborg RP, Eshed I, Krabbe S, Glinatsi D, et al. Whole-body magnetic resonance imaging in psoriatic arthritis, rheumatoid arthritis, and healthy controls: Interscan, intrareader, and interreader agreement and distribution of lesions. J Rheumatol (2021) 48(2):198–206. doi: 10.3899/jrheum.200084
65. Østergaard M, Eshed I, Althoff CE, Poggenborg RP, Diekhoff T, Krabbe S, et al. Whole-body magnetic resonance imaging in inflammatory arthritis: Systematic literature review and first steps toward standardization and an OMERACT scoring system. J Rheumatol (2017) 44(11):1699–705. doi: 10.3899/jrheum.161114
66. Althoff CE, Sieper J, Song IH, Weiß A, Diekhoff T, Haibel H, et al. Comparison of clinical examination versus whole-body magnetic resonance imaging of enthesitis in patients with early axial spondyloarthritis during 3 years of continuous etanercept treatment. J Rheumatol (2016) 43(3):618–24. doi: 10.3899/jrheum.150659
67. Krabbe S, Eshed I, Sorensen IJ, Jensen B, Moller JM, Balding L, et al. Whole-body magnetic resonance imaging inflammation in peripheral joints and entheses in axial spondyloarthritis: Distribution and changes during adalimumab treatment. J Rheumatol (2020) 47(1):50–8. doi: 10.3899/jrheum.181159
68. Krabbe S, Eshed I, Sorensen IJ, Moller J, Jensen B, Madsen OR, et al. Novel whole-body magnetic resonance imaging response and remission criteria document diminished inflammation during golimumab treatment in axial spondyloarthritis. Rheumatol (Oxford) (2020) 59(11):3358–68. doi: 10.1093/rheumatology/keaa153
69. Song IH, Hermann K, Haibel H, Althoff CE, Listing J, Burmester G, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): A 48-week randomised controlled trial. Ann Rheum Dis (2011) 70(4):590–6. doi: 10.1136/ard.2010.139667
70. Poggenborg RP, Pedersen SJ, Eshed I, Sorensen IJ, Moller JM, Madsen OR, et al. Head-to-toe whole-body MRI in psoriatic arthritis, axial spondyloarthritis and healthy subjects: first steps towards global inflammation and damage scores of peripheral and axial joints. Rheumatol (Oxford) (2015) 54(6):1039–49. doi: 10.1093/rheumatology/keu439
71. Weber U, Lambert RG, Rufibach K, Maksymowych WP, Hodler J, Zejden A, et al. Anterior chest wall inflammation by whole-body magnetic resonance imaging in patients with spondyloarthritis: Lack of association between clinical and imaging findings in a cross-sectional study. Arthritis Res Ther (2012) 14(1):R3.
72. Benjamin M, Bydder GM. Magnetic resonance imaging of entheses using ultrashort TE (UTE) pulse sequences. J Magn Reson Imaging (2007) 25(2):381–9. doi: 10.1002/jmri.20825
73. Chen B, Zhao Y, Cheng X, Ma Y, Chang EY, Kavanaugh A, et al. Three-dimensional ultrashort echo time cones (3D UTE-cones) magnetic resonance imaging of entheses and tendons. Magn Reson Imaging (2018) 49:4–9. doi: 10.1016/j.mri.2017.12.034
74. Dallaudière B, Trotier A, Ribot E, Verdier D, Lepreux S, Miraux S, et al. Three-dimensional ultrashort echo time (3D UTE) MRI of Achilles tendon at 4.7T MRI with comparison to conventional sequences in an experimental murine model of spondyloarthropathy. J Magn Reson Imaging (2019) 50(1):127–35. doi: 10.1002/jmri.26569
75. de Hooge M, van den Berg R, Navarro-Compán V, van Gaalen F, van der Heijde D, Huizinga T, et al. Magnetic resonance imaging of the sacroiliac joints in the early detection of spondyloarthritis: no added value of gadolinium compared with short tau inversion recovery sequence. Rheumatol (Oxford) (2013) 52(7):1220–4. doi: 10.1093/rheumatology/ket012
76. Maksymowicz H, Kowalewski K, Lubkowska K, Zołud W, Sąsiadek M. Diagnostic value of gadolinium-enhanced MR imaging of active sacroiliitis in seronegative spondyloarthropathy. Pol J Radiol (2010) 75(2):58–65.
77. Ramalho M, Ramalho J. Gadolinium-based contrast agents: Associated adverse reactions. Magn Reson Imaging Clin N Am (2017) 25(4):755–64. doi: 10.1016/j.mric.2017.06.006
78. Mathew AJ, Krabbe S, Eshed I, Gandjbakhch F, Bird P, Pedersen SJ, et al. The OMERACT MRI in enthesitis initiative: Definitions of key pathologies, suggested MRI sequences, and a novel heel enthesitis scoring system. J Rheumatol (2019) 46(9):1232–8. doi: 10.3899/jrheum.181093
79. Mathew AJ, Krabbe S, Eshed I, Lambert RG, Laredo JD, Maksymowych WP, et al. Atlas of the OMERACT heel enthesitis MRI scoring system (HEMRIS). RMD Open (2020) 6(1):e001150. doi: 10.1136/rmdopen-2019-001150
80. Kleinrensink NJ, Foppen W, Ten Katen I, van der Veen PH, de Klerk B, Diepstraten SCE, et al. Comparison of the heel enthesitis MRI scoring system (HEMRIS) with clinical enthesitis and local metabolic activity on PET-CT. RMD Open (2020) 6(3):e001424 . doi: 10.1136/rmdopen-2020-001424
Keywords: spondyloarthritis, enthesitis, screening, ultrasound, magnetic resonance imaging
Citation: Wu X, Liu D, Li Y, Xie Y, Tu L, Zhang Y, Zhang X, Fang L, Luo X, Lin Z, Liao Z, Rong L, Ren J, Zhou Y, Yang N, Xu J, Zhang H, Xu B, Wu Z, Zhan F, Li Z, Xiao W, Liu S, Zhou Y, Ye S, Lv Q, Zhang L, Zhao D, He S, Zhao L, Wu L, Lin H, Zhu Y, Guo D, Yang Z, Liu B, Yang K and Gu J (2022) A clinical practice guideline for the screening and assessment of enthesitis in patients with spondyloarthritis. Front. Immunol. 13:978504. doi: 10.3389/fimmu.2022.978504
Received: 26 June 2022; Accepted: 11 August 2022;
Published: 12 September 2022.
Edited by:
Michele Maria Luchetti Gentiloni, Marche Polytechnic University, ItalyReviewed by:
Niccolò Possemato, Local Health Authority of Reggio Emilia (IRCCS), ItalyCopyright © 2022 Wu, Liu, Li, Xie, Tu, Zhang, Zhang, Fang, Luo, Lin, Liao, Rong, Ren, Zhou, Yang, Xu, Zhang, Xu, Wu, Zhan, Li, Xiao, Liu, Zhou, Ye, Lv, Zhang, Zhao, He, Zhao, Wu, Lin, Zhu, Guo, Yang, Liu, Yang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieruo Gu, Z3VqaWVydW9AMTYzLmNvbQ==; Kehu Yang, a2VodXlhbmdlYm0yMDA2QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.