- 1First People’s Hospital of Shangqiu, Henan Province, Shangqiu, China
- 2Department of Pharmacy, the First Affiliated Hospital of Hainan Medical University, Haikou, China
- 3Department of Pharmacology, School of Basic Medicine and Life Sciences, Hainan Medical University, Haikou, China
Immune dysfunction has been proposed as a factor that may contribute to disease progression. Emerging evidence suggests that immunotherapy aims to abolish cancer progression by modulating the balance of the tumor microenvironment. 4-1BB (also known as CD137 and TNFRS9), a member of tumor necrosis factor receptor superfamily, has been validated as an extremely attractive and promising target for immunotherapy due to the upregulated expression in the tumor environment and its involvement in tumor progression. More importantly, 4-1BB-based immunotherapy approaches have manifested powerful antitumor effects in clinical trials targeting 4-1BB alone or in combination with other immune checkpoints. In this review, we will summarize the structure and expression of 4-1BB and its ligand, discuss the role of 4-1BB in the microenvironment and tumor progression, and update the development of drugs targeting 4-1BB. The purpose of the review is to furnish a comprehensive overview of the potential of 4-1BB as an immunotherapeutic target and to discuss recent advances and prospects for 4-1BB in cancer therapy.
Introduction
Tumor immunotherapy exerts antitumor efficacy through the interaction of the host immune system with tumor-associated antigens (1). It can restore or enhance the body’s immune system’s natural defenses against tumors, which typically targets specific biomolecules on the surface of cancer cells, exemplified by tumor-associated antigens (2). Immunotherapy including immune checkpoint inhibitor (ICI) and CAR-T therapy has made breakthroughs in tumor treatment, but the overall response rate is not high, and many patients cannot benefit from it (3–6). Therefore, the development of new immune checkpoints and biomarkers and expansion of the beneficiary population from immunotherapy are urgent problems to be solved.
Neoantigen epitopes generated by somatic mutations in cancer cells play an important role in T-cell immune responses, which have become an important driver of immune checkpoint discovery in immunotherapy. 4-1BB, also termed 4-1BB and TNFRSF9, was identified in 1989 and originally described as an inducible gene, which was expressed in T lymphocytes (7). 4-1BB exhibited an important effect in various cells and participated in the activation of multiple immune cells, such as CD8 T cells and cytotoxic T lymphocytes (CTL) (8). Emerging evidence has demonstrated that targeting 4-1BB is a uniquely attractive strategy for tumor immunotherapy (9–13). In this review, we discuss the recent advances and prospects of the cancer immunotherapy checkpoint 4-1BB from the aspects of structure, expression, role in tumor microenvironment, development of clinical drugs targeting 4-1BB, and their combination with traditional treatment methods.
Structure of 4-1BB and its ligand
4-1BB, a glycosylated type I membrane protein, contains four cysteine-rich pseudo repeats, which contribute to the formation of a cytoplasmic signaling domain, extracellular domain, and short helical transmembrane domain (7). An elongated structure was generally formed by the extracellular domain of TNFR (variation range: 1 to 4 CRDs). Based on this, antibodies can bind to these molecules through many modalities. Efficient binding of 4-1BB L to 4-1BB results in rapid receptor activation in response to antigenic stimulation. 4-1BBL (TNFSF9), a type II membrane protein of the TNF ligand superfamily, is the binding partner of 4-1BB (14, 15). TNFSF members, typically expressed on the cell membrane, exist in a homotrimeric complex (16–18), which can be divided into three parts: (a) LTα, TNF, RANKL, LIGHT, Apo2L/TRAIL, and CD40L (19, 20); (b) BAFF, APRIL, and EDA; and (c) GITRL, 4-1BBL, and OX40L, among which OX40L and GITRL exhibit a flatter conformation (19, 21). The sequences of 4-1BBL were poorly conserved in human and mouse.
As a member of the tumor necrosis factor superfamily, 4-1BB is mostly expressed on the surface of activated T cells but also on B cells, NK cells, and DC cells (22, 23). 4-1BB is widely distributed on various tumor cells (such as lung tumor cells, and leukemia cells) and has been identified in tissues (such as liver cancer tissue, and tumor vessel walls). Alfaro et al. found that 4-1BB is also expressed in tonsil and lymph node follicular structures. Thence, a comprehensive analysis of its distribution helps uncover potential roles and functions.
Role of 4-1BB in the tumor microenvironment
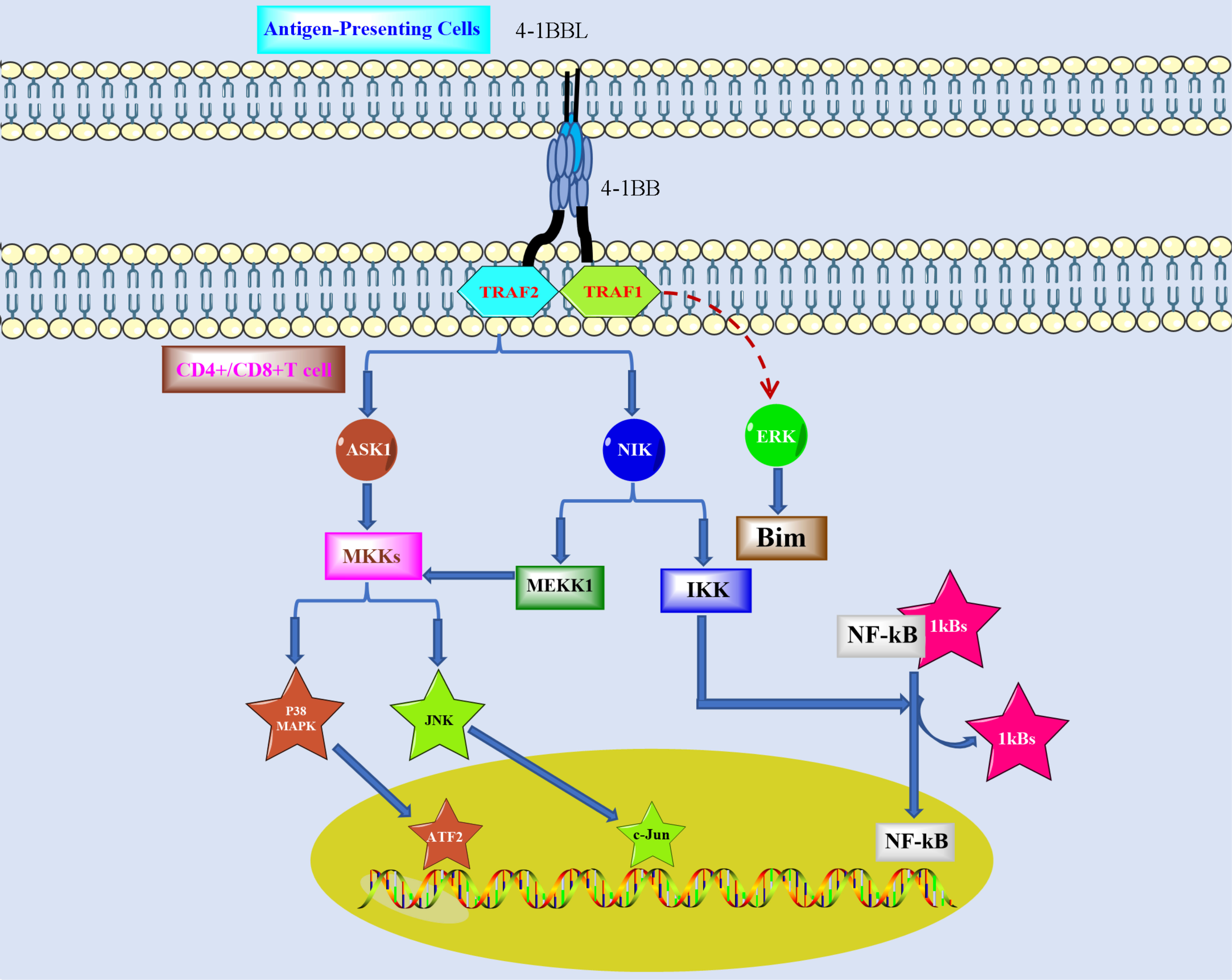
As shown in Figure 1, both IL-15 and IL-2 can promote the expression of 4-1BB on NK cells, which stimulates the proliferation of NK cells and produces IFN-γ, thus leading to the activation of T cells (24). 4-1BB facilitates the proliferation of CD8+ T cells to produce memory T (Tm) cells (25, 26). Stimulation by 4-1BB will upregulate the expression IL-2 and IFN-γ in CD4+ and CD8+ T cells. However, 4-1BB expresses a controversial effect in T regulatory cells (Treg), which leads to Treg proliferation but alters Treg for cytotoxic or helper effects (27, 28). 4-1BBL inhibits the conversion of CD4+FOXP3- cells to CD4+FOXP+ (29). 4-1BB is also expressed in monocytes, and it promotes upregulation of IL-8 and TNF-α but downregulation of IL-10. The differentiation of monocytes into dendritic cells can be promoted by 4-1BB, and dendritic cells then secrete IL-6 and IL-12 (30). However, 4-1BB stimulation differentiates monocytes into M2 macrophages and accelerates B-cell apoptosis, which also promotes the expression of TNF-α/β in B cells (31).
4-1BB in cancer progression
Through the PI3K/AKT/mTOR pathway, expression of 4-1BB was induced by EBV protein LMP1 to facilitate immune evasion in Hodgkin and Reed–Sternberg cells (32). Low levels of the soluble form of 4-1BBL in patients with AML were associated with better prognosis, especially longer disease-free survival (33). 4-1BB L and 4-1BB were abnormally expressed in tumor cells in hematopoietic malignancies, and their interaction promotes tumor growth in cutaneous T-cell lymphoma (34). Overexpression of 4-1BB on leukemic cells was significantly related to poor prognosis (35). Antitumor activity was enhanced in 4-1BB-knockout mice (36). Similarly, the tumor growth was seriously blocked in 4-1BB knockout mice subcutaneously injected with CT26 cells (37). The findings further proved the critical role of 4-1BB-4-1BBL in tumor development.
4-1BB-targeted drug development
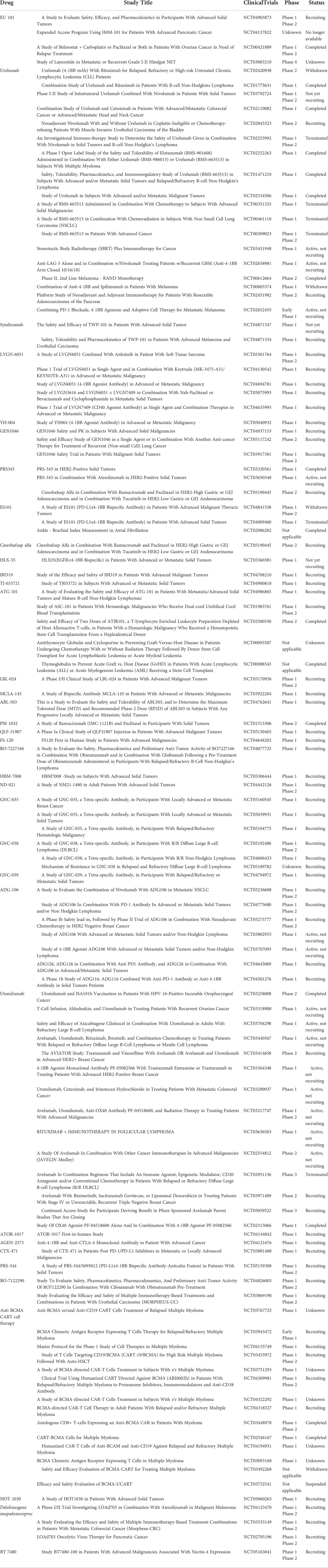
The efficacy of the 4-1BB antibody in preventing cancer in animals has prompted clinical development. The use of monoclonal antibodies to treat cancer has achieved great success over the past few decades, many of which have been under evaluation in different clinical trials, as shown in Table 1.
Urelumab (BMS-663513), the first 4-1BB-targeted therapy to enter clinical trials developed by Bristol–Myers Squibb, is a human IgG4 human monoclonal antibody, which will not inhibit the interaction between 4-1BB with its ligand (38). Preliminary clinical results in phase 1/2 disclosed in 2008 showed encouraging efficacy, but further development was hindered by liver toxicity (39). Urelumab reentered clinical trials in 2012, which was combined with nivolumab, cetuximab, rituximab, and elotuzumab, respectively (12). However, hepatotoxicity of the antibody emerged shortly thereafter, causing the urelumab development program to be shelved. Currently, urelumab, a potent agonist mAb, is still under different clinical trials (Table 1), and strategies to avoid hepatotoxicity and achieve appropriate drug exposure levels are worth investigating. Utomilumab (PF-05082566) is a 4-1BB-humanized IgG2 monoclonal antibody developed by Pfizer (40). Compared with urelumab, it has a higher safety profile and is currently undergoing multiple clinical trials (41).
To reduce the hepatotoxicity of systemic 4-1BB agonists, the development of bispecific antibodies against 4-1BB has been recognized as a viable strategy, and some bispecific antibodies, including GEN1046 (PD-L1/4-1BB) and PRS343 (HER2/4-1BB), are currently being evaluated in different clinical trials (Table 1) (42, 43). ES101 (INBRX-105), a first-in-class tetravalent bispecific antibody targeting PD-L1/4-1BB, originally developed by Inhibrx, was introduced into its Greater China rights by Kewan Pharmaceuticals (44). It contains four domains, and two of them target PD-L1 while the other two target 4-1BB, which can alleviate PD-1/PD-L1-mediated immune checkpoint inhibition. The 4-1BB-binding domain may drive the aggregation of 4-1BB molecules on the surface of T cells, so that 4-1BB-mediated immune activation can be concentrated on T cells near the tumor, effectively reducing the potential off-target toxicity.
In addition to double-antibody drugs, the development of 4-1BB targets has been extended to tertiary and tetraspecific antibodies. NM21-1480 is a monovalent trispecific antibody fragment molecule against PD-L1, 4-1BB, and human serum protein (HSA) (45). NM21-1480 exerts a synergistic effect of 4-1BB agonism and PD-L1 blockade and shows an extended half-life by binding to HSA, thereby reducing the frequency of dosing. GNC-035 is a four-antibody drug targeting PD-L1/CD3/4-1BB/ROR1 while GNC-039 targets PD-L1/4-1BB/CD3/EGFR. In terms of design, both GNC-035 and GNC-039 build symmetrical tetraspecific antibodies based on IgG with three scFvs in series. Among them, PD-L1, 4-1BB, and CD3 are immunoregulatory functions, and the fourth target is tumor antigen. Both drugs are undergoing evaluation in different clinical trials (Table 1).
Future directions
Immunotherapy is known as the fourth cancer treatment after surgery, radiotherapy, and chemotherapy, which has changed the treatment patterns of patients with advanced tumors (46). However, only a minority of cancer patients can benefit from it. Treatment methods such as surgery, chemotherapy, radiotherapy, and targeted therapy can synergize with immunotherapy to enhance the curative effect. Guillerey et al. found that anti-4-1BB mAb combined with chemotherapy could prevent MM relapse and prolong survival in MM mice (47). A study undertaken by Newcomb et al. demonstrated that radiation could synergistically enhance the antitumor effect of anti-4-1BB therapy in a mouse glioma model (48). Moreover, anti-4-1BB mAbs could enhance the efficacy of other antitumor Abs (such as cetuximab, rituximab, and trastuzumab) and exert synergistic effects. Taken together, combination therapy for tumors may also be the future direction of tumor therapy.
Conclusion
To summarize, existing studies support immunotherapies targeting the 4-1BB pathway for the treatment of cancer. In the study, we have summarized the structure of 4-1BB and its ligand as well as the expression in various immune cells and tumor cells. More importantly, we discuss the role of 4-1BB in the microenvironment and tumor progression. Furthermore, the development of drug-targeted 4-1BB was summarized and updated, which exhibited tremendous potential in clinical trials. Although the anti-4-1BB therapy provides hope for cancer treatment, the effectiveness of drugs targeting 4-1BB in clinical antitumor therapy alone or in combination with other antitumor therapies still needs to be investigated in the future.
Author contributions
Y-TW, K-FC and Q-BL conceived the review. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (No. 81960663).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol (2006) 90:51–81. doi: 10.1016/S0065-2776(06)90002-9
2. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest (2015) 125:3335–7. doi: 10.1172/JCI83871
3. Wang R, Liu H, He P, An D, Guo X, Zhang X, et al. Inhibition of PCSK9 enhances the antitumor effect of PD-1 inhibitor in colorectal cancer by promoting the infiltration of CD8+ T cells and the exclusion of treg cells. Front Immunol (2022) 13:947756. doi: 10.3389/fimmu.2022.947756
4. Xu C, Ju D, Zhang X. Cell membrane-derived vesicle: A novel vehicle for cancer immunotherapy. Front Immunol (2022) 13:923598. doi: 10.3389/fimmu.2022.923598
5. Duan LJ, Wang Q, Zhang C, Yang DX, Zhang XY. Potentialities and challenges of mRNA vaccine in cancer immunotherapy. Front Immunol (2022) 13:923647. doi: 10.3389/fimmu.2022.923647
6. Xu C, Ju D, Zhang X. Chimeric antigen receptor T-cell therapy: challenges and opportunities in lung cancer. Antib Ther (2022) 5:73–83. doi: 10.1093/abt/tbac006
7. Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U.S.A. (1989) 86:1963–7. doi: 10.1073/pnas.86.6.1963
8. Vinay DS, Kwon BS. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep (2014) 47:122–9. doi: 10.5483/bmbrep.2014.47.3.283
9. Chu DT, Bac ND, Nguyen KH, Tien NLB, Thanh VV, Nga VT, et al. An update on anti-CD137 antibodies in immunotherapies for cancer. Int J Mol Sci (2019) 20:1822. doi: 10.3390/ijms20081822
10. Ye L, Jia K, Wang L, Li W, Chen B, Liu Y, et al. CD137, an attractive candidate for the immunotherapy of lung cancer. Cancer Sci (2020) 111:1461–7. doi: 10.1111/cas.14354
11. Wong HY, Schwarz H. CD137/CD137 ligand signalling regulates the immune balance: A potential target for novel immunotherapy of autoimmune diseases. J Autoimmun (2020) 112:102499. doi: 10.1016/j.jaut.2020.102499
12. Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood (2018) 131:49–57. doi: 10.1182/blood-2017-06-741041
13. Etxeberria I, Glez-Vaz J, Teijeira A, Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open (2020) 4:e000733. doi: 10.1136/esmoopen-2020-000733
14. Armitage RJ. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol (1994) 6:407–13. doi: 10.1016/0952-7915(94)90119-8
15. Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol (1993) 23:2631–41. doi: 10.1002/eji.1830231037
16. Lum L, Wong BR, Josien R, Becherer JD, Erdjument-Bromage H, Schlondorff J, et al. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem (1999) 274:13613–8. doi: 10.1074/jbc.274.19.13613
17. Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble fas ligand and potentiates epithelial cell apoptosis. Curr Biol (1999) 9:1441–7. doi: 10.1016/s0960-9822(00)80113-x
18. Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature (1997) 385:729–33. doi: 10.1038/385729a0
19. Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure (2006) 14:1321–30. doi: 10.1016/j.str.2006.06.015
20. Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci (2002) 27:19–26. doi: 10.1016/s0968-0004(01)01995-8
21. Chattopadhyay K, Ramagopal UA, Mukhopadhaya A, Malashkevich VN, Dilorenzo TP, Brenowitz M, et al. Assembly and structural properties of glucocorticoid-induced TNF receptor ligand: Implications for function. Proc Natl Acad Sci U.S.A. (2007) 104:19452–7. doi: 10.1073/pnas.0709264104
22. Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol (2009) 9:271–85. doi: 10.1038/nri2526
23. Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther (2012) 11:1062–70. doi: 10.1158/1535-7163.MCT-11-0677
24. Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol (2002) 169:4230–6. doi: 10.4049/jimmunol.169.8.4230
25. Wortzman ME, Clouthier DL, McPherson AJ, Lin GH, Watts TH. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunol Rev (2013) 255:125–48. doi: 10.1111/imr.12086
26. Willoughby JE, Kerr JP, Rogel A, Taraban VY, Buchan SL, Johnson PW, et al. Differential impact of CD27 and 4-1BB costimulation on effector and memory CD8 T cell generation following peptide immunization. J Immunol (2014) 193:244–51. doi: 10.4049/jimmunol.1301217
27. Akhmetzyanova I, Zelinskyy G, Littwitz-Salomon E, Malyshkina A, Dietze KK, Streeck H, et al. CD137 agonist therapy can reprogram regulatory T cells into cytotoxic CD4+ T cells with antitumor activity. J Immunol (2016) 196:484–92. doi: 10.4049/jimmunol.1403039
28. Zhang P, Gao F, Wang Q, Wang X, Zhu F, Ma C, et al. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand J Immunol (2007) 66:435–40. doi: 10.1111/j.1365-3083.2007.01994.x
29. Madireddi S, Schabowsky RH, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H. SA-4-1BBL costimulation inhibits conversion of conventional CD4+ T cells into CD4+ FoxP3+ T regulatory cells by production of IFN-γ. PloS One (2012) 7:e42459. doi: 10.1371/journal.pone.0042459
30. Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol (2002) 14:275–86. doi: 10.1093/intimm/14.3.275
31. Zhang X, Voskens CJ, Sallin M, Maniar A, Montes CL, Zhang Y, et al. CD137 promotes proliferation and survival of human b cells. J Immunol (2010) 184:787–95. doi: 10.4049/jimmunol.0901619
32. Aravinth SP, Rajendran S, Li Y, Wu M, Yi Wong AH, Schwarz H. Epstein-Barr Virus-encoded LMP1 induces ectopic CD137 expression on Hodgkin and reed-sternberg cells via the PI3K-AKT-mTOR pathway. Leuk Lymphoma (2019) 60:2697–704. doi: 10.1080/10428194.2019.1607330
33. Schmohl JU, Nuebling T, Wild J, Kroell T, Kanz L, Salih HR, et al. Expression of 4-1BB and its ligand on blasts correlates with prognosis of patients with AML. J Investig Med (2016) 64:1252–60. doi: 10.1136/jim-2016-000081
34. Kamijo H, Miyagaki T, Shishido-Takahashi N, Nakajima R, Oka T, Suga H, et al. Aberrant CD137 ligand expression induced by GATA6 overexpression promotes tumor progression in cutaneous T-cell lymphoma. Blood (2018) 132:1922–35. doi: 10.1182/blood-2018-04-845834
35. Kaban K, Greiner SM, Holzmayer S, Tandler C, Meyer S, Hinterleitner C, et al. Immunoprofiling of 4-1BB expression predicts outcome in chronic lymphocytic leukemia (CLL). Diagnostics (Basel) (2021) 11:2041. doi: 10.3390/diagnostics11112041
36. Choi BK, Kim YH, Kim CH, Kim MS, Kim KH, Oh HS, et al. Peripheral 4-1BB signaling negatively regulates NK cell development through IFN-gamma. J Immunol (2010) 185:1404–11. doi: 10.4049/jimmunol.1000850
37. Kang SW, Lee SC, Park SH, Kim J, Kim HH, Lee HW, et al. Anti-CD137 suppresses tumor growth by blocking reverse signaling by CD137 ligand. Cancer Res (2017) 77:5989–6000. doi: 10.1158/0008-5472.CAN-17-0610
38. Segal NH, Logan TF, Hodi FS, McDermott D, Melero I, Hamid O, et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res (2017) 23:1929–36. doi: 10.1158/1078-0432.CCR-16-1272
39. Gordon MS, Sweeney CS, Mendelson DS, Eckhardt SG, Anderson A, Beaupre DM, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res (2010) 16:699–710. doi: 10.1158/1078-0432.CCR-09-1365
40. Fisher TS, Kamperschroer C, Oliphant T, Love VA, Lira PD, Doyonnas R, et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother (2012) 61:1721–33. doi: 10.1007/s00262-012-1237-1
41. Gopal A, Bartlett N, Levy R, Houot R, Smith S, Segal N, et al. A phase I study of PF-05082566 (anti-4-1BB) + rituximab in patients with CD20+ NHL. J Clin Oncol (2015) 33:3004–4. doi: 10.1200/jco.2015.33.15_suppl.3004
42. Mayes PA, Hance KW, Hoos A. The promise and challenges of immune agonist antibody development in cancer. Nat Rev Drug Discovery (2018) 17:509–27. doi: 10.1038/nrd.2018.75
43. Goebeler ME, Bargou RC. T Cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol (2020) 17:418–34. doi: 10.1038/s41571-020-0347-5
44. Hashimoto K. CD137 as an attractive T cell Co-stimulatory target in the TNFRSF for immuno-oncology drug development. Cancers (Basel) (2021) 13:2288. doi: 10.3390/cancers13102288
45. Park DE, Cheng J, McGrath JP, Lim MY, Cushman C, Swanson SK, et al. Merkel cell polyomavirus activates LSD1-mediated blockade of non-canonical BAF to regulate transformation and tumorigenesis. Nat Cell Biol (2020) 22:603–15. doi: 10.1038/s41556-020-0503-2
46. Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med (2021) 9:20503121211034366. doi: 10.1177/20503121211034366
47. Guillerey C, Nakamura K, Pichler AC, Barkauskas D, Krumeich S, Stannard K, et al. Chemotherapy followed by anti-CD137 mAb immunotherapy improves disease control in a mouse myeloma model. JCI Insight (2019) 5:e125932. doi: 10.1172/jci.insight.125932
Keywords: 4-1BB, immunotherapy, cancer, immune checkpoint inhibitor, clinical trials
Citation: Wang Y-T, Ji W-D, Jiao H-M, Lu A, Chen K-F and Liu Q-B (2022) Targeting 4-1BB for tumor immunotherapy from bench to bedside. Front. Immunol. 13:975926. doi: 10.3389/fimmu.2022.975926
Received: 22 June 2022; Accepted: 29 August 2022;
Published: 16 September 2022.
Edited by:
Xuyao Zhang, Fudan University, ChinaCopyright © 2022 Wang, Ji, Jiao, Lu, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-Bing Liu, cWliaW5nLmxpdUBoYWlubWMuZWR1LmNu; Kun-Feng Chen, Y2hlbmt1bmZlbmcxOTcyQDEyNi5jb20=
†These authors have contributed equally to this work
 Ya-Tao Wang
Ya-Tao Wang Wei-Dong Ji1†
Wei-Dong Ji1† Qi-Bing Liu
Qi-Bing Liu