- 1Department of Oncology, The Fifth Medical Center of PLA General Hospital, Beijing, China
- 2Department of Oncology, The Seventh Medical Center of PLA General Hospital, Beijing, China
- 3Department of Oncology, The Fourth Medical Center of PLA General Hospital, Beijing, China
- 4Department of Oncology, The First Medical Center of PLA General Hospital, Beijing, China
Background: The efficacy of immune checkpoint inhibitors (ICIs) in pretreated EGFR-mutated non-small cell lung cancer (NSCLC) patients is controversial. We conducted this multicenter retrospective study to examine the efficacy of ICIs in a real world setting.
Patients and methods: We collected 116 consecutive NSCLC patients with sensitive EGFR mutations who received ICIs alone or in combination after failure to respond to EGFR tyrosine kinase inhibitors (EGFR-TKIs), and 99 patients were included for final analysis. The impacts of ICIs on the patients’ objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) were assessed. The relationships between outcomes and clinical characteristics were analyzed.
Results: The ORR in patients with target lesions was 31.25% (95% CI: 22.18-41.52), and the DCR in all patients was 65.66% (95% CI: 55.44-74.91). The overall median PFS was 5.0 months (95% CI: 3.0-6.6), and the median OS was 15.9 months (95% CI: 10.8-23.8). The outcomes were better in patients receiving combination therapy with ECOG scores of 0-1 and no more than 2 lines of prior therapy, with a median PFS of 7.4 months (95% CI: 3.0-13.3) and a median OS of 29.0 months (95% CI: 11.7-NE). Primary EGFR mutation type and treatment mode were found to have a notable impact on clinical outcomes. Both median PFS and OS in patients with EGFR L858R mutation were significantly shorter than those in patients with EGFR exon 19 deletion (19del) (PFS: 2.5 versus 6.7 months, HR: 1.80, log-rank P=0.011; OS: 9.8 versus 26.9 months, HR: 2.48, log-rank P=0.002). Patients receiving combination therapy had notably longer median PFS and OS than those receiving monotherapy (PFS: 5.2 versus 3.0 months, HR: 0.54, log-rank P=0.020; OS: 19.0 versus 7.4 months, HR: 0.46, log-rank P=0.009).
Conclusions: Our study suggests that ICI-based combination therapy is a potential strategy for EGFR-mutated NSCLC patients after EGFR-TKI failure. The efficacy may differ according to EGFR subtypes.
1 Introduction
The discovery of epidermal growth factor receptor (EGFR) mutations and the advent of EGFR-tyrosine kinase inhibitors (TKIs) have dramatically shifted the therapeutic landscape of non-small cell lung cancer (NSCLC) from traditional chemotherapy to molecular targeted therapy. Characterized by low toxicity and high efficiency, EGFR-TKIs have become the standard of care as first-line treatment for patients with sensitive mutations (1, 2). However, resistance to targeted therapy is inevitable (3, 4). Although the development of third-generation EGFR-TKIs has overcome approximately 60% of acquired resistance due to the EGFR T790M mutation (5, 6), challenges still exist in treatments after failure of third-generation TKIs or first- and second-generation TKIs without T790M. Progression-free survival (PFS) with traditional chemotherapy in the subsequent lines of treatment is a disappointing length of 4.4-5.4 months (7, 8). New strategies are urgently needed to improve the prognosis of patients who are resistant to EGFR-TKI therapy.
In recent years, tremendous advances in immune checkpoint inhibitors (ICIs) have significantly improved the overall survival of advanced NSCLC patients without driver mutations, while the efficacy of programmed death 1 (PD-1) axis inhibition in EGFR-positive patients is still controversial. As programmed cell death-ligand 1 (PD-L1) levels have been reported to be significantly higher in EGFR-mutated NSCLC cell lines, especially in EGFR TKI-resistant cells (9–11), and EGFR-TKI treatment is considered to be associated with an increase in TMB and PD-1 expression (12), PD-1/PD-L1 blockade is considered a promising approach in NSCLC with EGFR mutations, especially in patients with acquired resistance to EGFR-TKIs. However, data from clinical trials have not shown substantial survival benefits of single-agent ICIs in pretreated EGFR-mutated NSCLC (13–19). Furthermore, the high rate of interstitial pneumonitis discontinued the attempt to combine of EGFR-TKI and PD-1 blockade (20).
With the further understanding of the synergistic mechanism of chemotherapy and immunotherapy, as well as the release of data from the clinical trial Impower150, more studies are focusing on the efficacy of immunotherapy-based combination treatment in EGFR-mutated NSCLC after EGFR-TKI resistance. In phase II trials, toripalimab and tislelizumab were reported to have objective response rates (ORRs) of 50.0% and 59.4% and disease control rates (DCRs) of 87.5% and 90.6%, respectively, in combination with chemotherapy as second-line treatment in pretreated EGFR-mutated advanced NSCLC (21, 22). Several phase III trials are ongoing. However, some limitations exist. First, their sample sizes were small. Second, it was not clear which patients would benefit most from this immunotherapy-based combination treatment. Finally, comparisons between single-agent ICIs and combination therapy were absent. To overcome these problems, we conducted this study to show the efficacy of ICIs in pretreated advanced EGFR-mutated NSCLC in a real world setting.
2 Methods
2.1 Study population
This was a multicenter, retrospective, cohort analysis of consecutive advanced NSCLC patients with sensitive EGFR mutations who received ICIs after resistance to EGFR-TKIs. Sensitive EGFR mutations included EGFR exon 19 deletion (19del), L858R, exon 19 insertion, L861Q, G719X and S768I. Data were collected from patients treated in the First, Fourth and Fifth Medical Center of PLA General Hospital between 2018 and 2021. Patients who were histologically confirmed to have advanced NSCLC with a sensitive EGFR mutation identified by polymerase chain reaction (PCR) testing or next generation sequencing (NGS) were eligible for inclusion. They must have received at least one line of EGFR-TKI therapy before receiving ICIs and experienced either disease progression or toxicity requiring a change in systemic therapy. Patients who received ICIs with biopsy-proven small cell transformation after progression or without complete clinical information were excluded. The Ethics Committee of PLA General Hospital approved this study (S2018-092-01).
2.2 Data collection
Data were collected from the electronic medical record system in each medical center. Demographic, clinicopathological and treatment data were collected with uniform database templates to ensure consistent data collection. For ICIs, including anti-PD-1/PD-L1 antibody, information on the specific drug, treatment time, reason for discontinuation, and grade 3 and above treatment-related adverse events (TRAEs) or any grade immune-related adverse events (irAEs) were collected. According to the response evaluation criteria in solid tumors (RECIST 1.1), radiographic responses were classified into four categories for patients with target lesions: complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). And for patients had only non-target lesions, the assessments were classified into CR, NonCR/NonPD or PD.
2.3 Study outcomes
The main objective of this study was to evaluate the efficacy of ICIs in NSCLC patients with sensitive EGFR mutations after failure to respond to EGFR-TKI therapy. The impacts of ICIs on ORR, DCR, PFS, and overall survival (OS) were assessed. ORR was defined as the proportion of patients with target lesions who achieved CR or PR as the best radiographic response. The DCR included the proportion of all the patients without PD. PFS was calculated from the initiation of ICI-based therapy until disease progression, death or the last follow-up, while OS was calculated until death or the last follow-up. Patients data were limited to the date of last dose if they discontinued treatment because of nonprogressive disease causes without further disease assessment.
2.4 Statistical analysis
Data were analyzed using STATA statistical software version 17.0. Clinical characteristics and safety data were summarized by descriptive statistical analysis. The differences in tumor response (objective response and disease control) in different subgroups were assessed using the chi-square test or Fisher’s exact test. Analyses of PFS and OS were performed using the Kaplan-Meier method. Univariate analysis and multivariate analysis were conducted to assess predictive factors associated with PFS or OS via Cox proportional hazard modeling. Variables that were selected for multivariate analysis included age, gender, Eastern Cooperative Oncology Group (ECOG) score, smoking history, primary EGFR mutation type, prior treatment of third-generation EGFR-TKI, status of brain metastasis, status of liver metastasis, status of bone metastasis, prior lines of therapy and treatment mode, and a backward stepwise regression procedure was applied. The hazard ratio (HR) was estimated to compare survival according to the factor of interest and significance was determined by the log-rank test. P values <0.05 were considered to be statistically significant.
3 Results
3.1 Patient characteristics and treatment
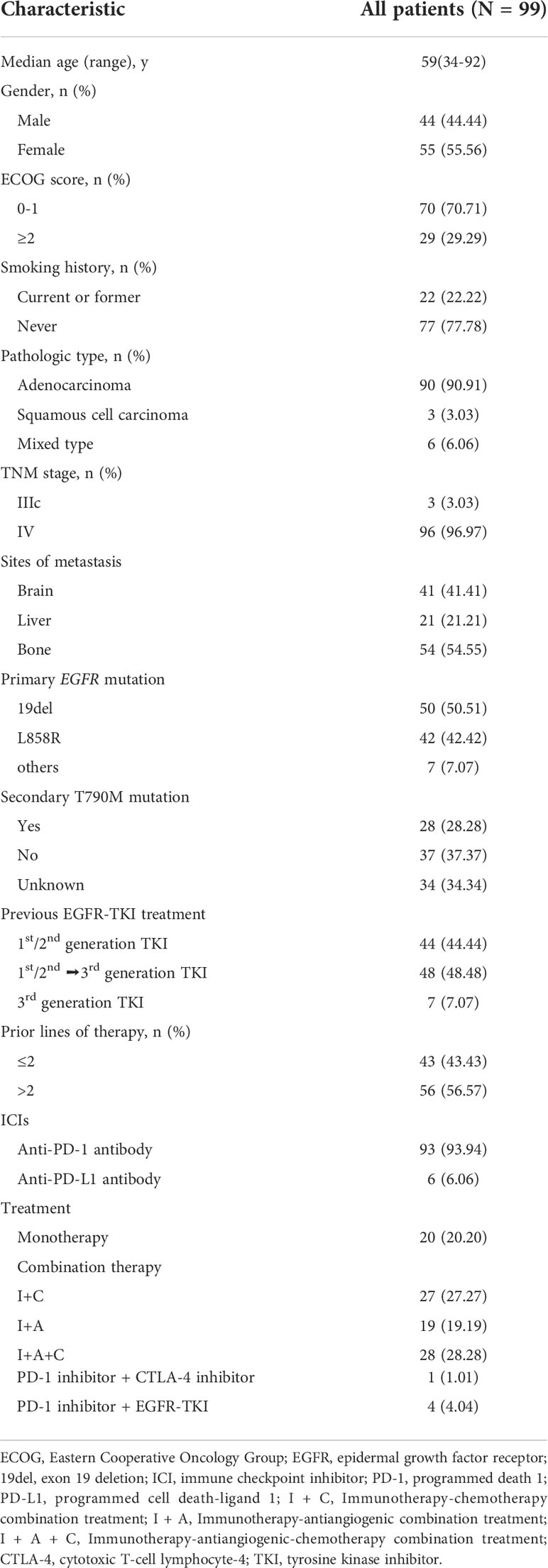
One hundred and sixteen NSCLC patients harboring sensitive EGFR mutations, who were treated with ICIs after EGFR TKI progression were identified from three institutions. Five patients were excluded for biopsy-proven small cell transformation after progression, and 12 were excluded for insufficient clinical information. Finally, a total of 99 patients were enrolled in this study for further analysis (Supplementary Figure S1). The median age at initiation of treatment with ICIs was 59 years (range from 34 to 92) and 44 were male. Primary EGFR mutations included 50 cases of 19del, 42 of L858R mutation, 3 of L861Q, 3 of G719X and 1 of S768I. Ninety-three patients received anti-PD-1 antibody and 6 received anti-PD-L1 antibody. Twenty patients (20.20%) received monotherapy while 79 (79.80%) received combination therapy. Combination therapy included immunotherapy-chemotherapy combination treatment (I+C: n=27; 27.27%), immunotherapy-antiangiogenic combination treatment (I+A: n=19; 19.19%), immunotherapy-antiangiogenic-chemotherapy combination treatment (I+A+C: n=28; 28.28%), PD-1 inhibitor combined with cytotoxic T-cell lymphocyte-4 (CTLA-4) inhibitor (n=1; 1.01%), and PD-1 inhibitor combined with EGFR TKI (n=4; 4.04%). The detailed characteristics of these patients are listed in Table 1.
3.2 Efficacy
At the time of data cutoff (February 11, 2022), the median duration of follow-up was 20.3 months (95% CI: 13.6-22.8) and 43 of 99 patients were still alive or lost to follow-up. A total of 81 patients had experienced disease progression. Thirty patients achieved PR as the best radiographic response, while 34 showed PD at the time of the first evaluation. No patients achieved CR. To compare with data in previous prospective studies, the patients receiving combination therapy with ECOG score 0-1 and no more than 2 lines of prior therapy were selected for analyze, and 26 patients met these criteria.
3.2.1 ORR
Three patients had only non-target lesions. The ORR was 31.25% (30 of 96 patients; 95% CI: 22.18-41.52) in all patients with target lesions and 37.50% (9 of 24 patients; 95% CI: 18.80-59.41) in the selected patients. Further analyses were conducted to compare tumor response in different subgroups, and the results are presented in Supplementary Table S1. A significant difference in ORR was found in subgroups of different primary EGFR mutation types (EGFR 19del versus L858R versus others: 40.43% versus 16.67% versus 57.14%; P=0.012).
3.2.2 DCR
The DCR was 65.66% (65 of 99 patients; 95% CI: 55.44-74.91) in all patients and 76.92% (95% CI: 56.35-91.03) in the selected 26 patients. The DCRs were significantly different according to age (<65 versus ≥65: 58.82% versus 80.65%; P=0.034) and treatment mode (monotherapy versus combination therapy: 40.00% versus 72.15%; P=0.007; Supplementary Table S1).
3.2.3 PFS
The median PFS was 5.0 months (95% CI: 3.0-6.6) in all patients (Figure 1A) and 7.4 months (95% CI: 3.0-13.3) in the selected 26 patients (Figure 1C). PFS was found to be significantly correlated with primary EGFR mutation, treatment mode, objective response and disease control (Figure 2). In the multivariate model, primary EGFR mutation type and treatment mode demonstrated a significant association with PFS (Figure 4).
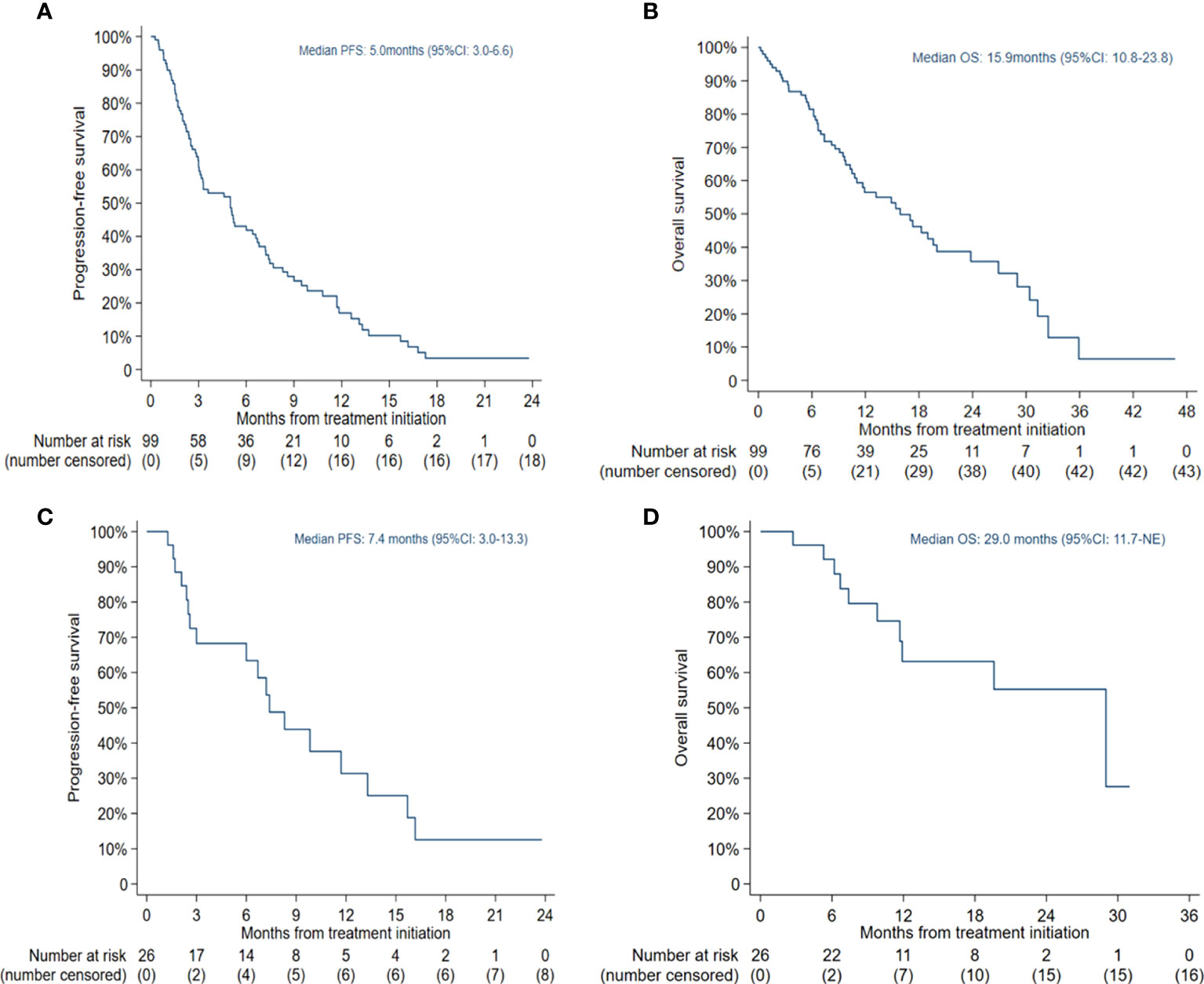
Figure 1 Kaplan-Meier estimates of progression-free survival (PFS) and overall survival (OS): (A) PFS in all patients; (B) OS in all patients; (C) PFS in patients receiving combination therapy with Eastern Cooperative Oncology Group (ECOG) score 0-1 and no more than 2 lines of prior therapy; (D) OS in patients receiving combination therapy with ECOG score 0-1 and no more than 2 lines of prior therapy.
The median PFS was 2.5 months (95% CI: 1.9-5.0) in patients with EGFR L858R mutation versus 6.7 months (95% CI: 3.6-8.6) in patients with EGFR 19del mutation (HR=1.80, 95% CI: 1.14-2.86, log-rank P=0.011) (Figure 5A). In patients receiving combination therapy, the median PFS was 5.2 months (95% CI: 3.1-7.4), compared with 3.0 months (95% CI: 1.3-3.3) in patients receiving monotherapy (HR=0.54, 95% CI: 0.32-0.92, log-rank P=0.020; Figure 6A). To investigate the impact of the combination mode on PFS, the differences were further compared, and the results are shown in Supplementary Figure S2A. The I+A+C mode appeared to be superior. However, no significant differences were detected among the three combination groups.
3.2.4 OS
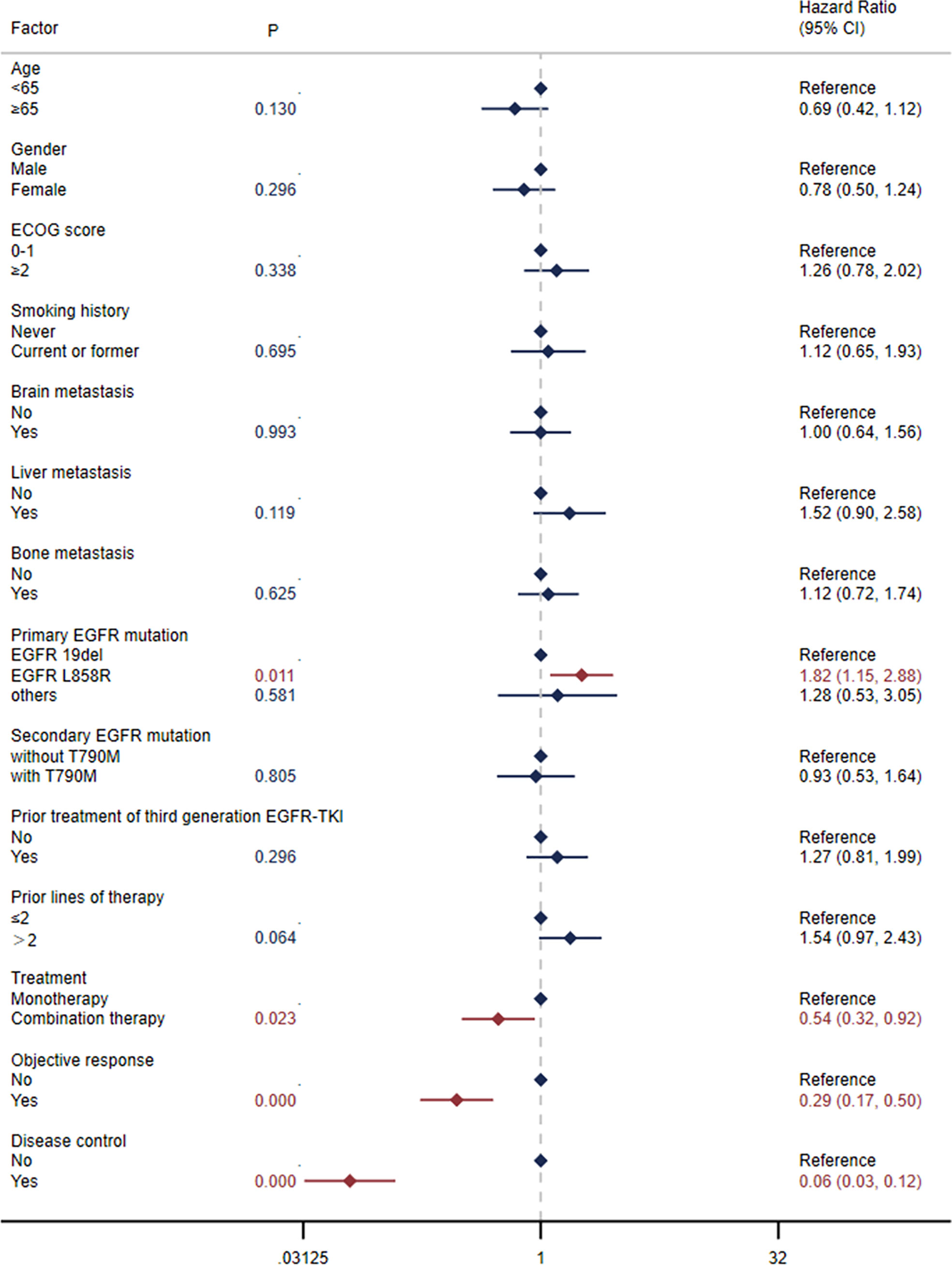
The median OS was 15.9 months (95% CI: 10.8-23.8) in all patients (Figure 1B) and 29.0 months (95% CI: 11.7-NE) in the selected 26 patients (Figure 1D). OS was significantly correlated with primary EGFR mutation, treatment mode, status of liver metastasis, status of bone metastasis, objective response and disease control (Figure 3). In the multivariate model, primary EGFR mutation type, treatment mode, and status of bone metastasis and brain metastasis demonstrated a significant association with OS (Figure 4).
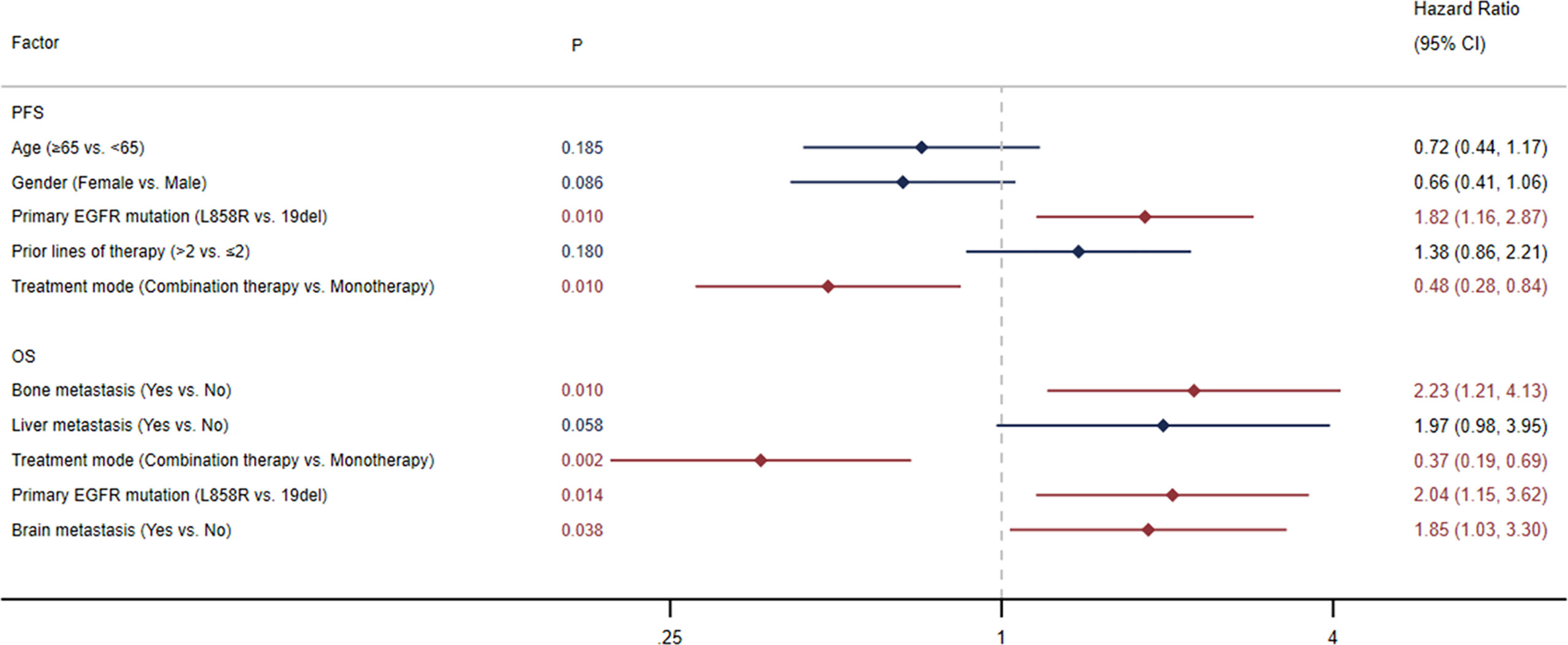
Figure 4 Multivariate Cox regression analysis for progression-free survival (PFS) and overall survival (OS).
The median OS was 9.8 months (95% CI: 6.4-17.3) in patients with an EGFR L858R mutation versus 26.9 months (95% CI: 15.9-31.3) in patients with an EGFR 19del mutation (HR=2.48, 95% CI: 1.37-4.51, log-rank P=0.002; Figure 5B). In patients receiving combination therapy, the median OS was 19.0 months (95% CI: 11.7-29.0) compared with 7.4 months (95% CI: 5.7-15.4) in patients receiving monotherapy (HR=0.46, 95% CI: 0.26-0.83, log-rank P=0.009; Figure 6B). The differences among different combination modes were further compared, and the results are shown in Supplementary Figure S2B. Similar to the result for PFS, the I+A+C mode appears to be superior, but no significant differences were detected among the three combination groups.
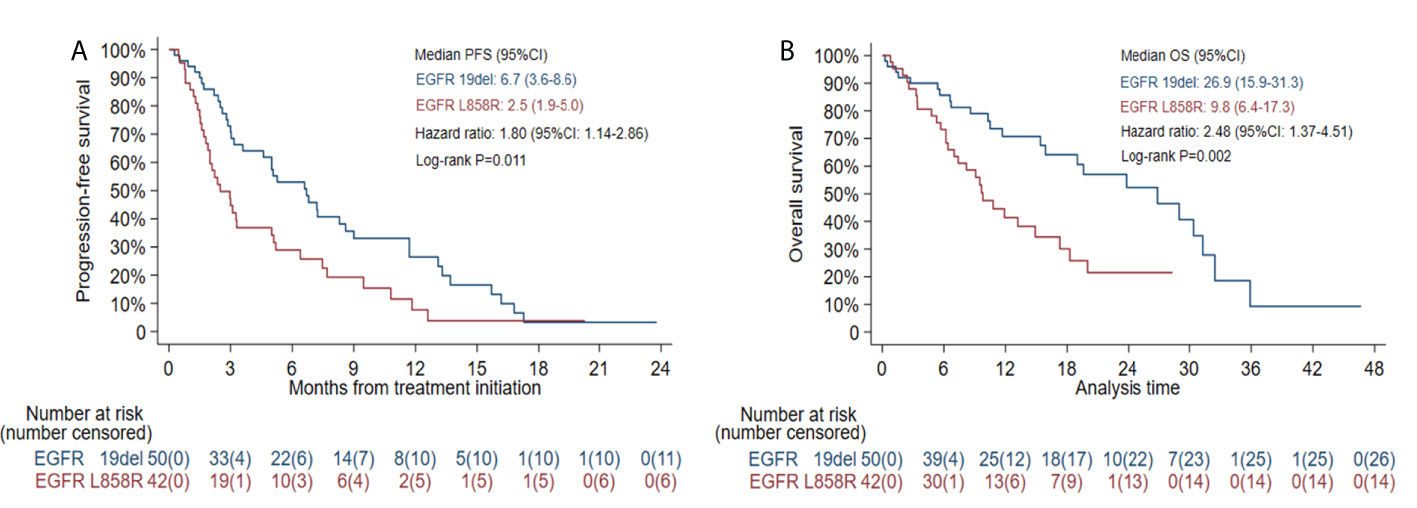
Figure 5 Kaplan-Meier estimates of progression-free survival (PFS) (A) and overall survival (OS) (B) in patients with EGFR exon 19 deletion (19del) or EGFR L858R mutation.
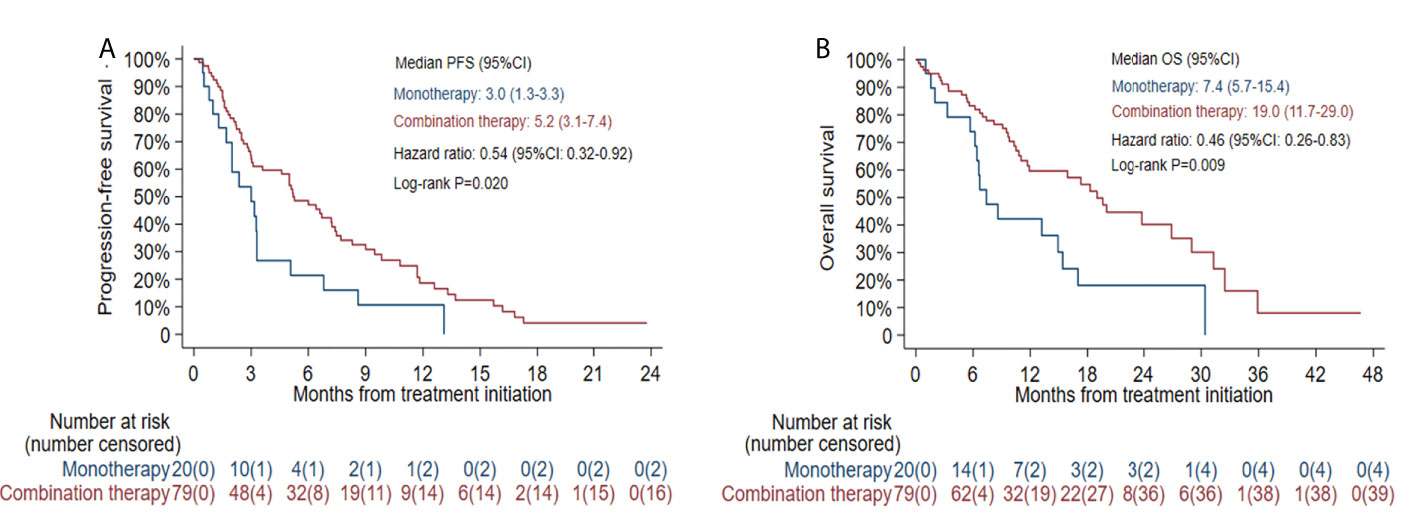
Figure 6 Kaplan-Meier estimates of progression-free survival (PFS) (A) and overall survival (OS) (B) according to different treatment modes.
3.3 Adverse events
Thirty-two of 99 (32.32%) patients experienced Grade 3 and above TRAEs, including fatigue (n=15; 15.15%), neutropenia (n=13; 13.13%), thrombocytopenia (n=5; 5.05%), anemia (n=4; 4.04%), pneumonitis (n=3; 3.03%), vomiting (n=2; 2.02%), rash (n=2; 2.02%), increased alanine aminotransferase (n=1; 1.01%), and hypophysitis (n=1; 1.01%). The any grade irAEs were pneumonitis (n=8; 8.08%), hypothyroidism (n=5; 5.05%), rash (n=3; 3.03%), diarrhea (n=3; 3.03%), myositis (n=2; 2.02%), increased alanine aminotransferase (n=2; 2.02%), myocarditis (n=1; 1.01%), and hypophysitis (n=1; 1.01%). Grade 3 and above irAEs only occurred in 7 (7.07%) patients, including 3 pneumonitis (3.03%), 2 rash (2.02%), 1 increased alanine aminotransferase (1.01%) and 1 hypophysitis (1.01%). Two patients discontinued treatment due to irAEs. No treatment related death was observed.
4 Discussion
Few of real-world data focusing on ICI-based therapy in EGFR-TKI-resistant NSCLC have been reported. Our multicenter study with a relatively large sample size demonstrated a substantial benefit from ICI-based therapy in pretreated EGFR-mutated NSCLC in a real world setting. We found that the prognosis was significantly better in patients carrying the EGFR 19del mutation and receiving combination therapy than in those carrying the EGFR L858R mutation and receiving monotherapy.
EGFR-mutated NSCLC has been reported to be more likely to have an uninflamed tumor microenvironment (23), and PD-1 axis inhibition was once considered a blunt sword in this setting due to its poor effectiveness. However, on the basis of IMpower150, atezolizumab-bevacizumab-carboplatin-paclitaxel (ABCP) has been approved for EGFR/ALK-positive NSCLC after failure with TKIs in Europe. Immunotherapy has begun to show its edge in this setting and several clinical trials are ongoing. In IMpower 150, ABCP was reported to achieve a median OS of 27.8 months in sensitizing EGFR-mutated patients with prior TKI therapy (24). Recently, the phase-II trial of toripalimab plus chemotherapy as a second-line treatment in previously EGFR-TKI-treated patients with EGFR-mutant advanced NSCLC showed results with an overall ORR of 50.0% and DCR of 87.5%. The median PFS and OS were 7.0 and 23.5 months, respectively, in that study, which were superior to historical data of traditional chemotherapy (21). Tislelizumab combined with chemotherapy has been reported to achieve an overall ORR of 59.4% and DCR of 90.6% in a phase II trial (22). Compared with the results of previous studies on single-agent ICIs, these data from prospective clinical trials suggest that combination therapy may significantly improve prognosis despite a lack of direct comparison. In our study, we performed a comparison by using multicenter real-world data and our results support this hypothesis. However, whether differences exist among different combination modes needs to be explored. In addition, although no significant difference was detected according to different ECOG scores and prior lines of therapy, the median PFS and median OS in the selected patients receiving combination therapy with ECOG scores of 0-1 and no more than 2 lines of prior therapy were 7.4 months and 29.0 months, respectively, which is much better than the results in unselected patients. The longer PFS in selected patients suggests that ICI-based combination therapy might be more beneficial if it was applied as early as possible after EGFR-TKI resistance when patients had a better performance status.
Mounting evidence has supported the differences among EGFR-mutant subtypes. Although both 19del and L858R are classic EGFR mutations, they have different biological behaviors and responses to TKI treatment, as well as different resistance mechanisms to TKIs (25, 26). The differences in ICI efficacy between cases with EGFR 19del and those with EGFR L858R mutations are controversial. Our study indicated that patients with EGFR 19del could get more benefits than those with EGFR L858R. This difference might be explained by the potential differences in PD-L1 expression, tumor mutation burden (TMB) and the tumor microenvironment, such as CD8+ T-cell infiltration (18, 27, 28). Takada’s study retrospectively examined the relationship between PD-L1 expression and EGFR status in 441 surgically resected primary lung adenocarcinomas. They found that the prevalence of PD-L1 TPS 5-49% was higher among patients with an EGFR 19del than with an EGFR L858R mutation (28). On the other hand, Hastings’ study examined the molecular and clinical features of 171 EGFR mutant lung cancer cases treated with anti-PD-(L)1 alone or in combination with anti-CTLA-4 and found that outcomes were worse in patients with EGFR 19del but similar for EGFR L858R compared with EGFR wild-type NSCLC. They further assessed the relationship between TMB and specific EGFR alterations in a separate cohort of 383 EGFR mutant lung cancer cases irrespective of treatment exposure and found that EGFR 19del had a lower TMB than EGFR L858R lung tumors, which might account for the different response to ICIs (18). However, a study with 9649 Chinese primary NSCLC patients reported no significant differences in PD-L1 expression, TMB level or CD8+ T-cell infiltration between these two EGFR subtypes irrespective of treatment exposure (29). Notably, several studies have shown the impact of EGFR-TKI treatment on PD-L1 expression, TMB and the tumor immune microenvironment in EGFR-mutated NSCLC (30, 31). Isomoto et al. indicated that PD-L1 expression in tumor cells was significantly increased and TMB tended to be increased after resistance to EGFR-TKI treatment (30). Nishii et al. suggested that pretreatment with EGFR-TKI can induce CD8+ T-cell responses in EGFR-mutant NSCLC tumors, which was further pronounced by sequential dual blockade of PD-1 and vascular endothelial growth factor receptor 2 (31). All the above evidences demonstrate that the tumor immune microenvironment in EGFR-mutated NSCLC is dynamic. As a result, in order to explore the differences in ICI efficacy between EGFR subtypes, it is essential to detect potential predictive biomarkers at the same time point.
Some limitations exist in this study. First, this is a retrospective study, and some clinical data, such as PD-L1 expression are lacking. Although PD-L1 expression status is considered predictive for benefit from PD-1 or PD-L1 blockade therapy, it alone is insufficient in determining which patients should be offered PD-1 or PD-L1 blockade therapy (32). The PD-L1 expression during TKI treatment is dynamic just as above discussed. Further investigations are needed to examine this relationship and the potential mechanism. Second, various biases on the conditions of the patients exist, such as treatment mode, prior treatment lines and the performance status of the patients. The retrospective design and the sample size might lead to these biases. More large-scale studies are needed to establish the best combination mode. Third, there is not enough data to explore the relationship between comutations with EGFR and ICI efficacy in this kind of patient, as our previous study indicated comutation of EGFR and PI3K signaling had interaction effects on ICI treatment in nonsquamous NSCLC (33).
5 Conclusion
In conclusion, our study suggests that ICI-based combination therapy is a promising strategy in EGFR-mutated NSCLC after EGFR-TKI failure. The efficacy might be better in patients with EGFR 19del than in those with EGFR L858R.
Data availability statement
The relevant data supporting the conclusions of this article will be available by contacting the corresponding authors upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of PLA General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study design: XL, JM, and YH. Data collection: all authors. Data analysis: JH and DH. Manuscript writing: JH and YW. Manuscript editing and approval: all authors.
Acknowledgments
Thanks to PLA General Hospital for supporting the study. Thanks all the patients and their families, and all the investigators involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.975246/full#supplementary-material
References
1. Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic therapy for stage IV non-Small-Cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol (2017) 35(30):3484–515. doi: 10.1200/JCO.2017.74.6065
2. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN guidelines insights: Non-small cell lung cancer, version 1. 2020. J Natl Compr Canc Netw (2019) 17(12):1464–72. doi: 10.6004/jnccn.2019.0059
3. Minari R, Bordi P, Tiseo M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance. Transl Lung Cancer Res (2016) 5(6):695–708. doi: 10.21037/tlcr.2016.12.02
4. Levantini E, Maroni G, Del Re M, Tenen DG. EGFR signaling pathway as therapeutic target in human cancers. Semin Cancer Biol (2022) 12:S1044–579X(22)00096-7. doi: 10.1016/j.semcancer.2022.04.002
5. John T, Akamatsu H, Delmonte A, Su WC, Lee JS, Chang GC, et al. EGFR mutation analysis for prospective patient selection in AURA3 phase III trial of osimertinib versus platinum-pemetrexed in patients with EGFR T790M-positive advanced non-small-cell lung cancer. Lung Cancer (2018) 126:133–8. doi: 10.1016/j.lungcan.2018.10.027
6. Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer (2018) 17(1):38. doi: 10.1186/s12943-018-0777-1
7. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 376(7):629–40. doi: 10.1056/NEJMoa1612674
8. Nishimura T, Okano T, Naito M, Iwanaka S, Ohiwa A, Sakakura Y, et al. Second-line therapy with first- or second-generation tyrosine kinase inhibitors in EGFR-mutated non-small cell lung cancer patients with T790M-negative or unidentified mutation. Thorac Cancer (2021) 12(7):1067–73. doi: 10.1111/1759-7714.13870
9. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: Implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol (2015) 10(6):910–23. doi: 10.1097/JTO.0000000000000500
10. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol (2014) 25(10):1935–40. doi: 10.1093/annonc/mdu242
11. Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, et al. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer (2019) 18(1):165. doi: 10.1186/s12943-019-1073-4
12. Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res (2020) 26(8):2037–46. doi: 10.1158/1078-0432.CCR-19-2027
13. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
14. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
15. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
16. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol (2017) 12(2):403–7. doi: 10.1016/j.jtho.2016.10.007
17. Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol (2018) 19(4):521–36. doi: 10.1016/S1470-2045(18)30144-X
18. Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol (2019) 30(8):1311–20. doi: 10.1093/annonc/mdz141
19. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol (2019) 30(8):1321–8. doi: 10.1093/annonc/mdz167
20. Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf (2017) 16(4):465–9. doi: 10.1080/14740338.2017.1300656
21. Jiang T, Wang P, Zhang J, Zhao Y, Zhou J, Fan Y, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther (2021) 6(1):355. doi: 10.1038/s41392-021-00751-9
22. Han B, Tian P, Zhao Y, Yu X, Guo Q, Yu Z, et al. 148P a phase II study of tislelizumab plus chemotherapy in EGFR mutated advanced non-squamous NSCLC patients failed to EGFR TKI therapies: First analysis. Ann Oncol (2021) 32:S1443–4. doi: 10.1016/annonc/annonc787
23. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology (2017) 6(11):e1356145. doi: 10.1080/2162402X.2017.1356145
24. Nogami N, Barlesi F, Socinski MA, Reck M, Thomas CA, Cappuzzo F, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol (2022) 17(2):309–23. doi: 10.1016/j.jtho.2021.09.014
25. Lee CK, Wu YL, Ding PN, Lord SJ, Inoue A, Zhou C, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: A meta-analysis. J Clin Oncol (2015) 33(17):1958–65. doi: 10.1200/JCO.2014.58.1736
26. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-lung 3 and LUX-lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol (2015) 16(2):141–51. doi: 10.1016/S1470-2045(14)71173-8
27. Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res (2019) 25(3):1063–9. doi: 10.1158/1078-0432.CCR-18-1102
28. Takada K, Toyokawa G, Tagawa T, Kohashi K, Shimokawa M, Akamine T, et al. PD-L1 expression according to the EGFR status in primary lung adenocarcinoma. Lung Cancer (2018) 116:1–6. doi: 10.1016/j.lungcan.2017.12.003
29. Lei Y, Wang K, Liu Y, Wang X, Xiang X, Ning X, et al. Various subtypes of EGFR mutations in patients with NSCLC define genetic, immunologic diversity and possess different prognostic biomarkers. Front Immunol (2022) 13:811601. doi: 10.3389/fimmu.2022.811601
30. Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI Treatment on the Tumor Immune Microenvironment in EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin Cancer Res (2022) 26(8):2037–2046. doi: 10.1158/1078-0432.CCR-19-2027
31. Nishii K, Ohashi K, Tomida S, Nakasuka T, Hirabae A, Okawa S, et al. CD8+ T-cell responses are boosted by dual PD-1/VEGFR2 blockade after EGFR inhibition in egfr-mutant lung cancer. Cancer Immunol Res (2022) cir.21.0751. doi: 10.1158/2326-6066.CIR-21-0751
32. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ (2018) 362:k3529. doi: 10.1136/bmj.k3529
Keywords: immune checkpoint inhibitor, epidermal growth factor receptor (EGFR) mutation, epidermal growth factor receptor tyrosine kinase inhibitor (EGFR) TKI, non-small cell lung cancer, combination therapy
Citation: Hu J, Huang D, Wang Y, Li D, Yang X, Fu Y, Du N, Zhao Y, Li X, Ma J and Hu Y (2022) The efficacy of immune checkpoint inhibitors in advanced EGFR-Mutated non-small cell lung cancer after resistance to EGFR-TKIs: Real-World evidence from a multicenter retrospective study. Front. Immunol. 13:975246. doi: 10.3389/fimmu.2022.975246
Received: 22 June 2022; Accepted: 11 August 2022;
Published: 09 September 2022.
Edited by:
Jiajun Du, Shandong Provincial Hospital, ChinaReviewed by:
Chengzhi Zhou, Clinical Management Department of National Respiratory Medical Center, ChinaHiroshige Yoshioka, Kansai Medical University, Japan
Weitao Zhuang, Guangdong Provincial People’s Hospital, China
Copyright © 2022 Hu, Huang, Wang, Li, Yang, Fu, Du, Zhao, Li, Ma and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaosong Li, bGl4aWFvc29uZ0Bob3RtYWlsLmNvbQ==; Junxun Ma, bWFqdW54dW43NEAxMjYuY29t; Yi Hu, aHV5aTMwMXpseGJAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
 Jia Hu
Jia Hu Di Huang
Di Huang Yanrong Wang1†
Yanrong Wang1† Nan Du
Nan Du Yi Hu
Yi Hu