- Georgia Cancer Center and Department of Pediatrics, Medical College at Georgia (GA), Augusta, GA, United States
Lymphocytes in tumor tissue are called tumor-infiltrating lymphocytes (TILs), and they play a key role in the control and treatment of tumor diseases. Since the discovery in 1987 that cultured TILs can kill tumor cells more than 100 times more effectively than T-cells cultured from peripheral blood in melanoma, it has been confirmed that cultured TILs can successfully cure clinical patients with melanoma. Since 1989, after we investigated TIL isolation performance from solid tumors, we modified some procedures to increase efficacy, and thus successfully established new TIL isolation and culture methods in 1994. Moreover, our laboratory and clinicians using our cultured TILs have published more than 30 papers. To improve the efficacy of TILs, we have been carrying out studies of TIL efficacy to treat solid tumor diseases for approximately 30 years. The three main questions of TIL study have been “How do TILs remain silent in solid tumor tissue?”, “How do TILs attack homologous and heterologous antigens from tumor cells of solid tumors?”, and “How do TILs infiltrate solid tumor tissue from a distance into tumor sites to kill tumor cells?”. Research on these three issues has increasingly answered these questions. In this review I summarize the main issues surrounding TILs in treating solid tumors. This review aims to study the killing function of TILs from solid tumor tissues, thereby ultimately introducing the optimal strategy for patients suffering from solid tumors through personalized immunotherapy in the near future.
Introduction
Tumor-infiltrating lymphocyte (TIL) therapy, one of the best adoptive immunotherapies (AITs) or adoptive cell therapies (ACTs), was discovered about 35 years ago, when Dr Steven Rosenberg used TILs to treat melanoma in 1988 (1–3). At a similar time, we were studying TIL cytotoxicity and the killing of tumor cells from solid tumors (4–10). After studying TIL cytotoxicity primarily related to T-cell isolation from solid tumors, we modified procedures and successfully used TIL isolation from solid tumors to treat several hundred patients suffering from solid tumors. We have produced more than 30 related publications since 1994 (11–18).
Although TIL immunotherapy has been broadly accepted to treat melanoma, variable responses mean that the efficacy of TILS in treating solid tumor diseases is still questioned (19, 20). Despite the fact that TILs are increasingly seen to have benefits that make them applicable for cancer treatment in some clinical laboratories, two conflicting opinions on efficacy still remain with regard to TIL immunotherapy to treat solid tumors. The first is that, even if TIL responses are powerful, undergoing a 1,000-fold expansion after activation (1), some reports show that TIL immunotherapy could not reliably produce effective responses in treating solid tumors in other clinical laboratories, especially in some early research reports (21, 22). The second issue is variable results to assay the cytotoxicity of tumor-specific lymphocytes to tumor cells for different tumor diseases (23). According to several laboratories’ reports, the anti-tumor cytotoxic activity of TILs can be identified in tumor samples of more than 80% of patients with melanoma, whereas the results from solid tumor diseases fluctuate (24, 25). However, two reports suggest that TILs have promising prospects; for example, they report TILs specifically traveling into the site of a solid tumor mass to kill tumor cells and TILs maintaining their therapeutic efficacy for many years after initial treatment (26, 27).
Because TILs actively killing human tumor cells from solid tumors remains controversial, and to address the auguring, this review presents three issues regarding the efficacy of TILs from solid tumors. Finally, I briefly present the future of adoptive TIL immunotherapy as strategies of personalized immunotherapy (28).
Why do TILs have variable efficacy in the treatmeant of solid tumors?
After Dr Rosenberg discovered that TILs can be cultured with the aid of the cytokine interleukin (IL)-2 and induced TIL-exhibited cytotoxic activity against melanoma cells in vitro, TILs isolated from tumor samples were studied in the earliest trials of ACT conducted at the surgical branch of the National Cancer Institute (NCI) in 1988 (29, 30). At that time, objective responses were observed in 11 out of 20 patients with metastatic melanoma. Five out of the 29 (17%) responses were complete responses (CRs), with a median duration of response of 4 months in these early studies (31). After more than 30 years of research and development (R&D), TILs have shown promising overall response rates (38%) for the treatment of recurrent/refractory melanoma according to clinical trial results presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting (32). Now, patients are first treated with cyclophosphamide (or fludarabine) (lymph depletion) for 1 week, followed by TIL infusion and then six doses of adjuvant IL-2. The TIL manufacturing process takes about 22 days (33).
Through studying TIL cytotoxicity mechanisms to autologous tumor cells from solid tumors during that early period, we discovered that TILs had variable efficacy in the treatment of patients with solid tumors (6, 34). To address how to increase TIL efficacy in the treatment of solid tumors, we began to study all procedures regarding TIL purification and culture from solid tumors. After spending approximately 30 years studying this, here I summarize the three issues discovered through the investigations as Silencing TILs in the tumor location, Heterogeneous immune response from heterogeneous tumor antigens, and Infiltrating TIL into solid tumors. I refer to these three issues as the “SHI” phenomenon (“SHI” in Chinese translates as “STONE”, whereas in English it is the same as the traditional Chinese medicine term for “tumor disease”).
Silencing TILs
In 1995, after analyzing TIL proliferation, cytotoxicity, and phenotype from 83 solid tumor cases, we reported that TILs have quiescent status if the TIL is located in solid tumor tissues (35). Although we discovered freshly isolated TIL-containing activated T-cell surface markers such as Human Leukocyte Antigen – DR isotype (HLA DR) and IL-2 receptors, the cells did not have strong cytotoxicity against tumor cells. In this early study, results regarding silencing TILs showed that the supernatants of TIL culture media contained inhibiting factors, including some things from autologous tumor cells. To overcome the inhibiting factors, we studied them and modified our methods, such as eliminating autologous tumor cells during TIL culture procedures, so that in this early period we achieved good responses to increasing cytotoxicity to autologous tumor cells from solid tumors (36). After a long period of study, now clinical scientists and clinical immunologists understand that there are two major factors in TIL quiescence influencing efficacy: internal factors and external factors.
Internal mechanism
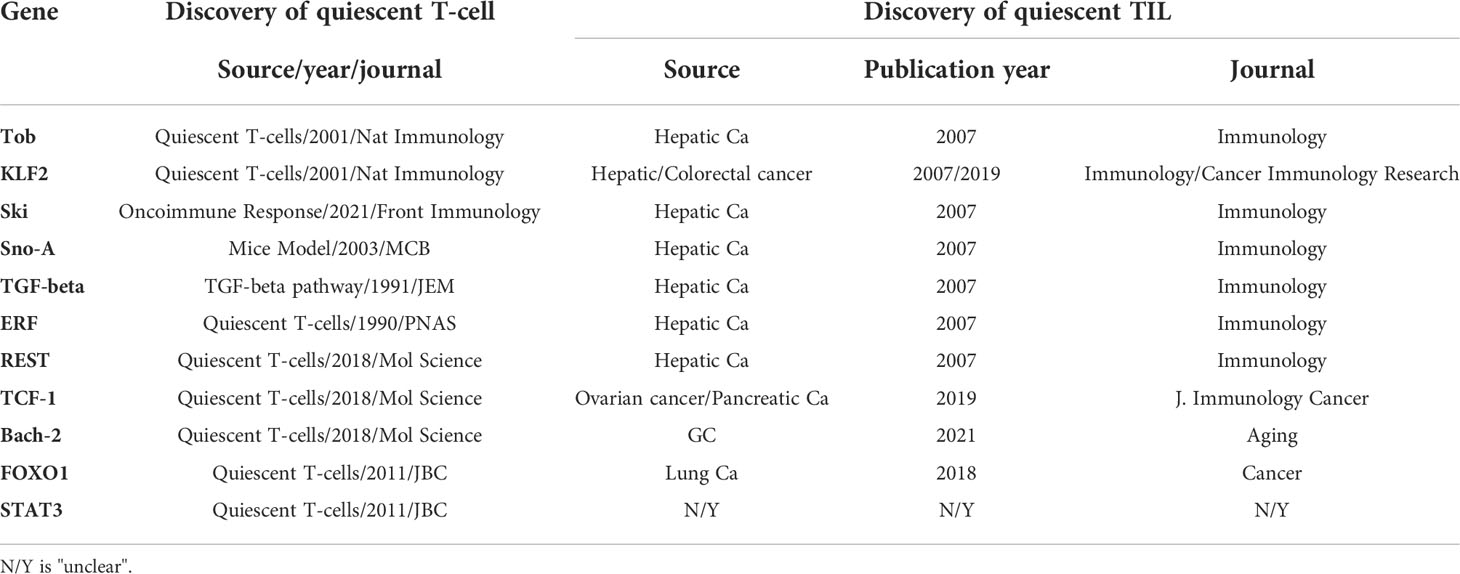
Silencing T-lymphocytes (quiescent lymphocytes) are a group of T-cells that display no spontaneous proliferation and a low metabolic rate (37). In silencing lymphocytes, quiescence reduces consumption of resources to maintain the vast repertoire of lymphocytes. Only a small fraction of native lymphocytes will be clonally selected by antigens during the lifetime of the host. From observation of lymphocyte quiescence, six mechanisms have been proposed to produce quiescence of lymphocytes (38–42): (1) thymus negative selection, (2) peripheral clonal deletion, (3) peripheral-induced anergy, (4) T-cell ignorance/indifference, (5) T-cell suppression, and (6) T-cell senescence (exhaustion). Lymphocyte quiescence obtained from solid tumors is caused by one or more factors of the six mechanisms, and, therefore, TIL quiescence may or may not produce immune cytotoxicity to tumor cells. To elucidate TIL quiescence from solid tumors, we screened and harvested quiescent TILs from those of hepatic cell cancers (HCCs). Before the genomic era (1996–2004), we used a single-cell messenger ribonucleic acid (mRNA) display system to find a profile from the quiescent cluster of differentiation 8 (CD8) TILs (43–45). After that, because microarray and ribonucleic acid sequencing (RNA-seq) began to be applied for single-cell genomic analyses, we carried out further studies of the set of quiescent genes by single-cell RNA-seq, as mentioned below. Now, most of the TIL quiescent genes have been confirmed by using single-cell quantitative reverse transcription polymerase chain reaction (RT-PCR) from both single-cell mRNA displays and single-cell RNA-seqs. The genes comprised at least seven genes, including Tob, LKLF (lung Krüppel-like factor), transforming growth factor (TGF)-beta, Sno, Ski, RE1 silencing transcriptor (REST) and ETS2 repressor factor (ERF). Nowadays, several laboratories have increasingly supported the results; as shown in Table 1, more genes, such as BTB Domain and CNC Homolog 2 (BACH2), Forkhead Box O1 (FOXO1) and Signal Transducer and Activator Of Transcription 3 (STAT3), have been found to be involved in quiescent lymphocytes. This finding comes after more than two decades of studying.
As shown in Table 1, the data for which come from different research laboratories, TOB (transducer of ERBB2) is a negative regulator of IL-2 transcription and T-cell for T-cell proliferation, which inhibits the Ag-MHCII pathway (46). LKLF (or KLF2) is a zinc finger-containing transcription factor that plays a negative regulatory role in cytotoxic T-lymphocyte (CTL), killing tumor cells by blocking the mimicry of IL2, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ (47). Ski, Sno and TGF-β are involved in the TGF-β pathway to maintain T-cell quiescence (48, 49). ERF negatively regulates TIL infiltration, migration, and migration to tumor sites (50). The REST gene, like PD-1 and CTLA-4, is involved in blocking the PI3K pathway (51). There are increasing reports of The transcription factor T cell factor 1 (TCF-1), Bach-2, FOXO1 and STAT3 as Krüppel-like Factor 2 (KLF2), which can inhibit T-cells blocking the Cytotoxic T lymphocytes (CTL) pathway (52).
External factors
As mentioned above, in 1994 we reported that TIL proliferation and cytotoxicity during TIL culture were inhibited by autologous tumor cells from solid tumors (35). To increase the expansion and cytotoxicity of TILs, we performed clean procedures during the isolation and culture of TILs, such as the adhesion process that clears autologous tumor cells from HCC and lung cancer (36). After two decades of effort, it was discovered that the so-called tumor microenvironment (TME) suppression phenomenon can maintain TIL quiescence and affect TIL cytotoxicity during TIL culture (53). The TME consists of three components (54–60): (1) a tissue called the extracellular matrix (ECM) with epithelium, basal and endothelium; (2) regulatory cells including tumor-associated macrophages (TAMs), neutrophils (tumor-related neutrophils-2, TAN-2), cancer-associated fibroblasts (CAFs), and myeloid-derived suppressor cells (MDSCs); and (3) signaling molecules by releasing extracellular signals, promoting tumor angiogenesis, promoting tumor cell growth and affecting tumor growth. Lymphocyte quiescence of the TME has been extensively reported in vivo, in vitro, and ex vivo. All ECMs have been shown to produce TGF-β, which blocks TIL function, including the inhibition of TIL growth and cytotoxicity (61). In addition, some signaling molecules can affect the growth of TILs by releasing extracellular signal pathway, such as adenosine (ADO) signaling molecules and indole 2,3-dioxygenase (IDO) regulatory molecules (62, 63). Adenosine triphosphate (ATP) is a popular molecule that plays a vital role as a universal energy currency within cells. ATP induces immunogenic cell death (ICD) of tumor cells at the tumor site and promotes immune surveillance in the TME, whereas ADO increases the lead to ADO immune dysfunction in T-cells, NK cells, and B cells at the tumor site. A secondary regulatory pathway that impedes T-cell proliferation in the TME is the IDO pathway. Dendritic cells (DCs,) myeloid-derived suppressor cells (MDSCs) and tumor cells can produce IDO, which breaks down tryptophan and produces kynurenine, resulting in tryptophan deprivation and production of its metabolites to inhibit the expansion of clonal T-cells.
Heterogeneous immune response from heterogeneous tumor antigens
To learn more about the immune response of TILs to autologous tumor cells, we have spent about 15 years studying the different TIL responses with their genes from solid tumors through single-cell gene expressions and individual immune responses in networks. After these studies from our experiments or from other data, we learned that TILs will produce responses to autologous tumor cells, including self-tolerance to homologous antigens, heterogeneous responses, and heterogeneous networks from TILs to autologous tumor cells.
Self-tolerance against homologous antigens
Heterogeneous immune responses and homologous immune responses are some of the key issues in addressing the efficacy of TILs on autologous tumor cells (64). Effective TIL CD8 cytotoxicity against autologous tumor cells results in TIL efficacy (65). Human tumor antigens are divided into two main types: shared tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs). TAAs include (1) cancer-testis (CT) antigens, (2) differentiation antigens (DAs) and (3) onco-fetal antigens (OFAs). TSAs include neo-antigens and tumor viral antigens such as hepatitis B (HBV), Epstein-Barr Virus (EBV), cytomegalovirus (CMV), and human papillomavirus (HPV) E6/E7 proteins.
CT antigens (such as MAGE-1) are present for some time during the spermatogenesis and placental stages, and they are also more highly expressed in different types of neoplastic diseases (66). Normally they are silent in adult tissues, and, therefore, the transcriptional machinery is stimulated in certain tumor types. Although many types of tumors express CT antigens at high levels and corresponding normal tissues at low levels, they are sometimes expressed at high levels in normal tissues. Differentiation antigens (such as gp100) are encoded by genes that are expressed in a tissue-specific manner (67). These proteins are usually produced in very low amounts, but their production is dramatically increased in tumor cells to activate immune responses. An example of such a protein is tyrosine, which is required for melanin production. They also share antigens between tumor cells and corresponding normal cells. Most of these antigens, such as gp100 glycoprotein and MART-1, are mainly present in metastatic melanoma in solid tissues (68, 69). Carcinoembryonic antigens are the third important TAA tumor antigens, such as alpha fetoprotein (AFP) and carcinoembryonic antigen (CEA) (70, 71). AFP is highly expressed in hepatocellular carcinoma (HCC) the most common type of primary liver cancer , and CEA is often highly expressed in colon cancer and other tumors such as NSCLC (non-small cell lung cancer). CT antigen, differentiation antigen, and onco-fetal antigen are produced early in the embryonic development stage and disappear when the adult immune system is fully developed, so self-tolerance may result in quiescent TILs against these antigens (72).
Heterogeneity from T-cell neo-antigens
Neo-antigens caused by genetic instability during carcinogenesis appear in non-coding and coding regions, whereas amino acid sequence changes caused by mutations in coding regions can generate antigens that do not exist in normal cells (73). Neo-antigens can also be induced by viral infection, such as alternative splicing and gene rearrangements (74). These variant antigens can be recognized by immune cells, such as TILs, and are, theoretically, specific immune responses to tumor cells, without autoimmunity to autologous normal cells.
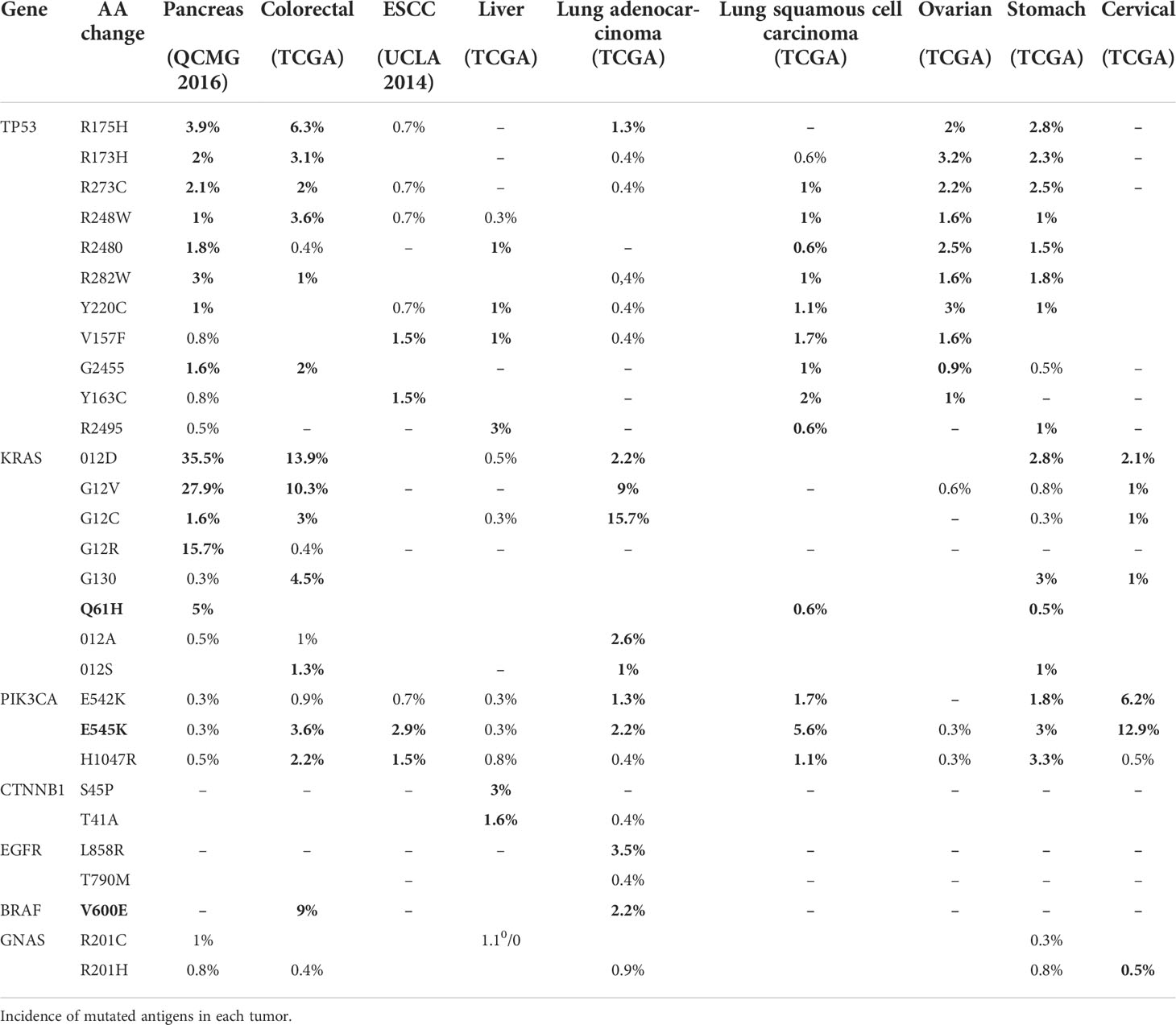
There are two types of neo-antigens: shared neo-antigens and personalized neo-antigens (75, 76). Shared neo-antigens refer to mutated antigens that are common in different cancer patients and not present in the normal genome, have a high immunogenic potential for screening, and are now used as broad-spectrum therapeutic cancer vaccines or adoptive immunotherapy for cancer patients, as shown in Table 2. Personalized neo-antigens refer to mutated antigens that differ from patient to patient (77, 78). Therefore, personalized neo-antigens can be targeted specifically for each patient, which can be used to design personalized immunotherapy.
Different types of neo-antigens (TSAs) and different amounts of TAAs can exist in the same tumor in different individuals, resulting in individual immune responses, so TIL administration should be developed into an individualized design for immunotherapy. Based on personalized information such as individual genomic data, personalized TIL therapy can increase the intensity and durability of antitumor effects, improve survival and quality of life, and ultimately improve cancer treatment outcomes for patients. For most patients with solid tumors, personalized TIL therapy is expected to be more feasible and safe.
Heterogeneous network response
To investigate individualized immune responses in different patients, we have studied TIL responses with distinct networks, even if their TILs were derived from patients with nearly similar clinical and pathological outcomes. Under analysis of their pathways and networks, through achieving gene expression patterns by single-cell gene expressions and analyzing molecular expressions from the heterogeneous responses of TIL CD8 cells, we found that small gene expression changes between two TILs can reveal individual immune responses in the network. A network study (79) of two TIL CD8 cells from two patients showed that Tob-1 gained some similar higher expressions, suggesting a common immune response in its quiescent network. Once one or more proteins are added to the network, changes in the network can occur. For example, TGFB1 and ERF underwent a greater increase in sample 1, whereas Sno-A and REST underwent a greater expression in sample 2, so, eventually, the granzyme B and perforin in CTL expressed were completely different. The results demonstrate that TILs can reveal individual immune responses in the network, and thus, artificial intelligence network analysis can be used in TIL personalized immunotherapy.
Infiltrating TILs into tumor tissue to attack tumor cells
The infiltration of TILs into tumor tissue to kill tumor cells has been studied in two areas (1): TIL CD8 cells should have intact signaling molecules after harvesting TILs from tumor tissue (4–6, 80), and (2) how ex vivo-cultured TILs specifically enter tumor tissue from circulating blood after reinfusion (81). The first question has been studied by our colleagues for more than 30 years.
CTL signal intactness
Earlier, we found changeable results based on Dr Rosenberg’s tumor disaggregation (with triple enzymes by collagenase type-IV, hyaluronidase, and DNase) to harvest and culture TILs for clinical treatment if we used the disaggregation procedures from solid tumors. Considering the TIL results in experimental and clinical work to treat solid tumors, we studied the TIL functions such as TIL proliferation, cytotoxicity, and phenotype with three enzymes under their conditions (37°C) (5). Finally, after modifying the enzymes’ condition, once collagenase IV under very moderate digestion condition the TILs’ function can be kept with an optimal proliferation, activity, and cytotoxicity to kill autogenous tumor cells. As stated in earlier publications (5, 6, 82), we had isolated and cultured TILs from solid tumors using the mild enzymatic digestion (cold enzymatic digestion with collagenase IV only), and the results showed that 65% of TILs proliferated more than 1,000 fold. The (3)TdR incorporation rate peaked at 45–75 days. Cytotoxicity to tumor cells was maintained for 56 days. The phenotypes of TILs after IL-2 induction were CD3 80 ± 21%, CD4 37 ± 21%, CD8 44 ± 18%, and HLA DR 69 ± 24%. CD3 and CD8 were significantly higher than in other clinical laboratories. Our clinical trials to treat solid tumors had shown better treatment outcomes than data from other clinical laboratories (7). Since then, most clinical laboratories chose collagenase IV only to disaggregate tumor tissues to harvest TILs. After 30 years’ study, we discovered that current collagenase IV products still contain other trace protease functions, such as trypsin-like activity. Collagenase IV products influence intact molecules on CD8 cells because abundant molecules are kept on the surface of TIL CD8 cells. Now, we are going to study the collagenase structure to influence TIL functions on CD8 cells. Hopefully, the functionally or genetically modified collagenase IV will delete trypsin-like activity. All in all, even if the trypsin activity of collagenase products is very low, abundant molecules on the surface of TIL CD8 cells, which are influenced by collagenase, are important for the adoptive TIL immunotherapy to solid tumors (4, 83).
TIL moving, migration, and infiltrating
Regardless of whether TILs can enter tumor tissue after isolation, culture, and infusion of TILs for immunotherapy, in early events, TILs cannot be fully characterized as tumor-specific T-cells in vivo. The mobilization of TILs into tumor tissue has become increasingly accepted after two experiments: Dr Torcellan’s in vivo TIL light-labeling technique in 2017 and Bai’s use of on-site antigen presentation for clonal expansion in 2001 (84, 85).
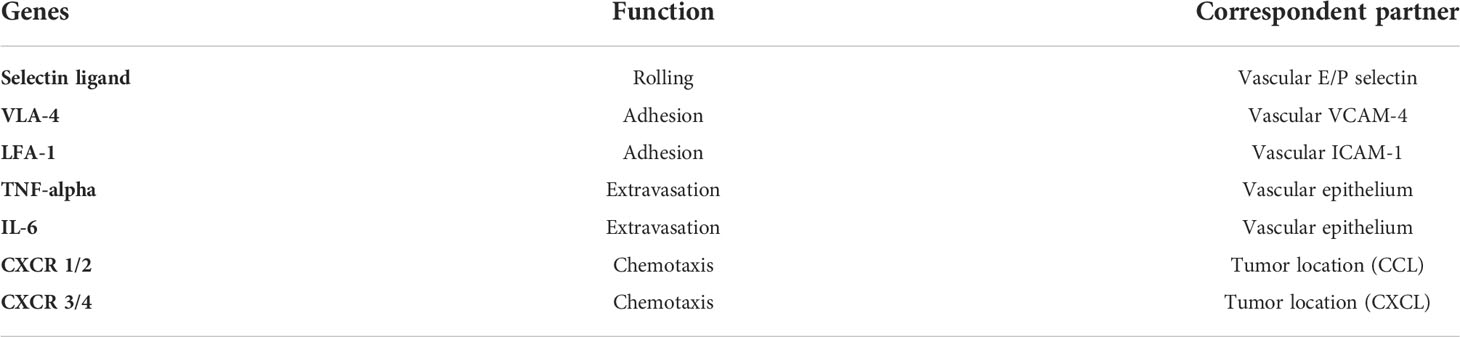
Despite the fact that T-cells play an important role at the tumor site, the specific transport capacity of TILs is dependent on dynamic processes such as rolling, adhesion, extravasation, and chemotaxis, as shown in Table 3 (86, 87). Because successfully killing tumor cells in solid tumors relies on a high frequency of TILs, TILs that target tumor cells may also include TIL homing processes influenced by immunosuppression and abnormal vasculature (88, 89).
Now, CXCR2 (the receptor for CXCL1 secreted by tumor cells), CCR4 (CCL17, the ligand for CCR4), and others have been modified to enhance T-cell trafficking to the tumor site (90–92). These studies suggest that future combination therapy may enhance the efficacy and homing ability of adoptively transferred T-cells in patients.
How can TILs have stable efficacy in the treatment of solid tumors?
As mentioned above, we discovered in our early research that TILs have variable efficacy in the treatment of solid tumors: we used some strategies to resolve some questions, as shown in Figure 1, in a clinical laboratory, and, as shown in Figure 2, in clinical TIL application (93–100).
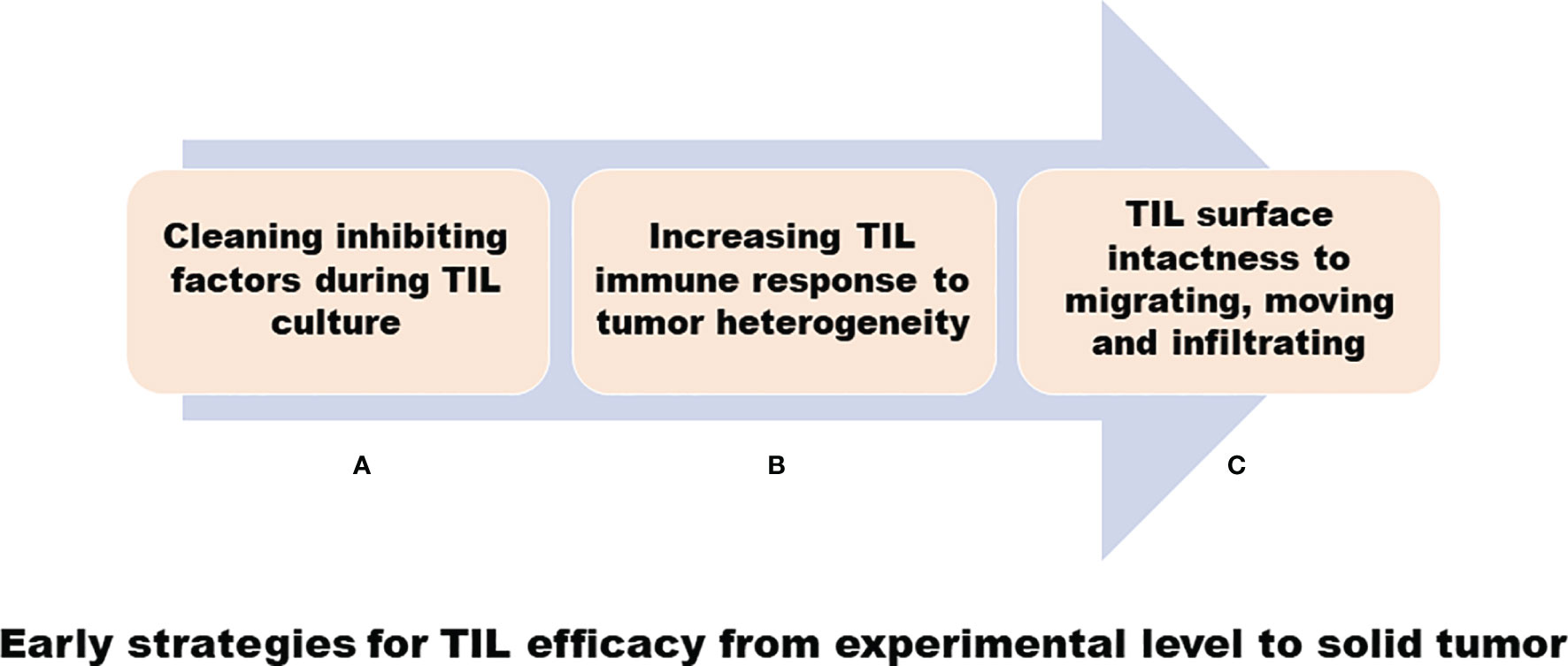
Figure 1 Early strategies for TIL efficacy from experimental level to solid tumor. (A) A cleaning inhibiting factor such as removing tumor cells during TIL cultures. (B) Increasing TIL immune response to tumor cells, including transducing TNA-α gene into TIL for heterogeneous tumor antigens. (C) Mild collagenase IV digestion to disaggregate tumor tissues to harvest TIL to keep the lymphocyte surface intact.

Figure 2 Clinical strategies for TIL efficacy. (A) A lympho-depleting regimen adding TIL treatment to increase TIL efficacy. (B) A clinical procedure combining TILs with sensitive chemotherapeutic agents, which were screened using chemo-sensitivity assay (CSA) from TIL cytotoxicity experiment of patients’ autogenous tumor cell to increase treatment response.
Cleaning inhibiting factors
As mentioned above, the results of silencing TILs showed that the supernatant of TILs’ medium and mixed autologous tumor cells contained inhibitory factors. To overcome these inhibitory factors, we investigated some cleaning methods, such as removing autologous tumor cells during TIL culture to obtain a good response to increasing cytotoxicity against autologous tumor cells derived from solid tumors (36). After a long period of research, now clinical scientists and clinical immunologists understand that there are at least two broad categories to keep TIL quiescence – internal factors and external factors, as described above – which are required to block, such as the PD-1 antibody blocker (101–105).
Increasing immune response to tumor cells
In our early research, to increase immune responses to kill tumor cells, we studied transduced retroviral vectors with TNF-α genes in TILs to increase TIL cytotoxicity function (93–96). To increase immune responses to tumor heterogeneous antigens, some clinical experiments remodeled tumor neo-antigens to T-cells, whereas we studied TIL responses with tumor antigen response with distinct networks (79). For example, after profiling gene expressions by establishing single-cell gene expressions and analyzing molecular expressions of heterogeneous responses of TIL CD8 cells, we found silent gene expressions by TIL CD8 cells such as Tob-1, KLF2, TGFB1, and ERF in solid tumors, which will be discussed below.
Increasing TIL contact tumor cells
Although adoptive cell therapy using ex vivo-activated autologous TIL intravenous infusion is considered one of the promising approaches, early adoptive T-cell immunization is considered to be effective only in some clinical patients with solid tumors. To increase TIL for efficient contact with tumor cells, two studies were performed on TIL-contacted tumor cells. As mentioned above, we have found that early triple enzymes (collagenase IV, hyaluronidase V, and Dnase I) disaggregating solid tumors will influence TIL function; we then modified formulations such as collagenase IV only under mild digestion conditions to keep T-cell intactness from solid tumors (4–7, 106–108). Moreover, my colleagues also used cultured TILs by directly injecting them into a patient’s tumor site to increase TIL-contact tumor cells (14–18). In the analysis of 68 patients with ovarian cancer and other female malignant tumors, intravenous injection of TILs could induce immunity by activating cellular immunity, thereby improving 1-year survival; the anticancer effect of the local injecting group was higher than that of the intravenous group compared with the intravenous and local injection groups of TILs (109, 110).
Increasing TIL efficacy to tumor cells in clinical application
As mentioned above, TIL treatment of melanoma achieved only a 17% complete remission in an early NCI study, whereas the addition of lymphatic depletion can greatly increase the response ratio (31, 32, 111). Clinically, physicians used a lympho-depleting chemotherapy regimen resulting in a 48% response rate in a melanoma clinical trial from NCI (Figure 2A) (33, 112).
To increase TIL efficacy, we have also developed a clinical procedure to combine TILs with sensitive chemotherapeutic agents, which were screened by chemo-sensitivity testing (CST) or chemo-sensitivity assay (CSA) from a patient’s autogenous tumor cell (7, 113), as shown in Figure 2B.
New generation of TIL therapy-personalized immunotherapy
As noted above, there are at least three main reasons for the variable efficacy of TILs in the treatment of solid tumor diseases. Now, more and more methods and issues of T-lymphocyte treatment will be discovered for the treatment of solid tumors, as shown in Figure 3. Moreover, after the development of genomics and proteomics to clinical immunotherapy, more and more targeted drugs with their mechanisms have been discovered for the treatment of tumor diseases. Now is a good time to develop personalized TIL therapies for solid tumor diseases (113). In particular, three achievements, as described above, will lead to personalized TIL R&D. First, single-cell technology has matured, as reported by most clinical laboratories. In addition, we have developed the single-cell techniques for over 15 years from single tumor cells and single TILs from solid tumors, and have identified some genes associated with TIL silencing (114, 115). Second, Dr Rosenberg has identified different SNPs in certain genes in clinical trials from different clinical responses (116). Third, we also used the TIL pathway and artificial intelligence analysis to address the clinical administration of solid cancer (117–119). All in all, following clinical genomics with single-cell technology and artificial intelligence networks, personalized T-cell therapy can be seen in Figure 4.
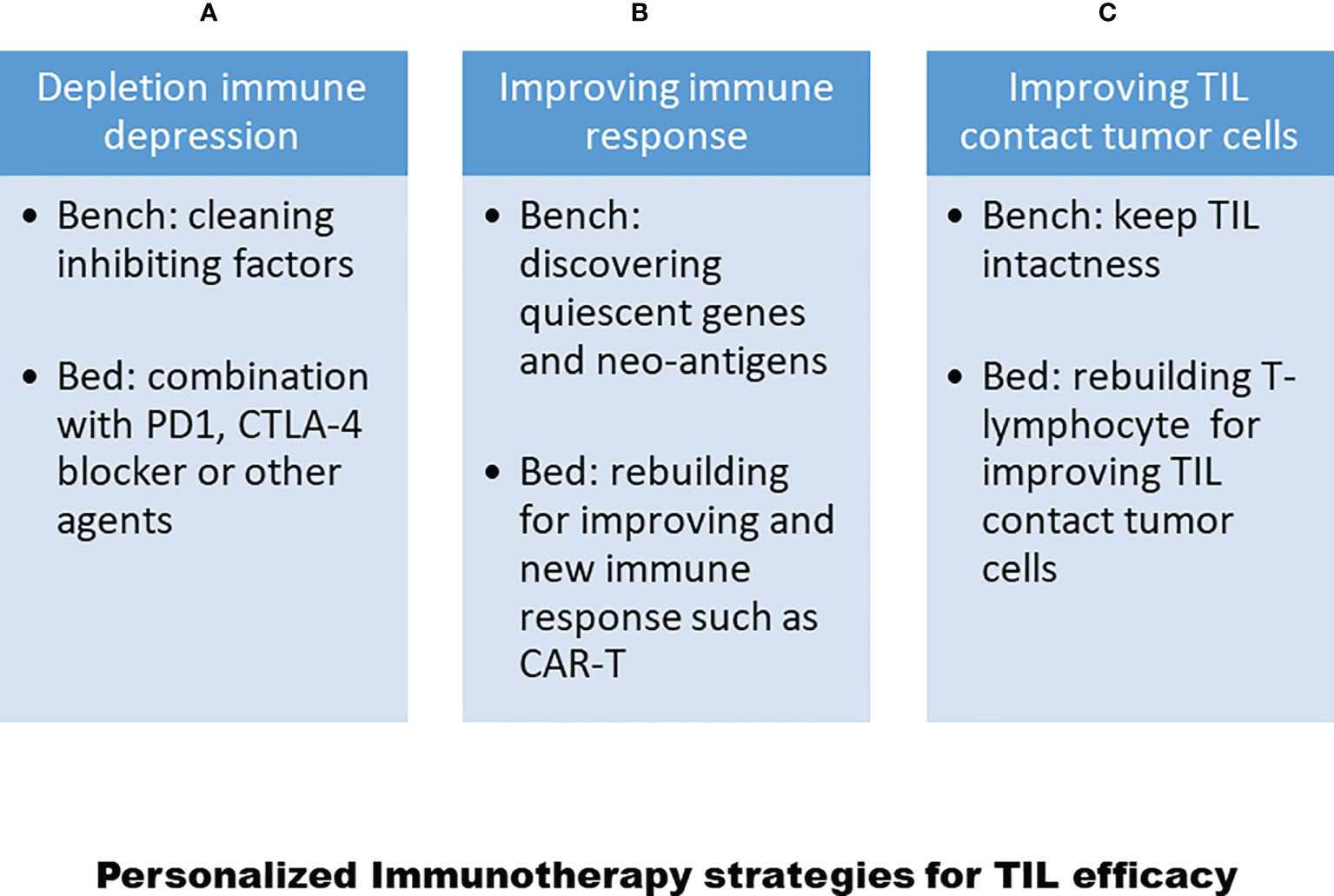
Figure 3 Strategies for personalized TIL therapy to increase TIL efficacy. (A) Depleting immune-depression regimen including clean immune depression in experiments and add PD1 or CTLA4 blocker. (B) Improving TIL immune response in experiments such as discover quiescent genes for TIL and neo-antigens from tumor cells, and rebuilding immune responses for TILs or setting up Car-T or TCT-T cells. (C) Maintaining T-cell intactness and improving T-cell homing into tumor sites.

Figure 4 Personalized TIL therapy or personalized T-cell therapy. (A) Harvesting tumor cells from solid tumor tissues to run RNA-seq to discover neo-antigen or other related genes. (B) Harvesting TILs from solid tumor tissues to run RNA-seq to discover quiescent genes, heterogamous response genes, and infiltrating and homing genes. A pink arrow represents primary tumor cell procedures for performing personalized immunotherapy and a dark arrow represents TIL procedures for performing personalized TIL immunotherapy. Red represents running RNA-seq for both TIL and primary tumor cells.
A good immunotherapy should have the characteristics of high efficacy, few side effects and low economic cost for every tumor patient. Based on those considerations, these developments in future immunotherapy require the development of affordable treatments, tolerable side effects and responsible effects for each patient in optimal cell culture and correct clinical treatment. To be sure, individualized T-cells are required for optimal culture in the laboratory and the correct treatment conditions in the clinic. Finally, all efforts in adoptive T-cell immunotherapy focus on establishing optimal T-cell cultures in the laboratory, choosing the correct mode of administration in the clinic, considering affordability, and improving availability to each patient (120, 121).
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
Study TIL and primary tumor cell more than 30 years so that the works were funded as different funds such as the National Natural Science Foundation of China(No.39370706), USA IRG-91-022-09 for single cell TIL genomic analysis and tumor cell genomics analysis (PO-1 75606-3) co-work with Dr. Preisler and RO1 CA60086 and so on.
Acknowledgments
I have studied and set up TIL cultures from solid tumors forclinical application for more than 30 years. All acknowledgements are for my colleagues. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science (1986) 233(4770):1318–21. doi: 10.1126/science.3489291
2. Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol (1987) 138(3):989–95.
3. Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary Rep N Engl J Med (1988) 319(25):1676–80. doi: 10.1056/NEJM198812223192527
4. Li B, Tong SQ, Hu BY, Zhu YM, Zhang XH, Wu JH, et al. Study on the influence of enzymatic digestion upon tumor-infiltrating lymphocytes. Acta Biologiae Experimentalis Sin (Shi Yan Sheng Wu Xue Bao. Chinese) (1994) 27(1):103–7.
5. Li B, Tong SQ, Zhang XH, Zhu YM, HU BW, Lu DY, et al. Research on TIL proliferation, phenotype from human malignant solid tumors. Modern Immunol (Chinese) (1994) 14(5):257–60.
6. Li B, Tong SQ, Zhu YM, HU BW, Zhang XH, Lu J, et al. Establishment of a method for separation of tumor infiltrating lymphocytes with high vitality. J Immunol (Chinese). (1994) 10(1):44–7.
7. Li B, Tong SQ, Zhang XH, Lu J, Gu QL, Lu DY. A new experimental and clinical approach of combining usage of highly active tumor-infiltrating lymphocytes and highly sensitive antitumor drugs for the advanced malignant tumor. Chin Med J (English). (1994) 107(11):803–7.
8. Li HF, Lu J, Hua ZD, Li B, Tong SQ, Lu DY. Study on the killing activity of TIL cells in ovarian cancer. Shanghai Med (Chinese) (1995) 18(5):268–70.
9. Lu J, Li B, Hua ZD, Zhu YM, Tong SQ. Research on TIL yield and vitality of different materials. Immunol J (Chinese) (1995) 11(3):182–4.
10. Lu J, Hu L LW, Hua ZD, Li B, Tong SQ. Analysis of the therapeutic effects of different therapeutic approaches for TIL. Chin J Cancer Biotherapy (Chinese) (1996) 3(2):127–9.
11. Gu QL, Lin YZ, Yin HR, Li B, Zhu YM, Hu BY. Phenotype and cytotoxic activity of infiltrating lymphocytes in gastrointestinal tumors. Acta UNIVERSITATIS MEDICINALIS SECONDAE SHANGHAI (Chinese) (1996) 3:153–5.
12. Gu QL, Lin YZ, Yin HR, Li B, Zhu YM, Hu BY. Preliminary study on cryopreservation of tumor infiltrating lymphocytes. J Immunol (Chinese) (1995) 04:251–2.
13. Gu QL, Lin YZ, Yin HR, Li B, Zhu YM, Hu BY. Electron microscopic observation of human gastric cancer TIL cells in vitro killing MKN45 gastric cancer cell lines. Acta UNIVERSITATIS MEDICINALIS SECONDAE SHANGHAI (Chinese) (1995) 15(4):331–4.
14. Hua ZD, Lu J, Li HF, Li B, Zhu YM, Tong SQ. Clinical study of tumor infiltrating lymphocytes in ovarian cancer. Chin J Obstetrics Gynecology (Chinese) (1996) 31(9):55–7.
15. Lu J, Li B, Hua ZD, Zhu YM, Tong SQ. Research on TIL yield and vitality of different materials. J Immunol (Chinese) (1995) 11(3):182–5.
16. Lu J, Hua ZD, Li HF, Li B, Zhu YM, Tong SQ. In vitro study of ovarian cancer TIL shanghai medical journal. (Chinese) (1995) 18(6):325–7.
17. Cai XM, Lu J, Hua ZD, Li B, Tong SQ. Clinical application of TIL from different sources. J Immunol (Chinese) (1996) 12(4):251–4.
18. Lu J, Hua ZD, Li B, Zhu YM, Tong SQ. Preliminary observation of biological characteristics of cord blood lymphocytes. Acta UNIVERSITATIS MEDICINALIS SECONDAE SHANGHAI (Chinese) (1995) 15(1):31–5.
19. Knochelmann HM, Rivera-Reyes AM, Wyatt MM, Smith AS, Chamness R, Dwyer CJ, et al. Modeling ex vivo tumor-infiltrating lymphocyte expansion from established solid malignancies. Oncoimmunology (2021) 10(1):1959101. doi: 10.1080/2162402X.2021.1959101
20. Cervera-Carrascon V, Quixabeira DCA, Havunen R, Santos JM, Kutvonen E, Clubb JHA, et al. Comparison of clinically relevant oncolytic virus platforms for enhancing T cell therapy of solid tumors. Mol Ther Oncolytics. (2020) 17:47–60. doi: 10.1016/j.omto.2020.03.003
21. Stötter H, Wiebke EA, Tomita S, Belldegrun A, Topalian S, Rosenberg SA, et al. Cytokines alter target cell susceptibility to lysis. II. Eval tumor infiltrating lymphocytes. J Immunol (1989) 142(5):1767–73.
22. Haas GP, Solomon D. Rosenberg SA.Tumor-infiltrating lymphocytes from nonrenal urological malignancies. Cancer Immunol Immunother. (1990) 30(6):342–50. doi: 10.1007/BF01786883
23. Ollé Hurtado M, Wolbert J, Fisher J, Flutter B, Stafford S, Barton J, et al. Tumor infiltrating lymphocytes expanded from pediatric neuroblastoma display heterogeneity of phenotype and function. PloS One (2019) 14(8):e0216373. doi: 10.1371/journal.pone.0216373
24. Junker N, Thor Straten P, Andersen MH, Svane IM. Characterization of ex vivo expanded tumor infiltrating lymphocytes from patients with malignant melanoma for clinical application. J Skin Cancer. (2011) 574695:1–6. doi: 10.1155/2011/574695
25. Junker N, Andersen MH, Wenandy L, Dombernowsky SL, Kiss K, Sørensen CH, et al. Bimodal ex vivo expansion of T cells from patients with head and neck squamous cell carcinoma: a prerequisite for adoptive cell transfer. Cytotherapy (2011) 13(7):822–34. doi: 10.3109/14653249.2011.563291
26. Economou JS, Belldegrun AS, Glaspy J, Toloza EM, Figlin R, Hobbs J, et al. Moen RC.In vivo trafficking of adoptively transferred interleukin-2 expanded tumor-infiltrating lymphocytes and peripheral blood lymphocytes. results of a double gene marking trial. J Clin Invest. (1996) 97(2):515–21. doi: 10.1172/JCI118443
27. Smazynski J, Webb JR. Resident memory-like tumor-infiltrating lymphocytes: Latest players in the immuno-oncology repertoire. Front Immunol (2018) 9:1741. doi: 10.3389/fimmu.2018.01741
28. Li B, Li S, Larson A. Personalized immunotherapy for tumor diseases and beyond. (Singapore:Bentham Science Publishers) (2020). p. 284.
29. Kawakami Y, Nishimura MI, Restifo NP, Topalian SI, O’Neil BH, Shilyansky J, et al. T-Cell recognition of human melanoma antigens. J immunother emphasis tumor immunol. author manuscript; available in PMC 2008 sep 29. J Immunother Emphasis Tumor Immunol (1993) 14(2):88–93. doi: 10.1097/00002371-199308000-00002
30. Rosenberg SA. Immersion in the search for effective cancer immunotherapies. Mol Med (2021) 27:63. doi: 10.1186/s10020-021-00321-3
31. Kawakami Y, Nishimura MI, Restifo NP, Topalian SL, O'Neil BH, Shilyansky J, et al. T-Cell recognition of human melanoma antigens. J Immunother Emphasis Tumor Immunol (1993) 14(2):88–93. doi: 10.1097/00002371-199308000-00002
32. Weaver CH. Tumor-infiltrating lymphocyte therapy for melanoma . Available at: https://news.cancerconnect.com/melanoma/tumor-infiltrating-lymphocyte-therapy-for-melanoma.
33. Rohaan MW, van den Berg JH, Kvistborg P, Haanen JBAG. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. (2018) 6(1):102. doi: 10.1186/s40425-018-0391-1
34. Li B. Biobank for personalized immunotherapy. chapter 13. In: Li B, Li S, Larson A, editors. Personalized immunotherapy for tumor diseases and beyond. (Singapore:Bentham Science Publishers) (2020). p. 224–54.
35. Li B, Shen DH. Preliminary study on the resting status of tumor-infiltrating lymphocytes. Chin Microbiol Immunol (Chinese) (1994) 14(6):399–402.
36. Zhu YM, Zhang XH, Li B, Hu BY, Wu JH, Tong SQ. Removal of tumor-doped tumor cells in tumor infiltrating lymphocyte culture. Acta UNIVERSITATIS MEDICINALIS SECONDAE SHANGHAI (Chinese) (1995) 15(2):144–7.
37. Melero I, Arina A, Cen L. Many sounds of T lymphocyte silence. Immunol Res (2005) 33:135–48. doi: 10.1385/IR:33:2:135
38. Koba F, Akiyoshi T, Tsuji H. Depression of the generation of cell-mediated cytotoxicity in regional lymph nodes of patients with gastric carcinoma. J Clin Lab Immunol (1987) 22:181–4.
39. Monsurro V, Wang E, Yamano Y, Migueles SA, Panelli MC, Smith K, et al. Quiescent phenotype of tumor-specific CD8+ T cells following immunization. Blood (2004) 104:1970–8. doi: 10.1182/blood-2004-02-0525
40. Marrack P, Mitchell T, Hildeman D, Kedl R, Teague TK, Bender J, et al. Genomic-scale analysis of gene expression in resting and activated T cells. Curr Opin Immunol (2000) 12:206–9. doi: 10.1016/S0952-7915(99)00075-8
41. James P, Did S. Lung krüpple-like factor: a quintessential player in T cell quiescence. Nat Immunol (2001) 2:667–8. doi: 10.1038/90598
42. Tzachanis D, Freeman GJ, Hirano N, van Puijenbroek AA, Delfs MW, Alla BLM, et al. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat Immunol (2001) 2:1174–82. doi: 10.1038/ni730
43. Li B. A strategy to identify genomic expression profiles at single-t-cell level and a small number of cells (review paper). J Biotechnol (2005) 8(1)71–82.
44. Li B, Perabekam S, Liu G, Yin M, Song S, Larson A. Experimental and bioinformatics comparison of gene expression between T cells from TIL of liver cancer and T cells from UniGene. J Gastroenterol (2002) 37(4):275–82. doi: 10.1007/s005350200035
45. Li B. Identification of mRNAs expressed in tumor-infiltrating lymphocytes by a strategy for rapid and high throughput screening. GENE (2000) 255:273–9. doi: 10.1016/S0378-1119(00)00330-9
46. Konrad MA, Zúñiga-Pflücker JC. The BTG/TOB family protein TIS21 regulates stage-specific proliferation of developing thymocytes. Eur J Immunol (2005) 35(10):3030–42. doi: 10.1002/eji.200526345
47. García-Palma L, Horn S, Haag F, Diessenbacher P, Streichert T, Mayr GW, et al. Up-regulation of the T cell quiescence factor KLF2 in a leukaemic T-cell line after expression of the inositol 5'-phosphatase SHIP-1. Br J Haematol (2005) 131(5):628–31. doi: 10.1111/j.1365-2141.2005.05811.x
48. Batlle E, Massagué J. Transforming growth factor-beta signaling in immunity and Cancer.Immunity. Immunity (2019) 50(4):924–40. doi: 10.1016/j.immuni.2019.03.024
49. Zhang S, Takaku M, Zou L, Gu AD, Chou WC, Zhang G, et al. Reversing SKI-SMAD4-mediated suppression is essential for T(H)17 cell differentiation. Nature (2017) 551(7678):105–9. doi: 10.1038/nature24283
50. Evans RL, Wall DW, Platsoucas CD, Siegal FP, Fikrig SM, Testa CM, et al. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. (1981) 78(1):544–8. doi: 10.1073/pnas.78.1.544
51. Mortazavi A, Leeper Thompson EC, Garcia ST, Myers RM, Wold B. Comparative genomics modeling of the NRSF/REST repressor network: from single conserved sites to genome-wide repertoire. Genome Res (2006) 16(10):1208–21. doi: 10.1101/gr.4997306
52. Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and bim in response to IL-2. J Immunol (2002) 168(10):5024–31. doi: 10.4049/jimmunol.168.10.5024
53. Toor SM, Sasidharan Nair V, Decock J, Elkord E. Immune checkpoints in the tumor microenvironment. Semin Cancer Biol (2019) 579X(19):30123–3. doi: 10.1016/j.semcancer.2019.06.021
54. Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Kuo CJ, et al. Organoid modeling of the tumor immune microenvironment. Cell (2018) 175(7):1972–1988.e16. doi: 10.1016/j.cell.2018.11.021
55. Bai Y, Wang Y, Zhang X, Fu J, Xing X, Wang C, et al. Potential applications of nanoparticles for tumor microenvironment remodeling to ameliorate cancer immunotherapy. Int J Pharm (2019) 570:118636. doi: 10.1016/j.ijpharm.2019.118636
56. Brooks EA, Galarza S, Gencoglu MF, Cornelison RC, Munson JM, Peyton SR, et al. Applicability of drug response metrics for cancer studies using biomaterials. Philos Trans R Soc Lond B Biol Sci (2019) 374(1779):20180226. doi: 10.1098/rstb.2018.0226
57. Mehla K, Singh PK. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. (2019) 5(12):822–34. doi: 10.1016/j.trecan.2019.10.007
58. Ferrari SM, Fallahi P, Galdiero MR, Ruffilli R, Elia G, Ragusa F, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci (2019) 20(18):E4413. doi: 10.3390/ijms20184413
59. Baglieri J, Brenner DA, Kisseleva T. The role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma. Int J Mol Sci (2019) 20(7):E1723. doi: 10.3390/ijms20071723
60. Tian X, Shen H, Li Z, Wang T, Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol (2019) 12(1):84. doi: 10.1186/s13045-019-0772-z
61. Masson N, Keeley TP, Giuntoli B, White MD, Puerta ML, Perata P, et al. Conserved n-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science (2019) 365(6448):65–9. doi: 10.1126/science.aaw0112
62. Shi L, Yang L, Wu Z, Xu W, Song J, Guan W. Adenosine signaling: Next checkpoint for gastric cancer immunotherapy? Int Immunopharmacol. (2018) 63:58–65. doi: 10.1016/j.intimp.2018.07.023
63. Whiteside TL. Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert Rev Anticancer Ther (2017) 17(6):527–35. doi: 10.1080/14737140.2017.1316197
64. Algarra I, Garrido F, Garcia-Lora AM. MHC heterogeneity and response of metastases to immunotherapy. Cancer Metastasis Rev (2021) 40(2):501–17. doi: 10.1007/s10555-021-09964-4
65. Chen J, Jiang H. Current challenges and strategies for chimeric antigen receptor-T-Cell therapy for solid tumors. Crit Rev Immunol (2021) 41(1):1–12. doi: 10.1615/CritRevImmunol.2020036178
66. Smet C, Lurquin C, Bruggen P, Plaen E, Brasseur F, Boon T. Sequence and expression pattern of the human MAGE2 gene. Immunogenetics (1994) 39(2):121–9. doi: 10.1007/BF00188615
67. Ugolini A, Nuti M. CD137+ T-cells: Protagonists of the immunotherapy revolution. Cancers (Basel). (2021) 13(3):456. doi: 10.3390/cancers13030456.68
68. Bakker BABH, Schreurs MWJ, Boer AJD, Kawakami Y, Rosenberg SA, Adema GJ, et al. Melanocyte lineage-specific antigen gpl00 is recognized by melanoma derived tumor-infiltrating lymphocytes. J Exp Med (1994) 179(3):1005–9. doi: 10.1084/jem.179.3.1005
69. Kawakami BY, Eliyahu S, Sakaguchi K, Rivoltini L, Yannelli JR, Appella E, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med (1994) 180(1):1–6. doi: 10.1084/jem.180.1.347
70. Warnes TW, Smith A. Tumour markers in diagnosis and management. Baillieres Clin Gastroenterol (1987) 1(1):63–89. doi: 10.1016/0950-3528(87)90034-0
71. Basu S, Parghane RV. Designing and developing PET-based precision model in thyroid carcinoma: The potential avenues for a personalized clinical care. PET Clin (2017) 12(1):27–37. doi: 10.1016/j.cpet.2016.08.007
72. Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst (1995) 87(13):982–90. doi: 10.1093/jnci/87.13.982
73. Wang X, Wang Q. Alpha-fetoprotein and hepatocellular carcinoma immunity. Can J Gastroenterol Hepatol (2018) 2018:9049252. doi: 10.1155/2018/9049252
74. von Witzleben A, Wang C, Laban S, Savelyeva N, Ottensmeier CH. HNSCC: Tumour antigens and their targeting by immunotherapy. Cells (2020) 9(9):2103. doi: 10.3390/cells9092103
75. Ping Y, Liu C, Zhang Y. T-Cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell (2018) 9(3):254–66. doi: 10.1007/s13238-016-0367-1
76. Zhao W, Wu J, Chen S, Zhou Z. Shared neoantigens: ideal targets for off-the-shelf cancer immunotherapy. Pharmacogenomics (2020) 21(9):637–45. doi: 10.2217/pgs-2019-0184
77. Kunert A, Straetemans T, Govers C, Lamers C, Mathijssen R, Sleijfer S, et al. TCR-engineered T cells meet new challenges to treat solid tumors: choice of antigen, T cell fitness, and sensitization of tumor milieu. Front Immunol (2013) 4:363. doi: 10.3389/fimmu.2013.00363
78. McCormack E, Adams KJ, Hassan NJ, Kotian A, Lissin MN, Sami M, et al. Bi-specific TCR anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1 positive tumors. Cancer Immunol Immunother. (2013) 62(4):773–85. doi: 10.1007/s00262-012-1384-4
79. Li B, Liu G, Hu HL, Ding JQ, Zheng J, Tong A. Biomarkers analysis for heterogeneous immune responses of quiescent CD8+cells -a clue for personalized immunotherapy. iMedPub Journals (2015) 1(3):1–12.
80. Li B, Ding JQ, Larson A, Song SW. Tissue recycling-a new combination therapy for solid tumor: experimental and preliminarily clinical research. Anticancer (IN VIVO) (1999) 13(5):1–6.
81. Penter L, Dietze K, Ritter J, Lammoglia Cobo MF, Garmshausen J, Aigner F, et al. Localization-associated immune phenotypes of clonally expanded tumor-infiltrating T cells and distribution of their target antigens in rectal cancer. Oncoimmunology (2019) 8(6):e1586409. doi: 10.1080/2162402X.2019.1586409
82. Yan LH, Jiang WH. MHC and cancer Immunotherapy.Chapter 1. In: Personalized immunotherapy for tumor diseases and beyond (Book). (Singapore.:Bentham Science Publishers) (2020). p. 1–19.
83. Li S, Perabekam S, Devemy E, Li B. Genetically modified T-cells affinity to tumor cells-development of adoptive T-cell immunotherapy. chapter 11. In: Personalized immunotherapy for tumor diseases and beyond (Book). (Singapore:Bentham Science Publishers) (2020). p. 174–96.
84. Torcellan T, Hampton HR, Bailey J, Tomura M, Brink R, Chtanova T. In vivo photolabeling of tumor-infiltrating cells reveals highly regulated egress of T-cell subsets from tumors. Proc Natl Acad Sci U S A. (2017) 114(22):5677–82. doi: 10.1073/pnas.1618446114
85. Bai XF, Gao JX, Liu J, Wen J, Zheng P, Liu Y. On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res (2001) 61(18):6860–7.
86. Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, et al. Tumor infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol (2013) 36:224–31. doi: 10.1097/COC.0b013e3182467d90
87. Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PO, et al. Elevated CD3þ and CD8þ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol (2013) 264:71–83. doi: 10.1016/j.jneuroim.2013.08.013
88. Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol (2013) 3:231. doi: 10.3389/fonc.2013.00231
89. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
90. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
91. Sasada T, Suekane S. Variation of tumor-infiltrating lymphocytes in human cancers: controversy on clinical significance. Immunotherapy (2011) 3:1235–51. doi: 10.2217/imt.11.106
92. Palmer DC, Balasubramaniam S, Hanada K-i, Wrzesinski C, Yu Z, Farid S, et al. Vaccine-stimulated, adoptively transferred CD8þT cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol (2004) 173:7209–16. doi: 10.4049/jimmunol.173.12.7209
93. Li B, Ding JQ. Gene therapy using cytokine-transduced tumor vaccine: Molecular and clinical aspects. In cast (1998):37–42.
94. Tao J, Zhang GQ, Ding JQ, Zhang XH, Li B, Tong SQ. Experimental study of receptor-mediated TNFα gene transfer. Acta UNIVERSITATIS MEDICINALIS SECONDAE SHANGHAI (Chinese) (2000) 20(1):32–4.
95. Li B, Xu W, Qian GX, Zhang XH, Dong SQ, Chen SS. Methodology of TNF gene transduction of tumor infiltrating lymphocytes. Acta UNIVERSITATIS MEDICINALIS SECONDAE SHANGHAI (Chinese) (1995) 15(3):185–9.
96. Ding JQ, Qian GX, Li B, Xu W, Zhu YM, Hu L, et al. A preliminary study of tumor necrosis factor gene transduction of tumor infiltrating lymphocytes application. Chin J Cancer Biotherapy (Chinese) (1995) 1:11–5.
97. Wang JH, Tong SQ, Li B, Ding JQ, Hu BY, Zhu YM, et al. Immunological character of TIL in ovarian carcinoma. Chin J Cancer Res (Chinese) (2000) 12(2):99–104. doi: 10.1007/BF02983432
98. Hu BC, Li GW, Cheng W, Shen JK, Lin D, Li B. Clinical application of infiltrating lymphocytes in malignant brain tumors. J Immunol (Chinese) (1997) 2:1–2.
99. Tong SQ, Wang JH, Li B, Ding JQ, Hu BY, Zhu YM, et al. Biological characteristics of TIL from ovarian cancer. Chin J Immunol (Chinese) (2000) 16(3):131–4.
100. Hu BY, Wang JH, Tong SQ, Li B, Zhu YM, Lu DY, et al. Immunological characteristics of TIL from ovarian cancer. Chin J Cancer (Chinese). (1999) 4:1–5.
101. Simsek H, Klotzsch E. The solid tumor microenvironment-breaking the barrier for T cells: How the solid tumor microenvironment influences T cells: How the solid tumor microenvironment influences T cells. Bioessays (2022) 8:e2100285. doi: 10.1002/bies.202100285
102. Revenko A, Carnevalli LS, Sinclair C, Johnson B, Peter A, Taylor M, et al. Direct targeting of FOXP3 in tregs with AZD8701, a novel antisense oligonucleotide to relieve immunosuppression in cancer. J Immunother Cancer. (2022) 4):e003892. doi: 10.1136/jitc-2021-003892
103. Budczies J, Kluck K, Beck S, Ourailidis I, Allgäuer M, Menzel M, et al. Homologous recombination deficiency is inversely correlated with microsatellite instability and identifies immunologically cold tumors in most cancer types. J Pathol Clin Res (2022). doi: 10.1002/cjp2.271
104. Mangaonkar AA, Patnaik MM. Role of the bone marrow immune microenvironment in chronic myelomonocytic leukemia pathogenesis: novel mechanisms and insights into clonal propagation. Leuk Lymphoma. (2022) 4:1–9. doi: 10.1080/10428194.2022.2056175
105. Shi W, Zhang F, Chen X, Wang S, Zhang H, Yang Z, et al. Tumor-derived immunoglobulin like transcript 5 induces suppressive immunocyte infiltration in colorectal cancer. Cancer Sci (2022) 4. doi: 10.1111/cas.15360
106. Wang ZH, Hu HL, Zheng J, LI B. Gene expression and pathway analysis of quiescent CD8+ T cells from liver cancer, liver sinusoid and peripheral blood - study on toxicogenomics and prevention targeting. BIBE (2011):72–6. doi: 10.1109/BIBE.2011.18
107. Li DYLB, Tong SQ, Zhang XH, Zhu YM, Wu BY. Development of adoptive T-cell immunotherapy -future of personalized immunotherapy. chapter 9. In: Personalized immunotherapy for tumor diseases and beyond. (Singapore:Bentham Science Publishers) (2020). p. 126–45.
108. Huang HL, Hsing HW, Lai TC, Chen YW, Lee TR, Chan HT, et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J BioMed Sci (2010) 17(1):36. doi: 10.1186/1423-0127-17-36
109. Möller P, Wittig B, Schadendorf D. Intratumoral adoptive immunotherapy with tumor infiltrating lymphocytes (TIL) in a melanoma patient leading to regression of local tumor mass. a case report. Anticancer Res (1998) 2B):1237–41.
110. Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. (1999) 45(2):141–57. doi: 10.1023/a:1006293606710
111. Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J Clin Oncol (2021) 39(24):2656–66. doi: 10.1200/JCO.21.00612
112. Trembath DG, Davis ES, Rao S, Bradler E, Saada AF, Midkiff BR, et al. Brain tumor microenvironment and angiogenesis in melanoma brain metastases. Front Oncol (2021) 10:604213. doi: 10.3389/fonc.2020.604213
113. Held W, Speiser DE. Not all tumor-infiltrating CD8 (+) T cells are created equal. Cancer Cell (2021) 39(2):145–7. doi: 10.1016/j.ccell.2021.01.015
114. Li B, Hu HL, Ding JQ, Yan D, Yang LM. Functional cell-proliferation and differentiation by system modeling for cell therapy. IJLSRST (2015):1–11.
115. Xu YB, Hu HL, Zheng J, Li B. Feasibility of whole RNA sequencing from single-TIL cell mRNA amplification. Genet Res Int (2013) 4:1. doi: 10.1155/2013/724124
116. Turcotte S, Gros A, Hogan K, Tran E, Hinrichs CS, Wunderlich JR, et al. Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy. J Immunol (2013) 191(5):2217–25. doi: 10.4049/jimmunol.1300538
117. Zhang W, Ding JQ, Qu Y, Hu HL, Lin MH, Datta A, et al. Genomic expression analysis of quiescent CD8 T-cells from tumor-infiltrating lymphocytes of in vivo liver tumor by single-cell mRNA differential display. Immunology (2009) 127(1):83–90. doi: 10.1111/j.1365-2567.2008.02926.x
118. Li B. Breakthrough of 2015-personalized immunotherapy. iMedPub Journals (2015) 1(4):1–2. doi: 10.21767/2472-1646.100008
119. Li B, Liu G, Hu HL, Ding JQ, Zheng J and Tong A. Biomarkers analysis for heterogeneous immune responses of quiescent CD8+cells -a clue for personalized immunotherapy. iMedPub Journals (2015) 1(3):1–12. doi: 10.21767/2472-1646.100003
120. Paria BC, Levin N, Lowery FJ, Pasetto A, Deniger DC, Parkhurst MR, et al. Rapid identification and evaluation of neoantigen-reactive T-cell receptors from single cells. J Immunother. (2021) 44(1):1–8. doi: 10.1097/CJI.0000000000000342
Keywords: TILs (tumor infiltrating lymphocytes), solid tumor, TIL quiescence, TIL attacking heterogeneous antigen, TIL infiltration, personalized immunotherapy
Citation: Li B (2022) Why do tumor-infiltrating lymphocytes have variable efficacy in the treatment of solid tumors? Front. Immunol. 13:973881. doi: 10.3389/fimmu.2022.973881
Received: 20 June 2022; Accepted: 14 September 2022;
Published: 21 October 2022.
Edited by:
Anna Pasetto, Karolinska Institutet (KI), SwedenReviewed by:
Daniele Fanale, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyLianghao Ding, University of Texas Southwestern Medical Center, United States
Copyright © 2022 Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biaoru Li, YmxpQGF1Z3VzdGEuZWR1
 Biaoru Li
Biaoru Li