- Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Deceased donor kidney transplantation (DDKT) is a major therapeutic option for patients with end-stage renal diseases. Although medical techniques improved in recent years, acute or chronic rejection after DDKT is not uncommon and often results in poor graft survival. Therefore, the determination of risk factors is very important to stratify patients and to improve outcomes. This study aims to evaluate the risk factors for treated rejection (TR) of patients after DDKT.
Methods: Clinical data of deceased donors and corresponding recipients were retrospectively collected. The primary outcome was TR defined as the treatment for rejection within 24 months after DDKT. Univariate comparisons of baseline characteristics were performed with Chi-square test, t-test, and Mann–Whitney U test. Logistic regression was constructed to analyze potential risk factors. Receiver operating characteristic (ROC) curve and Jordan index were generated to determine the optimal cutoff value. The association between continuous variables and TR was examined and visualized by using restricted cubic spline (RCS) models.
Results: Data of 123 deceased donors and 246 recipients were obtained and analyzed. The median age was 41 (4–62) years for recipients and 39 (1–65) years for donors. The recipients who died or suffered graft loss during the follow-up period were 8 (3.3%) and 12 (4.9%), respectively. After univariate analysis and subsequent multivariate analysis, the preoperative serum uric acid (OR, 2.242; 95% CI, 1.037–4.844; P = 0.040), platelet (OR, 2.163; 95% CI, 1.073–4.361, P = 0.031), absolute neutrophil count (OR, 2.183; 95% CI, 1.025–4.649; P = 0.043), and HLA-DQ mismatch (OR, 2.102; 95% CI, 1.093–4.043; P = 0.026) showed statistical significance. RCS models showed that patients with higher levels of uric acid had increased risk of TR.
Conclusions: Serum uric acid and other three indicators were found to be the independent risk factors for TR, which may contribute to stratify patients and develop personalized regimen in perioperative period.
Introduction
Worldwide, kidney transplantation from deceased donors is a major therapeutic option for patients with end-stage kidney diseases (1). With the improvement of medical techniques in recent years, long-term survival of recipients after deceased donor kidney transplantation (DDKT) appears to be possible. Thus, studies on complications related to graft failure and mortality in organ transplantation are essential (2). Although several risk factors for DDKT outcomes have been documented before, current evidence reveals that short-term adverse events including acute rejection and delayed graft function (DGF) are not rare, leading to increased incidence of graft loss, re-transplantation, and even death (3, 4).
On the other hand, it is reported that kidneys from deceased donors may suffer from various injurious factors during the donation (5). Among them, the source of donor kidney has been suggested as an important factor related to different outcomes of transplantation (5). Kidneys from the donation after cardiac death (DCD) donors may be susceptible to injury by warm ischemia and subsequently experience a higher incidence of DGF, and kidneys from the donation after brain-stem death (DBD) could incur greater metabolic disturbance and inflammatory response (6, 7). However, limited data and inconsistent opinions on the risk factors of graft survival in DDKT still exist (8, 9). Therefore, elements on graft survival require further investigation.
Indeed, the association of rejection in DBD with inferior graft outcomes has been established previously (6). Meanwhile, the definition, diagnosis, and treatment of rejection have been refined (10). However, the outcomes of DDKT can be affected by complicated factors, including specific allocation, healthcare, and surgical procedures, producing inevitable confound factors and statistical bias. Thus, a comprehensive assessment of risk factors based on paired donors and recipients might be conducive to reduce bias and make correct allocation and adequate preparation for transplants. Taken together, the determination of risk factors is important to stratify patients and improve graft survival (1). This study aims to evaluate the risk factors for treated rejection (TR) after DDKT basing on the data of paired kidneys.
Methods
Patients and data collection
We retrospectively collected the data of both donors and recipients performed DDKT between 2015 and 2018 in West China Hospital. The end of follow-up period in this study is December 2020. After the exclusion of eight donors and 16 paired recipients due to lack of major preoperative information, insufficient follow-up data, dual organ transplantation, and donor age older than 65, we included 123 donors and 246 recipients and analyzed their data (Supplementary Figure 1). Baseline characteristics included transplantation-related features and recipient and donor characteristics (Tables 1–3). Recipient age was not restricted, and donor–recipient characteristics were matched correspondingly to analyze covariates and control confounding variables better. For each recipient, clinical and laboratory information within 2 years after transplantation was obtained.
Clinical outcome and definitions
The primary clinical outcome was TR defined as the treatment for rejection within 24 months after the DDKT. DGF was defined as receiving dialysis within 1 week after the transplantation. Overall graft loss means regaining permanent dialysis after the transplantation or death with functional graft by any cause. Cold ischemic time (CIT) was established by the time from cold storage to reperfusion following implantation. Serum creatinine of recipients at implantation and within 24 months after transplantation was available for evaluating renal function and graft performance. Human leukocyte antigen (HLA) mismatch according to the UK allocation policy was defined as the incongruity of HLA-A, HLA-B, HLA-DR, HLA-DQ, HLA-Type I, and HLA-Type II between donor and recipient. Baseline characteristics of the included 123 pairs and transplantation were assessed as covariant for exploring potential risk factors.
Statistical analysis
Statistical analyses in this study were performed using SPSS 23.0 (SPSS, Inc., Chicago, USA). Mean value and standard deviation or median with ranges were calculated for continuous variables. Rates or proportions were calculated for categorical variables. The Kolmogorov–Smirnov test was used to indicate the distribution of variables. Univariate comparisons between transplants with rejection versus without rejection were made with Chi-square tests, t-test, and Mann–Whitney U test when appropriate. Meanwhile, variables were deemed to be statistically significant at P-values less than 0.10 in the correlation analyses, which might be conducive to seek potential correlation and risk factors. Logistic regression model was constructed to analyze potential risk factors. Significant variables in correlation analyses were incorporated into the regression model. In addition, estimated glomerular filtration rate (eGFR) was included in multivariate analysis for adjusting the effect of renal function on outcomes.
In multivariate analysis, the stepwise regression method was chosen to prevent multicollinearity among variables. Receiver operating characteristic (ROC) curve and corresponding Jordan index (subtract 1 from the sum of sensitivity and specificity) were generated to determine the optimal cutoff value for continuous variables in the regression model. The area under the curve (AUC) in ROC analysis is considered as a predictor to distinguish patients with or without TR. The AUC between 0.8 and 0.9 represents a high-rank results test, and the AUC between 0.5 and 0.6 represents a poor test. The peak point locating at where the sensitivity and specificity were maximized simultaneously was highest cutoff point. The association of variables on continuous scales with primary outcome was examined through the use of restricted cubic spline (RCS) regression models with multifactorial adjustment. RCSs with equally spaced percentiles (25th, 50th, 75th, and 95th) were used to capture the association, to visualize the relationship, and to test the potential non-linearity. In regression analysis, P-values were two-sided, and the statistical significance was defined as P < 0.05.
Results
Characteristics of study population and their relevance with TR
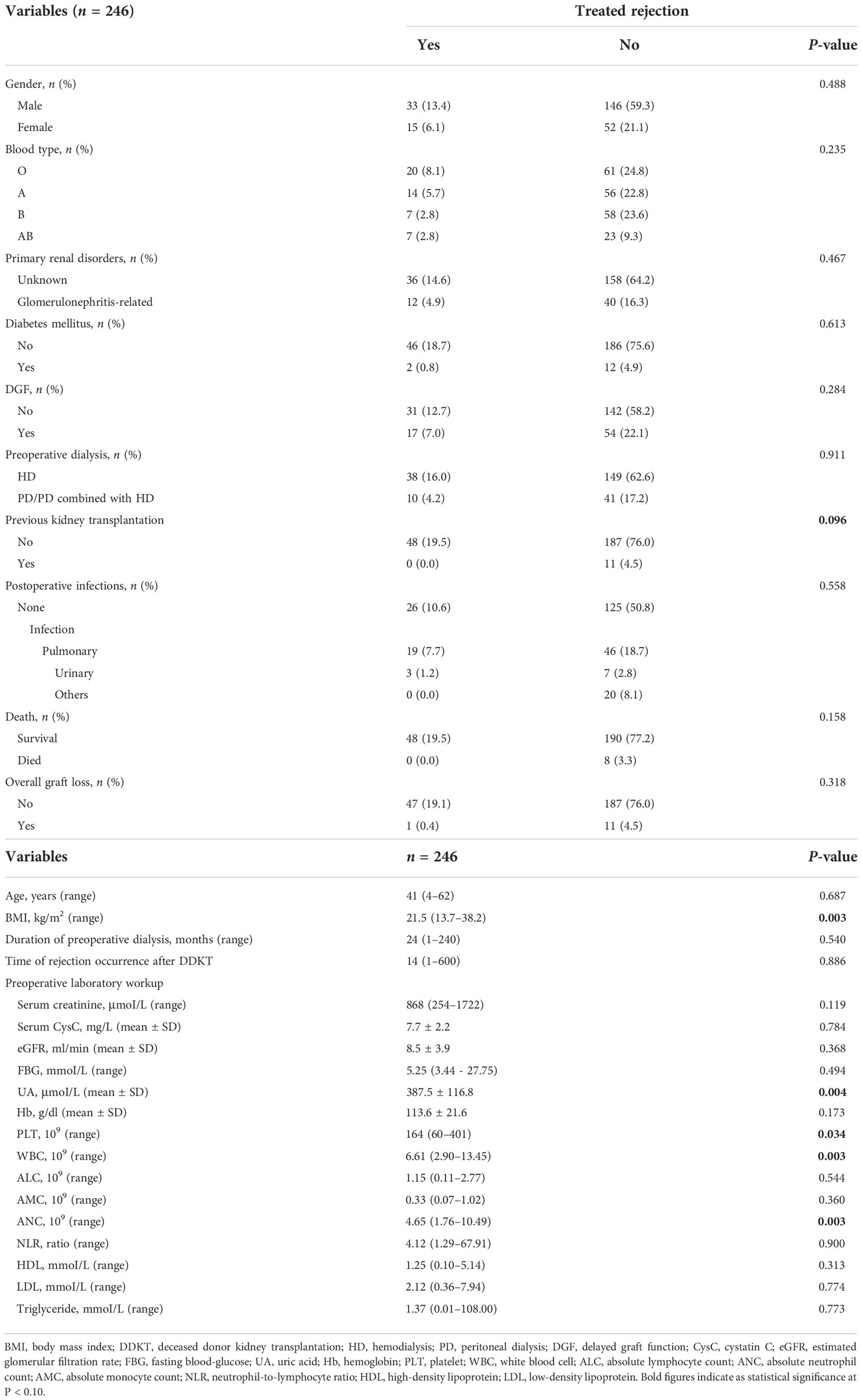
Of 246 DDKT recipients, 128 (52.0%) were from donors suffered severe craniocerebral injury, 84 (34.2%) from intracranial hemorrhage, and 34 (13.8%) from other diseases such as intracranial tumors. The median (range) age was 41 (4–62) years for recipients and 39 (1–65) years for donors. The recipients who died or suffered graft loss during the follow-up period were 8 (3.3%) and 12 (4.9%), respectively. Maintenance immunosuppressive therapy of recipients was the combination of tacrolimus, mycophenolate mofetil (MMF), and prednisone (97%) or the combination of cyclosporin, MMF, and prednisone (3%). Recipients who suffered from cardiovascular events, hyperparathyroidism, diabetes, anemia, and psychiatric disorders following transplantation were 6 (2.4%), 5 (2.0%), 5 (2.0%), 2 (0.8%), and 3 (1.2%), respectively. Of 246 recipients, 48 (19.5%) were diagnosed as TR. Overall, 26 (10.6%) were antibody-mediated rejection, 18 (7.3%) were T-cell–mediated rejection, and 4 (1.6%) were mixed form. Their levels of serum creatinine and cystatin C seem to be higher compaed with non-TR at same time within 24 months, indicating the adverse effect of TR on renal function (Supplementary Figure 2).
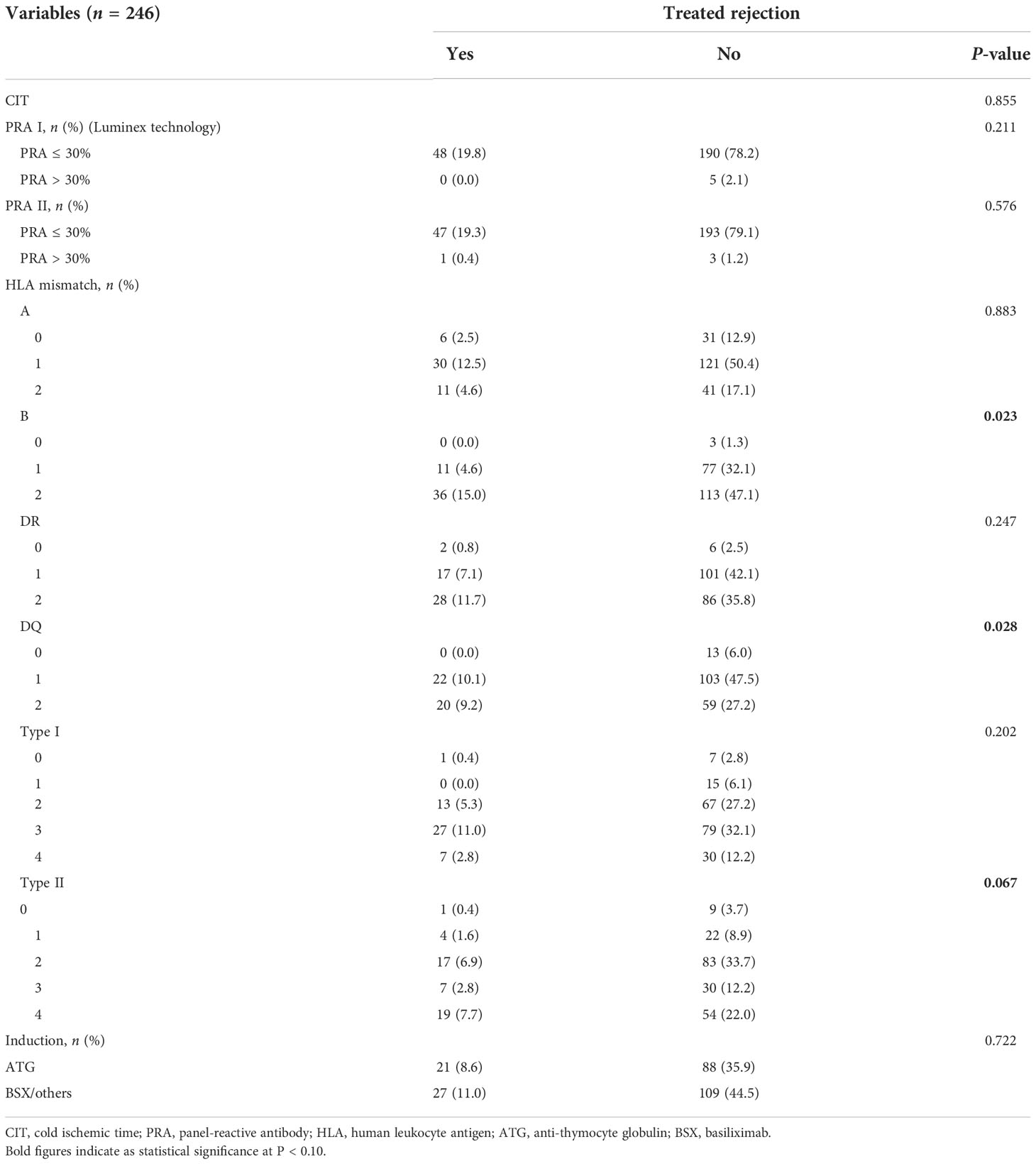
The median (range) body mass index (BMI) of recipients was 21.5 (13.7–38.2) kg/m2, which showed potential correlation with TR (P = 0.003). Of transplantation-related variables, HLA-B mismatch, HLA-DQ mismatch, and HLA-Type II showed significant correlation with TR (P = 0.023, 0.028, and 0.067, respectively). Unexpectedly, several parameters of preoperative laboratory workup showed robust association with TR. The uric acid (UA) (P = 0.004), platelet (PLT) (P = 0.034), white blood cell (WBC) (P = 0.003), and absolute neutrophil count (ANC) (P = 0.003) showed statistical significance of association with TR. All characteristics of transplants and their relevance to TR are shown in Tables 1–3.
Variables and risk fact of TR
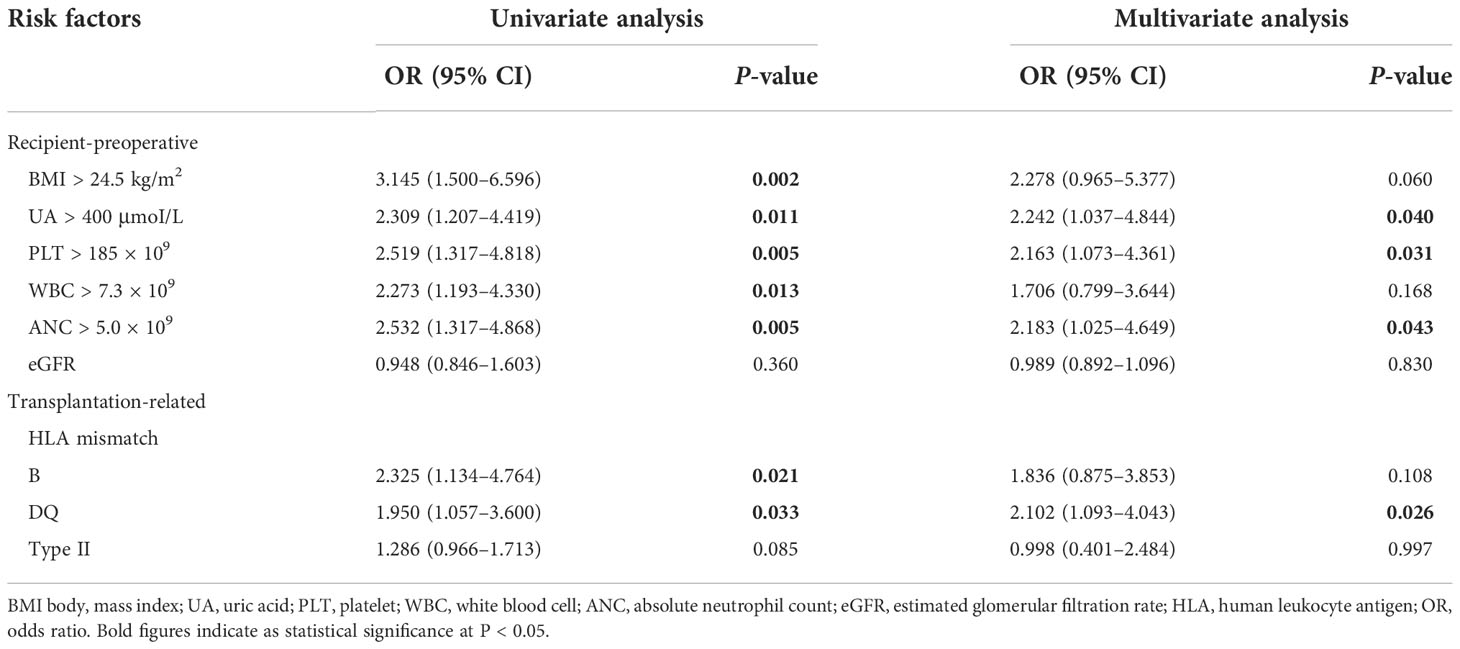
Optimal cutoff values of meaningful continuous variables in preliminary correlations analyses were confirmed via ROC curve and corresponding Jordan index (Figure 1). After that, dichotomous variables were generated and entered into univariate and multivariate analysis to explore independent risk factors. In univariate analysis, several variables of recipients and transplantation were strongly associated with TR (Table 4). Among them, BMI (OR, 3.145; 95% CI, 1.500–6.596; P = 0.002), preoperative UA (OR, 2.309; 95% CI, 1.207–4.419; P = 0.011), PLT (OR, 2.519; 95% CI, 1.317–4.818; P = 0.005), WBC (OR, 2.273; 95% CI, 1.193–4.330; P = 0.013), and absolute lymphocyte count (ALC) (OR, 2.532; 95% CI, 1.317–4.868; P = 0.005) of recipients demonstrated the potential risk for TR. Meanwhile, HLA-B mismatch (OR, 2.325; 95% CI, 1.134–4.764; P = 0.021) and HLA-DQ mismatch (OR, 1.950; 95% CI, 1.057–3.600; P = 0.033) might be risk factors for TR. Previous kidney transplantation did not acquire statistical assignment, perhaps due to the small sample (n = 11).
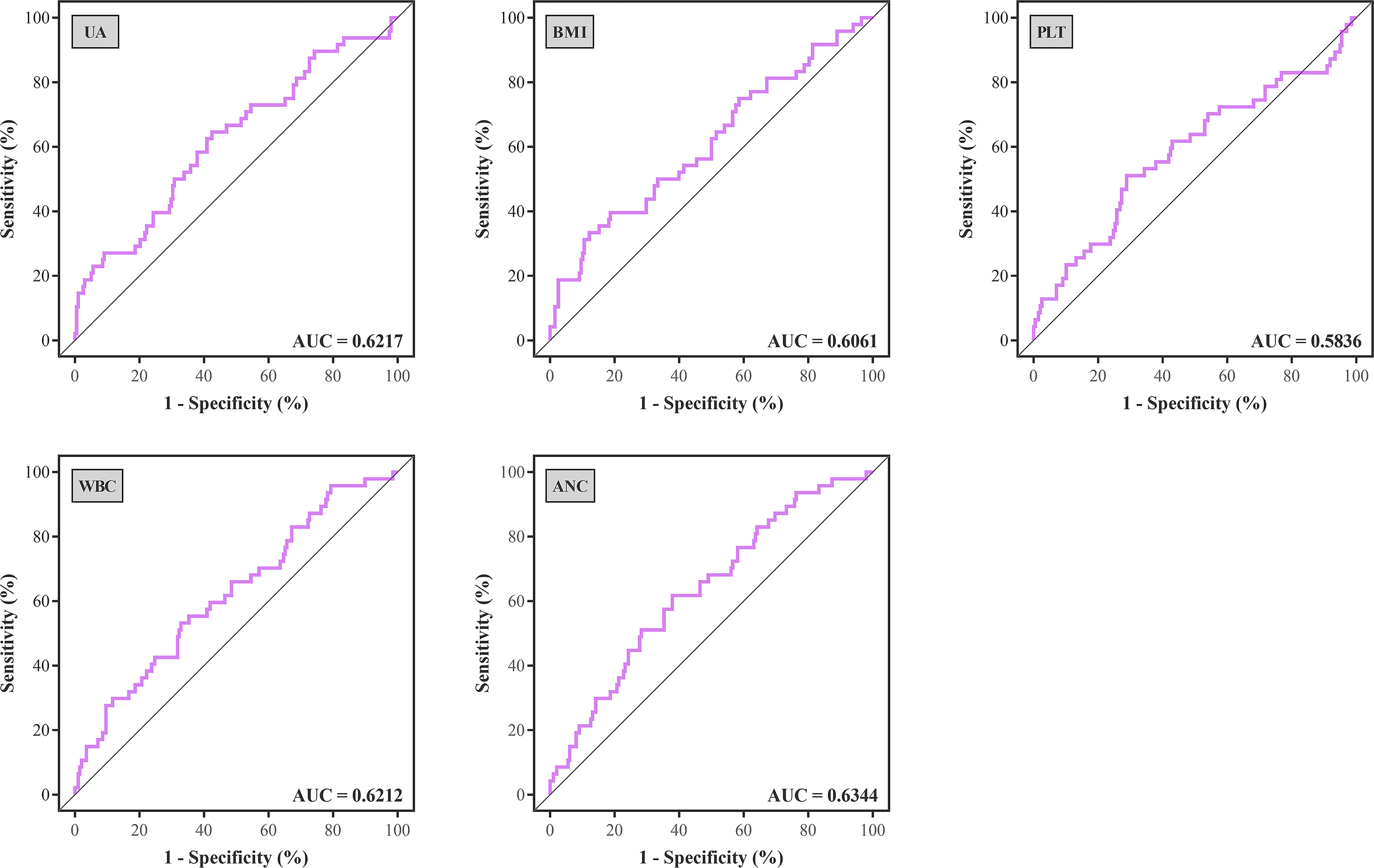
Figure 1 ROC curves were generated to determine the optimal cutoff value of UA, BMI, PLT, WBC, and ANC for treated rejection. ROC, receiver operating characteristic; AUC, area under the curve; UA, uric acid; BMI, body mass index; PLT, platelet; WBC, white blood cell; ANC, absolute neutrophil count.
In the multivariate analysis, UA > 400 μmoI/L (OR, 2.242; 95% CI, 1.037– 4.844; P = 0.040), platelet > 185 × 109 (OR, 2.163; 95% CI, 1.073–4.361; P = 0.031), ANC > 5.0 × 109 (OR, 2.183; 95% CI, 1.025–4.649; P = 0.043), and HLA-DQ mismatch (OR, 2.102; 95% CI, 1.093–4.043; P = 0.026) still showed statistical relevance and could be considered as the independent predictors for TR (Table 4). On the other hand, after multifactorial adjustment for covariates, RCS regression models indicated that patients with higher levels of preoperative serum UA had increased risk of rejection with some non-linearity (P < 0.001) (Figure 2A). The count of PLT, WBC, and ANC showed similar relationships with rejection (P < 0.001) (Figures 2B–D). Of note, RCSs examined the association of WBC and ANC with rejection on a more linear scale (Figures 2C, D).
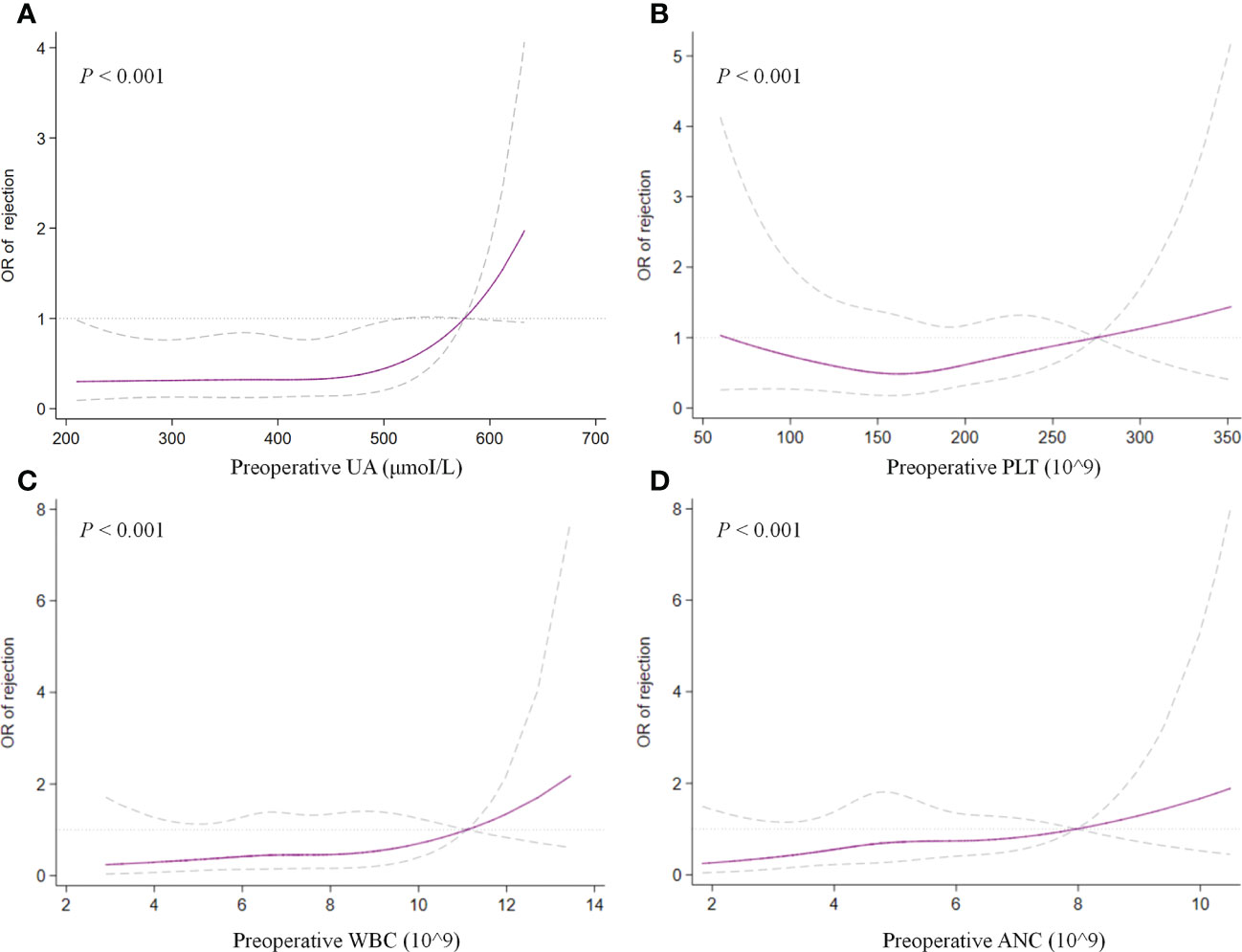
Figure 2 Restricted cubic splines examined the association of preoperative UA (A), PLT (B), WBC (C), and ANC (D) with treated rejection. UA, uric acid; PLT, platelet; WBC, white blood cell; ANC, absolute neutrophil count.
Discussion
We conducted the comprehensive analysis of 123 donors and 246 recipients and detected several possible risk factors for TR. In this study, donors’ profiles were matched with corresponding recipients for reducing potential selection biases in the evaluation of risk factors. Given the great infectious risk from over-immunosuppression caused by imbalance between immunosuppressive protocols and immunological rejection, appropriate stratification of recipients is important (11). Not only could the induction regimen individually tailored for each recipient, but also immunosuppression medication be personalized in line with immunological hazard. Hence, the assessment of risk factors for rejection would be beneficial to improve graft survival and long-term prognosis of patients.
Re-transplantation, grafts from deceased donors, and high level of panel-reactive antibody (PRA) have been reportedly associated with increased risk of graft loss and rejection after transplantation (12, 13). In the current cohort, these risk factors were also evaluated, and none of them demonstrated significant relevance to rejection in multivariate analysis, which might be attributed to the short-term follow-up and small population size that is not enough to prove these immunological associations. Graft matching remains a key element in allocation algorithm and selection of immunosuppressive agents, and poor HLA matching is associated with unwanted transplant outcomes (14). However, apart from HLA-DQ mismatch as an independent predictor of rejection that was confirmed (OR, 2.197; 95% CI, 1.119–4.317; P = 0.022), we also noted that several unexpected indicators from hemato-biochemical work-up of recipients showed statistical significance in the regression model. Precisely, UA with cutoff value of 400 μmoI/L, PLT with 185 × 109, and ANC with 5.0 × 109 exhibited robust association with TR and maybe considered as the risk factors for it.
Platelet and neutrophil have been deemed as the surrogates for inflammatory severity that is positively correlated prognosis in several diseases (15, 16). It is estimated that these indicators or ratios could reflect the systemic inflammation that might have adverse effects on hematologic cell lines and subsequently result in alteration of their ratios (16, 17). Current study demonstrated an independent positive correlation of both PLT and ANC with TR (OR, 2.202; 95% CI, 1.051–4.617; P = 0.037; and OR, 2.164; 95% CI, 1.018–4.599; P = 0.045, respectively) (Figures 2B, D). Our hypothesis is that elevated preoperative PLT and ANC of recipients maybe represent robust inflammatory response or over-activated immune system by any cause, which may drive the pathogenesis of rejection.
Another unexpected finding of our analysis was that preoperative serum UA levels revealed an independent association with TR in multivariate analysis (OR, 2.132; 95% CI, 1.016–4.476; P = 0.045). To test potential non-linearity, RCS regression models were used, which showed that the risk of rejection was relatively flat until UA was around 450 μmoI/L and then began to increase rapidly afterward, supporting the association between UA and TR. However, the comprehensive effect of UA on graft outcomes still remains controversial in published studies (18). Previous studies with complete and lengthy follow-up found an association between UA and outcomes of kidney transplantation (19, 20). Lower or normal levels of serum UA after transplantation might be an independent predictor of better graft outcomes in the long run (20). Recently, hyperuricemia was found to be related to increased death-censored graft loss but not an independent factor for long-term allograft function (21). Similarly, this study found no obvious correlation between preoperative serum UA and long-term renal function of allograft (Supplementary Figure 3).
It is unknown why UA could be an independent risk factor for TR in our study. Although hyperuricemia could result in deterioration of renal disease by inducing endothelial dysfunction and inflammatory dysregulation, it is hard to identify that UA is an immediate cause of renal disease due to unclear causal link between elevated UA and impaired renal function (22). Nevertheless, to our knowledge, the association of decreased UA with reduced graft-versus-host disease (GVHD) in allogeneic stem cell transplantation (allo-SCT) has been verified by animal models, and the level of serum UA could be used as a predictor for allo-SCT outcome (23). We therefore assume that the higher level of UA might initiate non-infectious inflammation and contribute to immune reconstitution, which increases the risk of rejection. Meanwhile, it is not unusual that hyperuricemia could be concomitant with end-stage renal diseases. Thus, although further studies are needed to confirm our results, it is necessary to address the hyperuricemia and decrease the UA during perioperative period.
In addition to retrospective design, this study has several inherent limitations. On the one hand, although the characteristics of donors were matched with recipients to control potential confounding factors, regression residual is a significant and iterative element in observational studies. On the other hand, for minimizing the negative effects of multicollinearity, the stepwise method was adopted in the regression model, which might marginalize some variables due to those with more statistical weight. Finally, our data from a single center may limit the potentially clinical application in other settings. However, although this retrospective study based on a single-center cohort and the results need further validation, the heterogeneity of large dataset from multicenter or even transnational registry could be significantly reduced in the current analysis.
Conclusions
Our study revealed that several preoperative parameters might be the independent risk factors for TR after DDKT. In contrast to the previous studies, we found that serum UA may represent an independent risk factor for TR. Although further validation is required, decreasing preoperative UA of recipients might be beneficial to reduce rejection and improve graft survival in DDKT. Moreover, the determination of these inexpensive and potentially modifiable indicators may contribute to stratify patients and develop personalized regimen in perioperative period.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. This study was approved by the Institutional Ethical Committee of the West China Hospital.
Author contributions
FXZ: project development, data collection, data analysis, and manuscript writing. JL: project development, data analysis, and data collection. YX: data analysis. FZ: data analysis. KW: data collection. WW: data collection. JY: project development and data analysis. TL and XW: project development, data analysis, manuscript correction, and manuscript editing. All named authors meet the ICMJE criteria for authorship in this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was collectively supported by grants from the National Natural Science Foundation of China (81870513); the Sichuan Science and Technology Program (2019YJ0133); the Chengdu Science and Technology Program (2019-YF05-00084-SN); and 1.3.5 Project for Disciplines of Excellence – Clinical Research Incubation Project, West China Hospital, Sichuan University (2018HXFH049, ZY2016104, ZYJC18004).
Acknowledgments
The authors would like to thank JY and Feng Qin in project development. This study had been posted in Research Square as the title “Risk factors for rejection after deceased donor kidney transplantation: A mono-institutional analysis of paired kidneys”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.973425/full#supplementary-material
Supplementary Figure 1 | Flow chart.
Supplementary Figure 2 | Changes of serum creatinine (A) and Cys C (B) in patients grouped by rejection. * means significant difference of cross-sectional data. Data are means ± SD.
Supplementary Figure 3 | Changes of serum creatinine (A) and Cys C (B) in patients grouped by preoperative serum uric acid (400 μmoI/L). * means significant difference of cross-sectional data. Data are means ± SD.
References
1. Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, et al. Analysis of factors that aff ect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet (2010) 376(9749):1303–11. doi: 10.1016/S0140-6736(10)60827-6
2. Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation (2014) 97(3):258–64. doi: 10.1097/01.TP.0000437178.48174.db
3. Troppmann C, Gillingham KJ, Benedetti E, Almond PS, Gruessner RW, Najarian JS, et al. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. the multivariate analysis. Transplantation (1995) 59(7):962–8. doi: 10.1097/00007890-199504150-00007
4. Zens TJ, Danobeitia JS, Leverson G, Chlebeck PJ, Zitur LJ, Redfield RR, et al. The impact of kidney donor profile index on delayed graft function and transplant outcomes: A single-center analysis. Clin Transplant (2018) 32(3):e13190. doi: 10.1111/ctr.13190
5. Summers DM, Watson CJ, Pettigrew GJ, Johnson RJ, Collett D, Neuberger JM, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int (2015) 88(2):241–9. doi: 10.1038/ki.2015.88
6. Pratschke J, Wilhelm MJ, Kusaka M, Beato F, Milford EL, Hancock WW, et al. Accelerated rejection of renal allografts from brain-dead donors. Ann Surg (2000) 232(2):263–71. doi: 10.1097/00000658-200008000-00017
7. Zitur LJ, Chlebeck PJ, Odorico SK, Danobeitia JS, Zens TJ, Van Kooten C, et al. Brain death enhances activation of the innate immune system and leads to reduced renal metabolic gene expression. Transplantation (2019) 3(9):1821–33. doi: 10.1097/TP.0000000000002744
8. Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant (2007) 7(7):1797–807. doi: 10.1111/j.1600-6143.2007.01852.x
9. Barlow AD, Metcalfe MS, Johari Y, Elwell R, Veitch PS, Nicholson ML, et al. Case-matched comparison of long-term results of non-heart beating and heart-beating donor renal transplants. Br J Surg (2009) 96(6):685–91. doi: 10.1002/bjs.6607
10. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant (2008) 8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x
11. Roberts MB, Fishman JA. Immunosuppressive agents and infectious risk in transplantation: Managing the "net state of immunosuppression. Clin Infect Dis (2021) 73(7):e1302–17. doi: 10.1093/cid/ciaa1189
12. Betjes MGH, Sablik KS, Otten HG, Roelen DL, Claas FH, de Weerd A. Pretransplant donor-specific anti-HLA antibodies and the risk for rejection-related graft failure of kidney allografts. J Transplant (2020) 2020:5694670. doi: 10.1155/2020/5694670
13. Dunn TB, Noreen H, Gillingham K, Maurer D, Ozturk OG, Pruett TL, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant (2011) 11(10):2132–43. doi: 10.1111/j.1600-6143.2011.03640.x
14. Alfaro R, Martínez-Banaclocha H, Llorente S, Jimenez-Coll V, Galián JA, Botella C, et al. Computational prediction of biomarkers, pathways, and new target drugs in the pathogenesis of immune-based diseases regarding kidney transplantation rejection. Front Immunol (2021) 12:800968. doi: 10.3389/fimmu.2021.800968
15. Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol (2015) 111(7):868–74. doi: 10.1002/jso.23900
16. Naranjo M, Agrawal A, Goyal A, Margonis G, Luo T, Ejaz A, et al. Neutrophil-to-Lymphocyte ratio and platelet-to-Lymphocyte ratio predict acute cellular rejection in the kidney allograft. Ann Transplant (2018) 23:467–74. doi: 10.12659/AOT.909251
17. Vartolomei MD, Kimura S, Ferro M, Vartolomei L, Foerster B, Abufaraj M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? a meta-analysis of 4385 patients. World J Urol (2018) 36(7):1019–29. doi: 10.1007/s00345-018-2235-5
18. Kim ED, Famure O, Li Y, Kim SJ. Uric acid and the risk of graft failure in kidney transplant recipients: a re-assessment. Am J Transplant (2015) 15(2):482–8. doi: 10.1111/ajt.13000
19. Dahle DO, Jenssen T, Holdaas H, Leivestad T, Vårdal M, Mjøen G, et al. Uric acid has a J-shaped association with cardiovascular and all-cause mortality in kidney transplant recipients. Clin Transplant (2014) 28(1):134–40. doi: 10.1111/ctr.12290
20. Kim DG, Choi HY, Kim HY, Lee EJ, Huh KH, Kim MS, et al. Association between post-transplant serum uric acid levels and kidney transplantation outcomes. PloS One (2018) 13(12):e0209156. doi: 10.1371/journal.pone.0209156
21. Isakov O, Patibandla BK, Shwartz D, Mor E, Christopher KB, Hod T, et al. Can uric acid blood levels in renal transplant recipients predict allograft outcome? Ren Fail (2021) 43(1):1240–9. doi: 10.1080/0886022X.2021.1969246
22. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int (2005) 67(5):1739–42. doi: 10.1111/j.1523-1755.2005.00273.x
23. Penack O, Peczynski C, van der Werf S, Finke J, Ganser A, Schoemans H, et al. Association of uric acid levels before start of conditioning with mortality after allogeneic hematopoietic stem cell transplantation - a prospective, non-interventional study of the EBMT transplant complication working party. Haematologica (2020) 15(7):1977–83. doi: 10.3324/haematol.2019.228668
Keywords: kidney transplantation, donor, recipient, rejection, risk factor
Citation: Zhang F, Liang J, Xiong Y, Zhang F, Wu K, Wang W, Yuan J, Lin T and Wang X (2022) Serum uric acid as a risk factor for rejection after deceased donor kidney transplantation: A mono-institutional analysis of paired kidneys. Front. Immunol. 13:973425. doi: 10.3389/fimmu.2022.973425
Received: 16 September 2022; Accepted: 23 November 2022;
Published: 12 December 2022.
Edited by:
Mohamed Ghoneim, Mansoura University, EgyptReviewed by:
Ernesto Paoletti, San Martino Hospital (IRCCS), ItalyManuel Muro, Hospital Universitario Virgen de la Arrixaca, Spain
Copyright © 2022 Zhang, Liang, Xiong, Zhang, Wu, Wang, Yuan, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianding Wang, Zm9vbHNvb24uY0AxNjMuY29t
†These authors have contributed equally to this work
 Fuxun Zhang
Fuxun Zhang Jiayu Liang
Jiayu Liang Yang Xiong
Yang Xiong Fan Zhang
Fan Zhang Kan Wu
Kan Wu Wei Wang
Wei Wang Jiuhong Yuan
Jiuhong Yuan Xianding Wang
Xianding Wang