- 1Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden
- 2Department of Clinical Chemistry, National Liver Institute, Menoufia University, Menoufia, Egypt
- 3Department of Immunology and Transfusion Medicine, Karolinska University Hospital, Stockholm, Sweden
Allogeneic Hematopoietic stem cell transplantation (allo-HCT) is a curative platform for several hematological diseases. Despite its therapeutic benefits, the profound immunodeficiency associated with the transplant procedure remains a major challenge that renders patients vulnerable to several complications. Today, It is well established that a rapid and efficient immune reconstitution, particularly of the T cell compartment is pivotal to both a short-term and a long-term favorable outcome. T cells expressing a TCR heterodimer comprised of gamma (γ) and delta (δ) chains have received particular attention in allo-HCT setting, as a large body of evidence has indicated that γδ T cells can exert favorable potent anti-tumor effects without inducing severe graft versus host disease (GVHD). However, despite their potential role in allo-HCT, studies investigating their detailed reconstitution in patients after allo-HCT are scarce. In this review we aim to shed lights on the current literature and understanding of γδ T cell reconstitution kinetics as well as the different transplant-related factors that may influence γδ reconstitution in allo-HCT. Furthermore, we will present data from available reports supporting a role of γδ cells and their subsets in patient outcome. Finally, we discuss the current and future strategies to develop γδ cell-based therapies to exploit the full immunotherapeutic potential of γδ cells in HCT setting.
Introduction
Over the past 5 decades, allogeneic hematopoietic stem cell transplantation (allo-HCT) has evolved rapidly; over 1 million HCTs have been performed worldwide since the first successful allo-HCT in 1959 (1). This remarkable progress was the result of several factors including the introduction of less toxic conditioning regimens and improved understanding of the immune system (2). Besides being established as an efficient therapeutic option for several hematological and non-hematological disorders, allo-HCT has also served as a platform to develop novel personalized cell therapies (3, 4).
Despite the tremendous improvement in allo-HCT outcome over the past years, several challenges remain, preventing the full therapeutic benefit. Among these challenges, primary disease relapse, graft versus host disease (GVHD), and infectious complications represent the main leading causes of transplant-related morbidity and mortality (5, 6). These complications are linked to the profound immunocompromised state encountered after allo-HCT (7). Therefore, efficient restoration of a functional immune system is central for beneficial outcome (8). In line with this, several studies have indicated that T cells are of key importance in allo-HCT outcome as prolonged lymphopenia after allo-HCT is associated with severe adverse effects (5, 8–10). Proper understanding of T cell reconstitution kinetics and the factors affecting this process following allo-HCT are crucial to improve HCT outcome.
Following allo-HCT, the T cell compartment is restored through a complex and dynamic process that involves two distinct pathways. During the early phase after transplantation T cells recover mainly through the homeostatic proliferation of donor-derived mature T cells co-infused with the stem cell graft (11). This process is also known as the thymic-independent pathway to distinguish it from the thymic-dependent pathway which involves de novo generation of naïve T cells from donor progenitor stem cells (12, 13).
Although homeostatic proliferation offers a faster route to replenish the virtually empty T cell pool, the T cells reconstituted in this fashion are relatively inferior to their de novo generated T cell counterparts. It has been shown that T cells originating from donor-derived mature T cells offer limited protection against infectious pathogens possibly because of their limited TCR diversity (14–16). Furthermore, they are more prone to activation-induced cell death (17), and can most likely induce GVHD as they are potentially alloreactive (18). On the contrary, de-novo generated T cells undergo TCR rearrangement and the stringent selection steps in the thymus resulting in a more diverse repertoire of cells which are self-tolerant (19–21). However, thymopoiesis is a slow and age-dependent process; thus it can take up to several months or years especially in elderly patients. Furthermore, the thymic stroma is extremely sensitive to transplant-related insults such as GVHD, infections, and conditioning regimen (20, 22, 23).
Although T cell reconstitution has been extensively investigated, most studies have focused on the role of conventional αβ T cells while unconventional T cells remain under scrutinized. During the past decades, a subset of T cells known as gamma delta (γδ) T cells has become the focus of increasing interest due to their proven role in stress immunosurveillance (24), as well as their emerging roles in tissue homeostasis, wound healing, and heat regulation (25–29). However, their detailed reconstitution after allo-HCT remains poorly understood. In the next sections we shed light on their main reconstitution features, the factors affecting them, and their role after allo-HCT.
Immunobiological features of gamma delta (γδ) T cells
Gamma delta (γδ) T cells comprise a distinct lineage of T lymphocytes that can be distinguished from conventional T cells through their TCR that encompasses γ and δ chains instead of the α and β chains (30, 31). Together with T and B lymphocytes, γδ T cells have been conserved across species for millions of years (32). Similar to their αβ T cell counterparts, the development of γδ T cells within the thymus entails somatic rearrangement of the V(D)J segments of their TCR CDR3 region by the recombination activating gene (33). Human γδ TCR is encoded by two distinct gene segments: TRG gene segment located on chromosome 7 encoding 6 functional Vγ genes, and the TRD gene segment embedded within the TRA locus at chromosome 14 encoding 8 functional Vδ genes (31). Based on the Vδ chain usage γδ T cells are classified into two major subsets; the Vδ2+ subset and the non-Vδ2 subset of which Vδ1+ γδ T cells are the predominant fraction (34). In the peripheral blood, 1-10% of total T cells are of γδ T cell lineage, the vast majority of which express the semi-invariant phosphoantigen reactive Vγ9Vδ2 TCR (35, 36). The non-Vδ2 subsets are found predominantly in epithelial tissue compartments such as skin, intestine, and reproductive system and unlike their Vδ2+ counterparts their activating ligands and the underlying recognition mechanisms have, so far, remained incompletely understood (37).
Whether γδ T cells align best with innate or adaptive biology remains undecided. Classically, γδ T cells were described as an interface between innate and adaptive immunity as they share features of both systems (38). Nonetheless, fundamental data from recent studies support distinct innate-like and adaptive-like immunobiological paradigms (39, 40). For instance, several recent studies revealed an antigen-driven clonotypic focusing of certain γδ T cells subsets upon encountering their cognate antigen, alongside transition to an effector memory phenotype supporting adaptive-like paradigm (41–43).
Receptors and effector functions of γδ T cells
Unlike αβ T cells, the majority of γδ T cells do not express CD4 or CD8 molecules and recognize their antigens in an HLA unrestricted manner (30). Apart from TCR-mediated activation, γδ T cells can be activated in a TCR-independent mechanism (44). Beside toll-like receptors, γδ T cells also express a wide array of NK receptors such as NKG2D, DNAM-1, and the natural cytotoxicity receptors, allowing γδ T cells to react rapidly in response to “altered-self” signals. These features placed γδ T cells in the front line of defense against pathogens and transformed/tumor cells (41, 45).
Upon activation, γδ T cells exert their effector functions either directly or indirectly. The direct cytotoxic effects can be executed through the death receptors (FAS/FAS ligand and TRAIL/TRAIL receptor) triggering target cell apoptosis and/or through perforin/granzyme mediated tumor cell lysis (34, 46). Additionally, γδ T cells express the FcRγIII (CD16) that can mediate direct target cell lysis via antibody-dependent cellular cytotoxicity (ADCC) (33, 44). Indirect mechanisms are mediated mainly through cytokines secreted by γδ T cells such as IFN-γ, TNFα, IL-17, and IL-22 (47). These cytokines allow γδ T cells to interact with and modulate the activity of other immune cells. In addition, γδ T cells can promote dendritic cell maturation, take up, process and cross-present antigens to other immune cells, and enhance anti-infectious activities of other immune cells such as NK cells and macrophages (48–50).
Reconstitution of γδ T cells after allo-HCT: Current understanding
The rate of immune cell recovery after allo-HCT varies from one cell type to another. In general, cells of the innate immune system such as neutrophils, monocytes, and NK cells recover earlier (within weeks), while the recovery of the adaptive immune cells is more prolonged, taking months or even years to entirely recover (51, 52). Only a limited number of studies have focused on the reconstitution of γδ T cells following allo-HCT. In this regard, numerous studies consistently demonstrated that γδ T cell reconstitution occurs in the initial few weeks after transplantation (1, 11, 53–55). In pediatric haplo-identical HCT patients that received αβ T cell-depleted (TCD) grafts, Airoldi et al. showed that γδ T cells expand quickly, reaching up to 90% of the initial T cell pool, and subsequently decline as αβ T cells start to recover. Further characterization of the γδ T cell composition one month post HCT showed that Vδ2 cells were the predominant fraction, indicating that γδ T cell composition did not differ significantly from that present in peripheral blood of the donors or healthy adults (56). Likewise, studying γδ T cell reconstitution at the clonal level showed that reconstituted γδ T cell repertoire remained very stable over time after transplantation (54, 57).
However, it remains unclear whether reconstituted γδ T cells in allo-HCT patients have originated from the peripheral expansion of donor γδ T cells infused within the stem cell grafts or whether they have originated from thymopoiesis. The quick reconstitution of γδ T cells shortly after allo-HCT suggests a peripheral expansion of donor-derived γδ T cells. Corroborating this, γδ T cell reconstitution has been shown to be delayed in patients that received OKT3 TCD grafts (58). Furthermore, by examining the CDR3 size distribution pattern in 23 allo-HCT recipients, Hirokawa et al. identified 2 γδ T cell clones in the donor grafts that were found as well in the recipient blood after allo-HCT, but not before (54). Consistently, chimerism analysis in the first 2-3 weeks after haplo-identical HCT indicated donor-origin of the reconstituted γδ T cells (56). Altogether, these data strongly support that initial γδ T cell reconstitution occurs mainly through the homeostatic peripheral expansion of donor-derived mature γδ T cells.
On the other hand, it has been shown that the proportion of naïve γδ T cells increases as early as 2-3 months after HCT (59). In a recent study, Raven et al. used high throughput RNA-based next generation sequencing to in-depth evaluate the TCR repertoires of 6 allo-HCT patients and their corresponding donors. Their results indicated the presence of heterogenous overlapping sequences in donor/recipient pairs, yet most of patient/donor pairs displayed no specific correlation of their repertoires, supporting de novo generation of γδ T cells (57). Altogether, these data suggest that γδ T cell reconstitution involves both thymic-dependent and independent mechanisms, even though γδ T cells rely principally on homeostatic proliferation during the early phase post allo-HCT.
Some questions remain which warrant further investigation. To what extent does the thymus contribute to γδ T cell reconstitution especially in long-term survivors and do de-novo generated γδ T cells provide a more favorable patient outcome?
Factors affecting γδ T cell reconstitution
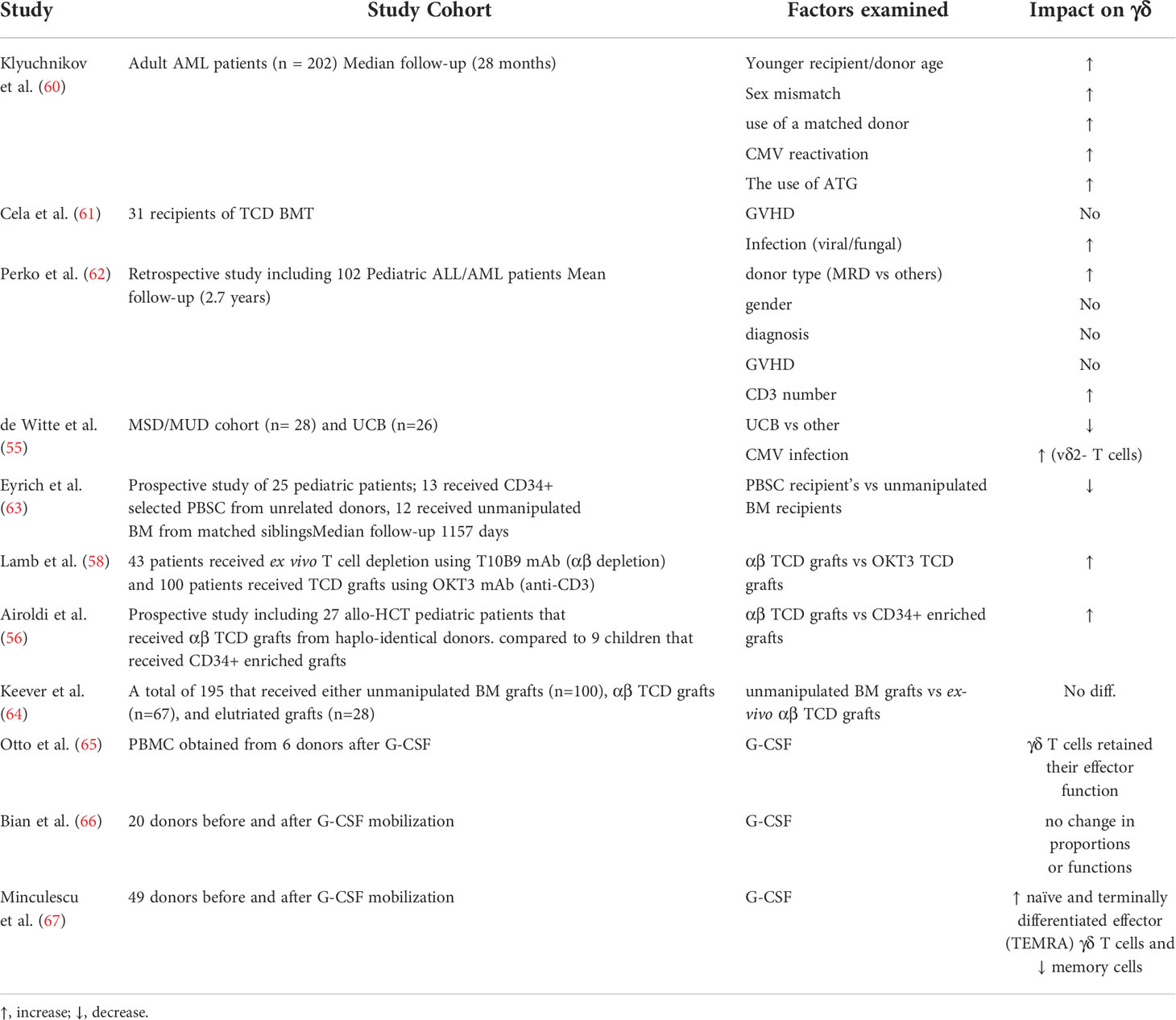
Unlike αβ T cells, the impact of different transplant-related factors, such as graft source, GVHD, conditioning regiment etc. on γδ T cell reconstitution and TCR diversity remains largely unknown (Table 1). In a recent retrospective study of 202 adult AML patients, Klyuchnikov and colleagues identified several transplant-related factors associated with γδ T cell reconstitution after allo-HCT (60). Their results indicated that younger recipient/donor age, sex mismatch, use of a matched donor, and CMV reactivation were factors associated with faster γδ T cell reconstitution. Additionally, they showed that the use of post-transplantation cyclophosphamide was associated with lower levels of γδ T cells compared to ATG (60). Of note, the impact of cytomegalovirus (CMV) infection/reactivation on γδ T cell reconstitution and TCR repertoire after allo-HCT has been discussed thoroughly in multiple studies including a recent review by our group (68).
Whether graft source impacts the rate of γδ T reconstitution after allo-HCT has only been adequately investigated in a handful of publications (Table 1). Perko et al. examined different variables associated with γδ T cells reconstitution post allo-HCT in 102 pediatric patients with acute leukemia. Their results indicated significant impact of donor source on γδ T cells reconstitution (62). Another study by De witte et al. showed that γδ T cells were significantly fewer in umbilical cord blood recipients compared to recipients of HLA matched siblings or unrelated donors (55). Furthermore, in allo-HCT pediatric patients, the recovery of γδ T cells was delayed in recipients of CD34+ selected PBSC compared to recipients of unmanipulated BM grafts from HLA identical siblings (63, 69). However, it is difficult to conclude whether this is the effect of the graft source per se or due to the manipulation of PBSC graft.
To further examine the impact of graft manipulation on immune reconstitution after HCT, Lamb et al. assessed γδ T cell reconstitution after ex vivo T cell depletion using T10B9 mAb (αβ depletion) and OKT3 mAb (anti-CD3). They showed better γδ T cell reconstitution in patients that received αβ TCD grafts (58). In line with this, Airoldi et al. showed that γδ T cells were significantly higher in haplo-identical allo-HCT pediatric patients that received αβ TCD grafts compared to patients that received CD34+ enriched grafts (56). Conversely, results by Keever-Taylor et al. indicated no differences in γδ T cell recovery between allo-HCT patients that received unmanipulated BM grafts or αβ TCD grafts, although they showed that the reconstitution of NK cells was faster in the TCD group (64).
The effect of stem cell mobilization using granulocyte colony stimulating factor (G-CSF) on γδ T cells has been scarcely investigated. Otto et al. showed that γδ T cell effector functions were not impaired in G-CSF mobilized stem cell grafts (65). In line with this, Bian et al. examined phenotypic characteristics of γδ T cells in the peripheral blood of 20 donors before and after G-CSF mobilization. They showed that γδ T cells retained their homeostatic proportions and IFN-γ secreting capabilities following G-CSF (66). Conversely, a more detailed analysis by Minculescu et al. showed that G-CSF preferentially mobilized naïve and terminally differentiated effector (TEMRA) γδ T cells over memory cells with a preferential increase in the non-Vδ2 subset while also increasing the proportion of HLA-DR expressing γδ T cells (67). Regarding the impact on TCR repertoire, it has been shown that G-CSF was associated with TCR repertoire disturbances in the form of alteration of distribution and clonality of some TRG and TRD subfamilies, suggesting a potential immune modulatory effect (70). Further investigations are required to elucidate the detailed impact of G-CSF mobilization on different γδ T cell subsets.
γδ T cell reconstitution and clinical outcome after allo-HCT
Multiple studies have highlighted the importance of conventional αβ T cell recovery and its impact on clinical outcome post allo-HCT, but the role of γδ T cells has not been well described. Whether γδ T cells are beneficial or not especially in the context of GVHD has been a matter of debate, particularly among earlier studies that showed controversial results. For instance, Viale et al. showed that percentages and total numbers of γδ T cells were increased in patients that developed acute GVHD up to 3 months after allo-HCT (71). Likewise, other studies in mice have suggested a role for γδ T cells in GVHD pathogenesis (72–74). For instance, using an experimental GVHD murine model, Maeda et al, showed that host γδ T cells exacerbate GVHD by enhancing the alloreactive capacity of DCs in a cell contact dependent manner (74). However, several lines of evidence from both murine and human studies support that γδ T cells are not involved in the initiation or severity of GVHD (44, 53, 75, 76). In their study, Drobyski et al. found that lethally irradiated mice that were infused with large doses of γδ T cells did not develop GVHD (75). Likewise, similar findings in human studies were reported. Cela et al. didn’t find any significant correlation between γδ T cells and the incidence of GVHD in the first 12 months posttransplant (61). Corroborating this, Lamb et al. showed that donor-derived γδ T cells were able to recognize and lyse primary ALL blasts but did not proliferate when cultured with allogeneic cells in a mixed lymphocyte reaction, suggesting a graft vs leukemia (GVL) activity in the absence of an allogeneic response (76). Additionally, several studies showed decreased incidence of GVHD in recipients of αβ TCD grafts.
In the same context, the impact of GVHD on γδ T cell reconstitution has not been adequately addressed after allo-HCT. Although previous studies have shown that GVHD and/or its treatment can impair thymic function and thus T cell recovery (77, 78), it remains unclear to what extent γδ T cell reconstitution is affected by GVHD. In an earlier study that included 31 recipients of TCD BM grafts no significant impact of GVHD on the pattern of γδ T cell recovery was found (61). Of note, in-depth analysis of γδ T cell reconstitution might reveal a potential impact of GVHD on γδ T cells at functional or clonal level.
It was not long before the anti-tumorous capabilities of γδ T cells were confirmed in human studies after it was first reported in an experimental mice model (79). In fact, the ability of γδ T cells to exhibit potent anti-tumor responses in the absence of the unwanted alloreactive immune response as well as their potential to bypass tumor immune evasion mechanisms (e.g. HLA down regulation) have made γδ T cells a subject of great interest in the field of allo-HCT (80). Several research groups have shown that elevated γδ T cells early post-transplantation was associated with improved leukemia-free survival and overall survival (81, 82). In fact, the earliest clinical report that suggested favorable γδ T cell role post allo-HCT, in terms of enhanced GVL effect and favorable survival outcome, was published in 1996 by Lamb et al. (81). In this study that included 43 leukemia patients that received αβ TCD grafts from partially mismatched related donors, the authors showed that disease-free survival was significantly improved in patients that developed high proportion (> 10%) of γδ T cells during the first 6 months after transplantation compared to patients with normal γδ T cell proportion. In a subsequent follow up (3 years) of this study, the authors confirmed the previous results and further showed that Vδ 1 cells were the predominant γδ T cell population in patients with high γδ T cell proportion (58). In an extension of this study with additional patients and longer follow up (up to 8 years), Godder at al. confirmed the long-term survival advantage with no increased incidence of acute GVHD in patients with higher numbers of γδ T cells (82). Likewise, Perko et al. reported decreased incidence of infection and improved event-free survival in patients with elevated γδ T cells (62). Corroborating these findings in pediatric setting, a long-term prospective study of children with acute leukemia that underwent αβ T- and B-cell–depleted haplo-HCT after a myeloablative regimen showed decreased incidence of both acute and chronic GVHD as well as, reduced incidence of none relapse mortality, conceivably due to the spared γδ T cells and NK cells transferred with the graft (83). Similarly, in pediatric haplo-identical HCTs with αβ TCD grafts, Park et al. showed improved relapse-free survival in acute leukemia patients that recovered with high percentage of γδ T cells at day 30 compared to those with low percentage of γδ T cells (84).
In the context of a potential pathogen protective role, it has been confirmed in multiple studies that CMV reactivation induces clonal expansion of non-Vδ2+ T cells (85, 86). Likewise, several studies have highlighted the role of Vδ1 T cells in CMV immunosurveillance after allo-HCT (85, 87). Furthermore, a more recent study in pediatric patients indicated that patients with a low percentage (≤21%) of γδ T cells at day 30 had significantly higher incidence of CMV reactivation compared to patients with a high percentage (84). Additionally, a possible role for γδ T cells in EBV immune response was suggested by De Paoli et al. (88). In a recent systematic review and meta-analysis we addressed whether γδ T cell reconstitution is associated with clinical outcome after allo-HCT. Out of 2412 studies, 11 studies (919 patients, median follow-up of 30 months) met the eligibility criteria for the meta-analysis. Results of the meta-analysis confirmed the benefit of having higher levels of γδ T-cells in peripheral blood after HCT in terms of less disease relapse, fewer viral infections, and improved overall and disease-free survivals, whereas there was no association with the incidence of acute GVHD (89).
Given that donor γδ T cells co-infused within the stem cell (SC) grafts substantially contribute to the reconstituted γδ T cell pool after allo-HCT, investigating γδ T cells graft composition can provide insight into clinical outcome. In this context, an earlier study showed increased cumulative incidence of acute GVHD II-IV in patients that received PBSC grafts with higher γδ T cells content (90). However, in this study γδ T cells were examined in total and the impact of different subsets was not investigated. Given that γδ T cells comprise heterogenous subsets, we recently analyzed the composition of γδ cell subsets in 105 donor stem cell grafts. We found that patients that received SC grafts containing higher proportions of CD8+γδ T cells had an increased cumulative incidence of acute GVHD II-III, suggesting a potential alloreactive role of this subset (91). In the same study, we found an inverse correlation between CD27+ γδ T cell graft content and the incidence of both relapse and CMV reactivation post allo-HCT (91). Though we have not assessed the functional properties of this subset, previous studies on murine γδ T cells have shown that CD27 demarcates IFNγ producing γδ cells (92). Altogether, these data suggest that the composition of intra-graft γδ T cell subsets can impact patient outcome after allo-HCT.
In addition to γδ T cell proportion, γδ TCR repertoire has been suggested to play a role in clinical outcome as it has been shown that γδ T cells expressing polyclonal TCR repertoire comprise different affinities and hence different functional capabilities (46). In line with this assumption, Grunder et al. showed that Vγ9Vδ2 T cells with a polyclonal TCR repertoire are inferior to cells that express a monoclonal repertoire (93). In a recent study, we examined the impact of intra-graft γδ T cell repertoire composition on the outcome of 20 adult AML patients that underwent allo-HCT. Analysis of the TCRγ repertoire by NGS indicated that grafts given to non-relapsed patients featured a more public repertoire and an increased presence of long sequence clonotypes. Further analysis of the amino acid sequences showed that 12 public and 4 private sequences were exclusively found in high frequencies in grafts given to non-relapsed patients most of these sequences were 42-54 amino acid long (94). Altogether, these data suggest that specific γδ TCR clonotypes might play important role in patient outcome after transplantation, adding another layer of complexity to the already intricate landscape.
Concluding remarks and future directions
In general, most of the ongoing and future directions to harness γδ T cells in allo-HCT fall under two main categories: strategies that aim to enhance γδ T cell reconstitution, and strategies that aim to exploit their anti-tumor properties by redirecting their immune responses against tumors. We briefly highlight some of these approaches. For more comprehensive information the readers are directed to these reviews (30, 46, 48, 95, 96).
The notion that γδ T cell-enriched grafts are associated with better γδ T cell reconstitution and more favorable outcome has encouraged researchers to develop strategies that enhance γδ T cell recovery post-HCT. Beside the use of αβ TCD grafts, another strategy that has been investigated is the adoptive transfer of autologous or allogeneic γδ T cells. In this strategy γδ T cells are enriched and re-infused either directly or after in vitro expansion. An example of such strategy is the use of αβ TCD donor lymphocyte infusion. This strategy would have the benefit of retaining higher numbers of γδ T and NK cells without the unfavorable alloreactive donor T cells. Our group has investigated the use of αβ TCD grafts as post-transplant boosters to treat secondary graft failure in 5 allo-HCT patients. The results were promising as there was no signs of GVHD or other side effects (97). A subsequent follow up of this study with more patients and longer follow up time supported the same conclusion (98).
Accumulating evidence indicates that the thymus is, to a certain extent, implicated in the reconstitution of γδ T cells after allo-HCT (57). Therefore, it is reasonable to assume that enhancing thymic regenerative capacity would positively improve γδ T cell recovery. Even though the clinical benefit of γδ T cells reconstituted through the thymic-dependent pathway is still unknown, enhancing the thymic function would still be beneficial for conventional T cell reconstitution. There are several reviews that have discussed the different strategies that are investigated to enhance thymic regenerative capacity, and the reader is referred to some publications for further information (20, 99, 100).
Since the first observation of the ability of aminobisphosphonate to selectively expand Vδ2Vγ9 T cells in multiple myeloma patients (101), several clinical trials have been conducted in solid and hematological cancers using zoledronate (ZOL) and low dose IL-2 for either in vivo or ex vivo expansion of Vδ2Vγ9 cells (30, 49). Although the use of Zol/IL-2 in these trials proved to be safe and tolerable, the resulting outcome was limited. This could, in part, be due to the functional plasticity, the infusion of polyclonal TCR repertoire, and/or cell exhaustion, emphasizing the need for further optimization of current protocols. In this regard, several strategies have been suggested to enhance the proliferative and functional capacity of ex-vivo expanded γδ T cells. For instance, a recent study showed that γδ T cell expansion was more efficient when a bisphosphonate prodrug was used. Furthermore, several studies have investigated the role of common γ-chain family cytokines such as IL-15. Results showed that γδ T cells expanded in presence of IL-15 displayed enhanced cytotoxic capabilities (102), and upregulated CD56, a marker associated with better cytotoxic effector function (103). Although TGF-β is generally regarded as an immunoregulatory cytokine, recent reports showed enhanced cytotoxicity and IL-9 secretion in TGF-β-treated, phosphoantigen activated γδ T cells (103). Besides, the administration of vit C during γδ T cell expansion has been recently explored (104).
Unlike Vδ2Vγ9 cells, protocols for Vδ1+ expansion have not been explored until recently due to the lack of specific agonist for Vδ1+ cells. So far, only a limited number of large-scale protocols for Vδ1+ expansion have been described (105, 106). In this regard, Wu et al. developed a protocol for preferential expansion of Vδ1+cells from PB of healthy donors and colon cancer patients using phytohemagglutinin (PHA) and IL7. To develop a GMP-compatible protocol that can be clinically adapted, Almeida et al. described a 3 week expansion protocol known as delta one T cell (DOT). Unlike previous protocols they did not use mitogenic stimulators like PHA, instead they used TCR stimulation and a defined cytokine cocktail. This protocol resulted in up to 2000-fold expansion of Vδ1+ cells that displayed antitumor capabilities with no IL17 production (107).
The redirection of T-cell responses against specific tumor antigens represents one of the mainstays of modern personalized precision medicine. Although the use of γδ T cells has lagged behind αβ T cells in this field, they offer an attractive alternative due to their rapid innate like response and lower alloreactivity. In this regard, engineering γδ T cells to express a chimeric antigen receptor (CAR) is currently under clinical development. In fact, γδ T cells that express a first-generation CAR directed against GD2 were first described in 2004 (108). Another interesting approach is the use of T cells engineered with defined γδ TCRs (TEG). Unlike the CAR γδ T cells, in this strategy αβ T cells are transduced to express a high-affinity Vγ9Vδ2 TCR providing features of both conventional and unconventional immune cells (109). A phase I trial is currently ongoing to test the safety of TEG in patients with a relapsed/refractory AML, high-risk Myelodysplastic Syndrome (MDS) or relapsed/refractory Multiple Myeloma (MM) (NTR 6541). Finally, redirecting γδ T cell against tumor antigens can be achieved using bispecific antibodies. These are nano constructs that comprise 2 single chain (sc) Fv domains where one scFv binds to the effector and the other binds its target (e.g. tumor antigen). In this regard, [HER2 ×Vγ9] bispecific T cell engager (BiTE) has been developed and tested by Oberg et al. (110). The same group have also developed and tested a Tribody [(HER2)2 × CD16] that redirect CD16-expressing γδ and NK cells against HER2-expressing cancer cells. The new tribody was shown to be effective in enhancing γδ T cell and natural killer cell cytotoxicity (111).
In conclusion, the inevitable favorable role of γδ T cells in allo-HCT setting has stimulated researchers to exploit their full immunotherapeutic benefits. However, as we discussed above, caution should be paid as to which subset and what function it may exert. Therefore, a better understanding of the functional heterogeneity of the γδ T cell compartment, mechanisms of antigen recognition, and γδ TCR ligands are fundamental to exploit the full therapeutic benefit of γδ T cells.
Author contributions
AG, LA, and MU designed the study. AG drafted the manuscript. LA and MU reviewed and approved the final manuscript.
Acknowledgments
This work was funded by grants from the Swedish Cancer Society, the Swedish Childhood Cancer Fund, the Swedish Research Council, and by grants provided by Stockholm County Council.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chaudhry MS, Velardi E, Malard F, van den Brink MR. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: Time to T up the thymus. J Immunol (2017) 198(1):40–6. doi: 10.4049/jimmunol.1601100
2. Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol (2015) 2(3):e91–100. doi: 10.1016/S2352-3026(15)00028-9
3. Gratwohl A, Baldomero H, Passweg J. Hematopoietic stem cell transplantation activity in Europe. Curr Opin Hematol (2013) 20(6):485–93. doi: 10.1097/MOH.0b013e328364f573
4. Chabannon C, Kuball J, Bondanza A, Dazzi F, Pedrazzoli P, Toubert A, et al. Hematopoietic stem cell transplantation in its 60s: A platform for cellular therapies. Sci Trans Med (2018) 10(436):eaap9630. doi: 10.1126/scitranslmed.aap9630
5. Arnaout K, Patel N, Jain M, El-Amm J, Amro F, Tabbara IA. Complications of allogeneic hematopoietic stem cell transplantation. Cancer Invest (2014) 32(7):349–62. doi: 10.3109/07357907.2014.919301
6. Jaglowski SM, Devine SM. Graft-versus-host disease: Why have we not made more progress? Curr Opin Hematol (2014) 21(2):141–7. doi: 10.1097/MOH.0000000000000026
7. Morris ES, Hill GR. Advances in the understanding of acute graft-versus-host disease. Br J Haematol (2007) 137(1):3–19. doi: 10.1111/j.1365-2141.2007.06510.x
8. Ho CM, McCarthy PL, Wallace PK, Zhang Y, Fora A, Mellors P, et al. Immune signatures associated with improved progression-free and overall survival for myeloma patients treated with AHSCT. Blood Adv (2017) 1(15):1056–66. doi: 10.1182/bloodadvances.2017005447
9. Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother (2016) 22(8):505–14. doi: 10.1016/j.jiac.2016.05.006
10. Arruda LCM, Malmegrim KCR, Lima-Junior JR, Clave E, Dias JBE, Moraes DA, et al. Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients. Blood Adv (2018) 2(2):126–41. doi: 10.1182/bloodadvances.2017011072
11. Dekker L, de Koning C, Lindemans C, Nierkens S. Reconstitution of T cell subsets following allogeneic hematopoietic cell transplantation. Cancers (2020) 12(7):1974. doi: 10.3390/cancers12071974
12. Abo T. Extrathymic pathways of T cell differentiation. Archivum Immunol Et Therapiae Experimentalis (2001) 49(2):81–90.
13. Bains I, Thiebaut R, Yates AJ, Callard R. Quantifying thymic export: combining models of naive T cell proliferation and TCR excision circle dynamics gives an explicit measure of thymic output. J Immunol (2009) 183(7):4329–36. doi: 10.4049/jimmunol.0900743
14. Krenger W, Blazar BR, Hollander GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood (2011) 117(25):6768–76. doi: 10.1182/blood-2011-02-334623
15. Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol (1996) 156(12):4609–16.
16. Mackall CL, Hakim FT, Gress RE. T-Cell regeneration: all repertoires are not created equal. Immunol Today (1997) 18(5):245–51. doi: 10.1016/S0167-5699(97)81664-7
17. Jimenez M, Ercilla G, Martinez C. Immune reconstitution after allogeneic stem cell transplantation with reduced-intensity conditioning regimens. Leukemia (2007) 21(8):1628–37. doi: 10.1038/sj.leu.2404681
18. Przybylski GK, Kreuzer KA, Siegert W, Schmidt CA. No recovery of T-cell receptor excision circles (TRECs) after non-myeloablative allogeneic hematopoietic stem cell transplantation is correlated with the onset of GvHD. J Appl Genet (2007) 48(4):397–404. doi: 10.1007/BF03195239
19. Gaballa A, Sundin M, Stikvoort A, Abumaree M, Uzunel M, Sairafi D, et al. T Cell receptor excision circle (TREC) monitoring after allogeneic stem cell transplantation; A predictive marker for complications and clinical outcome. Int J Mol Sci (2016) 17(10):1705. doi: 10.3390/ijms17101705
20. Gaballa A, Clave E, Uhlin M, Toubert A, Arruda LCM. Evaluating thymic function after human hematopoietic stem cell transplantation in the personalized medicine era. Front Immunol (2020) 11(1341). doi: 10.3389/fimmu.2020.01341
21. Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again. Tissue Antigens (2012) 79(2):83–9. doi: 10.1111/j.1399-0039.2011.01820.x
22. Pido-Lopez J, Imami N, Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin Exp Immunol (2001) 125(3):409–13. doi: 10.1046/j.1365-2249.2001.01640.x
23. Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood (2001) 97(5):1458–66. doi: 10.1182/blood.V97.5.1458
24. Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity (2009) 31(2):184–96. doi: 10.1016/j.immuni.2009.08.006
25. Agerholm R, Bekiaris V. Evolved to protect, designed to destroy: IL-17-producing gammadelta T cells in infection, inflammation, and cancer. Eur J Immunol (2021) 51(9):2164–77. doi: 10.1002/eji.202049119
26. Munoz LD, Sweeney MJ, Jameson JM. Skin resident gammadelta T cell function and regulation in wound repair. Int J Mol Sci (2020) 21(23):9286. doi: 10.3390/ijms21239286
27. Ciofani M. Tgammadelta17 cells build up the nerve. Nat Immunol (2020) 21(4):367–8. doi: 10.1038/s41590-020-0642-4
28. Otto G. Gammadelta T cells turn up the heat. Nat Rev Immunol (2018) 18(6):359. doi: 10.1038/s41577-018-0017-3
29. Nielsen MM, Witherden DA, Havran WL. Gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol (2017) 17(12):733–45. doi: 10.1038/nri.2017.101
30. Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with γδ T cells: Many paths ahead of us. Cell Mol Immunol (2020) 17:925–39. doi: 10.1038/s41423-020-0504-x
31. Chien YH, Iwashima M, Kaplan KB, Elliott JF, Davis MM. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. Nature (1987) 327(6124):677–82. doi: 10.1038/327677a0
32. Willcox BE, Willcox CR. Gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol (2019) 20(2):121–8. doi: 10.1038/s41590-018-0304-y
33. Minculescu L, Sengelov H. The role of gamma delta T cells in haematopoietic stem cell transplantation. Scand J Immunol (2015) 81(6):459–68. doi: 10.1111/sji.12289
34. Silva-Santos B, Serre K, Norell H. Gammadelta T cells in cancer. Nat Rev Immunol (2015) 15(11):683–91. doi: 10.1038/nri3904
35. Wang H, Henry O, Distefano MD, Wang YC, Raikkonen J, Monkkonen J, et al. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells. J Immunol (2013) 191(3):1029–42. doi: 10.4049/jimmunol.1300658
36. Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity (2014) 40(4):490–500. doi: 10.1016/j.immuni.2014.03.003
37. Lafont V, Sanchez F, Laprevotte E, Michaud HA, Gros L, Eliaou JF, et al. Plasticity of gammadelta T cells: Impact on the anti-tumor response. Front Immunol (2014) 5:622. doi: 10.3389/fimmu.2014.00622
38. SL T, Reddy M, Durbaka P, Rachamallu A, Reddanna P, Lomada D. γδ T cell mediated immune responses in disease and therapy. Front Immunol (2014) 5. doi: 10.3389/fimmu.2014.00571
39. Willcox CR, Mohammed F, Willcox BE. The distinct MHC-unrestricted immunobiology of innate-like and adaptive-like human γδ T cell subsets–nature's CAR-T cells. Immunol Rev (2020) 298(1):25–46. doi: 10.1111/imr.12928
40. Foord E, Arruda LCM, Gaballa A, Klynning C, Uhlin M. Characterization of ascites- and tumor-infiltrating gammadelta T cells reveals distinct repertoires and a beneficial role in ovarian cancer. Sci Trans Med (2021) 13(577):eabb0192. doi: 10.1126/scitranslmed.abb0192
41. Davey MS, Willcox CR, Baker AT, Hunter S, Willcox BE. Recasting human Vdelta1 lymphocytes in an adaptive role. Trends Immunol (2018) 39(6):446–59. doi: 10.1016/j.it.2018.03.003
42. Davey MS, Willcox CR, Hunter S, Oo YH, Willcox BE. Vdelta2(+) T cells-two subsets for the price of one. Front Immunol (2018) 9:2106. doi: 10.3389/fimmu.2018.02106
43. Gaballa A, Arruda LCM, Radestad E, Uhlin M. CD8(+) gammadelta T cells are more frequent in CMV seropositive bone marrow grafts and display phenotype of an adaptive immune response. Stem Cells Int (2019) 2019:6348060. doi: 10.1155/2019/6348060
44. Lamb LS Jr., Lopez RD. Gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant (2005) 11(3):161–8. doi: 10.1016/j.bbmt.2004.11.015
45. Serrano R, Wesch D, Kabelitz D. Activation of human gammadelta T cells: Modulation by toll-like receptor 8 ligands and role of monocytes. Cells (2020) 9(3):713. doi: 10.3390/cells9030713
46. Sebestyen Z, Prinz I, Dechanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov (2020) 19(3):169–84. doi: 10.1038/s41573-019-0038-z
47. Zheng J, Liu Y, Lau YL, Tu W. Gammadelta-T cells: An unpolished sword in human anti-infection immunity. Cell Mol Immunol (2013) 10(1):50–7. doi: 10.1038/cmi.2012.43
48. Silva-Santos B, Mensurado S, Coffelt SB. Gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer (2019) 19(7):392–404. doi: 10.1038/s41568-019-0153-5
49. Zou C, Zhao P, Xiao Z, Han X, Fu F, Fu L. Gammadelta T cells in cancer immunotherapy. Oncotarget (2017) 8(5):8900–9. doi: 10.18632/oncotarget.13051
50. Kabelitz D. Human gammadelta T cells: From a neglected lymphocyte population to cellular immunotherapy: A personal reflection of 30years of gammadelta T cell research. Clin Immunol (Orlando Fla) (2016) 172:90–7. doi: 10.1016/j.clim.2016.07.012
51. Cavazzana-Calvo M, Andre-Schmutz I, Dal Cortivo L, Neven B, Hacein-Bey-Abina S, Fischer A. Immune reconstitution after haematopoietic stem cell transplantation: obstacles and anticipated progress. Curr Opin Immunol (2009) 21(5):544–8. doi: 10.1016/j.coi.2009.08.001
52. Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol (2016) 7:507. doi: 10.3389/fimmu.2016.00507
53. Hu Y, Cui Q, Luo C, Luo Y, Shi J, Huang H. A promising sword of tomorrow: Human gammadelta T cell strategies reconcile allo-HSCT complications. Blood Rev (2016) 30(3):179–88. doi: 10.1016/j.blre.2015.11.002
54. Hirokawa M, Horiuchi T, Kawabata Y, Kitabayashi A, Miura AB. Reconstitution of gammadelta T cell repertoire diversity after human allogeneic hematopoietic cell transplantation and the role of peripheral expansion of mature T cell population in the graft. Bone Marrow Transplant (2000) 26(2):177–85. doi: 10.1038/sj.bmt.1702478
55. de Witte MA, Sarhan D, Davis Z, Felices M, Vallera DA, Hinderlie P, et al. Early reconstitution of NK and gammadelta T cells and its implication for the design of post-transplant immunotherapy. Biol Blood Marrow Transplant (2018) 24:1152–62. doi: 10.1016/j.bbmt.2018.02.023
56. Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, et al. Gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood (2015) 125(15):2349–58. doi: 10.1182/blood-2014-09-599423
57. Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L, et al. Human gamma delta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol (2017) 18(4):393–+. doi: 10.1038/ni.3686
58. Lamb LS Jr., Gee AP, Hazlett LJ, Musk P, Parrish RS, O'Hanlon TP, et al. Influence of T cell depletion method on circulating gammadelta T cell reconstitution and potential role in the graft-versus-leukemia effect. Cytotherapy (1999) 1(1):7–19. doi: 10.1080/0032472031000141295
59. Airoldi I, Prigione I, Bertaina A, Cocco C, Pagliara D, Zorzoli A, et al. Recovery of Gamma/Delta plus T cells after transplantation with alpha-Beta+/CD19+Lymphocyte depleted hematopoietic stem cells from HLA-haploidentical donors. Blood (2013) 122(21):3245. doi: 10.1182/blood.V122.21.3245.3245
60. Klyuchnikov E, Badbaran A, Massoud R, Fritsche-Friedland U, Janson D, Ayuk F, et al. Enhanced immune reconstitution of γδ T cells after allogeneic stem cell transplantation overcomes the negative impact of pretransplantation minimal residual disease-positive status in patients with acute myelogenous leukemia. Transplant Cell Ther (2021) 27(10):841–50. doi: 10.1016/j.jtct.2021.06.003
61. Cela ME, Holladay MS, Rooney CM, Richardson S, Alexander B, Krance RA, et al. Gamma delta T lymphocyte regeneration after T lymphocyte-depleted bone marrow transplantation from mismatched family members or matched unrelated donors. Bone Marrow Transplant (1996) 17(2):243–7.
62. Perko R, Kang GL, Sunkara A, Leung W, Thomas PG, Dallas MH. Gamma delta T cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol Blood Marrow Transplant (2015) 21(1):130–6. doi: 10.1016/j.bbmt.2014.09.027
63. Eyrich M, Leiler C, Lang P, Schilbach K, Schumm M, Bader P, et al. A prospective comparison of immune reconstitution in pediatric recipients of positively selected CD34+ peripheral blood stem cells from unrelated donors vs recipients of unmanipulated bone marrow from related donors. Bone Marrow Transplant (2003) 32(4):379–90. doi: 10.1038/sj.bmt.1704158
64. Keever-Taylor CA, Wagner JE, Kernan NA, Small TN, Carter SL, Thompson JS, et al. Comparison of immune recovery in recipients of unmanipulated vs T-cell-depleted grafts from unrelated donors in a multicenter randomized phase II–III trial (T-cell depletion trial). Bone Marrow Transplant (2010) 45(3):587–9. doi: 10.1038/bmt.2009.170
65. Otto M, Barfield RC, Iyengar R, Gatewood J, Muller I, Holladay MS, et al. Human gammadelta T cells from G-CSF-mobilized donors retain strong tumoricidal activity and produce immunomodulatory cytokines after clinical-scale isolation. J Immunother (2005) 28(1):73–8. doi: 10.1097/00002371-200501000-00009
66. Bian Z, Xu LP, Fu Q, Huo M, Liu L, Zhao X, et al. Homeostatic gammadelta T cell contents are preserved by granulocyte colony-stimulating factor priming and correlate with the early recovery of gammadelta T cell subsets after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2018) 24(2):252–9. doi: 10.1016/j.bbmt.2017.10.027
67. Minculescu L, Sengelov H, Marquart HV, Ryder LP, Fischer-Nielsen A, Haastrup E. Granulocyte colony-stimulating factor effectively mobilizes TCR gammadelta and NK cells providing an allograft potentially enhanced for the graft-Versus-Leukemia effect for allogeneic stem cell transplantation. Front Immunol (2021) 12:625165. doi: 10.3389/fimmu.2021.625165
68. Gaballa A, Alagrafi F, Uhlin M, Stikvoort A. Revisiting the role of gammadelta T cells in anti-CMV immune response after transplantation. Viruses (2021) 13(6):1031. doi: 10.3390/v13061031
69. de Koning C, Plantinga M, Besseling P, Boelens JJ, Nierkens S. Immune reconstitution after allogeneic hematopoietic cell transplantation in children. Biol Blood Marrow Transplant (2016) 22(2):195–206. doi: 10.1016/j.bbmt.2015.08.028
70. Xuan L, Wu X, Zhang Y, Fan Z, Ling Y, Huang F, et al. Granulocyte colony-stimulating factor affects the distribution and clonality of TRGV and TRDV repertoire of T cells and graft-versus-host disease. J Transl Med (2011) 9:215. doi: 10.1186/1479-5876-9-215
71. Viale M, Ferrini S, Bacigalupo A. TCR gamma/delta positive lymphocytes after allogeneic bone marrow transplantation. Bone Marrow Transplant (1992) 10(3):249–53.
72. Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Barrett TA, Bluestone JA, Vallera DA. Lethal murine graft-versus-host disease induced by donor gamma/delta expressing T cells with specificity for host nonclassical major histocompatibility complex class ib antigens. Blood (1996) 87(2):827–37. doi: 10.1182/blood.V87.2.827.bloodjournal872827
73. Huang Y, Cramer DE, Ray MB, Chilton PM, Que X, Ildstad ST. The role of alphabeta- and gammadelta-T cells in allogenic donor marrow on engraftment, chimerism, and graft-versus-host disease. Transplantation (2001) 72(12):1907–14. doi: 10.1097/00007890-200112270-00007
74. Maeda Y, Reddy P, Lowler KP, Liu C, Bishop DK, Ferrara JL. Critical role of host gammadelta T cells in experimental acute graft-versus-host disease. Blood (2005) 106(2):749–55. doi: 10.1182/blood-2004-10-4087
75. Lamb LS Jr., Musk P, Ye Z, van Rhee F, Geier SS, Tong JJ, et al. Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant (2001) 27(6):601–6. doi: 10.1038/sj.bmt.1702830
76. Drobyski WR, Majewski D, Hanson G. Graft-facilitating doses of ex vivo activated gammadelta T cells do not cause lethal murine graft-vs. -host disease Biol Blood Marrow Transplant (1999) 5(4):222–30. doi: 10.1053/bbmt.1999.v5.pm10465102
77. Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood (2009) 113(25):6477–84. doi: 10.1182/blood-2008-09-176594
78. Krenger W, Hollander GA. The immunopathology of thymic GVHD. Semin Immunopathol (2008) 30(4):439–56. doi: 10.1007/s00281-008-0131-6
79. Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Sci (New York NY) (2001) 294(5542):605–9. doi: 10.1126/science.1063916
80. Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol (2013) 13(2):88–100. doi: 10.1038/nri3384
81. Lamb LS Jr., Henslee-Downey PJ, Parrish RS, Godder K, Thompson J, Lee C, et al. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother (1996) 5(5):503–9. doi: 10.1089/scd.1.1996.5.503
82. Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, Abhyankar S, et al. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant (2007) 39(12):751–7. doi: 10.1038/sj.bmt.1705650
83. Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and b-cell depletion. Blood (2017) 130(5):677–85. doi: 10.1182/blood-2017-04-779769
84. Park M, Im HJ, Lee YJ, Park N, Jang S, Kwon SW, et al. Reconstitution of T and NK cells after haploidentical hematopoietic cell transplantation using alpha beta T cell-depleted grafts and the clinical implication of gamma delta T cells. Clin Transplant (2018) 32(1):e13147. doi: 10.1111/ctr.13147
85. Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood (2010) 116(12):2164–72. doi: 10.1182/blood-2010-01-255166
86. Prinz I, Thamm K, Port M, Weissinger EM, Stadler M, Gabaev I, et al. Donor Vdelta1+ gammadelta T cells expand after allogeneic hematopoietic stem cell transplantation and show reactivity against CMV-infected cells but not against progressing b-CLL. Exp Hematol Oncol (2013) 2(1):14. doi: 10.1002/eji.201343759
87. Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia (2013) 27(6):1328–38. doi: 10.1038/leu.2012.374
88. De Paoli P, Gennari D, Martelli P, Cavarzerani V, Comoretto R, Santini G. Gamma delta T cell receptor-bearing lymphocytes during Epstein-Barr virus infection. J Infect Dis (1990) 161(5):1013–6. doi: 10.1093/infdis/161.5.1013
89. Arruda LCM, Gaballa A, Uhlin M. Impact of gammadelta T cells on clinical outcome of hematopoietic stem cell transplantation: Systematic review and meta-analysis. Blood Adv (2019) 3(21):3436–48. doi: 10.1182/bloodadvances.2019000682
90. Pabst C, Schirutschke H, Ehninger G, Bornhauser M, Platzbecker U. The graft content of donor T cells expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of acute graft versus host disease after transplantation of allogeneic peripheral blood stem cells from unrelated donors. Clin Cancer Res an Off J Am Assoc Cancer Res (2007) 13(10):2916–22. doi: 10.1158/1078-0432.CCR-06-2602
91. Gaballa A, Stikvoort A, Onfelt B, Mattsson J, Sundin M, Watz E, et al. T-Cell frequencies of CD8(+) gammadelta and CD27(+) gammadelta cells in the stem cell graft predict the outcome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant (2019) 54(10):1562–74. doi: 10.1038/s41409-019-0462-z
92. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol (2009) 10(4):427–36. doi: 10.1038/ni.1717
93. Grunder C, van Dorp S, Hol S, Drent E, Straetemans T, Heijhuurs S, et al. gamma9 and delta2CDR3 domains regulate functional avidity of T cells harboring gamma9delta2TCRs. Blood (2012) 120(26):5153–62. doi: 10.1182/blood-2012-05-432427
94. Arruda LCM, Gaballa A, Uhlin M. Graft gammadelta TCR sequencing identifies public clonotypes associated with hematopoietic stem cell transplantation efficacy in acute myeloid leukemia patients and unravels cytomegalovirus impact on repertoire distribution. J Immunol (2019) 202(6):1859–70. doi: 10.4049/jimmunol.1801448
95. Handgretinger R, Schilbach K. The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood (2018) 131(10):1063–72. doi: 10.1182/blood-2017-08-752162
96. Fisher J, Anderson J. Engineering approaches in human gamma delta T cells for cancer immunotherapy. Front Immunol (2018) 9:1409. doi: 10.3389/fimmu.2018.01409
97. Radestad E, Wikell H, Engstrom M, Watz E, Sundberg B, Thunberg S, et al. Alpha/Beta T-cell depleted grafts as an immunological booster to treat graft failure after hematopoietic stem cell transplantation with HLA-matched related and unrelated donors. J Immunol Res (2014) 2014:578741. doi: 10.1155/2014/578741
98. Rådestad E, Sundin M, Törlén J, Thunberg S, Önfelt B, Ljungman P, et al. Individualization of hematopoietic stem cell transplantation using Alpha/Beta T-cell depletion. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.00189
99. Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev (2005) 205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x
100. Velardi E, Dudakov JA, van den Brink MR. Clinical strategies to enhance thymic recovery after allogeneic hematopoietic stem cell transplantation. Immunol Lett (2013) 155(1-2):31–5. doi: 10.1016/j.imlet.2013.09.016
101. Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. New Engl J Med (1999) 340(9):737–8. doi: 10.1056/NEJM199903043400914
102. Van Acker HH, Anguille S, Willemen Y, Van den Bergh JM, Berneman ZN, Lion E, et al. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J Hematol Oncol (2016) 9(1):101. doi: 10.1186/s13045-016-0329-3
103. Peters C, Meyer A, Kouakanou L, Feder J, Schricker T, Lettau M, et al. TGF-beta enhances the cytotoxic activity of Vdelta2 T cells. Oncoimmunology (2019) 8(1):e1522471. doi: 10.1080/2162402X.2018.1522471
104. Kouakanou L, Xu Y, Peters C, He J, Wu Y, Yin Z, et al. Vitamin c promotes the proliferation and effector functions of human gammadelta T cells. Cell Mol Immunol (2020) 17(5):462–73. doi: 10.1038/s41423-019-0247-8
105. Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y, Felizardo TC, et al. Human Vdelta1 gammadelta T cells expanded from peripheral blood exhibit specific cytotoxicity against b-cell chronic lymphocytic leukemia-derived cells. Cytotherapy (2011) 13(6):753–64. doi: 10.3109/14653249.2011.553595
106. Zhou J, Kang N, Cui L, Ba D, He W. Anti-gammadelta TCR antibody-expanded gammadelta T cells: A better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol Immunol (2012) 9(1):34–44. doi: 10.1038/cmi.2011.16
107. Almeida AR, Correia DV, Fernandes-Platzgummer A, da Silva CL, da Silva MG, Anjos DR, et al. Delta one T cells for immunotherapy of chronic lymphocytic leukemia: Clinical-grade Expansion/Differentiation and preclinical proof of concept. Clin Cancer Res (2016) 22(23):5795–804. doi: 10.1158/1078-0432.CCR-16-0597
108. Rischer M, Pscherer S, Duwe S, Vormoor J, Jurgens H, Rossig C. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol (2004) 126(4):583–92. doi: 10.1111/j.1365-2141.2004.05077.x
109. Straetemans T, Kierkels GJJ, Doorn R, Jansen K, Heijhuurs S, Dos Santos JM, et al. GMP-grade manufacturing of T cells engineered to express a defined gammadeltaTCR. Front Immunol (2018) 9:1062. doi: 10.3389/fimmu.2018.01062
110. Oberg HH, Kellner C, Gonnermann D, Peipp M, Peters C, Sebens S, et al. Gammadelta T cell activation by bispecific antibodies. Cell Immunol (2015) 296(1):41–9. doi: 10.1016/j.cellimm.2015.04.009
111. Oberg HH, Kellner C, Gonnermann D, Sebens S, Bauerschlag D, Gramatzki M, et al. Tribody [(HER2)2xCD16] is more effective than trastuzumab in enhancing gammadelta T cell and natural killer cell cytotoxicity against HER2-expressing cancer cells. Front Immunol (2018) 9:814. doi: 10.3389/fimmu.2018.00814
Keywords: gamma delta, immune reconstitution, immunotherapeutic, hct, allogeneic
Citation: Gaballa A, Arruda LCM and Uhlin M (2022) Gamma delta T-cell reconstitution after allogeneic HCT: A platform for cell therapy. Front. Immunol. 13:971709. doi: 10.3389/fimmu.2022.971709
Received: 17 June 2022; Accepted: 09 August 2022;
Published: 29 August 2022.
Edited by:
Stefan Nierkens, Princess Maxima Center for Pediatric Oncology, NetherlandsReviewed by:
Pietro Merli, Bambino Gesù Children’s Hospital (IRCCS), ItalyRichard O’Reilly, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2022 Gaballa, Arruda and Uhlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Gaballa, YWhtZWQuZ2FiYWxsYUBraS5zZQ==
 Ahmed Gaballa1,2*
Ahmed Gaballa1,2* Lucas C. M. Arruda
Lucas C. M. Arruda Michael Uhlin
Michael Uhlin