
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 06 October 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.969998
This article is part of the Research Topic Alloimmunity and Immunotherapy in Renal Allograft Fibrosis View all 3 articles
 Pierre Guy1
Pierre Guy1 Audrey Delas2
Audrey Delas2 Laure Esposito1
Laure Esposito1 Olivier Cointault1
Olivier Cointault1 Magali Colombat2,3
Magali Colombat2,3 Nicolas Congy-Jolivet4
Nicolas Congy-Jolivet4 Marc Raynaud5
Marc Raynaud5 Nassim Kamar1,3,6
Nassim Kamar1,3,6 Arnaud Del Bello1,3,6*
Arnaud Del Bello1,3,6*Recent large meta-analyses suggested a poorer long-term patients’ and grafts’ outcomes after ABO incompatible (ABOi) living-donor kidney transplantation (LDKT) compared to ABO compatible LDKT. However, little is known about the long-term histological pattern after ABOi LDKT. We compared the histological features observed on protocol biopsies from 03/11 to 11/19 in 94 ABOi LDKT (including 14 with preformed Donor Specific Antibodies, pDSAs), 27 LDKT ABO compatible (ABOc) with pDSAs, and 21 ABOc without pDSAs) during the first five years post transplantation. During the first 5 years post-transplantation, a progression of chronic lesions (patients with a ci >0 raised from 11% to 65%, p<0.0001, patients with a ct >0 raised from 29% to 78%, p<0.0001) was observed in ABOi LDKT without pDSAs. Histological patterns of evolution were comparable to those observed in ABOc kidney transplant patients. Microvascular inflammation was lower in ABOi LDKT without pDSAs compared to those with pDSAs (ABOi or ABOc). At last follow-up, 28 months, IQR (15-48) post-transplantation, 29 patients (36%) had a severe graft dysfunction (defined by a CKD-epi eGFR < 30 mL/min/1.73m²). The donor age was a predictive factor for the development of severe kidney allograft dysfunction at last follow-up (HR= 1.05, 95% CI [1.05-1.10], p= 0.03).
Hence, long-term histological analysis of ABOi LDKT shows only an increase of chronic interstitial and tubular atrophy changes, without active lesions. These data confirm that ABOi LDKT programs can be securely developed.
ABO incompatible (ABOi) living donor kidney transplantation (LDKT) was developed to overcome organ shortage. Although ABOi transplantation is considered to be a safe alternative to ABO compatible (ABOc) kidney transplantation (1, 2), it is associated to a higher mortality in the early period post transplantation, and a lower graft survival at one year (1, 3). While the over-mortality rate after ABOi LDKT was attributed to increased severe infection rates, the higher risk of early graft loss was associated to higher risks of surgical complications (such as surgical site bleedings), acute rejection, and polyomavirus associated nephropathy (PVAN) (4–8). Moreover, recent large meta-analyses suggested a poorer long-term patients’ and grafts’ outcomes after ABOi LDKT compared to ABOc LDKT (3, 9).
Protocol biopsies performed during the first year post transplantation, showed similar results in ABOi and ABOc LDKT (10–15). However, little is known about the long-term histology in ABOi kidney transplantation. In the present study, we describe the histological findings observed up to five years after ABOi living donors kidney transplantation transplanted and followed in our center (Nephrology and Organ transplant Department, CHU de Toulouse, France). We compared these histological features to those observed in a group of ABOc LDKT patients with preformed donor specific antibodies (DSAs) and to another group of ABOc kidney-transplant patients without DSAs. Finally, we aimed to compare the observed death-censored graft survival after one year with that was predicted by the iBox score system (an allograft survival prediction system).
Between March 2011 and November 2019, 94 ABOi LDKT (including 14 with preformed DSAs) and 41 ABOc LDKT with preformed DSAs were performed in our institution (Nephrology and Organ transplant Department, CHU de Toulouse, France). ABO compatible kidney transplant patients without DSAs didn’t undergo protocol biopsies in our institution except those grafted for IgA nephropathy between 2010-2014. During this interval, 21 patients were transplanted (6 from living and 15 for deceased donors) and histological findings were compared to ABOi and ABOc with preformed DSAs LDKT. Patients’ characteristics are presented in Table 1.
According to French law (Loi Jardé), anonymous retrospective studies do not require Institutional Review Board approval. However, as recommended it was registered in the register of retrospective studies of the Toulouse University Hospital (RnIPH 2021-94) and covered by the MR-004 (CNIL number: 2206723 v 0), (Supporting Document 1).
For ABOi LDKT, recipients had undergone an apheresis (immunoadsorption or double filtration plasmapheresis) to obtain a pre-operative IgG titer <1/8. In addition, they were given rituximab (375 mg/m2 once for ABOi without preformed DSA and twice for those with preformed DSAs). ABOc LDKT with preformed DSAs were also treated with apharesis and 2 infusions of rituximab. Patients with preformed DSAs were all given induction therapy with polyclonal antibodies while others received either polyclonal antibodies or basiliximab. Maintenance immunosuppression was started 15 days before transplantation and included tacrolimus (target trough level 10-12 g/mL during the first 3 months post-transplantation, and 8-10 ng/mL thereafter), mycophenolic sodium (Myfortic® 720 mg b.i.d until day 15 post-transplantation) and steroids. Since 2017, a switch from mycophenolic sodium to everolimus (Certican® 1.5 mg b.i.d) was initiated after 15 days post transplantation. Patients from the control group who were grafted for IgA nephropathy were also given tacrolimus, mycophenolic acid and steroids. Valgancyclovir prophylaxis was given according to donor/recipient status and type of induction therapy for 3 or 6 months. All patients were given cotrimoxazole prophylaxis for 1 year.
All patients were screened for de novo DSAs at month 3, and 12 post-transplantation, and thereafter every year. The presence of preformed or de novo DSAs was assessed by Luminex assays. Luminex assays determined the specificity of class I HLAs in A/B and class II in DR/DQ IgG antibodies in the recipients’ sera (centrifuged at 10,000g for 10 min) using Labscreen single Ag HLA class-I and class-II detection tests (One Lambda, Canoga Park, CA), according to the manufacturer’s instructions. The presence and specificity of antibodies were then detected using a Labscan 100®, and the mean fluorescence (baseline) value for each sample in each bead was evaluated. The baseline value was calculated as follows: (raw sample mean fluorescence intensity [MFI] − raw negative serum control MFI) – (negative-bead raw MFI sample − negative-bead raw MFI negative serum control). A baseline value of >1000 was considered positive.
Isoagglutinins were detected with a tube hemagglutination technique, according to the previously described technique (16).
In the present study, only protocol biopsies were analyzed, and reviewed by two pathologists specialized in kidney transplantation. For ABOi patients, these were scheduled at months 1 and 6, and thereafter at 1, 2, and 5 years post-transplantation. In ABOi LDKT patients without preformed DSAs (n=80), 226 biopsies were performed. The median number of biopsies/patient was 3 (IQR 2; 4). Sixty-one (76.3%), 52 (65.0%), 54 (67.5%), 41 (51.3%), and 18 (22.5%) patients had undergone a protocol biopsy at months 1, 6, and 1, 2 and 5 years, respectively (Supporting Document 2). The others were not done either due to medical reasons or patients’ refusal.
For ABOc patients with preformed DSAs, protocol biopsies were scheduled at month 6 and 1, 2, and 5 years. 27 out of 41 patients had at least one interpretable kidney biopsy during follow up. For ABOc patients without DSA grafted for IgA nephropathy, protocol biopsies were performed at 1 month, 1 year and 5 years post-transplant. Seven (33.3%), 7 (33.3%), and 16 (76.2%) patients had undergone a protocol biopsy that fitted to the standard analyses criteria at months 1, 1 year and 5 years post-transplantation, respectively. Histological findings were classified by our local, transplant renal pathologist according to the Banff 2017 classification (17). C4d staining was done by immunofluorescence technique and was scored, as positive or negative.
We aimed to compare the observed graft survival after one year and that was predicted by the iBox score system, an allograft survival prediction system. The observed death-censored graft survival was assessed at 3, 5 and 7 years post transplantation. Individual allograft survival probabilities were determined using the iBox scoring system (18). Data from patients completing the 1-year visit including a kidney biopsy were included for generation of iBox risk-prediction scores. The iBox scores were generated using eight parameters: time from transplant to biopsy, eGFR and urine protein/creatinine ratio at biopsy, presence of circulating DSA, and histological findings (glomerulitis, peritubular capillaritis, interstitial inflammation, tubulitis, transplant glomerulopathy, and interstitial fibrosis/tubular atrophy) classified according to Banff score.
Reported values represent the means (± SD) or medians (ranges). Quantitative variables were compared using the Mann–Whitney, or Kruskal-Wallis (if appropriated) non-parametric test. Categorical variables are expressed as percentages and compared between groups using the chi-squared tests or if appropriated the Fisher’s exact test. Cumulative probability of survival was calculated using the Kaplan-Meier method. Severe allograft dysfunction was defined by an estimated CKD-EPI glomerular filtration rate lower than 30 mL/min/1.73m². A cox-regression analysis was performed to identify predictive factors for graft survival without severe dysfunction (defined by an estimated CKD-EPI glomerular filtration rate lower than 30 mL/min/1.73m²). Variables with a p-value <0.2 in the univariate analysis were included into stepwise multivariate models. Statistical analyses were performed using the graphPad Prism 7 (San Diego, CA, USA) and Xlstat softwares (Addisoft, Paris, France).
The outcome of histological lesions is presented in Figure 1 and Table 2. Overall, interstitial fibrosis score (ci), and tubular atrophy (ct) increased overtime. Sixty-five out the of 80 patients (81%) had at least two interpretable kidney biopsies during the follow-up. The median times between transplantation and the first analyzed biopsy and between the first and last biopsy were 1 IQR (1;1) months and 23 IQR (11; 59) months, respectively. Between the first and last analyzed biopsy, an increase of ci (patients with a ci >0 raised from 11% to 65%, p<0.0001), ct (patients with a ct >0 raised from 29% to 78%, p<0.0001), and i-IFTA scores (patients with i-IFTA>0 raised from 31% to 54%, p= 0.01) was observed. To note, three patients with a diagnosis of PVAN that received a follow-up biopsy post diagnosis presented a ci>0 and ct>0. Patients with i-IFTA scores > 0 at last biopsy, presented lower graft function at last follow-up in comparison with patients with a i-IFTA score =0 or patients that did not present IFTA (Supplementary Document 3). Only 6 patients had an i-IFTA score > 2 at last biopsy: 3 had PVAN, 1 had recurrent IgA nephropathy and 2 patients had chronic active TCMR. Chronic AMR was observed in 3 patients (4.6%) at first biopsy and 6 (9.2%) at last biopsy (p= 0.49). No occurrence of acute AMR or acute TCMR rejection was observed at last biopsy.
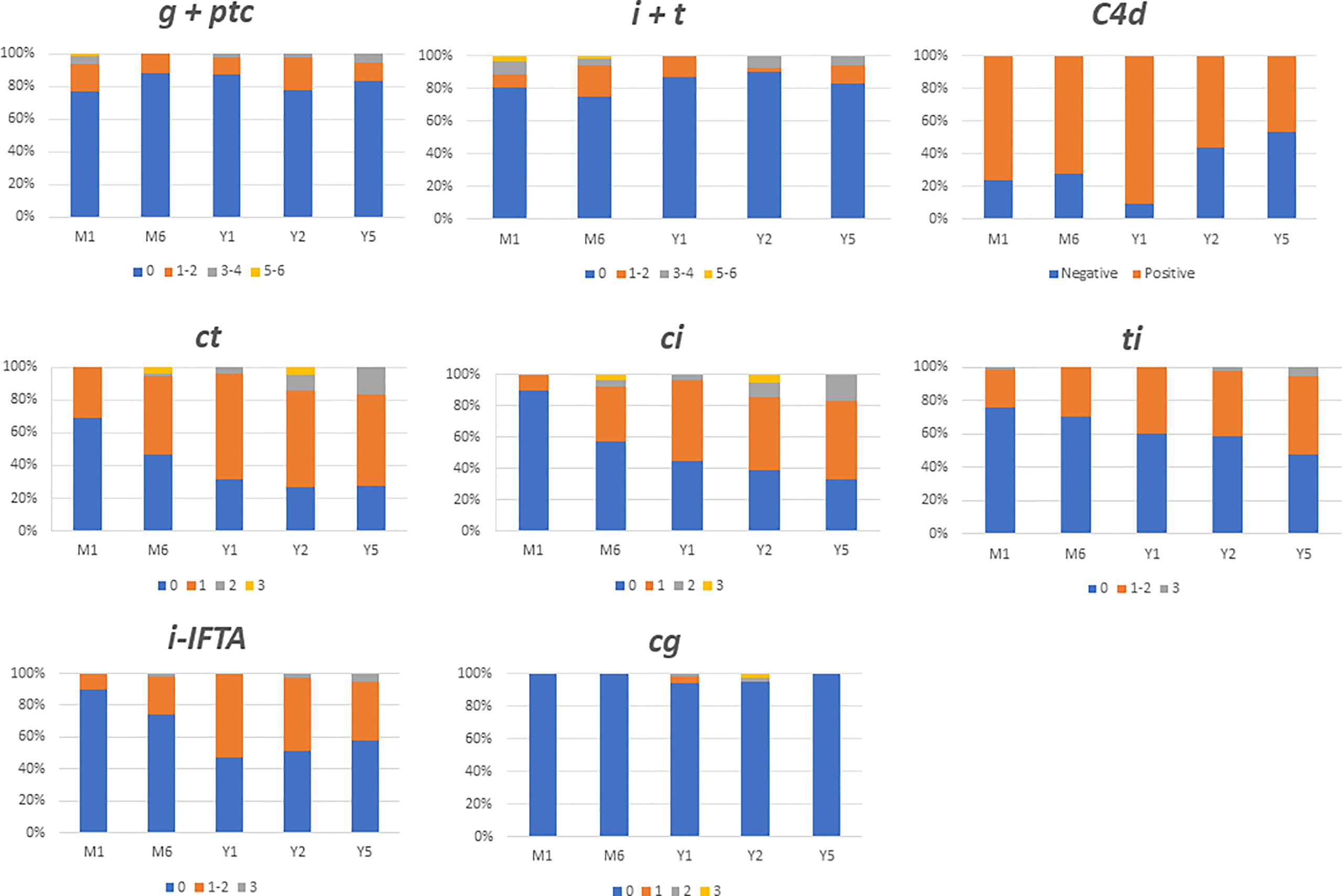
Figure 1 Main histological findings during 5 years follow up after ABO incompatible kidney transplantation. Data are expressed in each colomn with the percentage of patients presenting the same banff score. i, interstitial inflammation; t, tubulitis; g, glomerulitis; ptc, peritubular capilaritis; ci, chronic interstial fibrosis; ct, tubular atrophy; cg, glomerular basement membrane double coutours.
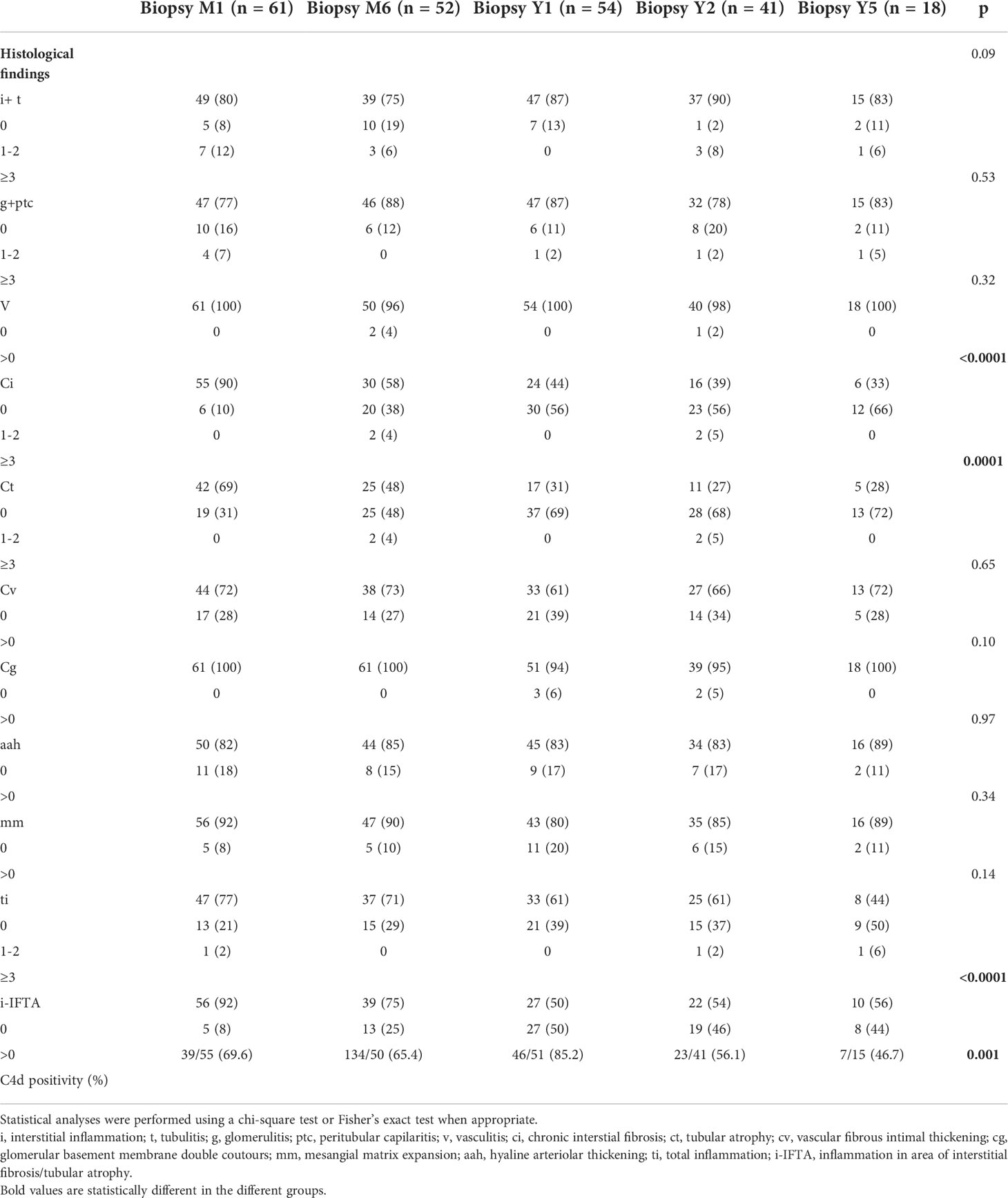
Table 2 Results of follow-up biopsies during the 5 years post transplantation after ABO incompatible kidney transplantation without preformed DSA.
In the 18 ABOi LDKT patients who had undergone kidney biopsies at month 1 and 5 years, there were also an increase in interstitial fibrosis (patients with a ci >0 raised from 22% to 67%) and tubular atrophy (patients with a ct >0 raised from 39% to 72%).
The progression of interstitial fibrosis and tubular atrophy was similar between ABOi and ABOc recipients during the follow-up. At year 5 post-transplantation, 67% of ABOi and 71% of ABOc recipients presented a ci score >0, and a (p> 0.99) ct score >0 was noted in 72% of ABOi and 71% of ABOc recipients (p> 0.99), (Figure 2; Supplementary Document 4).
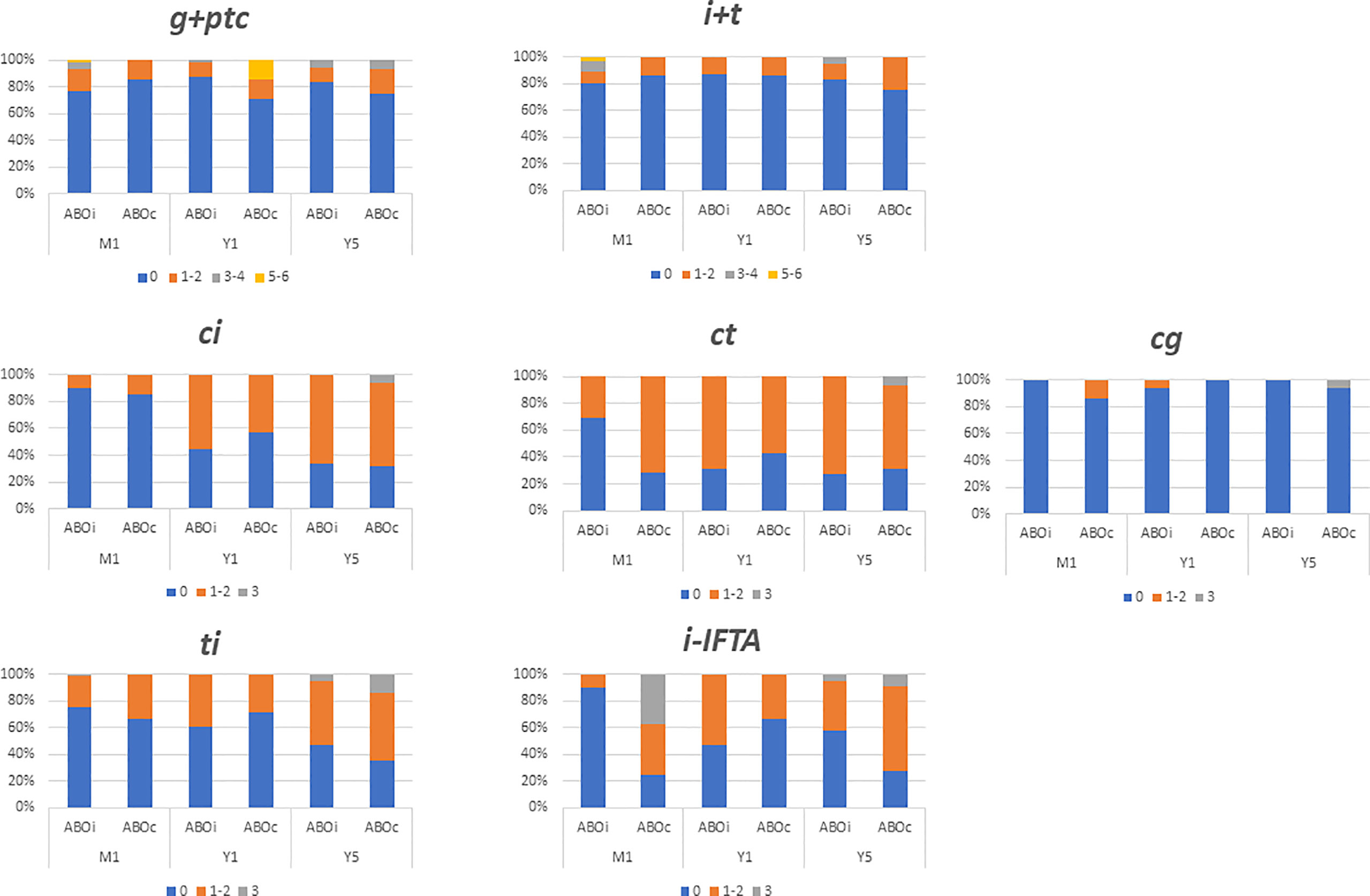
Figure 2 Comparison of biopsy finding at month one (M1), year one (Y1) and years five (Y5) between ABO incompatible (ABOi) and ABO compatible (ABOc) recipients. Data are expressed in each colomn with the percentage of patients presenting the same banff score. i, interstitial inflammation; t, tubulitis; g, glomerulitis; ptc, peritubular capilaritis; ci, chronic interstial fibrosis; ct, tubular atrophy; cg, glomerular basement membrane double coutours.
The microvascular inflammation remained low and stable during the follow-up in ABOi LDKT without DSAs (23%, 13%, and 17% at month 1, 12 and year 5 respectively presented a g+ptc>0) chi-square test p= 0.37). Conversely, at 5 years post-transplantation, micro-vascular inflammation was higher in patients with preformed DSA. A g+ptc>0 was found in 88% of ABOc with preformed DSA, 57% of ABOi with preformed DSAs, and 17% in ABOi without preformed DSA (p= 0.002), (Supplementary Document 5). Futhermore, glomerular basement double contours was significantly higher in ABOc with preformed DSAs recipients compared to ABOi without preformed DSAs (cg score >0 50% and 0% for ABOc with preformed DSA, and ABOi without preformed DSAs, p= 0.005).
During the follow-up (median time 28 months, IQR (15-48)), 9 (11.3%) patients died, and eleven (13.8%) other patients lost their grafts. The median time between transplantation and graft failure was 17 IQR (2; 26) months. The causes for graft failure were PVAN (n=4), vascular graft thrombosis during the first week (n=3), ABMR (n=2), surgical complication (n=1) and reoccurrence of initial nephropathy (n=1).
For the 54 ABOi LDKT without preformed DSAs who had undergone a one-year allograft biopsy, we used the iBOX score to predict the death-censored graft survival. The obtained mean iBOX score at each time post transplantation was compared with the observed graft survival. The observed graft survival at 3, 5 and 7 years did not differ significantly from the predicted ones (Figure 3).
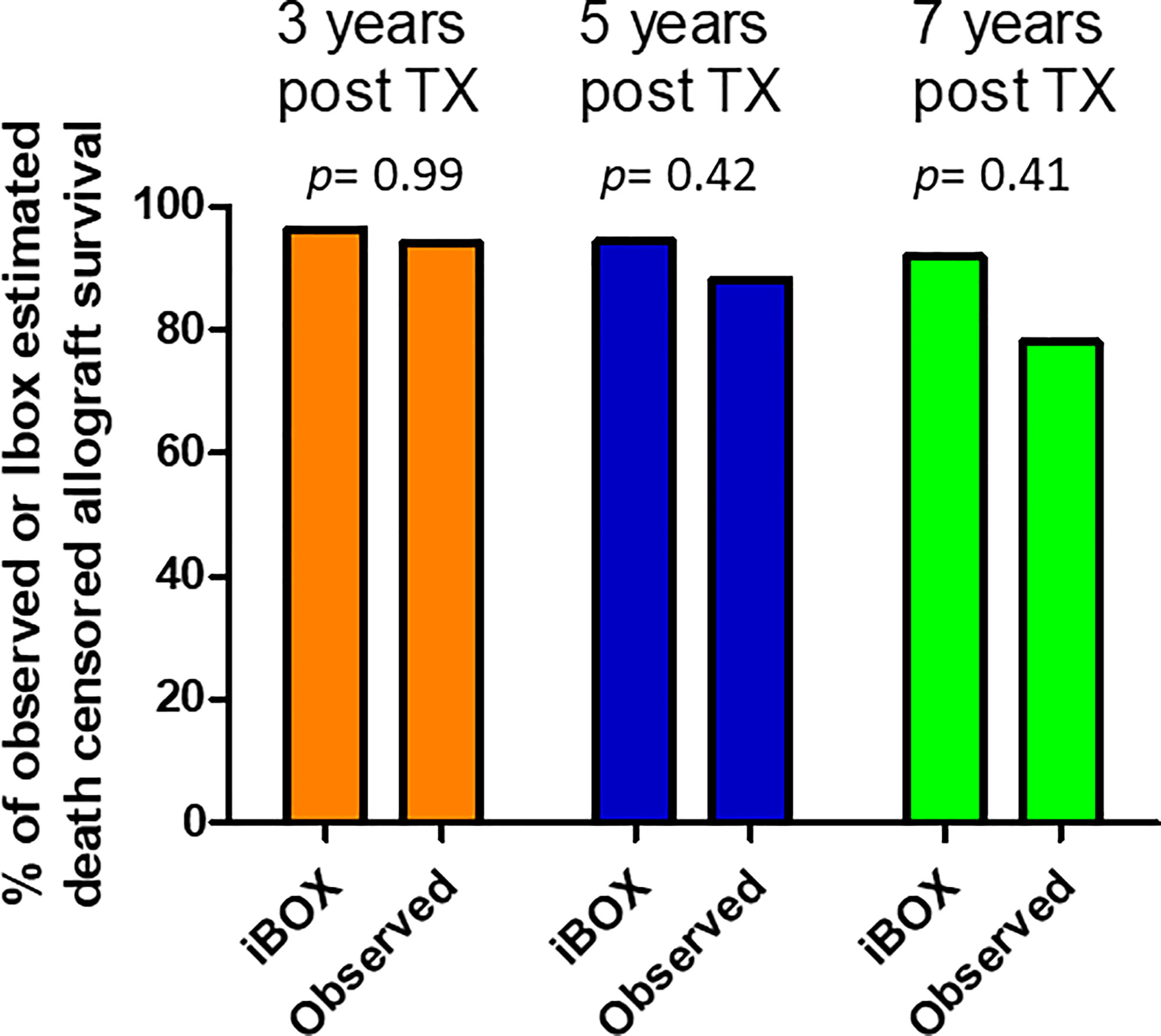
Figure 3 Comparison of Ibox predicted and observed death censored allograft survival at years 3, 5 and 7 post transplantation. The obtained mean iBOX score at each time post transplantation was compared with the observed graft survival.
During the first year, 17 patients experienced an acute rejection episode, 11 considered as TCMR and 6 ABMR. Subclinical rejection occurred in 3 patients (2 TCMR, 1 ABMR), at month 1 post transplantation. All TCMR episodes received steroid pulses. ABMR recipients received steroid pulses (n=2), plasmapheresis (n=5), eculizumab (n=3), or rituximab infusion (n=1). No acute rejection was observed beyond one year. None developed a de novo DSA.
Kidney function at 1 year was at 54.2 ± 21.0 ml/min/1.73m². At last follow-up, 28 months, IQR (15-48) post-transplantation, 29 patients (36%) were considered to have a severe graft dysfunction defined by a CKD-EPI eGFR lower than 30 mL/min/1.73m² (Table 3; Supporting Document 6A). In univariate analysis, the presence of delayed graft function and the donor age were associated with a severe kidney allograft dysfunction at last follow-up (Delayed graft function: 27.6% vs 7.8% in patients presenting or not a severe kidney allograft dysfunction at last follow-up, p=0.02; donor age: 55.2 ± 7.2 years, vs 48.7 ± 12.3 years in patients presenting or not a severe kidney allograft dysfunction at last follow-up, p= 0.008). After multivariate analysis, donor age (coded as a continuous variable) remained a slight predictive factor for the development of severe graft dysfunction after ABOi LDKT (HR= 1.05, 95% CI [1.05-1.10], p= 0.03). Interestingly, neither an history of acute rejection nor the pre-desensitization isoagglutinin titer, nor initial or follow-up histological lesions were associated with severe graft dysfunction.
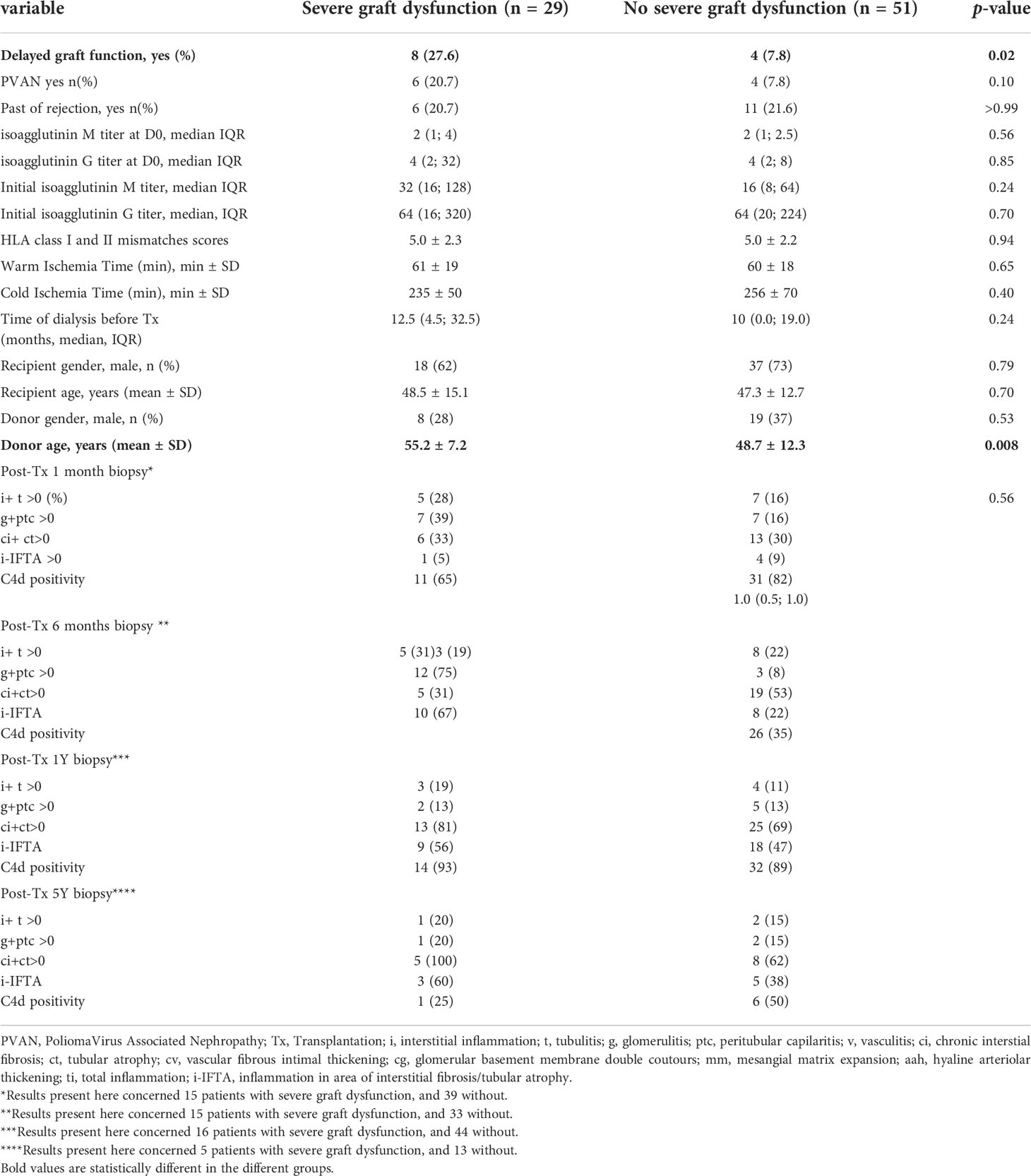
Table 3 Comparison of ABOi recipients without DSA according to the development of severe graft dysfunction (defined by a CKD-Epi eGFR<30 mL/min/1.73m²) at last follow-up.
To note, while the urine albumin/creatinine ratio remained stable in ABOi LDKT without preformed DSAs during the first five years post transplantation (median urine albumin/creatinine ratio: from 11.0 mg/g, IQR (0.0; 60.0) at one year to 26.0 mg/g, IQR (3.0; 100.0) at five years, p=0.28), an increase of proteinuria was observed in ABOi LDKT with preformed DSAs (from 0.0 mg/g, IQR (0.0; 0.0) at one year to 1000.0 mg/g, IQR (2.5; 2000.0) at five years, p=0.004), (Supplementary Document 6B).
Herein, we assessed the long-term histological pattern after ABOi LDKT. Overall, our study showed few histological modifications. During the five years post-transplantation, only chronic lesions (chronic interstitial fibrosis, “ci”, and chronic tubular atrophy, “ct”) worsened in ABOi LDKT. Similar results were observed by Masutani and colleagues who performed protocol biopsies at three and 12 months post-transplantation in 226 ABO compatible without preformed DSAs and 101 ABOi LDKT (10). In a retrospective study performed between 1999 and 2006, Bentall and collegues compared histological patterns at one- and five year post-transplant between LDKT patients with preformed DSAs (Positive crossmatches, n=102), ABOi without preformed DSAs (n=73), and 652 ABOc patients (19). They found higher rates of chronic injuries and inflammation in patients with positive crossmatch at transplantation compared to ABOi and ABOc transplants. Nonetheless, they also observed higher rates of chronic histological changes such as chronic glomerulopathy in ABOi group comparing with ABOc LDKT. However, this later study was done before the era of sensitive antibodies detection methods, modern immunosuppression, and Banff classification. This prompted us to investigate the long-term histological outcome post ABOi transplantation. For this purpose, we compared histological lesions in a group of ABOi LDKT and a group of ABOc without preformed DSAs patients transplanted for an IgA nephropathy. Interestingly, we did not observe any difference between both groups at 5 years after transplantation. We did not observe active microvascular inflammation in protocol biopsies. The occurrence of acute antibody mediated rejection is a classical and severe complication of the early transplant period after ABOi transplantation (20), possibly due to a hemagglutination assays limitations (21, 22) or complement control limitation (23). After this period, mechanisms of accommodation develop, preventing long-term acute and chronic rejection. Tasaki and colleagues demonstrated that the production of donor-specific blood group antibodies was downregulated in patients with stable graft function (24). ABO carbohydrate antigen recognition is a T-cell independent immunological response, in which innate B cells are considered to be the source of Isoagglutinines (2). However, these cells possess intrinsic regulatory function through the release of IL-10, depending on the site and environment (25). It was previously observed higher blood level of TGFβ and IL-10 in ABOi stable patients comparing with ABOc transplantation (24, 26). Others mechanisms are suspected such as intra-graft complement regulation could also participate (23). The absence of microvascular inflammation observed five years after transplantation in our study reinforces the role of accommodation in this setting. Moreover, the lack of development of transplant vasculopathy observed in our study could be explained by the absence of expression of ABO antigens by kidney smooth muscular cells (2). This is in starck contrast with transplantation with anti-HLA donor specific antibodies where chronic rejection remains a major cause of graft loss (27). As expected, in our study we found a progression of microvascular inflammation, and chronic rejection after transplantation with preformed DSA, regardless of the ABO compatibility. These results confirm the efficacy of current desensitization strategies and maintenance immunosuppression to overcome the ABO barrier.
In ABOi LDKT without preformed DSAs, we observed an increase of i-IFTA scores during the follow-up. As expected (28), at last follow-up, a lower glomerular filtration rates was observed in patients with i-IFTA compared to those without i-IFTA. Isolated i-IFTA is not specific lesion and can be observed in case of chronic TCMR, ABMR and poliomavirus-associated nephropathy.
Conflicting results were previously published concerning the long term kidney function after ABO incompatible kidney transplantation, comparing with ABO compatible transplantation (3, 9, 29, 30). However, early patient death from infection related to desensitization, and early antibody mediated rejection are responsible for the observed difference concerning graft survival. Similar results after one year post transplantation with ABO compatible LDKT is consistent with the comparable histological changes observed in our study between ABOi and ABOc patients (30).
This study has several limitations. First, this is a single-center study with small sample. However, even if some adjustments were made (with the use of mTOR inhibitors in place of MMF/MPA), patients had comparable maintenance immunosuppression strategy and histological pattern was re-analyzed by two specialized pathologists and classified according to the Banff classification. Second, to assess the histological changes after ABO incompatible transplantation, we compared kidney biopsies to those obtained in a group of patients transplanted for IgA nephropathy that had undergone protocol biopsies. We cannot exclude an overestimation of chronic lesions in our control group due to the reoccurrence of initial glomerular disease. Nevertheless, it has been shown that five-years histology and graft survival did not differ between patients grafted for IgA nephropathy and those transplanted for other reasons (31). In addition, we didn’t observe active lesions between ABOc and ABO-incompatible LDKT.
In conclusion, long-term histological analysis of ABO incompatible kidney transplantation shows only an increase of chronic interstitial and tubular atrophy changes, without active lesions. Histological pattern is comparable to the one observed in ABO-compatible kidney transplant recipients. These data corroborate the finding that ABOi LDKT programs can be securely developed.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Toulouse University Hospital (RnIPH 2021-94). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Each author mentioned have participated in the work as follow: AB, NK, and PG designed the study, analyzed and interpreted the data, and wrote the paper. MR analyzed the data, revised the manuscript and provided intellectual content of critical importance. OC, LE, AD, MC, NC-J revised the manuscript, and provided intellectual content of critical importance. All authors contributed to the article and approved the submitted version.
We sincerely thank all nurses involved in our department.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.969998/full#supplementary-material
ABOi, ABO incompatible; ABOc, ABO compatible; DSAs, Donor-Specific Antibodies; LDKT, Living Donor Kidney Transplant Recipients; PVAN, PoliomaVirus Associated Nephropathy; TCMR, T Cell Mediated Rejection; ABMR, Antibody Mediated Rejection.
1. Massie AB, Orandi BJ, Waldram MM, Luo X, Nguyen AQ, Montgomery RA, et al. Impact of ABO-incompatible living donor kidney transplantation on patient survival. Am J Kidney Dis (2020) 76(5):616–23. doi: 10.1053/j.ajkd.2020.03.029
2. Böhmig GA, Farkas AM, Eskandary F, Wekerle T. Strategies to overcome the ABO barrier in kidney transplantation. Nat Rev Nephrol (2015) 11(12):732–47. doi: 10.1038/nrneph.2015.144
3. de Weerd AE, Betjes MGH. ABO-incompatible kidney transplant outcomes: A meta-analysis. Clin J Am Soc Nephrol (2018) 13(8):1234–43. doi: 10.2215/CJN.00540118
4. Lonze BE, Bae S, Kraus ES, Holechek MJ, King KE, Alachkar N, et al. Outcomes and risk stratification for late antibody-mediated rejection in recipients of ABO-incompatible kidney transplants: a retrospective study. Transpl Int (2017) 30(9):874–83. doi: 10.1111/TRI.12969
5. Bentall A, Neil D, Sharif A, Ball S. ABO-incompatible kidney transplantation is a novel risk factor for BK nephropathy. Transplantation (2015) 99(2):e8–9. doi: 10.1097/TP.0000000000000483
6. Sharif A. Who benefits from ABO-incompatible kidney transplantation in the contemporary era? Am J Kidney Dis (2020) 76(5):607–9. doi: 10.1053/J.AJKD.2020.05.020
7. Sharif A, Alachkar N, Kraus E. Incompatible kidney transplantation: A brief overview of the past, present and future. QJM (2012) 105(12):1141–50. doi: 10.1093/qjmed/hcs154
8. Marlu R, Bennani HN, Seyve L, Malvezzi P, Janbon B, Noble J, et al. Effect of immunoadsorption alone or combined with membrane filtration on hemostasis parameters. J Clin Apher (2020) 35(5):444–52. doi: 10.1002/jca.21825
9. Scurt FG, Ewert L, Mertens PR, Haller H, Schmidt BMW, Chatzikyrkou C. Clinical outcomes after ABO-incompatible renal transplantation: A systematic review and meta-analysis. Lancet (2019) 393(10185):2059–72. doi: 10.1016/S0140-6736(18)32091-9
10. Masutani K, Tsuchimoto A, Kurihara K, Okabe Y, Kitada H, Okumi M, et al. Histological analysis in ABO-compatible & ABO-incompatible kidney transplantation by performance of 3- & 12-month protocol biopsies. Transplantation (2017) 101(6):1416–22. doi: 10.1097/TP.0000000000001324
11. Gloor JM, Cosio FG, Rea DJ, Wadei HM, Winters JL, Moore SB, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant (2006) 6(8):1841–7. doi: 10.1111/j.1600-6143.2006.01416.x
12. Chow KV, Hons M, Flint SM, Shen A, Landgren A, Finlay M, et al. Histological and extended clinical outcomes after ABO-incompatible renal transplantation without splenectomy or rituximab. Transplantation (2017) 101(6):1433–40. doi: 10.1097/TP.0000000000001415
13. Habicht A, Bröker V, Blume C, Lorenzen J, Schiffer M, Richter N, et al. Increase of infectious complications in ABO-incompatible kidney transplant recipients — a single centre experience. Nephrol Dial Transplant. (2011) 26, 4124–31. doi: 10.1093/ndt/gfr215.
14. Dorje C, Mjøen G, Strøm EH, Holdaas H, Jenssen T, Øyen O, et al. One-year protocol biopsies from ABO-incompatible renal allografts compared with a matched cohort of ABO-compatible allografts. Clin Transplant (2015) 29(3):268–76. doi: 10.1111/ctr.12515
15. Ushigome H, Okamoto M, Koshino K, Nobori S, Okajima H, Masuzawa N. Findings of graft biopsy specimens within 90 days after ABO blood group incompatible living donor kidney transplantation compared with ABO-identical and non-identical transplantation. Clin Transplant (2010) 24:16–21. doi: 10.1111/j.1399-0012.2010.01278.x
16. Kumlien G, Wilpert J, Säfwenberg J, Tydén G. Comparing the tube and gel techniques for ABO antibody titration, as performed in three European centers. Transplantation (2007) 84. doi: 10.1097/01.tp.0000296019.85986.af
17. Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. The banff 2017 kidney meeting report: Revised diagnostic criteria for chronic active T cell–mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant (2018) 18(2):293–307. doi: 10.1111/ajt.14625
18. Loupy A, Aubert O, Orandi BJ, Naesens M, Bouatou Y, Raynaud M, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ (2019) 366. doi: 10.1136/bmj.l4923
19. Bentall A, Herrera LP, Cornell LD, Gonzales MAM, Dean PG, Park WD, et al. Differences in chronic intragraft inflammation between positive crossmatch and ABO-incompatible kidney transplantation. Transplantation (2014) 98(10):1089–96. doi: 10.1097/TP.0000000000000188
20. Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the united states. Transplantation (2012) 93(6):603–9. doi: 10.1097/TP.0B013E318245B2AF
21. Tasaki M, Tateno H, Sato T, Tomioka A, Kaji H, Narimatsu H, et al. A novel method of CD31-combined ABO carbohydrate antigen microarray predicts acute antibody-mediated rejection in ABO-incompatible kidney transplantation. Transpl Int (2022). doi: 10.3389/TI.2022.10248
22. Bentall A, Jeyakanthan M, Braitch M, Cairo CW, Lowary TL, Maier S, et al. Characterization of ABH-subtype donor-specific antibodies in ABO-a-incompatible kidney transplantation. Am J Transplant (2021) 21(11):3649–62. doi: 10.1111/AJT.16712
23. Iwasaki K, Miwa Y, Ogawa H, Yazaki S, Iwamoto M, Furusawa T, et al. Comparative study on signal transduction in endothelial cells after anti-A/B and human leukocyte antigen antibody reaction: Implication of accommodation. Transplantation (2012) 93(4):390–7. doi: 10.1097/TP.0B013E3182424DF3
24. Tasaki M, Saito K, Nakagawa Y, Imai N, Ito Y, Aoki T, et al. Acquired downregulation of donor-specific antibody production after ABO-incompatible kidney transplantation. Am J Transplant (2017) 17(1):115–28. doi: 10.1111/AJT.13937
25. Grasseau A, Boudigou M, Le Pottier L, Chriti N, Cornec D, Pers JO, et al. Innate b cells: the archetype of protective immune cells. Clin Rev Allergy Immunol (2020) 58(1):92–106. doi: 10.1007/S12016-019-08748-7
26. Schlößer HA, Thelen M, Dieplinger G, von Bergwelt-Baildon A, Garcia-Marquez M, Reuter S, et al. Prospective analyses of circulating b cell subsets in ABO-compatible and ABO-incompatible kidney transplant recipients. Am J Transplant (2017) 17(2):542–50. doi: 10.1111/AJT.14013
27. Sellarés J, De Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant (2012) 12(2):388–99. doi: 10.1111/j.1600-6143.2011.03840.x
28. Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am J Transplant (2010) 10(9):2066–73. doi: 10.1111/J.1600-6143.2010.03240.X
29. Opelz G, Morath C, Süsal C, Tran TH, Zeier M, Döhler B. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: Results from 101 centers. Transplantation (2015) 99(2):400–4. doi: 10.1097/TP.0000000000000312
30. de Weerd AE, van den Brand JAJG, Bouwsma H, de Vries APJ, Dooper I (Ph).MM, Sanders JSF, et al. ABO-incompatible kidney transplantation in perspective of deceased donor transplantation and induction strategies: A propensity-matched analysis. Transpl Int (2021) 34(12):2706–19. doi: 10.1111/TRI.14145
Keywords: ABOi, ABO incompatible, histological evaluation, long - term effect, rejection, chronic antibody-mediated rejection (cABMR)
Citation: Guy P, Delas A, Esposito L, Cointault O, Colombat M, Congy-Jolivet N, Raynaud M, Kamar N and Del Bello A (2022) Progression of histological lesions after ABO incompatible kidney transplantation. Front. Immunol. 13:969998. doi: 10.3389/fimmu.2022.969998
Received: 15 June 2022; Accepted: 06 September 2022;
Published: 06 October 2022.
Edited by:
Min Gu, Nanjing Medical University, ChinaReviewed by:
Ross Francis, Princess Alexandra Hospital, AustraliaCopyright © 2022 Guy, Delas, Esposito, Cointault, Colombat, Congy-Jolivet, Raynaud, Kamar and Del Bello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arnaud Del Bello, ZGVsYmVsbG8uYUBjaHUtdG91bG91c2UuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.