
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Immunol., 26 August 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.967658
 Liudan Tu1
Liudan Tu1 Minjing Zhao1
Minjing Zhao1 Xiaohong Wang2
Xiaohong Wang2 Qingcong Kong2
Qingcong Kong2 Zena Chen1
Zena Chen1 Qiujing Wei1
Qiujing Wei1 Qiuxia Li1
Qiuxia Li1 Qinghong Yu3
Qinghong Yu3 Zhizhong Ye4
Zhizhong Ye4 Shuangyan Cao1
Shuangyan Cao1 Zhimin Lin1
Zhimin Lin1 Zetao Liao1
Zetao Liao1 Qing Lv1
Qing Lv1 Jun Qi1
Jun Qi1 Ou Jin1
Ou Jin1 Yunfeng Pan1
Yunfeng Pan1 Jieruo Gu1*
Jieruo Gu1*Objective: To investigate the efficacy and safety of clinical, magnetic resonance imaging (MRI) changes in active ankylosing spondylitis (AS) patients with etanercept and celecoxib alone/combined treatment.
Methods: A randomized controlled trial was conducted in three medical centers in China. Adult AS patients with BASDAI ≥4 or ASDAS ≥2.1, CRP >6 mg/L, or ESR 28 mm/1st hour were randomly assigned (1:1:1 ratio) to celecoxib 200 mg bid or etanercept 50 mg qw or combined therapy for 52 weeks. The primary outcomes were SPARCC change of the sacroiliac joint (SIJ) and spine and the proportion of patients achieving ASAS20 response at 52 weeks.
Results: Between September 2014 and January 2016, we randomly assigned 150 patients (mean age, 32.4 years; mean disease duration, 109 months), and 133 (88.6%) completed the study. SPARCC inflammation scores of the SIJ and spine decreased in the three groups, and significant differences were found between the combined group and the celecoxib group [between-group difference: −6.33, 95% CI (−10.56, −2.10) for SIJ; −9.53, 95% CI (−13.73, −5.33) for spine] and between the etanercept group and the celecoxib group [between-group difference: −5.02, 95% CI (−9.29, −0.76) for SIJ; −5.80, 95% CI (−10.04, −1.57) for spine]. The ASAS20 response rates were 44%, 58%, and 84% in the celecoxib, etanercept, and combined groups, respectively, and a significant difference was only found between the combined and the celecoxib groups.
Conclusion: Etanercept with or without celecoxib decreases inflammation detected by MRI at 1 year compared to celecoxib alone in active AS patients. The combination of etanercept and celecoxib was superior to celecoxib alone for the primary clinical response.
Clinical Trial Registration: ClinicalTrials.gov, identifier NCT01934933.
Ankylosing spondylitis (AS) is a chronic inflammatory disease that mainly affects the sacroiliac (SI) joint and spine. New bone formation in the SI joint and spine, which may cause permanent impairment in spinal mobility and function, is an important pathological feature during disease development (1). Since AS peaks at the second to the third decade, Economic burden is huge from patient and society’s perspective (2). Therefore, treatments that can improve clinical symptoms, reduce inflammation, prevent structural damage, and maintain physical function are crucial.
Non-steroidal anti-inflammatory drugs (NSAIDs) are recommended as first-line therapy for AS, and a Cochrane study (3) showed that NSAIDs were efficacious in relieving pain and improving function among axial spondyloarthritis (axSpA) patients when compared with placebo. A single randomized control trial (RCT) also demonstrated that celecoxib might retard radiographic progression in AS patients with elevated C-reactive protein (CRP) (4). However, evidence of NSAIDs’ positive effect upon the Assessment of SpondyloArthritis International Society (ASAS) 20 response rate or MRI inflammation in the long term is limited. Tumor necrosis factor inhibitor (TNFi) was widely used in inflammatory diseases (5) and showed excellent ability in reducing pain and MRI inflammation (6, 7) when compared with placebo, with an ASAS20 response rate of 57%–78% after 12 or 24 weeks of treatment compared to 20%–30% in placebo (8, 9). TNFi and NSAIDs reduce pain and inflammation in different mechanisms, and whether combined TNFi and NSAID therapy has a superior effect on AS patients compared with solo treatment is still unknown. Although NSAIDs were applied in several RCTs comparing the efficacy of TNFi and placebo, the optional use of NSAIDs, of different categories and doses, was allowed.
So far, no clinical trial compares the effect of NSAIDs and TNFi alone/combined treatment on inflammatory change detected with MRI in active AS patients directly. The aim of this study is to compare the clinical and inflammatory change with etanercept and celecoxib alone/combined treatments in active AS patients over 52 weeks.
This multicenter, open-label, randomized clinical trial was conducted at three centers in China from September 2014 to February 2016. Patients were screened by rheumatologists in clinic visits and assessed by researchers in the three centers. Ethics approval was reviewed and approved by the medical research ethics committee of three medical centers separately. The patients/participants provided their written informed consent to participate in this study.
We included patients who met the 1984 modified New York criteria of AS (10), who had more than two and less than 16 syndesmophytes between the cervical and lumber spine detected by X-ray. Patients were included if their Bath Ankylosing Spondylitis Disease Activity Index (11) (BASDAI) ≥4 or Ankylosing Spondylitis Disease Activity Score (ASDAS) (12) ≥2.1, CRP >6 mg/L, or erythrocyte sedimentation rate (ESR) >28 mm/1st hour at baseline. The details of the inclusion and exclusion criteria are shown in the Supplementary Appendix.
Participants in each site would be randomly assigned to etanercept or celecoxib or combined treatment in a ratio of 1:1:1 based on computer-generated random numbers. Allocation concealment was confirmed by a central automated distribution procedure that was independent of the investigators. This is an open-label study so that physicians and patients were aware of the allocated treatment groups after randomization. Follow-up assessments were done by trained research nurses who were masked to the allocated treatment groups.
Participants in the three groups were given one of the following three treatments: celecoxib 200 mg bid, etanercept 50 mg qw, and combined therapy for 52 weeks. During the study period, a certain concomitant treatment such as sulfasalazine or methotrexate with a stable dose (remains stable for 12 weeks prior to the study) would be allowed. NSAIDs (except celecoxib) and corticosteroids were prohibited during the study. NSAIDs should be stopped 7 days or five half-lives, and oral corticosteroids should be stopped 4 weeks prior to the administration of the study.
The primary outcomes of this trial were changes in the Spondyloarthritis Research Consortium of Canada (SPRACC) scores of the SI joint (13) and spine (14) and ASAS20 response rate at week 52. The key secondary outcome measures were ASAS20 at other time points; ASAS40, 50, 70, 5/6 response rate; ASAS partial remission; BASDAI and ASDAS at each visit; the change of modified Stoke Ankylosing Spondylitis Spine Score (mSASSS); and SPRACC SI joint structural score from baseline to week 52. Additional physician-assessed secondary outcomes include physician global assessment of disease activity, Bath Ankylosing Spondylitis Metroloty Index (BASMI) (15), Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), and tender joint count and swollen joint count at each visit. Patient-completed outcomes were visual analog scale scores (including patient global assessment of disease activity, pain, and back pain), AS-specific quality of life (ASQoL), and the Bath Ankylosing Spondylitis Functional Index (BASFI) at each visit. More details about the secondary outcomes appear in the Supplementary Appendix.
The MRI and radiographs of the SI joint and spine were assessed at baseline, 24 weeks, and 52 weeks. Scoring inflammation and structural damage in both the SI joint and spine are based on the SPARCC MRI scoring system (available on http://www.carearthritis.com/). mSASSS (16) was used to measure the quantification of chronic spinal changes by conventional spinal X-rays. More details about the MRI and radiographic scoring are seen in the Supplementary Appendix.
Two independent readers scored the images with masking to the treatment group, time sequence, and clinical data. The mean scores of the two readers were used for analysis unless a discrepancy exists. Discrepant situations included one reader considered the imaging to be unreadable, or if the scores moved in different directions (one positive, one negative), or differed by >5 points for SPARCC spinal and by >3 points for SPARCC SIJ (17) and by >5 points for the change of mSASSS (18). In those cases, a third reader (adjudicator) was required to assess the images, and the mean of the adjudicator’s score and the closest score of the two primary readers was considered as the final score. The two readers and one adjudicator were selected based on their experience with musculoskeletal image reading. The reliability of the SPARCC and mSASSS scores between readers was assessed using intraclass correlation coefficients (ICCs). The ICCs for all SI joint, spine SPARCC score, and mSASSS score were 0.82, 0.75, and 0.64, respectively. The ICCs for total change scores between time points were 0.71 for SIJ SPARCC scores, 0.74 for spine SPARCC scores, and 0.68 for mSASSS scores, respectively.
According to a previous study evaluating the efficacy of adalimumab on decreasing spinal and SI joint inflammation of MRI (7), a median change of SPARCC spinal score of 6.3 (SD = 6) and 0.5 (SD = 1) in the adalimumab and placebo groups and a median change of SPARCC SI joint score of 3.6 (SD = 3) and 1.1 (SD = 1) in the adalimumab and placebo groups were reported. We calculated that a sample size of 20 evaluable patients per group (a total sample size of 60 participants) would provide 90% power to detect differences of spinal and SI SPRACC between the three treatment groups at the two-sided 5% significance level. For the co-primary outcome (ASAS20 at 52 weeks), under the assumption of a 70% response rate in the combined treatment group and 40% response rate in the celecoxib treatment group, it will provide at least 80% power to detect a 30% difference. To allow for a 20% dropout rate, a total of 150 patients (50 per group) were recruited.
Categorical variables were presented as counts and percentages. The Kruskal–Wallis rank test was used for comparison between groups using the full analysis set (all patients receiving ≥1 dose of the study drug). Non-responder imputation was used for missing ASAS20 responses. Continuous variables including the SPARCC score for the SI joint and spine were presented as means (SD) and analyzed as the change from baseline with a repeated-measure mixed model with terms for age, sex, body mass index, treatment, and trial center. The correlation within the repeated measures was addressed by using individual participant identification as a random effect. The effect of treatment was evaluated by the month × treatment interaction. Stata version 15.0 was used for all the analyses, and a P-value <0.05 (two-tailed) was regarded as statistically significant.
Figure 1 shows the flow of the study participants. A total of 215 participants were screened for eligibility from September 2014 to January 2016, and 150 (69.7%) were enrolled and randomly assigned (1:1:1) to one of the three treatment groups: celecoxib 200 mg bid (group A), etanercept 50 mg qw (group B), and combined therapy (group C) for 52 weeks. In group A, 40 (80%) of 50 patients completed the treatment and follow-up to week 52, and 46 (92%) and 47 (94%) did so in groups B and C, respectively. No significant difference was found between patients who completed and the dropouts (Table S1). No patients were found to be ineligible for the trial post-randomization. More patients (10 vs. 4 vs. 3) discontinued early from the trial in group A compared with the other two groups mainly due to lack of efficacy with celecoxib treatment.
The mean (SD) age of the participants in this study was 32.4 (8.37) years, 86% were men, and the mean disease duration was 109 (70) months. Patients included were in high disease activity with a mean BASDAI score of 5.2 (2.2) and a mean ASDAS-C score of 3.6 (0.87). Meanwhile, the mean syndesmophyte number detected by X-ray was 4.64 (3.22) and the mean mSASSS score was 12.12 (6.48). Participants’ demographic characteristics were comparable at baseline between the three groups (Table 1). Sixteen of 50 celecoxib-treated patients (32%), 17 of 50 etanercept-treated patients (34%), and 19 of 50 combined-treated patients (38%) had an SIJ SPARCC score of 0 at baseline, and no significant difference was found between the groups. All patients scored larger than 0 in the spinal SPARCC score at baseline.
Changes in the SPARCC scores of the SI joint and spine are presented in Table 2. At baseline, the mean (SD) values of the SIJ and spinal SPARCC scores were 8.25 (11.5) and 24.51 (14.64) in group A, 8.28 (13.65) and 23.83 (10.70) in group B, and 9.63 (13.08) and 27.32 (13.34) in group C. The SIJ and spinal SPARCC scores decreased over 24 and 52 weeks in all the three groups, (Figure 2). At week 52, there were significant differences in the changes of the SIJ and spinal SPARCC scores between group C and group A and between group B and group A in the mixed-effect model. More improvement in the SIJ and spinal SPARCC scores was found in group C than in group B, but this difference did not reach statistical significance (Table 2). For the SIJ SPARCC score, a significant difference was found within group B [−7.44 (95% CI, −10.24 to −4.64), P < 0.001] and within group C [−8.75 (95% CI, −11.49 to −6.01), P < 0.001] from week 52 to baseline, while no significant difference was found within group A [−2.42 (95% CI, −5.64 to 0.80), P = 0.14] from week 52 to baseline. For the spinal SPARCC score, there were significant differences among the three groups from week 52 to baseline [group A: −13.71 (95% CI, −16.94 to −10.48), P < 0.001; group B: −19.51 (95% CI, −22.25 to −16.77), P < 0.001; group C: −23.23 (95% CI, −25.91to −20.56), P < 0.001]. At week 24, similar results were found in the changes of the SIJ SPARCC score between groups, while a significant difference was found between group C and group B in the spinal SPARCC score [between-group C–B difference, −4.06 (95% CI, −7.78 to −0.34), P = 0.03]. For within-group difference, the results of week 24 were similar to week 52 in both the SIJ and spinal SPARCC scores (Figure 2).
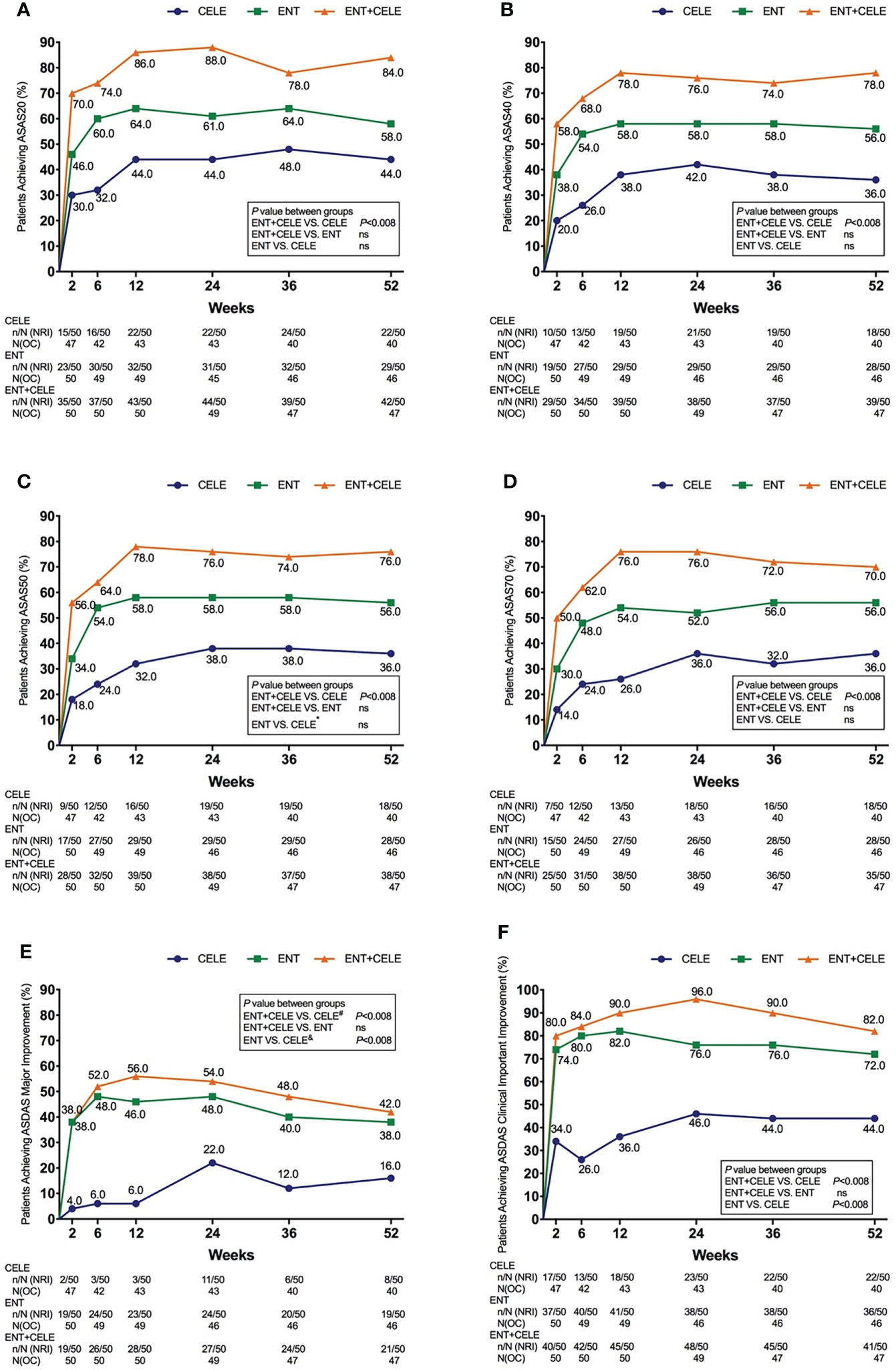
Figure 2 Mean change from baseline for (A) SPARCC MRI SIJ score and (B) SPARCC MRI spinal score. The mixed-effect model was used for the comparison between groups. *P < 0.05. Mean (SD) baseline values: (A) 8.63 (11.67) for CELE, 8.28 (13.65) for ENT, and 9.63 (13.08) for ENT+CELE; (B) 24.61 (14.05) for CELE, 24.14 (10.76) for ENT, and 27.80 (13.26) for ENT+CELE. Within-group P-value between baseline and week 52 from the mixed-effect model: <0.0001 for all in the SPARCC MRI SIJ and SPARCC MRI spinal scores. Within-group P-value between baseline and week 24 from the mixed-effect model: <0.0001 for all in the SPARCC MRI spinal score, <0.0001 for ENT and ENT+CELE in the SPARCC MRI SIJ score, and no significant difference for CELE in the SPARCC MRI SIJ score. ENT, etanercept; CELE, celecoxib; SIJ, sacroiliac joint; SPARCC, Spondylitis Research Consortium of Canada.
A total of 93/150 (51.28%) patients achieved ASAS20 using NRI by week 52.44% in group A, 58% in group B, and 84% in group C, and a significant difference was only found between group A and group C (P = 0.003) (Figure 3). Although no significant difference was found between group B and group C or between group B and group A, a slightly higher ASAS20 response rate was observed in group C than in group B at any time point.
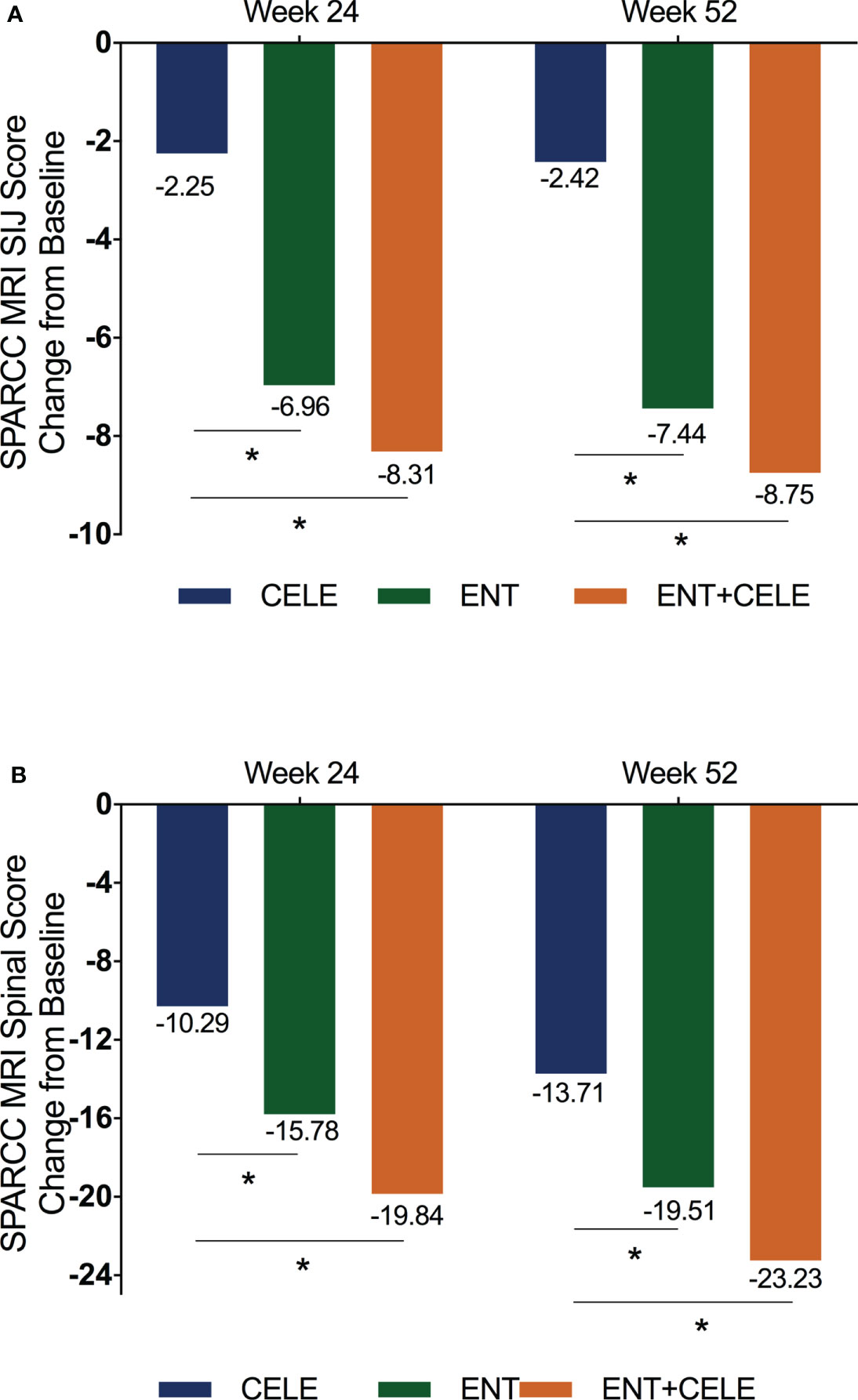
Figure 3 Proportion of patients achieving (A) ASAS20 response, (B) ASAS40 response, (C) ASAS50 response, (D) ASAS70 response, (E) ASDAS major improvement, and (F) ASDAS clinical important improvement in the three groups over 52 weeks. Population is modified intention to treat, non-responder imputation (NRI). The actual number of patients is shown as the observed case (OC). P-values for differences in the results between groups at any timepoint are from the Kruskal–Wallis test, and the adjusted P-value for significance is 0.008 in multiple comparisons between groups. ASAS, Assessment of SpondyloArthritis International Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; ENT, etanercept; CELE, celecoxib; ns, non-significant. *, significant difference was found between the etanercept and celecoxib groups only at week 2. #, no significant difference was found between the etanercept + celecoxib and celecoxib groups at week 52. &, significant differences were found between etanercept and celecoxib groups at weeks 2, 6, 12, and 36.
The proportions of all patients achieving ASAS40, ASAS50, ASAS70, ASAS5/6, and ASAS partial remission after 52 weeks of treatment were 56.7%, 56%, 54%, 52%, and 42.86%, respectively. At week 52, more patients in group C achieved an ASAS40, ASAS50, ASAS70, ASAS5/6 remission, and ASAS partial remission than in groups B and A (Figure 3 and Figure S1), but a significant difference was only found between group C and group A.
More patients in groups C and B achieved ASDAS major improvement compared with group A over 52 weeks although a significant difference was observed at some time points (Figure 3). Over the course of treatment, groups C and B were significantly more effective than group A at achieving ASDAS clinical important improvement. For BASDAI, BASFI, ASDAS-C, and back pain, significant improvements were found between group C and group A and group B and group A (Table 2).
The changes in the structural damages in the SIJ are presented in Table 2. Erosion decreased significantly in groups C and B after 52 weeks of treatment, and significant differences between group C and group A [between-group difference, −1.94 (95% CI, −3.16 to −0.72), P = 0.002] and between group B and group A [between-group difference, −1.79 (95% CI, −3.03 to −0.55), P = 0.004] were found. No significant difference was found between groups in terms of fat metaplasia, backfill, ankylosis, and mSASSS scores over 52 weeks (Table 2).
Adverse events were reported in 55 (36.6%) of the 150 patients, and no significant difference was found between the three treatment groups (P = 0.09) (Table 3). No death occurred except for one serious adverse event that happened in this trial. One patient was admitted to the hospital because of drug dermatitis (not caused by etanercept). In all the 55 adverse events reported, the most commonly reported were infections, hepatobiliary disorders, and gastrointestinal upset. The AEs of the combined treatment were comparable with etanercept or celecoxib alone.
This study is the first to compare the SIJ and spinal SPARCC scores in active AS patients over 52 weeks in the three treatment groups consisting of celecoxib, etanercept, and combined treatment. The combination of etanercept and celecoxib was superior to celecoxib alone for the primary clinical response and MRI-based inflammation scores over 52 weeks. Etanercept with or without celecoxib decreases inflammation detected by MRI at 1 year compared to celecoxib alone.
MRI assessments provide an objective measure of detecting inflammation, and the SPARCC scoring system has been used widely and validated in AS patients (13, 14). TNFi has been known to reduce MRI inflammation in the SI joint and spine (6, 19), and improvement may be sustained 1 or 2 years or even longer (17, 20). Patients with higher BASDAI, CRP levels, and MRI inflammation are the most likely to respond to TNF blockers (21, 22). However, in most of the studies published before, MRI assessment of the SI joint and spine was not the primary endpoint of the original studies, and most of them compared TNFi with placebo. Even though the use of NSAIDs was allowed, the category and dose were not consistent. On the other hand, studies about the effect of NSAIDs on the inflammation of MRI in SpA/AS patients were limited (23, 24), and studies with long-term follow-up, appropriate control group, and larger sample size are needed. To our knowledge, this is the first study comparing NSAIDs with TNFi in long-term efficacy on MRI inflammation in active AS patients with an RCT design. We found that etanercept with or without celecoxib significantly improved SIJ and spinal SPARCC scores when compared with celecoxib over 52 weeks. No significant difference in the change of SIJ and spinal SPARCC scores was found when comparing combined treatment with etanercept alone. For celecoxib treatment, a significant difference in the change of SIJ SPARCC score was not found until week 52, which may indicate the slower process of celecoxib in reducing inflammation. The baseline SIJ or spinal SPARCC score was higher in our study, and it may be because the patients included in other studies were SpA patients with shorter disease duration (25, 26) or only BASDAI ≥4 was required (7).
Previous studies reported that the ASAS20 response rate varied from 29.5% (27) to 64.7% (28) after NSAID treatment in AS patients when compared with placebo in a relatively short term. TNFi was shown to be effective in achieving an even higher ASAS20 response rate when compared with placebo (8) with most of the patients using NSAIDs in both groups. However, no head-to-head comparison in ASAS20 response rate between TNFi and NSAIDs was found. Our study showed that the ASAS20 response rate was higher in groups C and B compared with that in group A (84% vs. 58% vs. 44%, P < 0.001) at 52 weeks, and a significant difference was only found between group C and group A (P = 0.003). The combination of celecoxib and etanercept treatment may be beneficial for sign and symptom remission with different mechanisms. Nevertheless, the possible complication of the long-term use of NSAIDs is an important concern that clinicians would advise to discontinue NSAID intake once symptoms improve or disappear with anti-TNF therapy. In a multicenter, randomized double-blind, placebo-controlled SPARSE study, the use of etanercept can significantly reduce NSAID intake (ASAS-NSAIDs score) compared with placebo over 8 weeks (29). The concept of combined TNFi and NSAID therapy may have an effect on retarding the radiographic progression of AS when inflammation is suppressed at the same time. While the protocol for a planned study comparing golimumab versus golimumab plus NSAID has been published, the results (assessing the retardation of radiographic progression in the spine) are not yet available (30). More well-designed studies with a long-term follow-up are needed to determine the potential structural benefits of NSAIDs and TNFi.
Prior studies of TNFi in AS have generally employed plain radiographs of the spine to assess radiographic progression that requires long periods of follow-up and large numbers of study subjects due to slow rates of damage accumulation on radiographs. In contrast, we assessed structural lesions using MRI. More attention was paid to structural lesions detected with the T1 sequence of MRI in recent years. Erosion on SIJ MRI was reported to be a highly specific lesion in patients with SpA (31) and to enhance the diagnostic utility in early SpA (32). Erosion may occur in SpA without radiographic change and even in the absence of SIJ bone marrow edema on MRI (33). A study demonstrated that etanercept was associated with a significantly greater reduction in erosion and an increase in backfill at 12 weeks compared with placebo (34), while no difference was observed between adalimumab and placebo for structural lesions (35). In this study, erosion was found to be decreased more in the combined as well as in the etanercept treatment when compared with celecoxib treatment, while no significant difference was found in the other structural lesions like fat metaplasia, backfill, and ankylosis. The possible reason may be that the morphology of erosion may change as the inflammation resolves and the evolution of erosion to fat metaplasia or backfill may take time following inflammation resolution.
The limitations of this trial include the open-label design, which might have caused unintentional bias in favor of the etanercept or combined treatment group, and even though we used masked nurses to conduct the assessments, this problem may still be existing. The structural changes of the radiograph, with a minimum interval time of 2 years, were not designed to be the primary outcome, so only post-hoc analysis can be conducted in this study.
In summary, the results of this trial demonstrate that etanercept with or without celecoxib is clinically effective and has an excellent anti-inflammatory effect assessed by MRI over 52 weeks in patients with active AS compared with celecoxib treatment. This novel study, which allows for a direct comparison of NSAIDs and TNFi on active AS patients, provides clear evidence for the efficiency of anti-inflammatory in the three treatment options.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This study was reviewed and approved by the Third Affiliated Hospital of Sun Yat-sen University, ZhuJiang Hospital of Southern Medical University and Fourth People's Hospital of Shenzhen City. The patients/ participants provided their written informed consent to participate in this study.
JG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JG, LT, MZ, QY, and ZY. Acquisition, analysis, or interpretation of data: all authors. Drafting the manuscript: LT, MZ, and JG. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: LT, ZC, and JG. All authors contributed to the article and approved the submitted version.
This study was funded by Pfizer Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
We especially thank the participants who made this study possible, and we gratefully acknowledge the role of the study staff and volunteers in collecting the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.967658/full#supplementary-material
1. Landewe R, Dougados M, Mielants H, van der Tempel H, van der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheumatic Diseases (2009) 68(6):863–7. doi: 10.1136/ard.2008.091793
2. Tu L, Rai JC, Cao S, Lin Z, Hu Z, Gu J. Costs and work limitation of patients with ankylosing spondylitis in China. Clin Exp Rheumatol (2014) 32(5):661–6.
3. Kroon FP, van der Burg LR, Ramiro S, Landewe RB, Buchbinder R, Falzon L, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev (2015) 7):CD010952. doi: 10.1002/14651858.CD010952.pub2
4. Wanders A, Heijde D, Landewe R, Behier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: A randomized clinical trial. Arthritis Rheumatism (2005) 52(6):1756–65. doi: 10.1002/art.21054
5. Murdaca G, Colombo BM, Cagnati P, Gulli R, Spano F, Puppo F. Update upon efficacy and safety of TNF-alpha inhibitors. Expert Opin Drug Saf (2012) 11(1):1–5. doi: 10.1517/14740338.2012.630388
6. Braun J, Baraliakos X, Hermann KG, van der Heijde D, Inman RD, Deodhar AA, et al. Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo- controlled GO-RAISE study. Ann Rheumatic Dis (2012) 71(6):878–84. doi: 10.1136/annrheumdis-2011-200308
7. Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheumatism (2007) 56(12):4005–14. doi: 10.1002/art.23044
8. Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: Results of a randomised, controlled trial. Ann Rheumatic Dis (2014) 73(3):587–94. doi: 10.1136/annrheumdis-2012-202533
9. Landewe R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheumatic Dis (2014) 73(1):39–47. doi: 10.1136/annrheumdis-2013-204231
10. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheumatism (1984) 27(4):361–8. doi: 10.1002/art.1780270401
11. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: The bath ankylosing spondylitis disease activity index. J Rheumatol (1994) 21(12):2286–91.
12. van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheumatic Dis (2009) 68(12):1811–8. doi: 10.1136/ard.2008.100826
13. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis research consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheumatism (2005) 53(5):703–9. doi: 10.1002/art.21445
14. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Krishnananthan R, Stone M, et al. Spondyloarthritis research consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheumatism (2005) 53(4):502–9. doi: 10.1002/art.21337
15. Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The bath AS metrology index. J Rheumatol (1994) 21(9):1694–8.
16. Creemers MC, Franssen MJ, van’t Hof MA, Gribnau FW, van de Putte LB, van Riel PL. Assessment of outcome in ankylosing spondylitis: An extended radiographic scoring system. Ann Rheumatic Dis (2005) 64(1):127–9. doi: 10.1136/ard.2004.020503
17. Maksymowych WP, Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, et al. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheumatic Dis (2016) 75(7):1328–35. doi: 10.1136/annrheumdis-2015-207596
18. van der Heijde D, Salonen D, Weissman BN, Landewe R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther (2009) 11(4):R127. doi: 10.1186/ar2794
19. Pedersen SJ, Sorensen IJ, Hermann KG, Madsen OR, Tvede N, Hansen MS, et al. Responsiveness of the ankylosing spondylitis disease activity score (ASDAS) and clinical and MRI measures of disease activity in a 1-year follow-up study of patients with axial spondyloarthritis treated with tumour necrosis factor alpha inhibitors. Ann Rheumatic Dis (2010) 69(6):1065–71. doi: 10.1136/ard.2009.111187
20. Song IH, Hermann KG, Haibel H, Althoff CE, Poddubnyy D, Listing J, et al. Consistently good clinical response in patients with early axial spondyloarthritis after 3 years of continuous treatment with etanercept: Longterm data of the ESTHER trial. J Rheumatol (2014) 41(10):2034–40. doi: 10.3899/jrheum.140056
21. Davis JC Jr., van der Heijde DM, Dougados M, Braun J, Cush JJ, Clegg DO, et al. Baseline factors that influence ASAS 20 response in patients with ankylosing spondylitis treated with etanercept. J Rheumatol (2005) 32(9):1751–4.
22. Rudwaleit M, Listing J, Brandt J, Braun J, Sieper J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheumatic Dis (2004) 63(6):665–70. doi: 10.1136/ard.2003.016386
23. Varkas G, Jans L, Cypers H, Van Praet L, Carron P, Elewaut D, et al. Brief report: six-week treatment of axial spondyloarthritis patients with an optimal dose of nonsteroidal antiinflammatory drugs: Early response to treatment in signal intensity on magnetic resonance imaging of the sacroiliac joints. Arthritis Rheumatol (2016) 68(3):672–8. doi: 10.1002/art.39474
24. Tang M, Xue L, Shen Y, Bo L, Yang R, Wen J, et al. Efficacy of long-term nonsteroidal antiinflammatory drug treatment on magnetic resonance imaging-determined bone marrow oedema in early, active axial spondyloarthritis patients. Clin Rheumatol (2018) 37(1):245–50. doi: 10.1007/s10067-017-3666-x
25. Dougados M, van der Heijde D, Sieper J, Braun J, Maksymowych WP, Citera G, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: A multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol (2014) 66(8):2091–102. doi: 10.1002/art.38721
26. Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: Results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheumatic Dis (2013) 72(6):815–22. doi: 10.1136/annrheumdis-2012-201766
27. Cinar M, Dinc A, Simsek I, Erdem H, Koc B, Pay S, et al. Evaluation of the short-term efficacy of NSAIDs on patients with active ankylosing spondylitis in daily practice: A 3-month, longitudinal, observational study. Rheumatol Int (2010) 30(3):331–40. doi: 10.1007/s00296-009-0963-y
28. van der Heijde D, Baraf HS, Ramos-Remus C, Calin A, Weaver AL, Schiff M, et al. Evaluation of the efficacy of etoricoxib in ankylosing spondylitis: results of a fifty-two-week, randomized, controlled study. Arthritis Rheumatism (2005) 52(4):1205–15. doi: 10.1002/art.20985
29. Dougados M, Wood E, Combe B, Schaeverbeke T, Miceli-Richard C, Berenbaum F, et al. Evaluation of the nonsteroidal anti-inflammatory drug-sparing effect of etanercept in axial spondyloarthritis: results of the multicenter, randomized, double-blind, placebo-controlled SPARSE study. Arthritis Res Ther (2014) 16(6):481. doi: 10.1186/s13075-014-0481-5
30. Proft F, Muche B, Listing J, Rios-Rodriguez V, Sieper J, Poddubnyy D. Study protocol: COmparison of the effect of treatment with nonsteroidal anti-inflammatory drugs added to anti-tumour necrosis factor a therapy versus anti-tumour necrosis factor a therapy alone on progression of StrUctural damage in the spine over two years in patients with ankyLosing spondylitis (CONSUL) - an open-label randomized controlled multicenter trial. BMJ Open (2017) 7(6):e014591. doi: 10.1136/bmjopen-2016-014591
31. Wick MC, Weiss RJ, Jaschke W, Klauser AS. Erosions are the most relevant magnetic resonance imaging features in quantification of sacroiliac joints in ankylosing spondylitis. J Rheumatol (2010) 37(3):622–7. doi: 10.3899/jrheum.090602
32. Weber U, Lambert RG, Pedersen SJ, Hodler J, Ostergaard M, Maksymowych WP. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res (Hoboken) (2010) 62(12):1763–71. doi: 10.1002/acr.20312
33. Maksymowych WP, Wichuk S, Dougados M, Jones H, Szumski A, Bukowski JF, et al. MRI Evidence of structural changes in the sacroiliac joints of patients with non-radiographic axial spondyloarthritis even in the absence of MRI inflammation. Arthritis Res Ther (2017) 19(1):126. doi: 10.1186/s13075-017-1342-9
34. Maksymowych WP, Wichuk S, Dougados M, Jones HE, Pedersen R, Szumski A, et al. Modification of structural lesions on MRI of the sacroiliac joints by etanercept in the EMBARK trial: A 12-week randomised placebo-controlled trial in patients with non-radiographic axial spondyloarthritis. Ann Rheumatic Dis (2018) 77(1):78–84. doi: 10.1136/annrheumdis-2017-211605
35. Pedersen SJ, Poddubnyy D, Sorensen IJ, Loft AG, Hindrup JS, Thamsborg G, et al. Course of magnetic resonance imaging-detected inflammation and structural lesions in the sacroiliac joints of patients in the randomized, double-blind, placebo-controlled danish multicenter study of adalimumab in spondyloarthritis, as assessed by the berlin and spondyloarthritis research consortium of canada methods. Arthritis Rheumatol (2016) 68(2):418–29. doi: 10.1002/art.39434
Keywords: spondyloarthritis, biological therapies, inflammation, NSAIDs, MRI
Citation: Tu L, Zhao M, Wang X, Kong Q, Chen Z, Wei Q, Li Q, Yu Q, Ye Z, Cao S, Lin Z, Liao Z, Lv Q, Qi J, Jin O, Pan Y and Gu J (2022) Etanercept/celecoxib on improving MRI inflammation of active ankylosing spondylitis: A multicenter, open-label, randomized clinical trial. Front. Immunol. 13:967658. doi: 10.3389/fimmu.2022.967658
Received: 13 June 2022; Accepted: 04 August 2022;
Published: 26 August 2022.
Edited by:
Nicole Horwood, University of East Anglia, United KingdomReviewed by:
Maria Sole Chimenti, Rheumatology, Allergology and Clinical Immunology University of Rome Tor Vergata, ItalyCopyright © 2022 Tu, Zhao, Wang, Kong, Chen, Wei, Li, Yu, Ye, Cao, Lin, Liao, Lv, Qi, Jin, Pan and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieruo Gu, Z3VqaWVydW9AMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.