- 1Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China
Current treatments for central nervous system (CNS) inflammatory demyelinating diseases (IDDs) include corticosteroids, plasma exchange, intravenous immunoglobulin, and immunosuppressant drugs. However, some patients do not respond well to traditional therapies. In recent years, novel drugs, such as monoclonal antibodies, targeting the complement component C5, CD19 on B cells, and the interleukin-6 (IL-6) receptor, have been used for the treatment of patients with refractory CNS IDDs. Among these, tocilizumab and satralizumab, humanized monoclonal antibodies against the IL-6 receptor, have shown beneficial effects in the treatment of this group of diseases. In this review, we summarize current research progress and prospects relating to anti-IL-6 therapies in CNS IDDs.
Introduction
Central nervous system (CNS) inflammatory demyelinating diseases (IDDs) comprise a heterogeneous group of disorders that mainly includes clinically isolated syndrome, multiple sclerosis (MS), neuromyelitis spectrum disorders (NMOSDs), myelin-oligodendrocyte glycoprotein antibody-associated disease (MOGAD), and acute disseminated encephalomyelitis. These disorders are characterized by myelin loss and axonal damage associated with inflammatory lesions (1–5).
Current treatments for CNS IDDs are primarily aimed at relieving acute attacks and preventing relapse. High-dose corticosteroids, plasma exchange, and intravenous immunoglobulin are frequently employed for the treatment of acute attacks in CNS IDDs (6–14), whereas immunosuppressant drugs, such as oral corticosteroids, azathioprine (AZA), and mycophenolate mofetil, are commonly used for relapse prevention in NMOSDs and MOGAD (15–19). Disease-modifying drugs, such as IFN-β, dimethyl fumarate, and glatiramer acetate, are recommended as mainstream treatments for MS (20, 21). However, some patients do not respond well to these traditional therapies. Over recent years, novel drugs, such as monoclonal antibodies targeting the complement C5 protein, CD19 on B cells, and the interleukin-6 (IL-6) receptor have been used for the treatment of patients with refractory CNS IDDs (22–24).
IL-6 is a cytokine that plays a key role in host defenses, and dysregulated IL-6 signaling is implicated in various autoimmune and inflammatory diseases (25–27). IL-6 signals are transmitted via two routes, namely, the classical and trans-signaling pathways. In the classical pathway, IL-6 binds to the membrane-bound IL-6 receptor, forming a complex that recruits glycoprotein 130. In the trans-signaling pathway, IL-6 binds to the soluble form of the IL-6 receptor, and then to membrane-anchored glycoprotein 130 (28, 29). During the adaptive immune response, IL-6 exerts its effects via stimulating B-lymphocyte differentiation, promoting antibody production, modulating blood–brain barrier permeability, and enhancing T-lymphocyte activation (30–32). Studies demonstrate that serum and cerebrospinal fluid (CSF) IL-6 levels are significantly increased in patients with NMOSD, whereas IL-6 inhibition is shown to mitigate disease progression (33–38). Several studies have also identified a positive association between serum IL-6 receptor levels and the risk for MS (39–41). Accordingly, IL-6 receptor blockade may represent a novel therapeutic approach for the prevention of relapse in CNS IDDs. Tocilizumab and satralizumab, humanized monoclonal antibodies against the IL-6 receptor, have recently been shown to elicit beneficial clinical effects in the treatment of CNS IDDs (42–46). In this review, we summarize current studies regarding the effects of tocilizumab and satralizumab in the treatment of these disorders.
Tocilizumab
Tocilizumab in NMOSD
In 2013, Araki et al. reported the case of a 36-year-old woman who experienced an improvement in her disability burden and neuropathic pain 6 months after tocilizumab therapy (44). The same group described another case series involving seven patients with NMOSD who received monthly injections of tocilizumab (8 mg/kg) for 12 months. The authors reported a significant reduction in the annualized relapse rate (ARR), the Expanded Disability Status Scale (EDSS) score, neuropathic pain, and general fatigue among the patients. The anti-AQP4 antibody titers were also decreased 6 and 12 months after tocilizumab treatment (45).
In a German retrospective observational study in which the patients were followed up for a mean of 30.9 months, eight patients who received tocilizumab displayed a marked decrease in ARR and EDSS scores. Their AQP4 antibody titers and pain levels were also significantly reduced during tocilizumab treatment (46). Subsequently, data from a clinical study confirmed the long-term efficacy and safety of tocilizumab. Nineteen patients were given tocilizumab monthly, and the number of relapses decreased in all cases. Among 15 patients who received tocilizumab for more than 1 year, the EDSS score, ARR, neuropathic pain, and general fatigue were all significantly improved (47). Similar results were reported for 12 NMOSD patients who received at least 6 months of subcutaneous tocilizumab (48). In the same study, both median and annualized relapse rates were significantly decreased (from 2 to 0). The efficacy of subcutaneous tocilizumab appears to render it an alternative to infusion for patients with NMOSD. In Spain, an observational, retrospective study analyzed the effectiveness and safety of tocilizumab in five NMOSD patients who failed to respond to other immunosuppressant drugs. The authors reported that the mean ARR was reduced by 88.9% during the first year of treatment (from 1.8 ± 1.3 to 0.2 ± 0.4, P<.05) (49).
The TANGO trial (NCT03350633) was a randomized, open-label phase II trial that included 118 patients who were followed up for 90 weeks at six hospitals in China (50). The patients were randomly given tocilizumab (8 mg/kg every 4 weeks) or AZA (2–3 mg/kg per day). The median time to first relapse was longer in the patients treated with tocilizumab than in those administered AZA (78.9 weeks vs. 56.7 weeks; P=.0026). In the per-protocol analysis, 89% of the patients treated with tocilizumab were relapse-free after 60 weeks of treatment compared with 56% for AZA treatment (P<.0001).
A meta-analysis of five clinical trials that included 89 patients reported that the ARR was significantly reduced in patients treated with tocilizumab. Moreover, a significant correlation was found between the proportion of relapse-free patients and tocilizumab treatment (51). Another meta-analysis involving 775 patients from seven randomized controlled trials found that patients treated with tocilizumab or satralizumab exhibited significantly lower EDSS scores compared with patients treated with other monoclonal antibodies (52). Meanwhile, in a meta-analysis comprising a total of 202 patients with NMOSD from nine studies, Kharel et al. found that 76% of the patients treated with tocilizumab were relapse-free and the ARR was significant reduced (mean difference: −2.6) at follow-up (ranging from 12 to 31.8 months) (53).
Recently, Du et al. undertook a retrospective study on the effects of tocilizumab in 19 NMOSD patients with moderate-to-severe myelitis. The authors found that disease disability scores were significantly improved in patients treated with tocilizumab when compared with those in patients treated with steroids at 3 months. In addition, compared with steroids, tocilizumab treatment led to a significantly lower ARR and risk of relapse (54). A longitudinal study investigated retinal damage in 50 patients with NMOSD who received disease-modifying drugs and reported that, compared with AZA, tocilizumab and rituximab could delay macular ganglion cell complex thinning in the eyes of patients without a history of optic neuritis (55).
Tocilizumab In MOGAD
In 2019, Novi et al. reported that a 20-year-old patient with MOGAD experienced clinical deterioration despite receiving rituximab treatment, following which he received tocilizumab infusion every 4 weeks. At the 24-month follow-up, the patient was relapse-free, and a spinal MRI showed a reduction in cervical and thoracic lesions (56). Subsequently, Hayward-Koennecke et al. described the case of a 59-year-old man with recurrent optic neuritis who had received high-dose steroids, plasmapheresis, rituximab, natalizumab, and cyclophosphamide due to disease deterioration. Following further relapse, the patient tested positive for anti-MOG antibodies. Tocilizumab was initiated for 12 months and then tapered to an application every 6 weeks. No further relapses occurred (57).
Masuccio et al. reported a patient with MOGAD who experienced severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection during tocilizumab therapy. Given the high risk of relapse, the patient continued taking tocilizumab, and the symptoms stabilized. The patient also retained walking ability (58).
A retrospective study analyzed seven patients with inflammatory CNS disorder (four with NMOSD and positive for anti-AQP4 antibodies and three with MOGAD) who were treated with tocilizumab. The median follow-up period was 23 months (4–50 months). All the patients were relapse-free throughout tocilizumab treatment (59). Similarly, a single-center report involving 10 patients with relapsing MOGAD who received intravenous or subcutaneous tocilizumab found that none of the patients had clinical or radiographic recurrence over an average treatment duration of 28.6 months (60).
Recently, a retrospective study described 57 patients, including 14 with MOGAD, 36 with AQP4-IgG-seropositive NMOSD, and seven with AQP4-IgG-negative NMOSD who switched to tocilizumab from other immunotherapies. The authors reported that 60% of all the patients were relapse-free (79% for patients with MOGAD) during tocilizumab treatment. For MOGAD, the median ARR decreased from 1.75 to 0, and the inflammatory activity on MRI also decreased significantly under tocilizumab treatment. Eleven of the patients with MOGAD who received tocilizumab for more than 12 months had reduced ARR, and 73% of these were relapse-free (61).
Tocilizumab in MS
To date, relatively few studies have focused on tocilizumab therapy in MS, and the efficacy of tocilizumab as a treatment for this disease remains unclear. In 2014, Sato et al. reported the case of a 53-year-old Japanese woman with MS and rheumatoid arthritis (RA) who received tocilizumab and was relapse-free for more than 5 years (62). Moreover, in 2020, the neurological condition of a Japanese boy who was diagnosed with MS with a tumefactive lesion in the cervical spine deteriorated when his oral prednisolone dose was tapered off. After tocilizumab treatment, the prednisolone dose was reduced without symptom exacerbation, and the EDSS score effectively improved from 8.5 to 5.0 points (63).
Satralizumab
Satralizumab in NMOSD
Satralizumab is another IL-6 receptor-targeting monoclonal antibodies. It has better pharmacokinetic properties and a longer half-life than tocilizumab, resulting from modifications in the constant and variable regions of the antibody (64–66).
SAkuraStar (NCT02028884) and SAkuraSky (NCT02073279) are two randomized, double-blind, placebo-controlled phase III studies that compared the efficacy and safety of satralizumab as, respectively, an add-on treatment to other immunosuppressants and as monotherapy in patients with NMOSD (67, 68). In the two trials, 120 mg subcutaneous satralizumab or placebo were administered at weeks 0, 2, and 4 and then every 4 weeks thereafter. The primary endpoint was time to the first protocol-defined relapse. Secondary endpoints were changes in the Functional Assessment of Chronic Illness Therapy–Fatigue score and visual analog scale pain score.
In the SAkuraStar trial, the rate of relapse (8/41, 20%) was lower in the satralizumab group than in the group given the placebo (18/42, 43%). Additionally, 89% and 78% of patients treated with satralizumab were still relapse-free after 48 and 96 weeks, respectively. Among 55 AQP4-IgG-seropositive patients, the relapse rate was 11% in the satralizumab group versus 43% in the placebo group. No significant difference between satralizumab and placebo was observed in the AQP4-IgG seronegative subgroup (67). In the SAkuraSky trial, satralizumab elicited a 55% reduction in the relapse risk compared with the placebo. At 48 and 96 weeks, 76% and 72% of patients were relapse-free in the satralizumab group compared with 62% and 51%, respectively, in the placebo group. For the AQP4-IgG-seropositive subgroup, relapse occurred in 22% of patients who received satralizumab versus 57% for the placebo. No significant difference was observed between satralizumab and placebo in the AQP4-IgG-negative subgroup (68). Similarly, no significant differences in neuropathic pain or fatigue were detected in either study.
Based on the positive results of these two phase III clinical trials, on June 1, 2020, Health Canada approved satralizumab for use in the treatment of AQP4-IgG-seropositive NMOSD in adults and children aged ≥2 years. Subsequently, satralizumab was also approved in Japan on June 29, 2020, and in Switzerland on July 13, 2020 (69).
Recently, a Japanese study reported a patient with AQP4-IgG-seropositive NMOSD whose painful tonic seizures disappeared after 6 months of satralizumab treatment (70).
Adverse events
Overall, anti-IL-6 therapy is well-tolerated by patients with NMOSD. Infections, anemia, leukocytopenia, and hypercholesterolemia are the main adverse events associated with tocilizumab treatment in NMOSD (44); however, most are mild, and serious adverse events are rarely reported. Although a meta-analysis found that two patients with NMOSD died during tocilizumab therapy, neither death was related to tocilizumab treatment (50). Most adverse events related to satralizumab therapy are mild to moderate. The most commonly reported adverse events are upper respiratory tract infections, urinary tract infections, nasopharyngitis, and headaches. No deaths occurred during satralizumab treatment (67, 68, 71, 72).
Perspectives and challenges
Are IL-6 receptor antagonists also suitable as treatments for MOGAD and MS, in addition to NMOSD?
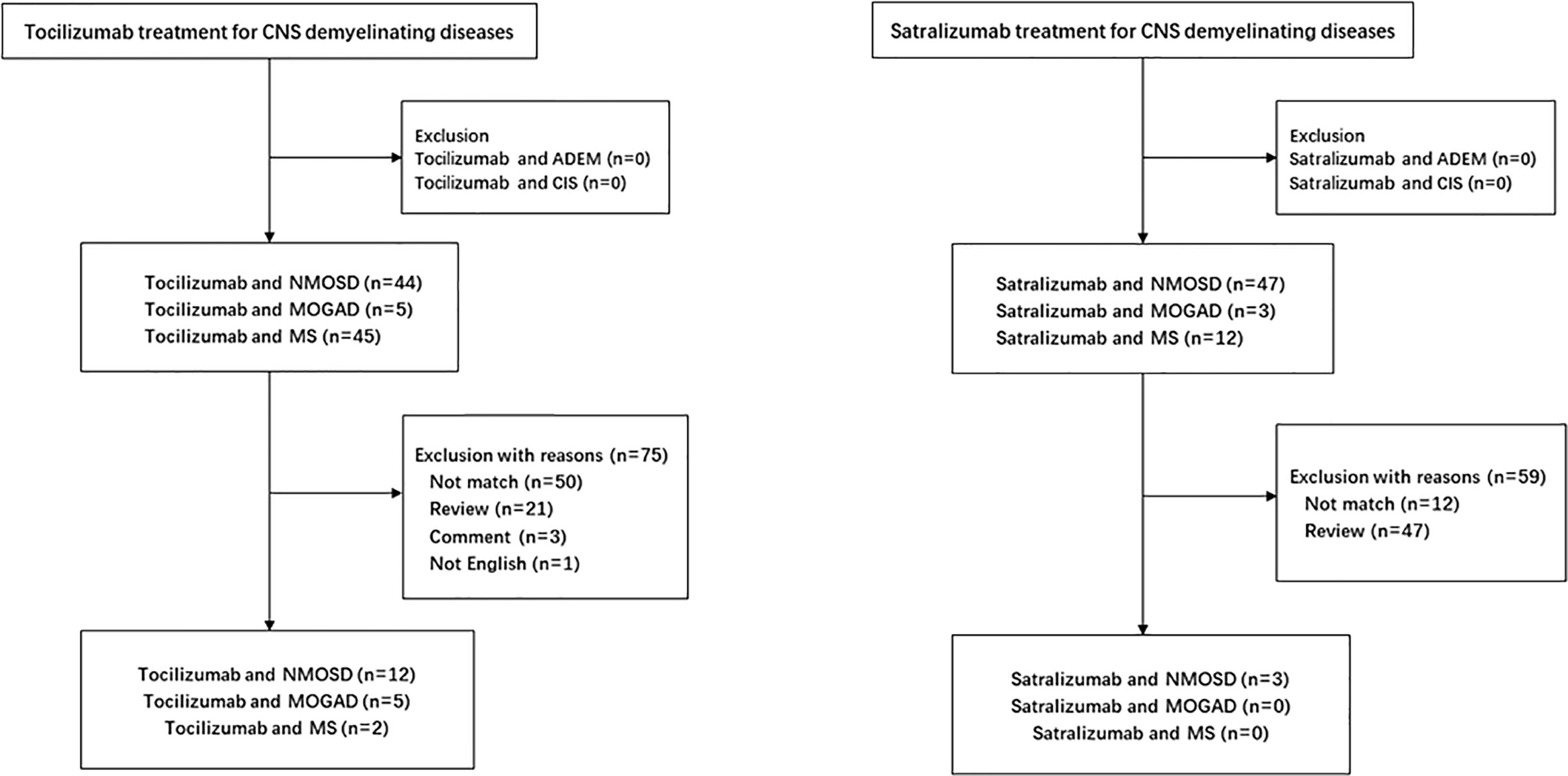
Growing evidence supports that IL-6 plays a key role in the pathophysiology of NMOSD. In vitro, dysregulated IL-6 activity leads to reduced blood–brain barrier function, increased leukocyte migration, enhanced chemokine production, and activation of AQP4 antibody secretion (32, 63, 73). In vivo, serum and CSF levels of IL-6 were found to be elevated in relapsing patients with NMOSD (33, 36, 66). Several studies report the efficacy and safety of IL-6 receptor inhibitor therapy in the treatment of NMOSD (Figure 1, Table 1).
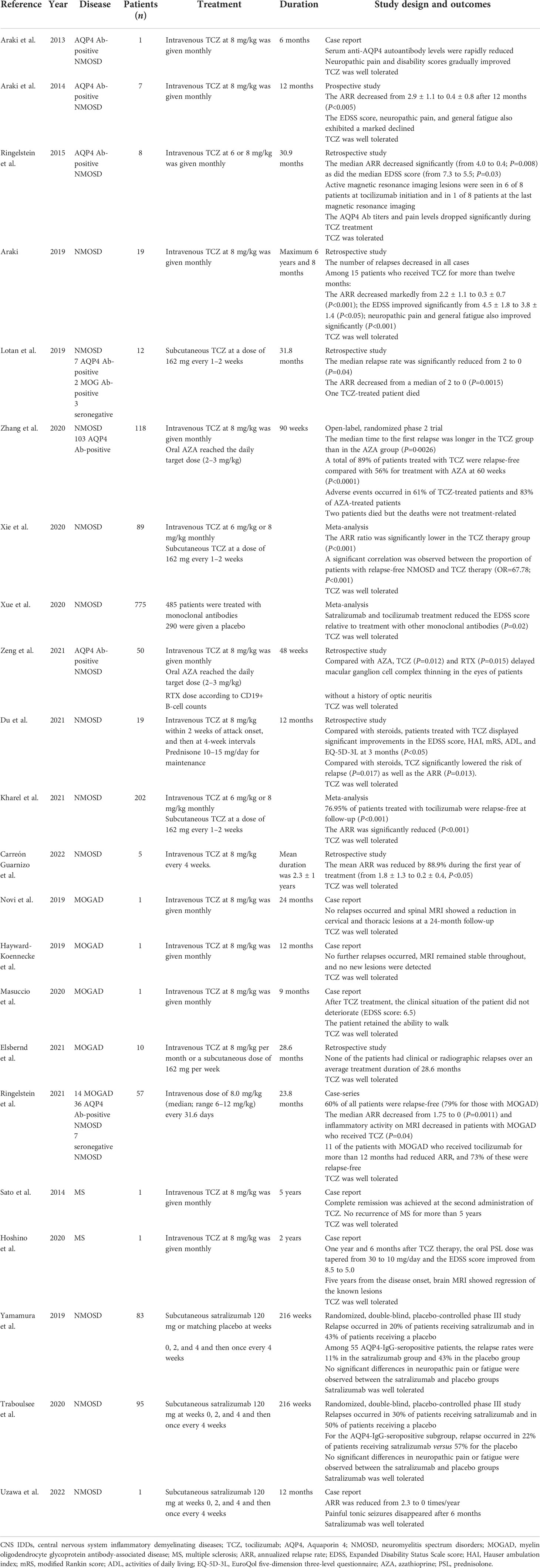
Table 1 Summary of the main clinical trials on tocilizumab and satralizumab in central nervous system inflammatory demyelinating diseases.
The pathogenic effect of IL-6 signaling in MS may be exerted through the induction of IL-17-producing T cells. The effect of lL-6 inhibition in MS treatment is unclear. Evidence from mouse models of MS (experimental autoimmune encephalomyelitis) indicates that Th17 cells play an important role in MS progression (74, 75). In the mouse, IL-6 mainly induce the differentiation of naive CD4-positive T cells into Th17 cells, whereas anti-IL-6 therapy effectively suppresses the onset of experimental autoimmune encephalomyelitis via the inhibition of the development of autoantigen-specific Th17 cells. In humans, the data are limited to a few studies. One patient with RA and MS received tocilizumab for more than 5 years without exacerbation of MS (62), whereas another RA patient developed MS during tocilizumab treatment (76). Although this result indicates that IL-6 inhibition might trigger inflammatory demyelination in the CNS, elevated levels of the soluble IL-6 receptor, an indirect marker for reduced IL-6 signaling, were found to be significantly correlated with a reduced risk of MS. This strongly suggests that IL-6 receptor inhibitor therapy may be suitable for use in MS treatment (77). Additionally, there are two recent case reports of patients with MS who have been successfully treated with tocilizumab (62, 63) (Figure 1, Table 1). Accordingly, the role of IL-6 in MS pathogenesis and the efficacy of IL-6 receptor inhibitors in MS treatment merit further evaluation.
Biopsy and autopsy data demonstrate complement and immunoglobulin deposition in demyelinating lesions of patients with MOGAD, indicative of a significant humoral immune component similar to that seen in AQP4 antibody-positive NMOSD (5, 15, 78). Although NMOSD and MOGAD have different underlying pathogenic mechanisms, they are both characterized by elevated levels of IL-6 in serum and CSF, especially during relapses (57, 79–83). The similarity in CSF cytokine profiles provides promising therapeutic options for NMOSD and MOGAD using IL-6 inhibitors. To date, relatively few reports have assessed the efficacy of tocilizumab treatment on patients with MOGAD (48, 53–57, 82, 83), (Figure 1, Table 1), and further studies are needed to confirm the efficacy and safety of anti-IL-6 receptor therapy in the treatment of this disease.
Intravenous or subcutaneous tocilizumab
Lotan et al. (48) reports 12 NMOSD patients who received at least 6 months of subcutaneous tocilizumab. The ARR decreased from a median of 2 (5.75–1.29) before subcutaneous tocilizumab to 0 (1.0–0) (P=.0015) after treatment. The efficacy of subcutaneous tocilizumab in NMOSD was similar to that of the intravenous formulation.
One case series reports seven patients (four with NMOSD and three with MOGAD), all of whom first received tocilizumab by intravenous injection; subsequently, three patients switched to the subcutaneous form of administration. None of these patients relapsed during tocilizumab treatment (59). Another case series describes 10 patients with relapsing MOGAD treated with tocilizumab, including six by intravenous injection, two by subcutaneous injection, and two who changed from intravenous to subcutaneous injection. All the patients were relapse-free during 28.6 months of follow-up (60), indicating that intravenous and subcutaneous tocilizumab have similar clinical efficacy. Similar results were reported by Lotan et al. (48).
The use of subcutaneous injection should be encouraged given its advantage of at-home administration. Prospective studies of subcutaneous tocilizumab treatment for CNS IDDs are warranted.
The usage of IL-6 receptor antagonists in pregnancy
Hoeltzenbein et al. undertook an analysis of a global safety database containing data for 399 women exposed to tocilizumab shortly before or during pregnancy, 288 of whom reported pregnancy outcomes. Of these 288 pregnancies, 60.6% resulted in live births, 21.7% in spontaneous abortions, 17.2% in elective terminations, and one in stillbirth. The rate of deformity was 4.5%. Although the rate of preterm birth increased (31.2%) compared with the general population, no substantial increase in the risk for deformity was observed (84). In a retrospective analysis from Japan, the authors analyzed pregnancy outcomes in patients with rheumatic disease exposed to tocilizumab. No increased risk of spontaneous abortion or congenital malformation was found in 61 pregnancies (85).
Data regarding the safety of tocilizumab during pregnancy and breastfeeding among patients with RA are limited, and these patients are advised to stop tocilizumab treatment 3 months before conception (86). In NMOSD, experts advise that tocilizumab can be used by pregnant women with severe disease, whereas breastfeeding may be considered, but only under close monitoring (87). The effects of satralizumab treatment on pregnancy outcomes are unknown.
Analysis of the cost effectiveness of IL-6 receptor antagonists
A cost-utility analysis of tocilizumab in the treatment of patients with RA in Japan showed that quality-adjusted life years (QALYs) and lifetime costs of tocilizumab were approximately 1.3- and 1.5-fold higher, respectively, compared with those for methotrexate. The incremental cost per QALY for tocilizumab treatment was reported to be USD 49,359, which was below the assumed cost-effectiveness threshold of USD 50,000 per QALY (88). These findings indicated that tocilizumab may be cost-effective in the treatment of RA.
A cost-effectiveness analysis of tocilizumab in patients with RA from the United Kingdom, Greece, and Italy showed similar results; namely, that tocilizumab, used either as a first-line biologic monotherapy or an addition to the standard treatment strategy, can be a cost-effective option for the treatment of patients with RA who have not adequately responded to conventional drugs (89–91).
No economic evaluation of tocilizumab and satralizumab therapy in the treatment of CNS IDDs has been undertaken to date. An assessment of the cost-effectiveness of IL-6 receptor inhibitors is warranted in the near future.
Finally, the cost of satralizumab in the United States is USD 219,231 for the first year, subsequently decreasing to USD 190,000 per year (92). The high costs of these new drugs limit their usage in low-income countries, and finding ways of providing these drugs at more reasonable prices constitutes a major challenge for the governments of these countries.
Author contributions
LJ conceived and wrote the manuscript, SG read and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 82072079).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol (2019) 15:89–102. doi: 10.1038/s41582-018-0112-x
2. Mader S, Kümpfel T, Meinl E. Novel insights into pathophysiology and therapeutic possibilities reveal further differences between AQP4-IgG- and MOG-IgG-associated diseases. Curr Opin Neurol (2020) 33:362–71. doi: 10.1097/WCO.0000000000000813
3. Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e163. doi: 10.1212/NXI.0000000000000163
4. Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients part 1: Frequency, syndrome specifcity, infuence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinfammation. (2016) 13:279. doi: 10.1186/s12974-016-0717-1
5. Höftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. (2020) 139:875–92. doi: 10.1007/s00401-020-02132-y
6. Barnes MP, Bateman DE, Cleland PG, Dick DJ, Walls TJ, Newman PK, et al. Intravenous methylprednisolone for multiple sclerosis in relapse. J Neurol Neurosurg Psychiatry (1985) 48:157–9. doi: 10.1136/jnnp.48.2.157
7. Durelli L, Cocito D, Riccio A, Barile C, Bergamasco B, Baggio GF, et al. High-dose intravenous methylprednisolone in the treatment of multiple sclerosis: clinical-immunologic correlations. Neurology (1986) 36:238–43. doi: 10.1212/WNL.36.2.238
8. Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. clinical effects. J Neurol Neurosurg Psychiatry (1987) 50:511–6. doi: 10.1136/jnnp.50.5.511
9. Sellebjerg F, Frederiksen JL, Nielsen PM, Olesen J. Double-blind, randomized, placebo-controlled study of oral, high-dose methylprednisolone in attacks of MS. Neurology (1998) 51:529–34. doi: 10.1212/WNL.51.2.529
10. Weiner HL, Dau PC, Khatri BO, Petajan JH, Birnbaum G, McQuillen MP, et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosupression for acute attacks of multiple sclerosis. Neurology (1989) 38:1143–9. doi: 10.1212/WNL.39.9.1143
11. Weinshenker BG, O'Brien PC, Petterson TM, Noseworthy JH, Lucchinetti CF, Dodick DW, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol (1999) 46:878–86. doi: 10.1002/1531-8249(199912)46:6<878::AID-ANA10>3.0.CO;2-Q
12. Llufriu S, Castillo J, Blanco Y, Ramió-Torrentà L, Río J, Vallès M, et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology (2002) 58:143–6. doi: 10.1212/WNL.58.1.143
13. Feasby T, Banwell B, Benstead T, Bril V, Brouwers M, Freedman M, et al. Guidelines on the use of intravenous immune globulin for neurologic conditions. Transfus Med Rev (2007) 21:S57–S107. doi: 10.1016/j.tmrv.2007.01.002
14. Elsone L, Panicker J, Kerry M, Boggild M, Appleton R, Jacob A. Role of intravenous immunoglobulin in the treatment of acute relapses of neuromyelitis optica: experience in 10 patients. Mult Scler J (2014) 20:501–4. doi: 10.1177/1352458513495938
15. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: Acute, preventive, and symptomatic. Curr Treat Options Neurol (2016) 18:2. doi: 10.1007/s11940-015-0387-9
16. Kimbrough DJ, Fujihara K, Jacob A, Jacob A, Lana-Peixoto MA, Leite MI, et al. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord (2012) 1:180–7. doi: 10.1016/j.msard.2012.06.002
17. Prasad S, Chen J. What you need to know about AQP4, MOG, and NMOSD. Semin Neurol (2019) 39:718–31. doi: 10.1055/s-0039-3399505
18. Borisow N, Mori M, Kuwabara S, Scheel M, Paul F. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol (2018) 9:888. doi: 10.3389/fneur.2018.00888
19. Torres J, Pruitt A, Balcer L, Galetta S, Markowitz C, Dahodwala N. Analysis of the treatment of neuromyelitis optica. J Neurol Sci (2015) 351:31–5. doi: 10.1016/j.jns.2015.02.012
20. Sato D, Callegaro D, Lana-Peixoto MA, Fujihara K. Treatment of neuromyelitis optica: an evidence based review. Arq Neuropsiquiatr. (2012) 70:59–66. doi: 10.1590/S0004-282X2012000100012
21. Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin cases (2015) 3:545–55. doi: 10.12998/wjcc.v3.i7.545
22. Collongues N, Ayme-Dietrich E, Monassier L, de Seze J. Pharmacotherapy for neuromyelitis optica spectrum disorders: Current management and future options. Drugs (2019) 79:125–42. doi: 10.1007/s40265-018-1039-7
23. Paul F, Murphy O, Pardo S, Levy M. Investigational drugs in development to prevent neuromyelitis optica relapses. Expert Opin Investig Drugs (2018) 27:265–71. doi: 10.1080/13543784.2018.1443077
24. Kleiter I, Gold R. Present and future therapies in neuromyelitis optica spectrum disorders. Neurotherapeutics (2016) 13:70–83. doi: 10.1007/s13311-015-0400-8
25. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
26. Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias GD, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med (1998) 187:461–8. doi: 10.1084/jem.187.4.461
27. Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol (2000) 164:4878–82. doi: 10.4049/jimmunol.164.9.4878
28. Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discovery (2018) 17:395–412. doi: 10.1038/nrd.2018.45
29. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity (2019) 50:1007–23. doi: 10.1016/j.immuni.2019.03.026
30. Tanaka T, Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci (2012) 8:1227–36. doi: 10.7150/ijbs.4666
31. Zhang J, Sadowska GB, Chen XD, Park SY, Kim JE, Bodge CA, et al. Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J (2015) 29:1739–53. doi: 10.1096/fj.14-258822
32. Chihara N, Aranami T, Sato W, Miyazaki YS, Miyake S, Okamoto T, et al. Interleukin 6 signaling promotes anti–aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci USA (2011) 108:3701–6. doi: 10.1073/pnas.1017385108
33. Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. (2010) 16:1443–52. doi: 10.1177/1352458510379247
34. Içöz S, Tüzün E, Kürtüncü M, Durmuş H, Mutlu M, Eraksoy M, et al. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int J Neurosci (2010) 120:71–5. doi: 10.3109/00207450903428970
35. Wei YZ, Chang HX, Li XD, Wang HB, Du L, Zhou H, et al. Cytokines and tissue damage biomarkers in first-onset neuromyelitis optica spectrum disorders: Significance of interleukin-6. Neuroimmuno- modulation. (2018) 25:215–24. doi: 10.1159/000494976
36. Uzawa A, Mori M, Sawai S, Masuda S, Muto M, Uchida T, et al. Cerebrospinal fluid interleukin-6 and glial fibrillary acidic protein levels are increased during initial neuromyelitis optica attacks. Clin Chim Acta (2013) 421:181–3. doi: 10.1016/j.cca.2013.03.020
37. Wang H, Wang K, Zhong XN, Dai YQ, Qiu W, Wu A, et al. Notable increased cerebrospinal fluid levels of soluble interleukin-6 receptors in neuromyelitis optica. Neuroimmunomodulation (2012) 19:304–8. doi: 10.1159/000339302
38. Uzawa A, Mori M, Ito M, Uchida T, Hayakawa S, Masuda S, et al. Markedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosis. J Neurol (2009) 256:2082–4. doi: 10.1007/s00415-009-5274-4
39. Bongioanni P, Mosti S, Romano MR, Lombardo F, Moscato G, Meucci G. Increased T-lymphocyte interleukin-6 binding in patients with multiple sclerosis. Eur J Neurol (2000) 7:291–7. doi: 10.1046/j.1468-1331.2000.00075.x
40. Chen YC, Yang X, Miao L, Liu ZG, Li W, Zhao ZX, et al. Serum level of interleukin-6 in Chinese patients with multiple sclerosis. J Neuroimmunol. (2012) 249:109–11. doi: 10.1016/j.jneuroim.2012.04.015
41. Kallaur AP, Oliveira SR, Colado Simao AN, Delicato de Almeida ER, Kaminami Morimoto H, Lopes J, et al. Cytokine profile in relapsing-remitting multiple sclerosis patients and the association between progression and activity of the disease. Mol Med Rep (2013) 7:1010–20. doi: 10.3892/mmr.2013.1256
42. Kieseier BC, Stüve O, Dehmel T, Goebels N, Leussink VI, Mausberg AK, et al. Disease amelioration with tocilizumab in a treatment resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol (2013) 70:390–3. doi: 10.1001/jamaneurol.2013.668
43. Ayzenberg I, Kleiter I, Schröder A, Hellwig K, Chan A, Yamamura T, et al. Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol (2013) 70:394–7. doi: 10.1001/jamaneurol.2013.1246
44. Araki M, Aranami T, Matsuoka T, Nakamura M, Miyake S, Yamamura T. Clinical improvement in a patient with neuromyelitis optica following therapy with the anti–IL-6 receptor monoclonal antibody tocilizumab. Mod Rheumatol (2013) 23:827–31. doi: 10.3109/s10165-012-0715-9
45. Araki M, Matsuoka T, Miyamoto K, Kusunoki S, Okamoto T, Murata M, et al. Efficacy of the anti–IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology (2014) 82:1302–6. doi: 10.1212/WNL.0000000000000317
46. Ringelstein M, Ayzenberg I, Harmel J, Lauenstein AS, Lensch E, Stogbauer F, et al. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol (2015) 72:756–63. doi: 10.1001/jamaneurol.2015.0533
47. Araki M. Blockade of IL-6 signaling in neuromyelitis optica. Neurochem Int (2019) 130:104315. doi: 10.1016/j.neuint.2018.10.012
48. Lotan I, Charlson RW, Ryerson LZ, Levy M, Kister I. Effectiveness of subcutaneous tocilizumab in neuromyelitis optica spectrum disorders. Mult Scler Relat Disord (2019) 39:101920. doi: 10.1016/j.msard.2019.101920
49. Carreón Guarnizo E, Hernández Clares R, Castillo Triviño T, Meca Lallana V, Arocas Casañ V, Iniesta Martínez F, et al. Experience with tocilizumab in patients with neuromyelitis optica spectrum disorders. Neurologia (2022) 37:178–83. doi: 10.1016/j.nrleng.2018.12.021
50. Zhang C, Zhang M, Qiu W, Ma HS, Zhang XH, Zhu ZL, et al. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open-label, multicentre, randomised, phase 2 trial. Lancet Neurol (2020) 19:391–401. doi: 10.1016/S1474-4422(20)30070-3
51. Xie Q, Zheng T, Sun M, Sun J, Wang M. A meta-analysis to determine the efficacy and safety of tocilizumab in neuromyelitis optica spectrum disorders. Mult Scler Relat Disord (2020) 45:102421. doi: 10.1016/j.msard.2020.102421
52. Xue T, Yu JH, Chen SJ, Wang ZL, Yang YB, Chen ZQ, et al. Different targets of monoclonal antibodies in neuromyelitis optica spectrum disorders: A meta-analysis evidenced from randomized controlled trials. Front Neurol (2020) 11:604445. doi: 10.3389/fneur.2020.604445
53. Kharel S, Shrestha S, Ojha R, Guragain N, Ghimire R. Safety and efficacy of interleukin-6-receptor inhibitors in the treatment of neuromyelitis optica spectrum disorders: a meta-analysis. BMC Neurol (2021) 21:458. doi: 10.1186/s12883-021-02488-y
54. Du C, Zeng P, Han JR, Zhang TX, Jia D, Shi FD, et al. Early initiation of tocilizumab treatmentagainst moderate-to-severe myelitis in neuromyelitis optica spectrum disorder. Front Immunol (2021) 12:660230. doi: 10.3389/fimmu.2021.660230
55. Zeng P, Du C, Zhang R, Jia DM, Jiang F, Fan ML, et al. Optical coherence tomography reveals longitudinal changes in retinal damage under different treatments for neuromyelitis optica spectrum disorder. Front Neurol (2021) 12:669567. doi: 10.3389/fneur.2021.669567
56. Novi G, Gastaldi M, Franciotta D, Pesce G, Benedetti L, Uccelli A. Tocilizumab in MOG-antibody spectrum disorder: a case report. Mult Scler Relat Disord (2019) 27:312–4. doi: 10.1016/j.msard.2018.11.012
57. Hayward-Koennecke H, Reindl M, Martin R, Schippling S. Tocilizumab treatment in severerecurrent anti-MOG-associated optic neuritis. Neurology (2019) 92:765–7. doi: 10.1212/WNL.0000000000007312
58. Masuccio FG, Lo Re M, Bertolotto A, Capobianco M, Solaro C. Benign SARS-CoV-2 infection in MOG-antibodies associated disorder during tocilizumab treatment. Mult Scler Relat Disord (2020) 46:102592. doi: 10.1016/j.msard.2020.102592
59. Rigal J, Pugnet G, Ciron J, Lépine Z, Biotti D. Off-label use of tocilizumab in neuromyelitis optica spectrum disorders and MOG-antibody-associated diseases: A case-series. Mult Scler Relat Disord (2020) 46:102483. doi: 10.1016/j.msard.2020.102483
60. Elsbernd PM, Hoffman WR, Carter JL, Wingerchuk DM. Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): A case-series and review. Mult Scler Relat Disord (2021) 48:102696. doi: 10.1016/j.msard.2020.102696
61. Ringelstein M, Ayzenberg I, Lindenblatt G, Fischer K, Gahlen A, Novi G, et al. Interleukin-6 receptor blockade in treatment-refractory MOG-IgG-Associated disease and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. (2021) 9:e1100. doi: 10.1212/NXI.0000000000001100
62. Sato H, Kobayashi D, Abe A, Ito S, Ishikawa H, Nakazono K, et al. Tocilizumab treatment safety in rheumatoid arthritis in a patient with multiple sclerosis: a case report. BMC Res Notes. (2014) 7:641. doi: 10.1186/1756-0500-7-641
63. Hoshino H, Shirai Y, Konishi H, Yamamura T, Shimizu N. Efficacy of tocilizumab for fulminant multiple sclerosis with a tumefactive cervical lesion: a 12-year-old boy. Mult Scler Relat Disord (2019) 37:101460. doi: 10.1016/j.msard.2019.101460
64. Fujihara K, Bennett JL, de Seze J, Haramura M, Kleiter I, Weinshenker BG, et al. Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e841. doi: 10.1212/NXI.0000000000000841
65. Uzawa A, Mori M, Kuwabara S. Cytokines and chemokines in neuromyelitis optica: pathogenetic and therapeutic implications. Brain Pathol (2014) 24:67–73. doi: 10.1111/bpa.12097
66. Melamed E, Levy M, Waters PJ, Sato DK, Bennett JL, John GR, et al. Update on biomarkers in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e134. doi: 10.1212/NXI.0000000000000134
67. Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med (2019) 381:2114–24. doi: 10.1056/NEJMoa1901747
68. Traboulsee A, Greenberg BM, Bennett JL, Szczechowski L, Fox E, Shkrobot S, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlledphase 3 trial. Lancet Neurol (2020) 19:402–12. doi: 10.1016/S1474-4422(20)30078-8
69. Paton DM. Satralizumab: an interleukin-6 (IL-6) receptor antagonist for the treatment of neuromyelitis optica spectrum disorders. Drugs Today (Barc). (2021) 57:209–18. doi: 10.1358/dot.2021.57.3.3251715
70. Uzawa A, Mori M, Iwai Y, Masuda H, Kuwabara S. Complete relief of painful tonic seizures in neuromyelitis optica spectrum disorder by satralizumab treatment. Intern Med (2022) 61:2785–87. doi: 10.2169/internalmedicine.9036-21
71. Duchow A, Bellmann-Strobl J. Satralizumab in the treatment of neuromyelitis optica spectrum disorder. Neurodegener Dis Manage (2021) 11:49–59. doi: 10.2217/nmt-2020-0046
72. Duchow A, Paul F, Bellmann-Strobl J. Current and emerging biologics for the treatment of neuromyelitis optica spectrum disorders. Expert Opin Biol Ther (2020) 20:1061–72. doi: 10.1080/14712598.2020.1749259
73. Carnero Contentti E, Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflammat (2021) 18:208. doi: 10.1186/s12974-021-02249-1
74. Fujimoto M, Serada S, Mihara M, Uchiyama Y, Yoshida H, Koike N, et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheumatol (2008) 58:3710–9. doi: 10.1002/art.24126
75. Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. (2008) 105:9041–6. doi: 10.1073/pnas.0802218105
76. Beauchemin P, Carruthers R. MS arising during tocilizumab therapy for rheumatoid arthritis. Mult Scler. (2016) 22:254–6. doi: 10.1177/1352458515623862
77. Zhang HH, Wang T, Han ZF, Liu GY. Mendelian randomization study to evaluate the effects of interleukin-6 signaling on four neurodegenerative diseases. Neurol Sci (2020) 41:2875–82. doi: 10.1007/s10072-020-04381-x
78. Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain (2020) 143:1431–46. doi: 10.1093/brain/awaa102
79. Kwon YN, Kim B, Ahn S, Seo J, Kim SB, Yoon SS, et al. Serum level of IL-1β in patients with inflammatory demyelinating disease: Marked upregulation in the early acute phase of MOG antibody associated G disease (MOGAD). J Neuroimmunol. (2020) 348:577361. doi: 10.1016/j.jneuroim.2020.577361
80. Kaneko K, Sato DK, Nakashima I, Ogawa R, Akaishi T, Takai Y, et al. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: a cross-sectional study and potential therapeutic implications. J Neurol Neurosurg Psychiatry (2018) 89:927–36. doi: 10.1136/jnnp-2018-317969
81. Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology (2014) 82:474–81. doi: 10.1212/WNL.0000000000000101
82. Breu M, Glatter S, H¨oftberger R, Freilinger M, Kircher K, Kasprian G, et al. Two cases of pediatric AQP4-antibody positive neuromyelitis optica spectrum disorder successfully treated with tocilizumab. Neuropediatrics (2019) 50:193–6. doi: 10.1055/s-0039-1684004
83. Schwake C, Hellwig K, Gold R, Ayzenberg I. Reader response: comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann Neurol (2020) 88:430. doi: 10.1002/ana.25805
84. Hoeltzenbein M, Beck E, Rajwanshi R, Gøtestam Skorpen C, Berber E, Schaefer C, et al. Tocilizumab use in pregnancy: Analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheumatol (2016) 46:238–45. doi: 10.1016/j.semarthrit.2016.05.004
85. Nakajima K, Watanabe O, Mochizuki M, Nakasone A, Ishizuka N, Murashima A. Pregnancy outcomes after exposure to tocilizumab: a retrospective analysis of 61 patients in Japan. Mod Rheumatol (2016) 26:667–71. doi: 10.3109/14397595.2016.1147405
86. Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol (2016) 12:937–44. doi: 10.1080/1744666X.2016.1184973
87. Mao-Draayer Y, Thiel S, Mills EA, Chitnis T, Fabian M, Katz SI, et al. Neuromyelitis optica spectrum disorders and pregnancy: therapeutic considerations. Nat Rev Neurol (2020) 16:154–70. doi: 10.1038/s41582-020-0313-y
88. Tanaka E, Inoue E, Hoshi D, Shimizu Y, Kobayashi A, Sugimoto N, et al. Cost-effectiveness of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, versus methotrexate in patients with rheumatoid arthritis using real-world data from the IORRA observational cohort study. Mod Rheumatol (2015) 25:503–13. doi: 10.3109/14397595.2014.1001475
89. Diamantopoulos A, Finckh A, Huizinga T, Sungher DK, Sawyer L, Neto D, et al. Tocilizumab in the treatment of rheumatoid arthritis: a cost-effectiveness analysis in the UK. Clin Exp Rheumatol (2018) 36:479–85. doi: 10.1007/s40273-014-0165-7
90. Athanasakis K, Tarantilis F, Tsalapati K, Konstantopoulou T, Vritzali E, Kyriopoulos J. Cost-utility analysis of tocilizumab monotherapy in first line versus standard of care for the treatment of rheumatoid arthritis in Greece. Rheumatol Int (2015) 35:1489–95. doi: 10.1007/s00296-015-3253-x
91. Diamantopoulos A, Benucci M, Capri S, Berger W, Wintfeld N, Giuliani G, et al. Economic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in Italy. J Med Econ (2012) 15:576–85. doi: 10.3111/13696998.2012.665110
Keywords: IL-6, central nervous system inflammatory demyelinating diseases, tocilizumab, satralizumab, monoclonal antibodies
Citation: Jiao L and Guo S (2022) Anti-IL-6 therapies in central nervous system inflammatory demyelinating diseases. Front. Immunol. 13:966766. doi: 10.3389/fimmu.2022.966766
Received: 11 June 2022; Accepted: 20 September 2022;
Published: 27 October 2022.
Edited by:
Raphaela Goldbach-Mansky, National Institutes of Health (NIH), United StatesReviewed by:
Honghao Wang, Guangzhou First People’s Hospital, ChinaVictor Rivera, Baylor College of Medicine, United States
Copyright © 2022 Jiao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shougang Guo, c2hvdWdhbmdndW8xMTI0QDE2My5jb20=
 Li Jiao1
Li Jiao1 Shougang Guo
Shougang Guo