- Department of Dermatology, Hunan Key Laboratory of Medical Epigenomics, The Second Xiangya Hospital, Central South University, Changsha, China
The aryl hydrocarbon receptor was previously known as an environmental receptor that modulates the cellular response to external environmental changes. In essence, the aryl hydrocarbon receptor is a cytoplasmic receptor and transcription factor that is activated by binding to the corresponding ligands, and they transmit relevant information by binding to DNA, thereby activating the transcription of various genes. Therefore, we can understand the development of certain diseases and discover new therapeutic targets by studying the regulation and function of AhR. Several autoimmune diseases, including systemic lupus erythematosus (SLE), have been connected to AhR in previous studies. SLE is a classic autoimmune disease characterized by multi-organ damage and disruption of immune tolerance. We discuss here the homeostatic regulation of AhR and its ligands among various types of immune cells, pathophysiological roles, in addition to the roles of various related cytokines and signaling pathways in the occurrence and development of SLE.
1 Introduction
The aryl hydrocarbon receptor (AhR) was first studied as an environmental toxicant sensor (1). It is a cytoplasmic receptor and transcription factor that relies primarily on ligand activation and subsequently regulates the transcription of many genes by binding to DNA. The AhR pathway is critical in the regulation of innate and adaptive immunity, it’s well established that both AhR agonists and antagonists can affect the immune system. Previous studies have shown that AhR is associated with many autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, SLE, and so on (2). By elucidating the role of AhR in autoimmune diseases, researchers can enhance their understanding of the pathogenesis of autoimmune diseases and develop new therapeutic targets. SLE is a classic autoimmune disease characterized by a dysregulation of immune tolerance leading to excessive inflammatory response and multi-organ damage. In this paper, we summarize the homeostatic regulation and pathophysiological roles of AhR and its ligands among various types of immune cells, along with the roles of various related cytokines and signaling pathways in the development of SLE.
2 About AhR
2.1 Structure and function of AhR
AhR is a class of ligand-dependent activating transcription factors belonging to the basic helix-loop-helix Per-Arnt-Sim (bHLH/PAS) superfamily that regulates the transcriptional expression of genes. The inactive AhR forms a complex with the 90-kDa heat shock protein (Hsp90) (3), the hepatitis B virus X-associated protein 2 (XAP2) (4), the protein tyrosine kinase c-Src (5) and the cochaperone p23 (6) in the cytoplasm. These molecular chaperones help to localize inactive AhR in the cytoplasm, protect it from degradation, and allow ligand binding to AhR to occur easily and consistently. As AhR binds to the ligand, conformational changes occur, and they form complexes with aryl hydrocarbon receptor nuclear transporter protein(ARNT), allowing them to enter the nucleus. The genomic regulatory region of AhR target genes contains specific DNA sequences (5’-TNGCTGG-3’) called dioxin or xenobiotic response elements (XRE). In the nucleus, the AhR-ARNT complex recognizes XRE, it controls the expression of multiple target genes through interaction with other transcription factors, such as CYP1A1, CYP1B1, and aryl hydrocarbon receptor repressor (AhRR) (7). AhR also controls gene expression through non-XRE DNA-responsive elements by interacting with other transcription factors in a similar way, such as nuclear factor-κB (NF-κB), c-Maf, retinoic acid receptor, estrogen receptor (ER), and retinoblastoma (Rb). AhR acts as a regulator of their activity, and with it the expression of their target genes (8). In conclusion, AhR promotes the expression of genes in regulatory regions, in addition to XRE, by recruiting new DNA sequences or by interacting with certain proteins (See Figure 1 on the AhR signaling pathway for details).
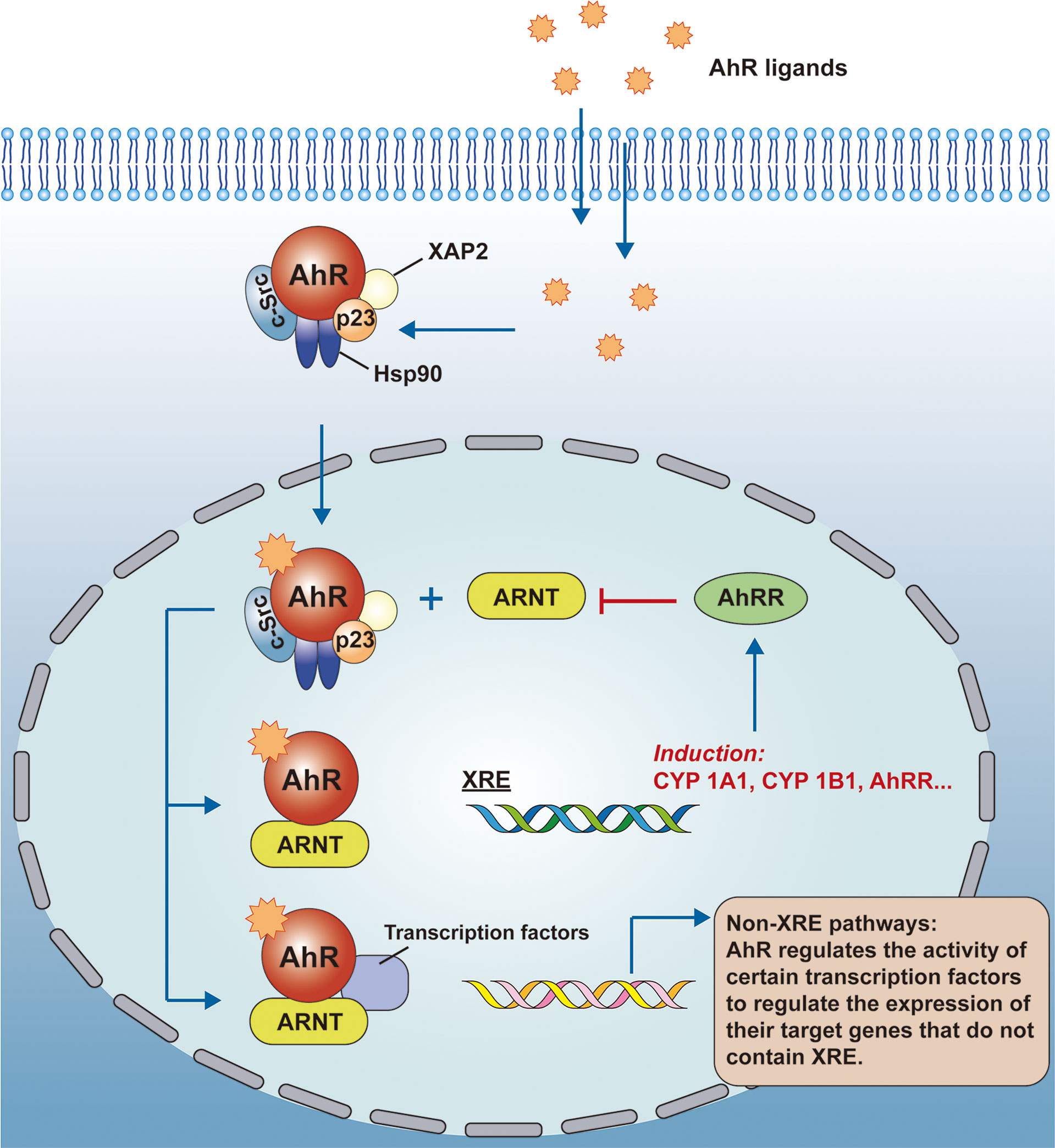
Figure 1 AhR signaling pathway. AhR is a ligand-activated transcription factor and inactive AhR forms a complex with Hsp90, XAP2, c-Src, and p23 in the cytoplasm. As AhR binds to the ligand, conformational changes occur, and they form complexes with the ARNT to enter the nucleus. the genomic regulatory region of AhR target genes contains the XRE, and in the nucleus, besides recognizing the XRE, the AhR-ARNT complex also interacts with other transcriptional regulators to control the expression of multiple target genes. AhR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon receptor nuclear transport protein; XRE, exogenous response element; CYP, cytochrome P450.
2.2 AhR is affected by multiple endogenous and exogenous factors
AhR is known as an environmental receptor and its ligands are very diverse that can be broadly classified into exogenous and endogenous according to their sources. Exogenous ligands are mainly from environmental toxins, including dioxins (9), tetrachlorodibenzo-p-dioxin(TCDD), polychlorinated biphenyl (PCB), polycyclic aromatic hydrocarbon (PAHs) (10), etc. The binding of PAHs to AhR/ARNT induces the expression of several CYPs that convert PAHs into genotoxic pro-electron derivatives. Foods are also rich in natural ligands (e.g., polyphenols, resveratrol (11), quercetin (12), flavonoids (13), etc.) and also include drugs such as cyproheptadine (14), leflunomide (15), and omeprazole (16).
The endogenous ligands mainly include tryptophan derivatives, such as FICZ (17), indole-3-carbinol (I3C) (18), indole-3-acetic acid (IAA) (19) and 2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) (20). Also, there are prostaglandins (PGB3, PGD3, PGF3alpha, PGG2, PGH1, PGH2) (21), indocyanine and indigo (22), bilirubin and biliverdin (23), etc. Tryptophan and its derivatives provide a large number of ligands for AhR, and we are familiar with kynurenine (Kyn) as an agonist of AhR (24), while AhR regulates the expression and activation of indoleamine 2,3-dioxygenase (IDO), tryptophan 2,3-dioxygenase (TDO2), kynurenase (KYNU), etc. These enzymes regulate the metabolism of Kyn, which precisely forms a feedback loop. Among them, FICZ, fully known as 6-formylindolo[3,2-b]carbazole, is the endogenous agonist that binds most tightly to AhR (25). See Table 1 on the classification of factors that can affect AhR for details. Notably, Kyn and the entire tryptophan family are regulated by the mechanistic target of rapamycin (mTOR), a serine/threonine kinase. mTOR is named after rapamycin, an antifungal macrolide antibiotic. mTOR activation and its pro-inflammatory effects profoundly influence the development of SLE, and there is an extensive link between mTOR and AhR signaling pathway, which will be described in 5.5.
3 Relevance of AhR to immune cells
3.1 AhR in dendritic cells
Dendritic cells (DCs) are the major antigen-presenting cells in human body. Monocytes enter tissues and differentiate into macrophages or dendritic cells. This differentiation process is regulated by several transcription factors. In the presence of AhR activation, monocyte-derived DCs differentiation is promoted through induction of BLIMP-1 (33). However, AhR is controversial in inducing the differentiation of DCs, which have several subsets: conventional DCs (cDCs) and plasmacytoid dendritic cells (pDCs) (34). TCDD can induce AhR expression and activation in DCs to enhance DCs differentiation (35), these experiments also demonstrated that the AhR pathway functioned as an important signaling pathway for the activation of indoleamine 2,3-dioxygenase 1(IDO1) and indoleamine 2,3-dioxygenase 2(IDO2) expression. IDO1 and tryptophan 2,3-dioxygenase-2 (TDO2) promote the production of the endogenous AhR ligand Kyn and generate DCs. In another study, in the presence of lipopolysaccharide or CpG, AhR negatively regulates DCs-mediated immune responses through a Kyn-dependent mechanism, which in turn affects the differentiation of primary T cells to regulatory T (Treg) cells and T helper 17 (Th17) cells (36).
AhR can also affect the antigen-presenting function of DCs (37). Jaishree Bankoti et al. reported that the AhR ligand TCDD decreased the expression of CD11c on the surface of bone marrow-derived dendritic cells (BMDCs) but increased the levels of MHC II and CD86 on BMDCs. The binding of CD86 molecules on DCs to CTLA-4 on T cells can impair T cell responses (38). As a consequence of AhR activation, CTLA-4 can be expressed on T cells, which binds tightly to CD86 on DCs, impairing T cell responses (39). As such, the attachment of CD86 on DCs to CTLA-4 on T cells, which is triggered by AhR activation, may contribute to immunosuppression. Comparatively, FICZ and ITE induced phenotypic changes similar to those seen in TCDD in BMDCs, indicating that DCs lack a particular response to AhR ligands.
3.2 AhR in macrophages
Macrophages are important components of intrinsic immunity, with roles such as phagocytosis and killing of pathogens, participation in the inflammatory response, and involvement in the regulation of adaptive immunity. Macrophages produce pro-inflammatory cytokines, such as IL-6, IL-12, and TNF-α, which activate T cells and induce their differentiation (40). Researchers have demonstrated that AhR suppresses the differentiation of monocytes and bone marrow-derived macrophages (33, 41). There are two subtypes of macrophages: M1 and M2 macrophages (42). FICZ can affect the balance between M1 and M2 macrophages by altering macrophage polarization through the activation of AhR (43). Yang et al. further demonstrated that AhR activation can reduce macrophage differentiation through the AhR-miR-142a-IRF1/HIF-1α pathway to reduce M1 macrophage polarization and promote M2 macrophage polarization (44).
In addition to affecting differentiation, AhR has other regulatory effects on macrophages, as Shinde et al. reported that macrophages exposed to apoptotic cells could activate AhR on their surface and promote the production of the immunosuppressive cytokine IL-10, thereby limiting the development of SLE in mice (45). After treatment with the endogenous AhR ligand FICZ and the exogenous ligand benzo-alpha-picryl, the abundance, ubiquitination, and phosphorylation of a variety of proteins in macrophages can be affected (46).
3.3 AhR in mast cells
Mast cells (MCs) contribute to the regulation of mucosal immune and allergic responses. Mast cells can be activated and release inflammatory mediators and cytokines by a variety of stimuli and have a surface that expresses a large number of IgE Fc receptors, which when bound to IgE and cross-linked to antigens can lead to a variety of pathophysiological events.
AhR is an important factor in mast cell activation. Exposure to the AhR ligand FICZ leads to activity reduction of the SHP-2 gene, enhancing the activation of mast cells by IgE (47, 48). Notably, different doses of FICZ stimulation lead to different changes in MCs. Sibilano R et al. reported that repeated exposure to FICZ inhibited MCs’ degranulation, the mechanism is that the release of histamine is FICZ dose-dependent, but is attenuated by repeated activation of AhR (49). Kyn can also activate mast cells by activating AhR to promote a series of reactions such as degranulation and leukotriene release (50, 51). Meanwhile, although AhR can regulate the function of MCs, experiments by Caroline Pilz et al. support that AhR does not have much role in regulating the number of MCs in mice (52).
The intricate relationship between MCs and T cells can serve as a fulcrum for regulating the balance between various types of T cells. On the other hand, in the presence of activated MCs, the Tregs/Th17 balance is tilted towards Th17 accompanied by a decrease in IL-6 abundance and a lack of Th1/Th2 cytokines (53), and this also study showed that MCs, as a source of inflammatory mediators, could counteract the suppressive effect of Treg cells on effector T cell-mediated immunity.
3.4 AhR in natural killer cells
Natural killer cells (NK cells) are derived from bone marrow lymphoid stem cells and, upon maturation, settle mainly in the spleen and liver (54). NK cells are innate lymphocytes capable of producing inflammatory cytokines that nonspecifically kill tumor cells and virally infected cells. In terms of NK cell development and differentiation, treatment of human embryonic stem cells (hESCs) with the AhR antagonist StemReginin-1(SR-1) and the AhR agonist TCDD, respectively, revealed that SR-1 increased the differentiation of NK cells while TCDD inhibited the development of them (55).
AhR has many important effects on NK cell function. Petr Bachleda et al. reasonably hypothesized that the effect of resveratrol on NK cytotoxicity is related to the partial agonistic activity of resveratrol on AhR (56), this hypothesis is based on the fact that resveratrol enhances perforin expression and NK cell cytotoxicity through the NKG2D-dependent pathway (57). Kyn can also enhance NK cell cytotoxicity by activating AhR (58). During infection, NK cells are one of the main sources of IL-10, and AhR activation is required for maximal IL-10 production (59). In hematologic cancer, AhR activation using FICZ not only improves the ability of NK cells to produce IFN-γ and cytolytic activity, but also enhances the ability of NK cells to inhibit cancer growth in an AhR-dependent manner (60).
3.5 AhR in T cells
3.5.1 AhR and CD4- CD8 -T (DN T) cells
The role between AhR and DN T has received less attention previously, but there is also a link between the two. Maturation of T cells from DN T to TCRαβ+ or TCRγδ+ cells occurs in the thymus, and the frequency of fetal DN TCRγδ+ cells is higher after activation of AhR using TCDD, suggesting that TCDD plays an important role in the differentiation and lineage commitment of DN T cells (61). Kyn stimulates the activation of mTOR complex 1 (mTORC1) in DN T cells of SLE patients (62).
3.5.2 AhR in T helper 1 (Th1) cells, T helper 2 (Th2) cells
It is believed that Th1 cells and Th2 cells antagonize each other in mediating immunity: Th1 mediates cellular immunity and promotes inflammation; Th2 cells mediate humoral immunity and inhibit inflammation (63).
AhR and Th1 cells are of interest in the body’s response to certain bacterial, viral, and parasitic infections. Th1-related immunity is initiated in vivo after Trypanosoma cruzi infection, and some AhR ligands have therapeutic effects, but Laura Fernanda Ambrosio et al. found that there is a threshold for AhR activation in this process. The use of higher affinity ligands (above threshold activation) limits CD8+ T cell development and promotes Treg cell development, enabling an earlier suppression of Th1-type responses. Conversely, the use of low-affinity ligands (below-threshold activation) promotes early inflammatory Th1-type responses, thereby limiting parasite replication. Thus, AhR induces multiple regulatory pathways that ultimately affect parasite replication and infection outcomes (64). Eliseu et al. reported that both AhR agonist Kyn and AhR antagonist CH223191 decreased the number of Th1 cells in a mice pulmonary fungal infection model, while agonist FICZ resulted in the expansion of all CD4+ T cell subsets (Th1, Th2, Th17, Th22, and Treg) (65). In addition, AhR-deficient mice displayed a decrease in Th1, Th22, and other immune cells in their lungs (66).
AhR is important as an environmental sensor in regulating the exposure of the organism to exogenous substances, a process that is closely related to Th2. V J Schulz et al. found that activation of AhR with TCDD can suppress Th2-mediated allergic responses by inducing differentiation of CD4+ T cell subpopulations to Treg cells (67). Exposure to ambient particulate matter (PM) (PAHs, dioxins, and heavy metals are the main components) can promote or exacerbate allergic responses by activating AhR in Th2 (68, 69) and enhanced Th2-mediated allergic response enhances the sensitivity of patients to allergens (70).
The imbalance between Th1/Th2 cells has been considered one of the pathogenesis of autoimmune diseases. AhR can regulate the Th1/Th2 balance by activating Th0 cells, and a synthetic anti-allergy agent M50367 (as AhR ligand) exerts anti-allergic effects by suppressing the differentiation of Th0 cells into Th2 cells in vitro, tilting the Th1/Th2 balance in favor of Th1 (71). Kakutani et al. demonstrated that low-dose, prolonged exposure to TCDD resulted in a dose-dependent significant increase in Th1 and Th2 lymphocyte responses, similarly shifting the Th1/Th2 balance towards Th1 (72).
3.5.3 AhR in Th17 and Treg cells
Th17 and Treg cells share a common precursor CD4+ T cell, and both require tumor growth factor (TGF)-β to induce differentiation. Nevertheless, their functions are opposite: Th17 cells tend to exacerbate autoimmune and inflammatory responses, while Treg cells suppress autoimmunity and maintain immune homeostasis (73).
AhR is expressed at the highest levels in human Th17 cells, and substantial evidence shows that AhR is necessary for IL-22 production and Th17 differentiation. It is important to note that AhR is unnecessary for the initial differentiation of Th17 cells, but is necessary to promote their expansion and production of IL-22 (74, 75). Many AhR ligands exhibit inhibitory effects on Th17 development and differentiation, such as TCDD, Kyn, I3C and diindolylmethane (DIM) (76–78). Some ligands block Th17-induced immune responses, such as curcumin and naphthoflavone (79, 80). It is thoroughly studied that FICZ can promote the differentiation of Th17 cells (81). Notably, Kyn has a double role, in addition to suppressing differentiation, it also promotes the differentiation of Th17 cells in a ligand-dependent manner, this may be related to the expression of the System L transporter on cells (82). I3C, although recognized to inhibit Th17 cell production and promote Treg production, increased Th17 cell expression in small intestinal cells in the non-obese diabetic (NOD) mice (83). Moreover, though AhR is unnecessary for Th17 differentiation, its activation promotes further function and differentiation of Th17, leading to the development of various autoimmune diseases dependent on Th17 cells.
Transcription factor Foxp3 drives the differentiation and function of Treg cells. AhR not only induced FoxP3 expression directly (76), but also stabilized FoxP3 expression by increasing enhancer activity (84). TCDD, I3C, DIM, ITE, and Kyn, as well as the recent discovery of baicalein and norisoboldine (NOR) (35, 74, 76, 85, 86), have been shown to promote Treg differentiation.
The regulation of the Treg/Th17 homeostasis by most ligands is unidirectional, that is, promoting one side and inhibiting the other (see Figure 2 for details). However, there are still some interesting results. TCDD activates AhR and induces the conversion of CD4+ Foxp3 T cells to functional Treg cells (76, 84). Interestingly, in the regulation of Th17, available evidence suggests that in vitro use of TCDD activates AhR to promote Th17 differentiation (87), but in vivo, TCDD leads to a decrease in Th17 numbers and suppresses experimental autoimmune encephalomyelitis (EAE) occurrence (76), this interesting phenomenon may be due to the fact that humans express the lower-affinity AhR (88), and the difference in affinity of AhR affects the amount of IL-17 and IL-22 produced by Th17 cells, and the number of these cytokines is significantly lower in the mouse model expressing the low affinity AhR. This different role of AhR in vivo and in vitro fully reflects the complexity of its pathway (87). The use of the AhR antagonist resveratrol interferes with the differentiation of both Treg and Th17 (76). Endogenous ligand FICZ promotes Th17 differentiation (74, 89), and its effect on Treg is controversial; some studies indicate that FICZ reduces the number of Treg cells (89), but more evidence suggests that FICZ promotes Treg production (66, 90). It was also shown that the tryptophan breakdown product Kyn (91) and the flavonoid compound alpinetin (92) increased Treg production but had no effect on Th17 cell production.
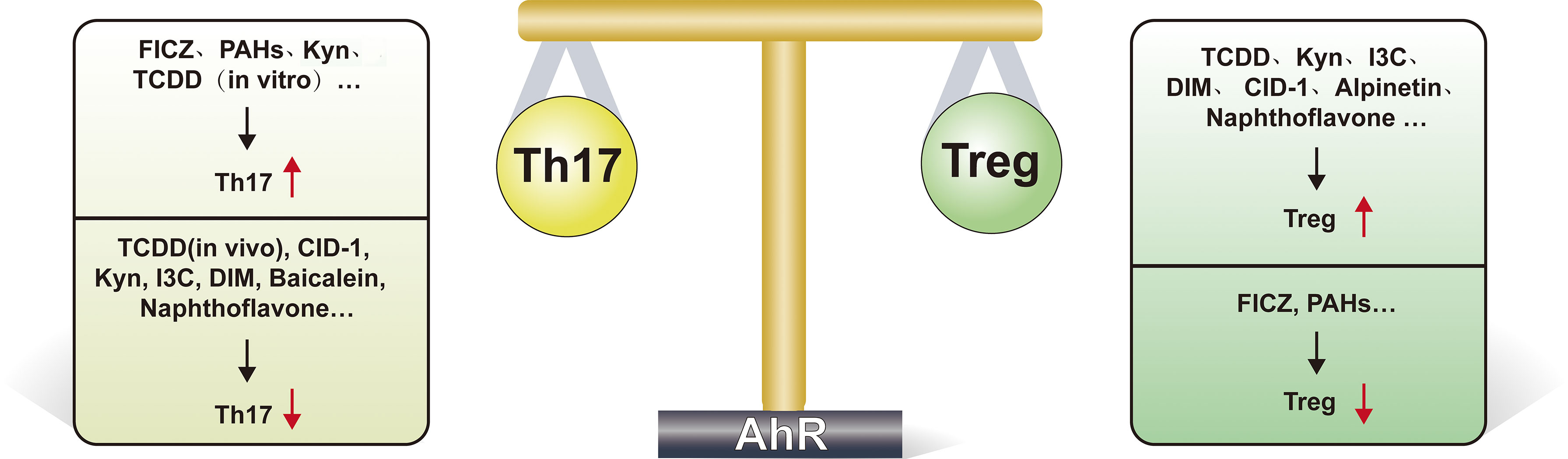
Figure 2 AhR regulates Th17/Treg cell homeostasis delicately. This figure highlights the important role of AhR in regulating Th17/Treg cell homeostasis. As shown, many ligands(such as TCDD, Kyn, I3C, DIM, CID-1, alpinetin and naphthoflavone) promote Treg differentiation after activation of AhR, while FICZ, PAHs suppress Treg differentiation. FICZ, PAHs, Kyn and TCDD (when acting in vitro) can promote Th17 cell differentiation, while TCDD (when acting in vivo), CID-1, Kyn, I3C, DIM, baicalein and naphthoflavone suppress Th17 differentiation. FICZ, 6-formylindolo[3,2-b]carbazole; Kyn, kynurenine; I3C, indole-3-carbinol; DIM, diindolylmethane; TCDD, Tetrachlorodibenzo-p-dioxin; PAH, Polycyclic aromatic hydrocarbons; CID-1, Cinnamtannin D1.
3.5.4 AhR and T helper 22 (Th22) cells
Th22 cells belong to the CD4+ T cell subpopulation and are important components of anti-microbial resistance in the mucosa. Th22 has an important impact on a variety of autoimmune diseases including Hashimoto’s thyroiditis (93), rheumatoid arthritis (94), Crohn’s disease (95), and leukoaraiosis (96). AhR deficiency suppresses Th22 expansion, and in a mouse model of pulmonary fungal disease, AhR also regulates the number of Th17 and Treg cells associated with Th22 (66). Th22 secretes IL-22 and AhR is the main transcription factor regulating IL-22 transcription (97), blocking AhR with the antagonist CH223191 decreases IL-22 production (65).
3.5.5 AhR and type 1 regulatory T (Tr1) cells
Tr1 cells are CD4+ regulatory T cells that suppress inflammation and autoimmunity by secreting the immunosuppressive cytokine IL-10. In human peripheral blood mononuclear cells (PBMCs), AhR promotes Tr1 cell differentiation and IL-10 production via granzyme B (84). IL-27 can promote the growth and differentiation of Tr1 cells (98). DCs secrete IL-27 after being stimulated by anti-CD3. IL-27R initiates the synthesis of AhR and the transcription factor c-maf, which interact to further expand Tr1 cells in vivo(99, 100). According to Gandhi R et al., activation of AhR using TCDD causes differentiation of Tr1 cells in vitro(84). Mascanfroni et al. demonstrated that AhR promotes hypoxia-inducible factor 1-α (HIF1-α) degradation and regulates Tr1 cell metabolism during the late stages of differentiation (101).
3.6 AhR and B cells
IL-4 regulates AhR expression and activation in B cells, according to Tanaka G et al. (102). Bharat Vaidyanathan et al. performed several experiments to clarify the role of AhR in B cell antibody class switching, differentiation into plasma cells, and generation of memory B cells. The impact of AhR in several developmental processes, AhR may therefore be a novel molecular target for the regulation of B cell immune response (103). It has been more thoroughly studied that the AhR agonist TCDD causes B cells to differentiate in a manner that suppresses antibody production, by reducing the expression of two transcription factors, EBF1 and PAX5 (104, 105). Bach2 is a transcriptional target of AhR in mouse B cell lines, and AhR-mediated transcriptional regulation of Bach2 is a mechanism through which TCDD suppresses B cells (106).
Regulatory B cells (Bregs) are capable of regulating immunity and inflammation, and AhR can promote the differentiation and function of Bregs (107).B10 cells are a specific subpopulation of Bregs, and PGE2 may induce B10 cell expansion through the AhR signaling pathway. Rosser EC et al. found that butyrate supplementation activated AhR by increasing levels of the serotonin-derived metabolite 5-hydoxyindoleacetic acid (5-HIAA), suppressing rheumatoid arthritis in a Bregs-dependent manner (27).
In particular, the numbers and differentiation of T cell subsets have been shown to be affected by cytokines produced by B-cell subsets. In this way, AhR appears to play a multifaceted regulatory role in immune cell differentiation, involving both direct and indirect interactions between immune cells.
The roles of AhR and its ligands in various types of immune cells are shown in Table 2.
4 The role of AhR in autoimmunity and autoimmune diseases
The incidence and prevalence of autoimmune diseases continue to increase and current studies have shown that genetic susceptibility accounts for approximately 30% of all autoimmune diseases, the remaining 70% is due to environmental factors (112), which are closely related to AhR. According to the previous section, we have learned that AhR is essential for immune function, and its role in autoimmunity is mainly achieved by regulating the differentiation and function of multiple immune cells, thus affecting autoimmune diseases. In particular, AhR is essential for maintaining the balance between Th17 and Treg cells, which plays a major role in autoimmune diseases (39), and also controls the differentiation and activity of specific T-cell subsets.
In the studies available to date, most of the evidence suggest that AhR activation suppresses inflammatory responses and alleviates autoimmune diseases. Multiple sclerosis (MS) is a demyelinating disease of the central nervous system, in which AhR also plays an active role. In a mouse model of EAE, microbial metabolites limit the pathogenic activity of microglia and astrocytes and suppress CNS inflammation through an AhR-mediated pathway (113). AhR can also be used to monitor disease activity, Rothhammer V et al. detected that AhR agonists are dynamically regulated during MS, and different levels of AhR agonists in serum have an important impact on the progression and prognosis of patients (114).
AhR is also an important regulator of systemic autoimmune diseases. Type 1 diabetes mellitus(T1D) develops due to severe destruction of pancreatic β-cells caused by islet cell autoantigen targeting. AhR activation attenuates the autoimmune response during the development of T1D (115). The most commonly used mouse model for T1D is the NOD mouse, which develops spontaneous disease similar to that of humans (116). Both Kerkvliet NI and Ehrlich AK et al. demonstrated effective suppression of T1D-related symptoms after treatment of NOD mice with AhR ligands (117, 118). Rheumatoid arthritis(RA) affects approximately 1% of the population and is characterized by chronic inflammation of the synovium and joint destruction. Its pathogenesis is unclear, and a variety of factors such as genetics, infection, smoking and environmental pollution may exacerbate the symptoms of RA (119). For example, smoking is one of the major risk factors. Cigarette smoke contains many AhR ligands, 3-MC, BaP and TCDD upregulate IL-1β mRNA in human-like synovial cell lines, and the AhR antagonist α-naphthoflavone inhibits the action of 3-MC (120). AhR has a critical impact on the development of Th17 cells, IL-6 induces Th17 cells and contributes to RA development. Nguyen NT et al. showed that the reduction of Th17 cells in a mouse model of RA by blocking IL-6 may be partially dependent on the inhibition of AhR expression and that AhR antagonists are therefore a promising therapeutic agent for RA (121). Taking all these evidence together, it could be said that AhR is indispensable for RA development.
The main character of this article, SLE, is also a very representative systemic autoimmune disease, and more details about AhR and SLE will be discussed below.
5 Role of AhR in the development of SLE
5.1 Environmental factors influence the development of SLE through AhR pathways
5.1.1 Gut microbiota affects SLE through AhR pathways
In recent years, the gut microbiota has been studied more intensively and can influence food and drug metabolism, human consciousness and behavior, the pregnancy process, and many other aspects. A growing number of studies have shown multiple associations of gut microbiota with AhR and autoimmune diseases.
On the one hand, the presence of dysregulated gut microbiota in many autoimmune diseases is associated with the activation of AhR signaling (122), and the regulation of gut flora by AhR is in turn modulated by multiple factors, which have been demonstrated in autoimmune diseases such as inflammatory bowel disease (IBD) (122–125). On the other hand, the gut microbiota itself can metabolize or produce some AhR ligands that affect the development of autoimmune responses (126–128).
There is no evidence that the gut microbiota plays an important role in the development of SLE through AhR pathways, and Adriana Cuervo’s group first demonstrated the presence of gut microbial dysbiosis in SLE patients (129), and it has even been suggested that new biomarkers of SLE may be found in the human microbiota (130). In a dietary investigation of SLE patients, the altered abundance of Lactobacillus and Bifidobacterium in the gut was directly correlated with the intake of flavonoid-rich apples and oranges. Some of these strains have immunomodulatory effects and promote Tregs/Th17 differentiation (31). Tryptophan has been reported to be catabolized differently in SLE patients (131). Brown J et al. demonstrated that intestinal flora promoted the production of tryptophan metabolites, including Kyn, which enhanced T cell activation in lupus mice (132). Feeding a high tryptophan diet (including an increase in Kyn) to lupus-prone TC mice resulted in dysbiosis of their intestinal flora and stimulated autoantibody production and produced lupus-like disease when their feces were transferred to germ-free kindred B6 mice (133). In conclusion, abnormal intestinal flora may contribute to the disease development in SLE through AhR pathways.
Although both gut microbiota and AhR are closely associated with the development of SLE, the interaction between the two does not seem to play a direct role in SLE development at present.
5.1.2 Ultraviolet affects the development of SLE through AhR pathways
Ultraviolet(UV) irradiation activates the AhR and promotes the production of several AhR ligands, such as FIZC (26). Among the environmental factors affecting the development of SLE, UVB is an important item. Ultraviolet B (UVB) can suppress DNA methyltransferase 1 (DNMT1) activity in CD4+ T cells of SLE patients and induce CD4+ T cells methylation-sensitive gene hypomethylation, thus exacerbating SLE. A study by Zhouwei Wu et al. confirmed that UVB can suppress SIRT1 expression through activation of AhR and subsequently suppress CD4+ T cells in SLE patients of DNMT1 activity (134). Propranolol, a potential lupus-inducing drug, induced stronger AhR activation in the PBMCs of SLE patients than in the control group, and signs of AhR activation were also shown in skin tissues related to lesion expression. Interestingly, its AhR agonist activity was increased by UVB exposure (135).
5.1.3 Environmental toxicants affect the development of SLE through AhR pathways
The sources of environmental toxins are very broad and can originate from vehicle exhaust, combustion, and industrial emissions, and the main components include PAHs, dioxins, and TCDD. A large body of evidence has demonstrated that environmental pollutants affect the development of SLE. Experiments by O’Driscoll CA et al. demonstrated that different doses of air particulate extract (SRM1650b PM, SRM2975 PM) affect the differentiation of Th17 and Treg in vitro, thus affecting the autoimmune response, and this regulatory effect depends on AhR (136). Long-term exposure to high levels of PCBs increased SLE morbidity and mortality in women in a long-term follow-up study in a Taiwanese population (137). However, contradictory results have been reported for different AhR ligands, Li J et al. demonstrated the immunosuppressive effect of TCDD on murine SLE (138), while Amjad Mustafa et al. reported that in lupus-like autoimmune SNF(1) mice, prenatal TCDD leads to an active postnatal immune response and exacerbates lupus-like responses (139).
Smoking is an important environmental factor, the main components of cigarette smoke are nicotine, PAHs, heterocyclic compounds, heavy metal elements, etc. Numerous studies have demonstrated that previous and current smoking increases the risk of SLE and exacerbates disease severity (140, 141). Marie Saghaeian Jazi et al. investigated the polymorphisms of AhR pathway genes in smoking-related SLE patients, in their study, xenobiotic-metabolizing genes CYP1A1 and AhRR are associated with xenobiotic susceptibility and disease severity in SLE. There was an association between polymorphisms in AhRR and CYP1A1 and SLE severity only in smokers, suggesting that smoking exposure requires significant effects of xenobiotic-metabolizing genes. Smoking is a definite environmental risk factor associated with the development of SLE (142), and the components of smoke include some AhR ligands (such as dioxins and dioxin-like compounds) that may be able to trigger the AhR/AhRR/CYP1A1/B1 axis (143).
5.2 AhR influences the development of SLE by modulating estrogen signaling
In the previous section on AhR signaling pathway we have described the non-genomic pathway of the AhR, which involves the ER. AhR and ER are both ligand-activated transcription factors that function in the nucleus and are involved in many physiological processes, including effects on endogenous estrogen metabolism and proteasomal degradation. The physiological roles of these two nuclear receptors and the complex crosstalk between their signaling pathways have potential implications for the development of certain diseases, including SLE, which is known to be more prevalent in women and may be attributed to the effects of estrogen on the immune system. Estrogen activates the ER and acts through two different receptors, ERα and ERβ. The effects of estrogen in SLE are complex and are mainly mediated by ERα (144, 145). It has been demonstrated that women during pregnancy (146) and taking postmenopausal estrogen therapy are at increased risk of SLE (147), whereas there is a little effect between oral contraceptives and the development of SLE (144) and no increase in the risk of exacerbations in women with stable SLE (148). Notably, high levels of estrogen cannot be simply assumed to be associated with SLE. S S Shabanova et al. studied 94 untreated women with SLE, 25% of whom had reduced estrogen levels (149).
There are multiple associations between AhR and estrogen; the AhR ligand TCDD leads to increased expression of CYP, which accelerates the metabolic degradation of estrogen (150); B Astroff et al. demonstrated that TCDD may produce anti-estrogenic effects by activating AhR (151). Whereas ARNT can also regulate estrogen signaling, ARNT can act as an estrogen receptor modulator alone, and in the presence of E2, ARNT is recruited to the estrogen response promoter, leading to an increase in ER transcription by an unknown mechanism (152). In another aspect, Ohtake et al. reported that the AhR-ARNT complex would interact directly with the estrogen receptor and bind to the estrogen response element (ERE) to activate transcription, while the AhR-ARNT complex also inhibits E2 binding to the ER, so that AhR also mediates some of the adverse estrogen-related effects (153). Meanwhile, AhR can function as an E3 ubiquitin ligase and promote ER degradation (154) (see Figure 3).
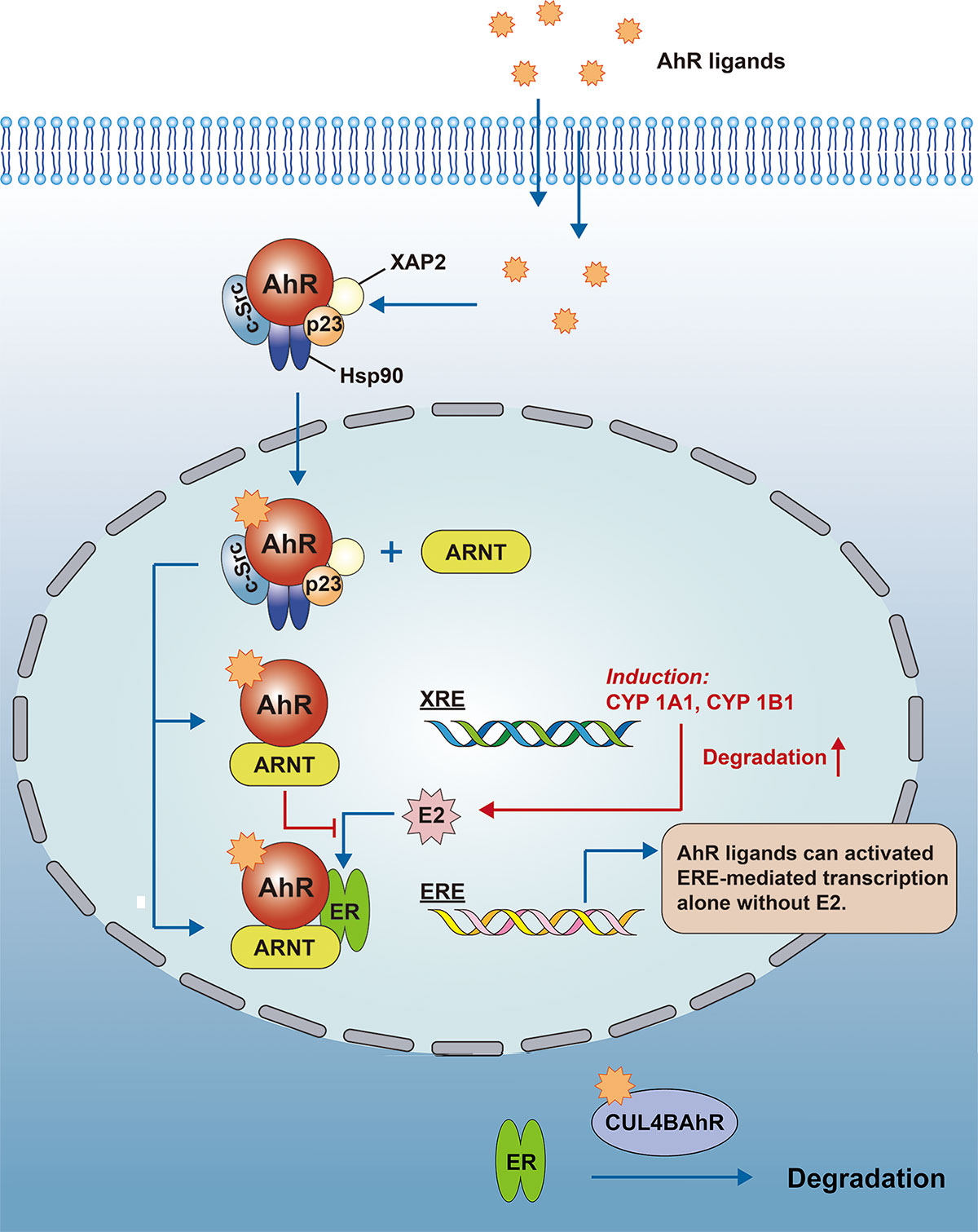
Figure 3 AhR regulates estrogen signaling. The AhR-ARNT complex would interact directly with the ER and bind to the ERE to activate transcription, while the AhR-ARNT complex also inhibits E2 binding to the ER. At the same time, AhR can function as an E3 ubiquitin ligase to promote the degradation of the sex hormone receptor (CUL4BAhR is a complex that consists of AhR, ARNT, CUL4B, TBL3, DDB1 and Rbx1, the AhR plays a role as an adapter for specific substrates).AhR, Aryl hydrocarbon receptor; ARNT, Aryl hydrocarbon receptor nuclear transport protein; XRE, xenobiotic response element; ERE, estrogen response element; CYP, Cytochrome P450; E2, estradiol; DDB1, damage-specific DNA binding protein 1; TBL3, transducin beta-like protein 3; Rbx1, ring-box 1.
Based on all the above evidence, it is reasonable to speculate that AhR can influence the occurrence and development of human SLE by regulating estrogen signaling.
5.3 AhR affects immune cells in SLE
Many immune cells are involved in AhR-induced SLE pathogenesis. According to the previous section, Th17 and Treg cells are key players in the immune response. The homeostasis of the Th17/Treg cell is critical to the pathogenesis of SLE (155), it has been shown that the proportion of Th17 cells is elevated and Treg cells are reduced in SLE patients (156, 157). In a case-control study, Haitao Yu et al. examined the relationship between the ratio of AhR in Th17 cells and the ratio of AhR in Treg cells and SLE skin lesions in SLE patients (158). In the analysis of PBMCs from SLE patients, AhR expression was more than three-fold higher than that in healthy controls, and patients in the high AhR ratio group had more extensive lesions and more decreased C3 levels compared to the low AhR ratio group.
Fcγ receptor IIb (FcgRIIb)-deficient (FcgRIIb-/-) mice can develop lupus-like disease and some environmental contaminants can activate AhR and cause amplify inflammatory responses (159). Kanyarat Udompornpitak et al (160) used the AhR agonist 1,4-chrysenequinone (1,4-CQ) to produce a strong response in macrophages of FcgRIIb-/- mice compared to macrophages of wild-type mice, and these activations led to a more severe inflammatory response, causing FcgRIIb-/- mice to develop lupus-like features (161).
5.4 AhR pathway affects SLE development in a ligand-dependent manner
As mentioned previously, a variety of environmental toxicants are ligands for the aryl hydrocarbon receptor and exposure to these environmental toxicants can exacerbate SLE. Other exogenous ligands, such as quercetin, curcumin and resveratrol as dietary components can bind to AhR and improve SLE symptoms (162–164). Leflunomide as an agonist of AhR suppresses the immune response and treats a variety of diseases, including SLE (165, 166).
As one of the endogenous ligands, I3C has been shown to be a beneficial factor in regulating the inflammatory response and cytokine expression in SLE. Saeed Mohammadi et al. showed (167) that I3C-mediated activation of AhR significantly downregulated the overexpression of inflammatory cytokines and also had an immunomodulatory effect on macrophages in SLE patients. Kyn, as an established endogenous ligand of AhR, can exert anti-inflammatory and immunomodulatory effects through activation of AhR (168) and mTOR (62), and we have previously mentioned that Kyn has a definite effect on SLE. In a quantitative metabolomic analysis of peripheral blood lymphocytes (PBL) from SLE patients, Andras Perl et al. found that Kyn was one of the most increased metabolites (62). Treatment with N-acetylcysteine (NAC) blocked mTOR (which is extensively linked with AhR, details in 5.5) in T lymphocytes and significantly reduced Kyn levels in patients, suggesting a therapeutic role in SLE (169).
It is important to note that we cannot simply attribute the effect of ligand activation of AhR on SLE to the agonist/antagonist activity of the ligand. For example, while resveratrol is an AhR antagonist and leflunomide is an AhR agonist, they both have a therapeutic effect on SLE. Just as two different AhR agonists, TCDD and FICZ, have opposite effects on EAE development (76). Since each ligand may have different effects on the disease, it is important to determine the detailed mechanisms by which each ligand affects AhR signaling.
5.5 AhR influences the development of SLE through multiple signaling pathways
In the previous description, we mentioned the mTOR signaling pathway, which is closely related to the pathogenesis of SLE (170). Moreover, the AhR and mTOR pathways have been extensively connected and AhR activation can stimulate mTOR pathway activation (171). Fangyi Shi et al. found that AhR enhanced activation of the PI3K/Akt/mTOR signaling pathway, thereby promoting cell survival (172). According to George Anderson et al (171), the link between mTOR and AhR activation is also widely present via metabolic pathways in the tumor microenvironment, the backbone of which is built up by oxidative phosphorylation and glycolysis regulated through the acetyl coenzyme A and melatonin pathways. The AhR can attenuate acetyl-CoA levels, with consequences for mTORC1 induction of amino acid transporter(LAT1) and glycolysis. Protein phosphatase 2A(PP2A) is recognised as a regulator of the tumour microenvironment (173), which can inhibit mTORC1-induced LAT1 and glycolysis, and there is a negative crosstalk between PP2A and mTORC1 (174). PP2A is also a regulator of the AhR (175). The involvement of mTOR activation in SLE was first mentioned by Fernandez DR et al (176). Furthermore, mTOR is involved in the proliferation and differentiation of immune cells and the production of inflammatory cytokines in SLE, and the AhR signaling pathway may be involved. The effect of mTOR blockade on cytokine production in SLE patients has been proved (177, 178). Tryptophan and its metabolites increase T cell metabolism and mTOR activation, and Kyn promotes IFN-γ production, all of which are associated with the development of lupus in mice (132). Overall, the pro-inflammatory effects of AhR stimulation by Kyn and the activation of mTOR by Kyn play an important role in the pathogenesis of SLE, there is likely to be a mutually reinforcing relationship between the two. For more links between mTOR, AhR and SLE please see Figure 4.
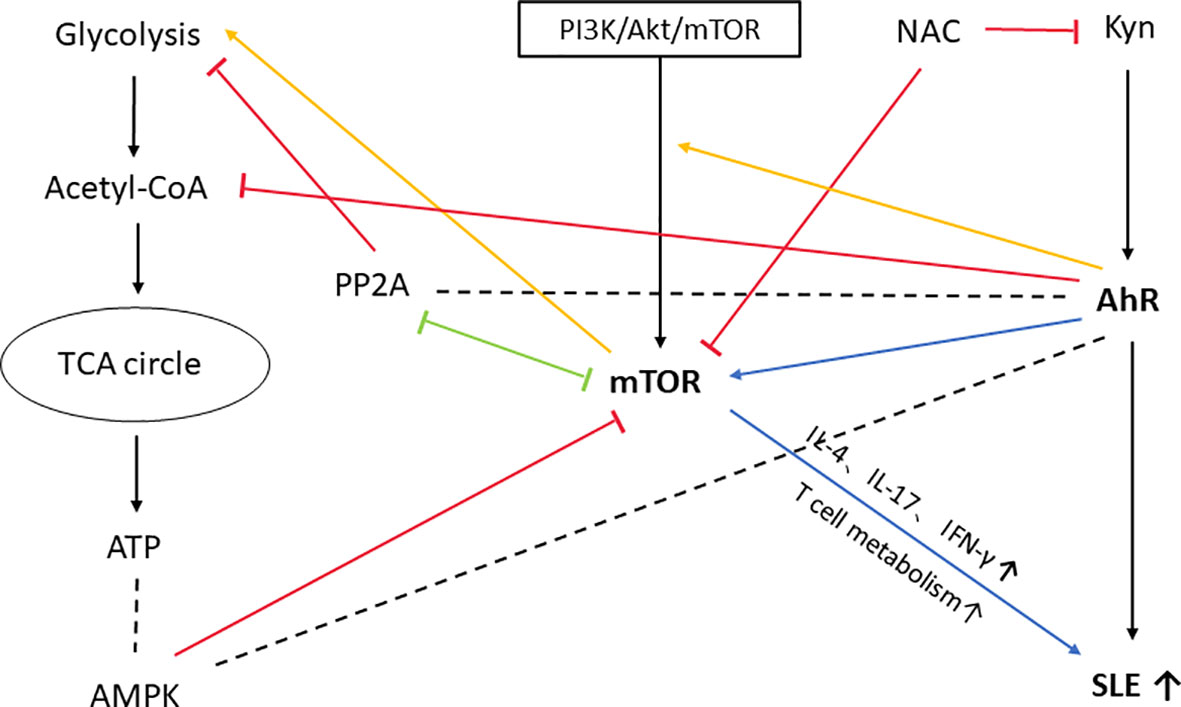
Figure 4 mTOR, an important molecule involved in the development of SLE by AhR. In this figure, we only show the possible association between themTOR and AhR pathways and their effects on SLE. AhR can reduce acetyl coenzyme A levels and thus have an effect onmTORC1-induced glycolysis. mTORC1-induced glycolysis is also inhibited by PP2A, which has a negative crosstalk with mTORC1 and is a regulator of AhR. AMP-activated protein kinase (AMPK) is regulated by ATP/ADP or AMP/ATP ratio in the cell. Activated AMPK inhibits the activity of mTORC1 (179). AMPK is also regulated by AhR-driven degradation of Synphilin-1, which can reduce AMPK production (180). So PP2A and AMPK may be importantmolecules linking mTOR to AhR. PI3K/Akt/mTOR pathway is one of the upstream pathways regulating mTOR, and AhR enhances the activation of PI3K/Akt/mTOR signaling pathway, thus promoting cell survival. Kyn itself has a powerful pro-inflammatory effect and can induce SLE development by activating AhR. Moreover, Kyn activation ofmTOR is also involved in the development of SLE, a process accompanied by production of cytokines such as IL-4, IL-17, IFN-γ, and increased T-cell metabolism. Treatment with NAC inhibits mTOR activation and significantly reduces Kyn levels in SLE patients. PP2A, protein phosphatase 2A; AMPK, AMP-activated protein kinase; PI3K, phosphatidylinositol-3-kinase; Akt, autologous tumor killing; NAC, N-acetylcysteine.
Kyn itself has a powerful pro-inflammatory effect and can induce SLE development by activating AhR. Moreover, Kyn activation of mTOR is also involved in the development of SLE, a process accompanied by production of cytokines such as IL-4, IL-17, IFN-γ, and increased T-cell metabolism. Treatment with NAC inhibits mTOR activation and significantly reduces Kyn levels in SLE patients.
Programmed death (PD)-1 signaling pathway contributes to the development of SLE. According to a recent report by Colleen S. Curran et al. (181), the PD-1 signaling cascade is regulated by the Toll-like receptor (TLR) pathway and the type I interferon (IFN) pathway by activating NF-κB and/or STAT1. Tyrosine kinase receptors (TAM) are the main regulators of these signals. In contrast, dysregulated cellular signaling in SLE can identify pathways involved in the control of PD-1 responses. The other two pathways mentioned before are also two key pathways affecting SLE pathogenesis (182, 183). FICZ, Kyn, and other AhR ligands increase or inhibit PD-1 and PD-L1 expression on cell surfaces, and as previously described, AhR is also expressed in various cells (such as Th17, Treg) that exhibit crosstalk with NF-κB and STAT1.
Also associated with SLE is the germinal center kinase-like kinase (GLK) pathway, which produces the cytokine IL-17A. The frequency of T cell overexpression of GLK shows a positive correlation with disease severity in SLE patients. Chuang HC and other investigators have found that in animal experiments, GLK signaling stimulates IL-17A production in mice Th17 cells by inducing the formation of the AhR-retinoic-acid-receptor-related orphan nuclear receptor γt (ROR-γt) complex, This process is highly selective and promotes autoimmunity (184). Their recent findings suggest (185) that the GLK-induced AhR-ROR-γt complex in Th17 may serve as a marker of IL-17A-mediated autoimmune disease and could be a new therapeutic target for human SLE and several diseases.
6 Summary
AhR is known as an environmental receptor, it’s a cytoplasmic receptor and transcription factor that is activated by binding to the corresponding ligands, and it transmits relevant information by binding to DNA, thus activating the transcription of various genes. There are many other interesting receptors similar to AhR in the human body, such as the retinoic acid-inducible gene I (RIG-I)-like receptor (RLR) and NOD-like receptor (NLR). RLR is a key sensor of viral infection that mediates transcriptional induction of type I interferons and other genes that co-establish antiviral host responses, both viral and host-derived RNAs can activate RLR (186). NLR is an intracellular innate immune sensor that sense intracellular microbial and non-microbial danger signals and form large cytoplasmic complexes called inflammasomes that link the sensing of microbial products and metabolic stress to the proteolytic activation of the proinflammatory cytokines IL-1beta and IL-18 (187). However, AhR differs from them in that it possesses ligands of diverse origin and is associated with much more different internal and external environmental factors, so AhR may have a broader role. It is necessary to study such receptors with sensor roles because they are often an important part of multiple pathophysiological responses. For this review, we focused on the role of AhR in the development of autoimmune diseases, mainly SLE, in order to provide clues for more effective and targeted prevention and treatment methods.
AhR contributes to many autoimmune diseases, and this review focuses on outlining the role of AhR in the development of different subtypes of immune cells and SLE. AhR ligands are mainly of exogenous and endogenous origin, but their effects are complex, with ligand-, dose- and environment-specific responses. For example, small doses of FICZ promote mast cell differentiation while large doses suppress it; some ligands have different effects in different environments, such as TCDD, which promotes Th17 cell differentiation in vitro but suppresses it in vivo. Moreover, what is clear about AhR ligands and autoimmunity is that most AhR ligands cause immunosuppression and improve autoimmune disease, while others exacerbate disease (especially environmental toxins, but which have both immunosuppressive and stimulatory effects). Different AhR ligands alter the balance between regulatory T cells and the outcome of autoimmune diseases. The specific mechanisms responsible for these complex, even contradictory effects may be as follows: First, the route of exposure and the degree and duration of AhR activation contribute to altering the effects of AhR ligands on autoimmunity; second, the lower affinity of AhR expressed in humans compared to animal models prevents the production of sufficient amounts of pro-inflammatory cytokines in vivo; and finally, AhR as a member of multiple signaling can be regulated by many different genes, substances, or environments, and subtle differences in cascade effects may make AhR act differently.
From the results of current studies on AhR and SLE, some experiments have demonstrated that AhR activation can exacerbate the development of SLE, such as the action of UV, environmental toxins, estrogen, etc. with AhR. However, there is still much evidence that ligand activation of AhR improves SLE symptoms, such as I3C and resveratrol, which suggests that AhR is a promising therapeutic target for autoimmune diseases. However, there are some interesting results, such as evidence that AhR activation can have different effects on lupus in mice and humans: Rahul Shinde et al. reported that blocking AhR activity in mice enhanced autoimmune responses and that activation of AhR improved lupus-like disease (45). However, increased AhR activity was found in human SLE patients (188, 189). Although the authors have not yet elucidated the reason, we speculate that this also seems to be related to the different affinities of AhR expressed in humans and mice as mentioned previously.
A variety of factors such as gut microbiota, environmental toxins (mainly PAHs, dioxins), and genetic susceptibility can influence SLE development through the AhR pathway. In addition to being involved in regulation in a ligand-specific manner, AhR itself is associated with a variety of intercellular signaling, and these factors together influence the development of SLE (Figure 5 summarizes the effect of multiple factors on the occurrence and development of SLE after activation of AhR). Therefore, in-depth research of the mechanisms of AhR in the development of SLE will hopefully inspire new ideas and targets for the prevention or treatment of SLE. However, we still face many problems: firstly, a further epidemiological investigation is needed to verify the relationship between AhR ligands and SLE; secondly, the number of studies related to AhR and SLE is small and the studies don't far enough, many mechanisms are still unexplored. At present, various diagnostic tools for SLE are developing rapidly, such as the application of bioinformatics, proteomics, biomechanics and functional analysis. Although the road to a brighter future is bumpy and winding, and it will take a long time to figure out the way forward, we believe that more AhR-related researches will also shine in SLE in the near future.
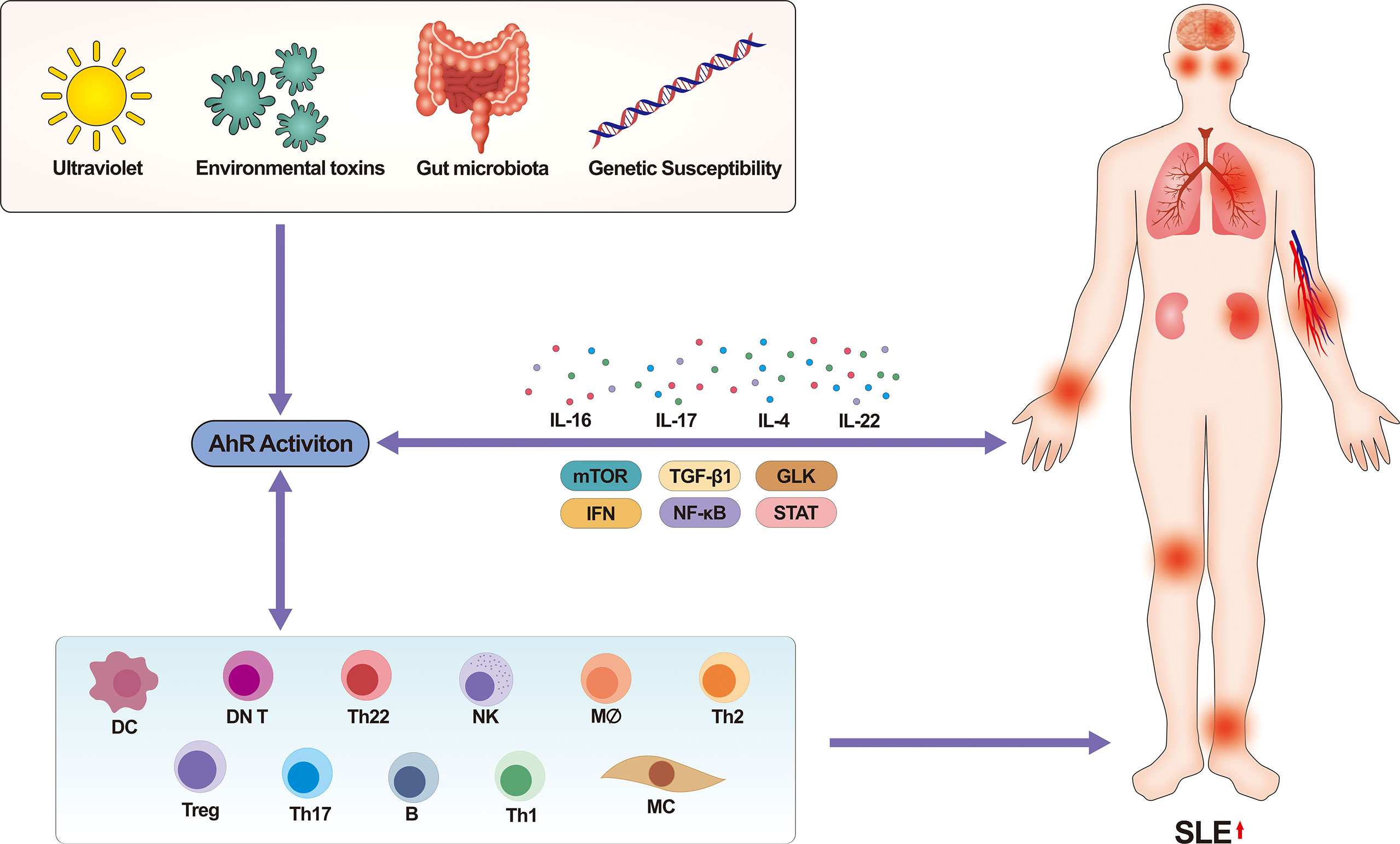
Figure 5 Activation of AhR by multiple factors promotes the occurrence and development of human SLE. On the one hand, factors such as ultraviolet, environmental toxins, and gut microbiota will mostly affect the binding of AhR to ligands. AhR regulates the pathophysiological functions of multiple immune cells in a ligand-specific manner, which in turn affects SLE. Meanwhile, many signaling pathways that regulate disease (e.g., mTOR, PD-1, NF-κB, STAT, TGF-β1, GLK signaling pathway, etc.) also involve activation of AhR, which promotes the development of SLE with the involvement of multiple cytokines. On the other hand, immune cells also secrete various pro-inflammatory substances and cytokines that influence the activation of certain signaling pathways. In conclusion, multiple pathways together contribute to the development of SLE through AhR. mTOR, mechanistic target of rapamycin, IFN, interferon, STAT, signal transducer and activator of transcription, TGF, transforming growth factor, GLK, germinal center kinase-like kinase, SLE, systemic lupus erythematosus.
Author contributions
JW wrote the manuscript and prepared the figures. HJ, MZ reviewed the manuscript. TP, ZL participated in reference search and discussion of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81874253), Excellent postdoctoral innovative talents of Hunan province in 2020 (No. 2020RC2014), Natural Science Foundation of Hunan Province China (No. 2021JJ40837). The 15th medium-term special grant of postdoctoral Science Foundation of China (2022T150742). The authors did not have financial support or benefits from commercial resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem (1976) 251:4936–46. doi: 10.1016/S0021-9258(17)33205-2
2. Shinde R, McGaha TL. The aryl hydrocarbon receptor: Connecting immunity to the microenvironment. Trends Immunol (2018) 39:1005–20. doi: 10.1016/j.it.2018.10.010
3. Perdew GH. Association of the ah receptor with the 90-kDa heat shock protein. J Biol Chem (1988) 263:13802–5. doi: 10.1016/S0021-9258(18)68314-0
4. Meyer BK, Perdew GH. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry (1999) 38:8907–17. doi: 10.1021/bi982223w
5. Dong B, Cheng W, Li W, Zheng J, Wu D, Matsumura F, et al. FRET analysis of protein tyrosine kinase c-src activation mediated via aryl hydrocarbon receptor. Biochim Biophys Acta (2011) 1810:427–31. doi: 10.1016/j.bbagen.2010.11.007
6. Tian J, Feng Y, Fu H, Xie HQ, Jiang JX, Zhao B. The aryl hydrocarbon receptor: A key bridging molecule of external and internal chemical signals. Environ Sci Technol (2015) 49:9518–31. doi: 10.1021/acs.est.5b00385
7. Dolwick KM, Swanson HI, Bradfield CA. In vitro analysis of ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci United States America (1993) 90:8566–70. doi: 10.1073/pnas.90.18.8566
8. Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophysics (2005) 433:379–86. doi: 10.1016/j.abb.2004.09.031
9. White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev (2009) 27:197–211. doi: 10.1080/10590500903310047
10. Mason GG. Dioxin-receptor ligands in urban air and vehicle exhaust. Environ Health Perspect (1994) 102 Suppl 4:111–6. doi: 10.1289/ehp.94102s4111
11. Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicological Sci an Off J Soc Toxicol (2009) 110:61–7. doi: 10.1093/toxsci/kfp079
12. Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J (1999) 340(Pt 3):715–22. doi: 10.1042/bj3400715
13. Goya-Jorge E, Jorge Rodríguez ME, Veitía MS, Giner RM. Plant occurring flavonoids as modulators of the aryl hydrocarbon receptor. Molecules (Basel Switzerland) (2021) 26(8):2315. doi: 10.3390/molecules26082315
14. Itkonen MK, Tornio A, Neuvonen M, Neuvonen PJ, Niemi M, Backman JT. Clopidogrel and gemfibrozil strongly inhibit the CYP2C8-dependent formation of 3-hydroxydesloratadine and increase desloratadine exposure in humans. Drug Metab Disposition: Biol Fate Chemicals (2019) 47:377–85. doi: 10.1124/dmd.118.084665
15. O'Donnell EF, Kopparapu PR, Koch DC, Jang HS, Phillips JL, Tanguay RL, et al. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PloS One (2012) 7:e40926. doi: 10.1371/journal.pone.0040926
16. Daujat M, Charrasse S, Fabre I, Lesca P, Jounaïdi Y, Larroque C, et al. Induction of CYP1A1 gene by benzimidazole derivatives during caco-2 cell differentiation. Evidence an Aryl-hydrocarbon Receptor-mediated Mechanism Eur J Biochem (1996) 237:642–52. doi: 10.1111/j.1432-1033.1996.0642p.x
17. Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, et al. Certain photooxidized derivatives of tryptophan bind with very high affinity to the ah receptor and are likely to be endogenous signal substances. J Biol Chem (1987) 262:15422–7. doi: 10.1016/S0021-9258(18)47743-5
18. Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci United States America (1991) 88:9543–7. doi: 10.1073/pnas.88.21.9543
19. Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, et al. Activation of the ah receptor by tryptophan and tryptophan metabolites. Biochemistry (1998) 37:11508–15. doi: 10.1021/bi980087p
20. Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci United States America (2002) 99:14694–9. doi: 10.1073/pnas.232562899
21. Seidel SD, Winters GM, Rogers WJ, Ziccardi MH, Li V, Keser B, et al. Activation of the ah receptor signaling pathway by prostaglandins. J Biochem Mol Toxicol (2001) 15:187–96. doi: 10.1002/jbt.16
22. Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem (2001) 276:31475–8. doi: 10.1074/jbc.C100238200
23. Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophysics (1998) 357:155–63. doi: 10.1006/abbi.1998.0814
24. Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature (2011) 478:197–203. doi: 10.1038/nature10491
25. Rannug A, Rannug U. The tryptophan derivative 6-formylindolo[3,2-b]carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation. Crit Rev Toxicol (2018) 48:555–74. doi: 10.1080/10408444.2018.1493086
26. Furue M, Uchi H, Mitoma C, Hashimoto-Hachiya A, Tanaka Y, Ito T, et al. Implications of tryptophan photoproduct FICZ in oxidative stress and terminal differentiation of keratinocytes. Giornale Italiano di Dermatologia e Venereologia Organo Ufficiale Societa Italiana di Dermatologia e sifilografia (2019) 154:37–41. doi: 10.23736/S0392-0488.18.06132-1
27. Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory b cells. Cell Metab (2020) 31:837–851.e10. doi: 10.1016/j.cmet.2020.03.003
28. Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol (1998) 56:197–206. doi: 10.1016/S0006-2952(98)00143-9
29. Amakura Y, Tsutsumi T, Sasaki K, Nakamura M, Yoshida T, Maitani T. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry (2008) 69:3117–30. doi: 10.1016/j.phytochem.2007.07.022
30. Wei ZF, Lv Q, Xia Y, Yue MF, Shi C, Xia YF, et al. Norisoboldine, an anti-arthritis alkaloid isolated from radix linderae, attenuates osteoclast differentiation and inflammatory bone erosion in an aryl hydrocarbon receptor-dependent manner. Int J Biol Sci (2015) 11:1113–26. doi: 10.7150/ijbs.12152
31. López P, González-Rodríguez I, Gueimonde M, Margolles A, Suárez A. Immune response to bifidobacterium bifidum strains support Treg/Th17 plasticity. PloS One (2011) 6:e24776. doi: 10.1371/journal.pone.0024776
32. Takamura T, Harama D, Fukumoto S, Nakamura Y, Shimokawa N, Ishimaru K, et al. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol (2011) 89:817–22. doi: 10.1038/icb.2010.165
33. Goudot C, Coillard A, Villani AC, Gueguen P, Cros A, Sarkizova S, et al. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity (2017) 47:582–96.e6. doi: 10.1016/j.immuni.2017.08.016
34. Liu H, Ramachandran I, Gabrilovich DI. Regulation of plasmacytoid dendritic cell development in mice by aryl hydrocarbon receptor. Immunol Cell Biol (2014) 92:200–3. doi: 10.1038/icb.2013.65
35. Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun (2008) 375:331–5. doi: 10.1016/j.bbrc.2008.07.156
36. Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA (2010) 107:19961–6. doi: 10.1073/pnas.1014465107
37. Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol (2010) 246:18–28. doi: 10.1016/j.taap.2010.03.013
38. Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med (1995) 182:459–65. doi: 10.1084/jem.182.2.459
39. Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol (Baltimore Md. 1950) (2005) 175:4184–8. doi: 10.4049/jimmunol.175.7.4184
40. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature (2006) 441:235–8. doi: 10.1038/nature04753
41. Riemschneider S, Kohlschmidt J, Fueldner C, Esser C, Hauschildt S, Lehmann J. Aryl hydrocarbon receptor activation by benzo(a)pyrene inhibits proliferation of myeloid precursor cells and alters the differentiation state as well as the functional phenotype of murine bone marrow-derived macrophages. Toxicol Lett (2018) 296:106–13. doi: 10.1016/j.toxlet.2018.07.050
42. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
43. Climaco-Arvizu S, Domínguez-Acosta O, Cabañas-Cortés MA, Rodríguez-Sosa M, Gonzalez FJ, Vega L, et al. Aryl hydrocarbon receptor influences nitric oxide and arginine production and alters M1/M2 macrophage polarization. Life Sci (2016) 155:76–84. doi: 10.1016/j.lfs.2016.05.001
44. Yang X, Liu H, Ye T, Duan C, Lv P, Wu X, et al. AhR activation attenuates calcium oxalate nephrocalcinosis by diminishing M1 macrophage polarization and promoting M2 macrophage polarization. Theranostics (2020) 10:12011–25. doi: 10.7150/thno.51144
45. Shinde R, Hezaveh K, Halaby MJ, Kloetgen A, Chakravarthy A, da Silva Medina T, et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat Immunol (2018) 19:571–82. doi: 10.1038/s41590-018-0107-1
46. Großkopf H, Walter K, Karkossa I, von Bergen M, Schubert K. Non-genomic AhR-signaling modulates the immune response in endotoxin-activated macrophages after activation by the environmental stressor BaP. Front Immunol (2021) 12:620270. doi: 10.3389/fimmu.2021.620270
47. Zhou Y, Tung HY, Tsai YM, Hsu SC, Chang HW, Kawasaki H, et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood (2013) 121:3195–204. doi: 10.1182/blood-2012-08-453597
48. Wang HC, Zhou Y, Huang SK. SHP-2 phosphatase controls aryl hydrocarbon receptor-mediated ER stress response in mast cells. Arch Toxicol (2017) 91:1739–48. doi: 10.1007/s00204-016-1861-1
49. Sibilano R, Frossi B, Calvaruso M, Danelli L, Betto E, Dall'Agnese A, et al. The aryl hydrocarbon receptor modulates acute and late mast cell responses. J Immunol (Baltimore Md. 1950) (2012) 189:120–7. doi: 10.4049/jimmunol.1200009
50. Kawasaki H, Chang HW, Tseng HC, Hsu SC, Yang SJ, Hung CH, et al. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy (2014) 69:445–52. doi: 10.1111/all.12346
51. Wang H, Do DC, Liu J, Wang B, Qu J, Ke X, et al. Functional role of kynurenine and aryl hydrocarbon receptor axis in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol (2018) 141:586–600.e6. doi: 10.1016/j.jaci.2017.06.013
52. Pilz C, Feyerabend T, Sonner J, Redaelli C, Peter K, Kunze A, et al. Normal mast cell numbers in the tissues of AhR-deficient mice. Exp Dermatol (2016) 25:62–3. doi: 10.1111/exd.12864
53. Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood (2009) 114:2639–48. doi: 10.1182/blood-2009-05-220004
54. Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol (2007) 7:703–14. doi: 10.1038/nri2154
55. Angelos MG, Ruh PN, Webber BR, Blum RH, Ryan CD, Bendzick L, et al. Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood (2017) 129:3428–39. doi: 10.1182/blood-2016-07-730440
56. Bachleda P, Vrzal R, Dvorak Z. Resveratrol enhances NK cell cytotoxicity: possible role for aryl hydrocarbon receptor. J Cell Physiol (2010) 225:289–90. doi: 10.1002/jcp.22233
57. Lu CC, Chen JK. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J Cell Physiol (2010) 223:343–51. doi: 10.1002/jcp.22043
58. Yang SL, Tan HX, Niu TT, Li DJ, Wang HY, Li MQ. Kynurenine promotes the cytotoxicity of NK cells through aryl hydrocarbon receptor in early pregnancy. J Reprod Immunol (2021) 143:103270. doi: 10.1016/j.jri.2020.103270
59. Wagage S, John B, Krock BL, Hall AO, Randall LM, Karp CL, et al. The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J Immunol (Baltimore Md. 1950) (2014) 192:1661–70. doi: 10.4049/jimmunol.1300497
60. Shin JH, Zhang L, Murillo-Sauca O, Kim J, Kohrt HE, Bui JD, et al. Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc Natl Acad Sci USA (2013) 110:12391–6. doi: 10.1073/pnas.1302856110
61. Majora M, Frericks M, Temchura V, Reichmann G, Esser C. Detection of a novel population of fetal thymocytes characterized by preferential emigration and a TCRgammadelta+ T cell fate after dioxin exposure. Int Immunopharmacol (2005) 5:1659–74. doi: 10.1016/j.intimp.2005.02.010
62. Perl A, Hanczko R, Lai ZW, Oaks Z, Kelly R, Borsuk R, et al. Comprehensive metabolome analyses reveal n-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics Off J Metabolomic Soc (2015) 11:1157–74. doi: 10.1007/s11306-015-0772-0
63. Saferding V, Blüml S. Innate immunity as the trigger of systemic autoimmune diseases. J Autoimmun (2020) 110:102382. doi: 10.1016/j.jaut.2019.102382
64. Ambrosio LF, Insfran C, Volpini X, Acosta Rodriguez E, Serra HM, Quintana FJ, et al. Role of aryl hydrocarbon receptor (AhR) in the regulation of immunity and immunopathology during trypanosoma cruzi infection. Front Immunol (2019) 10:631. doi: 10.3389/fimmu.2019.00631
65. de Araújo EF, Loures FV, Preite NW, Feriotti C, Galdino NA, Costa TA, et al. AhR ligands modulate the differentiation of innate lymphoid cells and T helper cell subsets that control the severity of a pulmonary fungal infection. Front Immunol (2021) 12:630938. doi: 10.3389/fimmu.2021.630938
66. de Araújo EF, Preite NW, Veldhoen M, Loures FV, Calich VLG. Pulmonary paracoccidioidomycosis in AhR deficient hosts is severe and associated with defective treg and Th22 responses. Sci Rep (2020) 10:11312. doi: 10.1038/s41598-020-68322-6
67. Schulz VJ, Smit JJ, Willemsen KJ, Fiechter D, Hassing I, Bleumink R, et al. Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model. Toxicological Sci an Off J Soc Toxicol (2011) 123:491–500. doi: 10.1093/toxsci/kfr175
68. Weng CM, Wang CH, Lee MJ, He JR, Huang HY, Chao MW, et al. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy (2018) 73:2192–204. doi: 10.1111/all.13462
69. Hong CH, Lee CH, Yu HS, Huang SK. Benzopyrene, a major polyaromatic hydrocarbon in smoke fume, mobilizes langerhans cells and polarizes Th2/17 responses in epicutaneous protein sensitization through the aryl hydrocarbon receptor. Int Immunopharmacol (2016) 36:111–7. doi: 10.1016/j.intimp.2016.04.017
70. Castañeda AR, Vogel CFA, Bein KJ, Hughes HK, Smiley-Jewell S, Pinkerton KE. Ambient particulate matter enhances the pulmonary allergic immune response to house dust mite in a BALB/c mouse model by augmenting Th2- and Th17-immune responses. Physiol Rep (2018) 6:e13827. doi: 10.14814/phy2.13827
71. Negishi T, Kato Y, Ooneda O, Mimura J, Takada T, Mochizuki H, et al. Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance. J Immunol (Baltimore Md. 1950) (2005) 175:7348–56. doi: 10.4049/jimmunol.175.11.7348
72. Kakutani H, Yuzuriha T, Nakao T, Ohta S. Long-term orally exposure of dioxins affects antigen-specific antibody production in mice. Toxicol Rep (2022) 9:53–7. doi: 10.1016/j.toxrep.2021.12.011
73. Lee GR. The balance of Th17 versus treg cells in autoimmunity. Int J Mol Sci (2018) 19(3):730. doi: 10.3390/ijms19030730
74. Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature (2008) 453:106–9. doi: 10.1038/nature06881
76. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of t(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature (2008) 453:65–71. doi: 10.1038/nature06880
77. Yang S, Tan L, Chen Y, Liu A, Hong M, Peng Z. DIM mitigates the development of experimental autoimmune encephalomyelitis by maintaining the stability and suppressive function of regulatory T cells. Cell Immunol (2020) 358:104238. doi: 10.1016/j.cellimm.2020.104238
78. Guo NH, Fu X, Zi FM, Song Y, Wang S, Cheng J. The potential therapeutic benefit of resveratrol on Th17/Treg imbalance in immune thrombocytopenic purpura. Int Immunopharmacol (2019) 73:181–92. doi: 10.1016/j.intimp.2019.04.061
79. Nishiumi S, Yoshida K, Ashida H. Curcumin suppresses the transformation of an aryl hydrocarbon receptor through its phosphorylation. Arch Biochem Biophysics (2007) 466:267–73. doi: 10.1016/j.abb.2007.08.007
80. Gasiewicz TA, Rucci G. Alpha-naphthoflavone acts as an antagonist of 2,3,7, 8-tetrachlorodibenzo-p-dioxin by forming an inactive complex with the ah receptor. Mol Pharmacol (1991) 40:607–12.
81. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol (2010) 10:479–89. doi: 10.1038/nri2800
82. Sinclair LV, Neyens D, Ramsay G, Taylor PM, Cantrell DA. Single cell analysis of kynurenine and system l amino acid transport in T cells. Nat Commun (2018) 9:1981. doi: 10.1038/s41467-018-04366-7
83. Kahalehili HM, Newman NK, Pennington JM, Kolluri SK, Kerkvliet NI, Shulzhenko N, et al. Dietary indole-3-Carbinol activates AhR in the gut, alters Th17-microbe interactions, and exacerbates insulitis in NOD mice. Front Immunol (2020) 11:606441. doi: 10.3389/fimmu.2020.606441
84. Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol (2010) 11:846–53. doi: 10.1038/ni.1915
85. Lv Q, Wang K, Qiao S, Yang L, Xin Y, Dai Y, et al. Norisoboldine, a natural AhR agonist, promotes treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis (2018) 9:258. doi: 10.1038/s41419-018-0297-3
86. Liu C, Li Y, Chen Y, Huang S, Wang X, Luo S, et al. Baicalein restores the balance of Th17/Treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediators Inflammation (2020) 2020:5918587. doi: 10.1155/2020/5918587
87. Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PloS One (2013) 8:e79819. doi: 10.1371/journal.pone.0079819
88. Connor KT, Aylward LL. Human response to dioxin: aryl hydrocarbon receptor (AhR) molecular structure, function, and dose-response data for enzyme induction indicate an impaired human AhR. J Toxicol Environ Health Part B Crit Rev (2006) 9:147–71. doi: 10.1080/15287390500196487
89. Liu X, Hu H, Fan H, Zuo D, Shou Z, Liao Y, et al. The role of STAT3 and AhR in the differentiation of CD4+ T cells into Th17 and treg cells. Medicine (2017) 96:e6615. doi: 10.1097/MD.0000000000006615
90. Jurado-Manzano BB, Zavala-Reyes D, Turrubiartes-Martínez EA, Portales-Pérez DP, González-Amaro R, Layseca-Espinosa E. FICZ generates human tDCs that induce CD4(+) CD25(high) Foxp3(+) treg-like cell differentiation. Immunol Lett (2017) 190:84–92. doi: 10.1016/j.imlet.2017.07.013
91. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol (Baltimore Md. 1950) (2010) 185:3190–8. doi: 10.4049/jimmunol.0903670
92. Lv Q, Shi C, Qiao S, Cao N, Guan C, Dai Y, et al. Alpinetin exerts anti-colitis efficacy by activating AhR, regulating miR-302/DNMT-1/CREB signals, and therefore promoting treg differentiation. Cell Death Dis (2018) 9:890. doi: 10.1038/s41419-018-0814-4
93. Bai X, Sun J, Wang W, Shan Z, Zheng H, Li Y, et al. Increased differentiation of Th22 cells in hashimoto's thyroiditis. Endocrine J (2014) 61:1181–90. doi: 10.1507/endocrj.EJ14-0265
94. Zhang L, Li JM, Liu XG, Ma DX, Hu NW, Li YG, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. J Clin Immunol (2011) 31:606–14. doi: 10.1007/s10875-011-9540-8
95. Fang L, Pang Z, Shu W, Wu W, Sun M, Cong Y, et al. Anti-TNF therapy induces CD4+ T-cell production of IL-22 and promotes epithelial repairs in patients with crohn's disease. Inflammatory Bowel Dis (2018) 24:1733–44. doi: 10.1093/ibd/izy126
96. Sugita S, Kawazoe Y, Imai A, Kawaguchi T, Horie S, Keino H, et al. Role of IL-22- and TNF-α-producing Th22 cells in uveitis patients with behcet's disease. J Immunol (Baltimore Md. (2013) 1950) 190:5799–808. doi: 10.4049/jimmunol.1202677
97. Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity (2012) 37:1061–75. doi: 10.1016/j.immuni.2012.08.024
98. Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin Immunol (2011) 23:438–45. doi: 10.1016/j.smim.2011.08.003
99. Wu HY, Quintana FJ, da Cunha AP, Dake BT, Koeglsperger T, Starossom SC, et al. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PloS One (2011) 6:e23618. doi: 10.1371/journal.pone.0023618
100. Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol (2010) 11:854–61. doi: 10.1038/ni.1912
101. Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med (2015) 21:638–46. doi: 10.1038/nm.3868
102. Tanaka G, Kanaji S, Hirano A, Arima K, Shinagawa A, Goda C, et al. Induction and activation of the aryl hydrocarbon receptor by IL-4 in b cells. Int Immunol (2005) 17:797–805. doi: 10.1093/intimm/dxh260
103. Vaidyanathan B, Chaudhry A, Yewdell WT, Angeletti D, Yen WF, Wheatley AK, et al. The aryl hydrocarbon receptor controls cell-fate decisions in b cells. J Exp Med (2017) 214:197–208. doi: 10.1084/jem.20160789
104. Sulentic CE, Kaminski NE. The long winding road toward understanding the molecular mechanisms for b-cell suppression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicological Sci an Off J Soc Toxicol (2011) 120 Suppl 1:S171–91. doi: 10.1093/toxsci/kfq324
105. Li J, Bhattacharya S, Zhou J, Phadnis-Moghe AS, Crawford RB, Kaminski NE. Aryl hydrocarbon receptor activation suppresses EBF1 and PAX5 and impairs human b lymphopoiesis. J Immunol (Baltimore Md. 1950) (2017) 199:3504–15. doi: 10.4049/jimmunol.1700289
106. De Abrew KN, Phadnis AS, Crawford RB, Kaminski NE, Thomas RS. Regulation of Bach2 by the aryl hydrocarbon receptor as a mechanism for suppression of b-cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol (2011) 252:150–8. doi: 10.1016/j.taap.2011.01.020
107. Piper CJM, Rosser EC, Oleinika K, Nistala K, Krausgruber T, Rendeiro AF, et al. Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-Producing regulatory b cells. Cell Rep (2019) 29:1878–1892.e7. doi: 10.1016/j.celrep.2019.10.018
108. Hwang WB, Kim DJ, Oh GS, Park JH. Aryl hydrocarbon receptor ligands indoxyl 3-sulfate and indole-3-carbinol inhibit FMS-like tyrosine kinase 3 ligand-induced bone marrow-derived plasmacytoid dendritic cell differentiation. Immune Netw (2018) 18:e35. doi: 10.4110/in.2018.18.e35
109. Ishimaru N, Takagi A, Kohashi M, Yamada A, Arakaki R, Kanno J, et al. Neonatal exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin causes autoimmunity due to the disruption of T cell tolerance. J Immunol (Baltimore Md. 1950) (2009) 182:6576–86. doi: 10.4049/jimmunol.0802289
110. Hew KM, Walker AI, Kohli A, Garcia M, Syed A, McDonald-Hyman C, et al. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2015) 45:238–48. doi: 10.1111/cea.12377
111. Villa M, Gialitakis M, Tolaini M, Ahlfors H, Henderson CJ, Wolf CR, et al. Aryl hydrocarbon receptor is required for optimal b-cell proliferation. EMBO J (2017) 36:116–28. doi: 10.15252/embj.201695027
112. Vojdani A, Pollard KM, Campbell AW. Environmental triggers and autoimmunity. Autoimmune Dis (2014) 2014:798029. doi: 10.1155/2014/798029
113. Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. Microglial control of astrocytes in response to microbial metabolites. Nature (2018) 557:724–8. doi: 10.1038/s41586-018-0119-x
114. Rothhammer V, Borucki DM, Garcia Sanchez MI, Mazzola MA, Hemond CC, Regev K, et al. Dynamic regulation of serum aryl hydrocarbon receptor agonists in MS. Neurology(R) Neuroimmunol Neuroinflamm (2017) 4:e359. doi: 10.1212/NXI.0000000000000359
115. Yue T, Sun F, Yang C, Wang F, Luo J, Yang P, et al. The AHR signaling attenuates autoimmune responses during the development of type 1 diabetes. Front Immunol (2020) 11:1510. doi: 10.3389/fimmu.2020.01510
116. Pearson JA, Wong FS, Wen L. The importance of the non obese diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun (2016) 66:76–88. doi: 10.1016/j.jaut.2015.08.019
117. Kerkvliet NI, Steppan LB, Vorachek W, Oda S, Farrer D, Wong CP, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy (2009) 1:539–47. doi: 10.2217/imt.09.24
118. Ehrlich AK, Pennington JM, Wang X, Rohlman D, Punj S, Löhr CV, et al. Activation of the aryl hydrocarbon receptor by 10-Cl-BBQ prevents insulitis and effector T cell development independently of Foxp3+ regulatory T cells in nonobese diabetic mice. J Immunol (Baltimore Md. 1950) (2016) 196:264–73. doi: 10.4049/jimmunol.1501789
119. Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet (London England) (2009) 373:659–72. doi: 10.1016/S0140-6736(09)60008-8
120. Tamaki A, Hayashi H, Nakajima H, Takii T, Katagiri D, Miyazawa K, et al. Polycyclic aromatic hydrocarbon increases mRNA level for interleukin 1 beta in human fibroblast-like synoviocyte line via aryl hydrocarbon receptor. Biol Pharm Bull (2004) 27:407–10. doi: 10.1248/bpb.27.407
121. Nguyen NT, Nakahama T, Kishimoto T. Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin Immunopathol (2013) 35:637–44. doi: 10.1007/s00281-013-0392-6
122. Chen L, Zhang W, Hua J, Hu C, Lok-Shun Lai N, Qian PY, et al. Dysregulation of intestinal health by environmental pollutants: Involvement of the estrogen receptor and aryl hydrocarbon receptor. Environ Sci Technol (2018) 52:2323–30. doi: 10.1021/acs.est.7b06322
123. Schoenroth LJ, Hart DA, Pollard KM, Fritzler MJ. The effect of the phytoestrogen coumestrol on the NZB/W F1 murine model of systemic lupus. J Autoimmun (2004) 23:323–32. doi: 10.1016/j.jaut.2004.09.004
124. Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology (2011) 141:237–48. doi: 10.1053/j.gastro.2011.04.007
125. Furumatsu K, Nishiumi S, Kawano Y, Ooi M, Yoshie T, Shiomi Y, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Digestive Dis Sci (2011) 56:2532–44. doi: 10.1007/s10620-011-1643-9
126. Liu JR, Miao H, Deng DQ, Vaziri ND, Li P, Zhao YY. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci CMLS (2021) 78:909–22. doi: 10.1007/s00018-020-03645-1
127. Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun (2018) 9:3294. doi: 10.1038/s41467-018-05470-4
128. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
129. Hevia A, Milani C, López P, Cuervo A, Arboleya S, Duranti S, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio (2014) 5:e01548–14. doi: 10.1128/mBio.01548-14
130. Proal AD, Albert PJ, Marshall T. Autoimmune disease in the era of the metagenome. Autoimmun Rev (2009) 8:677–81. doi: 10.1016/j.autrev.2009.02.016
131. Widner B, Sepp N, Kowald E, Ortner U, Wirleitner B, Fritsch P, et al. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology (2000) 201:621–30. doi: 10.1016/S0171-2985(00)80079-0
132. Brown J, Abboud G, Ma L, Choi SC, Kanda N, Zeumer-Spataro L, et al. Microbiota-mediated skewing of tryptophan catabolism modulates CD4(+) T cells in lupus-prone mice. iScience (2022) 25:104241. doi: 10.1016/j.isci.2022.104241
133. Choi SC, Brown J, Gong M, Ge Y, Zadeh M, Li W, et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci Trans Med (2020) 12(551):eaax2220. doi: 10.1126/scitranslmed.aax2220
134. Wu Z, Mei X, Ying Z, Sun Y, Song J, Shi W. Ultraviolet b inhibition of DNMT1 activity via AhR activation dependent SIRT1 suppression in CD4+ T cells from systemic lupus erythematosus patients. J Dermatol Sci (2017) 86:230–7. doi: 10.1016/j.jdermsci.2017.03.006
135. Dorgham K, Amoura Z, Parizot C, Arnaud L, Frances C, Pionneau C, et al. Ultraviolet light converts propranolol, a nonselective β-blocker and potential lupus-inducing drug, into a proinflammatory AhR ligand. Eur J Immunol (2015) 45:3174–87. doi: 10.1002/eji.201445144
136. O'Driscoll CA, Owens LA, Gallo ME, Hoffmann EJ, Afrazi A, Han M, et al. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Particle Fibre Toxicol (2018) 15:35. doi: 10.1186/s12989-018-0271-3
137. Tsai PC, Ko YC, Huang W, Liu HS, Guo YL. Increased liver and lupus mortalities in 24-year follow-up of the Taiwanese people highly exposed to polychlorinated biphenyls and dibenzofurans. Sci Total Environ (2007) 374:216–22. doi: 10.1016/j.scitotenv.2006.12.024
138. Li J, McMurray RW. Effects of chronic exposure to DDT and TCDD on disease activity in murine systemic lupus erythematosus. Lupus (2009) 18:941–9. doi: 10.1177/0961203309104431
139. Mustafa A, Holladay S, Witonsky S, Zimmerman K, Manari A, Countermarsh S, et al. Prenatal TCDD causes persistent modulation of the postnatal immune response, and exacerbates inflammatory disease, in 36-week-old lupus-like autoimmune SNF1 mice. Birth Defects Res Part B Dev Reprod Toxicol (2011) 92:82–94. doi: 10.1002/bdrb.20285
140. Ghaussy NO, Sibbitt WL Jr., Qualls CR. Cigarette smoking, alcohol consumption, and the risk of systemic lupus erythematosus: a case-control study. J Rheumatol (2001) 28:2449–53.
141. Costenbader KH, Karlson EW. Cigarette smoking and systemic lupus erythematosus: a smoking gun? Autoimmunity (2005) 38:541–7. doi: 10.1080/08916930500285758
142. Ekblom-Kullberg S, Kautiainen H, Alha P, Leirisalo-Repo M, Julkunen H. Smoking and the risk of systemic lupus erythematosus. Clin Rheumatol (2013) 32:1219–22. doi: 10.1007/s10067-013-2224-4
143. Muto H, Takizawa Y. Dioxins in cigarette smoke. Arch Environ Health (1989) 44:171–4. doi: 10.1080/00039896.1989.9935882
144. Sanchez-Guerrero J, Karlson EW, Liang MH, Hunter DJ, Speizer FE, Colditz GA. Past use of oral contraceptives and the risk of developing systemic lupus erythematosus. Arthritis Rheumatism (1997) 40:804–8. doi: 10.1002/art.1780400505
145. Shim GJ, Kis LL, Warner M, Gustafsson JA. Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci United States America (2004) 101:1720–4. doi: 10.1073/pnas.0307915100
146. Jara LJ, Medina G, Cruz-Dominguez P, Navarro C, Vera-Lastra O, Saavedra MA. Risk factors of systemic lupus erythematosus flares during pregnancy. Immunologic Res (2014) 60:184–92. doi: 10.1007/s12026-014-8577-1
147. Sánchez-Guerrero J, Liang MH, Karlson EW, Hunter DJ, Colditz GA. Postmenopausal estrogen therapy and the risk for developing systemic lupus erythematosus. Ann Internal Med (1995) 122:430–3. doi: 10.7326/0003-4819-122-6-199503150-00005
148. Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. New Engl J Med (2005) 353:2550–8. doi: 10.1056/NEJMoa051135
149. Shabanova SS, Ananieva LP, Alekberova ZS, Guzov II. Ovarian function and disease activity in patients with systemic lupus erythematosus. Clin Exp Rheumatol (2008) 26:436–41.
150. Tarnow P, Tralau T, Luch A. Chemical activation of estrogen and aryl hydrocarbon receptor signaling pathways and their interaction in toxicology and metabolism. Expert Opin Drug Metab Toxicol (2019) 15:219–29. doi: 10.1080/17425255.2019.1569627
151. Astroff B, Safe S. 2,3,7,8-tetrachlorodibenzo-p-dioxin as an antiestrogen: effect on rat uterine peroxidase activity. Biochem Pharmacol (1990) 39:485–8. doi: 10.1016/0006-2952(90)90054-O
152. Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology (2010) 268:132–8. doi: 10.1016/j.tox.2009.09.007
153. Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature (2003) 423:545–50. doi: 10.1038/nature01606
154. Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature (2007) 446:562–6. doi: 10.1038/nature05683
155. Shan J, Jin H, Xu Y. T Cell metabolism: A new perspective on Th17/Treg cell imbalance in systemic lupus erythematosus. Front Immunol (2020) 11:1027. doi: 10.3389/fimmu.2020.01027
156. Li D, Guo B, Wu H, Tan L, Chang C, Lu Q. Interleukin-17 in systemic lupus erythematosus: A comprehensive review. Autoimmunity (2015) 48:353–61. doi: 10.3109/08916934.2015.1037441
157. Zhu Y, Huang Y, Ming B, Wu X, Chen Y, Dong L. Regulatory T-cell levels in systemic lupus erythematosus patients: a meta-analysis. Lupus (2019) 28:445–54. doi: 10.1177/0961203319828530
158. Yu H, Jiang L, Liu R, Yang A, Yang X, Wang L, et al. Association between the ratio of aryl hydrocarbon receptor (AhR) in Th17 cells to AhR in treg cells and SLE skin lesions. Int Immunopharmacol (2019) 69:257–62. doi: 10.1016/j.intimp.2019.01.039
159. Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol (2019) 19:184–97. doi: 10.1038/s41577-019-0125-8
160. Udompornpitak K, Bhunyakarnjanarat T, Charoensappakit A, Dang CP, Saisorn W, Leelahavanichkul A. Lipopolysaccharide-enhanced responses against aryl hydrocarbon receptor in FcgRIIb-deficient macrophages, a profound impact of an environmental toxin on a lupus-like mouse model. Int J Mol Sci (2021) 22(8):4199. doi: 10.3390/ijms22084199
161. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukocyte Biol (2011) 90:1065–78. doi: 10.1189/jlb.0311114
162. Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L, et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Renal Nutr Off J Council Renal Nutr Natl Kidney Foundation (2012) 22:50–7. doi: 10.1053/j.jrn.2011.03.002
163. Wang ZL, Luo XF, Li MT, Xu D, Zhou S, Chen HZ, et al. Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PloS One (2014) 9:e114792. doi: 10.1371/journal.pone.0114792
164. Li W, Li H, Zhang M, Wang M, Zhong Y, Wu H, et al. Quercitrin ameliorates the development of systemic lupus erythematosus-like disease in a chronic graft-versus-host murine model. Am J Physiol Renal Physiol (2016) 311:F217–26. doi: 10.1152/ajprenal.00249.2015
165. Wu GC, Xu XD, Huang Q, Wu H. Leflunomide: friend or foe for systemic lupus erythematosus? Rheumatol Int (2013) 33:273–6. doi: 10.1007/s00296-012-2508-z
166. O'Donnell EF, Saili KS, Koch DC, Kopparapu PR, Farrer D, Bisson WH, et al. The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PloS One (2010) 5(10):e13128. doi: 10.1371/journal.pone.0013128
167. Mohammadi S, Memarian A, Sedighi S, Behnampour N, Yazdani Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity (2018) 51:199–209. doi: 10.1080/08916934.2018.1494161
168. Mondanelli G, Coletti A, Greco FA, Pallotta MT, Orabona C, Iacono A, et al. Positive allosteric modulation of indoleamine 2,3-dioxygenase 1 restrains neuroinflammation. Proc Natl Acad Sci United States America (2020) 117:3848–57. doi: 10.1073/pnas.1918215117
169. Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatism (2012) 64:2937–46. doi: 10.1002/art.34502
170. He J, Ma J, Ren B, Liu A. Advances in systemic lupus erythematosus pathogenesis via mTOR signaling pathway. Semin Arthritis Rheumatism (2020) 50:314–20. doi: 10.1016/j.semarthrit.2019.09.022
171. Anderson G. Tumour microenvironment: Roles of the aryl hydrocarbon receptor, O-GlcNAcylation, acetyl-CoA and melatonergic pathway in regulating dynamic metabolic interactions across cell types-tumour microenvironment and metabolism. Int J Mol Sci 22 (2020) 22(1):141. doi: 10.3390/ijms22010141
172. Shi F, Aloufi N, Traboulsi H, Trempe JF, Eidelman DH, Baglole CJ. Endogenous regulation of the akt pathway by the aryl hydrocarbon receptor (AhR) in lung fibroblasts. Sci Rep (2021) 11:23189. doi: 10.1038/s41598-021-02339-3
173. Ruvolo PP. Role of protein phosphatases in the cancer microenvironment. Biochim Biophys Acta Mol Cell Res (2019) 1866:144–52. doi: 10.1016/j.bbamcr.2018.07.006
174. Barthelemy C, Barry AO, Twyffels L, André B. FTY720-induced endocytosis of yeast and human amino acid transporters is preceded by reduction of their inherent activity and TORC1 inhibition. Sci Rep (2017) 7:13816. doi: 10.1038/s41598-017-14124-2
175. Shimoyama S, Kasai S, Kahn-Perlès B, Kikuchi H. Dephosphorylation of Sp1 at ser-59 by protein phosphatase 2A (PP2A) is required for induction of CYP1A1 transcription after treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin or omeprazole. Biochim Biophys Acta (2014) 1839:107–15. doi: 10.1016/j.bbagrm.2013.12.004
176. Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol (Baltimore Md. 1950) (2009) 182:2063–73. doi: 10.4049/jimmunol.0803600
177. Lai ZW, Borsuk R, Shadakshari A, Yu J, Dawood M, Garcia R, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol (Baltimore Md. 1950) (2013) 191:2236–46. doi: 10.4049/jimmunol.1301005
178. Lai ZW, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet (London England) (2018) 391:1186–96. doi: 10.1016/S0140-6736(18)30485-9
179. Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol (2012) 52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537
180. Li T, Liu J, Guo G, Ning B, Li X, Zhu G, et al. Synphilin-1 interacts with AMPK and increases AMPK phosphorylation. Int J Mol Sci (2020) 21(12):4352. doi: 10.3390/ijms21124352
181. Curran CS, Gupta S, Sanz I, Sharon E. PD-1 immunobiology in systemic lupus erythematosus. J Autoimmun (2019) 97:1–9. doi: 10.1016/j.jaut.2018.10.025
182. Celhar T, Fairhurst AM. Toll-like receptors in systemic lupus erythematosus: potential for personalized treatment. Front Pharmacol (2014) 5:265. doi: 10.3389/fphar.2014.00265
183. Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol (Baltimore Md. 1950) (2014) 192:5459–68. doi: 10.4049/jimmunol.1002795
184. Chuang HC, Tsai CY, Hsueh CH, Tan TH. GLK-IKKβ signaling induces dimerization and translocation of the AhR-RORγt complex in IL-17A induction and autoimmune disease. Sci Adv (2018) 4:eaat5401. doi: 10.1126/sciadv.aat5401
185. Chuang HC, Chen YM, Chen MH, Hung WT, Yang HY, Tseng YH, et al. AhR-ROR-γt complex is a therapeutic target for MAP4K3/GLK(high)IL-17A(high) subpopulation of systemic lupus erythematosus. FASEB J Off Publ Fed Am Societies Exp Biol (2019) 33:11469–80. doi: 10.1096/fj.201900105RR
186. Rehwinkel J, Gack MU. RIG-i-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol (2020) 20:537–51. doi: 10.1038/s41577-020-0288-3
187. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol (2009) 27:229–65. doi: 10.1146/annurev.immunol.021908.132715
188. Flaveny CA, Perdew GH. Transgenic humanized AHR mouse reveals differences between human and mouse AHR ligand selectivity. Mol Cell Pharmacol (2009) 1:119–23. doi: 10.4255/mcpharmacol.09.15
Keywords: aryl hydrocarbon receptor (Ah receptor or AhR), systemic lupus erythematosus (SLE), immunology, autoimmune disease (AD), AhR ligands
Citation: Wu J, Pang T, Lin Z, Zhao M and Jin H (2022) The key player in the pathogenesis of environmental influence of systemic lupus erythematosus: Aryl hydrocarbon receptor. Front. Immunol. 13:965941. doi: 10.3389/fimmu.2022.965941
Received: 10 June 2022; Accepted: 01 August 2022;
Published: 30 August 2022.
Edited by:
Andras Perl, Upstate Medical University, United StatesReviewed by:
Saeed Mohammadi, Golestan University of Medical Sciences, IranXiao-song Wang, First Affiliated Hospital of Anhui Medical University, China
Copyright © 2022 Wu, Pang, Lin, Zhao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Jin, amgyMDIwQGNzdS5lZHUuY24=
 Jingwen Wu
Jingwen Wu Tianyi Pang
Tianyi Pang Ziyuan Lin
Ziyuan Lin Ming Zhao
Ming Zhao Hui Jin
Hui Jin