- Department of Dermatology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan
Bullous pemphigoid (BP) is the most common autoimmune subepidermal blistering disease. Although the pathomechanism of BP onset has yet to be elucidated in detail, BP autoantibodies targeting two hemidesmosomal components, BP180 and BP230, are known to play a pivotal role in BP pathogenesis. Thus, the detection and measurement of BP autoantibodies are necessary for diagnosing BP and monitoring the disease activity. Immune assays such as immunofluorescence microscopy, immunoblotting, and ELISAs using BP180 and BP230 detect BP autoantibodies in most BP cases with high specificity; however, BP autoantibodies are sometimes detected in BP patients before the onset of this disease. BP autoantibodies that are detected in patients without typical tense blisters are defined as “preclinical BP autoantibodies”. These preclinical BP autoantibodies are detected even in a low percentage of normal healthy individuals. Although the importance of preclinical BP autoantibodies remains elusive, these autoantibodies might be a potential risk factor for subsequent BP development. Therefore, previous comparative epidemiological studies have focused on the prevalence of preclinical BP autoantibodies in populations susceptible to BP (e.g., the elderly) or in diseases with a higher risk of comorbid BP. This mini-review summarizes the literature on the prevalence of preclinical BP autoantibodies in patients with various conditions and diseases, and we discuss the significance of preclinical BP autoantibody detection.
Introduction
Bullous pemphigoid (BP), a major autoimmune blistering disease, is characterized clinically by tense blisters and/or urticarial erythema and histologically by subepidermal blisters with abundant eosinophilic infiltration (1, 2). Autoantibodies directed to the basement membrane zone (BMZ) play an essential role in BP pathogenesis. BP autoantibodies mainly target two hemidesmosomal components: BP180 and BP230, and anti-BP180 autoantibodies have been recognized as the major pathogenic autoantibodies in BP (3–5). Furthermore, the 16th non-collagenous (NC16A) domain has been identified as a major pathogenic epitope of BP autoantibodies because 80–90% of BP sera show positive reactivity to the NC16A domain within BP180 (6, 7).
Regarding BP diagnosis, the detection of BP autoantibodies is necessary in addition to clinical and histological findings (1, 8). However, the presence of BP autoantibodies is not a sufficient condition for diagnosis. In fact, the production of BP autoantibodies precedes clinical tense blister formation in certain BP cases (9). Therefore, previous studies have investigated the prevalence of preclinical BP autoantibodies as a potential risk for subsequent BP development in various conditions, including aging, pruritus, collagen diseases, and neurological disorders.
Detection methods for BP autoantibodies
BP autoantibodies are present in BP patients in two forms: tissue-bound autoantibodies in lesional skin and circulating autoantibodies in peripheral blood. The former is detected with direct immunofluorescence (DIF) microscopy; the latter is detected with indirect immunofluorescence (IIF) microscopy, ELISA, and immunoblotting. With DIF, which is the most sensitive method of finding BP autoantibodies, those antibodies show up as the linear deposition of IgG and/or C3 at the dermal-epidermal junction of lesional skin (10). Several studies suggested a new subset of IgG4-dominant BP, which predominantly has IgG4 autoantibodies without complement activation at the BMZ (11–13). However, the linear deposition of C3 at the BMZ is a promising diagnostic sign for BP (14). The limitation of DIF is that it is difficult to differentiate autoantibodies of BP from those of other subepidermal autoimmune blistering diseases.
IIF semi-quantitates the titration of circulating BP autoantibodies using skin or esophagus cryosections from a healthy individual. Using 1 M NaCl-split skin sections, IIF allows differentiation between BP and other autoimmune subepidermal blistering skin diseases. In 1 M NaCl-split skin IIF (ssIIF), reactivity against the epidermal side corresponds to BP, while reactivity against the dermal side corresponds to anti-laminin 332 mucous membrane pemphigoid, epidermolysis bullosa acquisita, and anti-p200 pemphigoid (also called anti-laminin gamma 1 pemphigoid). Immunoblotting using epidermal extracts or recombinant proteins of BP autoantigens is also available to detect circulating BP autoantibodies in limited facilities.
ELISA using the BP180 NC16A recombinant protein (BP180 NC16A ELISA) is a commercially available method of detecting and quantifying BP autoantibodies. BP180 NC16A ELISA has a sensitivity of 84.4% and a specificity of 98.9% in diagnosing BP (6). Furthermore, the titration of BP autoantibodies quantified by BP180 NC16A ELISA closely correlates with BP activity (15); thus, BP180 NC16A ELISA is also useful for monitoring BP. Also, an ELISA using tetrameric NC16A recombinant proteins is commercially available (16). A few laboratories have developed ELISAs that use non-NC16A regions of BP180 and full-length BP180 to diagnose BP. Hofmann et al. showed that 54/116 (46.6%) of BP sera reacted to BP180 COOH-terminal regions, i.e., aa1351–1497 (17). Using a random BP180 epitope library displayed on a bacteriophage, 31/57 (54.4%) of BP sera reacted to at least one additional region other than BP180 NC16A, aa490–562, within the extracellular (36.8%) or intracellular (28.1%) domains of BP180 (18). An ELISA using BP180 aa1080–1107 and aa1331–1404 detected anti-BP180 autoantibodies in 32/78 (41.0%) of BP sera (19). A multicenter prospective study showed that distinct epitopes of the BP180 ectodomain other than BP180 NC16A are recognized by 95.9% of BP sera (20). An ELISA using human full-length BP180 recombinant proteins (a full-length BP180 ELISA) was able to detect anti-BP180 autoantibodies targeting the NC16A domain as well as other regions of BP180 for 83.5% of BP sera (21).
BIOCHIP is a unique IIF-based serologic diagnostic assay for detecting autoantibodies of autoimmune blistering diseases (22). The BIOCHIP technique provides autoimmune blistering-related antibody profiles of patient sera in a single incubation on the mosaic slide. BIOCHIP contains a recombinant BP180 NC16A protein, and the sensitivity of anti-BP180 NC16A autoantibodies in BP detected by BIOCHIP is 55.3–100.0% (22–31).
Although IIF, WB, ELISA, and BIOCHIP can detect anti-BP180 antibodies, the results can differ. Among BP180 ELISA-positive BP patients, positive rates for IIF, immunoblot, and BIOCHIP were 77.3–83.3%, 69.0–95.5%, and 83.3–100.0%, respectively (25, 26, 29, 32, 33). Low-titer anti-BP180 autoantibodies detected by ELISA may be confirmed by IIF or immunoblotting.
Prevalence of preclinical anti-BP180 autoantibodies in normal healthy individuals
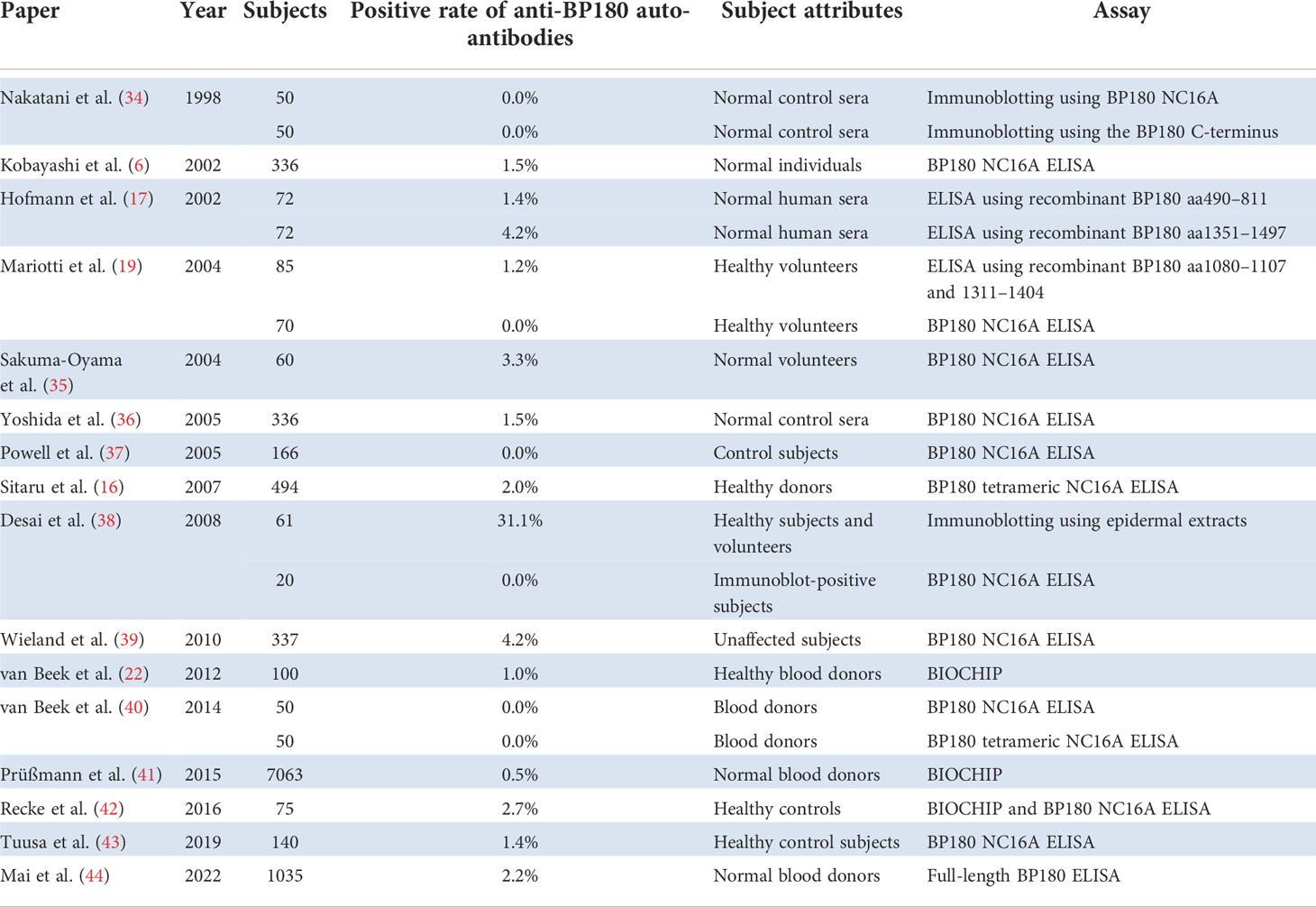
We reviewed the studies evaluating preclinical anti-BP180 autoantibodies in normal healthy individuals and listed representative studies whose populations exceeded 50 (Table 1). BP180 NC16A ELISA detected anti-BP180 autoantibodies in 0.0–4.2% of normal healthy individuals (6, 16, 19, 35–40, 42, 43). Wieland et al. showed that 14 (4.15%) of 337 unaffected subject sera were positive by BP180 NC16A ELISA, and the mean titer of ELISA-positive sera was 16.2 ± 6.3 (mean ± standard deviation, SD, cutoff value <9.0) (39). According to other studies, the mean titers of normal subjects by BP180 NC16A ELISA were 2.1–2.5 (35, 37, 43). The mean titer of ELISA-positive sera was higher in the Wieland et al. study than in the other studies. However, these ELISA-positive sera had no positive reactivities toward a monkey esophagus by IIF (39). ELISAs using the mid-portion (aa490–811) or the C-terminal domain (aa1351–1497) of BP180 detected anti-BP180 autoantibodies in 1.4% and 4.2% of sera from normal healthy individuals, respectively (17).
Regarding immunoblotting, none of the normal control sera showed positive reactivity against GST-fusion proteins containing 42 amino acids of the NC16A region nor 49 amino acids of the C-terminal region of BP180 (34). Immunoblotting using epidermal extracts detected BP autoantibodies in 31% of healthy individuals, while BP180 NC16A ELISA did not detect BP autoantibodies in a population of those who were seropositive to epidermal extracts (38). For BIOCHIP, 0.5–1.0% of healthy blood donors showed reactivity to the NC16A domain of BP180 (22, 41).
An observational study to evaluate the prevalence of anti-BP180 antibodies in healthy volunteers by using a full-length BP180 ELISA was conducted in Japan (44). 2.2% of serum samples from the healthy volunteers possessed anti-BP180 autoantibodies without the volunteers having any skin lesions. The mean titer ± SD of ELISA-positive sera was 13.9 ± 16.4 (cutoff value <4.64), whereas the mean titer of the parent population was 0.8 ± 3.2. In addition, 12/23 (52.1%) of ELISA-positive sera were positive by IIF using monkey esophagus. Intriguingly, the existence of anti-BP180 autoantibodies is associated with a history of bone fracture and the administration of anti-osteoporosis drugs (44).
We also compared the prevalence of preclinical anti-BP180 autoantibodies among the detection systems. The mean ± SD (range) prevalence for immunoblot, ELISA, and BIOCHIP was 10.4 ± 18.0 (0–31.1), 1.6 ± 1.4 (0.0–4.2), 1.4 ± 1.2 (0.5–2.7), respectively (6, 16, 17, 19, 22, 34–44). Although the high prevalence of anti-BP180 autoantibodies detected by immunoblotting in the Desai et al. study was not confirmed by ELISA (38), immunoblotting might detect preclinical anti-BP180 autoantibodies more efficiently than the other detection methods do.
Prevalence of preclinical anti-BP180 autoantibodies in BP-susceptible populations or in patients with diseases associated with comorbid BP
We also reviewed the studies evaluating preclinical anti-BP180 autoantibodies in various medical conditions and listed the representative studies whose populations exceeded 20 (Table 2).
Aging and pruritus
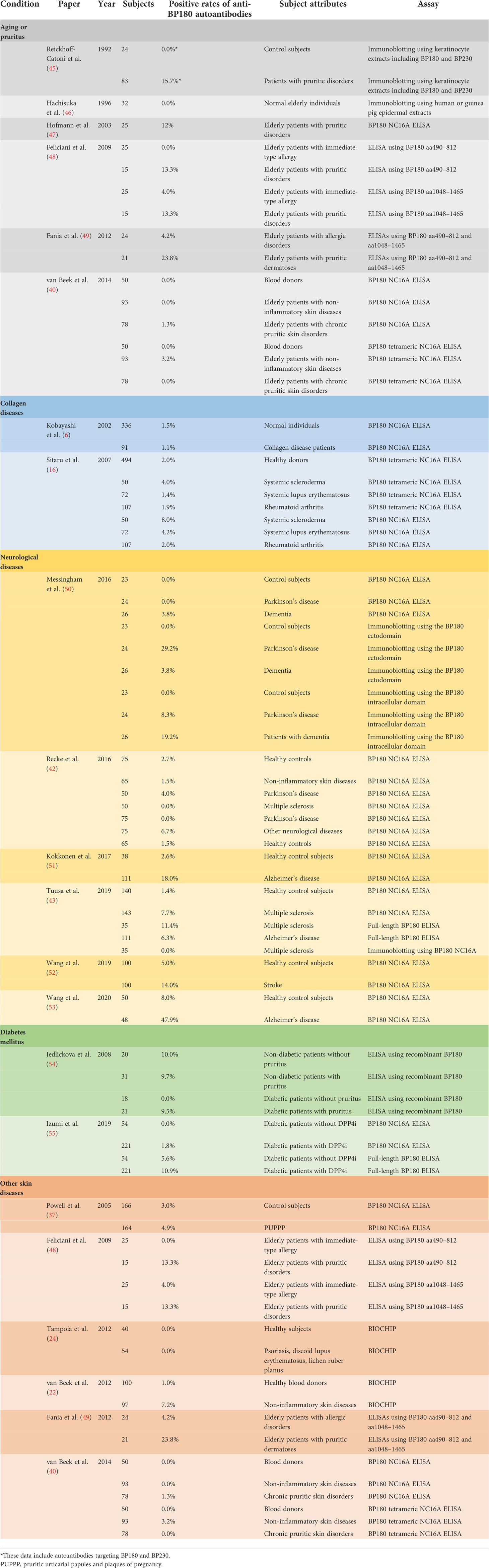
BP predominantly occurs in individuals over age 60 (56). In a retrospective observational study of 869 patients with BP in the United Kingdom, the median age at presentation for BP was 80 years (57). Two epidemiological studies found the incidence of BP to be significantly higher for people in their ninth or tenth decade than for people of other ages (58, 59). Thus, aging has been recognized as a significant risk factor for BP onset. Several studies investigated the prevalence of anti-BP180 autoantibodies in the elderly (40, 45–49, 60). Interestingly, Hachisuka et al. showed no preclinical anti-BP180 autoantibodies in 32 normal elderly individuals (46). In addition, large retrospective studies using unaffected subjects or a general population in Japan did not find a significant difference between age and the prevalence of anti-BP180 autoantibodies (39, 44).
Pruritus is a common problem among elderly people (61), and pruritus may develop in the early stage of BP (2, 62–64). Several studies reported the prevalence of preclinical anti-BP180 autoantibodies in elderly patients with pruritic disorders (40, 47–49). Hofmann et al. showed that three (12.0%) of 25 elderly patients with pruritic disorders had anti-BP180 autoantibodies, and the mean titer ± SD of these positive sera was 18.1 ± 4.6 (47). However, DIF, ssIIF, and immunoblotting using cultured keratinocyte extracts were negative among all elderly patients with pruritic disorders. Feliciani et al. investigated preclinical anti-BP180 autoantibodies using ELISAs with the mid-portion (aa490–812) or C-terminus (aa1048–1465) of BP180 in elderly patients with pruritic disorders (48). Both BP180 mid-portion and C-terminus ELISAs detected preclinical BP autoantibodies in two (13.3%) of 15 elderly patients with pruritic disorders, whereas one (4.0%) of 25 elderly patients with immediate-type allergy had preclinical anti-BP180 autoantibodies. Fania et al. showed a higher prevalence of preclinical anti-BP180 autoantibodies (23.8%) in elderly patients with pruritic disorders than that in elderly patients with allergic diseases (4.2%) by BP180 N-terminus and C-terminus ELISAs (49). In contrast, van Beek et al. found no significant difference in the prevalence of anti-BP180 autoantibodies among elderly patients with chronic disorders or non-inflammatory skin diseases and blood donors using a BP180 NC16A ELISAs (40). These results are conflicting, and further studies are needed.
Collagen disease
Several case reports showed that collagen diseases such as systemic lupus erythematosus, dermatomyositis, rheumatoid arthritis, or systemic sclerosis can co-exist with BP (65–73). Although it remains unclear whether collagen diseases are a risk factor for BP development, two studies explored preclinical anti-BP180 autoantibodies in a collagen disease population while evaluating the diagnostic performance of BP180 NC16A ELISAs (6, 16). These studies showed the prevalence of anti-BP180 autoantibodies to be 1.1–8.0% in collagen disease patients, which is comparable with the 1.5–2.0% in healthy controls.
Neurological disease
Previous epidemiological studies suggest that neurological diseases are a risk factor for BP development (74–76). A Finnish epidemiological study indicated that multiple sclerosis (MS) and Alzheimer’s disease increase the risk of BP development (77, 78). As neurological diseases are associated with BP, several studies examined the prevalence of preclinical anti-BP180 autoantibodies in patients with neurological disorders (42, 43, 50, 51).
Messingham et al. investigated the prevalence of BP autoantibodies in patients with Parkinson’s disease and dementia using a BP180 NC16A ELISA and immunoblotting using the BP180 ectodomain or intracellular domain (50). BP180 NC16A ELISA detected BP autoantibodies in 3.8% of the dementia patients but not in any of the Parkinson’s disease patients nor in the control subjects. Immunoblotting using the BP180 ectodomain found BP autoantibodies in 29.2% of the Parkinson’s disease patients, whereas immunoblotting using the BP180 intracellular domain detected BP autoantibodies in 19.2% of the dementia patients. Tuusa et al. indicated that BP autoantibodies were detected in 11.8% of MS sera with the full-length BP180 ELISA, while BP autoantibodies were detected in 53.8% of MS sera with immunoblotting using the full-length BP180, suggesting that BP autoantibodies from MS sera preferentially react to the denatured form of BP180 but not to its native form (43). A conflicting result was reported by Recke et al. (42). Although BIOCHIP, BP180 NC16A ELISA, BP230 ELISA, and immunoblotting with the extracellular matrix of cultured human keratinocytes were used to detect anti-BMZ antibodies in MS and Parkinson’s disease patients, MS and Parkinson’s disease showed no significant increased prevalence of BP autoantibodies (42). Wang et al. recently reported that anti-BP180 NC16A autoantibodies were found in 14 (14.0%) of 100 patients with stroke, and the mean titer ± SD was 19.2 ± 6.1 (52). Among these patients, 12 (85.7%) of the 14 positive sera were also positive by immunoblotting using BP180 recombinant proteins. The prevalence of BP autoantibodies detected by BP180 NC16A ELISA was found to be 18.0% in Alzheimer’s disease patients (51). Interestingly, subsequent analysis found the severity of dementia to correlate significantly with the titration of anti-BP180 NC16A autoantibodies in Alzheimer’s disease (51). In addition, Wang et al. reported that 20 of 48 (47.9%) patients with Alzheimer’s disease had anti-BP180 autoantibodies detected by BP180 NC16A ELISA, and the mean titer ± SD was 22.9 ± 20.9 (53). Among these patients, 9 (64.3%) of the 14 positive sera were also positive by immunoblotting using BP180 recombinant proteins.
Diabetes mellitus
BP may initially mimic other pruritic dermatoses, and it is known that DM is often associated with pruritic dermatoses (79). Therefore, the potential association between DM and the early phase of BP has been discussed. To address this issue, Jedlickova tested the seropositivity of anti-BMZ antibodies by IIF and ELISAs using recombinant BP180, BP230, and Laminin 332 (54). There were no significant differences in the seropositivity of anti-BMZ autoantibodies among non-diabetic patients or diabetic patients with or without pruritus; however, the prevalence of anti-BMZ autoantibodies in all groups detected by IIF was relatively high (12.2%). Recently dipeptidyl peptidase-IV inhibitors (DPP4i), medications for type 2 diabetes treatment, have attracted interest as a causative drug of BP (80–86). Izumi et al. investigated the prevalence of anti-BMZ autoantibodies among 275 DM patients with or without DPP4i administration (55). BP180 NC16A ELISA, BP230 ELISA, and full-length BP180 ELISA detected BP autoantibodies in 1.8%, 2.2%, and 10.9% of 221 DM patients with DPP4i treatment, respectively, whereas these assays detected BP autoantibodies in 0%, 7.4%, and 5.6% of 54 DM patients without DPP4i treatment, respectively. Among these patients, 54.2% of full-length BP180 ELISA-positive sera were also positive by ssIIF. Although there were significant differences in the prevalence of anti-full-length BP180 IgG between DM cases with and without DPP4i treatment in this study, the anti-full-length BP180 autoantibody-positive cases are likely to have been significantly older than the anti-full-length BP180 IgG-negative cases in the DM cases with DPP4i.
Other skin diseases
For the validation of anti-BP180 autoantibody detection, several studies performed the detection of anti-BP180 autoantibodies in patients with various skin diseases as a negative control for the detection system (16, 76). van Beek et al. reported that 7.2% of patients with non-inflammatory skin diseases, including leg ulcers, basal cell carcinoma, and squamous cell carcinoma, had anti-BP180 autoantibodies detected by BIOCHIP, although the details were not mentioned (22). For investigating the prevalence of anti-BP180 autoantibodies in elderly patients with pruritic disorders, elderly patients with allergic disorders or with non-inflammatory skin diseases were used as a control group (48, 49). In these studies, positive rates of anti-BP180 autoantibodies were 0.0–4.2% in elderly patients with allergic disorders detected by ELISAs using BP180 aa490–812 and aa1048–1465 (48, 49), and 1.3–3.2% in elderly patients with non-inflammatory skin diseases (40).
In addition, Powell et al. examined whether BP180 NC16A ELISA was able to differentiate pemphigoid gestationis, which is a blistering autoimmune disease mediated by anti-BP180 autoantibodies during pregnancy, from polymorphic urticarial papules and plaques of pregnancy (PUPPP) (37). In this study, 4.9% of the PUPPP patients showed seropositivity with BP180 NC16A ELISA.
Prodromal BP
The diagnosis of BP is usually made by the combination of clinical, histopathological, and immunological findings described above. Typical tense blisters are an essential clue in diagnosing BP, and it is challenging to diagnose BP in cases without blisters. A prospective nationwide cohort study found that BP was diagnosed an average of 6.1 months after onset of the first symptoms (87). Cohort studies showed that 17–20% of BP presented with no apparent blisters at the time of BP diagnosis (2, 87). There is the concept of prodromal BP (62, 88–90). Retrospective studies showed that 31.8% and 36.8% of BP patients had non-bullous lesions before the appearance of typical blisters (90, 91). The duration before blister development ranged from 2 weeks to 19 years: That duration was up to 12 months for 82% of the patients in the Sun et al. study, and the mean duration before blister appearance was 15.9 months in the Zhang et al. study (90, 91). This non-bullous stage of BP may lead to a delayed diagnosis.
Also, a subtype called non-bullous pemphigoid has been reported (63, 92–94). In a systematic review of non-bullous pemphigoid described by Lamberts et al., the most common clinical presentations of patients with non-bullous pemphigoid were erythematous, urticarial plaques and papules/nodules, and 9.8% of the non-bullous pemphigoid patients developed bullae during the reported follow-up (63). In addition, an IgM autoantibody-mediated pemphigoid disease called IgM pemphigoid has been reported (95–97), and IgM autoantibodies targeting BP180 might play a pathogenic role in IgM pemphigoid (95, 96). It is debatable whether non-bullous pemphigoid and IgM pemphigoid are just prodromes of BP or are distinct pemphigoid variants.
The detection of anti-BP180 autoantibodies might be an indicator for subsequent BP development. Wang et al. retrospectively analyzed medical records from 2005 to 2015 from a single center and found 208 BP autoantibody-positive patients with BP180 and/or BP230 ELISA but not with DIF (98). Dermatitis was the most common diagnosis among preclinical BP autoantibody-positive patients; of note, four patients had positive DIF results upon repeating the tests, and a diagnosis of BP was made during follow-up (98). Raneses et al. showed that 4 of 18 BP patients had positive ani-BP180 or anti-BP230 autoantibodies 79 months (range: 0.1 to 217 months) before the onset of BP symptoms (9).
Concluding remarks
Certain patients with various medical conditions such as aging with pruritus, neurological diseases, and diabetes mellitus with the administration of DPP4i can have anti-BP180 autoantibodies without BP development. However, it remains controversial whether the existence of anti-BP180 autoantibodies is a predictive factor for BP development. Further studies on preclinical BP autoantibodies promise to reveal whether the presence of anti-BP180 autoantibodies is a predictive marker for BP development and to identify the critical factors contributing to BP onset in the presence of BP autoantibodies.
Author contributions
YM, KI, and SM drafted the paper. HU supervised the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BP, bullous pemphigoid; BMZ, basement membrane zone; NC16A, the 16th non-collagenous A domain; ELISA, enzyme-linked immunosorbent assay; DIF, direct immunofluorescence analysis; IIF, indirect immunofluorescence analysis; ssIIF, 1M NaCl-split skin indirect immunofluorescence analysis; SD, standard deviation; MS, multiple sclerosis; DM, diabetes mellitus; DPP4i, dipeptidyl peptidase-IV inhibitor; DPP4i-BP, dipeptidyl peptidase-IV inhibitor-associated bullous pemphigoid; PUPPP, polymorphic urticarial papules and plaques of pregnancy.
References
1. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet (2013) 381:320–32. doi: 10.1016/s0140-6736(12)61140-4
2. Di Zenzo G, della Torre R, Zambruno G, Borradori L. Bullous pemphigoid: From the clinic to the bench. Clin Dermatol (2012) 30:3–16. doi: 10.1016/j.clindermatol.2011.03.005
3. Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, et al. Humanization of autoantigen. Nat Med (2007) 13:378–83. doi: 10.1038/nm1496
4. Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest (1993) 92:2480–8. doi: 10.1172/jci116856
5. Ujiie H, Shibaki A, Nishie W, Sawamura D, Wang G, Tateishi Y, et al. A novel active mouse model for bullous pemphigoid targeting humanized pathogenic antigen. J Immunol (2010) 184:2166–74. doi: 10.4049/jimmunol.0903101
6. Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci (2002) 30:224–32. doi: 10.1016/s0923-1811(02)00109-3
7. Matsumura K, Amagai M, Nishikawa T, Hashimoto T. The majority of bullous pemphigoid and herpes gestationis serum samples react with the NC16a domain of the 180-kDa bullous pemphigoid antigen. Arch Dermatol Res (1996) 288:507–9. doi: 10.1007/bf02505245
8. Ujiie H, Iwata H, Yamagami J, Nakama T, Aoyama Y, Ikeda S, et al. Japanese Guidelines for the management of pemphigoid (including epidermolysis bullosa acquisita). J Dermatol (2019) 46:1102–35. doi: 10.1111/1346-8138.15111
9. Raneses E, Simpson MM, Rosenberg A, Coffman M, Meyerle J. A retrospective review of autoimmune bullous disease antibody positivity prior to clinical symptoms. J Am Acad Dermatol (2021) 86:237–9. doi: 10.1016/j.jaad.2021.02.003
10. Sárdy M, Kostaki D, Varga R, Peris K, Ruzicka T. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol (2013) 69:748–53. doi: 10.1016/j.jaad.2013.07.009
11. Dainichi T, Chow Z, Kabashima K. IgG4, complement, and the mechanisms of blister formation in pemphigus and bullous pemphigoid. J Dermatol Sci (2017) 88:265–70. doi: 10.1016/j.jdermsci.2017.07.012
12. Dainichi T, Nishie W, Yamagami Y, Sonobe H, Ujiie H, Kaku Y, et al. Bullous pemphigoid suggestive of complement-independent blister formation with anti-BP180 IgG4 autoantibodies. Br J Dermatol (2016) 175:187–90. doi: 10.1111/bjd.14411
13. Yoshimoto N, Takashima S, Kawamura T, Inamura E, Sugai T, Ujiie I, et al. A case of non-bullous pemphigoid induced by IgG4 autoantibodies targeting BP230. J Eur Acad Dermatol (2021) 35:e282–5. doi: 10.1111/jdv.17044
14. Hammers CM, Stanley JR. Mechanisms of disease: Pemphigus and bullous pemphigoid. Annu Rev Pathol (2015) 11:1–23. doi: 10.1146/annurev-pathol-012615-044313
15. Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol (2000) 136:174–8. doi: 10.1001/archderm.136.2.174
16. Sitaru C, Dähnrich C, Probst C, Komorowski L, Blöcker I, Schmidt E, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol (2007) 16:770–7. doi: 10.1111/j.1600-0625.2007.00592.x
17. Hofmann SC, Thoma-Uszynski S, Stauber A, Schuler G, Hertl M, Hunziker T, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol (2002) 119:1065–73. doi: 10.1046/j.1523-1747.2002.19529.x
18. Di Zenzo G, Grosso F, Terracina M, Mariotti F, Mastrogiacomo A, Sera F, et al. Characterization of the anti-BP180 autoantibody reactivity profile and epitope mapping in bullous pemphigoid patients. J Invest Dermatol (2004) 122:103–10. doi: 10.1046/j.0022-202x.2003.22126.x
19. Mariotti F, Grosso F, Terracina M, Ruffelli M, Cordiali-Fei P, Sera F, et al. Development of a novel ELISA system for detection of anti-BP180 IgG and characterization of autoantibody profile in bullous pemphigoid patients. Br J Dermatol (2004) 151:1004–10. doi: 10.1111/j.1365-2133.2004.06245.x
20. Di Zenzo G, Thoma-Uszynski S, Fontao L, Calabresi V, Hofmann SC, Hellmark T, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol (2008) 128:415–26. doi: 10.1016/j.clim.2008.04.012
21. Izumi K, Nishie W, Mai Y, Wada M, Natsuga K, Ujiie H, et al. Autoantibody profile differentiates between inflammatory and noninflammatory bullous pemphigoid. J Invest Dermatol (2016) 136:2201–10. doi: 10.1016/j.jid.2016.06.622
22. van Beek N, Rentzsch K, Probst C, Komorowski L, Kasperkiewicz M, Fechner K, et al. Serological diagnosis of autoimmune bullous skin diseases: Prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis (2012) 7:49. doi: 10.1186/1750-1172-7-49
23. Damoiseaux J, van Rijsingen M, Warnemünde N, Dähnrich C, Fechner K, Tervaert JWC. Autoantibody detection in bullous pemphigoid: Clinical evaluation of the EUROPLUSTM dermatology mosaic. J Immunol Methods (2012) 382:76–80. doi: 10.1016/j.jim.2012.05.007
24. Tampoia M, Zucano A, Villalta D, Antico A, Bizzaro N. Anti-skin specific autoantibodies detected by a new immunofluorescence multiplex biochip method in patients with autoimmune bullous diseases. Dermatology (2012) 225:37–44. doi: 10.1159/000339776
25. Zarian H, Saponeri A, Michelotto A, Zattra E, Belloni-Fortina A, Alaibac M. Biochip technology for the serological diagnosis of bullous pemphigoid. ISRN Dermatol (2012) 2012:237802. doi: 10.5402/2012/237802
26. Özkesici B, Mutlu D, Dönmez L, Uzun S. The value of the BIOCHIP mosaic-based indirect immunofluorescence technique in the diagnosis of pemphigus and bullous pemphigoid in Turkish patients. Acta Dermatovenerol Croat (2017) 25:202–9.
27. Gornowicz-Porowska J, Seraszek-Jaros A, Bowszyc-Dmochowska M, Kaczmarek E, Pietkiewicz P, Bartkiewicz P, et al. Accuracy of molecular diagnostics in pemphigus and bullous pemphigoid: Comparison of commercial and modified mosaic indirect immunofluorescence tests as well as enzyme-linked immunosorbent assays. Postȩpy Dermatol Alergol (2017) 34:21–7. doi: 10.5114/ada.2017.65617
28. Yang A, Xuan R, Murrell DF. A new indirect immunofluorescence BIOCHIP method for the serological diagnosis of bullous pemphigoid: A review of literature. Australas J Dermatol (2019) 60:e173–7. doi: 10.1111/ajd.13034
29. Adaszewska A, Kalińska-Bienias A, Jagielski P, Woźniak K, Kowalewski C. The use of BIOCHIP mosaics in diagnostics of bullous pemphigoid: Evaluation and comparison to conventional multistep procedures. J Cutan Pathol (2020) 47:121–7. doi: 10.1111/cup.13591
30. Yang A, Xuan R, Melbourne W, Tran K, Murrell DF. Validation of the BIOCHIP test for the diagnosis of bullous pemphigoid, pemphigus vulgaris and pemphigus foliaceus. J Eur Acad Dermatol (2020) 34:153–60. doi: 10.1111/jdv.15770
31. Simpson K, Scardamaglia L, Kok Y, Vu M, Kidd D, Yap T, et al. Comparison of the EUROIMMUN dermatology profile ELISA to the novel BIOCHIP mosaic 7 for the diagnosis of immunobullous skin disease. Australas J Dermatol (2021) 62:314–22. doi: 10.1111/ajd.13611
32. Charneux J, Lorin J, Vitry F, Antonicelli F, Reguiai Z, Barbe C, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: A retrospective study of 138 patients. Arch Dermatol (2011) 147:286–91. doi: 10.1001/archdermatol.2011.23
33. Chan Y-C, Sun Y-J, Ng PP-L, Tan S-H. Comparison of immunofluorescence microscopy, immunoblotting and enzyme-linked immunosorbent assay methods in the laboratory diagnosis of bullous pemphigoid. Clin Exp Dermatol (2003) 28:651–6. doi: 10.1046/j.1365-2230.2003.01419.x
34. Nakatani C, Muramatsu T, Shirai T. Immunoreactivity of bullous pemphigoid (BP) autoantibodies against the NC16A and c-terminal domains of the 180 kDa BP antigen (BP180): Immunoblot analysis and enzyme-linked immunosorbent assay using BP180 recombinant proteins. Br J Dermatol (1998) 139:365–70. doi: 10.1046/j.1365-2133.1998.02396.x
35. Sakuma-Oyama Y, Powell AM, Oyama N, Albert S, Bhogal BS, Black MM. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol (2004) 151:126–31. doi: 10.1111/j.1365-2133.2004.06082.x
36. Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci (2006) 41:21–30. doi: 10.1016/j.jdermsci.2005.11.002
37. Powell AM, Sakuma-Oyama Y, Oyama N, Albert S, Bhogal B, Kaneko F, et al. Usefulness of BP180 NC16a enzyme-linked immunosorbent assay in the serodiagnosis of pemphigoid gestationis and in differentiating between pemphigoid gestationis and pruritic urticarial papules and plaques of pregnancy. Arch Dermatol (2005) 141:705–10. doi: 10.1001/archderm.141.6.705
38. Desai N, Allen J, Ali I, Venning V, Wojnarowska F. Autoantibodies to basement membrane proteins BP180 and BP230 are commonly detected in normal subjects by immunoblotting. Australas J Dermatol (2008) 49:137–41. doi: 10.1111/j.1440-0960.2008.00452.x
39. Wieland CN, Comfere NI, Gibson LE, Weaver AL, Krause PK, Murray JA. Anti–bullous pemphigoid 180 and 230 antibodies in a sample of unaffected subjects. Arch Dermatol (2010) 146:21–5. doi: 10.1001/archdermatol.2009.331
40. van Beek N, Dohse A, Riechert F, Krull V, Recke A, Zillikens D, et al. Serum autoantibodies against the dermal–epidermal junction in patients with chronic pruritic disorders, elderly individuals and blood donors prospectively recruited. Br J Dermatol (2014) 170:943–7. doi: 10.1111/bjd.12739
41. Prüßmann W, Prüßmann J, Koga H, Recke A, Iwata H, Juhl D, et al. Prevalence of pemphigus and pemphigoid autoantibodies in the general population. Orphanet J Rare Dis (2015) 10:63. doi: 10.1186/s13023-015-0278-x
42. Recke A, Oei A, Hübner F, Fechner K, Graf J, Hagenah J, et al. Parkinson Disease and multiple sclerosis are not associated with autoantibodies against structural proteins of the dermal–epidermal junction. Br J Dermatol (2016) 175:407–9. doi: 10.1111/bjd.14538
43. Tuusa J, Lindgren O, Tertsunen H-M, Nishie W, Kokkonen N, Huilaja L, et al. BP180 autoantibodies target different epitopes in multiple sclerosis or alzheimer’s disease than in bullous pemphigoid. J Invest Dermatol (2019) 139:293–9. doi: 10.1016/j.jid.2018.09.010
44. Mai Y, Izumi K, Sawada K, Akasaka E, Mai S, Sawamura D, et al. A 1,035-subject study suggesting a history of bone fracture as a possible factor associated with the development of anti-BP180 autoantibodies. J Invest Dermatol (2021) 142:984–987.e3. doi: 10.1016/j.jid.2021.11.028
45. Rieckhoff-Cantoni L, Bernard P, Didierjean L, Imhof K, Loës SK, Saurat JH. Frequency of bullous pemphigoid–like antibodies as detected by Western immunoblot analysis in pruritic dermatoses. Arch Dermatol (1992) 128:791–4. doi: 10.1001/archderm.1992.01680160075007
46. Hachisuka H, Kurose K, Karashima T, Mori O, Maeyama Y. Serum from normal elderly individuals contains anti–basement membrane zone antibodies. Arch Dermatol (1996) 132:1201–5. doi: 10.1001/archderm.1996.03890340061010
47. Hofmann SC, Tamm K, Hertl M, Borradori L. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol (2003) 149:910–2. doi: 10.1046/j.1365-2133.2003.05603.x
48. Feliciani C, Caldarola G, Kneisel A, Podstawa E, Pfütze M, Pfützner W, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol (2009) 161:306–12. doi: 10.1111/j.1365-2133.2009.09266.x
49. Fania L, Caldarola G, Müller R, Brandt O, Pellicano R, Feliciani C, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol (2012) 143:236–45. doi: 10.1016/j.clim.2012.02.003
50. Messingham KAN, Aust S, Helfenberger J, Parker KL, Schultz S, McKillip J, et al. Autoantibodies to collagen XVII are present in parkinson’s disease and localize to tyrosine-hydroxylase positive neurons. J Invest Dermatol (2016) 136:721–3. doi: 10.1016/j.jid.2015.12.005
51. Kokkonen N, Herukka SK, Huilaja L, Kokki M, Koivisto AM, Hartikainen P, et al. Increased levels of the bullous pemphigoid BP180 autoantibody are associated with more severe dementia in alzheimer’s disease. J Invest Dermatol (2017) 137:71–6. doi: 10.1016/j.jid.2016.09.010
52. Wang Y, Mao X, Wang D, Hammers CM, Payne AS, Wang Y, et al. Anti-BP180 autoantibodies are present in stroke and recognize human cutaneous BP180 and BP180-NC16A. Front Immunol (2019) 10:236. doi: 10.3389/fimmu.2019.00236
53. Wang YN, Hammers CM, Mao X, Jin H-Z, Yuan J, Li L. Analysis of the autoimmune response against BP180 in patients with alzheimer’s disease. Ann Transl Med (2020) 9:107–7. doi: 10.21037/atm-20-5343
54. Jedlickova H, Racovska J, Niedermeier A, Feit J, Hertl M. Anti-basement membrane zone antibodies in elderly patients with pruritic disorders and diabetes mellitus. Eur J Dermatol (2008) 18:534–8. doi: 10.1684/ejd.2008.0483
55. Izumi K, Nishie W, Beniko M, Shimizu H. A cross-sectional study comparing the prevalence of bullous pemphigoid autoantibodies in 275 cases of type II diabetes mellitus treated with or without dipeptidyl peptidase-IV inhibitors. Front Immunol (2019) 10:1439. doi: 10.3389/fimmu.2019.01439
56. Wertenteil S, Garg A, Strunk A, Alloo A. Prevalence estimates for pemphigoid in the united states: A sex-adjusted and age-adjusted population analysis. J Am Acad Dermatol (2019) 80:655–9. doi: 10.1016/j.jaad.2018.08.030
57. Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJP, West J. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. BMJ (2008) 337:160–3. doi: 10.1136/bmj.a180
58. Marazza G, Pham HC, Schärer L, Pedrazzetti PP, Hunziker T, Trüeb RM, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: A 2-year prospective study. Br J Dermatol (2009) 161:861–8. doi: 10.1111/j.1365-2133.2009.09300.x
59. Bertram F, Bröcker E, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in lower franconia, Germany. J Dtsch Dermatol Ges (2009) 7:434–9. doi: 10.1111/j.1610-0387.2008.06976.x
60. Bakker CV, Terra JB, Pas HH, Jonkman MF. Bullous pemphigoid as pruritus in the elderly: A common presentation. JAMA Dermatol (2013) 149:950–3. doi: 10.1001/jamadermatol.2013.756
61. Berger TG, Shive M, Harper GM. Pruritus in the older patient: A clinical review. JAMA (2013) 310:2443–50. doi: 10.1001/jama.2013.282023
62. Lamb PM, Abell E, Tharp M, Frye R, Deng J. Prodromal bullous pemphigoid. Int J Dermatol (2006) 45:209–14. doi: 10.1111/j.1365-4632.2004.02457.x
63. Lamberts A, Meijer JM, Jonkman MF. Nonbullous pemphigoid: A systematic review. J Am Acad Dermatol (2017) 78:989–995.e2. doi: 10.1016/j.jaad.2017.10.035
64. Cozzani E, Gasparini G, Burlando M, Drago F, Parodi A. Atypical presentations of bullous pemphigoid: Clinical and immunopathological aspects. Autoimmun Rev (2015) 14:438–45. doi: 10.1016/j.autrev.2015.01.006
65. Glover M, Leigh I. Dermatomyositis pemphigoides: A case with coexistent dermatomyositis and bullous pemphigoid. J Am Acad Dermatol (1992) 27:849–52. doi: 10.1016/0190-9622(92)70264-g
66. Yanagi T, Kato N, Yamane N, Osawa R. Bullous pemphigoid associated with dermatomyositis and colon carcinoma. Clin Exp Dermatol (2007) 32:291–4. doi: 10.1111/j.1365-2230.2007.02368.x
67. Huang C, Chen T. Bullous pemphigoid associated with systemic lupus erythematosus: the discrimination of antibasement membrane zone antibody. Int J Dermatol (1997) 36:40–2. doi: 10.1111/j.1365-4362.1997.tb03301.x
68. Loche F, Bernard P, Bazex J. Bullous pemphigoid associated with systemic lupus erythematosus. Br J Dermatol (1998) 139:927–8. doi: 10.1046/j.1365-2133.1998.02534.x
69. Yoshikawa N, Matsubara E, Yamamoto M, Yamazaki H, Uehara M, Kamata M, et al. Drug-induced bullous pemphigoid and lupus erythematosus occurring under anti-TNF-α and IL-6 therapy in a patient with rheumatoid arthritis. Internal Med (2020) 59:2611–8. doi: 10.2169/internalmedicine.4646-20
70. Giannini JM, Callen JP, Gruber GG. Bullous pemphigoid and rheumatoid arthritis. J Am Acad Dermatol (1981) 4:695–7. doi: 10.1016/s0190-9622(81)80202-2
71. Sant SM, O’Loughlin S, Murphy GM. Bullous pemphigoid and rheumatoid arthritis: Is there disease association? Ir J Med Sci (1997) 166:106–7. doi: 10.1007/bf02944199
72. Cozzani E, Cioni M, Gariazzo L, Gallo R, Parodi A. Bullous pemphigoid and systemic sclerosis: An incidental association? Eur J Dermatol (2017) 27:413–4. doi: 10.1684/ejd.2017.3012
73. Dahl MV. Bullous pemphigoid: Associated diseases. Clin Dermatol (1987) 5:64–70. doi: 10.1016/0738-081x(87)90051-4
74. Taghipour K, Chi C-C, Bhogal B, Groves RW, Venning V, Wojnarowska F. Immunopathological characteristics of patients with bullous pemphigoid and neurological disease. J Eur Acad Dermatol (2014) 28:569–73. doi: 10.1111/jdv.12136
75. Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: A population-based case–control study. J Invest Dermatol (2011) 131:631–6. doi: 10.1038/jid.2010.357
76. Jedlickova H, Hlubinka M, Pavlik T, Semradova V, Budinska E, Vlasin Z. Bullous pemphigoid and internal diseases – a case-control study. Eur J Dermatol (2010) 20:096–101. doi: 10.1684/ejd.2010.0805
77. Lai YC, Yew YW, Lambert WC. Bullous pemphigoid and its association with neurological diseases: A systematic review and meta-analysis. J Eur Acad Dermatol (2016) 30:2007–15. doi: 10.1111/jdv.13660
78. Försti A-K, Jokelainen J, Ansakorpi H, Seppänen A, Majamaa K, Timonen M, et al. Psychiatric and neurological disorders are associated with bullous pemphigoid – a nationwide Finnish care register study. Sci Rep (2016) 6:37125. doi: 10.1038/srep37125
79. Tseng HW, Ger LP, Liang CK, Liou HH, Lam HC. High prevalence of cutaneous manifestations in the elderly with diabetes mellitus: An institution-based cross-sectional study in Taiwan. J Eur Acad Dermatol (2015) 29:1631–5. doi: 10.1111/jdv.12664
80. Liu SD, Chen WT, Chi CC. Association between medication use and bullous pemphigoid. JAMA Dermatol (2020) 156:891–900. doi: 10.1001/jamadermatol.2020.1587
81. Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol (2012) 26:249–53. doi: 10.1111/j.1468-3083.2011.04062.x
82. Béné J, Moulis G, Bennani I, Auffret M, Coupe P, Babai S, et al. Centres the FA of RP. bullous pemphigoid and dipeptidyl peptidase IV inhibitors: A case–noncase study in the French pharmacovigilance database. Br J Dermatol (2016) 175:296–301. doi: 10.1111/bjd.14601
83. Lee SG, Lee HJ, Yoon MS, Kim DH. Association of dipeptidyl peptidase 4 inhibitor use with risk of bullous pemphigoid in patients with diabetes. JAMA Dermatol (2019) 155:172–7. doi: 10.1001/jamadermatol.2018.4556
84. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: Estimating the risk of the new agents and characterizing the patients. JAMA Dermatol (2018) 154:1152. doi: 10.1001/jamadermatol.2018.2352
85. Benzaquen M, Borradori L, Berbis P, Cazzaniga S, Valero R, Richard M-A, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: Retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol (2018) 78:1090–6. doi: 10.1016/j.jaad.2017.12.038
86. Varpuluoma O, Försti A-K, Jokelainen J, Turpeinen M, Timonen M, Huilaja L, et al. Vildagliptin significantly increases the risk of bullous pemphigoid: A Finnish nationwide registry study. J Invest Dermatol (2018) 138:1659–61. doi: 10.1016/j.jid.2018.01.027
87. della Torre R, Combescure C, Cortés B, Marazza G, Beltraminelli H, Naldi L, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: A prospective nationwide cohort. Br J Dermatol (2012) 167:1111–7. doi: 10.1111/j.1365-2133.2012.11108.x
88. Amato DA, Silverstein J, Zitelli J. The prodrome of bullous pemphigoid. Int J Dermatol (1988) 27:560–3. doi: 10.1111/j.1365-4362.1988.tb02405.x
89. Nakatani T, Inaoki M, Takehara K. Bullous pemphigoid with a prolonged prodrome. J Dermatol (2008) 35:433–6. doi: 10.1111/j.1346-8138.2008.00498.x
90. Zhang Y, Luo Y, Han Y, Tian R, Li W, Yao X. Non-bullous lesions as the first manifestation of bullous pemphigoid: A retrospective analysis of 181 cases. J Dermatol (2017) 44:742–6. doi: 10.1111/1346-8138.13782
91. Sun C, Chang B, Gu H. Non-bullous lesions as the first manifestation of bullous pemphigoid: A retrospective analysis of 24 cases. J Dermatol Treat (2009) 20:233–7. doi: 10.1080/09546630802683876
92. Lamberts A, Meijer JM, Pas HH, Diercks GFH, Horváth B, Jonkman MF. Nonbullous pemphigoid: Insights in clinical and diagnostic findings, treatment responses, and prognosis. J Am Acad Dermatol (2019) 81:355–63. doi: 10.1016/j.jaad.2019.04.029
93. Meijer JM, Diercks GFH, de Lang EWG, Pas HH, Jonkman MF. Assessment of diagnostic strategy for early recognition of bullous and nonbullous variants of pemphigoid. JAMA Dermatol (2019) 155:158–65. doi: 10.1001/jamadermatol.2018.4390
94. Strohal R, Rappersberger K, Pehamberger H, Wolff K. Nonbullous pemphigoid: Prodrome of bullous pemphigoid or a distinct pemphigoid variant? J Am Acad Dermatol (1993) 29:293–9. doi: 10.1016/0190-9622(93)70179-w
95. Boch K, Hammers CM, Goletz S, Kamaguchi M, Ludwig RJ, Schneider SW, et al. Immunoglobulin M pemphigoid. J Am Acad Dermatol (2021) 85:1486–1492. doi: 10.1016/j.jaad.2021.01.017
96. Hirano Y, Iwata H, Tsujuwaki M, Mai S, Mai Y, Imafuku K, et al. Super-resolution imaging detects BP180 autoantigen in immunoglobulin m pemphigoid. J Dermatol (2022) 49:374–8. doi: 10.1111/1346-8138.16260
97. Baardman R, Horváth B, Bolling MC, Pas HH, Diercks GFH. Immunoglobulin m bullous pemphigoid: An enigma. JAAD Case Rep (2020) 6:518–20. doi: 10.1016/j.jdcr.2020.04.008
Keywords: bullous pemphigoid, autoantibodies, BP180, anti-BP180 autoantibodies, autoimmune disease, aging, diabetes mellitus, neurological disease
Citation: Mai Y, Izumi K, Mai S and Ujiie H (2022) The significance of preclinical anti-BP180 autoantibodies. Front. Immunol. 13:963401. doi: 10.3389/fimmu.2022.963401
Received: 07 June 2022; Accepted: 18 July 2022;
Published: 08 August 2022.
Edited by:
Markus H. Hoffmann, University of Lübeck, GermanyReviewed by:
Christoph M. Hammers, University of Lübeck, GermanyXuming Mao, University of Pennsylvania, United States
Copyright © 2022 Mai, Izumi, Mai and Ujiie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kentaro Izumi, ay5penVtaUBtZWQuaG9rdWRhaS5hYy5qcA==
 Yosuke Mai
Yosuke Mai Kentaro Izumi
Kentaro Izumi Shoko Mai
Shoko Mai Hideyuki Ujiie
Hideyuki Ujiie