- 1Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Upper Airways Research Laboratory, Department of Otorhinolaryngology, Ghent University, Ghent, Belgium
- 3Division of Otorhinolaryngology Diseases, Department of Clinical Sciences, Intervention and Technology (CLINTEC), Karolinska Institute, Stockholm, Sweden
In the airway, IgE is traditionally regarded as a key mediator in allergic diseases, such as AR and allergic asthma. However, growing evidence demonstrates the importance of local IgE in airway inflammatory diseases, irrespective of the presence of allergy. In this review, we discuss the most recent evidence for IgE in chronic rhinosinusitis with nasal polyps(CRSwNP), including the local IgE’s characteristics, the modulation of its synthesis, and its function. The levels of local IgE are significantly elevated in polyps independently of IgE serum levels and atopic status. Local IgE, which is correlated with type 2 inflammation, is polyclonal and functional. IgE is produced by active B cells and is dependent on the class switch recombination(CSR). In NPs, this process is triggered by not only allergens but also microbial colonization, especially the superantigen- Staphylococcus aureus. The production of local IgE is modulated by lymphocytes(such as Tfh, ILC2s, iTreg), cytokines(such as IL-4, IL-13, IFN-γ, TGF-β, IL-2, IL-21), transcription factors, and B cell-intrinsic factor. Due to the central role of IgE in NPs, it is regarded as an ideal target for therapy and has been proved to be clinically successful. Based on this knowledge, we believe that exploring the trigger and regulatory factors for the activation of local B cells and CSR to IgE will provide more valuable information for us to recognize the pathological mechanisms of local IgE and offer the possible option for new therapeutic targets of nasal polyps.
Introduction
Immunoglobulin E(IgE) has an essential function in immunity to parasites and type I hypersensitivity. Although IgE is typically the least abundant isotype, it is one of the responsible antibodies for the classical adaptive immune response and is capable of triggering the most powerful inflammatory reactions after binding to its specific receptors (FcϵRI, the high-affinity IgE receptor; FcϵRII, also known as CD23, the low-affinity IgE receptor).
Traditionally, IgE is regarded as a key mediator in the pathophysiology of allergic diseases, such as allergic rhinitis(AR), allergic asthma, anaphylaxis, and food allergy. People with a tendency to develop allergic reactions when exposed to antigens initially produce antigen-specific IgE during sensitization. Subsequent exposure to its specific sensitizing allergen, IgE mediates antigen cross-linking of the high-affinity IgE receptor (FcϵRI) on a variety of cells (e.g. mast cells, basophils, dendritic cells, monocytes, and smooth muscle cells). Release of mediators by mast cells and basophils follows, triggering classic symptoms of allergy (nasal congestion, wheezing, sneezing, whealing, etc.)
However, our concept of the role of IgE in disease states is undergoing reassessment and re-evaluation., Growing evidence shows that IgE also plays a role in non-allergic airway diseases. Increased local IgE production was found in nasal polyps(NPs) regardless of systemic atopic status (1–4). Class switch recombination(CSR) to IgE was reported in the bronchial mucosa of both atopic and nonatopic patients with asthma (5). In addition, our clinical trials showed that omalizumab was effective in CRSwNP patients with or without asthma. independent of results of allergy tests (6). All these studies indicate the importance of local IgE in the airway, irrespective of the presence of allergy. Exploring the biological functions of IgE in these “non-allergic” diseases, therefore, is meaningful in terms of therapeutic interventions.
Even if IgE is involved in both allergic and nonallergic airway diseases, its role may vary in these diseases. In AR, IgE concentrations in serum and mucosal tissue homogenates are highly correlated, while they fail to show relevant correlations in chronic rhinosinusitis with nasal polyps(CRSwNP) and comorbid asthma (7). Although in both cases allergen-specific, IgE in AR and allergic asthma is often oligoclonal or monoclonal, while IgE in CRSwNP, often comorbid late-onset non-allergic asthma, is predominantly polyclonal (7). Furthermore, the formation of germinal center(GC)-like structures in NPs, has not been reported in the nasal mucosa of AR (8–10).
In this review, we focused on the role of IgE in NPs. We summarized the characteristics of local IgE in NPs, then analyzed the possible pathway of local IgE synthesis and its modulation, and finally discussed the function of IgE in NPs, including recent information from targeting IgE.
The characteristics of IgE in NPs
IgE is significantly increased
In NPs, tissue IgE appears to be increased in patients from all regions (11). The concentrations of total IgE are significantly increased in mucosal tissue compared with those in serum and they were also significantly elevated compared with those in non-NP tissue (7, 12, 13); this has been reported in both European and Asian populations (8, 14, 15). In addition, the IgE concentrations in mucosal tissue homogenates showed no correlation with those in serum, implying the possibility of local IgE production rather than the tissue IgE representing overspill from the peripheral blood. Moreover, IgE-positive plasma cells, IgE-positive B cells, secondary lymphoid tissue, and GC-like structures were found in polyp tissue, adding another argument for the local production of IgE in NPs (8, 10, 16, 17). Further evidence for local IgE synthesis within the polyp tissue has come from studies of cultured B cells derived from NPs secreting high levels of IgE antibodies in vitro (18). Interestingly, local hyper-immunoglobulinaemia E was present in both atopic and nonatopic patients (1, 2, 12) and unrelated to skin test results (3, 4). These data suggest that continuous local IgE production occurs, which is derived from many different B cell clones, even in the absence of a specific allergen stimulus or in the presence of a so far unknown allergen, to maintain IgE levels.
Local IgE is polyclonal and functional
IgE antibody in CRSwNP appear to be polyclonal and covers a broad spectrum of allergens/antigens rather than oligoclonal ones like in AR (7, 19). Local IgE in the nasal polyp mucosa is pro-inflammatory. The ability of polyclonal IgE to activate mast cells in polyps was first proposed after observations in tissue from patients with NP vs. AR. Then, mucosal IgE antibody in NP was found to induce mast cell degranulation in response to numerous inhalant allergens (7). Meanwhile, polyclonal IgE in polyp homogenates promoted the CD23-mediated pro-allergic responses and induced basophil activation and histamine release (19). Thus, distinguishing from the oligoclonal IgE in AR, which only reacts to specific antigens, the IgE in NPs is polyclonal, which reacts to multiple antigens and derives from many B cells; Then, the polyclonal IgE can initiate mediators releasing via interactions with FcϵRI on mast cells or FcϵRII(CD23) on basophil, finally contributing to the persistent local inflammation in CRSwNP.
Local IgE is involved in T2 inflammation
The Type2(T2) inflammation in NPs includes higher levels of local IgE, IL-5 (20), IL-4, IL-25, IL-33 (21), thymic stromal lymphopoietin(TSLP) (22), eotaxin-2, monocyte chemoattractant protein(MCP)-4 (23), and tissue eosinophilia. A T2 inflammation is typical in European CRSwNP, while less prevalent in patients from Asia (11, 24). In recent years, increasing T2-dominated profiles have been observed in Asian CRSwNP (25). The increased local IgE in nasal polyp tissue is correlated with increased Eosinophil cationic protein (ECP), IL-5, eosinophilic infiltration, and the presence of staphylococcal enterotoxin(SE)-IgE and asthma comorbidity (11, 12), implicating the role of IgE in the mediation of T2 inflammation. In the cluster analysis of CRS patients based on immunological biomarkers, the high IL-5 clusters showed increased levels of total IgE and SE-IgE in nasal tissues and also a higher frequency of comorbid asthma (26). Whereas 30-50% of CRSwNPs in Europe are associated with asthma, 57% of SE IgE-positive polyps and 64%-71% of polyps with high IgE levels show significantly higher comorbidity rates with asthma (27, 28).
The production of IgE in NPs
Local B cells are active in NPs
B cells differentiate into plasma cells and produce antibodies, so the differentiation and function of B cells in polyps directly influences the levels of local antibodies. In recent decades, the role of B cells and plasma cells has gained interest in CRSwNP(Figure 1). Early immunohistochemical and histopathologic research demonstrated that elevated expression of CD20 (naive B cell marker) and CD138 (plasma cell marker) were found in CRSwNP tissue vs inferior turbinate controls (8, 29), which were confirmed by a later flow cytometry study (30) and an immunofluorescence study (16). A recent report by Miljkovic D et al. demonstrated increased frequencies of naive and effector B–cell subtypes in the mucosa of patients with CRSwNP (31). Feldman S et al. found that CD19+CD27+CD38hi plasmablasts were dramatically increased in CRSwNPs, which also had a higher frequency of expression of Epstein Barr Virus-induced protein 2(EBI2), compared to tonsils samples (18). Furthermore, this report showed that nasal polyp-derived B cells secreted high levels of antibodies in vitro compared to tonsil-derived B cells, including IgG, IgA, and IgE antibodies, confirming the activation of B cells from NPs (32). This study was supported by elevated levels of all antibody isotypes in NPs, except IgD (13, 16, 30). These studies indicate the mechanisms of IgE overproduction in local polyp tissue. Nevertheless, the mechanisms that influence the local differentiation and function of B cells and antibody production remain unclear, and the pathways for the local activation of B cells are still controversial.
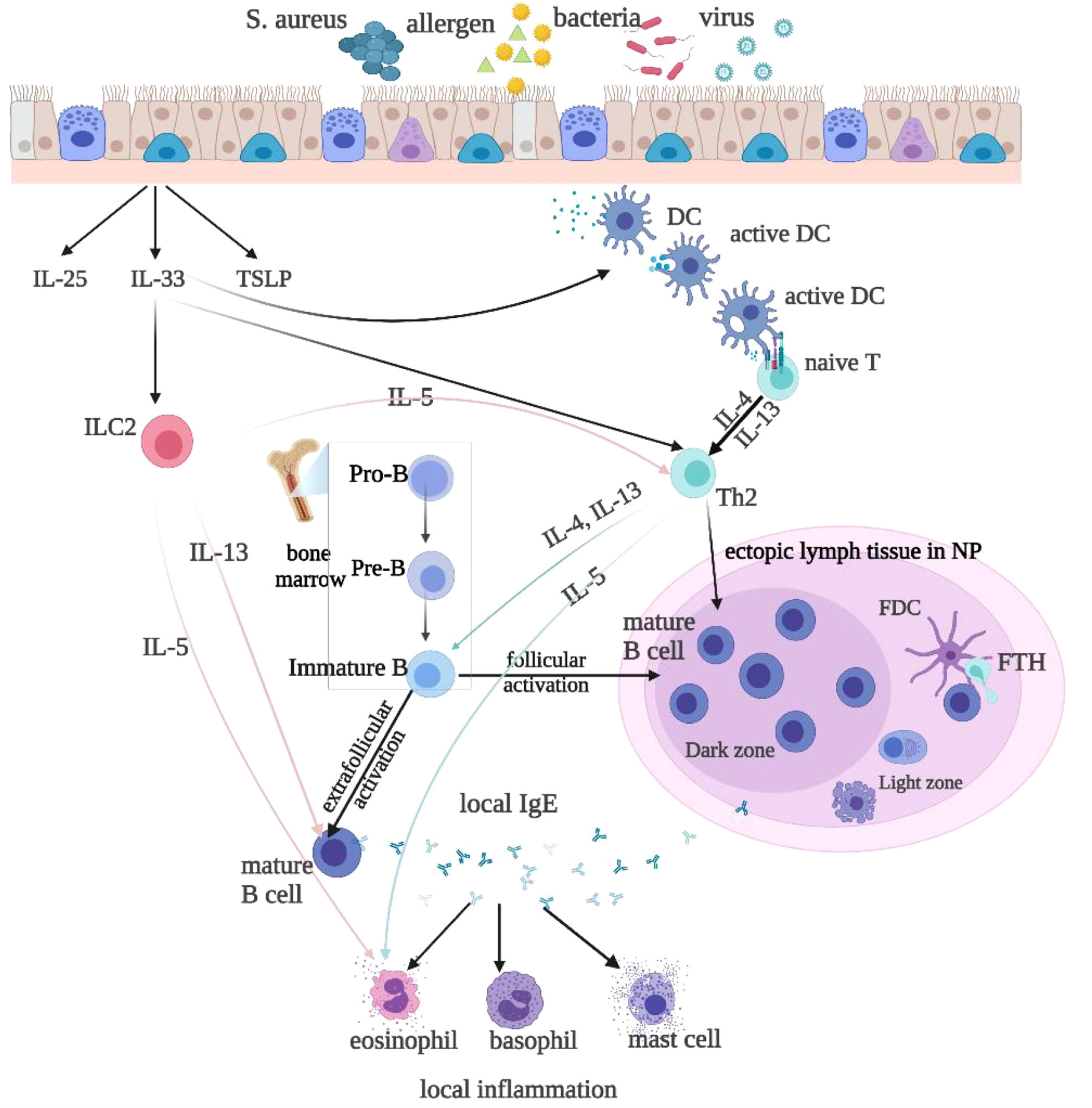
Figure 1 Local IgE is produced by follicular and extrafollicular activated B cells through class switch recombination. Microbial colonization, such as bacteria, rhinovirus, and allergens, especially the superantigen Staphylococcus aureus(SA), stimulate local IgE formation. The T follicular helper(Tfh) cells and Th2-like cells are helper cells for the B cell class switching to IgE. The Th2 cytokines IL-4 and IL-13 stimulate class switching to IgE and IgE formation. Type 2 innate lymphoid cells(ILC2s) produce Th2 cytokines, such as IL-4, IL-5, and IL-13, to promote type 2 inflammation, upon stimulation with epithelium-derived cytokines, such as IL-25, IL-33, and thymic stromal lymphopoietin(TSLP). IL-33 also activates DCs, which may via superantigens prime naive T cells to develop into Th2 cells. IL-4 and IL-13 then activate B-cells and initiate local IgE production. IL-5 released by ILC2s or Th2 cells activate eosinophils.
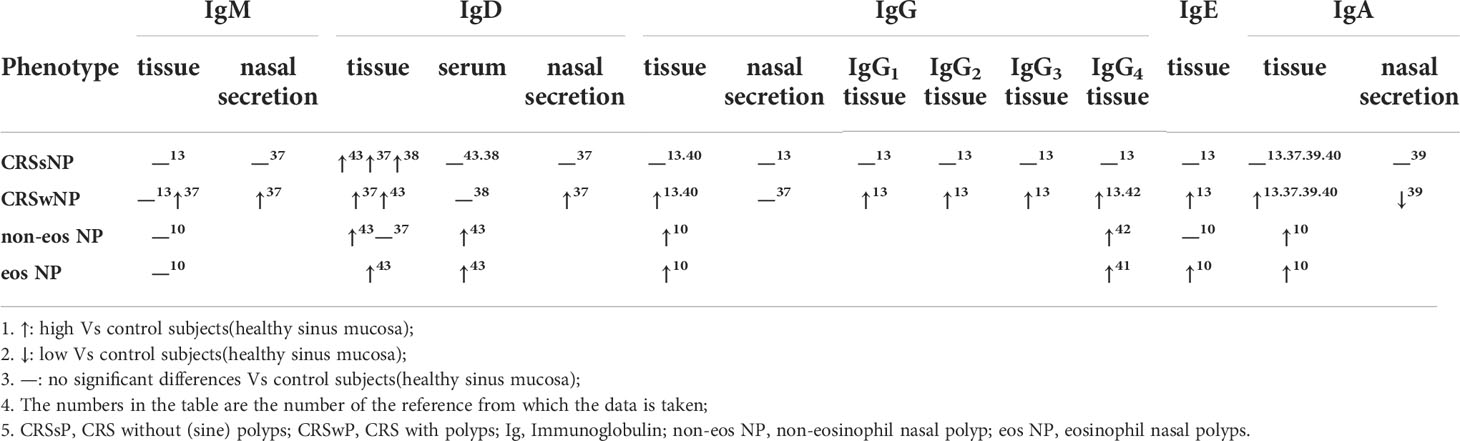
Local immunoglobulin production is enhanced in NPs
The activation of IgM- and IgD-producing B cells is followed by the generation of IgG-, IgE-, and IgA-producing B cells via class switching (Table 1). The levels of IgG, IgG subclasses(IgG1, IgG2, IgG3, and IgG4), IgM, IgE, and IgA were significantly increased in polyps compared with CRSsNP and controls samples, and showed little correlation with serum Ig levels (13). In addition, the levels of IgG, IgG4, and IgE were significantly increased in SAE IgE(+) NPs compared with SAE IgE(-) NP patients, while the levels of IgG2 were decreased in the SAE IgE(+) group (13). The number of IgM+, IgD+, and IgA+- plasma cells in tissue and the levels of IgM, IgD, IgA in nasal secretions were also significantly higher in CRSwNP than in CRSsNP, as confirmed by others (32–35). Also, significantly elevated IgE and IgG4 levels were observed in nasal polyp tissue from aspirin-exacerbated respiratory disease patients (36).
Classically, among these Ig isotypes, IgG4 and IgA can compete with IgE for binding to allergens, thus preventing the formation of allergen/IgE/receptor complexes. In NPs, recent studies also showed that locally elevated IgG could antagonize IgE-mediated proallergic inflammation (19, 37). Meanwhile, IgD-activated mast cells and PD-1highCXCR5-CD4+ T cells were found to participate in local immunoglobulin production independent of ectopic lymphoid tissues in patients with CRSwNP (38, 39). IL-5/Il-5α signaling was reported to enhance local B cell differentiation, proliferation, and survival, which could lead to increased generation of antibodies within the inflamed polyp tissue[41]. All these confirm the presence of locally generated IgE and locally activated B cells, although the complex mechanisms are not clear.
Local class switching to IgE is up-regulated in NPs
There is ample evidence that up-regulated class switching resulting in IgE production occurs in NPs. Local IgE levels and class switching to IgE were increased in polyp tissue compared with AR and controls tissues (16). Investigation of switch circle transcripts revealed ongoing local CSR to IgE, as evidenced by enhanced expression of key markers of the process, including IL-4, ϵ-germline gene transcripts(ϵ-GLT), ϵ-mRNA, and IgE protein. Increased local class switching to IgE and production of IgE were (17) also demonstrated to be significantly higher in terms of mRNA expression for ϵ-GLT, and enzyme activation-induced cytidine deaminase(AID) in NPs than in control tissues. In addition, the elevated expression of molecules required for Ig production, the generation of T follicular helper(Tfh) cells, and the production of IgE in eosinophilic polyps were shown (14). Furthermore, upon in vitro stimulation with Dermatophagoides pteronyssinus 1(Der p 1), a specific circle transcript of Iϵ-Cm (408 bp) produced during CSR from IgM to IgE was detected to be increased in eosinophilic NPs, meaning Ig class switching to IgE in eosinophilic polyps with ectopic lymphoid tissues (10). This evidence confirms the occurrence of local class switching to IgE in polyp tissue.
Modulation of local IgE production
Microbial colonization stimulates local IgE formation, especially SA
In nasal mucosa, local IgE production can be triggered not only by allergens (7, 40) but also by microbial pathogens, such as bacteria (40) and rhinovirus (41). Significantly higher levels of IgE antibodies specific for nasal bacteria, including Staphylococcus aureus(SA), Staphylococcus pyogenes, and Haemophilus influenzae, were found in polyps of patients with CRSwNP (42). Among these microbial pathogens, SA is one of the most important factors stimulating IgE formation in NPs. SA is a gram-positive bacterium and common human colonizer, and given the opportunity, it can become a dangerous pathogen (43, 44). Patients with NPs have been shown to have higher rates of SA colonization (63.6%) than those with CRSsNP(27.3%) and healthy adults(33%). Moreover, in the subgroup of NP with asthma and aspirin intolerance, SA colonization was present in 87% (45–47).
On the one hand, SA can act as an allergen and induce the formation of IgE with specificity for SEs in polyps, which is independent of serum total and specific IgE (12). Local SE-IgE has been demonstrated to rapidly degranulate mast cells and basophils upon contact with the specific antigen and to facilitate antigen binding to B cells (8, 19). Local SE-IgE is predictive of concomitant asthma and associated with severe local eosinophilic inflammation, suggesting the potential role of SE as a disease modifier (48). On the other hand, SA can release enterotoxins(i.e., SEs) that act as superantigens and effectively modify the functions of T and B cells, eosinophils, and other inflammatory as well as structural cells. Superantigens directly activate T cells by crosslinking the Vβ chain of T-cells to major histocompatibility complex class II on antigen-presenting cells(polyclonal activation), which is different from the T-cell activation induced by classical peptide antigens (46). Superantigens stimulate proliferation and effector function in Th2 cells, induce the release of IL-4 and IL-13, and stimulate class switching to IgE and finally IgE formation in CRSwNP (49). This activity is nonspecific and polyclonal and leads to massive inflammatory mediators release, resulting in inflammation. Meanwhile, staphylococcal protein A(SpA) produced by SA, can also act as a B-cell superantigen and induce multiclonal B cell activation, which also triggers the production of polyclonal IgE by plasma cells (49). Not only in nasal polyps, but also in asthma, specific IgE to SE-IgE can frequently be detected in serum and has been associated with severe asthma defined by hospitalizations, oral steroid use, and a decrease in lung function. Even serum SE-IgE was demonstrated to predict the development into severe asthma with exacerbations over the next decade.
Dysfunction and dysregulation of B cells affect antibody synthesis
In healthy nasal mucosa, B cells and their derived plasma cells are relatively rare (30), while expansion and activation of B cells occur in the nasal mucosa in patients with CRSwNP, which results in the overproduction of antibodies and local inflammation. Several recent reports have shown an immunodeficiency and specific antibody deficiency in CRS (50–53), suggesting that dysfunction and dysregulation of B cells play a pathogenic role in CRS. However, the mechanisms are still unclear.
B cell dysfunction is partially associated with genetic defects. CD19 functions with both, CD21 and CD81, to enhance signaling from the B cell receptor. Mutations in these receptors are associated with hypogammaglobulinemia, decreased memory B cells, and impaired specific antibody responses (54, 55). In addition, mutations in the receptors for B-cell activating factor (BAFF), BAFF receptor(BAFF-R) and transmembrane activator and calcium modulator and cyclophilin ligand interactor(TACI) lead to hypogammaglobulinemia (56–60) and TACI mutations are also related to sIgA deficiency (59, 60). B cell functions triggered by the B cell receptor are regulated by several germline-encoded receptors, including Toll-like receptors(TLRs) and complement receptors(CRs). In pathological conditions, the role of B cell-expressed TLRs and CRs is important in B cell dysfunction. For example, SA triggers B cell activation via SpA-induced sensitization of B cells to TLR2-active lipopeptides. Combined SpA- and TLR2-mediated B cell activation promotes B cell proliferation (61). The excessive activation of B cells via TLRs or CRs is associated with several diseases, including sepsis, B cell malignancies, asthma, and autoimmunity. In addition, autophagy and mitochondria also play roles in B cell development, activation, and differentiation (62).
In CRSwNP, factors promoting the activation of B cells include inflammatory cytokines [i.e., IL-5 (36), IL-6, BAFF, and APRIL(a proliferation-inducing ligand)], chemokines [C-X-C motif ligand 12(CXCL12), C-X-C motif ligand 13(CXCL13)], and complement pathway products(C3d) (63, 64). Our group previously described a significantly elevated terminal complement complex (C5b-9), and anaphylatoxins (C3a, C5a) are present in nasal polyp tissues (65). Studies demonstrated that Type-2 innate lymphoid cells(ILC2s) directly induced EBI2 expression on B cells and that some B cells might be activated via an extrafollicular environment, which is distinct from classic GC-mediated mechanisms (18). Recently, new strategies for B cell modulation were reported in autoimmune diseases. These therapies, such as anti-CD19 mAbs(inebilizumab, blinatumomab), anti-CD20 mAbs(rituximab, ofatumumab, ocrelizumab), and anti-CD22 mAbs(Epratuzumab), target B cell surface markers (CD19, CD20, CD22), activating factors(BAFF, TACI), or cytokines(IL-6, TNFα, IFNα) to modulate the function of B cells, which may open new therapeutic avenues to regulate B cell activation for the treatment of CRSwNPs (66–68).
CSR to IgE is regulated by lymphocytes, cytokines, transcription factors, and B cell-intrinsic factor
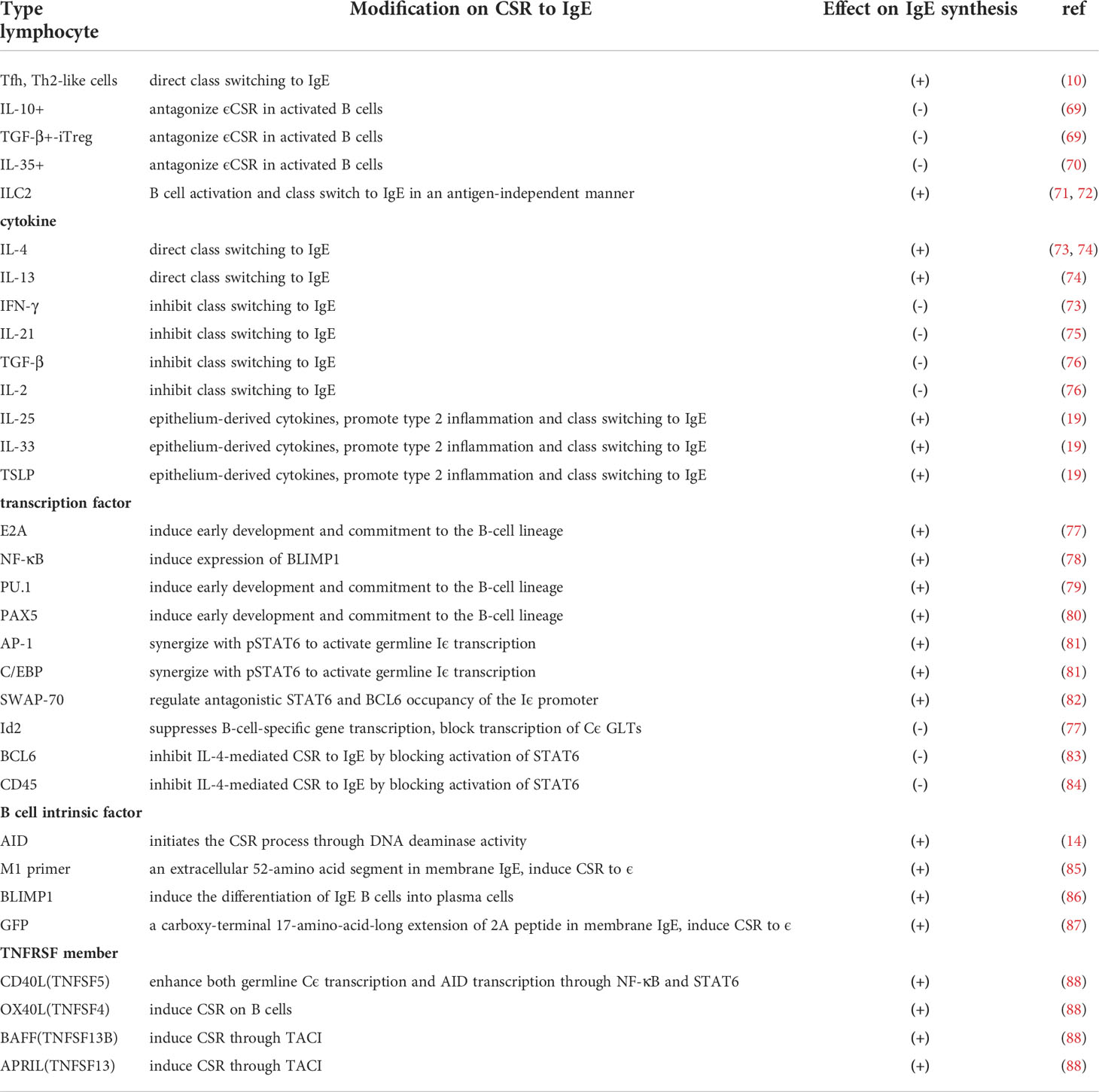
T cells and the related cytokines and numbers of other transcription factors play a major role in CSR (Table 2). Both Tfh and Th2-like cells are helper cells for B cell class switching to IgE, while IL-10+, IL-35+, and TGF-β+ inducible Treg(iTreg) cells can antagonize ϵCSR in activated B cells (10, 89, 90). In addition, ILC2s may directly drive B cell activation and class switching to IgE in an antigen-independent manner (91, 92). ILC2s produce type 2 cytokines to promote type 2 inflammation upon stimulation with epithelium-derived cytokines, such as IL-25, IL-33, and thymic stromal lymphopoietin(TSLP) (1). IL-33 also activates dendritic cells(DCs), which may prime naive T cells to develop into Th2 cells via superantigens (93, 94). The Th2 cytokines IL-4 and IL-13 direct class switching to IgE, whereas IFN-γ, TGF-β, IL-2, and IL-21 inhibit class switching to IgE (69–72). TGF-β induces class switching to IgA and IL-21 promotes the production of IgG1. In T cell-dependent class switching, T cell-derived cytokines regulate switching and direct T and B cell collaboration and normal class switching through CD40. However, in T cell-independent class switching, BCR signaling in concert with TLR signaling can induce class switching in the absence of T cell help in response to microbial pathogens, allergens, and vaccines (95).
Charcot-Leyden crystals (CLCs), composed of galectin-10 (Gal10), are proteins produced by eosinophils, basophils, and some T cells (96). They are typically found in the airways of patients with tenacious mucus, high tissue eosinophil counts, and high serum IgE concentrations (96). Recent research found that CLCs were closely related to IgE production in airway inflammation (73). The administration of CLCs with house dust mites(HDMs) boosted IgE synthesis in a mouse model compared with exposure to the presence of HDMs alone (73). In addition, crystal-dissolving antibodies suppressed airway inflammation, goblet cell metaplasia, bronchial hyperreactivity, and IgE synthesis induced by CLC and HDM inhalation in the model (73). Recent genome-wide genetic and epigenetic association studies also found a strong association of total IgE levels with hypomethylation at the CLC and LGALS10 gene loci, suggesting that crystalline Gal10 may also be a trigger for IgE synthesis in human type 2 immune disease (74, 75).
The binding of phosphorylated signal transducer and activator of transcription 6(pSTAT6) to the Iϵ promoter is essential for promoter activation. Transcription factors including E2A, NF-κB, PU.1, Pax5, AP-1, C/EBP and SWAP-70 synergizes with pSTAT6 to activate germline Iϵ transcription (76, 97–101). In contrast, the transcriptional inhibitor B cell lymphoma 6(BCL6) (77) and the transmembrane phosphatase CD45 can inhibit IL-4-mediated CSR to IgE in human B cells (78) by blocking the activation of signal transducer and activator of transcription 6(STAT6). In addition, the transcription factor inhibitor of DNA binding 2(Id2), induced downstream of TGF-β signaling, is also a negative regulator of the Iϵ promoter (76).
In studies focusing on B cell-intrinsic factors, three different IgE reporter mice were used to explore the regulation of IgE class switching and production. With M1 prime GFP knock-in IgE reporter mice, Talay O described that M1, an extracellular 52-amino-acid segment present in membrane IgE, induced CSR to ϵ (79). With Verigem IgE reporter mice, Yang Z described a predisposition for IgE-expressing germinal center B cells to differentiate into short-lived IgE plasma cells. In addition, B lymphocyte-induced maturation protein 1(BLIMP1), a plasma cell differentiation factor, was expressed at significantly higher levels in IgE B cells than in B cells producing other antibody types and inducing the differentiation of IgE B cells into plasma cells (80). With CϵGFP IgE reporter mice, He J. S reported that a GFP-tagged carboxy-terminal 17-amino-acid-long extension of the 2A peptide expressed in membrane IgE induced CSR to ϵ. Moreover, IgE plasma cells and IgE memory responses primarily arose from sequential class switching through an IgG1 B cell stage (81).
In addition, tumor necrosis factor receptor superfamily(TNFRSF) members play key roles in B cell development, maturation, homeostasis, activation, and differentiation, also influencing the ability of B cells to synthesize IgE antibodies and act as regulators of immune responses, such as CD40(TNFRSF5) and OX40L(TNFSF4), BAFF(TNFSF13B) and APRIL(TNFSF13) (82). BAFF, and the related cytokine APRIL, are important for B cell survival, proliferation, and T cell-independent class switch recombination.
In nasal mucosa, the production of IL-4 and IL-13 creates the possibility for class switching of B cells to IgE-positive B cells, and the maturation of B cells into IgE-producing plasma cells (83, 84). Blocking IL-4 and IL-13 attenuated the expression of ϵ RNA in nasal mucosa from AR patients, which indicated that allergen– dependent induction of ϵ GLT was mediated by locally produced IL-4 and IL-13 (85). In NPs, elevated levels of IL-4 and ϵ-GLT, ϵ-mRNA, and local IgE were detected, implying local CSR to IgE (16). Meanwhile, overproduction of BAFF in NPs has been demonstrated, and the subepithelial expression of BAFF was associated with increased numbers of B cells and plasma cells and increased production of IgA in patients with CRSwNP. These results suggest that BAFF might contribute to the pathogenesis of CRSwNPs via local induction of IgA (86, 87).
The relationship between IgE and atopy in NP is still unclear
Allergy has been recognized as a cause of NPs since the early 1930s (88). Research showed that 51-86% of patients with NP were sensitized to at least one aeroallergen (102, 103), and sinus disease could worsen during the allergen season (104). However, this suggestion was challenged later. A retrospective study by Settipane and Chafee (105) demonstrated that more NPs were found in the nonatopic group than in the atopic group. Subsequent studies demonstrated that multiple positive skin test responses were less common in patients with NPs than in the general population (3). In addition, elevated total serum IgE was observed in aspirin-exacerbated respiratory disease, even though atopy was not present (106).
As we all know, IgE is a key player in allergic airway diseases, such as AR and allergic asthma, while the biology of IgE is not limited to the mediation of allergic diseases. As previously described, growing evidence has indicated increased local IgE production in NPs regardless of systemic atopy and nonatopy (1–4). In atopic NP, local specific IgE production can be the effect of stimulation with allergen–HDM (107). However, local hyperimmunoglobulinaemia is also present in non-atopic patients. Higher levels of specific IgE for cockroach and plantain in NP and local antigen-specific IgE in 57% of non-atopic polyp tissue were observed (108), implying the role of localized mucosal specific IgE in NP without evidence of systemic atopy.
Thus, nasal polyposis is considered a “non-allergic” disease and local IgE is independent of atopy. Our group proposed that patients may develop sensitization and allergy early in life, and in contrast, NP is mostly a late-onset disease with a different IgE pattern. A NP patient may be skin prick test positive, independent of polyclonal IgE formation occurring later in life (109), or maybe SPT negative even in the presence of polyclonal IgE in NPs.
The function of IgE and anti-IgE treatment in NPs
The function of local IgE
IgE antibodies are best known for their role in inducing immediate hypersensitivity reactions. Polyclonal IgE is thought to contribute to chronic T2 inflammation in CRSwNP. Several publications have demonstrated the increased levels of IgE in NPs, and the function and specificity of local IgE have been proven. In a previous study, we showed that IgE from NP tissue homogenates mediated mast cell activation and basophil degranulation (7). Recently, Shamji MH et al. confirmed the role of local IgE antibodies in CRSwNP. Specific IgE antibodies in nasal polyp tissue promoted the CD23-mediated pro-allergic response and induced basophil activation and histamine release (19). These implicated IgE an important mediator of nasal polyposis pathology. Furthermore, the functionality of local specific SE-IgE antibodies from polyp homogenates has been proven. It promoted FcϵRI- and FcϵRII-mediated pro-allergic responses by rapidly degranulating mast cells and basophils upon contact with the specific antigen, functioning as an allergen in this situation, and facilitating antigen binding to B cells (7, 19). All these effects trigger the release of T2 cytokine(IL-4, IL-5, IL-13) and eosinophil infiltration, thus facilitating chronic T2 inflammation in NPs.
Anti-IgE treatment in NPs
Local IgE antibodies in patients with CRSwNP appear to be functional and involved in the regulation of chronic inflammation. Thus, as the important marker of NP, IgE is regarded as an ideal target for intervention. Omalizumab, a recombinant humanized monoclonal antibody against IgE, has been in use to treat allergic asthma for more than a decade. Recently, several studies were showing the effect of Omalizumab therapy on IgE-mediated multimorbidities in allergic asthma, including AR, rhinoconjunctivitis, atopic dermatitis, allergic bronchopulmonary aspergillosis, and so on (110). Omalizumab specifically binds circulating free IgE and inhibits the binding of IgE to the high-affinity receptor, thus decreasing free IgE levels and inhibiting the binding of the antibody to effector cells, consequently preventing antigen crosslinking of receptor-bound IgE and inhibiting the release of and chronic exposure to pro-inflammatory cytokines (111). Furthermore, it was known that the expression and stability of FcϵRI receptors on the surface of basophils, mast cells, and dendritic cells were regulated by IgE levels. Therefore, Omalizumab also leads to a decrease in cell surface density of IgE receptors (112). It has been proven in 2 Phase III trials to be clinically effective and safe in SE-IgE positive, allergic, and non-allergic CRSwNP and asthma. Omalizumab, adding on to intranasal corticosteroids, was associated with significant reductions in nasal polyp and CT scores, and also improved symptoms of upper and lower airways and quality of life, irrespective of the presence of allergy, which also confirmed the importance and functionality of local IgE in nasal polyposis physiopathology (6). Nowadays, anti-IgE treatment has been approved in many countries as add-on therapy with intranasal corticosteroids for the treatment of adults with severe CRSwNP for whom therapy with intranasal corticosteroids does not provide adequate disease control. Noticeably, although Omalizumab is useful in the treatment of CRSwNP, a recent network meta-analysis and systematic review showed that anti-IgE antibodies are less effective than anti-IL-4 receptor antibodies (113, 114)
Summary
In CRSwNP, IgE plays an important role in the pathophysiology and should be considered as the central mediator of T2 inflammation. In polyp tissue, the production and function of local IgE are characteristic. First, the concentrations of local IgE are significantly increased in NPs and show weak correlations with those in serum. Second, local IgE abs are polyclonal and functional. It can induce mast cell and basophil activation and histamine release. Finally, elevated local polyclonal IgE is correlated with increased ECP, IL-5, tissue eosinophilia, and the presence of SE-specific IgE, which are also associated with asthma comorbidity and recurrence after surgery. The synthesis of local IgE is triggered by not only allergens but also microbial pathogens, especially SA. Meanwhile, it is modulated by the activation of local B cells and CSR to IgE. These effects are partially related to genetic defects and are also regulated by T cell-related cytokines, transcription factors, and B cell- intrinsic factors. Due to the central role of IgE in the local chronic inflammation of CRSwNP, it is regarded as an ideal target for therapy and anti-IgE has been proved to be clinically successful. However, the cause and main pathway of IgE production in NPs remain unknown. The mechanisms of B cell function and regulation, which are correlated with CSR to IgE, are not elucidated. The triggers of B cell activation are still unclear, and these may be specific antigens, superantigens, or B-cell dysregulation-related inflammatory factors. Answering these questions will provide valuable information to understand local IgE in CRSwNP and possibly offer the option for new therapeutic targets.
What do we know?
1. Local IgE production occurs continuously in CRSwNP, resulting in elevated levels of IgE in polyp tissue.
2. Local IgE is polyclonal and functional, associated with Th2-inflammation in CRSwNP.
3. Local B cells are activated to produce Ig classes and class switching to IgE in CRSwNP.
4. Local synthesis of IgE is regulated by pathogen and allergen, such as SA, also B cell function, and class switching.
Future research needs
1. What are the characteristics of local Ig production in type 2 CRSwNP?
2. What are the origin and main pathways of local polyclonal IgE production?
3. What are the triggers and mechanisms for the activation and modulation of local B cells, which are correlated with CSR to IgE?
4. How about the possibility of monoclonal antibodies targeted against B cell surface markers, activating factors, or cytokines to become new treatment options in NP?
Author contributions
YS and NZ wrote the review and YS, YCY and SLH revised the review. CB guided the writing of the review. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China, Grant/Award Number: 81970864; Chongqing Talents Project, Grant/Award Number: cstc2021ycjh-bgzxm0080; Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (2020MSXM035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. De Schryver E, Devuyst L, Derycke L, Dullaers M, Van Zele T, Bachert C, et al. Local immunoglobulin e in the nasal mucosa: clinical implications. Allergy Asthma Immunol Res (2015) 7(4):321–31. doi: 10.4168/aair.2015.7.4.321
2. Suh KS, Park HS, Nahm DH, Kim YK, Lee YM, Park K. Role of IgG, IgA, and IgE antibodies in nasal polyp tissue: their relationships with eosinophilic infiltration and degranulation. J Korean Med Sci (2002) 17:375–80. doi: 10.3346/jkms.2002.17.3.375
3. Drake-Lee AB. Histamine and its release from nasal polyps: preliminary communication. J R Soc Med (1984) 77:120–4. doi: 10.1177/014107688407700210
4. Donovan R, Johansson SGO, Bennich H, Soothill JF. Immunoglobulins in nasal polyp fluid. Int Arch Allergy Appl Immunol (1970) 37:154–66. doi: 10.1159/000230229
5. Takhar P, Corrigan CJ, Smurthwaite L, O'Connor BJ, Durham SR, Lee TH, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol (2007) 119(1):213–8. doi: 10.1016/j.jaci.2006.09.045
6. Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol (2013) 131(1):110–116.e1. doi: 10.1016/j.jaci.2012.07.047
7. Zhang N, Holtappels G, Gevaert P, Patou J, Dhaliwal B, Gould H, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy (2011) 66:141–8. doi: 10.1111/j.1398-9995.2010.02448.x
8. Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy (2005) 60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x
9. Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J Immunol (2003) 171(10):5602–10. doi: 10.4049/jimmunol.171.10.5602
10. Song J, Wang H, Zhang YN, Cao PP, Liao B, Wang ZZ, et al. Ectopic lymphoid tissues support local immunoglobulin production in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol (2018) 141(3):927–37. doi: 10.1016/j.jaci.2017.10.014
11. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol (2016) 138:1344–53. doi: 10.1016/j.jaci.2016.05.041
12. Bachert C, Gevaert P, Holtappels G, Johansson SGO, Van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol (2001) 107:607–14. doi: 10.1067/mai.2001.112374
13. Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy (2007) 37:1840–7. doi: 10.1111/j.1365-2222.2007.02838.x
14. Sejima T, Holtappels G, Kikuchi H, Imayoshi S, Ichimura K, Bachert C. Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int (2012) 61:115–22. doi: 10.2332/allergolint.10-OA-0290
15. Shi J, Fan Y, Xu R, Zuo K, Cheng L, Xu G, et al. Characterizing T-cell phenotypes in nasal polyposis in Chinese patients. J Investig Allergol Clin Immunol (2009) 19(4):276–82.
16. Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy (2013) 68:55–63. doi: 10.1111/all.12054
17. Baba S, Kondo K, Toma-Hirano M, Kanaya K, Suzukawa K, Ushio M, et al. Local increase in IgE and class switch recombination to IgE in nasal polyps in chronic rhinosinusitis. Clin Exp Allergy (2014) 44:701–12. doi: 10.1111/cea.12287
18. Feldman S, Kasjanski R, Poposki J, Hernandez D, Chen JN, Norton JE, et al. Chronic airway inflammation provides a unique environment for b cell activation and antibody production. Clin Exp Allergy (2017) 47(4):457–66. doi: 10.1111/cea.12878
19. Shamji MH, Thomsen I, Layhadi JA, Kappen J, Holtappels G, Sahiner U, et al. Broad IgG repertoire in patients with chronic rhinosinusitis with nasal polyps regulates proinflammatory IgE responses. J Allergy Clin Immunol (2019) 143(6):2086–2094.e2. doi: 10.1016/j.jaci.2019.02.001
20. Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol (1997) 99:837–42. doi: 10.1016/S0091-6749(97)80019-X
21. Workman AD, Kohanski MA, Cohen NA. Biomarkers in chronic rhinosinusitis with nasal polyps. Immunol Allergy Clin North Am (2018) 38:679–92. doi: 10.1016/j.iac.2018.06.006
22. Takabayashi T, Schleimer RP. Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin. J Allergy Clin Immunol (2020) 145:740–50. doi: 10.1016/j.jaci.2020.01.027
23. Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med (2015) 192:682–94. doi: 10.1164/rccm.201412-2278OC
24. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol (2008) 122:961–8. doi: 10.1016/j.jaci.2008.07.008
25. Katotomichelakis M, Tantilipikorn P, Holtappels G, De Ruyck N, Feng L, Van Zele T, et al. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy (2013) 27:354–60. doi: 10.2500/ajra.2013.27.3922
26. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol (2016) 137(5):1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324
27. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol (2010) 126(5):962–8, 968.e1-6. doi: 10.1016/j.jaci.2010.07.007
28. Bachert C, Claeys SE, Tomassen P, van Zele T, Zhang N. Rhinosinusitis and asthma: A link for asthma severity. Curr Allergy Asthma Rep (2010) 10(3):194–201. doi: 10.1007/s11882-010-0096-0
29. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy (2006) 61(11):1280–9. doi: 10.1111/j.1398-9995.2006.01225.x
30. Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by b-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol (2013) 131:1075–83. 83 e1–7. doi: 10.1016/j.jaci.2013.01.043
31. Miljkovic D, Psaltis A, Wormald PJ, Vreugde S. Naive and effector b-cell subtypes are increased in chronic rhinosinusitis with polyps. Am J Rhinol Allergy (2018) 32(1):3–6. doi: 10.2500/ajra.2018.32.4496
32. Sokoya M, Ramakrishnan VR, Frank DN, Rahkola J, Getz A, Kingdom TT, et al. Expression of immunoglobulin d is increased in chronic rhinosinusitis. Ann Allergy Asthma Immunol (2017) 119(4):317–23. doi: 10.1016/j.anai.2017.07.024
33. Min JY, Nayak JV, Hulse KE, Stevens WW, Raju PA, Huang JH, et al. Evidence for altered levels of IgD in the nasal airway mucosa of patients with chronic rhinosinusitis. J Allergy Clin Immunol (2017) 140(6):1562–71. doi: 10.1016/j.jaci.2017.05.032
34. Hupin C, Rombaux P, Bowen H, Gould H, Lecocq M, Pilette C. Downregulation of polymeric immunoglobulin receptor and secretory IgA antibodies in eosinophilic upper airway diseases. Allergy (2013) 68(12):1589–97. doi: 10.1111/all.12274
35. Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol (2011) 128:1198–1206.e1. doi: 10.1016/j.jaci.2011.08.037
36. Buchheit KM, Dwyer DF, Ordovas-Montanes J, Katz HR, Lewis E, Vukovic M, et al. IL-5Rα marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol (2020) 145(6):1574–84. doi: 10.1016/j.jaci.2020.02.035
37. Koyama T, Kariya S, Sato Y, Gion Y, Higaki T, Haruna T, et al. Significance of IgG4-positive cells in severe eosinophilic chronic rhinosinusitis. Allergol Int (2019) 68(2):216–24. doi: 10.1016/j.alit.2018.09.002
38. Zhai GT, Wang H, Li JX, Cao PP, Jiang WX, Song J, et al. IgD-activated mast cells induce IgE synthesis in b cells in nasal polyps. J Allergy Clin Immunol (2018) 142(5):1489–99. doi: 10.1016/j.jaci.2018.07.025
39. Wang ZC, Yao Y, Chen CL, Guo CL, Ding HX, Song J, et al. Extrafollicular PD-1highCXCR5-CD4+ T cells participate in local immunoglobulin production in nasal polyps. J Allergy Clin Immunol (2022) 149(2):610–23. doi: 10.1016/j.jaci.2021.06.023
40. Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy (2014) 44:690–700. doi: 10.1111/cea.12304
41. Hamed A, Preston DC, Eschenbacher W, Khokhar D, Workman L, Steinke JW, et al. Nasal IgE production in allergic rhinitis: Impact of rhinovirus infection. Clin Exp Allergy (2019) 49(6):847–52. doi: 10.1111/cea.13372
42. Takeda K, Sakakibara S, Yamashita K, Motooka D, Nakamura S, El Hussien MA, et al. Allergic conversion of protective mucosal immunity against nasal bacteria in patients with chronic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol (2019) 143(3):1163–75.e15. doi: 10.1016/j.jaci.2018.07.006
43. Lowy F. Staphylococcus aureus infections. N Engl J Med (1998) 339(8):520–9. doi: 10.1056/NEJM199808203390806
44. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev (2015) 28(3):603–61. doi: 10.1128/CMR.00134-14
45. Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol (2004) 114:981–3. doi: 10.1016/j.jaci.2004.07.013
46. Bachert C, Gevaert P, Zhang N, van Zele T, Perez-Novo C. Role of staphylococcal superantigens in airway disease. Chem Immunol Allergy (2007) 93:214–36. doi: 10.1159/000100897
47. Nordengrün M, Michalik S, Völker U, Bröker BM, Gómez-Gascón L. The quest for bacterial allergens. Int J Med Microbiol (2018) 308(6):738–50. doi: 10.1016/j.ijmm.2018.04.003
48. Zhang N, Gevaert P, van Zele T, Perez-Novo C, Patou J, Holtappels G, et al. An update on the impact of staphylococcus aureus enterotoxins in chronic sinusitis with nasal polyposis. Rhinology (2005) 43(3):162–8.
49. Bachert C, Humbert M, Hanania NA, Zhang N, Holgate S, Buhl R, et al. Staphylococcus aureus and its IgE-inducing enterotoxins in asthma: Current knowledge. Eur Respir J (2020) 55(4):1901592. doi: 10.1183/13993003.01592-2019
50. Schwitzguebel AJ, Jandus P, Lacroix JS, Seebach JD, Harr T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: Systematic review of the literature and meta-analysis. J Allergy Clin Immunol (2015) 136(6):1523–31. doi: 10.1016/j.jaci.2015.07.016
51. Kashani S, Carr TF, Grammer LC, Schleimer RP, Hulse KE, Kato A, et al. Clinical characteristics of adults with chronic rhinosinusitis and specific antibody deficiency. J Allergy Clin Immunol Pract (2015) 3(2):236–42. doi: 10.1016/j.jaip.2014.09.022
52. Keswani A, Dunn NM, Manzur A, Kashani S, Bossuyt X, Grammer LC, et al. The clinical significance of specific antibody deficiency (SAD) severity in chronic rhinosinusitis (CRS). J Allergy Clin Immunol Pract (2017) 5:1105–11. doi: 10.1016/j.jaip.2016.11.033
53. Tan BK, Peters AT, Schleimer RP, Hulse KE. Pathogenic and protective roles of b cells and antibodies in patients with chronic rhinosinusitis. J Allergy Clin Immunol (2018) 141(5):1553–60. doi: 10.1016/j.jaci.2018.03.002
54. van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ, et al. An antibody deficiency syndrome due to mutations in the CD19 gene. N Engl J Med (2006) 354:1901–12. doi: 10.1056/NEJMoa051568
55. Thiel J, Kimmig L, Salzer U, Grudzien M, Lebrecht D, Hagena T, et al. Genetic CD21 deficiency is associated with hypogammaglobulinemia. J Allergy Clin Immunol (2012) 129:801–10e6. doi: 10.1016/j.jaci.2011.09.027
56. Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet (2005) 37:820–8. doi: 10.1038/ng1600
57. Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet (2005) 37:829–34. doi: 10.1038/ng1601
58. Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci USA (2009) 106:13945–50. doi: 10.1073/pnas.0903543106
59. Pieper K, Rizzi M, Speletas M, Smulski CR, Sic H, Kraus H, et al. A common single nucleotide polymorphism impairs b-cell activating factor receptor's multimerization, contributing to common variable immunodeficiency. J Allergy Clin Immunol (2014) 133:1222–5. doi: 10.1016/j.jaci.2013.11.021
60. Losi CG, Silini A, Fiorini C, Soresina A, Meini A, Ferrari S, et al. Mutational analysis of human BAFF receptor TNFRSF13C (BAFF-r) in patients with common variable immunodeficiency. J Clin Immunol (2005) 25:496–502. doi: 10.1007/s10875-005-5637-2
61. Bekeredjian-Ding I, Inamura S, Giese T, Moll H, Endres S, Sing A, et al. Staphylococcus aureus protein a triggers T cell-independent b cell proliferation by sensitizing b cells for TLR2 ligands. J Immunol (2007) 178(5):2803–12. doi: 10.4049/jimmunol.178.5.2803
62. Sandoval H, Kodali S, Wang J. Regulation of b cell fate, survival, and function by mitochondria and autophagy. Mitochondrion (2018) 41:58–65. doi: 10.1016/j.mito.2017.11.005
63. Min JY, Hulse KE, Tan BK. B-cells and antibody-mediated pathogenesis in chronic rhinosinusitis with nasal polyps. Adv Otorhinolaryngol (2016) 79:48–57. doi: 10.1159/000445129
64. Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol (2010) 125:397–403.e10. doi: 10.1016/j.jaci.2009.10.072
65. Van Zele T, Coppieters F, Gevaert P, Holtappels G, Van Cauwenberge P, Bachert C. Local complement activation in nasal polyposis. Laryngoscope (2009) 119:1753–8. doi: 10.1002/lary.20484
66. Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev (2010) 237:264–83. doi: 10.1111/j.1600-065X.2010.00945.x
67. Jordan N, Lutalo PM, D'Cruz DP. Novel therapeutic agents in clinical development for systemic lupus erythematosus. BMC Med (2013) 11:120. doi: 10.1186/1741-7015-11-120
68. Musette P, Bouaziz JD. B cell modulation strategies in autoimmune diseases: New concepts. Front Immunol (2018) 9:622. doi: 10.3389/fimmu.2018.00622
69. Finkelman FD, Holmes J, Katona IM, Urban JF Jr, Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol (1990) 8:303–33. doi: 10.1146/annurev.iy.08.040190.001511
70. Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human b cells. Proc Natl Acad Sci USA (1993) 90:3730–4. doi: 10.1073/pnas.90.8.3730
71. Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line cϵ transcription of IL-4-stimulated b cells. Blood (2002) 100:4565–73. doi: 10.1182/blood-2002-04-1115
72. He JS, Narayanan S, Subramaniam S, Ho WQ, Lafaille JJ, Curotto de Lafaille MA. Biology of IgE production: IgE cell differentiation and the memory of IgE responses. Curr Top Microbiol Immunol (2015) 388:1–19. doi: 10.1007/978-3-319-13725-4_1
73. Persson EK, Verstraete K, Heyndrickx I, Gevaert E, Aegerter H, Percier JM, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science (2019) 364(6442):eaaw4295. doi: 10.1126/science.aaw4295
74. Liang L, Willis-Owen SAG, Laprise C, Wong KCC, Davies GA, Hudson TJ, et al. An epigenome-wide association study of total serum immunoglobulin e concentration. Nature (2015) 520(7549):670–4. doi: 10.1038/nature14125
75. Virkud YV, Kelly RS, Croteau-Chonka DC, Celedón JC, Dahlin A, Avila L, et al. Novel eosinophilic gene expression networks associated with IgE in two distinct asthma populations. Clin Exp Allergy (2018) 48(12):1654–64. doi: 10.1111/cea.13249
76. Sugai M, Gonda H, Kusunoki T, Katakai T, Yokota Y, Shimizu A. Essential role of Id2 in negative regulation of IgE class switching. Nat Immunol (2003) 4(1):25–30. doi: 10.1038/ni874
77. Harris MB, Mostecki J, Rothman PB. Repression of an interleukin-4-responsive promoter requires cooperative BCL-6 function. J Biol Chem (2005) 280(13):13114–21. doi: 10.1074/jbc.M412649200
78. Loh RK, Jabara HH, Ren CL, Fu SM, Vercelli D, Geha RS. Role of protein tyrosine kinases and phosphatases in isotype switching: crosslinking CD45 to CD40 inhibits IgE isotype switching in human b cells. Immunol Lett (1995) 45(1-2):99–106. doi: 10.1016/0165-2478(94)00233-H
79. Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, et al. IgE+ memory b cells and plasma cells generated through a germinal-center pathway. Nat Immunol (2012) 13(4):396–404. doi: 10.1038/ni.2256
80. Yang Z, Sullivan BM, Allen CD. Fluorescent in vivo detection reveals that IgE(+) b cells are restrained by an intrinsic cell fate predisposition. Immunity (2012) 36(5):857–72. doi: 10.1016/j.immuni.2012.02.009
81. He JS, Meyer-Hermann M, Xiangying D, Zuan LY, Jones LA, Ramakrishna L, et al. The distinctive germinal center phase of IgE+ b lymphocytes limits their contribution to the classical memory response. J Exp Med (2013) 210(12):2755–71. doi: 10.1084/jem.20131539
82. Tafalla C, Granja AG. Novel insights on the regulation of b cell functionality by members of the tumor necrosis factor superfamily in jawed fish. Front Immunol (2018) 7(9):1285. doi: 10.3389/fimmu.2018.01285
83. Bradding P, Feather IH, Wilson S, Bardin PG, Heusser CH, Holgate ST, et al. Immuno]ocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. the mast cell as a source of lL-4, lL-5,and IL-6 in human allergic mucosal inflammation. J Immunol (1993) 151:3853–65.
84. Kleinlan A, Dijkstra MD, Boks SS, Severijnen LA, Mulder PG, Fokkens WJ. Increase in TL-8,IL-I0, IL-13, and RANTES mRNA levels (in situ hybridization) in the nasal mucosa after nasal allergen provocation [In process citation]. J Allergy Clin Immunol (1999) 103(3 Pt 1):441–50. doi: 10.1016/S0091-6749(99)70469-0
85. Cameron L, Gounni AS, Frenkiel S, Lavigne F, Vercelli D, Hamid Q, et al. S gamma switch circles in human nasal mucosa following ex vivo allergen challenge: Evidence for direct as well as sequential class switch recombination. J Immunol (2003) 171(7):3816–22. doi: 10.4049/jimmunol.171.7.3816
86. Yoon YH, Jin J, Kwon KR, Kim SH, Rha KS, Kim YM, et al. The role of b cell activating factor (BAFF) expression on pathogenesis of nasal polyp in chronic rhinosinusitis with nasal polyposis. Rhinology (2014) 52(4):390–6. doi: 10.4193/Rhin13.154
87. Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for b cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol (2008) 121(6):1385–1392.e13922. doi: 10.1016/j.jaci.2008.03.002
88. Kern RA, Schenck HP. Allergy, a constant factor in the etiology of socalled mucous nasal polyps. J Allergy (1933) 4:48597. doi: 10.1016/S0021-8707(33)90102-1
89. Palomares O, Akdis M, Martín-Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and b cells. Immunol Rev (2017) 278(1):219–36. doi: 10.1111/imr.12555
90. Shamji MH, Layhadi JA, Achkova D, Kouser L, Perera-Webb A, Couto-Francisco NC, et al. Role of IL-35 in sublingual allergen immunotherapy. J Allergy Clin Immunol (2019) 143(3):1131–1142.e4. doi: 10.1016/j.jaci.2018.06.041
91. Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat Med (2013) 19:977–9. doi: 10.1038/nm.3300
92. de Groot JC, Ten Brinke A, Bel EH. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res (2015) 1(1):00024–2015. doi: 10.1183/23120541.00024-2015
93. Lan F, Zhang N, Holtappels G, De Ruyck N, Krysko O, Van Crombruggen K, et al. Staphylococcus aureus induces a mucosal type 2 immune response via epithelial cell-derived cytokines. Am J Respir Crit Care Med (2018) 198(4):452–63. doi: 10.1164/rccm.201710-2112OC
94. Teufelberger AR, Nordengrün M, Braun H, Maes T, De Grove K, Holtappels G, et al. The IL-33/ST2 axis is crucial in type 2 airway responses induced by staphylococcus aureus-derived serine protease-like protein d. J Allergy Clin Immunol (2018) 141(2):549–559.e7. doi: 10.1016/j.jaci.2017.05.004
95. Egest JP, Lou Z, Lam T, Greenberg ML, Wang R, Xu Z, et al. B cell TLR1/2, TLR4, TLR7 and TLR9 interact in induction of class switch DNA recombination: Modulation by BCR and CD40, and relevance to T-independent antibody responses. Autoimmunity (2015) 48(1):1–12. doi: 10.3109/08916934.2014.993027
96. Gevaert E, Delemarre T, De Volder J, Zhang N, Holtappels G, De Ruyck N, et al. Charcot-Leyden crystals promote neutrophilic inflammation in patients with nasal polyposis. J Allergy Clin Immunol (2020) 145(1):427–430.e4. doi: 10.1016/j.jaci.2019.08.027
97. Stütz AM, Woisetschläger M. Functional synergism of STAT6 with either NF-kappa b or PU.1 to mediate IL-4-induced activation of IgE germline gene transcription. J Immunol (1999) 163(8):4383–91.
98. Thienes CP, De Monte L, Monticelli S, Busslinger M, Gould HJ, Vercelli D. The transcription factor b cell-specific activator protein (BSAP) enhances both IL-4- and CD40-mediated activation of the human epsilon germline promoter. J Immunol (1997) 158(12):5874–82.
99. Dryer RL, Covey LR. A novel NF-kappa b-regulated site within the human I gamma 1 promoter requires p300 for optimal transcriptional activity. J Immunol (2005) 175(7):4499–507. doi: 10.4049/jimmunol.175.7.4499
100. Shen CH, Stavnezer J. Activation of the mouse ig germline epsilon promoter by IL-4 is dependent on AP-1 transcription factors. J Immunol (2001) 166(1):411–23. doi: 10.4049/jimmunol.166.1.411
101. Audzevich T, Pearce G, Breucha M, Günal G, Jessberger R. Control of the STAT6-BCL6 antagonism by SWAP-70 determines IgE production. J Immunol (2013) 190(10):4946–55. doi: 10.4049/jimmunol.1203014
102. Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope (2013) 123 Suppl 7:S1–11. doi: 10.1002/lary.24418
103. Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol (2013) 131(5):1350–60. doi: 10.1016/j.jaci.2013.02.002
104. Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy (2009) 23(2):145–8. doi: 10.2500/ajra.2009.23.3284
105. Settipane GA, Chafee FH. Nasal polyps in asthma and rhinitis. a review of 6,037 patients. J Allergy Clin Immunol (1977) 59:17–21. doi: 10.1016/0091-6749(77)90171-3
106. Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy (2014) 28(4):287–9. doi: 10.2500/ajra.2014.28.4054
107. Kang JW, Nahm DH, Suh KS, Kim HY, Park HS. Local production of specific IgE antibody to house dust mite in nasal polyp tissues. J Asthma Allergy Clin Immunol (1998) 18:426–33.
108. Sheahan P, Ahn CN, Harvey RJ, Wise SK, Mulligan RM, Lathers DM, et al. Local IgE production in nonatopic nasal polyposis. J Otolaryngol Head Neck Surg (2010) 39:45–51.
109. Song WJ, Lee JH, Won HK, Bachert C. Chronic rhinosinusitis with nasal polyps in older adults: Clinical presentation, pathophysiology, and comorbidity. Curr Allergy Asthma Rep (2019) 19(10):46. doi: 10.1007/s11882-019-0880-4
110. Humbert M, Bousquet J, Bachert C, Palomares O, Pfister P, Kottakis I, et al. IgE-mediated multimorbidities in allergic asthma and the potential for omalizumab therapy. J Allergy Clin Immunol Pract (2019) 7(5):1418–29. doi: 10.1016/j.jaip.2019.02.030
111. Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, et al. Humanization of an antibody directed against IgE. J Immunol (1993) 151:2623–32.
112. MacGlashan DW Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzieWhite J, et al. D6wn-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol (1997) 158:1438–45.
113. Boechat JL, Silva D, Sousa-Pinto B, Delgado L. Comparing biologicals for severe chronic rhinosinusitis with nasal polyps: A network meta-analysis. Allergy (2022) 77(4):1299–306. doi: 10.1111/all.15205
Keywords: Immunoglobulin E, local B cell, nasal polyposis, Type2 inflammation, Staphylococcus aureus
Citation: Shen Y, Zhang N, Yang Y, Hong S and Bachert C (2022) Local Immunoglobulin E in nasal polyps: Role and modulation. Front. Immunol. 13:961503. doi: 10.3389/fimmu.2022.961503
Received: 04 June 2022; Accepted: 19 August 2022;
Published: 08 September 2022.
Edited by:
Rudi W. Hendriks, Erasmus University Rotterdam, NetherlandsReviewed by:
Gianenrico Senna, University of Verona, ItalyRalph Pries, University of Lübeck, Germany
Ludger Klimek, Centre for Rhinology and Allergology, Germany
Copyright © 2022 Shen, Zhang, Yang, Hong and Bachert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claus Bachert, Q2xhdXMuQmFjaGVydEBVR2VudC5iZQ==
 Yang Shen
Yang Shen Nan Zhang
Nan Zhang Yucheng Yang1
Yucheng Yang1 Claus Bachert
Claus Bachert