
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 14 December 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.959691
This article is part of the Research TopicInnate Immunity: A Top Player in Inflammatory Skin Diseases?View all 8 articles
Background: To date, evidences with high evidence-level evaluating the association between liver diseases and hidradenitis suppurativa was lacking. Given that inconsistency exists in some of the previous observational studies, evaluating the prevalence of liver diseases in HS patients could potentially serve as a reference of future guidelines for HS comorbidity screening. The aim of the current study was to evaluate potential association between hidradenitis suppurativa and liver diseases and provide integrated evidences.
Methods: A search in PubMed, Web of Science and Embase based on the syntaxes ‘‘hidradenitis suppurativa’’ or ‘‘acne inversa’’ with “comorbidities”, “liver diseases”, “fatty liver” or “hepatitis” was performed. Observational studies evaluating epidemiological association between hidradenitis suppurativa and the risk of all liver diseases, including specific diseases as non-alcoholic fatty liver disease, hepatitis B, hepatitis C were targeted to be extracted in this systematic review and meta-analysis.
Results: Within the initial 702 records, there were finally 8 real-world observational studies extracted. Results suggest that patients with HS are associated with all liver diseases (OR= 1.50; 95% CI, 1.27, 1.76), non-alcoholic fatty liver disease (OR= 1.78; 95% CI, 1.28, 2.48) and hepatitis B (OR=1.48; 95% CI, 1.12, 1.94), but not hepatitis C (OR= 1.27; 95% CI, 0.78, 2.07). HS patients were associated with significantly increased risk of liver diseases, especially the risk of non-alcoholic fatty liver disease and hepatitis B.
Conclusions: Clinicians should be alert to the clinical relationship while caring people with hidradenitis suppurativa and the screening of liver function should be recommended to HS patients.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022296034.
● Current meta-analysis suggested HS patients have higher risk of all liver diseases, non-alcoholic fatty liver disease and hepatitis B, but not hepatitis C
● Liver function screening should be recommended to HS patients for comorbidity screening in future guidelines
As a chronic disorder associated with many immunological comorbidities, hidradenitis suppurativa (HS) causes impairment to patients’ quality of life (1). The prevalence of HS was estimated to be 0.4% in Western countries (2). Comorbidities of HS including dermatological, cardiovascular and endocrinological events (3). Though the actual immunologic mechanism of HS has not be fully developed, it was reported that HS was involved in the increased secretion of inflammatory cytokines including IL-17 and TNF alpha (4).
Liver dysfunctions were thought to be involved in the pathophysiology of chronic inflammatory dermatologic diseases (5, 6). Presence of inflammatory skin diseases such as psoriasis and bullous pemphigoid were thought to influence the pathogenesis of viral hepatitis (7, 8). The interaction between non-alcoholic fatty liver diseases (NAFLD) and other inflammatory dermatological events has also long been discussed (5). Mechanisms including the increase of proinflammatory cytokines and related metabolic syndromes could play a role in the interaction between inflammatory skin diseases and liver diseases such as NAFLD and viral hepatitis (9–11).
Chronic inflammatory status could potentially trigger subsequent endocrinological events, leading to multi-systemic consequences (12–15). However, to date, evidences with high evidence-level evaluating the association between liver diseases and HS was lacking. A recent guideline indicated that since association between HS and non-alcoholic fatty liver disease (NAFLD) was unsure due to insufficient evidences, NAFLD screening in HS patients could not be recommended for comorbidity screening in HS pateints (3). Hence, it’s necessary to clarify the association between HS and NAFLD. Additionally, aside from NAFLD, other liver diseases were also reported to have high prevalence in HS patients (16, 17). Given that inconsistency exists in some of the results, evaluating the prevalence of liver diseases in HS patients could be practical and could potentially serve as a reference of future guidelines for HS comorbidity screening. Hereby, we conducted a systematic review and meta-analysis to evaluate potential association between HS and liver diseases and provide integrated evidences.
The current study abided by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (18). Observational studies evaluating epidemiological association between HS and the risk of all liver diseases, including specific diseases as NAFLD, hepatitis B, hepatitis C were targeted to be extracted in this systematic review and meta-analysis. This study project has been registered in PROSPERO with an ID number of CRD42022296034.
On December 12, 2021, we performed a search in PubMed, Web of Science and Embase based on the syntaxes ‘‘hidradenitis suppurativa’’ or ‘‘acne inversa’’ with “comorbidities”, “liver diseases”, “fatty liver” or “hepatitis”. In the searching process, characteristics of studies such as language or ethnics of study population was not limited. Searching protocol and detailed information of PICO (participants, intervention, comparison and outcome) could be found in the Supplementary files. Similar study design and study extraction criteria has been applied in published studies (19, 20).
Observational studies, including cohort studies, case-control studies and cross-sectional studies were regarded as having eligible study designs for extraction. Studies would be excluded in the screening process if meeting any of the following criteria: (1) Unrelated to HS (2) HS related but not evaluating comorbidity association (3) HS comorbidity related but not mentioning any HS-liver disease association (4) in vivo/in vitro studies or genetic studies (5) HS-liver disease related but not having appropriate comparative arms. To address publication bias, conference abstracts were not excluded.
After screening of studies, information regarding authors’ name, study design, year each study published, location each study conducted, study outcome of interest, definition of HS patients and outcome events, gender percentage, mean age and the number of participants were extracted from the studies meeting our eligibility criteria. Extracted characteristics were recorded in Table 1. Newcastle-Ottawa Scale was applied to evaluate study quality (21).
To perform qualitative synthesis, pooled odds ratio was calculated based on the data extracted from eligible observational studies. In all analysis models in the current study, random effect model was applied to address possible clinical heterogeneity. To evaluate the heterogeneity within pooled studies, value of I (2) was applied and would be presented in each forest plot. With a I (2) value greater than 75%, heterogeneity could be considerable (22). For the presentation of odds ratio, 95% confidence interval (95% CI) were applied. Review Manager 5.4 (Cochrane, London, UK) were utilized for statistical analyses.
As presented in Figure 1, within the initial 702 records, there were finally 8 real-world observational studies [1 cohort study (23), 1 case-control study (24) and 6 cross-sectional studies (1, 16, 17, 25–27)] included for data extraction after screening process. Most of the included studies were conducted in North America and Europe [4 studies conducted in United States (16, 23, 26, 27) and 2 in Spain (24, 25)], whereas one-third of the studies were conducted in Asia [1 study conducted in Korea (23) and 1 in Israel (1)]. Within the included studies, outcomes including NAFLD, hepatitis B, hepatitis C and all liver diseases had been extracted (Table 1).
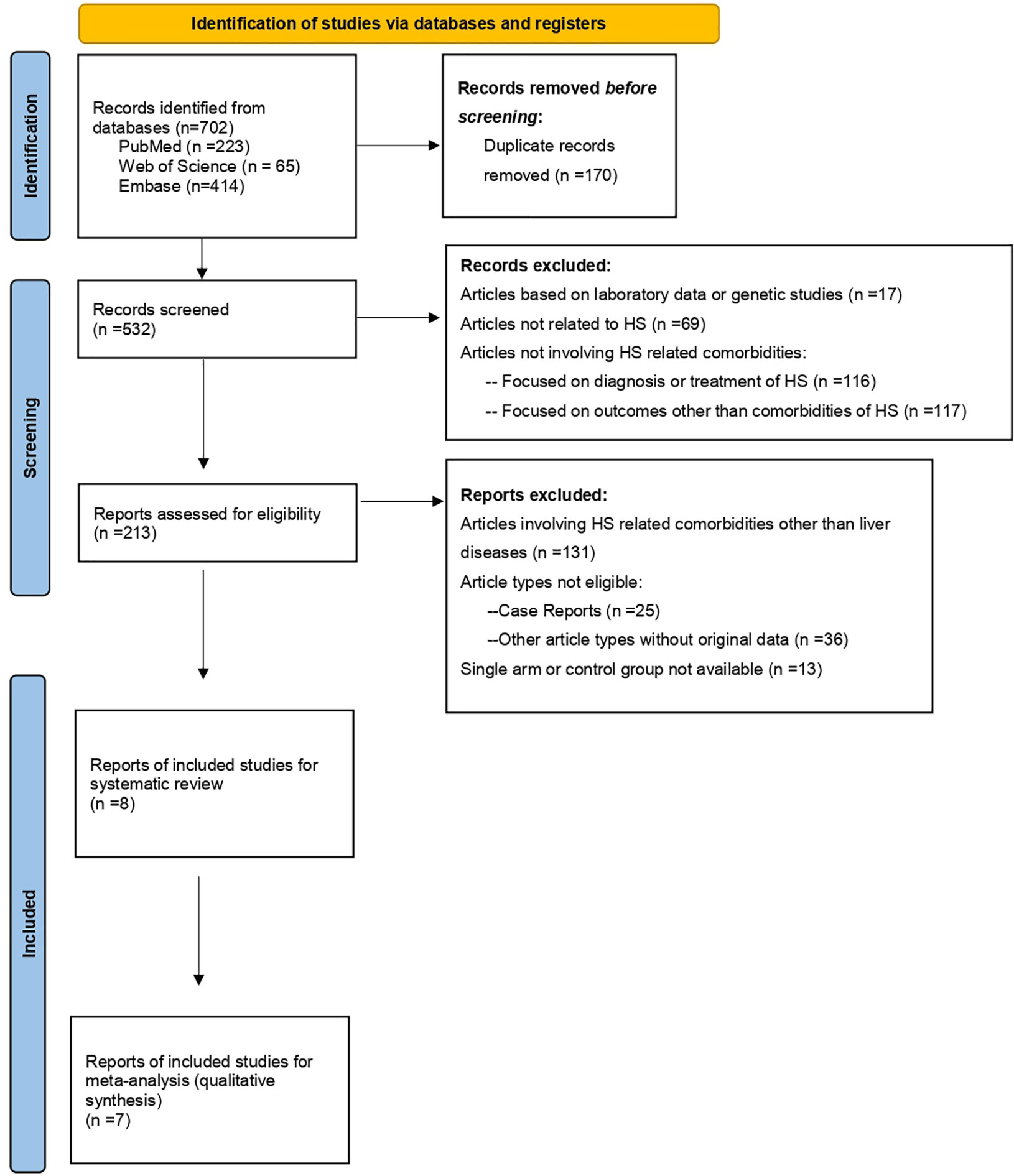
Figure 1 PRISMA Study Flowchart (18).
Within included studies risk of bias had been assessed by Newcastle-Ottawa Scale. (Figures S1A, B). Four cross-sectional studies (16, 17, 26, 27) had been evaluated to have unknown bias regarding the adequacy of case definition since dermatologist of specialists were not reported to be involved in the identification of cases. For case identification and diagnosis, provided information was insufficient or only the International Classification of Diseases, 9th Revision/10th Revision (ICD-9/10) was applied as the identification criteria. Two studies were evaluated to have unknown bias to non-response rate and same methods ascertaining controls and cases due to insufficient information. Publication bias could exist in the extracted studies due to asymmetric results in the funnel plot
Comparing with non-HS people, patients with HS were associated with higher risk of liver diseases (OR=1.50; 95% confidence interval, 1.27-1.76), with moderate heterogeneity between pooled studies (I2 = 66%) (Figure 2A). validate the HS-liver disease association. Given that representative of a population could be different between baseline of case-control studies and cross-sectional studies, a sensitivity model has been performed based on including cross-sectional studies in the analysis. The association remained statistically significant in the sensitivity analysis (Figure S2). Subgroup analyses was performed to evaluate risk of viral hepatitis in HS people (Figures 2B, C). Based on studies with low to moderate heterogeneity, HS patients had statistically significant risk for having hepatitis B. (OR=1.48, 95% CI, 1.12-1.94). For hepatitis C, the association was not statistically significant (p=.35).
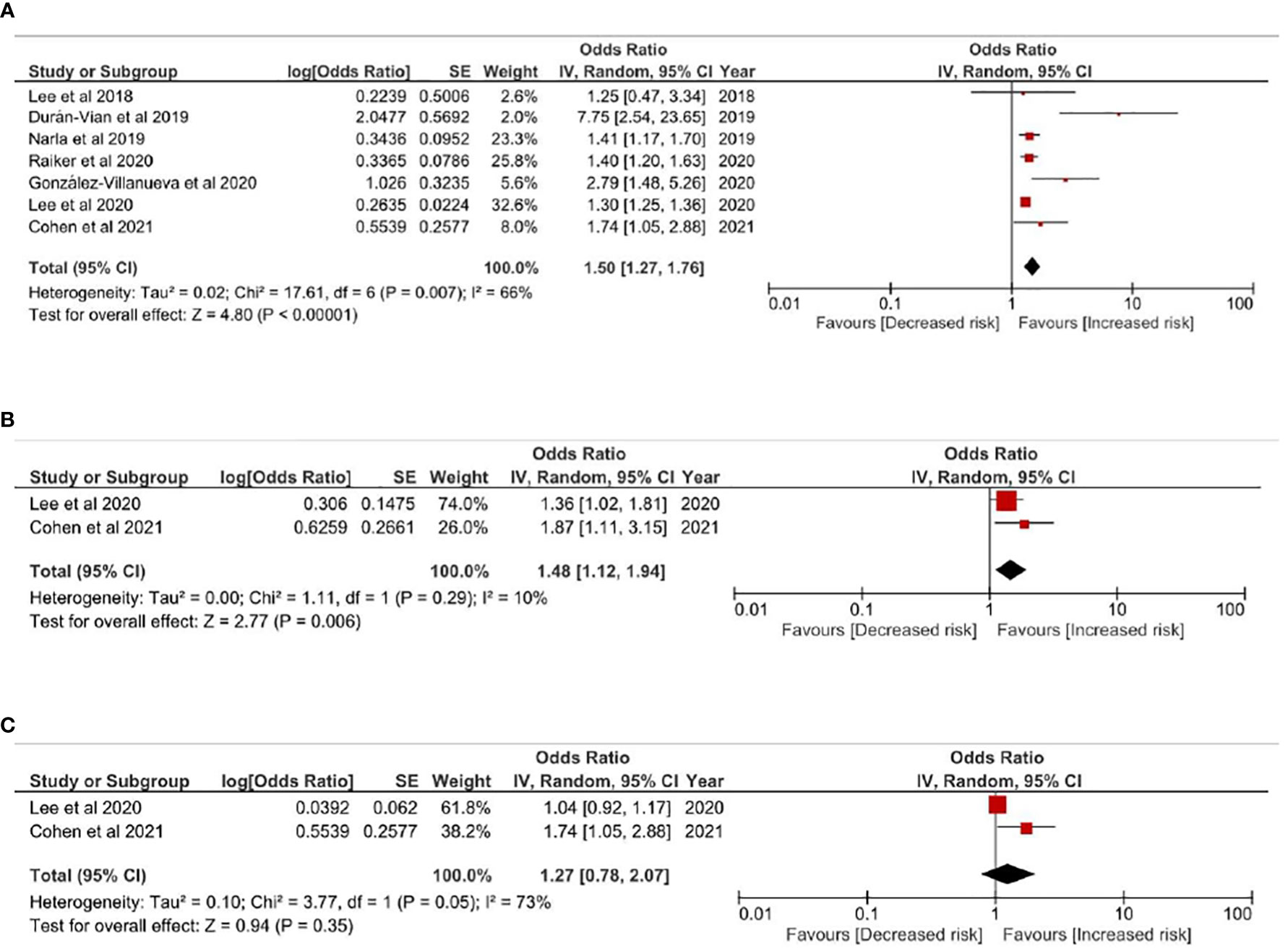
Figure 2 (A) Odds ratio of all pooled evidences of liver diseases in people with Hidradenitis Suppurativa (B) Odds ratio of hepatitis B in people with hidradenitis suppurativa (C) Odds ratio of hepatitis C in people with hidradenitis suppurativa.
In Figures 3A, B, risk of NAFLD in HS patients was evaluated. HS patients were associated with higher risk of NAFLD comparing with non-HS controls, with a pooled odds ratio of 1.78 (95% CI, 1.28-2.48) (Figure 3A). Given obesity was highly involved in both HS and NAFLD, obesity-related factors were critical of considering while evaluating HS-NAFLD association. Thereby, we performed a sensitivity analysis based on an additional model only including studies adjusting obesity related factors such as BMI or comorbidities related to metabolic syndrome in the qualitative synthesis. In this model, a 4.18-fold risk of having NAFLD was observed in people with HS (95% CI, 1.57-11.12) (Figure 3B). The observed association was validated in another sensitivity analysis including only cross-sectional studies (Figure S3). In all models evaluating HS-NALFD association, moderate heterogeneity was showed within included studies.
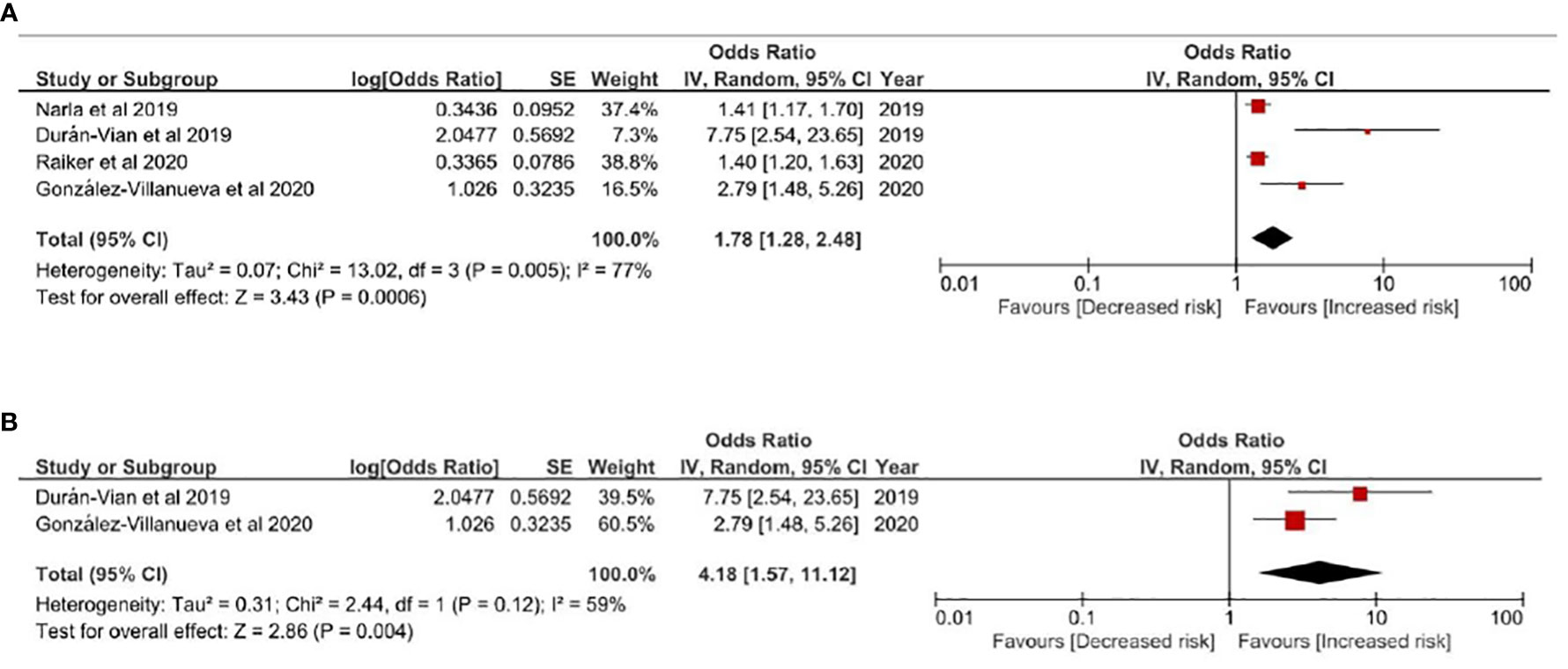
Figure 3 (A) Odds ratio of all pooled evidences of NAFLD in people with Hidradenitis Suppurativa (B) Sensitivity Analysis: Odds ratio of NAFLD in people with Hidradenitis Suppurativa based on results controlling obesity related factors Legends: Given obesity was highly involved in both HS and NAFLD, obesity-related factors were critical of considering while evaluating HS-NAFLD association. In this model, studies not adjusting obesity related factors such as BMI or metabolic syndrome comorbidities were excluded from the qualitative synthesis.
The present systematic review and meta-analysis provides integrated evidences of the increased risk of liver diseases, including NAFLD and hepatitis B in people with HS. To the best of our knowledge, this is the first study providing pooled odds ratio regarding the HS-liver disease association. Given that the current recommendation for HS comorbidity screening did not provide clear information regarding the liver comorbidities of HS (3), the results provided in this study could potentially serve as a critical reference of future guidelines for HS comorbidity screening.
Being viewed as a disease involving multiple organic system and highly associated with metabolic system, the term “Metabolic-associated Fatty Liver Disease (MAFLD)” was designed to clinically describe NAFLD (28). NAFLD and MAFLD were reported to share similar clinical status and long-term outcomes (29). Whichever term utilized, the status of fatty liver disease was thought to be massively influenced by the status of obesity and metabolic syndrome. Therefore, in studies evaluating NAFLD-related association, obesity and comorbidities status could potentially cause great confounding bias. In the current study, the sensitivity analysis considering BMI and metabolic-syndrome-related comorbidities information could serve as a validation of the HS-NAFLD association. In the obesity-adjusted model the risk was even higher than other models, presenting a more than 4-fold risk. Though current evidence was validated in three different sensitivity models, the analysis was limited to insufficient data, for only two studies was available for obesity adjustment (24, 25). Future eligible observational evidences considering the influence of obesity were necessary to make the integrated evidence more rigorous. However, the current three models could provide a critical reference regarding the significantly increased risk of NAFLD in HS patients.
The reported association between HS and liver diseases could potentially attributed to elevated proinflammatory cytokines and adipokines involved in the chronic inflammatory status of HS.
In the pathogenesis of inflammatory skin diseases, elevated cytokines such as tumor necrosis factor alpha (TNF alpha) or interleukin 1 (IL1) could potentially lead to impairment in liver function (5, 24, 25). Immunological links between inflammatory skin diseases, for instance, psoriasis, could also be potentially attributed to the role of low-grade chronic inflammation. In psoriasis patients, presence of chronic inflammation could lead to the elevation of TNF alpha, which could in turn increase the risk of insulin resistance and hepatic fibrogenesis (30). These consequences could serve as risk factors of NAFLD (30) (Figure 4). Additionally, recent real-world evidences also indicated that HS and psoriasis could have bidirectional association (5). Current studies had been finding the actual inflammatory pathway involved in the pathogenesis of HS. Though the full theory of HS-caused inflammation has not been well-developed, it was reported that Th17 family also played a great role in the HS inflammation pathway (11, 31). Medications against IL-17 were thought to be potential choice for HS treatments (32, 33). As for the development of NAFLD, mechanisms related to Th17 were also highly involved (10, 34). In viral hepatitis, high level of IL17 was also presented in people with HBV and HCV infection and could be associated with severer disease status (9, 35). In previous studies, dysregulation of serum Th17 cells and IL17 concentration were observed in patients with hepatitis B. Given that hepatitis B and HS were involved in common immunological pathways, the high prevalence could possibly be explained. Moreover, in people with HS, status of adipokines, including adiponectin, resistin and leptin were reported to be dysregulated (36). In the pathogenies of NAFLD, imbalanced adipokines were also thought to play a potential role (37, 38). Though common mechanisms in immunological pathways existed, actual mechanisms have not been completed developed to determine the HS-liver diseases relation. Further lab-based studies were needed to clarify the interaction between inflammatory system, cytokines, adipokines and liver function in HS patients.
The current study was limited to current available data. First, given that available observational data was insufficient to perform stratification analysis of age or gender, we were not able to evaluate whether age and gender could show difference on the HS-liver diseases association. Second, since in most of the included databased-based retrospective studies, information regarding severity indicators of HS such Hurley Stage were not available, we were not able to consider the severity of HS as common covariate in the pooled model. Third, for the identification of NAFLD, most studies did not clarify the difference between non-alcoholic steatohepatitis and non-alcoholic fatty liver, for the two subgroups of NAFLD referred to different disease severity. Third, in most studies, alcoholism status was not provided, which could lead to potential confounding bias. Lacking information regarding alcohol uptake could be a potential limitation in both studies evaluating HS-NAFLD association and comprehensive analyses based on these real-world studies. Fourth, the current study is limited due to the small amount of eligible evidences, and the extracted studies were less than 10 studies. However, it is not recommended to present publication bias via funnel plot when studies were less than 10 due to low evidence power (22).Readers should be cautious while interpreting the reported results of the current study.
As a conclusion, we report a significantly increased risk of liver diseases in HS patients, especially the risk of NAFLD and hepatitis B. Clinicians should be alert to the clinical relationship while caring people with HS. Future studies should focus on whether the difference between HS severity and duration would affect the extent of the observed association.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
All the authors involved in drafting or revising the article and approved of the submitted version. Study conception and design: S-YG, Y-PH, KM and M-CW. Data analysis and demonstration: S-YG and M-CW. Original draft preparation: S-YG and M-CW. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from Taichung Veterans General Hospital Research Foundation (TCVGH-1106501B; TCVGH-1116502C).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.959691/full#supplementary-material
1. Cohen JM, Kridin KK, Perez-Chada LM, Merola JF, Cohen AD. Hepatitis b and c among patients with hidradenitis suppurativa: A population-based study. Int J Dermatol (2021) 61(1):84–8. doi: 10.1111/ijd.15578
2. Jfri A, Nassim D, O’Brien E, Gulliver W, Nikolakis G, Zouboulis CC. Prevalence of hidradenitis suppurativa: A systematic review and meta-regression analysis. JAMA Dermatol (2021) 157(8):924–31. doi: 10.1001/jamadermatol.2021.1677
3. Garg A, Malviya N, Strunk A, Wright S, Alavi A, Alhusayen R, et al. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the us and canadian hidradenitis suppurativa foundations. J Am Acad Dermatol (2021) 86(5):1092–101. doi: 10.1016/j.jaad.2021.01.059
4. Schell SL, Sun H, Maczuga S, Kirby JS, Nelson AM. Cross-sectional study reveals reduced odds of allergies in people with hidradenitis suppurativa. J Am Acad Dermatol (2021) 85(1):232–34. doi: 10.1016/j.jaad.2020.08.014
5. Gau SY, Huang KH, Lee CH, Kuan YH, Tsai TH, Lee CY. Bidirectional association between psoriasis and nonalcoholic fatty liver disease: Real-world evidence from two longitudinal cohort studies. Front Immunol (2022) 13:840106. doi: 10.3389/fimmu.2022.840106
6. Bellinato F, Gisondi P, Mantovani A, Girolomoni G, Targher G. Risk of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis: An updated systematic review and meta-analysis of observational studies. J Endocrinol Invest (2022) 45(7):1277–88. doi: 10.1007/s40618-022-01755-0
7. Jang H, Jin YJ, Yoon CH, Kim CW, Kim L. Bullous pemphigoid associated with chronic hepatitis c virus infection in a hepatitis b virus endemic area: A case report. Med (Baltimore) (2018) 97(15):e0377. doi: 10.1097/MD.0000000000010377
8. Wang X, Zhang M, Chen Y, Liu Y, Yu Y, Huang X, et al. Risk for hepatitis b virus reactivation in patients with psoriasis treated with biological agents: A systematic review and meta-analysis. Dermatol Ther (Heidelb) (2022) 12(3):655–70. doi: 10.1007/s13555-022-00682-5
9. Paquissi FC. Immunity and fibrogenesis: The role of th17/il-17 axis in hbv and hcv-induced chronic hepatitis and progression to cirrhosis. Front Immunol (2017) 8:1195. doi: 10.3389/fimmu.2017.01195
10. Mirea AM, Tack CJ, Chavakis T, Joosten LAB, Toonen EJM. Il-1 family cytokine pathways underlying nafld: Towards new treatment strategies. Trends Mol Med (2018) 24(5):458–71. doi: 10.1016/j.molmed.2018.03.005
11. Fletcher JM, Moran B, Petrasca A, Smith CM. Il-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin Exp Immunol (2020) 201(2):121–34. doi: 10.1111/cei.13449
12. Gau SY, Huang JY, Wei JC. Higher risk of hyperthyroidism in people with asthma: Evidence from a nationwide, population-based cohort study. J Allergy Clin Immunol Pract (2022) 10(3):751–58.e1. doi: 10.1016/j.jaip.2021.09.021
13. Gau SY, Lai JN, Yip HT, Wu MC, Wei JC. Higher dementia risk in people with gastroesophageal reflux disease: A real-world evidence. Front Aging Neurosci (2022) 14. doi: 10.3389/fnagi.2022.830729
14. Huang SC, Gau SY, Huang JY, Wu WJ, Wei JC. Increased risk of hypothyroidism in people with asthma: Evidence from a real-world population-based study. J Clin Med (2022) 11(10). doi: 10.3390/jcm11102776
15. Lee YH, Tsou HK, Kao SL, Gau SY, Bai YC, Lin MC, et al. Patients with rheumatoid arthritis increased risk of developing osteoarthritis: A nationwide population-based cohort study in taiwan. Front Med (Lausanne) (2020) 7:392. doi: 10.3389/fmed.2020.00392
16. Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI. Associations of cutaneous and extracutaneous infections with hidradenitis suppurativa in u.S. children and adults. Br J Dermatol (2020) 182(2):327–34. doi: 10.1111/bjd.18093
17. Lee JH, Kwon HS, Jung HM, Kim GM, Bae JM. Prevalence and comorbidities associated with hidradenitis suppurativa in korea: A nationwide population-based study. J Eur Acad Dermatol Venereol (2018) 32(10):1784–90. doi: 10.1111/jdv.15071
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
19. Gau SY. Increased risk of renal diseases in people with hidradenitis suppurativa: A systematic review and meta-analysis. Int J Dermatol (2022). doi: 10.1111/ijd.16423
20. Gau SY, Lee CY, Kuan YH, Ma KS, Wei JC. Hyperthyroidism and hypothyroidism in patients with hidradenitis suppurativa: A systematic review and meta-analysis. Int J Dermatol (2022). doi: 10.1111/ijd.16484
21. Wells G SB, O’Connell D, Robertson J. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analysis (2018). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 21 March 2022).
22. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, London (2022). Available at: www.training.cochrane.org/handbook.
23. Kimball AB, Sundaram M, Gauthier G, Guérin A, Pivneva I, Singh R, et al. The comorbidity burden of hidradenitis suppurativa in the united states: A claims data analysis. Dermatol Ther (Heidelb) (2018) 8(4):557–69. doi: 10.1007/s13555-018-0264-z
24. Duran-Vian C, Arias-Loste MT, Hernandez JL, Fernandez V, Gonzalez M, Iruzubieta P, et al. High prevalence of non-alcoholic fatty liver disease among hidradenitis suppurativa patients independent of classic metabolic risk factors. J Eur Acad Dermatol Venereol (2019) 33(11):2131–36. doi: 10.1111/jdv.15764
25. Gonzalez-Villanueva I, DeGracia C, Planells M, Poveda I, Alvarez P, Schneller-Pavalescu L, et al. Hidradenitis suppurativa is associated with non-alcoholic fatty liver disease: A cross-sectional study. Acta Derm Venereol (2020) 100(15):adv00239. doi: 10.2340/00015555-3597
26. Narla S, Silverberg JI. Autoimmune comorbidities of hidradenitis suppurativa in us adults and children. J Am Acad Dermatol (2019) 81(4):AB25. doi: 10.1016/j.jaad.2019.06.129
27. Raiker R, Pakhchanian H, Yousaf A, Davis S, Gayam S, Zinn Z. 18858 examining the association between hidradenitis suppurativa and gastrointestinal disorders. J Am Acad Dermatol (2020) 83(6):AB223. doi: 10.1016/j.jaad.2020.06.981
28. Kuchay MS, Misra A. From non-alcoholic fatty liver disease (nafld) to metabolic-associated fatty liver disease (mafld): A journey over 40 years. Diabetes Metab Syndr (2020) 14(4):695–96. doi: 10.1016/j.dsx.2020.05.019
29. Younossi ZM, Paik JM, Al Shabeeb R, Golabi P, Younossi I, Henry L. Are there outcomes differences between non-alcoholic fatty liver disease (nafld) and metabolic associated fatty liver disease (mafld)? Hepatology (2022) 76(5):1423–37. doi: 10.1002/hep.32499
30. Prussick RB, Miele L. Nonalcoholic fatty liver disease in patients with psoriasis: A consequence of systemic inflammatory burden? Br J Dermatol (2018) 179(1):16–29. doi: 10.1111/bjd.16239
31. Thomi R, Cazzaniga S, Seyed Jafari SM, Schlapbach C, Hunger RE. Association of hidradenitis suppurativa with t helper 1/t helper 17 phenotypes: A semantic map analysis. JAMA Dermatol (2018) 154(5):592–95. doi: 10.1001/jamadermatol.2018.0141
32. Navrazhina K, Frew JW, Grand D, Williams SC, Hur H, Gonzalez J, et al. Il-17ra blockade by brodalumab decreases inflammatory pathways in hidradenitis suppurativa skin and serum. Br J Dermatol (2022) 187(2):223–33. doi: 10.1111/bjd.21060
33. Kashetsky N, Mufti A, Alabdulrazzaq S, Lytvyn Y, Sachdeva M, Rahat A, et al. Treatment outcomes of il-17 inhibitors in hidradenitis suppurativa: A systematic review. J Cutan Med Surg (2022) 26(1):79–86. doi: 10.1177/12034754211035667
34. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early th17 cell differentiation by interleukin-1 signaling. Immunity (2009) 30(4):576–87. doi: 10.1016/j.immuni.2009.02.007
35. Du WJ, Zhen JH, Zeng ZQ, Zheng ZM, Xu Y, Qin LY, et al. Expression of interleukin-17 associated with disease progression and liver fibrosis with hepatitis b virus infection: Il-17 in hbv infection. Diagn Pathol (2013) 8:40. doi: 10.1186/1746-1596-8-40
36. Malara A, Hughes R, Jennings L, Sweeney CM, Lynch M, Awdeh F, et al. Adipokines are dysregulated in patients with hidradenitis suppurativa. Br J Dermatol (2018) 178(3):792–93. doi: 10.1111/bjd.15904
37. Zheng YT, Xiao TM, Wu CX, Cheng JY, Li LY. Correlation of adiponectin gene polymorphisms rs266729 and rs3774261 with risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Front Endocrinol (Lausanne) (2022) 13:798417. doi: 10.3389/fendo.2022.798417
Keywords: hidradenitis suppurativa, liver diseases, non-alcoholic fatty liver disease, hepatitis B, hepatitis C
Citation: Gau S-Y, Hsiao Y-P, Liao W-C, Ma KS-K and Wu M-C (2022) Risk of liver dysfunction and non-alcoholic fatty liver diseases in people with hidradenitis suppurativa: A systematic review and meta-analysis of real-world evidences. Front. Immunol. 13:959691. doi: 10.3389/fimmu.2022.959691
Received: 02 June 2022; Accepted: 22 November 2022;
Published: 14 December 2022.
Edited by:
Paola Maura Tricarico, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyReviewed by:
Farida Benhadou, Hôpital Erasme, Université libre de Bruxelles, BelgiumCopyright © 2022 Gau, Hsiao, Liao, Ma and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng-Che Wu, d3VtZW5nY2hlQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.