- 1Anatomic Pathology Division, Children’s Hospital of Eastern Ontario (CHEO), Ottawa, ON, Canada
- 2Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada
- 3Department of Orthopedics, Tianyou Hospital, Wuhan University of Science and Technology, Wuhan, China
- 4Medical Imaging Department, Children’s Hospital of Eastern Ontario (CHEO), University of Ottawa, Ottawa, ON, Canada
Chronic recurrent and multifocal osteomyelitis (CRMO) is a nonsporadic autoinflammatory disorder. Currently, it is diagnosed based on clinical, radiologic, pathological, and longitudinal data. Numerous aspects should be highlighted due to increased knowledge in imaging and immunology. We emphasize the use of whole-body MRI, which is a non-invasive diagnostic strategy. A literature review was carried out on longitudinal studies. Commonly, the mean age at diagnosis is 11 years, ranging between 3 and 17. The most common sites are the long bone metaphysis, particularly femoral and tibial metaphysis. In addition, the pelvis, spine, clavicle, and mandible may be involved. In long bones, the radiologic appearance can show typical structure, mixed lytic and sclerotic, sclerotic or lytic. It is frequently metaphyseal or juxta-physeal, with hyperostosis or periosteal thickening. The involvement of the vertebral skeleton is often multifocal. Therefore, whole-body MRI is essential in identifying subclinical lesions. CRMO is a polymorphic disorder in which whole-body MRI is beneficial to demonstrate subclinical edema. Vertebral collapse requires long-term monitoring.
Introduction
Osteomyelitis is a common condition that involves the bone marrow. It may present with acute and chronic features and variable etiology. In most cases, acute osteomyelitis is due to hematogenous spread. Microorganisms can enter a bone in various ways, including in the bloodstream. Therefore, acute hematogenous osteomyelitis is a common diagnosis. It is often takes place after an episode of bacteremia in which the bacteria inoculate the bony tissue. The microorganisms frequently involved are Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae type b (1, 2).
On the other hand, chronic recurrent multifocal osteomyelitis (CRMO) is an inflammatory bone disorder lacking bacterial involvement. It is characterized by lytic, sclerotic, and hyperostotic lesions. Despite the chronicity character, CRMO often exhibits periodic flairs and phases of remission (3). CRMO is currently indicated as an autoinflammatory disease. It often affects the pediatric population, and the onset age is usually nine years, with a female to male ratio of 2:1. CRMO is occasionally labeled SAPHO, an acronym describing these patients’ clinical features, including synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome. Another name, which some rheumatologists also use, is chronic nonbacterial osteomyelitis (CNO), or simply nonbacterial osteomyelitis (4, 5). It has been suggested that SAPHO is often nonbacterial osteomyelitis with an autoinflammatory character of the adult, while CRMO should be limited to the pediatric age group (6–13).
Etio-pathogenesis
There is a substantial body of evidence that rejects any infection or colonization in CRMO, and despite some treatments, there is no positive result apart from single or anecdotal improvements in patients receiving macrolide therapy. Macrolides are also known to harbor anti-inflammatory activity (14, 15). It has been clarified that the anti-inflammatory properties of macrolides are linked to the chemical structure. Immunomodulatory effects have been detected with 14- (erythromycin, clarithromycin and roxithromycin) and 15- (azithromycin). No results with 16-member (josamycin) macrolides have been identified. The most frequently and consistently reported immunomodulatory effect of macrolides is decreased neutrophilic inflammation. A decrease of neutrophils and inhibition of neutrophilic function lead to lower IL-8 and neutrophil elastase rates, ultimately limiting tissue injury.
Similar to other autoimmune conditions, there is a remarkable imbalance of cytokines in patients affected by CRMO. It has been underlined that patients suffering from CRMO show a decreased production of anti-inflammatory cytokines (interleukins 9, 10, and 18; IL-9, IL-10, IL-18) and increased production of proinflammatory cytokines (IL-1b, IL-6, tumor necrosis factor-α, TNF-a) (4, 16–22). The cytokine imbalance is probably the basis of the bony manifestations of CRMO. In a mouse model, bone, cartilage, and skin inflammation of the extremities and ears have been described (23). These authors detected an L98P mutation in the Pstpip2 gene and commented that this genetic variation may be the cause of the autoinflammatory phenotype. Lukens et al. revised the critical role of the inflammasome in osteomyelitis (24) and showed that the bone disease in CRMO rodents may harbor NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3). It is inflammasome dependent and, of course, IL-1b mediated (4, 13, 17, 25–32). In some patients affected by CRMO, single-gene defects have been identified. Clinical geneticists have also identified some similarities with Majeed syndrome, which is extremely rare, being described in only 24 individuals from 10 families, and is characterized by recurrent episodes of fever and osteomyelitis, and dermatitis (33, 34).
LPIN2 (Lipin 2) is a Protein Coding gene. LPIN2 acts as a phosphatidate phosphatase enzyme, magnesium-dependent. The ultrastructural and biochemical investigations revealed that the reticulum endoplasmic membrane is critical. It catalyzes the conversion of phosphatidic acid to diacylglycerol during triglyceride, phosphatidylcholine, and phosphatidylethanolamine biosynthesis. LPIN2 is crucial to controlling fatty acid metabolism and is a nuclear transcriptional coactivator for PPARGC1A to modulate lipid metabolism. This gene seems to be a candidate gene for human lipodystrophy, characterized by fatty liver, body fat loss, hypertriglyceridemia, and insulin resistance. CRMO patients have been demonstrated to have LPIN2 gene mutations (35).
There is also DIRA, or deficiency of IL-1 Receptor Antagonist, which is a genetic disorder with an autosomal recessive pattern of inheritance and has autoinflammatory clinic-pathological characteristics caused by mutations in the IL1RN gene (36). These patients show a pustular rash and nonbacterial osteomyelitis within the first few postnatal weeks. It has been demonstrated that the IL1RN gene instructs for the codification of the interleukin 1 receptor antagonist, which is key in leading to unchecked inflammation. Another gene has been controversially discussed to be an integral part of CRMO. The Filamin-binding LIM protein 1 (FBLIM1) gene harboring bi-allelic variants has been described in CRMO patients, although recently, a new study seems to vindicate this gene in causing CRMO. FBLIM1 gene encodes filamin binding lin-11, islet-1, mec-3 (LIM) protein (37, 38). More recently, Yahara et al. (39) have indicated that the interaction between some haplotypes of killer cell immunoglobulin-like receptors and the human leukocyte antigen (HLA) may be critical. This interaction would be able to determine the HLA instability leading inexorably to autoimmunity in CRMO.
Diagnosis
The approach to CRMO is not a straightforward process because no specific biomarkers are available (17, 18, 40–45). Therefore, we need to rule out malignancies first, then infections and other inflammatory conditions. Thus, expertise in radio-diagnostics and pathology is key in ruling out osteosarcoma and Ewing sarcoma as primary bone tumors, metastases as secondary bony tumors, and leukemia or lymphoma in the setting of a generalized lymphoproliferative disorder (6, 46). Currently, there are two sets of diagnostic criteria, the Jansson and Bristol criteria (47, 48). According to the Jansson system, CRMO is validated by two major, or one major plus three minor criteria (49). In this system, major criteria are as follows: (1) Radiologically demonstrated osteolytic/osteosclerotic bony lesions, (2) Multifocality of the bony lesions, (3) Palmoplantar dermatosis (pustulosis psoriasis), and (4) Sterile osseous biopsy with signs of inflammation, fibrosis, or sclerosis. In the Jansson system, minor criteria include (1) Normal blood count and good general state of health, (2) Increase of C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (mild to moderate levels), (3) Time for more than half a year, (4) Hyperostosis, and (5) Positive family history with grade I or II relatives diagnosed by any autoimmune disease. In the Bristol system, diagnostic criteria include typical clinical findings (bony pain localized or swelling features of inflammation), typical radiological findings, and one of the following criteria: (1) >1 bone affected without significantly raised CRP (<30 mg/l), (2) Bony biopsy demonstrating inflammation with the recruitment of both chronic inflammatory cells (plasma cells) as well as osteoclasts and histologic evidence of fibrosis or sclerosis with no growth of microorganisms in a status without antibiotic therapy.
Clinico-laboratory data
In the clinical presentation, some features seem to be recurrent. They include painful inflammatory bony lesions that may affect any skeleton area. The analysis of several studies identified that the more often active regions include the metaphysis of long bones, the pelvis, and the shoulder girdle. Also, the clavicle, vertebral bodies, and lower jaw are often involved. The patients may present to the emergency department with vertebral body fracture, and a skilled radiologist should raise the differential diagnosis properly. Local inflammation may often be identified in the affected extremities (48, 50). Children experience pain, which is worse at night. In about half of the patients reported in the literature, symmetrical involvement of bony lesions is seen. The pain is usually insidious in origin and persists or is intermittent and can last for several years. The multilateral and multifocal aspects are often seen and need to be distinguished by the occurrence of juvenile rheumatoid arthritis (6, 51). Extraosseous features of CRMO include skin, gastrointestinal, ocular, and cardiac involvement. The pustular rash on the palms and soles is quite characteristic (50). Prognosis may be variable, but poor factors include prolonged time from the onset of symptoms to the final diagnosis, multifocality, and male gender (52). Laboratory tests may exhibit a mild to moderate increase of inflammatory markers (50, 53, 54), but lactate dehydrogenase, uric acid, or alkaline phosphatase may be abnormal. Importantly, low albumin may indicate a simultaneous inflammatory bowel disease. In a few patients, there have been reports of autoantibodies or HLA B27.
Imaging
X-rays might be unremarkable in the early stage of the disease and this does not exclude the diagnosis. Lytic, poorly define or well defined lesions can be seen early with progressive sclerosis or cortical thickening of the bone in the reparative phase (4, 10, 55–59). We and others have suggested fluorodeoxyglucose (FDG), positron emission tomography (PET)-computed tomography (CT)-scan, and bone scintigraphy as diagnostic tools (6). However, this kind of imaging is not recommended for children because of the use of radiation. Bone scintigraphy has been replaced by whole body magnetic resonance imaging (MRI) to assess multifocal and extent of involvement, soft tissue involvement and exclude other diagnoses. The most typical findings include bone marrow edema (increased signal on T2 weighted MRI images and reduced signal on T1 weighted MRI images) and characterized by an enhancement and periostitis/periosteal reaction (Figures 1, 2). Often associated with extensive soft tissue edema and inflammation. Joint involvement is sometimes seen with signs of synovitis on MRI. Three patterns have been described with the use of whole-body MRI: (1) Tibia-appendicular multifocal pattern with tibial lesions, multifocal and no clavicular involvement (Figure 1), (2) clavicular-spinal pauci-focal pattern: clavicular lesions and a few other predominantly spinal lesions without tibial involvement (Figure 2), and (3) Tibia-clavicular crossover pattern (60, 61). The long bone methaphysis, particularly clavicle (classic for CRMO)- and lower extremities (distal femur, proxima and distal tibia and distal fibular are most frequently affected (51, 62). Clavicle or mandible are the most common if a solitary site is involved, which is extremely rare.
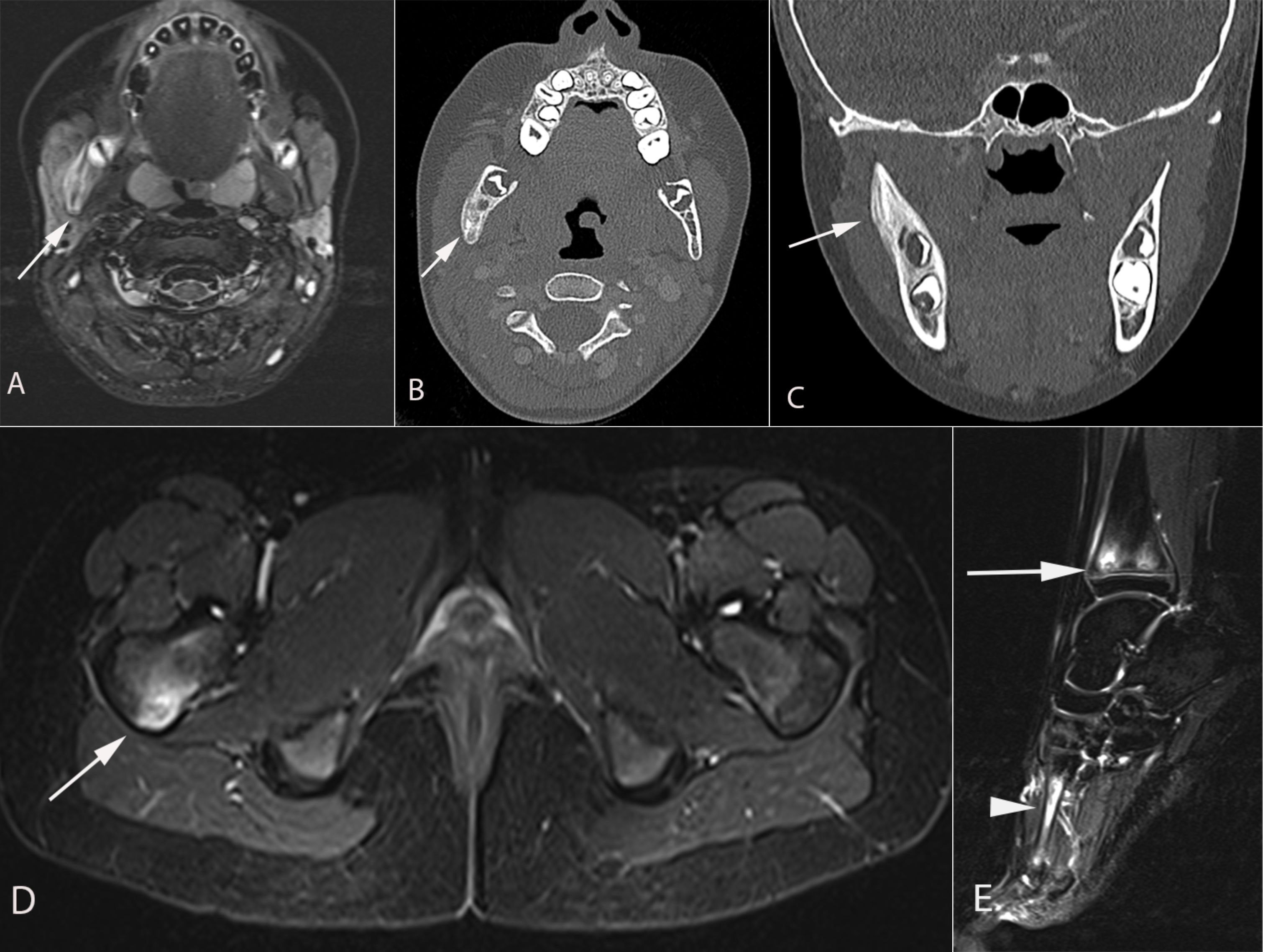
Figure 1 A 9-year-old girl presented with 10 days of right cheek swelling. (A) MRI Axial T2 STIR demonstrated asymmetric enlargement of the right masseter muscle with a heterogeneous hyperintense signal of the right mandibular ramus (arrow) in keeping with bone marrow edema. (B) axial computed tomography and (C) coronal computed tomography images demonstrate smooth periosteal reaction in the right hemi mandible. No associated soft tissue mass or periodontal abscess. Axial (D) and sagittal (E) MRI STIR images demonstrate another focus of hyperintensity in the posterior aspect of the right femoral neck (arrow in D), the distal tibial metaphyses (arrow in e), and the right first (arrowhead in e) and second metatarsals (not shown). Findings are consistent with CRMO following a Whole Body MRI.
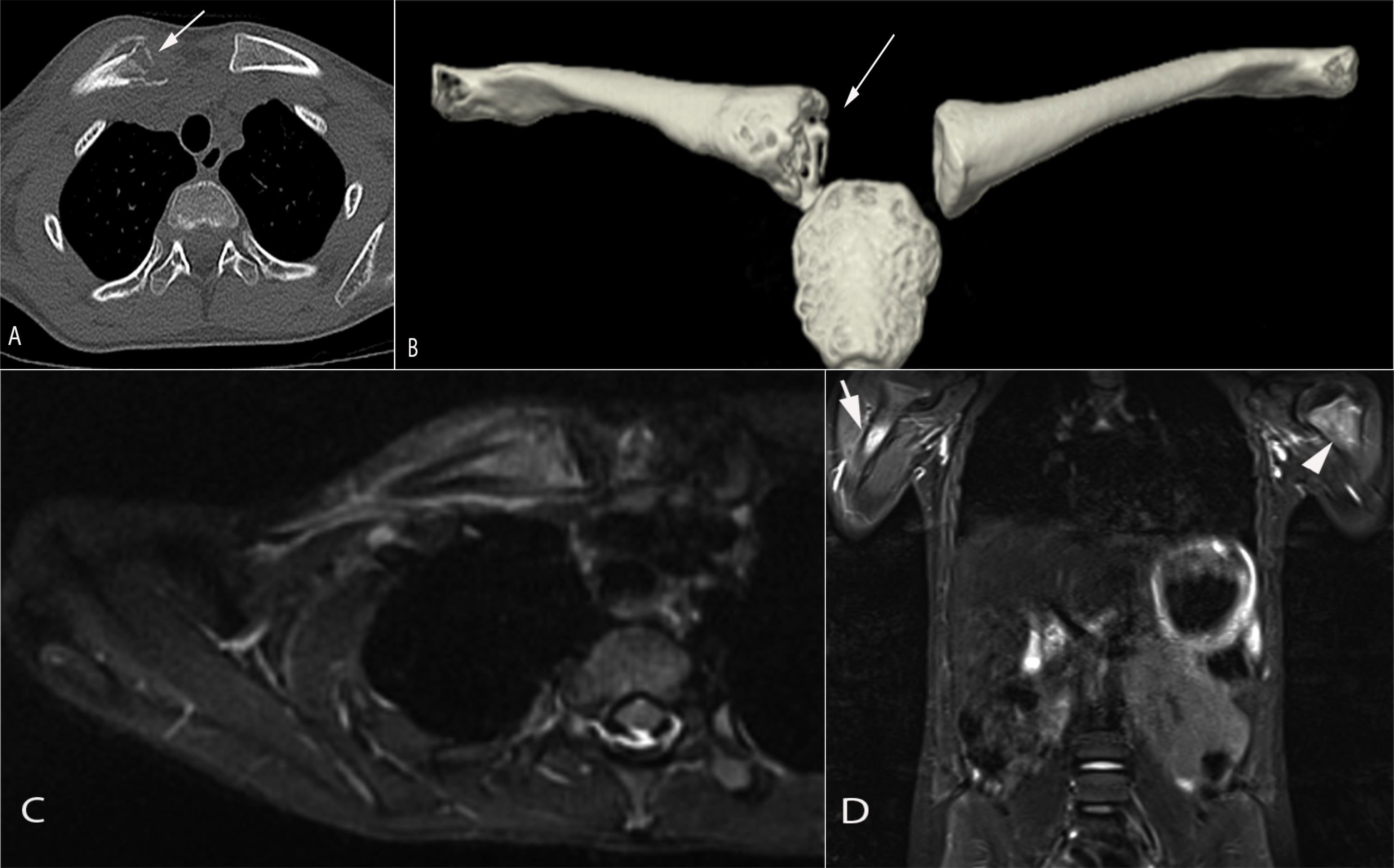
Figure 2 An 8-year-old girl presented with a painful lump on the right clavicle. Axial (A) and 3D reformats (B) computed tomography images demonstrate a destructive lesion in the medial aspect of the right clavicle associated with periosteal reaction and soft tissue swelling (arrow in a/b). Axial T2 STIR magnetic resonance image (C) showed a marked signal abnormality involving the medial aspect of the right clavicle with associated periosteal fluid and soft tissue edema. Total body MRI-STIR sequences (D) showed additional lesions in the proximal right (arrow) and left (arrowhead) humeral metaphysis, right iliac crest (not shown)and left greater trochanter (not shown). Findings are consistent with CRMO following a Whole Body MRI.
Pathology
A bone biopsy is a gold standard for diagnosis. Histologic sections of the bone include bony tissue showing areas of resorption with increased osteoclasts and cellular infiltrates, which may be highly dependent on the illness stage. In the beginning, there is a neutrophil predominance in the bone marrow cavities. An abscess can also be found, but Gram staining and other special stains fail to identify any microorganisms. Mast cell infiltrates have been described (9, 13, 63–66). Thus, the special stain tryptase and the immunohistochemical stain CD117 may be necessary for the bone pathology workup (Figure 3). In the late stages of CRMO, macrophages, lymphocytes, and plasma cells belong to the mixed inflammatory infiltrates often detected under the lens. Occasionally, noncaseating granulomas have been reported. In this setting, the differential diagnosis with sarcoidosis needs to be raised at this point. Ultimately, osteolysis, osteonecrosis, the recruitment of multinucleated giant cells, fibrosis, and, finally, sclerosis seem to be characteristically seen in the late stage of the disease. Of note, it is important to remember that the fibrosing end-stage may be detected already after a few years of active osteolysis with inflammation.
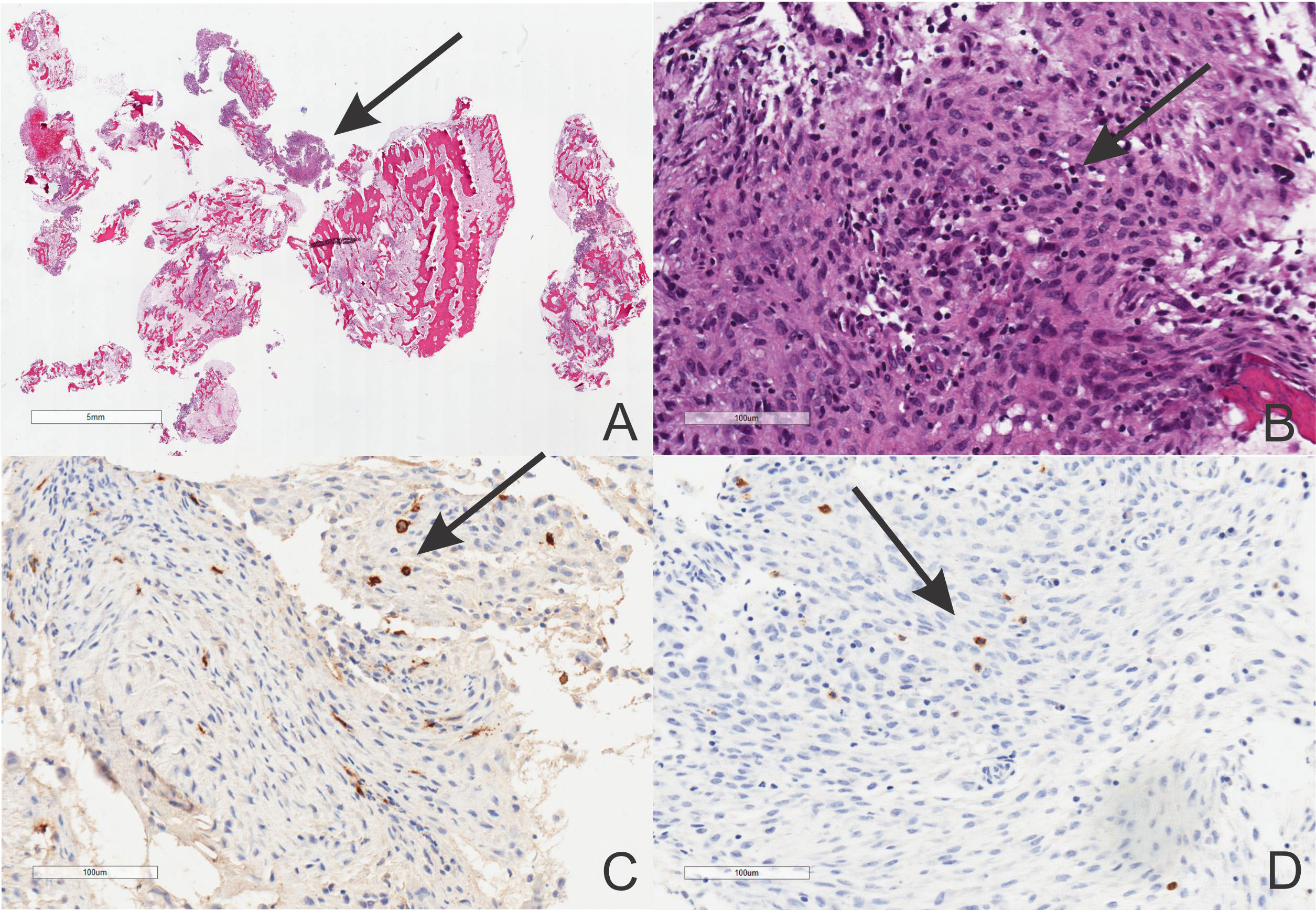
Figure 3 Histopathology of the CRMO showing low and high power magnification of a nonbacterial osteomyelitis (A, B, hematoxylin-eosin staining, scale bars included) with CD117 (C) and tryptase (D) positivities (Avidin-Biotin Complex immunostaining, scale bars included).
Treatment
CRMO is a complex and complicated disease, and a multidisciplinary approach to treating this illness is critical. Some of these patients associate cutaneous and gastrointestinal symptoms to chronic bony pain. The goal is to relieve pain and prevent disease progression with the ultimate aim to annihilate the permanent damage in the affected bones. Non-steroidal anti-inflammatory drugs are the first line of treatment, but they are often insufficient to control the disease’s pain and progression. Therefore, biorepositories may need to be implemented for clinical trials (67). The institutions of worldwide accessible biorepositories are vital due to the rarity ad variability of CRMO. Randomized controlled trials have been difficult to execute in places where a laboratory information system is not fully operative (68). The CNO/CRMO subgroup of the Childhood Arthritis and Rheumatology Research Alliance recently produced a consensus treatment plan with three treatment arms (29). These include (1) Conventional disease-modifying antirheumatic drugs (DMARDs): methotrexate or sulfasalazine; (2) Biologic DMARDs: TNF-a inhibitors with or without concomitant methotrexate; and (3) Bisphosphonates. In all regimens, short courses of glucocorticoids are permitted. Among TNF-a inhibitors, infliximab has shown improvement in many CRMO patients as it is used in patients with inflammatory bowel disease (69–75). Etanercept has demonstrated superb clinical outcomes involving improvement in skin manifestations, and it is well tolerated. Adalimubab and certolizumab pegol have been demonstrated to be very successful. They show complete remission of skin and musculoskeletal manifestations. Other biologics include IL-1 blockers, IL-23, and IL-17 inhibitors with relatively promising results (11, 36, 54, 76–79). Bisphosphonates are effective and well-tolerated. In children, pamidronate is the bisphosphonate of choice.
Prognosis
As indicated above, the outcome may be variable. Still, poor factors consist of prolonged time from symptoms to the final diagnosis, multifocality, and male gender (51). If treated early, CRMO may show a favorable outcome. Severe complications of this chronic bony disease include limb length discrepancy, deformity of the extremities, or vertebral fracture (4, 80). When matched with healthy children, children who were adequately diagnosed and treated did not demonstrate significant variations in objective amounts of physical activity and fitness (81).
Conclusion
CRMO is a rare autoinflammatory disease of the skeleton with an impressive debilitating burden for skeletal and extra-skeletal systems. Despite numerous efforts to convey biorepositories to identify biomarkers, the diagnosis remains of exclusion. Two methods of diagnosis are not incompatible, and some of the criteria overlap. Nevertheless, bone biopsy remains the gold standard for diagnosing CRMO. Several lines of therapy exist with the TNF-α inhibitors showing effectiveness and good compliance.
Author contributions
CS conceptualized the study. CS collected data, drafted the initial manuscript, and revised the manuscript. CS revised the data and performed the analysis, was responsible for the intramural funding, and revised the final draft of the manuscript. EM collected the imaging data, prepared the figures, and revised the manuscript. FS revised the manuscript and expanded on the genetics of the disorder. MZ revised the manuscript and was responsible for part of the funding. All authors meet the ICMJE requirements for authorship, approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This research has been funded by the generosity of the Children's Hospital of Eastern Ontario, University of Ottawa, Ontario, Canada, and the Department of Orthopedics, Tianyou Hospital, Wuhan University of Science and Technology, Wuhan, Hubei, P.R. China. The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Furuta A, Brokaw A, Manuel G, Dacanay M, Marcell L, Seepersaud R, et al. Bacterial and host determinants of group b streptococcal infection of the neonate and infant. Front Microbiol (2022) 13:820365. doi: 10.3389/fmicb.2022.820365
2. Masters EA, Ricciardi BF, Bentley KLM, Moriarty TF, Schwarz EM, Muthukrishnan G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol (2022) 20(7):385–400. doi: 10.1038/s41579-022-00686-0
3. Hedrich CM, Morbach H, Reiser C, Girschick HJ. New insights into adult and paediatric chronic non-bacterial osteomyelitis cno. Curr Rheumatol Rep (2020) 22(9):52. doi: 10.1007/s11926-020-00928-1
4. Moreno-Mateo F, Perea SH, Onel KB. Chronic recurrent multifocal osteomyelitis: Diagnosis and treatment. Curr Opin Pediatr (2021) 33(1):90–6. doi: 10.1097/MOP.0000000000000970
5. Sato TS, Watal P, Ferguson PJ. Imaging mimics of chronic recurrent multifocal osteomyelitis: Avoiding pitfalls in a diagnosis of exclusion. Pediatr Radiol (2020) 50(1):124–36. doi: 10.1007/s00247-019-04510-5
7. Ferjani Hanene L, Makhlouf Y, Maatallah K, Triki W, Ben Nessib D, Kaffel D, et al. Management of chronic recurrent multifocal osteomyelitis: Review and update on the treatment protocol. Expert Opin Biol Ther (2022) 22(6):781–7. doi: 10.1080/14712598.2022.2078161
8. Zhao M, Wu D, Yu K, Shen M. Clinical and genetic features of Chinese adult patients with chronic non-bacterial osteomyelitis: A single center report. Front Immunol (2022) 13:860646. doi: 10.3389/fimmu.2022.860646
9. O'Leary D, Wilson AG, MacDermott EJ, Lowry C, Killeen OG. Variability in phenotype and response to treatment in chronic nonbacterial osteomyelitis; the Irish experience of a national cohort. Pediatr Rheumatol Online J (2021) 19(1):45. doi: 10.1186/s12969-021-00530-4
10. Ziegeler K, Eshed I, Diekhoff T, Hermann KG. Imaging of joints and bones in autoinflammation. J Clin Med (2020) 9(12):4074. doi: 10.3390/jcm9124074
11. Hartland EL. A potential new target for autoinflammatory bone disease. J Biol Chem (2020) 295(11):3401–2. doi: 10.1074/jbc.H120.012867
12. Al-Mayouf SM, Almutairi A, Albrawi S, Fathalla BM, Alzyoud R, AlEnazi A, et al. Pattern and diagnostic evaluation of systemic autoinflammatory diseases other than familial Mediterranean fever among Arab children: A multicenter study from the pediatric rheumatology Arab group (Prag). Rheumatol Int (2020) 40(1):49–56. doi: 10.1007/s00296-019-04478-3
13. Young S, Sharma N, Lee JH, Chitu V, Neumeister V, Sohr E, et al. Mast cells enhance sterile inflammation in chronic nonbacterial osteomyelitis. Dis Model Mech (2019) 12(8):dmm040097. doi: 10.1242/dmm.040097
14. Crosbie PA, Woodhead MA. Long-term macrolide therapy in chronic inflammatory airway diseases. Eur Respir J (2009) 33(1):171–81. doi: 10.1183/09031936.00042208
15. Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front Immunol (2018) 9:302. doi: 10.3389/fimmu.2018.00302
16. Ramanan AV, Hampson LV, Lythgoe H, Jones AP, Hardwick B, Hind H, et al. Defining consensus opinion to develop randomised controlled trials in rare diseases using Bayesian design: An example of a proposed trial of adalimumab versus pamidronate for children with Cno/Crmo. PloS One (2019) 14(6):e0215739. doi: 10.1371/journal.pone.0215739
17. Hofmann SR, Kapplusch F, Girschick HJ, Morbach H, Pablik J, Ferguson PJ, et al. Chronic recurrent multifocal osteomyelitis (Crmo): Presentation, pathogenesis, and treatment. Curr Osteoporos Rep (2017) 15(6):542–54. doi: 10.1007/s11914-017-0405-9
18. Hofmann SR, Kubasch AS, Range U, Laass MW, Morbach H, Girschick HJ, et al. Serum biomarkers for the diagnosis and monitoring of chronic recurrent multifocal osteomyelitis (Crmo). Rheumatol Int (2016) 36(6):769–79. doi: 10.1007/s00296-016-3466-7
19. Hofmann SR, Kubasch AS, Ioannidis C, Rosen-Wolff A, Girschick HJ, Morbach H, et al. Altered expression of il-10 family cytokines in monocytes from crmo patients result in enhanced il-1beta expression and release. Clin Immunol (2015) 161(2):300–7. doi: 10.1016/j.clim.2015.09.013
20. Cassel SL, Janczy JR, Bing X, Wilson SP, Olivier AK, Otero JE, et al. Inflammasome-independent il-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci USA (2014) 111(3):1072–7. doi: 10.1073/pnas.1318685111
21. Hofmann SR, Morbach H, Schwarz T, Rosen-Wolff A, Girschick HJ, Hedrich CM. Attenuated Tlr4/Mapk signaling in monocytes from patients with crmo results in impaired il-10 expression. Clin Immunol (2012) 145(1):69–76. doi: 10.1016/j.clim.2012.07.012
22. Galeazzi M, Gasbarrini G, Ghirardello A, Grandemange S, Hoffman HM, Manna R, et al. Autoinflammatory syndromes. Clin Exp Rheumatol (2006) 24(1 Suppl 40):S79–85.
23. Ferguson PJ, Bing X, Vasef MA, Ochoa LA, Mahgoub A, Waldschmidt TJ, et al. A missense mutation in Pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone (2006) 38(1):41–7. doi: 10.1016/j.bone.2005.07.009
24. Lukens JR, Gross JM, Calabrese C, Iwakura Y, Lamkanfi M, Vogel P, et al. Critical role for inflammasome-independent il-1beta production in osteomyelitis. Proc Natl Acad Sci USA (2014) 111(3):1066–71. doi: 10.1073/pnas.1318688111
25. Scianaro R, Insalaco A, Bracci Laudiero L, De Vito R, Pezzullo M, Teti A, et al. Deregulation of the il-1beta axis in chronic recurrent multifocal osteomyelitis. Pediatr Rheumatol Online J (2014) 12:30. doi: 10.1186/1546-0096-12-30
26. Hofmann SR, Kapplusch F, Mabert K, Hedrich CM. The molecular pathophysiology of chronic non-bacterial osteomyelitis (Cno)-a systematic review. Mol Cell Pediatr (2017) 4(1):7. doi: 10.1186/s40348-017-0073-y
27. Cox AJ, Zhao Y, Ferguson PJ. Chronic recurrent multifocal osteomyelitis and related diseases-update on pathogenesis. Curr Rheumatol Rep (2017) 19(4):18. doi: 10.1007/s11926-017-0645-9
28. Cox AJ, Ferguson PJ. Update on the genetics of nonbacterial osteomyelitis in humans. Curr Opin Rheumatol (2018) 30(5):521–5. doi: 10.1097/BOR.0000000000000530
29. Zhao Y, Wu EY, Oliver MS, Cooper AM, Basiaga ML, Vora SS, et al. Consensus treatment plans for chronic nonbacterial osteomyelitis refractory to nonsteroidal antiinflammatory drugs and/or with active spinal lesions. Arthritis Care Res (Hoboken) (2018) 70(8):1228–37. doi: 10.1002/acr.23462
30. Andreasen CM, Jurik AG, Glerup MB, Host C, Mahler BT, Hauge EM, et al. Response to early-onset pamidronate treatment in chronic nonbacterial osteomyelitis: A retrospective single-center study. J Rheumatol (2019) 46(11):1515–23. doi: 10.3899/jrheum.181254
31. Dasari TK, Geiger R, Karki R, Banoth B, Sharma BR, Gurung P, et al. The nonreceptor tyrosine kinase syk drives caspase-8/Nlrp3 inflammasome-mediated autoinflammatory osteomyelitis. J Biol Chem (2020) 295(11):3394–400. doi: 10.1074/jbc.RA119.010623
32. Sergi CM, Chiu B. Targeting Nlrp3 inflammasome in an animal model for coronavirus disease 2019 (Covid-19) caused by the severe acute respiratory syndrome coronavirus 2 (Sars-Cov-2). J Med Virol (2021) 93(2):669–70. doi: 10.1002/jmv.26461
33. Chavan PP, Aksentijevich I, Daftary A, Panwala H, Khemani C, Khan A, et al. Majeed syndrome: Five cases with novel mutations from unrelated families in India with a review of literature. J Rheumatol (2021) 48(12):1850–5. doi: 10.3899/jrheum.201663
34. Chen Z, Cheng L, Feng G. Bone inflammation and chronic recurrent multifocal osteomyelitis. Eur Rev Med Pharmacol Sci (2018) 22(5):1380–6. doi: 10.26355/eurrev_201803_14482
35. Ferguson PJ, Chen S, Tayeh MK, Ochoa L, Leal SM, Pelet A, et al. Homozygous mutations in Lpin2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome). J Med Genet (2005) 42(7):551–7. doi: 10.1136/jmg.2005.030759
36. Kuemmerle-Deschner JB, Welzel T, Hoertnagel K, Tsiflikas I, Hospach A, Liu X, et al. New variant in the Il1rn-gene (Dira) associated with late-onset, crmo-like presentation. Rheumatol (Oxf) (2020) 59(11):3259–63. doi: 10.1093/rheumatology/keaa119
37. Cox AJ, Darbro BW, Laxer RM, Velez G, Bing X, Finer AL, et al. Recessive coding and regulatory mutations in Fblim1 underlie the pathogenesis of chronic recurrent multifocal osteomyelitis (Crmo). PloS One (2017) 12(3):e0169687. doi: 10.1371/journal.pone.0169687
38. Assmann G, Kohm M, Schuster V, Behrens F, Mossner R, Magnolo N, et al. Genetic variants in Fblim1 gene do not contribute to sapho syndrome and chronic recurrent multifocal osteomyelitis in typical patient groups. BMC Med Genet (2020) 21(1):102. doi: 10.1186/s12881-020-01037-7
39. Yahara H, Horita S, Yanamoto S, Kitagawa Y, Asaka T, Yoda T, et al. A targeted genetic association study of the rare type of osteomyelitis. J Dent Res (2020) 99(3):271–6. doi: 10.1177/0022034520901519
40. Jaramillo D, Dormans JP, Delgado J, Laor T, St Geme JW 3rd. Hematogenous osteomyelitis in infants and children: Imaging of a changing disease. Radiology (2017) 283(3):629–43. doi: 10.1148/radiol.2017151929
41. Averill LW, Hernandez A, Gonzalez L, Pena AH, Jaramillo D. Diagnosis of osteomyelitis in children: Utility of fat-suppressed contrast-enhanced mri. AJR Am J Roentgenol (2009) 192(5):1232–8. doi: 10.2214/AJR.07.3400
42. Darge K, Jaramillo D, Siegel MJ. Whole-body mri in children: Current status and future applications. Eur J Radiol (2008) 68(2):289–98. doi: 10.1016/j.ejrad.2008.05.018
43. Jaramillo D, Treves ST, Kasser JR, Harper M, Sundel R, Laor T. Osteomyelitis and septic arthritis in children: Appropriate use of imaging to guide treatment. AJR Am J Roentgenol (1995) 165(2):399–403. doi: 10.2214/ajr.165.2.7618566
44. Zhao DY, McCann L, Hahn G, Hedrich CM. Chronic nonbacterial osteomyelitis (Cno) and chronic recurrent multifocal osteomyelitis (Crmo). J Transl Autoimmun (2021) 4:100095. doi: 10.1016/j.jtauto.2021.100095
45. Hofmann SR, Bottger F, Range U, Luck C, Morbach H, Girschick HJ, et al. Serum interleukin-6 and Ccl11/Eotaxin may be suitable biomarkers for the diagnosis of chronic nonbacterial osteomyelitis. Front Pediatr (2017) 5:256. doi: 10.3389/fped.2017.00256
46. Zetouni NC, Sergi CM. Features of metastatic Ewing sarcoma. In: Sergi CM, editor. Metastasis. Brisbane (AU: Exon Publications (2022).
47. Roderick MR, Shah R, Rogers V, Finn A, Ramanan AV. Chronic recurrent multifocal osteomyelitis (Crmo) - advancing the diagnosis. Pediatr Rheumatol Online J (2016) 14(1):47. doi: 10.1186/s12969-016-0109-1
48. Bhat CS, Anderson C, Harbinson A, McCann LJ, Roderick M, Finn A, et al. Chronic non bacterial osteitis- a multicentre study. Pediatr Rheumatol Online J (2018) 16(1):74. doi: 10.1186/s12969-018-0290-5
49. Jansson A, Renner ED, Ramser J, Mayer A, Haban M, Meindl A, et al. Classification of non-bacterial osteitis: Retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatol (Oxford) (2007) 46(1):154–60. doi: 10.1093/rheumatology/kel190
50. Wobma H, Jaramillo D, Imundo L. When local bone pain is just the tip of the iceberg-a case report of three patients with chronic multifocal recurrent osteomyelitis and some red flags to help make the diagnosis. Front Pediatr (2019) 7:407. doi: 10.3389/fped.2019.00407
51. Girschick H, Finetti M, Orlando F, Schalm S, Insalaco A, Ganser G, et al. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: A series of 486 cases from the eurofever international registry. Rheumatol (Oxford) (2018) 57(8):1504. doi: 10.1093/rheumatology/key143
52. Girschick H, Finetti M, Orlando F, Schalm S, Insalaco A, Ganser G, et al. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: A series of 486 cases from the eurofever international registry. Rheumatol (Oxford) (2018) 57(7):1203–11. doi: 10.1093/rheumatology/key058
53. Arnoldi AP, Schlett CL, Douis H, Geyer LL, Voit AM, Bleisteiner F, et al. Whole-body mri in patients with non-bacterial osteitis: Radiological findings and correlation with clinical data. Eur Radiol (2017) 27(6):2391–9. doi: 10.1007/s00330-016-4586-x
54. Zhao Y, Iyer RS, Reichley L, Oron AP, Gove NE, Kitsch AE, et al. A pilot study of infrared thermal imaging to detect active bone lesions in children with chronic nonbacterial osteomyelitis. Arthritis Care Res (Hoboken) (2019) 71(11):1430–5. doi: 10.1002/acr.23804
55. Kopec M, Braszewska M, Jarosz M, Dylewska K, Kurylak A. Role of diagnostic imaging in chronic recurrent multifocal osteomyelitis (Crmo) in children: An observational study. Children (Basel) (2021) 8(9):792. doi: 10.3390/children8090792
56. Koryllou A, Mejbri M, Theodoropoulou K, Hofer M, Carlomagno R. Chronic nonbacterial osteomyelitis in children. Children (Basel) (2021) 8(7):551. doi: 10.3390/children8070551
57. Andronikou S, Kraft JK, Offiah AC, Jones J, Douis H, Thyagarajan M, et al. Whole-body mri in the diagnosis of paediatric Cno/Crmo. Rheumatol (Oxford) (2020) 59(10):2671–80. doi: 10.1093/rheumatology/keaa303
58. Bergeron A, Lewellen T, Joshi B. Radiographic changes of chronic recurrent multifocal osteomyelitis that persisted into adulthood. BMJ Case Rep (2020) 13(7):e232106. doi: 10.1136/bcr-2019-232106
59. Himuro H, Kurata S, Nagata S, Sumi A, Tsubaki F, Matsuda A, et al. Imaging features in patients with Sapho/Crmo: A pictorial review. Jpn J Radiol (2020) 38(7):622–9. doi: 10.1007/s11604-020-00953-1
60. Andronikou S, Mendes da Costa T, Hussien M, Ramanan AV. Radiological diagnosis of chronic recurrent multifocal osteomyelitis using whole-body mri-based lesion distribution patterns. Clin Radiol (2019) 74(9):737 e3– e15. doi: 10.1016/j.crad.2019.02.021
61. Gamalero L, Belot A, Zajc Avramovic M, Giani T, Filocamo G, Guleria S, et al. Chronic non-bacterial osteomyelitis: A retrospective international study on clinical manifestations and response to treatment. Clin Exp Rheumatol (2020) 38(6):1255–62.
62. Mahady S, Ladani A. Clinical and diagnostic considerations for atypical, adult onset presentation of chronic recurrent multifocal osteomyelitis (Crmo). Case Rep Rheumatol (2019) 2019:8206892. doi: 10.1155/2019/8206892
63. Monsour PA, Dalton JB. Chronic recurrent multifocal osteomyelitis involving the mandible: Case reports and review of the literature. Dentomaxillofac Radiol (2010) 39(3):184–90. doi: 10.1259/dmfr/23060413
64. Okay E, Ulu K, Demir F, Sari T, Zeynalov S, Toksoz Yildirim AN, et al. Chronic recurrent multifocal osteomyelitis: A multidisciplinary experience of 22 pediatric cases with a mean follow-up of 27 months. J Orthop Sci (2021) S0949-2658(21)00374-2. doi: 10.1016/j.jos.2021.11.009
65. Chang E, Vickery J, Zaiat N, Sallam E, Hanan A, Baker S, et al. Chronic recurrent multifocal osteomyelitis (Crmo): A study of 12 cases from one institution and literature review. Fetal Pediatr Pathol (2021) 20:1–12. doi: 10.1080/15513815.2021.1978602
66. Skrabl-Baumgartner A, Singer P, Greimel T, Gorkiewicz G, Hermann J. Chronic non-bacterial osteomyelitis: A comparative study between children and adults. Pediatr Rheumatol Online J (2019) 17(1):49. doi: 10.1186/s12969-019-0353-2
67. Sergi CM. Biorepository - a key component of research studies. Contemp Clin Trials (2022) 112:106655. doi: 10.1016/j.cct.2021.106655
68. Sergi CM. Implementing epic beaker laboratory information system for diagnostics in anatomic pathology. Risk Manag Healthc Policy (2022) 15:323–30. doi: 10.2147/RMHP.S332109
69. Clarke WT, Papamichael K, Vande Casteele N, Germansky KA, Feuerstein JD, Melmed GY, et al. Infliximab and adalimumab concentrations may vary between the enzyme-linked immunosorbent assay and the homogeneous mobility shift assay in patients with inflammatory bowel disease: A prospective cross-sectional observational study. Inflammation Bowel Dis (2019) 25(11):e143–e5. doi: 10.1093/ibd/izz202
70. Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis (2019) 10:2040622319838443. doi: 10.1177/2040622319838443
71. Cantarelli E, Baccelli F, Simonini G, Alvisi P. Chronic recurrent multifocal osteomyelitis associated with crohn disease: A potential role of exclusion diet? comment on starz et al. the modification of the gut microbiota Via selected specific diets in patients with crohn's disease. Nutrients (2021) 13(11):4005. doi: 10.3390/nu13114005
72. Dushnicky MJ, Beattie KA, Cellucci T, Heale L, Zachos M, Sherlock M, et al. Pediatric patients with a dual diagnosis of inflammatory bowel disease and chronic recurrent multifocal osteomyelitis. J Pediatr Gastroenterol Nutr (2021) 73(5):626–9. doi: 10.1097/MPG.0000000000003225
73. Moussa T, Bhat V, Kini V, Fathalla BM. Clinical and genetic association, radiological findings and response to biological therapy in seven children from Qatar with non-bacterial osteomyelitis. Int J Rheum Dis (2017) 20(9):1286–96. doi: 10.1111/1756-185X.12940
74. Marangoni RG, Halpern AS. Chronic recurrent multifocal osteomyelitis primarily affecting the spine treated with anti-tnf therapy. Spine (Phila Pa 1976) (2010) 35(7):E253–6. doi: 10.1097/BRS.0b013e3181c09601
75. Deutschmann A, Mache CJ, Bodo K, Zebedin D, Ring E. Successful treatment of chronic recurrent multifocal osteomyelitis with tumor necrosis factor-alpha blockage. Pediatrics (2005) 116(5):1231–3. doi: 10.1542/peds.2004-2206
76. Wipff J, Costantino F, Lemelle I, Pajot C, Duquesne A, Lorrot M, et al. A Large national cohort of French patients with chronic recurrent multifocal osteitis. Arthritis Rheumatol (2015) 67(4):1128–37. doi: 10.1002/art.39013
77. Timme M, Bohner L, Huss S, Kleinheinz J, Hanisch M. Response of different treatment protocols to treat chronic non-bacterial osteomyelitis (Cno) of the mandible in adult patients: A systematic review. Int J Environ Res Public Health (2020) 17(5):1737. doi: 10.3390/ijerph17051737
78. Eleftheriou D, Gerschman T, Sebire N, Woo P, Pilkington CA, Brogan PA. Biologic therapy in refractory chronic non-bacterial osteomyelitis of childhood. Rheumatol (Oxf) (2010) 49(8):1505–12. doi: 10.1093/rheumatology/keq122
79. Sulko J, Ebisz M, Bien S, Blazkiewicz M, Jurczyk M, Namyslak M. Treatment of chronic recurrent multifocal osteomyelitis with bisphosphonates in children. Joint Bone Spine (2019) 86(6):783–8. doi: 10.1016/j.jbspin.2019.06.005
80. Schnabel A, Range U, Hahn G, Berner R, Hedrich CM. Treatment response and longterm outcomes in children with chronic nonbacterial osteomyelitis. J Rheumatol (2017) 44(7):1058–65. doi: 10.3899/jrheum.161255
Keywords: osteomyelitis, chronicity, multilaterality, autoinflammatory, bone
Citation: Sergi CM, Miller E, Demellawy DE, Shen F and Zhang M (2022) Chronic recurrent multifocal osteomyelitis. A narrative and pictorial review. Front. Immunol. 13:959575. doi: 10.3389/fimmu.2022.959575
Received: 01 June 2022; Accepted: 27 July 2022;
Published: 22 August 2022.
Edited by:
Tomoyuki Mukai, Kawasaki Medical School, JapanReviewed by:
Hermann Girschick, Vivantes Hospital, GermanyMustafa Koyun, Akdeniz University Hospital, Turkey
Copyright © 2022 Sergi, Miller, Demellawy, Shen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Consolato M. Sergi, Y3NlcmdpQGNoZW8ub24uY2E=
 Consolato M. Sergi
Consolato M. Sergi Elka Miller
Elka Miller Dina El Demellawy1
Dina El Demellawy1 Fan Shen
Fan Shen