
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 25 August 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.958677
This article is part of the Research TopicEndothelial Cells as Innate Immune CellsView all 7 articles
 Xiaoxuan Ma
Xiaoxuan Ma Meiming Su
Meiming Su Qingze He
Qingze He Zhidan Zhang
Zhidan Zhang Fanshun Zhang
Fanshun Zhang Zhenghong Liu
Zhenghong Liu Lu Sun
Lu Sun Jianping Weng*†
Jianping Weng*† Suowen Xu*†
Suowen Xu*†Genome-wide association studies (GWAS) have recently identified phosphatase and actin regulator-1 (PHACTR1) as a critical risk gene associated with polyvascular diseases. However, it remains largely unclear how PHACTR1 is involved in endothelial dysfunction. Here, by mining published datasets of human stable and vulnerable/ruptured plaque tissues, we observed upregulated expression of PHACTR1 in vulnerable/ruptured plaques. Congruent with these data, we demonstrated increased Phactr1 gene expression in aortic endothelium from ApoE-/- mice fed a western type diet compared with that in normal C57BL/6J mice. Relevantly, PHACTR1 gene expression was upregulated by pro-inflammatory and pro-atherogenic stimuli, including TNF-α, IL-1β and oxidized LDL (oxLDL). By employing next-generation RNA sequencing, we demonstrate that PHACTR1 overexpression disrupts pathways associated with endothelial homeostasis. Cell biological studies unravel that PHACTR1 mediates endothelial inflammation and monocyte adhesion by activating NF-κB dependent intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) expression. In addition, overexpression of PHACTR1 also reduces the generation of nitric oxide (NO) by inhibiting Akt/eNOS activation. In-house compound screening of vasoprotective drugs identifies several drugs, including lipid-lowering statins, decreases PHACTR1 gene expression. However, PHACTR1 gene expression was not affected by another lipid-lowering drug-fenofibrate. We also performed a proteomic study to reveal PHACTR1 interacting proteins and validated that PHACTR1 can interact with heat shock protein A8 (HSPA8) which was reported to be associated with coronary artery disease and eNOS degradation. Further studies are warranted to confirm the precise mechanism of PHACTR1 in driving endothelial dysfunction. In conclusion, by using systems biology approach and molecular validation, we disclose the deleterious effects of PHACTR1 on endothelial function by inducing endothelial inflammation and reducing NO production, highlighting the potential to prevent endothelial dysfunction and atherosclerosis by targeting PHACTR1 expression. The precise role of endothelial cell PHACTR1 in polyvascular diseases remains to be validated in diseased conditions.
Coronary artery disease (CAD) has been a major cause of increasing morbidity and mortality of global human population (1). Genome-wide association studies (GWAS) have identified phosphatase and actin regulator-1 (PHACTR1) as a risk gene associated with coronary artery disease (CAD) recently (2). However, it remains largely unclear how these GWAS-associated genes affect the development of CAD. Endothelial dysfunction plays an important role in the initiation and progression of CAD (3, 4). Vascular endothelial dysfunction consists of chronic inflammation, impaired endothelium-dependent vasodilation, oxidative stress, leukocyte adhesion and hyperpermeability, and endothelial-to-mesenchymal transition (5).
ECs are the central and active players in the immune system and vasculature (6). Although ECs are not professional immune cells, they essentially execute immune functions by sensing and responding to inflammatory cytokines, viral and bacterial infection (7). Upon inflammation, ECs recruit immune cells, such as neutrophils and monocytes and direct the extravasation of leukocytes at the inflammatory site through inducing the adhesion molecules like vascular cell adhesion molecule 1 (VCAM1) or intercellular adhesion molecule 1 (ICAM1) and E-selectin (SELE) expression (8). We have previously demonstrated that GWAS-identified CAD risk gene JCAD/KIAA1462 regulates leukocyte adhesion and atherosclerosis in mice via regulating endothelial inflammation (9). It remains elusive whether other GWAS-CAD genes will also affect endothelial inflammation and dysfunction.
Emerging studies have implicated phosphatase and actin regulator 1 (PHACTR1) as another important GWAS-identified CAD risk gene associated with polyvascular disease, including CAD, migraine, hypertension, fibromuscular dysplasia and cervical artery dissection (10, 11). PHACTR1 encodes a protein that binds to actin and protein phosphatase 1 (PP1), which plays a role in endothelial survival and tube formation (12, 13). Recently, a comprehensive integrative genomics analysis has reported the top 25 prioritized candidate risk genes for CAD. PHACTR1 ranked the second among the list of 25 prioritized candidate causal genes (14). PHACTR1 is essential for macrophages efferocytosis capacity and facilitates M2 macrophage polarization to inhibit atherosclerosis in mice (15, 16). In addition, PHACTR1 regulates the proliferation, apoptosis, migration and tube formation of ECs (12, 13, 17). PHACTR1 also promotes vascular calcification in vascular smooth muscle cells (18). In ECs, PHACTR1 is inextricably linked with endothelial inflammation and endothelial dysfunction; however, the precise role of PHACTR1 in regulating endothelial function remain controversial. In one study, downregulation of PHACTR1 trigged pro-inflammatory and pro-atherogenic factors, including CD36, cadherin-13, PAR-1 and thrombin (19). In another study, the authors have showed that PHACTR1 drived oxidative stress and inflammation through interaction with NF-κB/p65 in human coronary artery endothelial cells (HCAECs) (20). However, the effect and molecular mechanism whereby PHACTR1 modulates endothelial function and homeostasis remain largely unknown.
Therefore, the purpose of the present study is to evaluate the effect and mechanism of PHACTR1 overexpression on endothelial dysfunction by focusing on inflammation and nitric oxide (NO) production. By utilizing unbiased RNA-sequencing and subsequent molecular characterizations, we demonstrate that PHACTR1 overexpression mediates endothelial inflammation and impairs endothelial NO production to render endothelial dysfunctional.
TNF-α (#300-01A, PEPROTECH, Rocky Hill, USA); IL-1β (#200-01B; PEPROTECH, Rocky Hill, USA); oxLDL (#H7980, Solarbio, Beijing, China); DMSO (#A100231, Sangon Biotech, Shanghai, China); Atorvastatin (#222412-82-0, Cayman Biochem, Ann Arbor, Michigan, USA); Simvastatin (#79902-63-9, TargetMol, Shanghai, China); Rosuvastatin (#287714-41-4, TargetMol, Shanghai, China); Fenofibrate (#49562-28-9,TargetMol, Shanghai, China); Empagliflozin (#864070-44-0,TargetMol, Shanghai, China); Troglitazone (#97322-87-7, MCE, Shanghai, China); Metformin (#1115-70-4,TargetMol, Shanghai, China); Cilostazol (#73963-72-1,TargetMol, Shanghai, China); Fluvoxamine (#61718-82-9,TargetMol, Shanghai, China); Riociguat (#625115-55-1,TargetMol, Shanghai, China); Sildenafil citrate (#171599-83-0,TargetMol, Shanghai, China).
C57BL/6J and ApoE-/- mice were purchased from Gempharmatech (Nanjing, China). ApoE-/- mice were fed with 1.25% High Cholesterol Diet (#D12108C, RESEARCH DIETS, NJ, USA) and C57BL/6J mice were fed with chow diet for 6 weeks. After then, mice were sacrificed and endothelium-enriched intimal RNA was collected. The Institutional Animal Care and Use Committee of University of Science and Technology of China (USTC) approved all animal care procedures. All experiments were performed in accordance with the relevant regulations by USTC.
After sacrifice, mouse hearts were fixed with 4% PFA (G1101, Servicebio, Wuhan, China) and then dehydrated with 30% sucrose solution. Heart tissues were embedded by OCT for whole night, and then frozen sections of the aortic sinus were performed with a thickness of 8 µm. Frozen sections were rinsed with 10% isopropyl alcohol (#A507048, Sangon Biotech, Shanghai, China) after rewarming. The aortic sinus was stained with 0.3% Oil Red O solution (#G1260, Solarbio, Wuhan, China) for 1 min, rinsed with 10% isopropyl alcohol for 10 s, and rinsed with PBS for several minutes. 10% Fast Green solution (#G1661, Solarbio, Wuhan, China) was applied for counterstaining for 10 s, and rinse with PBS for 1 min. Finally, slides were sealed with glycerin gelatin mounting media. The images were taken using Pannoramic MIDI (3D HISTECH, Hungary).
Human umbilical vein endothelial cells (HUVECs) were isolated from the umbilical cord of normal pregnant women as previously described (21). The clinical scheme of umbilical cord collection in this experiment was approved by the institutional review committee of the First Affiliated Hospital of University of Science and Technology of China (scheme No.: 2020-ky013). HUVEC cells within 3-9 generations from three to four different donors were used in this study. The culture temperature of these cells was 37 °C, and the gas environment contained 5%CO2. ECM medium was used to culture these cells, which consists of 1×endothelial cell growth supplement (ScienCell, Carlsbad, CA), 5% FBS and 1×penicillin/streptomycin antibiotic.
RNeasy kits (YiShan Biotech, Shanghai, China) were used to extract total RNA from cultured HUVECs. In addition, Reverse Transcription Kits (Takara, Dalian, China) or HiScript III RT SuperMix (Vazyme, Nanjing, China) were used to convert the total RNA of HUVECs into complementary cDNA. After the reverse transcription process, ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) was used in quantitative real-time PCR assay. Meanwhile, Roche LC96 real-time PCR detection system was used for detection, and GAPDH was used as loading control for relative mRNA quantification. Supplementary Table 1 lists the sequences of primers used.
Whole-cell lysates were extracted from HUVECs with sample buffer and boiled at 95 °C for 10 min. Samples were separated by SDS-PAGE, and then transferred to nitrocellulose membrane (Pall, New York, USA). The nitrocellulose membrane was incubated with blocking buffer at room temperature for 1 hour. After discarding the blocking buffer, the blots were incubated with primary antibodies at 4 °C overnight. The primary antibodies used were listed in Supplementary Table 2. 1 × Tris buffered saline with 0.1% Tween-20 (TBST) was used to wash the membrane three times for 10 minutes each time. Then, membranes were incubated with IRDye® 680RD Goat anti-Mouse IgG (H + L) or IRDye® 800CW Goat anti-Rabbit IgG (H + L) (1:10,000 dilution, LI-COR, Lincoln, Nebraska, USA) at room temperature for 1 hour. Finally, the Li-COR CLx infrared imaging system was used to visualize the blots.
HUVECs at sub-confluence were seeded in 12-well plate the day before transfection. The culture medium was changed into serum-free Opti-MEM medium with 350 μL per well. Dilution of transfection reagent lipofectamine2000 (lipo2000): before use, mix the lipo2000 transfection reagent gently, then take 3 μL of lipo2000 from each well. Serum free opti-MEM was used to dilute lipo2000. Dilution of siRNA (#SIGS0016696-1, si-PHACTR1#1, RIBOBIO, Guangzhou, China): Serum free opti-MEM was used to dilute control or PHACTR1 siRNA (20 nM). Fresh complete culture medium was added 4 h after transfection. Cells were cultured for 48 h since transfection before RNA or protein was collected.
HUVECs were seeded in 12-well plates. Stable-Lite Luciferase Assay System (#DD1202-01, Vazyme, Nanjing, China) was used to determine the NF-κB-luciferase activity. Ad-NF-κB-luc (Vector Biolabs, Malvern, PA, USA M.O.I. = 1) was used to transfect HUVECs for 48 h. Afterwards, HUVEC was treated with Ad-PHACTR1 (#NM_030948, WZ Biosciences, Shandong, China) or control adenovirus for 24 h and treated with TNF-α (10 ng/ml) for 3 h. Lysis buffer was added to treated cells, and normalized luciferase activity was measured with a microplate reader (Molecular Devices, iD3).
HUVECs were seeded in 12-well plates and were treated with Ad-PHACTR1 or control adenovirus for 24 h. Then, HUVECs were treated with TNF-α (10 ng/ml) for 6 h to activate inflammatory response. THP1 monocytic cells were seeded onto the monolayer of HUVECs at 1 × 105 cells/well for an additional 30 min. After three gentle rinses with ECM medium, non-adherent THP1 monocytes were removed. A Zeiss microscope was used to image adherent monocytes, and each well was photographed. Then, the number of adherent monocytes was obtained by counting cells in each well, and the value was calculated and presented.
DAF-FM DA dye (#S0019, Beyotime, Shanghai, China) was diluted with the DAF-FM DA diluent provided with the kit at a dilution of 1:1, 000 to reach a final concentration of 5 μM. After digestion, the cells were counted, centrifuged, and resuspended with diluted DAF-FM DA. Usually, the volume of diluted DAF-FM DA is 200 μL per each well in the 12-well plate. Cells were incubated with DAF-FM DA for 20 min at 37°C. After staining, the cells were washed three times with PBS (pH7.4). Fluorescence intensity was detected by a fluorimeter (SYNERGY H1, BioTek, USA) using excitation wavelength of 495 nm and emission wavelength of 515 nm.
HUVECs were treated with control adenovirus or Ad-PHACTR1 for 24 h before stimulation of TNF-α for 6 h. Cells were lysed in IP lysis buffer (150 mM NaCl, 2.5 mM KCl, 10 mM Tris, pH 7.5, 30 mM β-glycerophosphate, 0.5% Triton X-100, 0.5% Nonidet P-40, and 50 mM NaF) supplemented with 1% protease inhibitor cocktail. 10% of the lysate was saved as input, while the remaining lysate were incubated with Anti-flag® M2 affinity gel (#A2220, Sigma, Saint Louis, USA) overnight at 4°C. Beads were washed 10 times using IP lysis buffer before elution with loading buffer, followed by boiling with heated block (95°C, 10 min). SDS-PAGE gel was prepared and gel samples were cut at 1 cm from the top of the separation gel. Gel slices were sent for mass spectrometry analysis (PTM BIO, Hangzhou, China).
HUVECs were seeded in 0.1% gelatin-coated dishes the day before experiment. One day later, cells were treated with Ad-PHACTR1 (MOI=1, WZ Biosciences) or control adenovirus for 24 h. After treatment, an RNA-Easy Mini Plus kit (QIAGEN, Germany) was used to isolate the total RNA. RNA libraries were prepared for sequencing by BGI (Beijing Genomic Institute in ShenZhen, China) as previously described (22).
Gel slices were treated with 50% acetonitrile for decolorization, followed by 100% acetonitrile treatment for dehydration, and subject to vacuum drying for 15 min. Trypsin was added at the final concentration of 10 ng/μL to digest the gel overnight at 37°C. The peptides were separated by an ULTRA-performance liquid phase system and then injected into an NSI ion source for ionization and analysis by Q-EXactive ™ Plus mass spectrometry. Retrieval parameter settings were as follows: The annotated database was homo_sapiens_9606_SP_20210721.FASTA (20387 sequences), and the reverse database was added to calculate the false positive rate (FDR) caused by random matching. Common contamination database was added to eliminate the influence of contaminated proteins in identification results. The number of missing tangent position is set to 2; The minimum peptide length was set to 7 amino acid residues; The maximum modification number of peptide was set to 5. FDR for protein identification and PSM identification was set to 1%.
In this study, unless specified otherwise, means ± SD is used to represent data. Graphpad Prism software version 9.0 (Graphpad software, La Jolla, CA) is used for graphing and statistical analysis. When appropriate, students t-test or one-way analysis of variance (ANOVA) were used to test and analyze the data when appropriate. P value less than 0.05 is considered to be statistically significant.
PHACTR1 is a GWAS-identified gene associates with polyvascular diseases (11). We first mined data from dataset in GSE163154, which showed that gene expression of PHACTR1 is significantly increased in aortic plaque tissues from patients with intraplaque hemorrhage (IPH) vs those without IPH (non-IPH) (Figure 1A). Moreover, by mining another GEO datasets (GSE41571) (23), we found that PHACTR1 gene expression was upregulated in macrophage-rich regions of ruptured human atheromatous plaques (using laser micro-dissection), compared with that of stable plaques (Supplemental Figure 1). Meanwhile, we found that Phactr1 gene expression was upregulated in vascular intima of ApoE-/-mice fed with western diet for 6 weeks compared with C57BL/6J mice (Figures 1B, C). However, Phactr1 gene expression in media/adventitia from ApoE-/- mice and C57BL/6J mice was similar (Figure 1D). To understand the potential effects of PHACTR1 on endothelial function, we performed RNA sequencing of HUVECs with PHACTR1 overexpression. We obtained top 20 GO_p terms enrichment of Ad-PHACTR1-treated HUVECs. The differentially expressed genes upon PHACTR1 overexpression relate to cell migration, localization of cells, regulation of cell proliferation and motility, tube development and angiogenesis, and regulation of protein phosphorylation (Figure 1E). Coincidentally, there were several studies revealing the role of PHATCR1 on biological processes above (12, 13, 17). The analysis of differentially expressed genes showed that some genes associated with endothelial dysfunction were significantly upregulated, such as CD36, EDN1, ANGPT2 and so on. While genes maintaining endothelial homeostasis were downregulated, such as KLF4, KLF2, THBD and so on. This suggests that PHACTR1 overexpression may aggravate endothelial dysfunction (Figure 1F). Similarly, among ranking top 30 minimum P value genes, bule bubbles representing for upregulated genes were associated with endothelial dysfunction, while yellow bubbles representing for downregulated genes were associated with maintaining endothelial homeostasis (Figure 1G). Figure 1H showed the top 20 KEGG pathways enriched with upregulated and downregulated differential genes, respectively. Collectively, PHACTR1 may be an important CAD risk gene that mediates endothelial dysfunction.
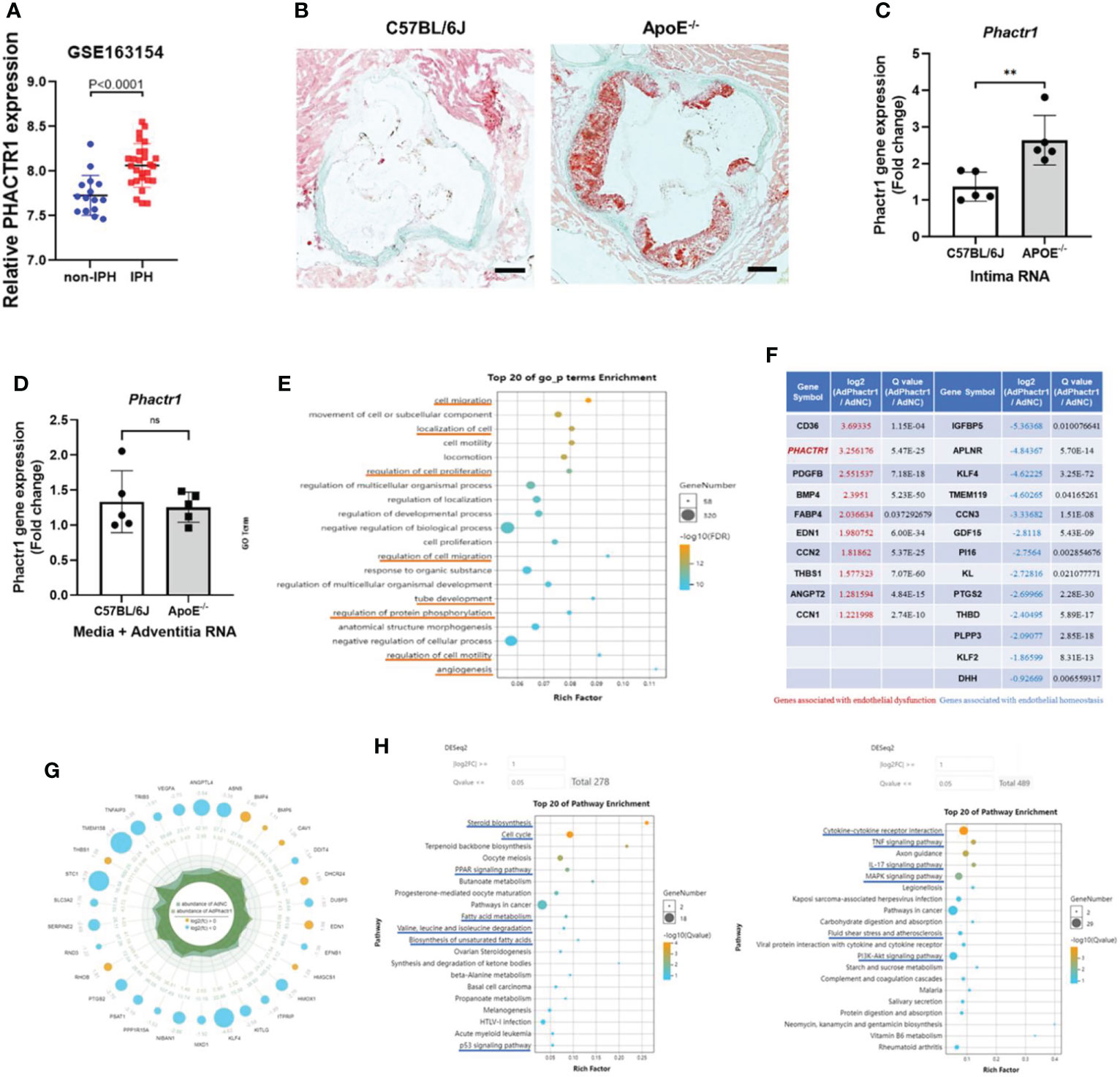
Figure 1 PHACTR1 is an important CAD risk gene that mediates endothelial dysfunction. (A) Expression of PHACTR1 mRNA levels in unstable plaques/intraplaque hemorrhage (IPH) (n = 27) and stable plaques (non-IPH, n = 16) from human plaques mined from GSE163154. (B) Oil Red O staining of lesions of the aortic root in male ApoE-/-mice following 6 weeks of western diet and male C57BL/6J WT mice fed with normal chow diet, n = 5. Scale bar = 200 μm. (C) Phactr1 gene expression in vascular intimal lysate of ApoE-/- mice compared with C57BL/6J WT mice (n = 5 mice per group), **P < 0.01. (D) Phactr1 gene expression in vascular media and adventitia of ApoE-/- mice compared with C57BL/6J WT mice (n = 5 mice per group) ns, non-significant. (E) Top 20 GO_p terms enrichment of Ad-PHACTR1-treated endothelial cells revealed by transcriptomic profiling. (F) Impact of PHACTR1 overexpression on selected expression of genes relevant to endothelial function dysfunction by transcriptomic profiling. (G) Differential genes with top 30 P value of Ad-PHACTR1-treated endothelial cells. (H) Top 20 KEGG upregulated or downregulated pathways enriched in Ad-PHACTR1-treated endothelial cells.
Oxidized low-density lipoprotein (ox-LDL) is considered as the main contributing factor to endothelial damage and atherosclerosis (24). HUVECs was treated with 50 μg/mL for 6 h, and then we found that PHACTR1 gene expression was upregulated by ox-LDL (Figure 2A). This piece of data agreed with a previous report by Reschen et al. who found that an intermediate length transcript of PHACTR1 was upregulated by ox-LDL (25). Moreover, tumor necrosis factor (TNF-α) and interleukin-1β (IL-1β), two additional inducers of inflammatory response by upregulated expression of endothelial adhesion molecules (26–28). These adhesion molecules (e.g., VCAM1 and ICAM1) regulate endothelial barrier function. These molecules also mediate leukocyte transendothelial migration and endothelial permeability during inflammation (29, 30). When HUVECs were treated with the TNF-α or IL-1β, we observed that both PHACTR1 mRNA and PHACTR1 protein expression were upregulated, suggesting that PHACTR1 responds to inflammatory stimulation (Figures 2B, C). Then, we examined the expression of adhesion molecules (VCAM1 and ICAM1) upon overexpressing or silencing PHACTR1 in HUVECs. The results showed that the protein expression of inflammatory markers VCAM1 and ICAM1 were upregulated through overexpression of PHACTR1 (Figures 2D, E). Meanwhile, knocking down PHACTR1 downregulated protein expression of VCAM1 and ICAM1 (Supplemental Figure 2 and Figure 2F). The above results demonstrated that PHACTR1 mediates endothelial inflammation. Next, we wanted to validate whether endothelial inflammation induced by PHACTR1 is triggered by typical pro-inflammatory NF-κB pathway. Through assessing the activation of NF-κB by luciferase reporter assay, we observed that the overexpression of PHACTR1 significantly activated NF-κB activity compared with control group (Figure 2G). After NF-κB activation, the adhesion of THP1 monocytes to ECs is also a vital mechanism of driving endothelial inflammation. We observed that the overexpression of PHACTR1 could aggravate THP1 cells adhesion to ECs under TNF-α stimulation (Figure 2H).
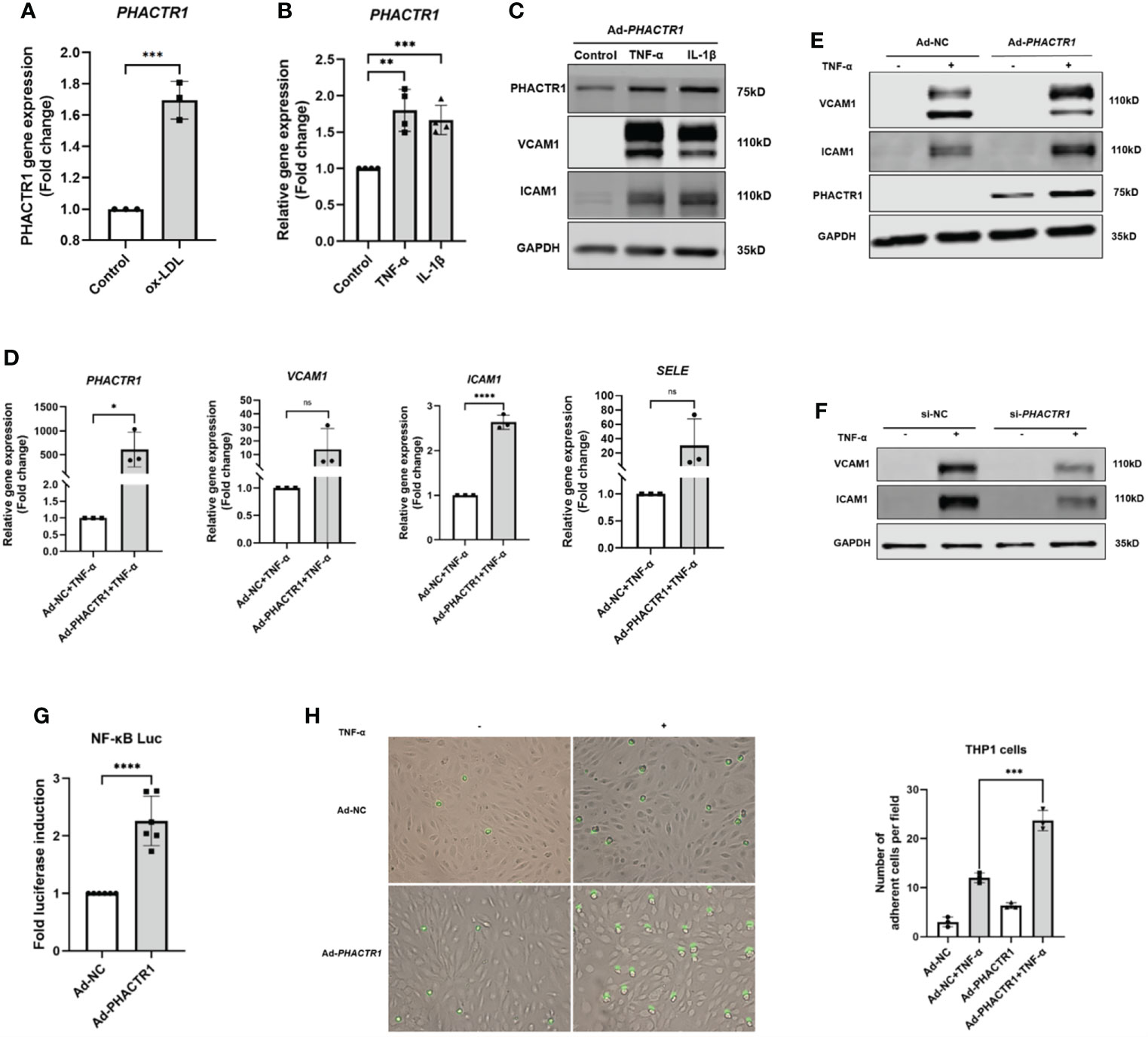
Figure 2 PHACTR1 mediates endothelial inflammation. (A) PHACTR1 mRNA level was upregulated by ox-LDL (50 μg/mL, 6 h) in HUVECs (n = 3). (B) PHACTR1 mRNA level was upregulated under TNF-α and IL-1β (10 ng/mL, 24 h) in HUVECs (n = 4). (C) PHACTR1 protein level was upregulated under TNF-α and IL-1β (10 ng/mL, 24 h) in HUVECs (n = 5). (D, E) PHACTR1 overexpression enhanced VCAM1, ICAM1 gene and protein expression under TNF-α stimulation (10 ng/mL, 6 h) in HUVECs (n = 3 or 6). (F) PHACTR1 silencing suppressed VACM1 and ICAM1 protein expression under TNF-α stimulation (10 ng/mL, 6 h) in HUVECs (n = 3). (G) PHACTR1 overexpression activated NF-κB luciferase activity (n = 6). (H) PHACTR1 overexpression aggravated the adhesion of THP1 cells under TNF-α stimulation (10 ng/mL, 6 h) in HUVECs (n = 3). ns, non-significant, *P < 0.05, **P < 0.01, ***P < 0.001, ***P < 0.0001 ****, P < 0.0001.
Endothelial dysfunction is characterized by reduced bioavailability of NO, which is an early event in the development of atherosclerosis (31). NO is a potent vasodilator and anti-inflammatory signal molecule, which is a gasotransmitter produced by endothelial NO synthases (eNOS) in a stepwise redox reaction from L-arginine (32). NO plays a variety of roles in maintaining vascular homeostasis (33). In contrast, endothelin 1 (EDN1) is the most potent vasoconstrictor which induces the expression of proinflammatory signals and promotes vasoconstriction (34). EDN1 could constrict blood vessels in inflamed areas to contribute to cardiovascular diseases (6).
Taking that into account, we evaluated the effect of PHACTR1 on eNOS expression after treatment with Ad-PHACTR1 in HUVECs. PHACTR1 overexpression decreased the level of eNOS gene and protein expression (Figures 3A, B). Also, the expression of eNOS phosphorylation at Ser1177 (p-eNOS-S1177) was decreased accordingly (Figure 3B). Similarly, the level of EDN1 gene expression was significantly elevated (Figure 3A). Next, we assessed the possible effect of PHACTR1 overexpression on NO production in HUVECs. We observed that NO production was significantly reduced by PHACTR1 overexpression in HUVECs (Figure 3C). To the best of our knowledge, this is the first study to assess the effect of PHACTR1 on endothelial NO production. It is known that Akt mediates eNOS phosphorylation and increases VEGF secretion, vasodilation, and angiogenesis in the cardiovascular system (35). We observed that the expression of Akt phosphorylation at Ser473 (p-Akt -Ser473) was decreased by PHACTR1 overexpression, rather than the total Akt protein expression (Figure 3D). At this point, PHACTR1 leads to eNOS downregulation and decreases NO production partially through inhibiting Ser473 phosphorylation of Akt protein to reduce eNOS activation in HUVECs. In this manner, PHACTR1 impairs endothelial NO production, which in turn leads to endothelial dysfunction.
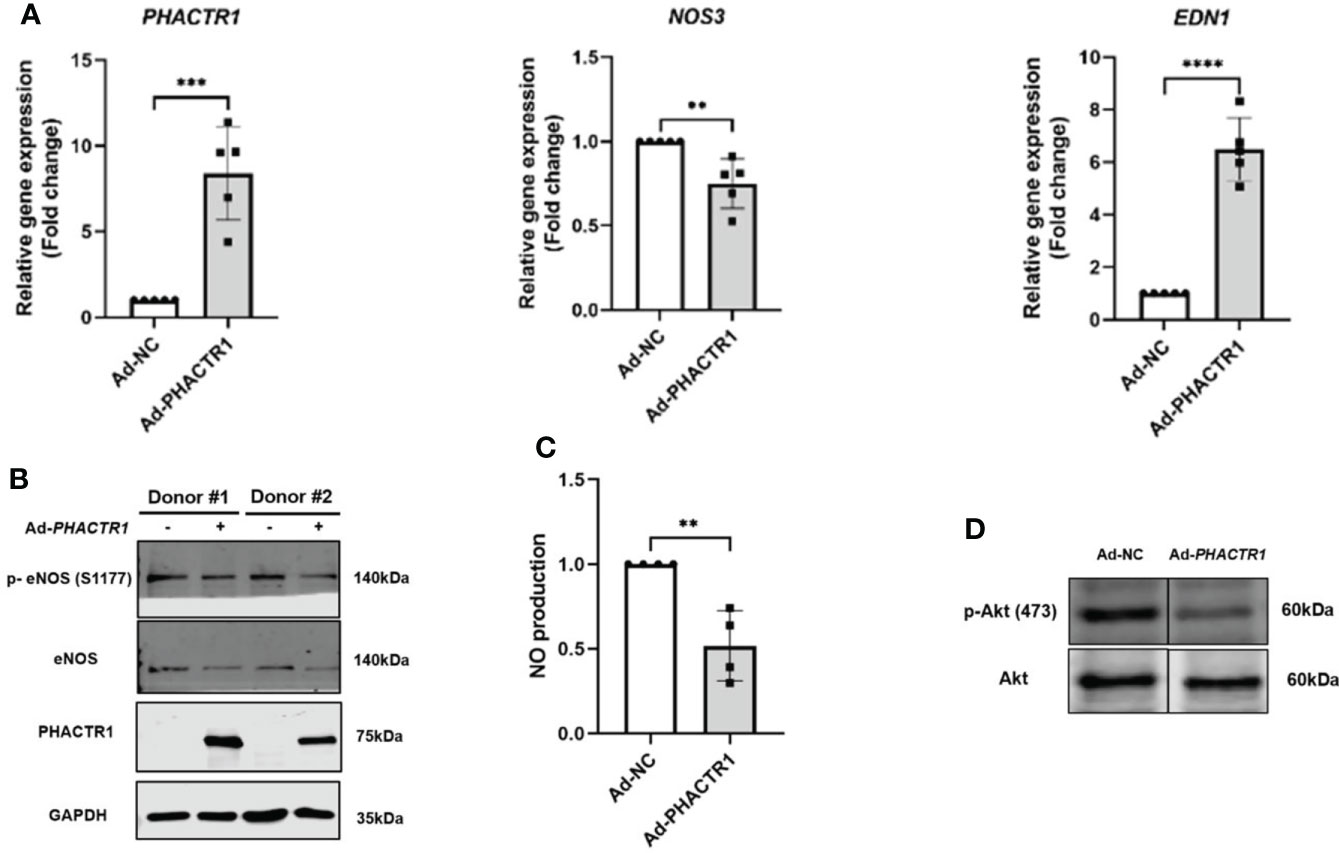
Figure 3 PHACTR1 overexpression leads to eNOS downregulation and decreased NO production. (A) PHACTR1 overexpression upregulated EDN1 (also known as ET-1) and downregulated eNOS mRNA expression in HUVECs (n = 5). (B) PHACTR1 overexpression can decrease eNOS total protein and eNOS phosphorylation at Ser1177 (n = 6). (C) PHACTR1 overexpression can reduce NO production in HUVECs (n = 4). (D) PHACTR1 overexpression decreased Akt phosphorylation at Ser473 (n = 3). Lanes presented were spliced from the same blots with uncropped images displayed in online Supplementary Material. **P < 0.01, ***P < 0.001, ***P < 0.0001 ****, P < 0.0001.
Since we have confirmed that PHACTR1 could mediate endothelial inflammation and impair endothelial NO production, we next asked whether several clinical agents with known cardiovascular actions could suppress PHACTR1 gene expression. We performed qPCR validation on 11 selected lipid-lowering, or hypoglycemic or cardiovascular protective drugs. The results showed that Statins, Empagliflozin, Riociguat and Sildenafil citrate had significant inhibitory effects on PHACTR1 gene expression (Figures 4A, B). Considering Atorvastatin has superior effects on lipid-lowering and cardiovascular protection, we focused on evaluating the effect of Atorvastain on PHACTR1 gene expression (36, 37). Firstly, we observed that only PHACTR3 was not expressed among four members of the PHACTR family in HUVECs (Figure 4C). Then, ECs were treated with different doses of Atorvastatin. The results demonstrated that PHACTR2 and PHACTR4 had no significant dose-response effects, while only PHACTR1 expression was decreased by Atorvastatin in a dose-dependent manner (Figure 4D). However, another lipid-lowering agent-Fenofibrate with an array of cardiovascular and renal pleiotropic beneficial activities (38), did not have the dose-dependent effects on PHACTR1 gene expression (Figure 4E). Therefore, Statins are likely to be a class of drugs that negatively regulate PHACTR1 gene expression in human ECs.
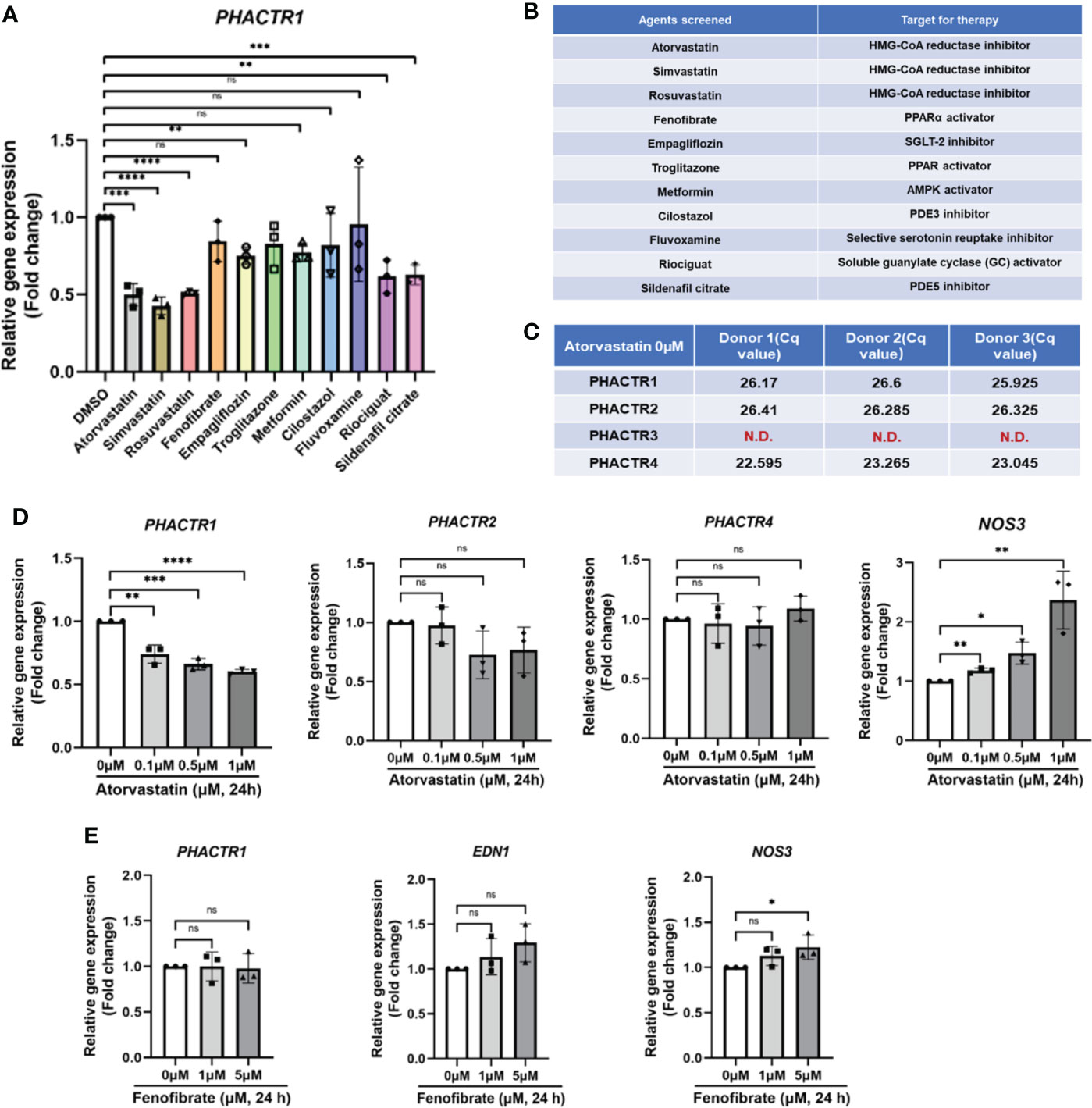
Figure 4 Identification of statin as a negative regulator of PHACTR1 gene expression. (A) Effect of selected 11 compounds in in-house library on PHACTR1 gene expression (n = 3). (B) Compound list and their molecular targets. (C) Gene expression of all PHACTR family members (PHACTR1 to PHACTR4) in HUVECs by real-time PCR (n = 3). (D) Effect of atorvastatin on PHACTR1-4 gene expression in HUVECs. NOS3 (eNOS) was used as the positive control (n = 3). (E) Effect of Fenofibrate on PHACTR1 gene expression in HUVECs (n = 3). ns, non-significant, N.D., non-detectable, *P < 0.05, **P < 0.01, ***P < 0.001, ***P < 0.0001, ****P < 0.0001.
Since statins are known activators of KLF2 and KLF4, an important class of transcription factors that maintain endothelial homeostasis (39, 40). Though overexpressing KLF2 and KLF4, we found that PHACTR1 gene expression was significantly downregulated, thus the result further demonstrated that PHACTR1 could possibly mediated the effects of KLF2 and KLF4 on endothelial homeostasis (Figures 5A, B). Considering our RNA-sequencing analysis that PHACTR1 overexpression decreased KLF2 mRNA expression (Figure 1F), it is plausible that the effects of eNOS gene expression downregulation by PHACTR1 overexpression may be related to KLF2 downregulation as demonstrated by the fact that activation of KLF2 in ECs induces eNOS gene expression and provides vasodilatory effect (41).
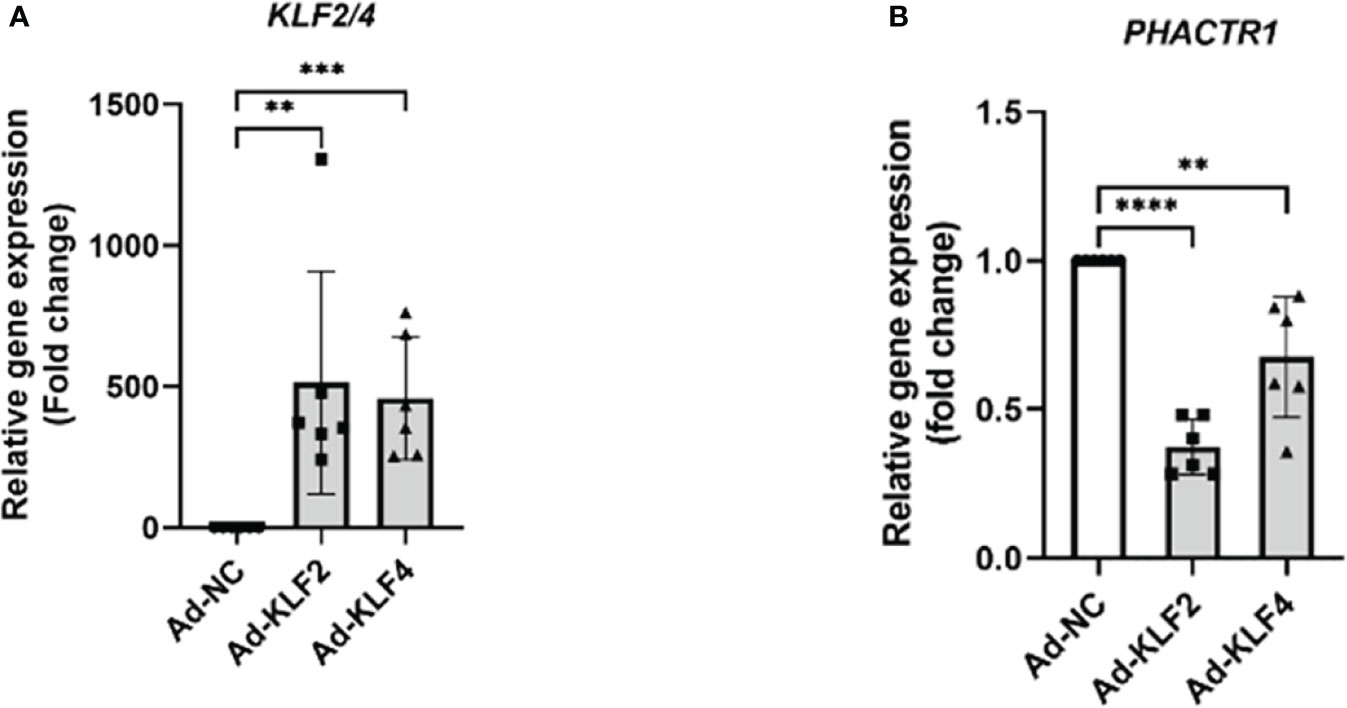
Figure 5 KLF2 and KLF4 suppress PHACTR1 gene expression. (A) Validation of KLF2 and KLF4 gene overexpression by adenovirus (n = 6). (B) PHACTR1 gene expression was suppressed by Ad-KLF2 and Ad-KLF4 after 24 h of transfection (n = 6). **P < 0.01, ***P < 0.001, ****P < 0.0001.
Next, we aim to explore the potential molecular mechanism whereby PHACTR1 triggers endothelial dysfunction. Figure 6A is scheme of co-immunoprecipitation (IP) study design. By the successful immunoprecipitation of PHACTR1 in TNF-α-induced HUVECs with PHACTR1 overexpression (Figure 6B), we found that most of the proteins binding to PHACTR1 were proteins with myosin head based on analysis of mass spectrometry (Figure 6C). According to the reported binding sites of PHACTR family protein sequence elements, RPEL1 and RPEL2 bind to actin protein, and C-terminal bind to PP1 protein (Figure 6D) (42). Three proteins, myosin heavy chain 10 (MYH10), protein phosphatase 1 catalytic subunit gamma (PPP1CC) and heat shock protein family A member 8 (HSPA8), were screened from the table of more than 70 proteins binding with PHACTR1 quantified by mass spectrometry (Figure 6E, Supplementary Table 3). Through immunoprecipitation-based verification, we observed HSPA8 (HSC70) bind with PHACTR1 (Figure 6F). It is well established that HSPA8 is a molecular chaperone that recognizes the non-natural decoupling strands of peptide chains and assists in the correct folding, assembly, transport and degradation of other protein polypeptide chains (43). There is a study demonstrating that HSPA8 and ICAM-1 functioned as damage-induced mediators of γδ T cell activation (44). Moreover, HSPA8 was the most significantly upregulated in the endometrial carcinoma tissues (45). Further studies are warranted to prove whether PHACTR1-HSPA8 interaction is essential for the effects of PHACTR1 on endothelial function, including inflammation and NO production.
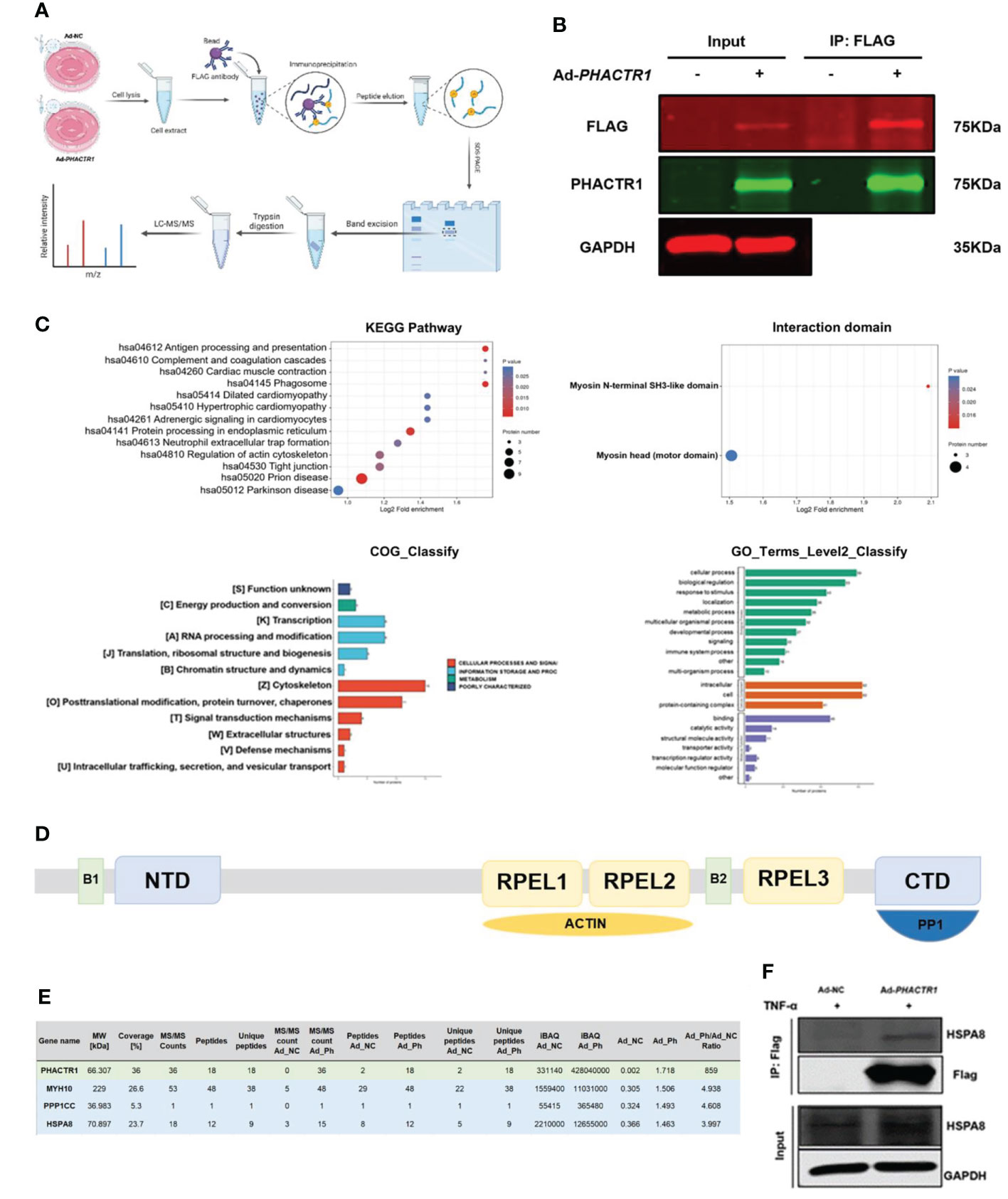
Figure 6 PHACTR1 interactomics in TNF-α-induced HUVECs. (A) Scheme of Co-IP study design created with Biorender.com. (B) Successful IP of PHACTR1 in TNF-α-treated (10 ng/mL, 6 h) HUVECs revealed by Li-Cor Infrared Imaging. (C) GO and KEGG analysis of PHACTR1 interactomics. (D) Schematic diagram of PHACTR family protein sequence elements. (E) PHACTR1 potential interacting protein were selected by Co-IP. (F) Co-IP of PHACTR1 and HSPA8 in HUVECs treated with TNF-α (10 ng/mL, 6 h).
ECs act as the gatekeeper of vascular health due to a semipermeable biomechanical barrier between blood flow and vascular wall. Endothelial dysfunction is considered a marker of many different polyvascular diseases in human, including atherosclerosis, hypertension, and diabetes (5).
It was previously reported that PHACTR1 silencing induced several factors associated with atherosclerotic events, as well as the expression of atherosclerotic biomarkers, and inhibited metabolic response pathways (AMPK/CREB/eNOS) in HUVECs (19). These findings suggest that PHACTR1 might play an anti-inflammatory and anti-atherosclerotic role in ECs (19). However, other evidence suggested that PHACTR1 played a pro-inflammatory and pro-atherogenic role in ECs. To be specific, oxLDL and TNF-α upregulated PHACTR1 intermediate transcripts (25). Knockdown of PHACTR1 decreased the expression of inflammatory markers ICAM1, VCAM1 and VE-cadherin induced by oxLDL in human coronary artery ECs. It also alleviates nuclear accumulation of NF-κB/p65 by decreasing its interaction with myocardin-related transcription factor (MRTF-A), thereby inhibiting oxidative stress and inflammation in ECs (20). In spite of above conflicting findings, our results illustrated that PHACTR1 positively regulates TNF-α-induced endothelial inflammation and impairs NO bioavailability by decreasing Akt phosphorylation and eNOS activation. The lack of L-arginine or the essential cofactor tetrahydrobiopterin or excessive oxidative stress, including chronic endothelial inflammation, could lead to eNOS uncoupling (46). In this case, eNOS uncoupling produces superoxide anion and reactive oxygen species (ROS), leading to atherosclerotic plaque formation (6, 47). Based on the above heterogeneity of studies, it may be necessary to study the transcript and genotype of PHACTR1 firstly and then explore the specific role of PHACTR1 in endothelial inflammation and dysfunction. For example, rs9349379-A/G is located in the non-coding region of PHACTR1 (48). The intermediate transcripts are pro-inflammatory and may differ in their transcriptional regulation levels. Certainly, we cannot totally exclude that the heterogeneity of the results stem from the heterogeneity of ECs from different vascular beds (25, 49).
Considering that the biological function of PHACTR1 itself as a PP1 binding protein, PHACTR1 can directly interact with PP1 and dephosphorylate substrates. Fedoryshchak et al. examined the structure of the PHACTR1/PP1 complex using biochemical methods and X-ray crystallography (50). The physical structure showed that the combination of PHACTR1 and PP1 created a new surface pocket. Further experiments showed that the pocket structure allowed the complex to show an order of magnitude enhanced dephosphorylation of its substrate protein compared to PP1 solely (50). However, no more studies have been reported the dephosphorylated role of PHACTR1/PP1 complex in ECs. It is plausible that PHACTR1/PP1 interaction is likely to dephosphorylate Akt at Ser473 or eNOS at Ser1177 to downregulate eNOS activity and decrease NO production. Moreover, HSPA8 (HSC70) belonging to Hsp70 family, acts as a chaperone and is likely to bind PP1 or PHACTR1 to help it assemble correctly for stabilizing its conformation. Moreover, Subramani et al. reported that chaperone HSPA8 interacted with cysteine residues of glutathionylated eNOS and shuttle to LAMP2A vesicles to amount chaperone-mediated autophagy in myocardial ischemia-reperfusion injury, leading to irreversible loss of eNOS and NO availability (51). In addition, another GWAS revealed that HSPA8 genetic variant (SNP rs2236659) is associated with coronary heart disease risk in a Chinese population (52). In platelets adhering to collagen, HSPA8 is completely dephosphorylated and dissociates from the Hsp90/PP1α/PP1M complex, suggesting that HSP and PP1 are involved in platelet adhesion (53). It remains to be elucidated whether PHACTR1, PP1 and HSPA8 (HSC70) constitute a trimeric complex to target substrate proteins for dephosphorylation.
Recently, Rubin et al. provided an important in vivo analysis showing that Phactr1 knockout separately in ECs or vascular smooth muscle cells do not lead to vascular phenotypes in models of non-atherosclerotic arteriopathies including cervical artery dissection or fibromuscular dysplasia (54). This finding is at variance with intronic variation rs9349379 of PHACTR1 increases cervical artery dissection and fibromuscular dysplasia risk (55). For noncoding GWAS variants at 6p24 locus, further experiments in atherosclerotic disease models will be required to establish a causal link between Phactr1 and atherosclerosis. Besides, Phactr1 has recently been demonstrated to play a key structural role within the vasculature rather than impacting endothelial function (56). To shed light on the role of PHACTR1 in polyvascular diseases, in vivo functional assays of Phactr1 in different vascular disease models will be required using tissue-specific transgenic or deficient mice.
In view of the important role of PHACTR1 in polyvascular diseases, it is vital to study its function and mechanism in the vasculature in future studies (11). Drug discovery with PHACTR1 as therapeutic target is expected and still needs further exploration. From our small-scale drug screening, we found that Statins, Empagliflozin, Riociguat and Sildenafil citrate can decrease expression of PHACTR1 gene. For genetic variant of rs9349379 (A/G) or mutations at other locus discovered in the future, CRISPR/Cas9 gene editing technology can be used to depict the role of these variants in endothelial function and vascular disorders. In addition, it has been reported that PHACTR1 gene expression was reduced by 35% by deleting the MEF2 binding site of PHACTR1 using the CRISPR/Cas9 gene editing technology in ECs carrying this deletion (57). Since KLF2 is transcriptionally regulated by MEF-2, it is also plausible that MEF-2 regulates PHACTR1 gene expression via KLF2.
In conclusion, our study demonstrates the role of PHACTR1 in endothelial dysfunction, in terms of endothelial inflammation and endothelial NO production by activating NF-κB and decreasing Akt/eNOS activity, respectively. A potential limitation of the current study is that we have not validated the assumption of the PHACTR1/PP1α/HSPA8 complex and whether PHACTR1 enhances the binding of HSPA8 to PP1α and orchestrated downstream dephosphorylation events. It also warrants further study whether endothelial cell-derived PHACTR1, unlike that macrophage-derived PHACTR1 (15, 16), can promotes endothelial dysfunction and vascular disease in vivo. Addressing these open questions will definitely contribute to our deepened understanding of cell type-specific role of PHACTR1 in endothelial inflammation, innate immunity and associated vascular disorders.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD034213 and Gene Expression Omnibus (GEO) repository with the accession number: GSE186761.
The studies involving human participants were reviewed and approved by Institutional review board of First Affiliated Hospital of University of Science and Technology of China. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Institutional Animal Care and Use Committee of University of Science and Technology of China (USTC).
SX and JW conceptualize and supervise whole study. XM, MS, ZZ, QH, ZL and FZ performed experiments and data analysis. LS provide insightful comments on the manuscript. All authors read, revised, and approved the final manuscript.
This study was supported by grants from the National Key R&D Program of China (No.2021YFC2500500), the National Natural Science Foundation of China (Grant Nos. 82070464, 81941022, 81530025), and the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB38010100). This work was also supported by the Program for Innovative Research Team of the First Affiliated Hospital of USTC (CXGG02), Anhui Provincial Key Research and Development Program (Grant No. 202104j07020051), Anhui Province Science Fund for Distinguished Young Scholars (Grant No. 2208085J08), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (Grant No. 2017BT01S131).
Scheme of Figure 6A was created using Biorender.com.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.958677/full#supplementary-material.
1. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol (2019) 234(10):16812–23. doi: 10.1002/jcp.28350
2. Paquette M, Dufour R, Baass A. PHACTR1 genotype predicts coronary artery disease in patients with familial hypercholesterolemia. J Clin Lipidol (2018) 12(4):966–71. doi: 10.1016/j.jacl.2018.04.012
3. Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int J Mol Sci (2021) 22(8):3850. doi: 10.3390/ijms22083850
4. Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis (2020) 11(9):776. doi: 10.1038/s41419-020-02985-x
5. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol Rev (2021) 73(3):924–67. doi: 10.1124/pharmrev.120.000096
6. Sturtzel C. Endothelial cells. Adv Exp Med Biol (2017) 1003:71–91. doi: 10.1007/978-3-319-57613-8_4
7. Stolarz AJ, Mu S, Zhang H, Fouda AY, Rusch NJ, Ding Z. Opinion: Endothelial cells - macrophage-like gatekeepers? Front Immunol (2022) 13:902945. doi: 10.3389/fimmu.2022.902945
8. Vestweber D. Relevance of endothelial junctions in leukocyte extravasation and vascular permeability. Ann N Y Acad Sci (2012) 1257:184–92. doi: 10.1111/j.1749-6632.2012.06558.x
9. Xu S, Xu Y, Liu P, Zhang S, Liu H, Slavin S, et al. The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis. Eur Heart J (2019) 40(29):2398–408. doi: 10.1093/eurheartj/ehz303
10. Klarin D, Zhu QM, Emdin CA, Chaffin M, Horner S, McMillan BJ, et al. Genetic analysis in UK biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet (2017) 49(9):1392–7. doi: 10.1038/ng.3914
11. Gupta RM, Hadaya J, Trehan A, Zekavat SM, Roselli C, Klarin D, et al. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell (2017) 170(3):522–33.e15. doi: 10.1016/j.cell.2017.06.049
12. Jarray R, Allain B, Borriello L, Biard D, Loukaci A, Larghero J, et al. Depletion of the novel protein PHACTR-1 from human endothelial cells abolishes tube formation and induces cell death receptor apoptosis. Biochimie (2011) 93(10):1668–75. doi: 10.1016/j.biochi.2011.07.010
13. Allain B, Jarray R, Leforban B, Dufour S, Liu WQ, et al. Neuropilin-1 regulates a new VEGF-induced gene, phactr-1, which controls tubulogenesis and modulates lamellipodial dynamics in human endothelial cells. Cell Signal (2012) 24(1):214–23. doi: 10.1016/j.cellsig.2011.09.003
14. Hao K, Ermel R, Sukhavasi K, Cheng H, Ma L, Li L, et al. Integrative prioritization of causal genes for coronary artery disease. Circ Genom Precis Med (2022) 15(1):e003365. doi: 10.1161/CIRCGEN.121.003365
15. Kasikara C, Schilperoort M, Gerlach B, Xue C, Wang X, Zheng Z, et al. Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis. J Clin Invest (2021) 131(8):e145275. doi: 10.1172/JCI145275
16. Li T, Ding L, Wang Y, Yang O, Wang S, Kong J, et al. Genetic deficiency of Phactr1 promotes atherosclerosis development via facilitating M1 macrophage polarization and foam cell formation. Clin Sci (Lond) (2020) 134(17):2353–68. doi: 10.1042/CS20191241
17. Jing Y, Yang L, Xu Z, Chen H, Ju S, Ding J. Phosphatase actin regulator-1 (PHACTR-1) knockdown suppresses cell proliferation and migration and promotes cell apoptosis in the bEnd.3 mouse brain capillary endothelial cell line. Med Sci Monit (2019) 25:1291–300. doi: 10.12659/MSM.912586
18. Aherrahrou R, Aherrahrou Z, Schunkert H, Erdmann J. Coronary artery disease associated gene Phactr1 modulates severity of vascular calcification in vitro. Biochem Biophys Res Commun (2017) 491(2):396–402. doi: 10.1016/j.bbrc.2017.07.090
19. Jarray R, Pavoni S, Borriello L, Allain B, Lopez N, Bianco S. Disruption of phactr-1 pathway triggers pro-inflammatory and pro-atherogenic factors: New insights in atherosclerosis development. Biochimie (2015) 118:151–61. doi: 10.1016/j.biochi.2015.09.008
20. Zhang Z, Jiang F, Feng L, Wang X, Tu S. PHACTR1 regulates oxidative stress and inflammation to coronary artery endothelial cells via interaction with NF-κB/p65. Atherosclerosis (2018) 278:180–9. doi: 10.1016/j.atherosclerosis.2018.08.041
21. Wu X, Xu M, Liu Z, Zhang Z, Liu Y, Luo S, et al. Pharmacological inhibition of IRAK1 and IRAK4 prevents endothelial inflammation and atherosclerosis in ApoE(-/-) mice. Pharmacol Res (2022) 175:106043. doi: 10.1016/j.phrs.2021.106043
22. Xu S, Liu Y, Ding Y, Luo S, Zheng X, Wu X, et al. The zinc finger transcription factor, KLF2, protects against COVID-19 associated endothelial dysfunction. Signal Transduct Target Ther (2021) 6(1):266. doi: 10.1038/s41392-021-00690-5
23. Wang H, Liu D, Zhang H. Investigation of the underlying genes and mechanism of macrophage-enriched ruptured atherosclerotic plaques using bioinformatics method. J Atheroscler Thromb (2019) 26(7):636–58. doi: 10.5551/jat.45963
24. Zhu Z, Liu J, Zhang X. Salidroside protects against ox-LDL-induced endothelial injury by enhancing autophagy mediated by SIRT1-FoxO1 pathway. BMC Complement Altern Med (2019) 19(1):111. doi: 10.1186/s12906-019-2526-4
25. Reschen ME, Lin D, Chalisey A, Soilleux EJ, O'Callaghan CA. Genetic and environmental risk factors for atherosclerosis regulate transcription of phosphatase and actin regulating gene PHACTR1. Atherosclerosis (2016) 250:95–105. doi: 10.1016/j.atherosclerosis.2016.04.025
26. Murdaca G, Spanò F, Cagnati P, Puppo F. Free radicals and endothelial dysfunction: potential positive effects of TNF-α inhibitors. Redox Rep (2013) 18(3):95–9. doi: 10.1179/1351000213Y.0000000046
27. Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev (2011) 22(4):189–95. doi: 10.1016/j.cytogfr.2011.10.001
28. Liu Y, Tie L. Apolipoprotein m and sphingosine-1-phosphate complex alleviates TNF-α-induced endothelial cell injury and inflammation through PI3K/AKT signaling pathway. BMC Cardiovasc Disord (2019) 19(1):279. doi: 10.1186/s12872-019-1263-4
29. Marcos-Ramiro B, García-Weber D, Millán J. TNF-induced endothelial barrier disruption: beyond actin and rho. Thromb Haemost (2014) 112(6):1088–102. doi: 10.1160/th14-04-0299
30. Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol (2002) 157(7):1233–45. doi: 10.1083/jcb.200112126
31. Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin d and endothelial function. Nutrients (2020) 12(2):575. doi: 10.3390/nu12020575
32. Siasos G, Tousoulis D, Antoniades C, Stefanadi E, Stefanadis C. L-arginine, the substrate for NO synthesis: an alternative treatment for premature atherosclerosis? Int J Cardiol (2007) 116(3):300–8. doi: 10.1016/j.ijcard.2006.04.062
33. Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit Care Clin (2020) 36(2):307–21. doi: 10.1016/j.ccc.2019.12.009
34. Fang Z, Li M, Ma Z, Tu G. Association of endothelin-1 gene polymorphisms with essential hypertension in a Chinese population. Genet Mol Res (2017) 16(3):gmr16037446. doi: 10.4238/gmr16037446.
35. Abeyrathna P, Su Y. The critical role of akt in cardiovascular function. Vascul Pharmacol (2015) 74:38–48. doi: 10.1016/j.vph.2015.05.008
36. Yamagishi S, Nakamura K, Matsui T, Inoue H. A novel pleiotropic effect of atorvastatin on advanced glycation end product (AGE)-related disorders. Med Hypotheses (2007) 69(2):338–40. doi: 10.1016/j.mehy.2006.11.054
37. Ray KK, Cannon CP. Atorvastatin and cardiovascular protection: a review and comparison of recent clinical trials. Expert Opin Pharmacother (2005) 6(6):915–27. doi: 10.1517/14656566.6.6.915
38. Balakumar P, Sambathkumar R, Mahadevan N, Muhsinah AB, Alsayari A, Venkateswaramurthy N, et al. Molecular targets of fenofibrate in the cardiovascular-renal axis: A unifying perspective of its pleiotropic benefits. Pharmacol Res (2019) 144:132–41. doi: 10.1016/j.phrs.2019.03.025
39. Novodvorsky P, Chico TJ. The role of the transcription factor KLF2 in vascular development and disease. Prog Mol Biol Transl Sci (2014) 124:155–88. doi: 10.1016/B978-0-12-386930-2.00007-0
40. Villarreal G Jr., Zhang Y, Larman HB, Gracia-Sancho J, Koo A, García-Cardeña G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun (2010) 391(1):984–9. doi: 10.1016/j.bbrc.2009.12.002
41. Turpaev KT. Transcription factor KLF2 and its role in the regulation of inflammatory processes. Biochem (Mosc) (2020) 85(1):54–67. doi: 10.1134/S0006297920010058
42. Allen PB, Greenfield AT, Svenningsson P, Haspeslagh DC, Greengard P. Phactrs 1-4: A family of protein phosphatase 1 and actin regulatory proteins. Proc Natl Acad Sci U.S.A. (2004) 101(18):7187–92. doi: 10.1073/pnas.0401673101
43. Stricher F, Macri C, Ruff M, Muller S. HSPA8/HSC70 chaperone protein: structure, function, and chemical targeting. Autophagy (2013) 9(12):1937–54. doi: 10.4161/auto.26448
44. Johnson MD, Otuki MF, Cabrini DA, Rudolph R, Witherden DA, Havran WL. Hspa8 and ICAM-1 as damage-induced mediators of γδ T cell activation. J Leukoc Biol (2022) 111(1):135–45. doi: 10.1002/JLB.3AB0420-282R
45. Shan N, Zhou W, Zhang S, Zhang Y. Identification of HSPA8 as a candidate biomarker for endometrial carcinoma by using iTRAQ-based proteomic analysis. Onco Targets Ther (2016) 9:2169–79. doi: 10.2147/OTT.S97983
46. Karbach S, Wenzel P, Munzel T, Daiber A, Karbach S. eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr Pharm Des (2014) 20(22):3579–94. doi: 10.2174/13816128113196660748
47. Xu H, Pritchard KA Jr. Targeted increases in endothelial cell superoxide anion production stimulate eNOS-dependent nitric oxide production, not uncoupled eNOS activity. Arterioscler Thromb Vasc Biol (2008) 28(9):1580–1. doi: 10.1161/ATVBAHA.108.171801
48. Ford TJ, Corcoran D, Padmanabhan S, Aman A, Rocchiccioli P, Good R, et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J (2020) 41(34):3239–52. doi: 10.1093/eurheartj/ehz915
49. Codina-Fauteux VA, Beaudoin M, Lalonde S, Lo KS, Lettre G. PHACTR1 splicing isoforms and eQTLs in atherosclerosis-relevant human cells. BMC Med Genet (2018) 19(1):97. doi: 10.1186/s12881-018-0616-7
50. Fedoryshchak RO, Přechová M, Butler AM, Lee R, O'Reilly N, Flynn HR, et al. Molecular basis for substrate specificity of the Phactr1/PP1 phosphatase holoenzyme. Elife (2020) 9:e61509. doi: 10.7554/eLife.61509
51. Subramani J, Kundumani-Sridharan V, Das KC. Chaperone-mediated autophagy of eNOS in myocardial ischemia-reperfusion injury. Circ Res (2021) 129(10):930–45. doi: 10.1161/CIRCRESAHA.120.317921
52. He M, Gou H, Yang X, Zhou L, Zhang X, Cheng L, et al. Genetic variations in HSPA8 gene associated with coronary heart disease risk in a Chinese population. PLoS One (2010) 5(3):e9684. doi: 10.1371/journal.pone.0009684
53. Polanowska-Grabowska R, Simon CG Jr, Falchetto R, Shabanowitz J, Hunt DF, Gear AR. Platelet adhesion to collagen under flow causes dissociation of a phosphoprotein complex of heat-shock proteins and protein phosphatase 1. Blood (1997) 90(4):1516–26. doi: 10.1182/blood.V90.4.1516
54. Rubin S, Bougaran P, Martin S, Abelanet A, Delobel V, Pernot M, et al. PHACTR-1 (Phosphatase and actin regulator 1) deficiency in either endothelial or smooth muscle cells does not predispose mice to nonatherosclerotic arteriopathies in 3 transgenic mice. Arterioscler Thromb Vasc Biol (2022) 42(5):597–609. doi: 10.1161/ATVBAHA.122.317431
55. Adlam D, Olson TM, Combaret N, Kovacic JC, Iismaa SE, Al-Hussaini A, et al. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol (2019) 73(1):58–66. doi: 10.1016/j.jacc.2018.09.085
56. Wood A, Antonopoulos A, Chuaiphichai S, Kyriakou T, Diaz R, Al Hussaini A, et al. PHACTR1 modulates vascular compliance but not endothelial function: a translational study. Cardiovasc Res (2022), cvac092. doi: 10.1093/cvr/cvac092
Keywords: endothelial dysfunction, inflammation, PHACTR1, cardiovascular disease, RNA sequencing
Citation: Ma X, Su M, He Q, Zhang Z, Zhang F, Liu Z, Sun L, Weng J and Xu S (2022) PHACTR1, a coronary artery disease risk gene, mediates endothelial dysfunction. Front. Immunol. 13:958677. doi: 10.3389/fimmu.2022.958677
Received: 31 May 2022; Accepted: 01 August 2022;
Published: 25 August 2022.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Tlili Barhoumi, King Abdullah International Medical Research Center (KAIMRC), Saudi ArabiaCopyright © 2022 Ma, Su, He, Zhang, Zhang, Liu, Sun, Weng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suowen Xu, c3h1MTk4NEB1c3RjLmVkdS5jbg==; Jianping Weng, d2VuZ2pwQHVzdGMuZWR1LmNu
†ORCID: Jianping Weng, orcid.org/0000-0002-7889-1697
Suowen Xu, orcid.org/0000-0002-5488-5217
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.