- 1Department of Rheumatology, The Second affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, China
- 2Division of Rheumatology, Huashan Hospital, Fudan University, Shanghai, China
- 3Department of Endocrinology, The Second affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, China
Gout is a common type of inflammatory arthritis characterized by the presence of monosodium urate crystals (MSU) in the joints. Macrophages are believed to be involved in gout flares. It has long been recognized that resident macrophage and monocyte derived macrophages are distinct subsets and there have been attempts to investigate their roles in acute gout, respectively. Previous studies revealed that resident macrophages initiate and drive the inflammation, while monocyte derived macrophages differentiated into M1-like macrophages in response to MSU crystals. With the advancement of technologies, subpopulations of synovial resident macrophages have been defined with the characteristics more accurately described. Resident macrophages in the synovial lining layer showed an anti-inflammatory effect in rheumatoid arthritis, but specific Trpv4 depletion of them reduced MSU crystals induced murine arthritis. CD14+ monocytes in the synovial fluid from patients with gout exhibit phenotypes of anti-inflammatory as well as pro-inflammatory characteristics. Here, we review the main aspects of macrophages in the initiation and resolution of acute gout and try to clarify the specific role of each subpopulation. Building a reliable diagram of the effect of monocytes and macrophages during MSU crystals induced arthritis will bring us closer to targeting macrophages for improving the management of gout.
Introduction
Acute gout is an auto-inflammatory disease characterized by self-limiting inflammation in response to the deposition of monosodium urate (MSU) crystals in the joints (1). Various immune cells are involved in the initiation, development and spontaneous resolution. The role of macrophages, neutrophils (2–5), mast cells (6, 7) and, less frequently mentioned previously, T cells (8–10) have been revealed by recent studies. The macrophage is one of the earliest recognized and well-studied immune cells that mediated the entire process of acute gout. However, there are several issues that have been debated. The ultimate one is the role of resident macrophages and monocyte-derived macrophages in acute gout. In the meantime, as the study of synovial macrophages has been advanced, methods like fate-mapping approaches and single-cell RNA sequencing (scRNAseq) have revealed more refined and distinct phenotypes and functions of the synovial resident macrophages (11–13). It has allowed a great progress in the study of rheumatoid arthritis (RA). However, in the field of acute gout, there is still no consensus, and this review attempts to elucidate the role of these two subgroups of macrophages in the process of acute gout by combining the recent studies.
The synovial resident macrophages are divided into three subpopulations
The synovium is a thin membrane encapsulating the joints and supports its mobility. It consists of two layers, the intimal lining layer composed of fibroblast-like synoviocytes and macrophage-like synoviocytes. And the sub lining layer is the underlying connective tissue composed of fibroblasts, fat cells, macrophages and blood vessels (14). Synovial tissue resident macrophages were previously identified by CD68highCD163high (15). Lining synovial macrophages with the capacity of cellular clearance were discriminated from sub-lining cells by the expression of FcγRIIIa (16, 17). However, contradictory findings (18–20) call for more adequate and precise markers to discriminate between the various subgroups of synovial resident macrophages.
A recent approach using fate mapping and reporter mice in conjunction with three-dimensional light sheet microscopy yielded important insights into the spatiotemporal composition, origin and biology of synovial resident macrophage subsets (13). Lining macrophages (the major resident group) selectively express CX3Cr1 and organize in dense membrane-like barrier structures. They also show an immune regulatory and highly phagocytic phenotype with the expression VSIG4 (21) and TREM2 (22). MHCII+ interstitial macrophages are in the sub lining layer and give rise to the CX3Cr1+ macrophages. A third population of RELMα+CD163+CD206+ interstitial macrophages, also generated from the MHCII+ interstitial macrophages, display many features of alternatively activated macrophages. Additional studies have subsequently demonstrated similar synovial macrophage phenotypes, from both human and murine perspectives (11, 12).
Synovial resident macrophages mainly initiate and drive MSU crystals induced inflammation
Wiliiam John Martin and colleagues confirmed resident macrophages initiating and driving inflammation in MSU crystals induced murine peritoneal models (23). This study served as a cornerstone for the role of resident macrophage in gout. For more than a decade thereafter, studies on both the mechanisms of acute gout and intervention strategies have been conducted on the basis of the latter research. However, the conclusion was sometimes challenged. From a macrophage classification perspective, the peritoneal cavity could somehow mimic the joint, but peritoneal macrophages are not equivalent to synovial macrophages. An investigation of synovial fluid mononuclear cells in patients with acute gout revealed that CD14+ infiltrated monocytes rather than tissue-resident macrophages produced pro-inflammatory cytokines (24). Although a sparse number of macrophages phenotypically representative of resident macrophages were present in the synovial fluid, they do not truly characterize the function of synovial resident macrophages, either.
What really makes the theory that resident macrophages initiate and drive MSU crystal-induced arthritis challenged is that multiple studies have indicated the synovial lining macrophages display an immune regulatory phenotype and effect. Depleting of the synovial CX3CR1+TREM2+ macrophages aggravated the arthritis in K/BxN STA murine models (13). Two synovial macrophage subpopulations (MerTKposTREM2high and MerTKposLYVE1pos) from patients with rheumatoid arthritis (RA) showed unique remission transcriptomic signatures enriched in negative regulators of inflammation (11). HUANG and colleagues observed that the synovial macrophage tissue–resident niche is necessary for suppression of chronic inflammation and may contribute to the pathogenesis of RA (12). All these studies were conducted to investigate the contribution of synovial lining macrophages in arthritis and invariably identified their anti-inflammatory and tissue stabilizing properties. The latter evidence leads us to wonder whether synovial resident macrophages actually initiate and drive acute gout, and whether the numerous studies on them as target cells were rational.
Notably, the above studies all described the role of synovial resident macrophages in chronic arthritis like RA. In contrast, gout is a crystal-induced acute arthritis. An important investigation by Zhou Lan et al. confirmed that TRPV4 is critically involved in the activation of macrophage NLRP3 inflammasome and production of interleukin(IL)-1β induced by MSU crystals (25). In the study, they established the macrophage-specific Trpv4 cKO (Cx3cr1CreERT; Trpv4f/f) murine model. Fate mapping studies have shown that tissue-resident macrophages retained their identity but the monocyte-derived macrophages were replaced with wild type macrophages produced by bone-marrow-derived progenitors in the inducible macrophage-specific Trpv4 cKOs four weeks after tamoxifen administration (25). The ankle swelling was significantly reduced in the Cre+Cx3cr1CreERT; Trpv4f/f mice when compared with their Cre- littermates. TPRV4 has been known to aggravate inflammation by activating NLPR3 inflammasome. And TPRV4 could not work if the CX3CR1+ synovial macrophages did not undergo a pro-inflammatory response with MSU crystals. Therefore this study provided sufficient evidence for people to believe that CX3CR1+ synovial resident macrophages initiate and drive MSU crystal-induced arthritis.
There has been no direct evidence to prove the involvement of synovial resident macrophage in the spontaneous resolution of acute gout. In our previous study (26), we observed that in rat gouty joints, the M2 (alternative macrophage polarization) was seen at an early stage at 2 hours after injection of MSU crystals. And in the murine peritoneal model, the anti-inflammatory cytokine IL-37 level also peaked at 2 hours after injection of MSU crystals. RELMα+CD163+CD206+ interstitial macrophages express genes associated with alternative activation, suggesting an immunosuppressive phenotype that is protective. We suppose they might be the source of M2 at an early stage of acute gout and involved in the initiation of the spontaneous resolution. However, the exact positioning of synovial RELMα+ interstitial macrophages requires further investigation by imaging and functional technologies.
Recruited monocytes may exhibit both pro-inflammatory and anti-inflammatory phenotypes in MSU induced arthritis
Macrophages were originally proposed to be derived from circulating monocytes (27). With the advancement of research tools, such as lineage tracing (28, 29), depleting strategies (30, 31), therapeutic targeting (28) and monitoring (32), it has been accepted that many tissue macrophage populations arise during embryonic development, are seeded well before birth and are thereafter maintained by local proliferation rather than monocyte recruitment (33). Circulating monocytes are completely or at least partly independent from resident macrophages. Consequently, when we consider the tissue inflammation, it is important to investigate the precise effect of resident macrophages and monocyte-derived macrophages, respectively. The classical hierarchical mononuclear phagocyte system proposed by Van Furth et al. in 1972 held the opinion that circulating monocytes from the bone marrow continuously migrated to peripheral tissues, where they differentiated into macrophages and then worked (34). However, many researchers discovered that the monocytes were also involved in the inflammatory responses (35–39).
Several studies have shown the phenotype of the circulating monocytes during MSU induced inflammatory responses (40–43). Only a few directly explored the role of monocytes in the joints during acute gouty inflammation. We have to recognize that cells with an intermediate immunophenotype are detected during the differentiation and maturation from monocytes to mature macrophages. The phenotype of monocyte-derived macrophages is affected by the phase of inflammation that affects the composition of macropahges with different maturation stages. Therefore, we need to pay great attention to the time point of the studies on monocyte phenotypes when discussing these researches.
By using the murine peritoneal model, William John Martin proved MSU crystal-recruited monocytes differentiate into pro-inflammatory M1-like macrophages in vivo (44). In this study, the most straightforward evidence was that the expression of pro-IL-1β, IL-1β, pro-caspase-1 and caspase-1 of the cells from the peritoneal fluid increased with time. However, what has been troubling us is that the expression of the above proteins was highest at 72 hours after MSU crystal injection, which meant a very late phase of acute gout. But at 18 hours, the inflammation in the peritoneal cavity had all resolved and was no longer occurring. How did these cells with high expression of inflammasome related proteins play a non-inflammatory role? Are these really monocyte-derived macrophages or just the resident macrophages which were previously attached to the peritoneum returning to the peritoneal cavity?
In patients with gout, CD14+ monocytes were markedly increased in the synovial fluid (24). The CD14+ monocytes showed the phenotypes of infiltrated monocytes rather than tissue-resident macrophages. Intracellular cytokine staining of the CD14+ monocytes showed considerable amounts of IL-8. However, there was no significant increase of pro-inflammatory cytokines after MSU re-stimulation. Compared with monocytes from patients with RA, monocytes of gouty patients produced more IL-10 significantly. Thus, the authors came to a conclusion that CD14+ cells of the synovial fluid from the patients with gout are infiltrated monocytes and exhibit phenotypes of anti-inflammatory as well as pro-inflammatory characteristics. Since the samples of joint fluid were obtained from patients with gout, the authors only stated that the patients were in active disease at the time of sampling, and there was no specific time point, so there was variability in the phenotype ofmonocytes from various patients. In our own study, we placed great emphasis on the phenotypic changes of monocyte-macrophages with time changes following MSU crystal intervention, we have also discovered that the iNOS/Arg-1, which represented M1/M2 ratio, of peritoneal monocytes increased with time post MSU crystals injection (26). It means that M1 and M2 co-existed in the inflamed tissue and the number of M1 exceeded M2 at the initial and progressing stages of acute gout.
Discussion
It is believed that macrophages initiate and drive the MSU induced arthritis. And a variety of new therapeutic targets focusing on macrophages have been constantly discovered. Our group has published articles describing the role and mechanism of Sirt1, IL-37 and leptin on macrophages in mediating acute gout (45–49). Also, we observed distinct macrophage polarizations in acute and chronic gout (26). It is with the accumulation and continuous progress of studies that we believe it is necessary to properly characterize the role of macrophages, especially the various subpopulations, in acute gout and the spontaneous resolution.
When we searched “gout and macrophage” on the Pubmed from 2007 to 2022, there were 336 articles. Only a few of them actually elucidate the role of resident macrophages, monocytes and monocyte-derived macrophages in acute gout. The fate-mapping approaches and scRNAseq have divided the synovial resident macrophages into three well-defined subpopulations, of which the major one is the lining CX3Cr1+ macrophages. The other two sub-populations are the MHCII+ interstitial and RELMα+CD163+CD206+ resident synovial macrophages in the sub-lining layer, see Figure 1. While studies have implicated the lining CX3Cr1+ macrophages in maintaining joint homeostasis and suppressing chronic arthritis, a recent study confirmed that they do initiate MSU crystals induced arthritis (25). Taking into account our own findings, we propose that RELMα+ CD163+CD206+ resident synovial macrophages may directly generate M2 macrophage polarization and mediate the spontaneous resolution, “ programmed” for remission at the onset of MSU crystal induced inflammatory responses. Although sparsely studied and in contrast to the role played in chronic arthritis, the available evidences all suggests that synovial resident macrophages initiate and drive the MSU crystal induced inflammatory responses, with a minor proportion, probably RELMα+CD163+CD206+ interstitial macrophages, mediating the resolution of inflammation at an early stage.
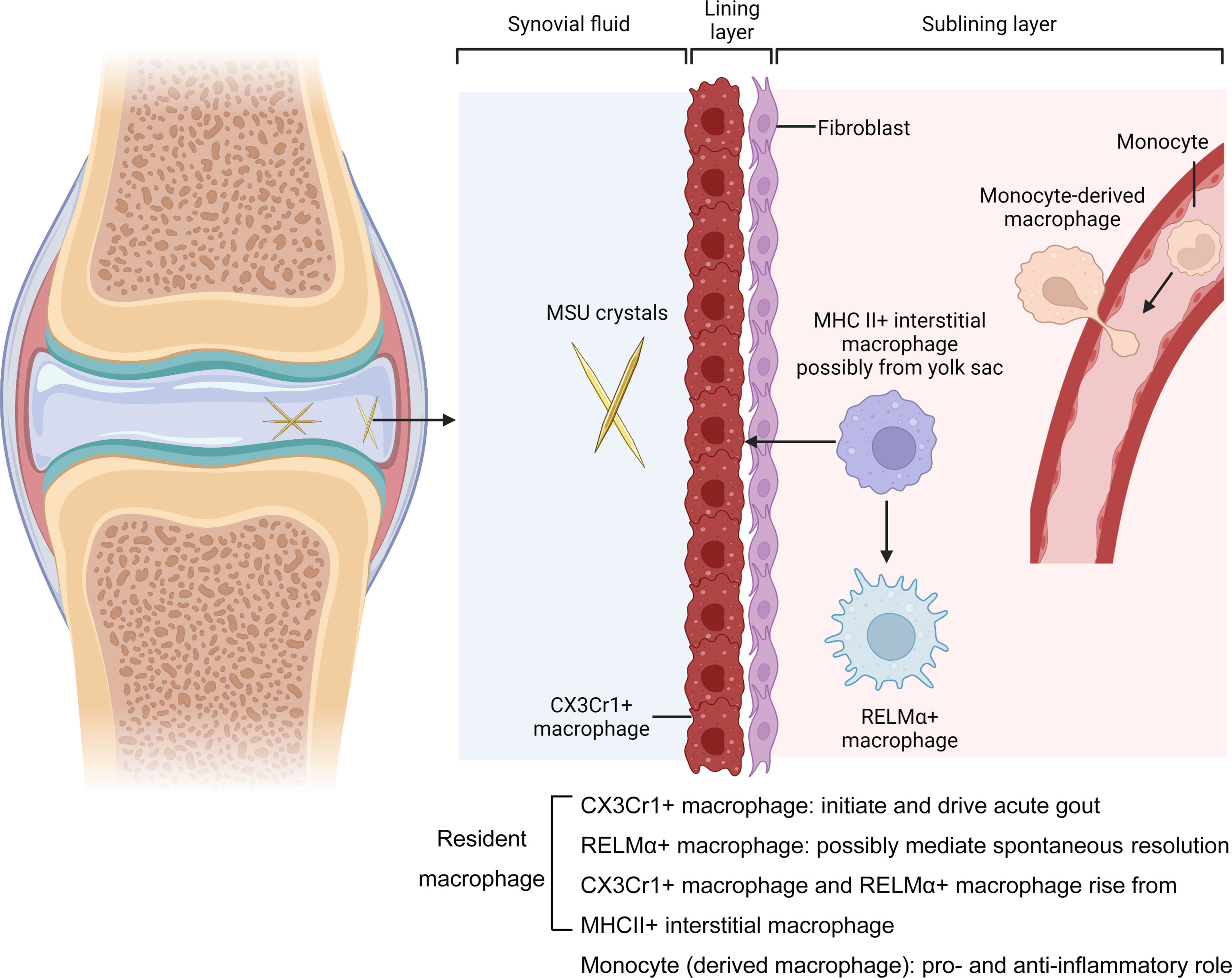
Figure 1 Macrophages in the MSU crytals induced arthritis. Joints are surrounded by a tissue called the synovium, which is formed from layers of cells called the lining and the sublining layers. The resident macrophages in the lining layer highly expressed CX3Cr1 and form a barrier adjacent to a layer of cells called fibroblasts. They initiate and drive acute gout. Barrier-forming macrophages arise from a subpopulation of MHCII+ macrophages called interstitial macrophages in the sublining layer. The MHCII+ macrophages aslo generate another subset called RELMα+ interstitial macrophages. They express genes associated with alternative activation (CD163+CD206+), suggesting an immunosuppressive phenotype that is protective. The other Non-resident macrophages enter the joint from blood vessels. These cells, which can drive and supress MSU crystals induced inflammation, derived from monocytes. Mr. Xiangyu Xu and Dr. Lei Liu, created with BioRender.com.
Studies on monocytes in acute gouty arthritis are even fewer and there seemed no consensus among the researchers on this issue. The most widely accepted finding comes from the MSU crystal-induced murine model of peritoneal inflammation (44). Although there were results suggesting that the recruited monocyte-macrophages expressed higher levels of both IL-1β and caspase-1 over time. The major problem was that the phenotype of the cells was not consistent with the overall phenotype of the murine model, and the inflammation in the peritoneal cavity had spontaneously resolved at the time when monocyte-macrophage expression of pro-inflammatory factors was at the highest level. We suppose that this part of the study may not be truly representative of the features of the recruited monocytes. A recent study explored the phenotype and function of CD14+ monocytes in the synovial fluid of patients with acute gout (24). What is indicative of the pro-inflammatory profile of the recruited monocytes is the elevated intracellular IL-8 level. But they did not secrete more pro-inflammatory cytokines than the control group when re-stimulated by MSU crystals in vitro. Interestingly, this group of cells also expressed the surface markers of M2, suggesting that the recruited monocytes might also play a role in suppressing inflammation in the joint. We have also observed M2 from the peritoneal fluid at the early stage of MSU crystals induced peritonitis (26).
In addition to the fact that direct observation of various subpopulations of macrophages in the joint is the most crucial challenge to clarify the topic, the lack of consensus on origin of macrophages and how to derive and activate them with MSU crystals is another important issue. THP-1 designates a spontaneously immortalized monocyte-like cell line, which has been widely used in studies of gout. Pam3CSK4 (50), PMA (51), LPS (52), and IL-6 (53) were all used for priming the THP-1 cells. Bone marrow derived monocytes (BMDM) are murine primary cells, for which priming is necessary as well. The colony-stimulating factors, macrophage colony-stimulating factor (M-CSF) and granulocyte macrophage-colony stimulating factor (GM-CSF), are known to prime or activate BMDM (54). However, they have substantial polarizing effects (55). Therefore, distinct protocols bring different effects on macrophages derived from primary monocytes or cell lines. This makes the macrophage status prior to the MSU crystal intervention vary and is poorly comparable across various studies. An important nomenclature and experimental guideline for macrophage activation and polarization was raised by Peter J. Murray and colleagues (56). In the fields of studies of gout, we hope standardization issues for priming and activating the monocytes could be established and accepted to avoid stunting progress. Importantly, in our studies, non-primed murine peritoneal resident macrophages and RAW264.7 cell lines exhibited a pro-inflammatory phenotype in response to MSU crystals (45, 47, 48). It has been proved that MSU crystals alone induce a metabolic-inflammatory transcriptional program in non-primed human and murine macrophages that is markedly distinct to that induced by LPS. Increased binding of JUN to the promoter of target genes through JNK signaling initiates the inflammatory-metabolic changes (57). Nonetheless, some studies still primed the macrophages and resulted in more robust inflammatory responses. It is also important to note that the inflammatory response of macrophages to MSU crystals is not equal to that of LPS and special regard should be paid when designing studies. Therefore, the consensus of primary monocytes and macrophages and the corresponding cell lines appear to be crucial for the future study of gout.
Although there are numerous studies on the anti-inflammatory treatment of gout, the only agents currently available for clinical use are colchicine, NSAIDs, glucocorticoids, and anakinra, of which, the side effects constrain their use in patients with cardiovascular, gastrointestinal, or renal comorbidities. Clinical translational medicine is inseparable from advances in disease mechanism research. Currently in the field of acute gout, the role of diverse subpopulations of macrophages, one of the most important targets of gout therapy, in the various stages of initiating, driving and spontaneous resolution have not well been understood. With the development of techniques, such as fate-mapping, scRNAseq and three-dimensional light-sheet fluorescence microscopy, we believe that people will eventually have a clear understanding of the relationship between macrophages and gout and obtain better ideas for the treatment of the disease.
Author Contributions
LL, YY, LJZ, and LZ drafted the manuscript. LS and YX supervised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China No. 81801597.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Mr. Xiangyu Xu for editing the figures with LL, and Dr. Luis Munoz for the figure drawing tool.
References
1. Steiger S, Harper JL. Mechanisms of Spontaneous Resolution of Acute Gouty Inflammation. Curr Rheumatol Rep (2014) 16(1):392. doi: 10.1007/s11926-013-0392-5
2. Yin C, Liu B, Li Y, Li X, Wang J, Chen R, et al. IL-33/ST2 Induces Neutrophil-Dependent Reactive Oxygen Species Production and Mediates Gout Pain. Theranostics (2020) 10(26):12189–203. doi: 10.7150/thno.48028
3. Garcia-Gonzalez E, Gamberucci A, Lucherini OM, Ali A, Simpatico A, Lorenzini S, et al. Neutrophil Extracellular Traps Release in Gout and Pseudogout Depends on the Number of Crystals Regardless of Leukocyte Count. Rheumatol (Oxford) (2021) 60(10):4920–8. doi: 10.1093/rheumatology/keab087
4. Zhong L, Li S, Wen Y, Zheng J, Liu F, Cao D, et al. Expansion of Polymorphonuclear Myeloid-Derived Suppressor Cells in Patients With Gout. Front Immunol (2020) 11:567783. doi: 10.3389/fimmu.2020.567783
5. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated Neutrophil Extracellular Traps Limit Inflammation by Degrading Cytokines and Chemokines. Nat Med (2014) 20(5):511–7. doi: 10.1038/nm.3547
6. Reber LL, Marichal T, Sokolove J, Starkl P, Gaudenzio N, Iwakura Y, et al. Contribution of Mast Cell-Derived Interleukin-1beta to Uric Acid Crystal-Induced Acute Arthritis in Mice. Arthritis Rheumatol (2014) 66(10):2881–91. doi: 10.1002/art.38747
7. Hoffmeister C, Silva MA, Rossato MF, Trevisan G, Oliveira SM, Guerra GP, et al. Participation of the TRPV1 Receptor in the Development of Acute Gout Attacks. Rheumatol (Oxford) (2014) 53(2):240–9. doi: 10.1093/rheumatology/ket352
8. Mansour AA, Raucci F, Saviano A, Tull S, Maione F, Iqbal AJ. Galectin-9 Regulates Monosodium Urate Crystal-Induced Gouty Inflammation Through the Modulation of Treg/Th17 Ratio. Front Immunol (2021) 12:762016. doi: 10.3389/fimmu.2021.762016
9. Cho YN, Jeong HS, Park KJ, Kim HS, Kim EH, Jin HM, et al. Altered Distribution and Enhanced Osteoclastogenesis of Mucosal-Associated Invariant T Cells in Gouty Arthritis. Rheumatol (Oxford) (2020) 59(8):2124–34. doi: 10.1093/rheumatology/keaa020
10. Wang J, Yang Q, Zhang Q, Yin C, Zhou L, Zhou J, et al. Invariant Natural Killer T Cells Ameliorate Monosodium Urate Crystal-Induced Gouty Inflammation in Mice. Front Immunol (2017) 8:1710. doi: 10.3389/fimmu.2017.01710
11. Alivernini S, MacDonald L, Elmesmari A, Finlay S, Tolusso B, Gigante MR, et al. Distinct Synovial Tissue Macrophage Subsets Regulate Inflammation and Remission in Rheumatoid Arthritis. Nat Med (2020) 26(8):1295–306. doi: 10.1038/s41591-020-0939-8
12. Huang QQ, Doyle R, Chen SY, Sheng Q, Misharin AV, Mao Q, et al. Critical Role of Synovial Tissue-Resident Macrophage Niche in Joint Homeostasis and Suppression of Chronic Inflammation. Sci Adv (2021) 7(2):eabd0515. doi: 10.1126/sciadv.abd0515
13. Culemann S, Gruneboom A, Nicolas-Avila JA, Weidner D, Lammle KF, Rothe T, et al. Locally Renewing Resident Synovial Macrophages Provide a Protective Barrier for the Joint. Nat (2019) 572(7771):670–5. doi: 10.1038/s41586-019-1471-1
14. Firestein GS. Invasive Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. Passive Responders or Transformed Aggressors? Arthritis Rheumatol (1996) 39(11):1781–90. doi: 10.1002/art.1780391103
15. Smolen JS, Aletaha D. Forget Personalised Medicine and Focus on Abating Disease Activity. Ann Rheum Dis (2013) 72(1):3–6. doi: 10.1136/annrheumdis-2012-202361
16. Araki Y, Mimura T. The Mechanisms Underlying Chronic Inflammation in Rheumatoid Arthritis From the Perspective of the Epigenetic Landscape. J Immunol Res (2016) 2016:6290682. doi: 10.1155/2016/6290682
17. Wallner S, Schroder C, Leitao E, Berulava T, Haak C, Beisser D, et al. Epigenetic Dynamics of Monocyte-to-Macrophage Differentiation. Epigenet Chromatin (2016) 9:33. doi: 10.1186/s13072-016-0079-z
18. Muller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, et al. Synovial Fibroblasts of Patients With Rheumatoid Arthritis Attach to and Invade Normal Human Cartilage When Engrafted Into SCID Mice. Am J Pathol (1996) 149(5):1607–15.
19. Dennis G Jr., Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, et al. Synovial Phenotypes in Rheumatoid Arthritis Correlate With Response to Biologic Therapeutics. Arthritis Res Ther (2014) 16(2):R90. doi: 10.1186/ar4555
20. Stephenson W, Donlin LT, Butler A, Rozo C, Bracken B, Rashidfarrokhi A, et al. Single-Cell RNA-Seq of Rheumatoid Arthritis Synovial Tissue Using Low-Cost Microfluidic Instrumentation. Nat Commun (2018) 9(1):791. doi: 10.1038/s41467-017-02659-x
21. Li J, Diao B, Guo S, Huang X, Yang C, Feng Z, et al. VSIG4 Inhibits Proinflammatory Macrophage Activation by Reprogramming Mitochondrial Pyruvate Metabolism. Nat Commun (2017) 8(1):1322. doi: 10.1038/s41467-017-01327-4
22. Kim SM, Mun BR, Lee SJ, Joh Y, Lee HY, Ji KY, et al. TREM2 Promotes Abeta Phagocytosis by Upregulating C/EBPalpha-Dependent CD36 Expression in Microglia. Sci Rep (2017) 7(1):11118. doi: 10.1038/s41598-017-11634-x
23. Martin WJ, Walton M, Harper J. Resident Macrophages Initiating and Driving Inflammation in a Monosodium Urate Monohydrate Crystal-Induced Murine Peritoneal Model of Acute Gout. Arthritis Rheumatol (2009) 60(1):281–9. doi: 10.1002/art.24185
24. Jeong JH, Hong S, Kwon OC, Ghang B, Hwang I, Kim YG, et al. CD14(+) Cells With the Phenotype of Infiltrated Monocytes Consist of Distinct Populations Characterized by Anti-Inflammatory as Well as Pro-Inflammatory Activity in Gouty Arthritis. Front Immunol (2017) 8:1260. doi: 10.3389/fimmu.2017.01260
25. Lan Z, Chen L, Feng J, Xie Z, Liu Z, Wang F, et al. Mechanosensitive TRPV4 is Required for Crystal-Induced Inflammation. Ann Rheum Dis (2021) 80(12):1604–14. doi: 10.1136/annrheumdis-2021-220295
26. Zhao L, Ye W, Zhu Y, Chen F, Wang Q, Lv X, et al. Distinct Macrophage Polarization in Acute and Chronic Gout. Lab Invest (2022). doi: 10.1038/s41374-022-00798-4
27. van Furth R, Cohn ZA. The Origin and Kinetics of Mononuclear Phagocytes. J Exp Med (1968) 128(3):415–35. doi: 10.1084/jem.128.3.415
28. Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, et al. RNAi Targeting Multiple Cell Adhesion Molecules Reduces Immune Cell Recruitment and Vascular Inflammation After Myocardial Infarction. Sci Transl Med (2016) 8(342):342ra80. doi: 10.1126/scitranslmed.aaf1435
29. Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Sci (2012) 336(6077):86–90. doi: 10.1126/science.1219179
30. Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, et al. Perivascular Macrophages Mediate the Neurovascular and Cognitive Dysfunction Associated With Hypertension. J Clin Invest (2016) 126(12):4674–89. doi: 10.1172/JCI86950
31. Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell (2017) 169(3):510–22 e20. doi: 10.1016/j.cell.2017.03.050
32. Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, et al. Ischemic Stroke Activates Hematopoietic Bone Marrow Stem Cells. Circ Res (2015) 116(3):407–17. doi: 10.1161/CIRCRESAHA.116.305207
33. Honold L, Nahrendorf M. Resident and Monocyte-Derived Macrophages in Cardiovascular Disease. Circ Res (2018) 122(1):113–27. doi: 10.1161/CIRCRESAHA.117.311071
34. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The Mononuclear Phagocyte System: A New Classification of Macrophages, Monocytes, and Their Precursor Cells. Bull World Health Organ (1972) 46(6):845–52.
35. Kapellos TS, Bonaguro L, Gemund I, Reusch N, Saglam A, Hinkley ER, et al. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol (2019) 10:2035. doi: 10.3389/fimmu.2019.02035
36. Radzyukevich YV, Kosyakova NI, Prokhorenko IR. Participation of Monocyte Subpopulations in Progression of Experimental Endotoxemia (EE) and Systemic Inflammation. J Immunol Res (2021) 2021:1762584. doi: 10.1155/2021/1762584
37. Auger JP, Rivest S, Benoit-Biancamano MO, Segura M, Gottschalk M. Inflammatory Monocytes and Neutrophils Regulate Streptococcus Suis-Induced Systemic Inflammation and Disease But Are Not Critical for the Development of Central Nervous System Disease in a Mouse Model of Infection. Infect Immun (2020) 88(3):e00787–19. doi: 10.1128/IAI.00787-19
38. Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernandez-Jimenez E, et al. Human Monocytes Undergo Functional Re-Programming During Sepsis Mediated by Hypoxia-Inducible Factor-1alpha. Immun (2015) 42(3):484–98. doi: 10.1016/j.immuni.2015.02.001
39. Kelly A, Gunaltay S, McEntee CP, Shuttleworth EE, Smedley C, Houston SA, et al. Human Monocytes and Macrophages Regulate Immune Tolerance via Integrin Alphavbeta8-Mediated TGFbeta Activation. J Exp Med (2018) 215(11):2725–36. doi: 10.1084/jem.20171491
40. Crisan TO, Cleophas MCP, Novakovic B, Erler K, van de Veerdonk FL, Stunnenberg HG, et al. Uric Acid Priming in Human Monocytes is Driven by the AKT-PRAS40 Autophagy Pathway. Proc Natl Acad Sci U S A (2017) 114(21):5485–90. doi: 10.1073/pnas.1620910114
41. Ma Q, Honarpisheh M, Li C, Sellmayr M, Lindenmeyer M, Bohland C, et al. Soluble Uric Acid Is an Intrinsic Negative Regulator of Monocyte Activation in Monosodium Urate Crystal-Induced Tissue Inflammation. J Immunol (2020) 205(3):789–800. doi: 10.4049/jimmunol.2000319
42. Lee JH, Yang JA, Shin K, Lee GH, Lee WW, Lee EY, et al. Elderly Patients Exhibit Stronger Inflammatory Responses During Gout Attacks. J Korean Med Sci (2017) 32(12):1967–73. doi: 10.3346/jkms.2017.32.12.1967
43. Wang X, Liu X, Qi Y, Zhang S, Shi K, Lin H, et al. High Level of Serum Uric Acid Induced Monocyte Inflammation is Related to Coronary Calcium Deposition in the Middle-Aged and Elder Population of China: A Five-Year Prospective Cohort Study. J Inflammation Res (2022) 15:1859–72. doi: 10.2147/JIR.S353883
44. Martin WJ, Shaw O, Liu X, Steiger S, Harper JL. Monosodium Urate Monohydrate Crystal-Recruited Noninflammatory Monocytes Differentiate Into M1-Like Proinflammatory Macrophages in a Peritoneal Murine Model of Gout. Arthritis Rheumatol (2011) 63(5):1322–32. doi: 10.1002/art.30249
45. Liu L, Zhu X, Zhao T, Yu Y, Xue Y, Zou H. Sirt1 Ameliorates Monosodium Urate Crystal-Induced Inflammation by Altering Macrophage Polarization via the PI3K/Akt/STAT6 Pathway. Rheumatol (Oxford) (2019) 58(9):1674–83. doi: 10.1093/rheumatology/kez165
46. Chen H, Zheng S, Wang Y, Zhu H, Liu Q, Xue Y, et al. The Effect of Resveratrol on the Recurrent Attacks of Gouty Arthritis. Clin Rheumatol (2016) 35(5):1189–95. doi: 10.1007/s10067-014-2836-3
47. Zhao L, Zhao T, Yang X, Cao L, Xu R, Liu J, et al. IL-37 Blocks Gouty Inflammation by Shaping Macrophages Into a non-Inflammatory Phagocytic Phenotype. Rheumatol (Oxford) (2022). doi: 10.1093/rheumatology/keac009
48. Liu L, Xue Y, Zhu Y, Xuan D, Yang X, Liang M, et al. Interleukin 37 Limits Monosodium Urate Crystal-Induced Innate Immune Responses in Human and Murine Models of Gout. Arthritis Res Ther (2016) 18(1):268. doi: 10.1186/s13075-016-1167-y
49. Yu Y, Yang J, Fu S, Xue Y, Liang M, Xuan D, et al. Leptin Promotes Monosodium Urate Crystal-Induced Inflammation in Human and Murine Models of Gout. J Immunol (2019) 202(9):2728–36. doi: 10.4049/jimmunol.1801097
50. ElSayed S, Jay GD, Cabezas R, Qadri M, Schmidt TA, Elsaid KA. Recombinant Human Proteoglycan 4 Regulates Phagocytic Activation of Monocytes and Reduces IL-1beta Secretion by Urate Crystal Stimulated Gout PBMCs. Front Immunol (2021) 12:771677. doi: 10.3389/fimmu.2021.771677
51. Luo C, Lian X, Hong L, Zou J, Li Z, Zhu Y, et al. High Uric Acid Activates the ROS-AMPK Pathway, Impairs CD68 Expression and Inhibits OxLDL-Induced Foam-Cell Formation in a Human Monocytic Cell Line, THP-1. Cell Physiol Biochem (2016) 40(3-4):538–48. doi: 10.1159/000452567
52. Takashiba S, Van Dyke TE, Amar S, Murayama Y, Soskolne AW, Shapira L. Differentiation of Monocytes to Macrophages Primes Cells for Lipopolysaccharide Stimulation via Accumulation of Cytoplasmic Nuclear Factor Kappab. Infect Immun (1999) 67(11):5573–8. doi: 10.1128/IAI.67.11.5573-5578.1999
53. Cochran FR, Finch-Arietta MB. Interleukin-6 can Prime THP-1 Macrophages for Enhanced Production of Tumor Necrosis Factor-Alpha in Response to LPS. Immunopharmacology (1992) 23(2):97–103. doi: 10.1016/0162-3109(92)90033-9
54. Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-Macrophage Colony-Stimulating Factor (CSF) and Macrophage CSF-Dependent Macrophage Phenotypes Display Differences in Cytokine Profiles and Transcription Factor Activities: Implications for CSF Blockade in Inflammation. J Immunol (2007) 178(8):5245–52. doi: 10.4049/jimmunol.178.8.5245
55. Lescoat A, Ballerie A, Augagneur Y, Morzadec C, Vernhet L, Fardel O, et al. Distinct Properties of Human M-CSF and GM-CSF Monocyte-Derived Macrophages to Simulate Pathological Lung Conditions In Vitro: Application to Systemic and Inflammatory Disorders With Pulmonary Involvement. Int J Mol Sci (2018) 19(3):894. doi: 10.3390/ijms19030894
56. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immun (2014) 41(1):14–20. doi: 10.1016/j.immuni.2014.06.008
Keywords: synovial resident macrophage, lining layer, gout, spontaneous resolution, monocyte derived macrophage
Citation: Liu L, Zhu L, Liu M, Zhao L, Yu Y, Xue Y and Shan L (2022) Recent Insights Into the Role of Macrophages in Acute Gout. Front. Immunol. 13:955806. doi: 10.3389/fimmu.2022.955806
Received: 29 May 2022; Accepted: 21 June 2022;
Published: 08 July 2022.
Edited by:
Yoshiro Kobayashi, Toho University, JapanReviewed by:
Satoshi Ueha, Tokyo University of Science, JapanCopyright © 2022 Liu, Zhu, Liu, Zhao, Yu, Xue and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhen Shan, ZW1pbHlkYW5Aemp1LmVkdS5jbg==
†These authors have contributed equally to this work
 Lei Liu
Lei Liu Lingjiang Zhu1†
Lingjiang Zhu1† Li Zhao
Li Zhao Yu Xue
Yu Xue Lizhen Shan
Lizhen Shan